Abstract
Parasitism and similar life styles such as carnivorous grazing or mucus feeding without killing the prey are important in marine gastropods. Some of the most diverse living gastropod families have this feeding behavior. Taxonomic uniformitarianism is the most important tool to infer parasitism or similar life styles in fossil gastropods. The extant family groups in question (Eulimidae, Epitoniidae, Pyramidellidae, Architectonicidae, Coralliophilinae, Ovulidae, Cerithiopsidae and Triphoridae) originate mostly in the Late Cretaceous (Cerithiopsidae in the Middle Jurassic) and Paleocene. They are performing an ongoing adaptive radiation and some of the mentioned families belong to the most diverse gastropod groups forming a considerable part of marine ecosystems regarding species richness and relative abundance. At the same time, origination and radiation of the carnivorous, commonly predatory Neogastropoda took place. This points to a trophic revolution in Gastropoda that forms an important aspect of the Mesozoic Marine Revolution. Most modern parasitic gastropods are small, high-spired, show high diversity and low disparity within families and belong to Apogastropoda. By analogy, some extinct gastropod families which show the same properties might have lived parasitic too (e.g., Pseudozygopleuridae, Zygopleuridae, Meekospiridae, Donaldinidae). However, this will remain speculative to a large degree until direct host associations are found. Direct evidence for parasitism is exceptional with the Palaeozoic platyceratid/crinoid interaction being one of the best studied examples. In Gastropoda, functional shell morphology may help to identify parasitism in the fossil record but this field is scarcely studied.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Among mollusks, most parasites are gastropods. Weinstein and Kuris (2016) estimated that parasitism has evolved convergently at least nine times within Gastropoda out of more than 200 times it evolved within Metazoa. Parasitic or carnivorous grazers more or less permanently attached to their hosts form some of the most diverse living groups of gastropods. An origin of a parasitic lifestyle from carnivorous relatives seems to be a common prerequisite in metazoans, particularly in gastropods (Takano and Kano 2014). Modern groups of parasitic gastropods belong to higher gastropods (Apogastropoda) i.e., Caenogastropoda and Heterobranchia. However, I am not aware of any living parasitic patellogastropods, vetigastropods or neritimorphs (Ponder and Lindberg 1997). The living gastropod family groups Pyramidellidae, Eulimidae, Epitoniidae, Architectonicidae, Mathildidae, Coralliophilinae, Aclididae, Pedicularidae, Caledoniellidae, Ovulidae, Triviidae, Colubrariidae, Cypraeidae, Cancellariidae, Marginellidae and Pseudosacculidae are considered to represent parasites in a strict sense or contain at least one or more parasitic species (e.g., Warén 1983, p. 27, Table 6; Lorenz 2005, Table 5.5). Several of the mentioned families contain few parasitic species and/or have a scarce fossil record and are not considered here in depth.
Eulimidae feed on echinoderms and Pyramidellidae are ectoparasites feeding on the body fluids of various invertebrates, especially polychaete worms and mollusks (e.g., Ankel 1949; Robertson and Mau-Lastovicka 1979). Epitoniidae (Janthinoidea), Architectonicidae, Mathildidae and Ovulidae feed on Cnidaria (anthozoans) either as parasites or as carnivorous grazers (e.g., Kohn 1983). Coralliophilinae (Muricidae, Neogastropoda) live in and feed on corals. Robertson (1970) concluded that adaptations for feeding on Cnidaria in unrelated gastropod families include cuticularized esophaguses and proboscises, ptenoglossate-like radular teeth and total loss of radulae.
The majority of parasitic gastropods are small and high-spired with a generally low shell morphological disparity within each of the families so that alpha taxonomy (species identification and discrimination) is usually very difficult. This also limits the fossil record of parasitic gastropods: Small tiny, mostly aragonitic gastropod shells are rarely preserved at all or in sufficient quality for an identification, especially in formations of high geological age (Paleozoic and Mesozoic) (cf. Roden et al. 2019) (Table 6.1). However, there are also numerous examples in each family where species and genera deviate strongly from the standard morphology so that low disparity within families is not without exceptions. The high diversity of some of these families can be interpreted as result of a pronounced host specificity and co-evolution—but although plausible, this has yet to be demonstrated in detail. Most species of the mentioned families are ectoparasites and are attached by mucous threads or by the proboscis/snout to their hosts. Endoparasitism does occur but is seemingly rare. However, direct observations on feeding and behavior are scarce in relation to the vast diversity of these snails. Parasitic gastropods may have reduced their radula or have adapted their radula for feeding on tissue of their host.
The highly diverse generally small-sized Cerithiopsidae and sinistrally coiled Triphoridae (Triphoroidea) feed on sponges obviously without necessarily killing their prey. These spongivorous gastropods are not considered to represent parasites in a strict sense but carnivorous grazers and their relationship to sponges resembles parasitism. It is also likely that they feed on microbial biofilms and mucous associated with the sponges. They probably encompass several thousand living species. Cerithiopsidae and Triphoridae were placed in the heterogeneous, probably not-monophyletic group Ptenoglossa together with Epitoniidae and Eulimidae (e.g., Nützel 1998). Takano and Kano (2014) showed based on a molecular phylogeny that Ptenoglossa in their traditional composition are polyphyletic and that Eulimidae are the sister group of carnivorous Vanikoridae.
The census of a marine area at Koumac, an intensively studied coastal site in New Caledonia, undertaken by Bouchet et al. (2002) shows how important parasitic gastropods are in modern tropical environments. These authors counted a total of 2187 mollusk species, 270 (ca. 12%) of which belong to the parasitic Eulimidae (the third most diverse family) and Pyramidellidae (the fourth most species-rich family). The spongivorous Triphoridae and Cerithiopsidae represent the second and fifth most diverse mollusk families. Similarly, a census from the coral triangle (Panglao, Philippines) resulted in the finding of at least 715 species of Pyramidellidae from an area of 15,000 ha (150 km2) and the real number there may exceed 1100 species (Bouchet 2009). Most of these species are small (41% have adult sizes equal or below 2 mm) (Bouchet 2009). Besides Pyramidellidae, the parasitic Eulimidae and sponge-associated Cerithiopsidae and Triphoridae belong the most diverse groups.
6.2 How to Infer Parasitism in Fossil Gastropods
There are several lines of evidence to infer parasitism (see also Boucot 1990; Nagler and Haug 2015): (1) direct evidence i.e., observation of parasite and prey/host associations or traces of parasitism on fossil prey, (2) taxonomic uniformitarianism i.e., the fossil record of extant parasitic gastropod groups, usually on the family or genus level, (3) functional shell morphology i.e., a shell morphology that is suggestive of parasitism, and connected with this (4) analogy of associated phenomena e.g., the observation that modern parasitic gastropod families generally consist of small, high-spired and highly diverse groups with low disparity can be transferred to extinct groups that show similar properties.
6.2.1 Direct Observations
Direct observations of parasitism in fossil gastropods are rare. Baumiller and Gahn (2002) reported about 30 cases of parasitism from the fossil record, seven of which involved gastropods as parasites. One of the most famous examples is the interaction of platyceratid (respectively orthonychiid) gastropods and crinoids (e.g., Bowsher 1955; Boucot 1990; Webster and Donovan 2012; Baumiller and Gahn 2018) (Fig. 6.1). The cap-shaped or coiled gastropods are found on the crowns of crinoids from the Ordovician to the Permian. These gastropods are attached firmly over or near the crinoid periproct (Baumiller and Gahn 2018). Traditionally, this interaction has been interpreted as coprophagy, not damaging to the crinoid host. However, this interpretation has shifted to kleptoparasitism that hampered growth of the crinoid hosts i.e., infested crinoids are generally smaller than non-infested ones (Baumiller and Gahn 2002, 2018; Baumiller 2003; Gahn and Baumiller 2006). Baumiller and Gahn (2018) observed that “in the rare instances in which snails are found on tubed crinoids, they are never attached over the periproct but rather at the base of the tube, near the crinoid foregut. In order to access nutrients, infesting platyceratids breached the tube by drilling a circular hole through the plates of the tegmen. The development of long tubes in crinoids, which occurred multiple times, and the drilling abilities of platyceratids may represent a case of evolutionary escalation.” Sutton et al. (2006) reported a Silurian gastropod with concretionary soft body preservation and assigned it to Platyceratidae. They concluded that the anatomy suggests a sedentary life style. Accordingly, the absence of a proboscis suggests a coprophagous rather than a cleptoparasitic mode of life.
Cap-shaped platyceratid gastropod Orthonychia varians attached to the crown of the crinoid Platycrinites wachsmuthi, one of the few direct evidences of fossil parasitism; Permian, Timor (from Wanner 1922)
The Ordovician genus Cyclonema that can be found attached to tegmens of crinoids is usually assigned to Platyceratidae but its shell morphology is quite distinct from Platyceras and according to Knight et al. (1960) Cyclonema has a nacreous shell which is unknown for both, Platyceras and Orthonychia. Therefore it is not unlikely that Cyclonema is not a platyceratid and that its possibly parasitic life style attached to crinoids has evolved convergently.
Another example of direct observation of parasitism is certain drill holes or attachment scars (Boucot 1990; Boucot and Poinar 2010). Boucot (1990, p. 72) summarized several holes and malformations on Cretaceous echinid tests likely produced by eulimid gastropods. Neumann and Wisshak (2009) reported the trace fossil (drill hole) Oichnus halo on the tests of Late Cretaceous (Campanian) to Early Paleocene holasteroid echinoids. In this case the drill holes look exactly the same as those produced by the recent cap-shaped eulimid genus Thyca. The drill holes are sufficiently complex (concentric scars surmount the hole) and together with an echinoderm being the prey it is reasonable to assume that an eulimid gastropod was the producer. In other cases, traces are not sufficiently complex, e.g. simple drill holes or galls. There are several modern examples of galls produced by parasitic gastropods in skeletons of their host for instance eulimid galls in an echinoderm spine (Warén 1983) and in principal, this has a fossilization potential but according to my knowledge there are few reports of such cases from the fossil record (Boucot 1990; Baumiller and Gahn 2002; Boucot and Poinar 2010). Breton et al. (2017) interpreted a bioerosion trace fossil on Cenomanian oysters as product of parasitic gastropods.
Hayami and Kanie (1980) reported large Late Cretaceous (Campanian) limpets (Gigantocapulus) in association with inoceramid bivalves. In analogy with some recent gastropod limpets of the family Capulidae, they interpreted this as parasitism (see also Baumiller and Gahn 2002). In the absence of protoconch and shell micro-structure the assignment to Capulidae of this limpet is doubtful. Beu (2007) noted that the systematic placement of Gigantocapulus is doubtful and placed it tentatively in the caenogastropod superfamily Vanikoroidea but stated it could also represent Monoplacophora. This author stated an epiparasitic mode of life was possible but a filter feeding sedentarily on bivalves was more likely.
Petit et al. (2014) reported gastropods attached to fossil fishes, but their attribution to scavengers or parasites is not straightforward. They putatively assigned the gastropod specimens to Aclis which is normally not associated with fishes.
6.2.2 Taxonomic Uniformitarianism
The most commonly used tool to infer parasitism in fossil gastropods is taxonomic uniformitarianism. If for instance all extant pyramidellids are ectoparasites, it is plausible and the most parsimonious assumption that fossil pyramidellid species were parasites too and that this feeding type is a synapomorphy of this clade. Difficulties arise when assignment of fossil species is uncertain and it may be impossible to establish the life style of fossil sister-groups of parasitic families or genera and hence the timing of the acquirement of the parasitic life style in evolutionary lineages. It is also generally true that taxonomic uniformitarianism becomes increasingly problematic the higher the geological age is. As stated above, modern groups of parasitic gastropods belong to ‘higher’ gastropods (Apogastropoda) i.e., Caenogastropoda and Heterobranchia and the mentioned families are usually present since the Late Cretaceous or Paleocene. Hence, it is evident that parasitism has been pervasive at least since the Paleocene.
In the following the most important parasitic families (or those having a similar lifestyle) are mentioned including their host and fossil record as currently known. This account is not meant to be complete but covers the most diverse groups.
6.2.2.1 Eulimidae
Eulimidae (Caenogastropoda) are mostly ectoparasites that feed on echinoderms (holothurians, sea urchins, sea stars). They are attached to their host with their proboscis or snout more or less permanently (Warén 1983) and can be embedded in the tissue of their host (endoparasitic). Many eulimids have reduced the radula (Aglossa) as result of their parasitic life style. Those eulimids that possess a radula have needle-shaped teeth (Warén 1983) and hence resemble the ptenoglossate radula of Janthinoidea including Epitoniidae. The majority of eulimid species are small, high-spired and have a glossy, extremely smooth shell (Fig. 6.2a, d). However, there is considerable variation regarding shell morphology within this family including cap-shaped or turbiniform morphologies and strongly ornamented forms (e.g., Warén 1983). Some eulimids have abandoned the shell entirely and became worm-like - one of the very few cases in Caenogastropoda (Ponder et al. 2008). Eulimids are very diverse but their alpha taxonomy is notoriously difficult because of their small size and the limited disparity of shell morphology among many species of this group. The smooth glossy shell can be interpreted as an adaption to the parasitic life style .
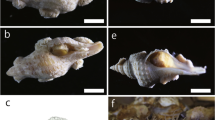
Modern eulimids (a, d) and similar Late Palaeozoic forms (b, c, e) representing Meekospiridae. Similar shell morphology, smooth shells and small size could suggest a similar mode of life i.e., parasitism. (a) Recent eulimid with twisted outline, Lizard Island, Queensland, Australia. (b) Ceraunocochlis fulminula, with likewise twisted shell axis, Pennsylvanian, Texas (Nützel et al. 2000). (c) Ceraunocochlis sp. Mississippian, Indiana (Nützel et al. 2000). (d) Recent eulimid, Lizard Island, Queensland, Australia. (e) Pennsylvanian Meekospira sp., Pennsylvanian, Texas (Nützel et al. 2000). Scale bars 1 mm
The number of living eulimid species is unknown but certainly >1000. The fossil record shows that the family Eulimidae has a minimum age of Campanian (Dockery 1993)—older reports are doubtful. Eulimids have become increasingly diverse and abundant throughout the Cenozoic (Warén 1983). Some eulimids display a pronounced convergence with Late Palaeozoic subulitoid shells representing the genera Meekospira and Ceraunocochlis (Fig. 6.2) but the Carboniferous species have a blunt apex/protoconch instead of a pointed larval shell as found in Eulimidae .
6.2.2.2 Epitoniidae
Epitoniidae (wenteltraps) is a highly diverse caenogastropod family that is associated with or feeds on cnidarians. They feed and live in association with anthozoans, such as species of Actiniaria, Zoantharia or Scleractinia (e.g., Gittenberger and Hoeksema 2013 and references therein). Most are considered to be ectoparasitic, others as carnivorous grazers respectively foraging predators or as symbionts (e.g., Robertson 1980; Gittenberger and Hoeksema 2013). Robertson (1980) observed that an Epitonium species lives in symbiosis with the Coelenterates feeding, together with another gastropod species representing Coralliophila, on mucous, nematocysts and zooxanthellae of its host bot not on its tissue of polyps. Bandel (1991) reported that larger epitoniids feed with their proboscis on anthozoans until their prey dies. Gittenberger and Gittenberger (2005) observed that epitoniids are attached to the underside of mushroom corals by mucous threads. They also found that epitoniid species have specialized on one or few host species .
More than 700 recent epitoniid species have been described validly (Brown and Neville 2015). The diversity may be much greater because unexplored cryptic radiations are likely (cf. Gittenberger and Gittenberger 2005). Epitoniids have small to medium sized shells commonly with lamellar axial ribs and a holostomatous aperture (Fig. 6.3a). Epitoniids are unusual for caenogastropods in having a calcitic shell. They possess the ptenoglossate radula with needle-shaped teeth. Epitoniidae have a smooth larval shell (Fig. 6.3a). By contrast, Nystiellidae, the likewise parasitic sister-group of Epitoniidae, have a larval shell with axial ribs.
(a) Recent epitoniid Cycloscala hyaline with smooth larval shell and uncoiled teleoconch with lamellar axial ribs (from Sasaki 2008, fig. 10b). (b) Pennsylvanian pseudozygopleurid Helminthozyga vermicula with axially ribbed larval shell and uncoiled teleoconch convergent to epitoniid in a—this could point to parasitism of this Palaeozoic species (from Nützel 1998). (c) Recent pyramidellid, with pronounced folds on inner lip of aperture, Sulawesi, Indonesia. (d) Late Triassic zygopleurid, possibly parasitic; Cassian Formation, Italy. Scale bars (a): 0.5 mm, (b): 0.3 mm, (c, d): 1 mm
Previous reports of a Middle Jurassic first occurrence of Epitoniidae (Cossmann 1912; Tracey et al. 1993) are doubtful because this species, Proacirsa inornata, has smooth whorls and its protoconch is unknown. Gründel (2012) placed Proacirsa in the heterobranch family Gordenellidae and hence outside Epitoniidae. The oldest known members of Epitoniida with smooth larval shell have a Campanian age (Dockery 1993). Guzhov (2006) reported a nystiellid from the Upper Jurassic of Russia. It has an axially ribbed protoconch and teleoconch and thus resembles the Triassic zygopleurid subfamily Ampezzopleurinae. Nützel (1998) suggested that Epitoniidae arose from Ampezzopleurinae (Zygopleuridae) via Nystiellidae.
6.2.2.3 Coralliophilinae (Muricidae)
Coralliophilinae, a subfamily of Muricidae (Bouchet et al. 2017), is a moderately diverse, small to medium-sized caenogastropod group with approximately 200–250 living species (Marshall and Olivero 2009). The shell morphology including ornamentation is quite variable. Coralliophilinae have reduced the radula and live parasitic on and in coelenterates (Robertson 1970). The genus Magilus lives within stony corals and builds a tube within it (Fig. 6.4). Gittenberger and Gittenberger (2011) reported a cryptic radiation of the coralliophilin endoparasitic genus Leptoconchus. The species live in parasitic association with mushroom corals.
Coralliophilinae are known since the Late Cretaceous (Sohl 1964). Lozouet and Renard (1998) reported one of the oldest members boring in corals from the Oligocene of the Paris Basin .
6.2.2.4 Pyramidellidae
Pyramidellidae are ectoparasites that feed on body fluids of their hosts and have reduced the radula (e.g. Robertson and Mau-Lastovicka 1979; Healey 1998a; Ponder and De Kayser 1998). Most of the verified hosts are polychaetes, gastropods and bivalves, but there also appear to be various minor host groups such as polyplacophorans and echinoderms (Robertson and Mau-Lastovicka 1979). Pyramidellidae are one of the most diverse gastropod families with more than 6000 living species (Lygre and Schander 2010). Bouchet (2009) reported that 715 species are present in the Philippines in an area of 15,000 ha (c. 150 km2) of coastal habitats to a depth of 150 m.
Most pyramidellids are small (<10 mm) and have a high-spired shell with one or more columellar plaits in the aperture (Fig. 6.3c). Shell shape and ornament are quite variable in this family (see Bouchet 2009 for some examples). As heterobranchs they have heterostrophic, sinistral protoconchs which help to separate them from convergent caenogastropods.
(a) Recent sinistral triphorid from Australia, Queensland, Lizard Island (from Nützel 1998). (b, c) Eocene sinistral triphorid Ogivia singularis (J13084) from the Paris Basin. (c) Detail of teleoconch ratchet sculpture of spirals cords, anteriorly rounded, gently sloping and posteriorly steep, arrow points in anterior direction . (d) Danian sinistral triphorid from Denmark with tube-like anterior and posterior siphonal canals. (e) Recent sinistral triphorid from Australia, Queensland, Lizard Island, with tube-like anterior and posterior siphonal canals (from Nützel 1998). (f) Danian cerithiopsid with preseved larval shell (from Nützel 1998). (g, h) Recent barrel-shaped cerithiopsid with slender, smooth larval shell from Australia, Queensland, Lizard Island. (h) Detail of teleoconch ratchet sculpture of spirals cords, anteriorly rounded, gently sloping and posteriorly steep, arrow points in anterior direction. Scale bars (a), (b), (d), (e), (g): 1 mm; (c), (h): 0.2 mm, (f): 0.3 mm
The fossil record of Pyramidellidae is hard to track; the earliest finds in the PBDB dating from the Palaeozoic and Triassic refer to the modern genus Chemnitzia and these assignments are all outdated—Chemnitzia was used as a waste basket taxon, especially in the nineteenth century. Based on Sohl (1964), Tracey et al. (1993) gave a Maastrichtian FAD. Kaim (2004) placed the Middle Jurassic Chrysallida minuera (Late Bathonian, Poland) in Pyramidellidae and the similarity to modern Chrysallida is indeed stunning although a distinct columellar fold has not been shown; but is has an incipient bulge similar to that of some Recent pyramidellid species. Nevertheless, a Late Bathonian age can be accepted as FAD for Pyramidellidae since folds are also absent or weak in some Recent Pyramidellidae. This relatively old fossil record is somewhat surprising given molecular studies that suggest that they are deeply rooted in Pulmonata (Dinapoli et al. 2011; Schrödl et al. 2011) which would be more congruent with a younger age. Amathinidae Ponder (1987) (Pyramidelloidea), that are considered to be parasites on bivalves today, were reported by Kaim (2004) from the Early Cretaceous .
6.2.2.5 Architectonicidae and Mathildidae
Architectonicidae are considered to be ectoparasites of colonial cnidarians such as zoantharian, scleractinian and antiparian corals (Healey 1998b and references therein). They show buccal and radular specializations for feeding on hexacorallian coelenterates such as stony corals, sea anemones and zoanthids (Bieler and Petit 2005). A large number of species names have been proposed but according to Bieler and Petit (2005) only about 140 extant species are valid. Their attractive shells are conical and comparatively low-spired (some even planispiral) with an umbilicated base—thus they are unlike most other parasitic gastropod according to shell morphology. Their shell morphology resembles that of Trochidae but they can be separated from them by having a heterostrophic larval shell and lacking nacre.
Bandel (1988) placed two Late Triassic (Carnian) genera (Amphitomaria and Rinaldoconchus) from the Cassian Formation in Architectonicidae which represents their first appearance date according to the current state of knowledge (Tracey et al. 1993). Attributions of Palaeozoic shells to Architectonicidae are erroneous (Bieler and Petit 2005).
Architectonicidae and the high-spired Mathildoidea are considered to be closely related and are sometimes placed in the same superfamily or as separate superfamilies. Mariottini et al. (2009) stated that “these mollusks are associated to cnidarians for feeding habit”. However, the feeding habit of this group is poorly known. If Mathildidae are parasitic or have a similar life style, it would be an important group representing this guild, especially in the Mesozoic. Mathildidae are richly diversified during the Mesozoic at least from the Late Triassic onward and are still present today but more in deeper water environments than in shallow water as it used to be earlier (Bandel 1995; Gründel and Nützel 2013; Bieler 1995). In the Late Triassic Cassian Formation, Mathildidae were much more diverse and abundant than in any site today .
6.2.2.6 Triphoridae and Cerithiopsidae: Carnivorous Grazers (Sponge Feeders)
Triphoridae and Cerithiopsidae are super-diverse gastropod groups that live on sponges (Fig. 6.5). They actually feed on sponge tissue of demosponges—stomach and fecal pellets may contain sponge spicules (Nützel 1998; Fig. 6.6). They also feed on mucous on the sponges. There are observations for some living species that individuals are attached to sponges with mucous threads and that they deposit spawn on or within sponges (Nützel 1998; Fernandes and Pimenta 2019; see Fig. 6.6). Lorenz (2005) reported cypraeid egg clusters in sponges. So in a wider sense, Triphoridae and Cerithiopsidae can be considered ectoparasites, living on their host without necessarily killing it. In any case, all available evidence suggests a close relationship of these gastropods to their host sponges. There are hints that they are not obligate sponge eaters but it seems that sponges are the preferred source of food. At least they belong, as Turridae and other gastropods, “in guilds of commensals, associates and parasites” (Bouchet et al. 2009). It must be emphasized that feeding observations are scarce covering few species so that generalizations must be considered with caution.
Spawn, stomach contents, fecal pellet and radula of Recent Triphoroidea (from Nützel 1998). (a–d): Egg chamber of Cerithiopsis sp. in the sponge Suberites domunculus, Recent, Mediterranean (a, b). Same view, surface of sponge showing sealed opening (dashed line) with an cerithiopsid embryonic shell emerging from the chamber (arrow). (c, d). Egg chamber in transverse section with several cerithiopsid embryonic shells. (e, f) Sponge spicules from a stomach of a cerithiopsid. (g) Shrinked fecal pellet of a triphorid containing sponge spicules. (h) Rhinioglossate radula of a Triphorinae species, R rachidian, L lateral, M1–M6 marginal teeth. Scale bars (a–d): 0.3 mm, (e): 50μm, (f): 3μm, (g): 30μm, (h): 10μm
Triphoridae and Cerithiopsidae are small (usually <10 mm), high-spired or barrel shaped and commonly have a constricted body whorl in fully-grown specimens (Fig. 6.5). The great majority of species in both families share a reticulate teleoconch ornament, commonly with asymmetrical knobby spiral cords that are rounded and shallowly sloping anteriorly and steeply sloping posteriorly (Fig. 6.5c, h). This represents probably a ratchet sculpture which facilitates penetration of the sponge tissue and prevents uncontrolled back-slipping. Signor (1982) reported similar ornaments for burrowing gastropods. Commonly cerithiopsids and triphorids have a constricted body whorl and/or a rounded base resulting in an overall elongated pear-shape which may have facilitated intrusion into the host sponge tissue (Fig. 6.5a, d, e, g). Triphorids and Cerithiopsidae sensu strictu have a minimum Paleogene age (see below).
6.2.2.6.1 Triphoridae
Triphoridae encompass at least 1000 living species, many of them undescribed. Fernandes and Pimenta (2017) counted more than 600 recent species based on the WORMS database (http://www.marinespecies.org/). Most species belong to the sinistrally coiled subfamily Triphorinae. They have a complex aperture with an anterior and a posterior siphonal canal, sometimes both are tube-like (Fig. 6.5d, e). The posterior siphonal canal forms the outlet for special tentacles emerging form the mantle cavity that may help to ventilate it (Nützel 1998). Triphoridae possess the rhinioglossate radula—the number of teeth per row varies from 5 to 63 (Marshall 1983) (Fig. 6.6h).
Undoubted sinistrally coiled Triphoridae (Triphorinae) are present since the Danian (e.g., Ravn 1933; Nützel 1998; Wesenberg and Schnetler 2014) (Fig. 6.5d) and few Late Cretaceous forms have been reported but those need confirmation (e.g., Kaunhowen 1897; Nützel 1998; Kaim 2004).
6.2.2.6.2 Cerithiopsidae
Cerithiopsidae is another highly diverse family of spongivorous small, high-spired caenogastropods. Cerithiopsidae may closely resemble non-parasitic small Cerithioidea for instance the genus Bittium and protoconch morphology is needed to separate both groups. However, protoconch preservation is rare, especially in fossil gastropods (e.g., Nützel 2014). Cerithiopsidae are taenioglossate having seven teeth per row. Within the group, the tooth morphology is extremely variable, some having strongly elongated marginal teeth (e.g. Marshall 1978; Nützel 1998). It is obvious that this represents an adaptation to the feeding on sponges but feeding mechanisms have not been studied.
Cerithiopsidae sensu strictu are at least as old as Danian and are richly diversified in the Danian Naesekalk from Denmark (Ravn 1933; Nützel 1998; Wesenberg and Schnetler 2014) (Fig. 6.5f). The higher category Triphoroidea (respectively Cerithiopsoidea) is older with a good fossil record from the Cretaceous and some Jurassic representatives with the earliest record from the Bathonian (Nützel 1998 and references therein). The Triassic Protorculidae (earliest record Early Triassic, Smithian) probably represent its fossil sister group (Nützel 1998; Nützel and Erwin 2002).
6.2.2.7 Ovulidae
According to Wilson (1998) Ovulidae feed on the polyps of cnidarians and belong thus to the guild of carnivorous grazers and they are considered to be parasites sometimes. They belong to Cypraeoidea and have the convolute shell with genera like Cypraea. They are of moderate diversity (400 species according to Lorenz 2005) and most species occur in the tropical Indo-Pacific.
Ovulidae originate at the latest in the Late Cretaceous according to Tracey et al. (1993).
6.2.3 Functional Shell Morphology
Functional shell morphology is a potential tool to infer parasitism in fossil gastropods. However, this has not been explored and functional morphology of the gastropod shell is generally not well understood. As outlined above, the ratchet sculpture of triphorids and cerithiopsids as well as their anteriorly constricted and rounded shell may facilitate intrusion into the tissue of the host (Fig. 6.5). The extremely smooth, polished shell with very shallow sutures of many eulimids (Fig. 6.2a, d) forms certainly an adaptation regarding the parasitic life style. This shell morphology is also present in Palaeozoic subulitoid gastropods, such as Ceraunocochlis (Fig. 6.2b, c); it is a reasonable speculation that such fossil gastropods were parasitic too (see below).
Gahn and Baumiller (2006) argued that the elongation of the anal tubes of Palaeozoic crinoids had the function to hinder infestations by parasitic platyceratids.
6.2.4 Analogy Based on Associated Phenomena
Taxonomic uniformitarianism fails if gastropod families are extinct, have a relatively high geological age and if the closest living relatives are unknown. Most extant parasitic and carnivorous grazer gastropods are ectosymbiotic and do not leave fossil traces. If, for instance, Pyramidellidae were an extinct Mesozoic or Paleozoic group, their parasitic life style would have remained unrecognized because there is no fossil evidence for it and their preferred host polychaetes have a relatively poor fossil record. The same is true for Triphoridae and Cerithiopsidae. On the other hand, it seems to be unlikely (though not impossible) that a parasitic or similar life style was only present in the mentioned extant highly diverse gastropod families whereas this life style was largely absent or rare in the Palaeozoic and Early Mesozoic. As mentioned, extant families exhibit several traits and if these traits can be found in fossil gastropods, a parasitic life style can be hypothesized by analogy. These traits are as follows:
-
belonging to Caenogastropoda or Heterobranchia (Apogastropoda)
-
high within family diversity
-
low shell disparity within families
-
mostly small
-
mostly high-spired
Needless to say that the above-mentioned traits are also present in some non-carnivorous or non-parasitic gastropod families (for instance Rissoiidae) and therefore do not represent unequivocal evidence but they do offer the possibility that certain extinct families which display them were parasitic. Potentially, discoveries of rare host associations or effects of parasitic on growth could potentially corroborate such hypotheses. The mentioned traits are present in the following fossil gastropod families which therefore could have had a parasitic life style:
Pseudozygopleuridae | Carboniferous (Tournaisian)–Early Triassic (Griesbachian) |
Zygopleuridae | Early Triassic (Smithian)–Cretaceous (Albian) |
Protorculidae | Triassic (Smithian)–Middle Jurassic |
Donaldinidae | Middle Devonian (Givetian)–Triassic (Carnian)a |
Streptacididae | Carboniferous (Tournaisian)–Cretaceous (Campanian) |
Meekospiridae | Carboniferous (Tournaisian)–Permian (Changhsingian) |
Pseudozygopleuridae are small caenogastropods with a worldwide distribution ranging from the Carboniferous to the Early Triassic (Nützel 1998; Foster et al. 2017). Most are high-spired and have a teleoconch ornament of axial ribs, and generally a low disparity with many species differing only in small details. The genus Helminthozyga shows uncoiling of teleoconch whorls and resembles extant members of Epitoniidae in this respect and could point to a parasitic lifestyle (Bandel 1991; Sasaki 2008) (Fig. 6.3a, b).
Zygopleuridae range from the Early Triassic (Nützel and Schulbert 2005; Foster et al. 2017) to the Cretaceous (Albian) (Kiel 2006). They closely resemble Palaeozoic Pseudozygopleuridae but differ in larval ornaments (Bandel 1991; Nützel 1998). Undoubtedly, both families are closely related to each other and are included in Pseudozygopleuroidea that may represent the “stem-group” of modern Ptenoglossa (Bandel 1991; Nützel 1998).
Protorculidae are known from the Early Triassic to the Middle Jurassic, especially from the Carnian Cassian Formation (Bandel 1991; Nützel 1998; Nützel and Erwin 2002; Kaim and Conti 2010). They have an anterior siphonal channel and may have a complex teleoconch ornamentation. They share an axially ribbed larval shell with other Zygopleuroidea as well as with living Nystiellidae and basal Cerithiopsidae and were identified as fossil sister group of cerithiopsids (Nützel 1998). It is possible that they had acquired a spongivorous lifestyle already.
Donaldinidae and Streptacididae are small, high-spired, sometimes very slender gastropods with a heterostrophic larval shell. They range from the Devonian to the Cretaceous but their shell morphology is very close to living genera such as Murchisoniella or Ebala and it thus might be that they persist until today (Bandel 2005; Warén 2013). Their small size, high diversity but low disparity may point to a parasitic life style similar to that of living Pyramidellidae.
Amongst the heterogeneous, largely Palaeozoic group Subulitoidea, there are some diverse genera and families which closely resemble extant parasitic eulimids. Amongst Meekospiridae (Carboniferous–Permian) the genera Ceraunocochlis and Meekospira closely resemble recent eulimid species (Fig. 6.2). They share the smooth surface, shallow suture and high-spired shape and in the case of Ceraunocochlis the bent spire producing an asymmetrical shape is also present in several eulimid genera.
6.3 Conclusions
Direct evidence for parasitism of gastropods such as preserved host/parasite associations or characteristic trace fossils in the host is anecdotic at best in relation with the vast diversity of this class. One of the few exceptions is the Palaeozoic platyceratid/crinoid interaction that has a fairly good record of examples where host and parasite are still attached to each other and additional borings. Most modern highly diverse parasitic (or carnivorous grazers) gastropod families have a Late Mesozoic/Paleocene origin and seem to undergo a major adaptive radiation. At present, taxonomic uniformitarism is the most important tool to infer the fossil record of gastropod parasites. This works for the Cenozoic and Late Mesozoic. Few lines can be traced into the Jurassic and Triassic with some confidence. Several diverse older, extinct families of tiny, high-spired Caenogastropoda and Heterobranchia might have been parasitic but this remains speculative to some degree at this point. Parasitism and similar feeding types are major trophic modes in modern gastropods and is present in as many as 10,000 extant species with Pyramidellidae being most diverse. This underlines the trophic diversity of gastropods which is most likely one of the major reasons for their evolutionary success.
References
Ankel WE (1949) Die Nahrungsaufnahme der Pyramidelliden. Verhandlungen der Deutschen Zoologen, Kiel 1948:478–484
Bandel K (1988) Repräsentieren die Euomphaloidea eine natürliche Einheit der Gastropoden? Geol-Paläont Inst Univ Hamburg 67:1–33
Bandel K (1991) Über triassische "Loxonematoidea" und ihre Beziehungen zu rezenten und paläozoischen Schnecken. Paläontol Z 65:239–268
Bandel K (1995) Mathildoidea (Heterostropha, Gastropoda) from the Upper Triassic St. Cassian formation. Scr Geol 111:1–83
Bandel K (2005) Living fossils among tiny allogastropoda with high and slender shell from the reef environment of the Gulf of Aquba with remarks on fossil and recent relatives. Geol-Paläont Inst Univ Hamburg 89:1–24
Baumiller TK, Gahn, FJ (2002) Fossil record of parasitism on marine invertebrates with special emphasis on the platyceratid-crinoid interaction. In: Kowalewski M, Kelley PH (eds) The fossil record of predation. The Paleontological Society Papers, 8. p 195–209
Baumiller TK, Gahn F (2018) Biotic interactions between platyceratid gastropods and crinoids: a review and new insights. Abstract. In: 5th international paleontological congress, Paris. p. 456
Baumiller TK (2003) Evaluating the interaction between platyceratid gastropods and crinoids: a cost-benefit approach. Palaeogeography, Palaeoclimatology, Palaeoecology 201:199–209
Beu AG (2007) The “Inoceramus limpet” Gigantocapulus problematicus (Nagao & Otatume, 1938) in New Zealand (Late Cretaceous Gastropoda or Monoplacophora, Gigantocapulidae n. fam). Paläontol Z 81:267–282
Bieler R (1995) Mathildidae from New Caledonia and the Loyalti Islands (Gastropoda: Heterobranchia). Mém Mus Natl Hist Nat 167:595–641
Bieler R, Petit RE (2005) Cataloque of recent and fossil taxa of the family Architectonicidae Gray, 1850 (Mollusca: Gastropoda). Zootaxa 1101:1–119
Bouchet P (2009) From specimens to data, and from seashells to molluscs: the Panglao Marine Biodiversity Project. Vita Malacol 8:1–8
Bouchet P, Lozouet P, Maestrati P, Heros V (2002) Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol J Linn Soc 75:421–436
Bouchet P, Lozouet P, Sysoev A (2009) An inordinate fondness for turrids. Deep-Sea Res 56:1724–1731
Bouchet P, Rocroi J-P, Hausdorf B, Kaim A, Kano Y, Nützel A, Parkhaev P, Schrödl M, Strong EE (2017) Revised classification, nomenclature and typification of gastropod and monoplacophoran families. Malacologia 61:1–526
Boucot AJ (1990) Evolutionary paleobiology of behavior and coevolution. Elsevier, Amsterdam, 725 p
Boucot AJ, Poinar GO Jr (2010) Fossil behavior compendium. CRC Press, Boca Raton, 391 p
Bowsher AL (1955) Origin and adaptation of platyceratid gastropods. KU Paleontol Contrib Mollusca 5:1–11
Breton G, Wisshak M, Néraudeau D, Morel N (2017) Parasitic gastropod bioerosion trace fossil on Cenomanian oysters from Le Mans, France and its ichnologic and taphonomic context. Acta Palaeontol Pol 62:45–57
Brown LG, Neville BD (2015) Catalog of the recent taxa of the families Epitoniidae and Nystiellidae (Mollusca: Gastropoda) with a bibliography of the descriptive and systematic literature. Zootaxa 3907:1–188
Cossmann M (1912) Essais de paléoconchologie comparée, vol 9. Published by the author and J. Lamarre and Cie, Paris, 215 p
Dinapoli A, Zinssmeister C, Klussmann-Kolb A (2011) New insights into the phylogeny of the Pyramidellidae (Gastropoda). J Molluscan Stud 77:1–7
Dockery DT (1993) The streptoneuran gastropods, exclusive of the Stenoglossa, of the Coffe Sand (Campanian) of northeastern Mississippi. Mississippi Department of Environmental Quality, Office of Geology, Bulletin 129:1–191
Fernandes MR, Pimenta AD (2019) Taxonomic review of Inella and Strobiligera (Gastropoda: Triphoridae) from Brazil. Zootaxa 4613:1–52
Foster WJ, Danise S, Twitchett RJ (2017) A silicified Early Triassic marine assemblage from Svalbard. J Syst Palaeontol 15:851–877
Fernandes MR, Pimenta AD (2017) Taxonomic overview of Triphoridae in the world: describing triphorids. Informativo SBMa, Ano 48, nº200 (jun/2017):4–10
Gahn JG, Baumiller TK (2006) Using platyceratid gastropod behaviour to test functional morphology. Historical Biology 18:397–404
Gittenberger A, Gittenberger E (2005) A hitherto unnoticed adaptive radiation: epitoniid species (Gastropoda: Epitoniidae) associated with corals (Scleractinia). Contrib Zool 74:125–203
Gittenberger A, Gittenberger E (2011) Cryptic, adaptive radiation of endoparasitic snails: sibling species of Leptoconchus (Gastropoda: Coralliophilidae) in corals. Org Divers Evol 11:21–41
Gittenberger A, Hoeksema BW (2013) Habitat preferences of coral-associated wentletrap snails (Gastropoda: Epitoniidae). Contrib Zool 82:1–25
Gründel J (2012) Neubearbeitung der von Laube 1867 beschriebenen Gastropodenfauna aus dem mittleren Jura von Balin/Polen. Ann Naturhist Mus Wien 114:193–288
Gründel J, Nützel A (2013) Evolution and classification of Mesozoic mathildoid gastropods. Acta Palaeontol Pol 58:803–826
Guzhov AV (2006) Liapinella, a new epitonioidean genus (Gastropoda) from the Upper Jurassic of European Russia. Ruthenica 16:1–4
Hayami I, Kanie Y (1980) Mode of life of a giant capulid gastropod from the Upper Cretaceous of Saghalien and Japan. Palaeontology 23:689–698
Healey JM (1998a) Superfamily Pyramidelloidea. In: Beesley PL, GJB R, Wells A (eds) Mollusca: the southern synthesis, part B. Fauna of Australia. CSIRO Publishing, Melbourne, pp 865–869
Healey JM (1998b) Superfamily Architectonicoidea. In: Beesley PL, GJB R, Wells A (eds) Mollusca: the southern synthesis, part B. Fauna of Australia. CSIRO Publishing, Melbourne, pp 858–862
Kaim A (2004) The evolution of conch ontogeny in Mesozoic open sea gastropods. Palaeontol Pol 62:1–182
Kaim A, Conti MA (2010) A problematic zygopleuroid gastropod Acanthostrophia revisited. Zitteliana A50:21–24
Kaunhowen F (1897) Die Gastropoden der Maestricher Kreide. Palaeontologische Abhandlungen, Neue Folge 4(der ganzen Reihe Band 8):1–132
Kiel S (2006) New and little-known gastropods from the Albian of the Mahajanga Basin, Northwestern Madagascar. J Paleontol 80:455–476
Knight JB, Cox LR, Keen AM, Smith AG, Batten RL, Yochelson EL, Ludbrook NH, Robertson R, Yonge CM, Moore RC (1960) Mollusca - General Features, Scaphopoda, Amphineura, Monoplacophora, Gastropoda. General Features, Archaeogastropoda and some (mainly Paleozoic) Caenogastropoda and Opisthobranchia. Geological Society of America & University of Kansas Press, Lawrence, xxiii +351 pp.
Kohn AJ (1983) Feeding biology of Gastropoda. In: Wilbur KM (ed) The Mollusca, vol. 5, physiology. Academic, New York, pp 1–64
Lorenz F (2005) Mollusca (molluscs). In: Rohde K (ed) Marine parasitology. CABI Publishing, Oxon, pp 240–245
Lozouet P, Renard P (1998) The Coralliophilidae, gastropoda from the oligocene and lower miocene of the aquitaine (southwestern france): systematics and details of coral hosts. Geobios 31:171–184
Lygre F, Schander C (2010) Six new species of pyramidellids (Mollusca, Gastropoda, Pyramidelloidea) fromWest Africa, introducing the new genus Kongsrudia. Zootaxa 2657:1–17
Mariottini P, Smriglio C, Di Giulio A (2009) Two new mathildids from the south-eastern coast of Africa (Gastropoda, Heterobranchia, Mathildidae). Basteria 73:69–76
Marshall BA (1978) Cerithiopsidae (Mollusca: Gastropoda) of New Zealand, and a provisional classification of the family. New Zealand J Zool 5:47–120
Marshall BA (1983) A revision of the recent Triphoridae of Southern Australia (Mollusca: Gastropoda). Rec Aust Mus Suppl 2:1–119
Marshall BA, Olivero M (2009) The recent Coralliophilinae of the New Zealand region, with descriptions of two new species (Gastropoda: Neogastropoda: Muricidae). Mollusc Res 29:155–173
Nagler C, Haug JT (2015) From fossil parasitoids to vectors: insects as parasites and hosts. In: De Baets K, Littlewood T (eds) Fossil parasites, advances in parasitology 90. Elsevier, Amsterdam, pp 137–200
Neumann C, Wisshak M (2009) Gastropod parasitism on Late Cretaceous to Early Paleocene holasteroid echinoids—evidence from Oichnus halo isp. n. Palaeogeogr Palaeoclimatol Palaeoecol 284:115–119
Nützel A (1998) Über die Stammesgeschichte der Ptenoglossa (Gastropoda). Berliner Geowissenschaftliche Abhandlungen. Reihe E 26:1–229
Nützel A (2014) Larval ecology and morphology in fossil gastropods. Palaeontology 57:479–503
Nützel A, Erwin DH, Mapes RH (2000) Identity and phylogeny of the late Paleozoic Subulitoidea (Gastropoda). J Paleontol 74:575–598
Nützel A, Schulbert C (2005) Gastropod lagerstätten in the aftermath of the end-Permian mass extinction—diversity and facies of two major Early Triassic occurrences. Facies 51:495–515
Nützel A, Erwin DH (2002) Battenizyga, a new early Triassic gastropod genus with a discussion on the gastropod evolution at the Permian/Triassic boundary. Paläontologische Zeitschrift 76:21–26
Petit G, Robin N, Zorzin R, Merle D (2014) Fossil gastropods (?Aclis aenigmaticus n. sp.) on a fish from the Pesciara of Bolca Lagerstätte (Eocene, Northern Italy): an enigmatic association. Studi e ricerche sui giacimenti terziari di Bolca, XV - Miscellanea paleontologica 12:129–136
Ponder WF (1987) The anatomy and relationships of the pyramidellacean limpet, Amathina tricarinata (Mollusca: Gastropoda). Asian Marine Biol 4:1–34
Ponder WF, Colgan DJ, Healy JM, Nützel A, Simone LRL, Strong EE (2008) Caenogastropoda. In: Ponder WF, Lindberg DL (eds) Phylogeny and evolution of the Mollusca. University of California Press, Berkeley, pp 331–383
Ponder WF, De Kayzer RG (1998) Superfamily Pyramidelloidea. In: Beesley PL, Ross GJB, Wells A (eds) Mollusca: the southern synthesis, part B. Fauna of Australia. CSIRO Publishing, Melbourne, pp 865–869
Ponder WF, Lindberg DR (1997) Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zool J Linnean Soc 119:83–265
Ravn JPJ (1933) Études sur les Pélécypodes et Gasteropodes Daniens. K dansk Vidensk Selsk Skr 9(2):1–71
Robertson R (1970) Review of the predators and parasites of stony corals with special reference to symbiotic prosobranch gastropods. Pac Sci 24:43–54
Robertson R (1980) Epitonium millecostatum and Coralliophila clathrata: two prosobranch gastropods symbiotic with Indo-Pacific Palythoa (Coelenterata: Zoanthidae). Pac Sci 34:1–17
Robertson R, Mau-Lastovicka T (1979) The ectoparasitism of Boonea and Fargoa (Gastropoda: Pyramidellidae). Biol Bull 157:320–333
Roden VJ, Hausmann IM, Nützel A, Seuss B, Reich M, Urlichs M, Hagdorn H, Kiessling W (2019) Towards an assessment of true biodiversity in fossil ecosystems—the role of liberation lagerstätten. Palaeontology 1–18. https://doi.org/10.1111/pala.12441
Sasaki T (2008) Micromolluscs in Japan: taxonomic composition, habitats, and future topics. Zoosymposia 1:147–232
Schrödl M, Jörger KM, Klussmann-Kolb A, Wilson NG (2011) Bye bye “Opisthobranchia”! A review on the contribution of mesopsammic sea slugs to euthyneuran systematics. Thalassas 27:101–112
Signor PW (1982) Constructional morphology of gastropod ratchet sculpture. Neues Jahrb Geol Palaontol Abh 163:349–368
Sohl NF (1964) Neogastropoda, Opisthobranchia, and Basommatophora of the Ripley, Owl Creek and Prairie Bluff formations. U S Geol Surv Prof Pap 331-B:153–344
Sutton MD, Briggs DEG, Siveter DJ, Siveter DJ (2006) Fossilized soft tissues in a Silurian platyceratid gastropod. Proc R Soc B Biol Sci 273(1590):1039–1044
Takano T, Kano Y (2014) Molecular phylogenetic investigations of the relationships of the echinoderm-parasite family Eulimidae within Hypsogastropoda (Mollusca). Mol Phylogenet Evol 79:258–269
Tracey S, Todd JA, Erwin DH (1993) Mollusca: Gastropoda. In: Benton MJ (ed) The fossil record, vol 2. Chapman & Hall, New York, pp 131–167
Wanner C (1922) XVIII. Die Gastropoden und Lamellibranchiaten der Dyas von Timor. In: Wanner J (ed) Paläontologie von Timor XI. Lieferung. Schweizerbart, Stuttgart, pp 1–82
Warén A (1983) A generic revision of the family Eulimidae. J Molluscan Stud Suppl 13:1–96
Warén A (2013) Murchisonellidae: who are they, where are they and what are they doing? (Gastropoda, lowermost Heterobranchia). Vita Malacol 11:1–14
Webster GD, Donovan SK (2012) Before the extinction—Permian platyceratid gastropods attached to platycrinitid crinoids and an abnormal four-rayed Platycrinites s.s. wachsmuthi (Wanner) from West Timor. Palaeoworld 21:153–159
Wesenberg B, Schnetler KI (2014) A catalogue of Danian gastropods from the Baunekule facies, Faxe Formation, Denmark. Geological Survey of Denmark and Greenland Bulletin 32:1–117
Weinstein SB, Kuris AM (2016) Independent origins of parasitism in Animalia. Biol Lett 12: 20160324. http://dx.doi.org/10.1098/rsbl.2016.0324
Wilson BW (1998) Superfamily Cypraeoidea. In: Beesley PL, GJB R, Wells A (eds) Mollusca: the southern synthesis, part B. Fauna of Australia. CSIRO Publishing, Melbourne, pp 780–786
Acknowledgments
I would like to thank Anders Warén and Philippe Bouchet for helpful discussions and providing images. I thank Kenneth de Baets and Andrzej Kaim who provided critical, helpful reviews.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Nützel, A. (2021). Gastropods as Parasites and Carnivorous Grazers: A Major Guild in Marine Ecosystems. In: De Baets, K., Huntley, J.W. (eds) The Evolution and Fossil Record of Parasitism. Topics in Geobiology, vol 49. Springer, Cham. https://doi.org/10.1007/978-3-030-42484-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-42484-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42483-1
Online ISBN: 978-3-030-42484-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)