Abstract
Albumin is widely conserved from vertebrates to invertebrates, and nature of mammalian albumins permit them to bind various endogenous ligands and drugs in the blood. It is known that at least two major ligand binding sites are present on the albumin molecule, which are referred to as Site I and Site II. These binding sites are thought to be almost completely conserved among mammals, even though the degree of binding to these sites are different depending on the physical and chemical properties of drugs and differences in the microenvironment in the binding pockets. In addition, the binding sites for medium and long-chain fatty acids are also well conserved among mammals, and it is considered that there are at least seven binding sites, including Site I and Site II. These bindings properties of albumin in the blood are also widely known to be important for transporting drugs and fatty acids to various tissues. It can therefore be concluded that albumin is one of the most important serum proteins for various ligands, and information on human albumin can be very useful in predicting the ligand binding properties of the albumin of other vertebrates.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Albumin is one of most widely studied proteins in body fluids. In 400 A.D., Hippocrates described the presence of foam in urine, which was subsequently proved to be albumin. After more than a thousand years, albumins from human to various mammals have now been identified. As of this writing, serum albumins from various vertebrate and invertebrate species have been identified and cloned. Vertebrate albumins are known to be the most abundant soluble protein in plasma. Albumins in mammals including human, bovine, dog, sheep, goat, cat and rabbit have been researched and their amino acid sequences (Allerton et al. 1962; Brown et al. 1989; Dixon and Sarkar 1974; Dugaiczyk et al. 1982; Hilger et al. 1996; Ho et al. 1993; Jacobs and Koj 1969; Lawn et al. 1981; Sala-Trepat et al. 1979a, b; Sargent et al. 1979; Weijers 1977) have been revealed and three-dimensional structures by X-ray crystallographic studies (Bujacz 2012; He and Carter 1992; Majorek et al. 2012; Yamada et al. 2016).
The bindings of various endogenous and exogeneous substrates in the blood control their pharmacokinetics (Fanali et al. 2012; Kragh-Hansen 1981, 1990, 2013; Peters 1970, 1985). Clinically, human albumin has been used for patients who are bleeding based on its function to maintain the colloid osmotic pressure of serum (Kobayashi 2006; Matejtschuk et al. 2000; Ohnishi et al. 2008). Since the 1970s, not only in humans, but also other mammalians such as bovine, horse and rabbit, the binding of fatty acids and various drugs to serum albumins has been investigated. These studies clearly indicate that these albumins have important roles in the delivery of endogenous and exogenous ligands in blood. In this chapter, the ligand binding properties, including fatty acid binding properties, are reviewed, focusing on albumin from mammalian species, especially, human albumin.
Human Albumin
Among the vertebrate and invertebrate albumin species, human albumin has been the most extensively studied for its function and structure (Chuang and Otagiri 2006; Fanali et al. 2012; Kragh-Hansen 1981, 1990, 2013; Kragh-Hansen et al. 2002; Otagiri 2005). Human albumin consists of 585 amino acid residues and there are no oligosaccharide chains attached to the molecule. The molecule has a heart-shaped structure, containing α-helices (67%) and no β-sheets. In addition, Human albumin is composed of three highly homologous domains, which are referred to as domain I, II and III, respectively. The physiological functions of the molecule other than ligand binding, include the maintenance of colloid osmotic pressure (Losowsky et al. 1962). Albumin has been administered to patients with hypoalbuminemia, hemorrhagic shock due to a loss of albumin (burn victims, the nephrotic syndrome, etc.) and decreased albumin biosynthesis (cirrhosis, etc.) (Reviewers 1998). Human albumin also has antioxidant properties (Anraku et al. 2001, 2003; Watanabe et al. 2017). This is due to the fact that, in addition to albumin being the most abundant molecule in blood, it contains a free cysteine residue at position 34 in the molecule. There is some evidence to show that this cysteine residue functions to scavenge reactive oxygen species that are generated during various inflammatory disease such as, for example, chronic renal and hepatic disease and diabetes (Anraku et al. 2011; Matsuyama et al. 2009; Mera et al. 2005; Nagumo et al. 2014; Oettl and Marsche 2010; Terawaki et al. 2004). It has been reported that the high glycemic state in blood during diabetes leads to the glycation of albumin molecules. This glycated albumin finally becomes an advanced glycation end product known as an AGE and this structure can exert various adverse effects (Baret et al. 2017; de Souza Pinto et al. 2012; Yamagishi et al. 2003). It has been also reported that such oxidation and glycation of human albumin also affects the drug binding capacity of the molecule (Bourdon et al. 1999; Collison et al. 2002; Keita et al. 1993; Murtiashaw and Winterhalter 1986; van Boekel et al. 1992; Yamazaki et al. 2005). Thus, human albumin acts as, not only an important ligand carrier, but also as a serum protein that can be used as a biomarker for certain disease states. Other functions, human albumin include enzymatic properties (esterase and thioesterase activities) (Diaz et al. 2001; Kurono et al. 1992; Watanabe et al. 2000). In fact, the angiotensin II receptor blocker, olmesartan medoxomil, was developed as prodrug that can be hydrolyzed by human serum albumin (Ma et al. 2005).
There are essentially two high-affinity drug binding sites on the HSA molecule, referred to as Site I and II, respectively. Most endogenous and exogenous ligands, which bind to human albumin, bind to either site in the range of 104–106 M−1. Until recently it has not been possible to predict which site a ligand binds to, based on the structural characteristics of the drug. In previous reports, Site I was reported to be formed as a pocket in subdomain IIA and drugs with multiple rings and bulky structures such as warfarin, phenylbutazone, acenocoumarol and indomethacin tend to bind to this site (Kragh-Hansen 1988; Yamasaki et al. 1996). In addition, the finding that ligands as large as bilirubin bind to Site I suggests that this Site is large (Brodersen and Stern 1980). This site is considered to be flexible and to have less structural specificity because Site I-binding drugs exhibit diverse structural characteristics. Furthermore, Site I is comprised of a broad area on the molecule, called Site Ia, Ib and Ic. These subsite regions are known to be overlapped (Petersen et al. 2000). A single mutation in amino acid residues in Site I (K199, W214, R218 and H242) affects the overall structure and thermal stability of Site I (Watanabe et al. 2001). Site I is located at the boundary between domain I and domain II. In a study using recombinants of each domain, it was found that the ligand binding ability of subsites Ia and Ib could not be detected in recombinant domain II, but the binding of ligands to subsite Ic in domain II was preserved (Matsushita et al. 2004).
An X-ray crystallographic study showed that Site II is located in subdomain IIIA (He and Carter 1992; Sudlow et al. 1975). NSAIDs containing aromatic carboxylic acids such as ketoprofen and ibuprofen bind to Site II (Chuang et al. 1999; Wanwimolruk et al. 1982). Thus, unlike Site I, Site II is considered to have a narrow binding pocket, and to be slightly rigid compared to Site I. In fact, a single mutation of Arg110 or Tyr411, both of which are involved in ligand binding in Site II, significantly induced a decrease in binding capacity (Watanabe et al. 2000).
Although there are several types of ligands, binding sites other than Site I and Site II have also been reported. Human albumin contains three metal binding sites (Peters 1966). The N-terminal binding site is one such site, where the binding of Cu (II), Co (II), Ni (II) can occur, and the binding involves Asp1, Ala2, His3 (Sadler et al. 1994). In particular, the binding of Cu (II) is very strong, and its dissociation constant is as low as 6.7 × 10−17 M. The second Site is located at Cys34. As described above, Cys34 is located in subdomain IA, and approximately 40% of this residue in circulation is present as the free form without binding of a low molecular thiol (cysteine and glutathione). Therefore, free ligands can bind covalently to Cys34 which contains not only endogenous substances, nitric oxide (Ishima et al. 2009), Ag (I), Cu (II), Au (I) and Pt (II) (Coffer et al. 1986; Peters 1966), but also thiol-containing drugs, such as N-acetyl-L-cysteine (Sengupta et al. 2001), D-penicillamine and captopril (Coffer et al. 1986; Narazaki et al. 1997). The binding capacity of these ligands has been reported to fluctuate during a disease state, suggesting that the redox state of Cys34 is susceptible during a disease state. The third metal binding site, referred to as a Co (II) binding site A and B respectively, binds Zn (II) and Cd (II) other than Co (II). X-ray absorption fine structure spectroscopy and modeling studies suggest that this binding involves His67, Asn99 (domain I), His247, Asp249 (domain II) (Blindauer et al. 2009).
The saturated long-chain fatty acids, lauric acid (C12), myristic acid (C14), palmitic acid (C16), and stearic acid (C18), have all been reported to form a three-dimensional complex structure with human albumin (Fig. 15.1) (Bhattacharya et al. 2000). Among them, myristic acid and palmitic acid have been extensively studied. Previous studies indicate that the albumin molecule contains five binding sites for myristic acid, one in subdomain IB, one at the interface between subdomain IA and subdomain IIA, two in IIIA and one in IIIB respectively (Peters 1966). Furthermore, it has been reported that the sixth Site is located at the boundary between subdomains IIA and IIB. These six fatty acid binding sites have also been identified in other long chain fatty acids (C12: 0, C16: 0 and C18: 0). In addition, it has also been reported that a seventh binding Site is present in subdomain IIA.
Structures of human albumin complexed with five different fatty acids. Numbers in molecules (1–9) represent the biding site of fatty acids respectively (Bhattacharya et al. J Mol Biol. 2000; 303: 721–732.)
The binding site of medium-chain fatty acids such as capric acid (C10) is also very similar to long-chain fatty acids. According to a report by Curry and colleagues there are an additional two binding sites with slightly lower affinity for the seven long-chain fatty acid binding sites (Bhattacharya et al. 2000; Petitpas et al. 2001). One is in the crevice between domain I-II and domain II-III and the other is in between domain I and III.
Although the binding site of the short-chain fatty acid octanoic acid and albumin has not been clarified, inhibition experiments using the previous Site II binding ligand suggest that it is Site II (Kragh-Hansen 1991). Furthermore, we recently reported that phenylbutyrate, a fatty acid derivative, binds strongly to Site II of the albumin molecule, and the short medium-chain fatty acid in the side chain is likely to bind to Site II (Sakurama et al. 2018, 2019). On the other hand, 2D NMR spectroscopy findings suggest that there are nine binding sites for fatty acids (Hamilton 2013), but the number of fatty acid binding sites of human albumin is still controversial. Similar to saturated fatty acids, unsaturated fatty acids, oleic acid (cis-form) and arachidonic acid have been also reported to bind to these seven binding sites, and the binding modes appear to be very similar to those of saturated fatty acids (Petitpas et al. 2001).
Regarding other endogenous ligands, it is well known that bilirubin, various metals, and uremic toxins (indoxyl sulfate; IS, 3-carboxy-4-methyl-5-propyl-2-furanpropanoate; CMPF, which are known to be elevated during renal dysfunction and are known to be associated with renal dysfunction) bind to human albumin (Sakai et al. 1995). Dicarboxylic acids such as bilirubin or CMPF bind to Site I (Brodersen and Stern 1980; Petersen et al. 2000; Sakai et al. 1995). It is known that ligands that contain an aromatic carboxylic acid such as L-tryptophan, indole acetic acid and indoxyl sulfuric acid bind to Site II (Irikura et al. 1991; Kragh-Hansen 1990).
Other Mammalian Albumins
The three-dimensional structures of albumins have been clarified by X-ray crystal structure analysis for bovine, dog, horse, sheep, goat, cat and rabbit albumins, and they show that amino acids sequences and structures of the these molecules are extremely homologous (71–76%) to human albumin. This suggests that albumins from these species also have drug and fatty acid binding properties that are equivalent to human albumin. In fact, there are reports that the binding properties of ligands, including fatty acids, are similar to those from humans and that binding pockets to similar to those for human albumins are also present in other mammalian albumins. Superposing the X-ray crystal structures of human and other mammalian albumins suggest that other animal albumins also have quite similar binding pockets in Site I and II. The positions of amino acid residues that are considered to be important for drug binding in human albumin are also very similar in other mammalian albumins (Fig. 15.2). The width and shape of the Site I entrance appears to be different between these albumins (Fig. 15.3). However, there is a little difference in the binding characteristics in site I among human and other mammalian albumins (Kosa et al. 1997). This may be due to the fact that the entrance of Site I is sufficiently large to bind bilirubin. On the other hand, the entrance to Site II appears to be much narrower than site I, and the charge state around the entrance of this site in human albumin appears to be similar to that of dogs but different from other animal species (Fig. 15.4). These slight differences in the microenvironment may have resulted in minor differences in drug binding between animal species. This section discusses the ligand binding properties of other mammalian albumins compared to that from humans.
Bovine
Bovine albumin is also one of the most widely studied albumin from a mammal species. X-ray crystallographic study data show that three-dimensional structure of this molecule is quite similar to that of humans. From substitution experiments using fluorescent probes that bind to Site I or Site II of human albumin, it has been reported that Site I are retained in bovine albumin (Kosa et al. 1997). On the other hand, it has been reported that Site II on bovine albumin is slightly different from that of human. The results of binding experiments suggest that this difference is due to changes in the microenvironment and is caused by differences in the size and/or hydrophobicity of the binding site, rather than differences in the amino acid sequences. Isothermal titration calorimetry assays (ITC) and various spectroscopic methods show, as in human albumin, there are two binding sites for metal cations such as Cu (II), Zn (II) and Ni (II), where one is at the N-terminal moiety, and another is cysteine residue at position 34 (Kolthoff and Willeford 1958; Peters and Blumenstock 1967).
Bovine albumin is also reported to have three primary binding sites for long chain fatty acids (Hamilton et al. 1991). NMR measurements performed using a pepsin albumin digest (1–306, 307–582) and a tryptic digest (377–582) suggest that three primary sites are present on the albumin molecule, among which two are located in residues 307–582 or residues 377–582, and one is in the N-terminal fragment (1–306). Furthermore, based on studies involving the chemical modification of bovine albumin, the modification of Lys 222 in subdomain IIA was inhibited by a C8 fatty acid (but not by medium chain, long chain fatty acids (C16, C18, C18:1) (Walker 1976). A possible binding site for short-chain fatty acids may be present near Lys222. In addition, photoaffinity labeling experiment showed Lys116, Lys349 and Lys473 were labeled with palmitate (Reed 1986).
Horse (Equine)
Similar to human albumin, equine albumin is also known to bind NSAID drugs. Detailed structure of complexes of diclofenac and naproxen with equine albumin have been revealed based on X-ray crystallography and differential scanning calorimetry data (Sekula and Bujacz 2015). Albumin is known to contain 2 binding sites for both diclofenac and naproxen respectively; sites for diclofenac are located in domain III, sites for naproxen are located in domain II and III, and the binding sites in domain III for each drug overlap. This site is considered to correspond to the Site II of human albumin. Therefore, similar to human albumin, with regard to Site II, equine albumin is also considered to contain a Site II for binding drugs.
Dog (Canine)
Representative drugs that bind to Site II of human albumin, warfarin and phenylbutazone, also bind to human, bovine, rabbit and rat albumin, but not dog albumin. However, drug binding experiments using Site II binding drugs, such as ibuprofen and diazepam, indicate that ibuprofen binds to mammalian species albumins (human, bovine, dog, rabbit and rat) to the same degree (1.0–3.0 × 106 M−1), but diazepam appears to have a high affinity only to human and dog albumin (Kosa et al. 1997). Furthermore, we recently reported that the antipsychotic drug, aripiprazole, which, similar to diazepam, contains a chlorine atom binds strongly only to human and dog albumins among mammalian species (Sakurama et al. 2018). This finding suggests that the microenvironment of the Site II binding pocket of human and dog albumin are similar. On the other hand, copper binding sites observed in human and bovine albumin are lacking (Appleton and Sarkar 1971).
Role of Albumin in the Transport of Fatty Acids
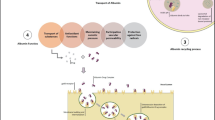
Albumin is known to be one of major carriers of fatty acids in the blood. Very low density lipoprotein (VLDL) and chylomicrons, fatty acid binding proteins (FABP) and fatty acid translocase (FAT/CD36), albumin binding protein (ABP) are also involved in the transport of long chain fatty acids from the blood to the intracellular cytosol and mitochondria (Fig. 15.5) (van der Vusse et al. 2002). The first step starts when fatty acids that are bound to albumin in blood pass to the apical membrane of vascular endothelial cells. Current knowledge of this indicates that the albumin-fatty acid complex interacts directly with the lipid bilayer membrane and during the transcytosis of this complex, fatty acids are thought to be released intracellularly (Kosa et al. 2007; Horie et al. 1988; Reed and Burrington 1989), whereas albumin binds to ABP on the membrane surface and the released fatty acids are then taken up by vascular endothelial cells (Luiken et al. 1999). On other hand, triglycerides (TG) and cholesterol ester (CE) in VLDL and chylomicrons are hydrolyzed to glycerol, cholesterol and fatty acids by lipoprotein lipases that are produced by endothelial cells (van der Vusse et al. 1992). The neonatal Fc receptor (FcRn) on the vascular endothelial cell membrane is considered to be a receptor for albumin and to be important for albumin recycling (Kim et al. 2006). Therefore, FcRn may be involved in the uptake of fatty acids that are bound to albumin. The fatty acids which pass through the vascular endothelial cells again bind to albumin in the intercellular space and are then transferred to the cell membrane of tissue, but the mechanism responsible for this is not well understood. Thereafter, the fatty acids transported into tissue cells bind to FABP followed by forming Acyl-CoA, which is used for the acylation of diacylglycerol, protein and signal transduction. In addition, acyl-CoA is transported into mitochondria and undergoes β-oxidation to become acetyl-CoA, which is a fuel for the citric acid cycle (van der Vusse et al. 2002). Thus, albumin plays a very important role in the transport of fatty acids in the blood, for not only the synthesis of various lipids, but also energy production. Furthermore, albumin not only plays an important role in fatty acid transport, it is also reported that fatty acids contribute to the structural stability and thermal stability of human albumin. Therefore, fatty acids may contribute to the structural stability of albumin, resulting in extending its retention time in the blood.
Conclusion
Albumin is one of the most extensively studied proteins, and past studies indicate that it is widely conserved from vertebrates to invertebrates. It is well known that the binding of drugs and fatty acids are a main functions of albumin, and that the amino acid sequences, three-dimensional structures and the drug binding properties (affinity and binding site) of mammalian albumins are all quite similar. In particular, the transport of fatty acids that are bound to albumin to tissues, where triglycerides and phospholipids are synthesized, is a very important process in terms of maintaining biological homeostasis. Therefore, albumin is a protein that plays an important role in a wide variety of species ranging from vertebrates to invertebrates, and data on human albumin is likely to be the useful information for many researchers.
References
Allerton SE, Elwyn D, Edsalljt SP (1962) Isolation and amino acid composition of dog plasma albumin. J Biol Chem 237:85–88
Anraku M, Yamasaki K, Maruyama T, Kragh-Hansen U, Otagiri M (2001) Effect of oxidative stress on the structure and function of human serum albumin. Pharm Res 18(5):632–639
Anraku M, Kragh-Hansen U, Kawai K, Maruyama T, Yamasaki Y, Takakura Y, Otagiri M (2003) Validation of the chloramine-T induced oxidation of human serum albumin as a model for oxidative damage in vivo. Pharm Res 20(4):684–692
Anraku M, Takeuchi K, Watanabe H, Kadowaki D, Kitamura K, Tomita K, Kuniyasu A, Suenaga A, Maruyama T, Otagiri M (2011) Quantitative analysis of cysteine-34 on the anitioxidative properties of human serum albumin in hemodialysis patients. J Pharm Sci 100(9):3968–3976. https://doi.org/10.1002/jps.22571
Appleton DW, Sarkar B (1971) The absence of specific copper (II)-binding site in dog albumin a comparative study of human and dog albumins. J Biol Chem 246(16):5040–5046
Baret P, Le Sage F, Planesse C, Meilhac O, Devin A, Bourdon E, Rondeau P (2017) Glycated human albumin alters mitochondrial respiration in preadipocyte 3T3-L1 cells. BioFactors (Oxford, England) 43(4):577–592. https://doi.org/10.1002/biof.1367
Bhattacharya AA, Grune T, Curry S (2000) Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303(5):721–732. https://doi.org/10.1006/jmbi.2000.4158
Blindauer CA, Harvey I, Bunyan KE, Stewart AJ, Sleep D, Harrison DJ, Berezenko S, Sadler PJ (2009) Structure, properties, and engineering of the major zinc binding site on human albumin. J Biol Chem 284(34):23116–23124
Bourdon E, Loreau N, Blache D (1999) Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J: Off Publ Fed Am Soc Exp Biol 13(2):233–244. https://doi.org/10.1096/fasebj.13.2.233
Brodersen R, Stern L (1980) Binding of bilirubin to albumin. CRC Crit Rev Clin Lab Sci 11(4):307–399
Brown WM, Dziegielewska KM, Foreman RC, Saunders NR (1989) Nucleotide and deduced amino acid sequence of sheep serum albumin. Nucleic Acids Res 17(24):10495. https://doi.org/10.1093/nar/17.24.10495
Bujacz A (2012) Structures of bovine, equine and leporine serum albumin. Acta Crystallogr D Biol Crystallogr 68(Pt 10):1278–1289. https://doi.org/10.1107/S0907444912027047
Chuang VT, Otagiri M (2006) Stereoselective binding of human serum albumin. Chirality 18(3):159–166. https://doi.org/10.1002/chir.20237
Chuang VT, Kuniyasu A, Nakayama H, Matsushita Y, Hirono S, Otagiri M (1999) Helix 6 of subdomain III A of human serum albumin is the region primarily photolabeled by ketoprofen, an arylpropionic acid NSAID containing a benzophenone moiety. Biochem Biophys Acta 1434(1):18–30. https://doi.org/10.1016/s0167-4838(99)00174-0
Coffer MT, Shaw CF III, Eidsness M, Watkins J, Elder R (1986) Reactions of auranofin and chloro (triethylphosphine) gold with bovine serum albumin. Inorg Chem 25(3):333–339
Collison KS, Parhar RS, Saleh SS, Meyer BF, Kwaasi AA, Hammami MM, Schmidt AM, Stern DM, Al-Mohanna FA (2002) RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J Leukoc Biol 71(3):433–444
de Souza Pinto R, Castilho G, Paim BA, Machado-Lima A, Inada NM, Nakandakare ER, Vercesi AE, Passarelli M (2012) Inhibition of macrophage oxidative stress prevents the reduction of ABCA-1 transporter induced by advanced glycated albumin. Lipids 47(5):443–450. https://doi.org/10.1007/s11745-011-3647-9
Diaz N, Suarez D, Sordo TL, Merz KM Jr (2001) Molecular dynamics study of the IIA binding site in human serum albumin: influence of the protonation state of Lys195 and Lys199. J Med Chem 44(2):250–260
Dixon JW, Sarkar B (1974) Isolation, amino acid sequence and copper(II)-binding properties of peptide (1-24) of dog serum albumin. J Biol Chem 249(18):5872–5877
Dugaiczyk A, Law SW, Dennison OE (1982) Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Natl Acad Sci USA 79(1):71–75. https://doi.org/10.1073/pnas.79.1.71
Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P (2012) Human serum albumin: from bench to bedside. Mol Aspects Med 33(3):209–290. https://doi.org/10.1016/j.mam.2011.12.002
Hamilton JA (2013) NMR reveals molecular interactions and dynamics of fatty acid binding to albumin. Biochim Biophys Acta (BBA)-Gen Subj 1830(12):5418–5426
Hamilton JA, Era S, Bhamidipati SP, Reed RG (1991) Locations of the three primary binding sites for long-chain fatty acids on bovine serum albumin. Proc Natl Acad Sci USA 88(6):2051–2054. https://doi.org/10.1073/pnas.88.6.2051
He XM, Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature 358(6383):209–215. https://doi.org/10.1038/358209a0
Hilger C, Grigioni F, Hentges F (1996) Sequence of the gene encoding cat (Felis domesticus) serum albumin. Gene 169(2):295–296
Ho JX, Holowachuk EW, Norton EJ, Twigg PD, Carter DC (1993) X-ray and primary structure of horse serum albumin (Equus caballus) at 0.27-nm resolution. European journal of biochemistry 215(1):205–212. https://doi.org/10.1111/j.1432-1033.1993.tb18024.x
Horie T, Mizuma T, Kasai S, Awazu S (1988) Conformational change in plasma albumin due to interaction with isolated rat hepatocyte. Am J Physiol 254(4 Pt 1):G465–G470. https://doi.org/10.1152/ajpgi.1988.254.4.G465
Irikura M, Takadate A, Goya S, Otagiri M (1991) 7-Alkylaminocoumarin-4-acetic acids as fluorescent probe for studies of drug-binding sites on human serum albumin. Chem Pharm Bull 39(3):724–728
Ishima Y, Kragh-Hansen U, Maruyama T, Otagiri M (2009) Albumin as a nitric oxide-traffic protein: characterization, biochemistry and possible future therapeutic applications. Drug Metab Pharmacokinet 24(4):308–317
Jacobs S, Koj A (1969) Amino acid composition of rabbit plasma albumin and fibrin. Anal Biochem 27(1):178–182
Keita Y, Kratzer W, Worner W, Rietbrock N (1993) Effect of free fatty acids on the binding kinetics at the benzodiazepine binding site of glycated human serum albumin. Int J Clin Pharmacol Ther Toxicol 31(7):337–342
Kim J, Bronson CL, Hayton WL, Radmacher MD, Roopenian DC, Robinson JM, Anderson CL (2006) Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces. Am J Physiol Gastrointest Liver Physiol 290(2):G352–G360. https://doi.org/10.1152/ajpgi.00286.2005
Kobayashi K (2006) Summary of recombinant human serum albumin development. Biol: J Int Assoc Biol Stand 34(1):55–59. https://doi.org/10.1016/j.biologicals.2005.08.021
Kolthoff I, Willeford B Jr (1958) The interaction of copper(II) with bovine serum albumin1. J Am Chem Soc 80(21):5673–5678
Kosa T, Maruyama T, Otagiri M (1997) Species differences of serum albumins: I. Drug binding sites. Pharm Res 14(11):1607–1612
Kosa T, Nishi K, Maruyama T, Sakai N, Yonemura N, Watanabe H, Suenaga A, Otagiri M (2007) Structural and ligand-binding properties of serum albumin species interacting with a biomembrane interface. J Pharm Sci 96(11):3117–3124. https://doi.org/10.1002/jps.20887
Kragh-Hansen U (1981) Molecular aspects of ligand binding to serum albumin. Pharmacol Rev 33(1):17–53
Kragh-Hansen U (1988) Evidence for a large and flexible region of human serum albumin possessing high affinity binding sites for salicylate, warfarin, and other ligands. Mol Pharmacol 34(2):160–171
Kragh-Hansen U (1990) Structure and ligand binding properties of human serum albumin. Dan Med Bull 37(1):57–84
Kragh-Hansen U (1991) Octanoate binding to the indole- and benzodiazepine-binding region of human serum albumin. Biochem J 273(Pt 3):641–644. https://doi.org/10.1042/bj2730641
Kragh-Hansen U (2013) Molecular and practical aspects of the enzymatic properties of human serum albumin and of albumin-ligand complexes. Biochem Biophys Acta 1830(12):5535–5544. https://doi.org/10.1016/j.bbagen.2013.03.015
Kragh-Hansen U, Chuang VT, Otagiri M (2002) Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull 25(6):695–704
Kurono Y, Kushida I, Tanaka H, Ikeda K (1992) Esterase-like activity of human serum albumin. VIII. Reaction with amino acid p-nitrophenyl esters. Chem Pharm Bull 40(8):2169–2172
Lawn RM, Adelman J, Bock SC, Franke AE, Houck CM, Najarian RC, Seeburg PH, Wion KL (1981) The sequence of human serum albumin cDNA and its expression in E. coli. Nucleic Acids Res 9(22):6103–6114. https://doi.org/10.1093/nar/9.22.6103
Losowsky M, Alltree E, Atkinson M (1962) Plasma colloid osmotic pressure and its relation to protein fractions. Clin Sci 22:249
Luiken JJ, Schaap FG, van Nieuwenhoven FA, van der Vusse GJ, Bonen A, Glatz JF (1999) Cellular fatty acid transport in heart and skeletal muscle as facilitated by proteins. Lipids 34(Suppl):S169–S175
Ma SF, Anraku M, Iwao Y, Yamasaki K, Kragh-Hansen U, Yamaotsu N, Hirono S, Ikeda T, Otagiri M (2005) Hydrolysis of angiotensin II receptor blocker prodrug olmesartan medoxomil by human serum albumin and identification of its catalytic active sites. Drug Metab Dispos: Biol Fate Chem 33(12):1911–1919. https://doi.org/10.1124/dmd.105.006163
Majorek KA, Porebski PJ, Dayal A, Zimmerman MD, Jablonska K, Stewart AJ, Chruszcz M, Minor W (2012) Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol Immunol 52(3–4):174–182. https://doi.org/10.1016/j.molimm.2012.05.011
Matejtschuk P, Dash CH, Gascoigne EW (2000) Production of human albumin solution: a continually developing colloid. Br J Anaesth 85(6):887–895. https://doi.org/10.1093/bja/85.6.887
Matsushita S, Isima Y, Chuang VT, Watanabe H, Tanase S, Maruyama T, Otagiri M (2004) Functional analysis of recombinant human serum albumin domains for pharmaceutical applications. Pharm Res 21(10):1924–1932
Matsuyama Y, Terawaki H, Terada T, Era S (2009) Albumin thiol oxidation and serum protein carbonyl formation are progressively enhanced with advancing stages of chronic kidney disease. Clin Exp Nephrol 13(4):308–315. https://doi.org/10.1007/s10157-009-0161-y
Mera K, Anraku M, Kitamura K, Nakajou K, Maruyama T, Otagiri M (2005) The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochem Biophys Res Commun 334(4):1322–1328. https://doi.org/10.1016/j.bbrc.2005.07.035
Murtiashaw MH, Winterhalter KH (1986) Non-enzymatic glycation of human albumin does not alter its palmitate binding. Diabetologia 29(6):366–370
Nagumo K, Tanaka M, Chuang VT, Setoyama H, Watanabe H, Yamada N, Kubota K, Tanaka M, Matsushita K, Yoshida A, Jinnouchi H, Anraku M, Kadowaki D, Ishima Y, Sasaki Y, Otagiri M, Maruyama T (2014) Cys34-cysteinylated human serum albumin is a sensitive plasma marker in oxidative stress-related chronic diseases. PLoS ONE 9(1):e85216. https://doi.org/10.1371/journal.pone.0085216
Narazaki R, Harada K, Sugii A, Otagiri M (1997) Kinetic analysis of the covalent binding of captopril to human serum albumin. J Pharm Sci 86(2):215–219. https://doi.org/10.1021/js960234+
Oettl K, Marsche G (2010) Redox state of human serum albumin in terms of cysteine-34 in health and disease. Methods Enzymol 474:181–195. https://doi.org/10.1016/s0076-6879(10)74011-8
Ohnishi K, Kawaguchi A, Nakajima S, Mori H, Ueshima T (2008) A comparative pharmacokinetic study of recombinant human serum albumin with plasma-derived human serum albumin in patients with liver cirrhosis. J Clin Pharmacol 48(2):203–208. https://doi.org/10.1177/0091270007310549
Otagiri M (2005) A molecular functional study on the interactions of drugs with plasma proteins. Drug Metab Pharmacokinet 20(5):309–323
Peters T (1966) All about Albumin: Biochemistry, Genetics, and Medical Application, Acad. Press, Orlando, FL:42
Peters T Jr (1970) Serum albumin. Adv Clin Chem 13:37–111
Peters T Jr (1985) Serum albumin. Adv Protein Chem 37:161–245
Peters T Jr, Blumenstock FA (1967) Copper-binding properties of bovine serum albumin and its amino-terminal peptide fragment. J Biol Chem 242(7):1574–1578
Petersen CE, Ha CE, Harohalli K, Feix JB, Bhagavan NV (2000) A dynamic model for bilirubin binding to human serum albumin. J Biol Chem 275(28):20985–20995. https://doi.org/10.1074/jbc.M001038200
Petitpas I, Grune T, Bhattacharya AA, Curry S (2001) Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J Mol Biol 314(5):955–960. https://doi.org/10.1006/jmbi.2000.5208
Reed RG (1986) Location of long chain fatty acid-binding sites of bovine serum albumin by affinity labeling. J Biol Chem 261(33):15619–15624
Reed RG, Burrington CM (1989) The albumin receptor effect may be due to a surface-induced conformational change in albumin. J Biol Chem 264(17):9867–9872
Reviewers CIGA (1998) Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ (Clin Res Ed) 317(7153):235–240. https://doi.org/10.1136/bmj.317.7153.235
Sadler PJ, Tucker A, Viles JH (1994) Involvement of a lysine residue in the N-terminal Ni2+ and Cu2+ binding site of serum albumins. Comparison with Co2+, Cd2+ and Al3+. Eur J Biochem 220(1):193–200. https://doi.org/10.1111/j.1432-1033.1994.tb18614.x
Sakai T, Takadate A, Otagiri M (1995) Characterization of binding site of uremic toxins on human serum albumin. Biol Pharm Bull 18(12):1755–1761
Sakurama K, Kawai A, Tuan Giam Chuang V, Kanamori Y, Osa M, Taguchi K, Seo H, Maruyama T, Imoto S, Yamasaki K, Otagiri M (2018) Analysis of the binding of aripiprazole to human serum albumin: the importance of a chloro-group in the chemical structure. ACS Omega 3(10):13790–13797. https://doi.org/10.1021/acsomega.8b02057
Sakurama K, Nishi K, Imoto S, Hashimoto M, Komatsu T, Morita Y, Taguchi K, Otagiri M, Yamasaki K (2019) Further evidence regarding the important role of chlorine atoms of aripiprazole on binding to the site II area of human albumin. J Pharm Sci 108(5):1890–1895. https://doi.org/10.1016/j.xphs.2018.11.045
Sala-Trepat JM, Dever J, Sargent TD, Thomas K, Sell S, Bonner J (1979a) Changes in expression of albumin and alpha-fetoprotein genes during rat liver development and neoplasia. Biochemistry 18(11):2167–2178. https://doi.org/10.1021/bi00578a006
Sala-Trepat JM, Sargent TD, Sell S, Bonner J (1979b) alpha-Fetoprotein and albumin genes of rats: no evidence for amplification-deletion or rearrangement in rat liver carcinogenesis. Proc Natl Acad Sci USA 76(2):695–699. https://doi.org/10.1073/pnas.76.2.695
Sargent TD, Wu JR, Sala-Trepat JM, Wallace RB, Reyes AA, Bonner J (1979) The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci USA 76(7):3256–3260. https://doi.org/10.1073/pnas.76.7.3256
Sekula B, Bujacz A (2015) Structural insights into the competitive binding of diclofenac and naproxen by equine serum albumin. J Med Chem 59(1):82–89
Sengupta S, Chen H, Togawa T, DiBello PM, Majors AK, Budy B, Ketterer ME, Jacobsen DW (2001) Albumin thiolate anion is an intermediate in the formation of albumin-S-S-homocysteine. J Biol Chem 276(32):30111–30117. https://doi.org/10.1074/jbc.M104324200
Sudlow G, Birkett DJ, Wade DN (1975) The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol 11(6):824–832
Terawaki H, Yoshimura K, Hasegawa T, Matsuyama Y, Negawa T, Yamada K, Matsushima M, Nakayama M, Hosoya T, Era S (2004) Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int 66(5):1988–1993. https://doi.org/10.1111/j.1523-1755.2004.00969.x
van Boekel MA, van den Bergh PJ, Hoenders HJ (1992) Glycation of human serum albumin: inhibition by diclofenac. Biochem Biophys Acta 1120(2):201–204. https://doi.org/10.1016/0167-4838(92)90270-n
van der Vusse GJ, Glatz JF, Stam HC, Reneman RS (1992) Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 72(4):881–940. https://doi.org/10.1152/physrev.1992.72.4.881
van der Vusse GJ, van Bilsen M, Glatz JF, Hasselbaink DM, Luiken JJ (2002) Critical steps in cellular fatty acid uptake and utilization. Mol Cell Biochem 239(1–2):9–15
Walker JE (1976) Lysine residue 199 of human serum albumin is modified by acetylsalicylic acid. FEBS Lett 66(2):173–175
Wanwimolruk S, Birkett DJ, Brooks PM (1982) Protein binding of some non-steroidal anti-inflammatory drugs in rheumatoid arthritis. Clin Pharmacokinet 7(1):85–92. https://doi.org/10.2165/00003088-198207010-00005
Watanabe H, Tanase S, Nakajou K, Maruyama T, Kragh-Hansen U, Otagiri M (2000) Role of arg-410 and tyr-411 in human serum albumin for ligand binding and esterase-like activity. Biochem J 349(Pt 3):813–819. https://doi.org/10.1042/bj3490813
Watanabe H, Kragh-Hansen U, Tanase S, Nakajou K, Mitarai M, Iwao Y, Maruyama T, Otagiri M (2001) Conformational stability and warfarin-binding properties of human serum albumin studied by recombinant mutants. Biochem J 357(Pt 1):269–274. https://doi.org/10.1042/0264-6021:3570269
Watanabe H, Imafuku T, Otagiri M, Maruyama T (2017) Clinical implications associated with the posttranslational modification-induced functional impairment of albumin in oxidative stress-related diseases. J Pharm Sci 106(9):2195–2203. https://doi.org/10.1016/j.xphs.2017.03.002
Weijers RN (1977) Amino acid sequence in bovine serum albumin. Clin Chem 23(7):1361–1362
Yamada K, Yokomaku K, Kureishi M, Akiyama M, Kihira K, Komatsu T (2016) Artificial blood for dogs. Sci Rep 6:36782. https://doi.org/10.1038/srep36782
Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M (2003) Advanced glycation end products inhibit de novo protein synthesis and induce TGF-beta overexpression in proximal tubular cells. Kidney Int 63(2):464–473. https://doi.org/10.1046/j.1523-1755.2003.00752.x
Yamasaki K, Maruyama T, Kragh-Hansen U, Otagiri M (1996) Characterization of site I on human serum albumin: concept about the structure of a drug binding site. Biochem Biophys Acta 1295(2):147–157. https://doi.org/10.1016/0167-4838(96)00013-1
Yamazaki E, Inagaki M, Kurita O, Inoue T (2005) Kinetics of fatty acid binding ability of glycated human serum albumin. J Biosci 30(4):475–481
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nishi, K., Yamasaki, K., Otagiri, M. (2020). Serum Albumin, Lipid and Drug Binding. In: Hoeger, U., Harris, J. (eds) Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins. Subcellular Biochemistry, vol 94. Springer, Cham. https://doi.org/10.1007/978-3-030-41769-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-41769-7_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41768-0
Online ISBN: 978-3-030-41769-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)