Abstract
Gene editing methods have been considered as one of the important approaches that have a huge impact on plant breeding. Currently, gene editing is considered as one of the most powerful techniques for crop improvement, which offers precise base editing in a predictable manner. Within a short span of time, genome editing based breeding has made remarkable advances in the area of crop improvement as well as on human lives, due to cost-effectiveness, reliability and easy to use. However, it has also raised plethora of ethical and legal debates among research organizations, funding agencies besides national and international policy makers. The bigger question is do the prevailing law, directives and regulations anticipate and/or address the societal concern about genome-edited crop? Here, we review the existing regulatory framework and ethical complications that may influence the adoption of technology and its acceptance. Additionally, perspective for an alternative regulatory system that appropriately matches the scientific progress is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Re-orientation of crop improvement strategy is a prerequisite to meet the requirement of an ever-growing global population under limiting resources and predicted climate change implications. Genetic gains achieved through plant breeding (crossing and selection) has made a substantial contribution in terms of development and dissemination of high yielding resilient crops (Ejeta 2009). However, the crossing and selection face limitations with polyploidy, heterozygosity, self-incompatibility and longer generation time. Subsequently, genetic variations through mutation breeding were adopted where selection and screening remain time-consuming and expensive. Mutation breeding is regarded as non-transgenic or non-genetic engineering technique and hence, was exempted from GM legislation annexure of Directive 2001/18/EC (Gracia 2005). However, mutation breeding through chemical or physical mutagens was not as successful as expected; mainly due to (i) low frequency of non-synonymous mutations, (ii) require extensive backcrossing for the removal of non-essential mutations (background mutations acquired due to mutagen), and (iii) crop ploidy remained a challenge as higher ploidy level reduces the chance of effective phenotype as well as required more number of individuals in a population to identify homozygous line.
Scientific progress and novelty have been always implemented to facilitate plant breeding in order to improve precision in genome alteration. Genome or gene editing is an addition in the continuum of plant breeding innovation. It allows to create precise genetic variation (exchange, insertion, deletion) within an existing gene pool. Genome editing techniques include protein-mediated techniques (viz. TALENS, ZFN), nucleic acid-mediated techniques (viz. ODM) and combination of these two (viz. CRISPR). It offers great opportunities for crop improvement and could lead to gene stacking (Puchta 2017) but it also created regulatory challenges across the world. In the polarised world, some countries have developed the mechanism to address the issue of regulatory status of genome-edited crops. However, the legal status remains unclear across many countries particularly, in case of CRISPR/Cas derived crops (Whelan and Lema 2015; Wolt et al. 2016). In the year 2007, European commission instituted “New Techniques Working Group (NTWG)” to evaluate new techniques of GM with reference to prevailing European Union (EU) legislation on genetically modified organism (GMO) (Lusser et al. 2012). The legal opinion made by NTWG has never been officially released. However, European Commission has exempted mutagenesis-derived plants from the directive, if no recombinant nucleic acid is incorporated.
In the USA, specific GMO legislation was not constituted. However, existing legislation which was taking care of plant protection, pest management and food safety was used to regulate the GMO (USDA 2017). Canada has set up special regulatory provision for the “plant with novel traits (PNTs)” where any technology derived plants have not been kept under legislation, rather development of novel traits trigger legislation (Custers 2017). However, the prevailing EU GM legislation (European Directive 2001/18/EC) is process-based provoked by the implementation of GM steps. As far as Germany is concerned, the BVL (Federal Office of Consumer Protection and Food Safety) do not consider CRISPR derived crop as recombinant. Similarly, the Swedish Board of Agriculture (SBA) considers CRISPR/Cas like mutagenesis provided no foreign DNA occurs in the final plant/product. Nevertheless, due to lack of data about the long-term effect and uniform guidelines across the world on the legal status of genome-edited plants. Till now, lack of clarity exists on the regulatory framework of genome-edited crops. There is no clear-cut demarcation that the regulatory framework should be executed on the process adopted (process-based approach) or product generated (product-based approach). Concurrently, whether the same legal status of crops generated through point mutation and spontaneous mutation will be followed or different, is also not crystal clear and hence, is a matter of discussion. Moreover, asynchronous regulations must be synchronized for satisfactory risk assessment. The current chapter has made an effort to highlight the provisions of directives, ethical issues as well as regulatory framework related to genome-edited crops.
9.2 Regulatory Scenario
Regulatory framework was developed to monitor the GM crops more than two decades ago on the basis of limpid distinction between transgenics products and conventional breeding products. However, dearth of continuum between genetic engineering and conventional plant breeding was observed at the time of prevailing EU directive (Directive 2001/18/EC).
According to EU legislation, the definition of GM crops is “any organism whose genetic material has been altered in such a way that does not originate by natural mating and/or natural recombination”. However, techniques involving nucleic acid were kept under the surveillance of regulation whereas mutagenesis was kept out of the regulations (European Parliament 2001). Thus, crops derived from genetic alterations were subject to follow stringent regulatory guidelines, while mutagenesis derived crops were excluded from EU regulatory guidelines. This stringent guideline somehow retarded the entry of GM crops into the market (Smyth 2014). Slow approval and long queue of pending cases within the EU countries further restricted the rate of commercialization of GM crops (European Commission 2015). It seems that the EU precautionary measures and genetic modification defined process increases the complexity of regulatory framework across the world (Bayer et al. 2010; Okeno et al. 2013). Other countries like Australia, Argentina and Brazil have positively instigated process-based regulatory approach which is relatively consistent compared to North America (Smyth and Phillips 2014). To date, even a single internationally agreed regulatory framework do not exist; nevertheless, there are many countries that are in the process of evaluation whether and/or to what extent the existing regulations are necessary for research work and to access the products of gene editing.
9.3 Current Status and Opinion
Various scientific communities and regulatory policy makers have reviewed the status of existing regulations of genome-edited crops (Breyer et al. 2009; Lusser and Davies 2013; Pauwels et al. 2014). There is no consensus, however, nature of DNA repair process, usage of phenotype developed and existing regulatory framework to release product are the focus point of discussion across the globe. EU scientists are exploring genome editing with engineered nucleases (GEEN) and new plant breeding technology for crop improvement and to avoid strict GM regulatory framework (Hartung and Schiemann 2014). There is hope within EU community of paradigm shift of product over process (Hartung and Schiemann 2014). Currently, the main objective is to ensure that GEEN and other technology which uses site-directed mutation should not fall under any regulation across the world, exception is Canada and its PNT regulation (International Life Science Institute 2013). With the emergence of genome editing techniques applied in crop development and regulatory framework, the three broad categories have been framed. The category 1 technique involves transient integration of rDNA into host genome (which does not integrate into plant genome) with subsequent removal (Pauwels et al. 2014). There are three famous examples of Category 1 technique, which includes site-specific point mutation, site-specific random mutation and site-specific mutation with oligonucleotides (OMM), by NHEJ (SDN1) and with DNA repair (SDN2) respectively (Wolt et al. 2016). The category 2 technique involves stable integration of rDNA into host genome. It also includes the intermediate steps involving expression of SDN1 and SDN2 (Lusser and Davies 2013). The category 3 technique involves stable integration of rDNA into host genome. In this category, GEEN technology is used to deliver the transgene/s through infusion of homologous recombination (SDN3). Site-directed stacking of transgene fall under this category (D’Halluin et al. 2013).
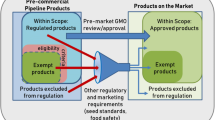
On the basis of various methodologies used for genome editing, it can be demonstrated that the regulatory consideration is a never-ending process across the world. Usually, decisions are made on the merits of the case through case by case study. Therefore, until the development of consensus among regulatory bodies and single uniform regulatory guideline, there will be uncertainty. Today there is scientific sentiment for different regulatory standards for gene-edited crops and transgenic crops. However, some NGO groups argue otherwise and show over-concern and do not want to give any regulatory benefits to gene-edited crops (Camacho et al. 2014). Finally, it can be concluded that plant developed through GEEN techniques will face various regulatory constraints based on interpretation of an obscure regulatory framework. In USA, USDA has specified a guideline to product developers which recommend that site-directed approaches leading to targeted deletion of endogenous nucleotide (SDN1) and transgenesis intermediary steps used to check the absence of transgenic element would not be regulated articles under USDA status, however, in case of site-directed oligonucleotide insertions or substitution, case-specific approval will be required (Camacho et al. 2014). In fact, the product derived from these new plant breeding technologies (NPBT) including genome-edited/GMOs are now in market because of strong support USDA (Fig. 9.1).

Source Original source Turner 2014; modified and adopted from a book chapter (https://www.ncbi.nlm.nih.gov/books/NBK424533/ or Doi: https://doi.org/10.17226/23395)
The role of regulatory agencies in US for the approval of GMOs/Genome-edited crop and some examples of approved products.
9.4 Public Understanding and Regulatory Implications
Plant biologists are constantly working towards improvising genome editing method which may get better social and regulatory acceptance over transgenic approaches. Today the biggest challenge is not only to develop the best scientific and technical methods; rather it is gaining confidence of public and regulatory authorities for easy approval of the edited crops (Chapoti and Wolt 2007). The restrictive regulations impact both product developers and public desire. The ability of US regulatory authority with regard to new breeding technologies appears challengeable, while their track record is exemplary well when it comes to transgenic regulation and safety (Camacho et al. 2014). Greater openness by US in the regulation of genome editing and innovation has outpaced Europe, who has led in the area till 2011. The major reason behind this setback was the incapability of EU to make an advancement in the adoption of GM crops and delayed in taking a position on regulation of genome editing (Funk 2015).
The general public opinion is not encouraging as far as food produced through biotechnological intervention is concerned. This has led to creating a hurdle that has delayed the implementation of the regulatory process. There is a huge difference among public opinions and scientific community on genome editing including CRISPR/Cas. According to a survey conducted by Pew Research Center, 2015, only 37% people responded positively on GM food; however 88% of scientific community recognizes GM food as safe. The greater discrepancy among public and scientific community indicate that the public may not accept food produced from genome editing technology unless transparent science-based campaign is conducted early. In fact, a section of scientist also argues that genome editing technology requires rigorous scrutiny in contrast to established technology such as transgenics (Araki and Ishii 2015). Additionally, civil society campaigns have also created doubt about the safety of GM foods (Paarlberg 2014) and hence, scientific community has to communicate more emphatically in order to translate the benefits of genome editing technology for greater purposes.
9.5 Need Within the Regulatory Community
New plant breeding technologies (NPBT) including CRISPR provides feasible alternative compared to transgenics. CRISPR offers new opportunities for innovation and it may be very useful in introducing novel traits in crops. The success of CRISPR and other GEEN technology will be limited like GMO if public opinion is not changed and regulatory procesess are broken in many parts of the world. To harness the benefit from these modern breeding technologies, the regulatory processes have to be made easy and uniform globally. The continued belief in process-based definitions and process-based language in public discourse reduced the aptly assessed approaches for the genome-edited crops. This will lead to public misunderstanding for crops derived from genome editing, which may create hurdle for regulators. Further, regulators may face pressure to evaluate the genome-edited crops within the existing biosafety framework. Thus, the focus for generation of novel phenotype/trait or trait staking is lost because of strict regulatory rules especially in the EU and other developing countries, resulted in closer of several research R&D units of the private seed companies which were focussed for improvisation of elite cultivars through NPBT. Fortunately, progress is being made across the globe by regulators in creating sensible, viable and pragmatic approaches towards implementation of genome editing tools for crop improvement. However, down the line product-based regulation should come for NPBT for a better world.
9.6 Conclusions and Perspectives
Although, the introduction of genome editing in plant breeding program has various benefits, however, the public observation and perception play a major role in commercialization of its product (Scheben et al. 2018). GMO food has lacked widespread public acceptance because of negative perception especially due to the public unawareness of the modern NPBT. Therefore, precaution has to be taken care of to avoid such negative public image about genome-edited crops (Frewer et al. 2004). Negative perception may create pressure on government to restrict the use of genome-edited crops, which may further limit the scientific innovation (Malyska et al. 2016). Therefore, public should be engaged in a honest dialogue regarding safety of genome-edited crops/foods. Public primary concern is the introduction of transgene which is not found in genome-edited crops; thus the chance of public acceptance is very high. There is also need for transparent legislation which can cover existing and future genome-edited based plant breeding program. The inconsistency in regulation of chemical and radiation mutagenesis which hold greater risk compared to genome editing (Urnov et al. 2018). Importantly, the potential risk of genome editing must be evaluated in conjunction with the benefits that the technology is expected to carry.
References
Araki M, Ishii T (2015) Towards social acceptance of plant breeding by genome editing. Trends Pl Sci 20:145–149
Bayer JC, Norton GW, Falck-Zepeda JB (2010) Cost of compliance with biotechnology regulation in the Philippines: implications for developing countries
Breyer D, Herman P, Brandenburger A, Gheysen G, Remaut E et al (2009) Commentary: genetic modification through oligonucleotide-mediated mutagenesis. A GMO regulatory challenge? Environmental Biosafety Res 8:57–64
Camacho A, Van Deynze A, Chi-Ham C, Bennett AB (2014) Genetically engineered crops that fly under the US regulatory radar. Nature Biotech 32:1087
Chapoti SM, Wolt JD (2007) Genetically modified crops for the bioeconomy: meeting public and regulatory expectations. Transgenic Res 16:675–688
Custers R (2017) The regulatory status of gene-edited agricultural products in the EU and beyond. Emerg Topics Life Sci 1:221–229
D’Halluin K, Vanderstraeten C, Van Hulle J, Rosolowska J, Van Den Brande I, Pennewaert A, Broadhvest J (2013) Targeted molecular trait stacking in cotton through targeted double-strand break induction. Plant Biotech J 11:933–941
Ejeta G (2009) Revitalizing agricultural research for global food security. Food Security 1:391
European Commission (2015) Proposal for a regulation of the European Parliament and of the Council amending regulation (EC) No 1829/2003 as regards the possibility for the Member States to restrict or prohibit the use of genetically modified food and feed on their territory
European Parliament (2001) Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC–Commission Declaration. Official J Euro Union 40:1–39
Frewer L, Lassen J, Kettlitz B, Scholderer J, Beekman V, Berdal KG (2004) Societal aspects of genetically modified foods. Food Chem Toxicol 42:1181–1193
Funk C, Rainie L (2015) Public and scientists’ views on science and society. Pew Research Center 29
García PR (2005) Directive 2001/18/EC on the Deliberate release into the environment of GMOs: an overview and the main provisions for placing on the market. Policy 263
Hartung F, Schiemann J (2014) Precise plant breeding using new genome editing techniques: opportunities, safety and regulation in the EU. Plant J 78:742–752
International Life Sciences Institute (2013) ILSI workshop on new breeding technologies (NBT). International Life Sciences Institute SEA Region Australasia, Canberra
Lusser M, Davies HV (2013) Comparative regulatory approaches for groups of new plant breeding techniques. New Biotech 30:437–446
Lusser M, Parisi C, Plan D, Rodríguez-Cerezo E (2012) Deployment of new biotechnologies in plant breeding. Nat Biotech 30:231
Malyska A, Bolla R, Twardowski T (2016) The role of public opinion in shaping trajectories of agricultural biotechnology. Trends Biotech 34:530–534
National Academies of Sciences, Engineering, and Medicine (2016) Genetically engineered crops: experiences and prospects. The National Academies Press, Washington, DC. https://doi.org/10.17226/23395
Okeno JA, Wolt JD, Misra MK, Rodriguez L (2013) Africa’s inevitable walk to genetically modified (GM) crops: opportunities and challenges for commercialization. New Biotech 30:124–130
Paarlberg R (2014) A dubious success: the NGO campaign against GMOs. GM Crops Food 5:223–228
Pauwels K, Podevin N, Breyer D, Carroll D, Herman P (2014) Engineering nucleases for gene targeting: safety and regulatory considerations. New Biotechnol 31:18–27
Puchta H (2017) Applying CRISPR/Cas for genome engineering in plants: the best is yet to come. Curr Opin Plant Biol 36:1–8
Scheben A, Edwards D (2018) Towards a more predictable plant breeding pipeline with CRISPR/Cas-induced allelic series to optimize quantitative and qualitative traits. Curr Opin Pl Biol 45:218–225
Smyth SJ (2014) The state of genetically modified crop regulation in Canada. GM Crops Food 5:195–203
Smyth SJ, Phillips PW (2014) Risk, regulation and biotechnology: the case of GM crops. GM Crops Food 5:170–177
Turner J (2014) Regulation of genetically engineered organisms at USDA–APHIS. Presentation to the National Academy of Sciences’ Committee on Genetically Engineered Crops: Past Experience and Future Prospects, December 10. Washington, DC
Urnov FD, Ronald PC, Carroll D (2018) A call for science-based review of the European court’s decision on gene-edited crops. Nat Biotech 36:800
Whelan AI, Lema MA (2015) Regulatory framework for gene editing and other new breeding techniques (NBTs) in Argentina. GM Crops Food 6:253–265
Wolt JD, Wang K, Yang B (2016) The regulatory status of genome-edited crops. Plant Biotech J 14:510–518
Acknowledgements
RK acknowledges Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India for the financial support. A.K sincerely thanks University Grants Commission (UGC) start-up grant (No.F.30-392/2017 (BSR) and Madhya Pradesh Council of Science and Technology (Endt. No. 3879/CST/R&D/BioSci/2018) for the funding to the laboratory.
Ethics Approval and Consent to Participate
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
AK, RK, NS and AM wrote the MS. All authors have read and approved the MS for publication.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kumar, A., Kumar, R., Singh, N., Mansoori, A. (2020). Regulatory Framework and Policy Decisions for Genome-Edited Crops. In: Bhattacharya, A., Parkhi, V., Char, B. (eds) CRISPR/Cas Genome Editing. Concepts and Strategies in Plant Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-42022-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-42022-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42021-5
Online ISBN: 978-3-030-42022-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)