Abstract
Surgical management of thyroid eye disease (TED) has two general aims: (1) relieve symptoms of orbital congestion and fibrosis, including possibly compressive optic neuropathy, and (2) reconstruct the periocular region to reduce deformity and renormalize appearance. Surgical management follows a sequence that is tailored to individual patient needs. The signs and symptoms of TED that can be addressed surgically include proptosis, orbital pressure/pain, orbital inflammatory signs, diplopia secondary to restrictive strabismus, ocular surface exposure secondary to lagophthalmos, and eyelid retraction. Because orbital decompression surgery can improve lagophthalmos and eyelid retraction, and can affect strabismus (positively or negatively), these issues are traditionally addressed sequentially by orbital decompression first, followed by strabismus surgery, and finally eyelid retraction repair, as needed. In this chapter, we focus primarily on orbital decompression surgery and eyelid surgery, although key aspects of strabismus surgery are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Orbital wall
- Orbital decompression
- Compartment syndrome
- Lateral decompression
- Medial decompression
- Floor decompression
- Balanced decompression
- Orbital septum
- Compressive optic neuropathy
- Proptosis
- Eyelid retraction
Introduction
Surgical management of thyroid eye disease (TED) has two general aims: (1) relieve symptoms of orbital congestion and fibrosis, including possibly compressive optic neuropathy , and (2) reconstruct the periocular region to reduce deformity and renormalize appearance. Surgical management follows a sequence that is tailored to individual patient needs. The signs and symptoms of TED that can be addressed surgically include proptosis, orbital pressure/pain, orbital inflammatory signs, diplopia secondary to restrictive strabismus, ocular surface exposure secondary to lagophthalmos, and eyelid retraction. Because orbital decompression surgery can improve lagophthalmos and eyelid retraction, and can affect strabismus (positively or negatively), these issues are traditionally addressed sequentially by orbital decompression first, followed by strabismus surgery, and finally eyelid retraction repair, as needed. In this chapter, we focus primarily on orbital decompression surgery and eyelid surgery, although key aspects of strabismus surgery are also discussed.
Thyroid eye disease follows a sequence that is tailored to patient needs. The key issues addressed by surgery include proptosis, orbital pressure/pain, orbital inflammatory signs, diplopia secondary to restrictive strabismus, ocular surface exposure secondary to lagophthalmos, and eyelid retraction. These are traditionally addressed sequentially by orbital decompression, strabismus surgery, and eyelid retraction repair surgery. The extent of surgical rehabilitation is patient specific and customized to the needs of each patient. Coordination and proper sequencing of surgical intervention is key to promoting good outcomes. In this chapter, we focus primarily on orbital decompression and eyelid surgery – the two main reconstructive surgeries performed by oculoplastic surgeons for patients with TED.
Orbital decompression surgery is performed to address the tight compartment caused by expansion of orbital soft tissues. The surgery essentially rebalances the contents to the size of the bony orbit, thereby reducing orbital pressure, proptosis, and, when present, compressive optic neuropathy. Surgical techniques include different approaches to removing orbital bone in order to enlarge the space within the orbital compartment, as well as removal of scarred fat and connective tissue to reduce the volume of orbital soft tissue.
Strabismus surgery is performed following orbital decompression surgery, but prior to eyelid retraction surgery. This is done for three reasons: (1) orbital decompression must be performed first because it can alter mechanical vectors of extraocular muscle function, leading to changes in eye alignment; (2) restrictive strabismus can lead to secondary eyelid retraction – essentially a pseudoretraction that can be reduced by releasing the extraocular muscle restriction; and (3) strabismus surgery can itself cause eyelid retraction, particularly of the lower eyelids, secondary to the relationship of the lower eyelid retractors to the inferior rectus and oblique muscles.
It is important to note that recession of fibrotic inelastic muscles frequently causes worsening proptosis [1]. Therefore, it is important to provide sufficient decompression to account for the expected increase in proptosis following strabismus surgery and to properly counsel patients.
Eyelid retraction repair is performed last – once proptosis is reduced to an acceptable level, any compressive issues are addressed, and strabismus is resolved. Upper eyelid retraction repair typically requires release of eyelid retractors, which undergo fibrosis as part of the orbitopathy process. This can be done via anterior or posterior surgical approaches and may involve levator muscle, Müller’s muscle, and/or conjunctiva. Lower eyelid retraction repair frequently requires a spacer graft, which can be autologous (e.g., hard palate, ear cartilage, dermis), allograft or xenograft (e.g., decellularized dermis), or alloplastic material.
In this chapter, we will describe the diagnostic process for customizing a surgical plan, perioperative planning, surgical techniques and postoperative considerations.
Orbital Decompression
History
Orbital decompression has evolved since its inception in the early twentieth century. Dollinger first described orbital bone decompression for TED in 1911 by removing the lateral wall [2, 3]. Orbital roof decompression was advocated by Naffziger, a neurosurgeon, in 1931. The procedure allowed access to both orbital apices in cases of bilateral optic neuropathy, though it provided minimal volumetric decompression [2]. Direct external ethmoidectomy for decompression of the medial wall was described by Sewall, an otolaryngologist, in 1936 [2, 4]. The combined approach to the medial wall and floor of the orbit was described by Walsh and Ogura, otolaryngologists, in 1957, and performed via a transantral approach [2, 4]. This was the procedure of choice until the early 1980s, when more direct orbital approaches, including the swinging eyelid, transconjunctival, transcaruncular, and endoscopic approaches to the lateral wall, floor, and medial wall were described [5,6,7,8,9]. Decompression of the orbital roof has generally been avoided due to its potential complications, including cerebrospinal fluid leak and pulsating proptosis from transmission of intracranial pressure changes [10].
Currently, the most common surgical approaches to orbital decompression surgery include (1) lateral decompression through an extended lateral upper eyelid crease or a lateral canthotomy approach to the lateral orbit, lateral roof, and lateral floor (avoiding injury to the lateral commissure); (2) medial transconjunctival approach to the orbit (allows access to the medial orbit and posterior floor); and (3) inferior fornix approach to the anterior floor and orbital fat [11,12,13,14]. Endonasal surgery is a reasonable option to access the medial wall and floor [15, 16], although this approach has several important limitations, including extended length of surgery [17], removal of tissue while moving toward the globe instead of away from the globe (increasing risk of inadvertent injury), and a more limited access to the orbital process of the palatine bone at the orbital apex [18].
With improved techniques and better fellowship training, the trend in surgical decompression has been toward more nuanced customizable techniques that enhance outcomes and decrease morbidity [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Each surgery for TED is individually tailored based on patient characteristics and surgeon experience. Given the lack of randomized controlled trials comparing surgical techniques for orbital decompression, no particular method has been deemed scientifically superior [35, 38]. However, with improved understanding of the goals of surgery, some techniques have been abandoned by most orbital surgeons. This includes the misguided technique of discarding the lateral orbital rim in the hope of providing additional decompression; this technique not only damages the lateral canthal tendon attachments, leading to disfigurement and eyelid instability, but also provides no decompression where it is most needed – at the orbital apex. Instead, improved understanding of how to deeply contour the diploic spaces within the sphenoid, frontal, and zygomatic bones has led to excellent results with dramatic improvements in proptosis reduction through smaller incisions and without damaging the lateral orbital rim. Indeed, many orbital surgeons no longer remove the orbital rim at all during a lateral decompression surgery, choosing instead to create the window to the temporalis fossa from within the orbit, prior to initiating removal of diploe for apical decompression.
Customization of orbital decompression can be accomplished through the number and choice of orbital walls removed, augmenting the decompression with excision of hypertrophied and fibrotic orbital fat, varying the extent of ethmoidectomy (medial decompression) or deep bone removal (lateral decompression), targeting the posterior inferomedial strut and orbital process of the palatine bone, and timing of surgery. The development of modern techniques has been made possible by advancements in noninvasive imaging [39, 40], preoperative analysis of the orbital anatomy [41, 42], stereotactic image-guided surgical navigation devices [43,44,45,46,47], piezoelectric technology that minimizes tissue damage [48], and an improved understanding of the spectrum of orbital pathology represented by thyroid eye disease (TED) [49,50,51]. Additionally, the expansion of accepted indications for decompression, the growing referral pattern of most advanced TED patients to academic medical centers, and the significant increase in the number of orbital surgery fellowships over the past decade have resulted in more orbital surgeons who have better surgical training and experience with decompression. Hence, the clinical threshold for performing orbital decompression surgery has decreased, leading to earlier and more robust interventions with good outcomes.
Surgical Timing
The acute phase of TED is marked by congestive and inflammatory signs, such as chemosis, caruncular edema, eyelid edema, orbital pain, and progressive strabismus. For many years, conventional wisdom ruled that orbital decompression should be avoided in patients in the acute phase of TED, limiting treatment to medical measures, including topical lubrication, salt restriction, head elevation, prism glasses, corticosteroids (oral, intravenous, or intraorbital), and low-dose external beam radiotherapy [52]. Details of medical therapy, including new potential therapeutic modalities, are described in the previous chapter. However, with the understanding that congestion and inflammation cannot be differentiated based on clinical exam, and the further understanding that orbital congestion leads to venous stasis and accumulation of cytokines within the orbit, some surgeons began to perform orbital decompression surgery for orbits with acute-phase signs and symptoms – with excellent results [53, 54]. These outcomes may be related to improved techniques that allow for excellent decompression of the orbital apex, where the ophthalmic venous system drains, through smaller incisions and less invasive surgical approaches. Since there are no good randomized clinical trials of orbital decompression techniques, surgeons are mostly left to their own device regarding the best approach to the timing and technique of orbital decompression surgery. However, operating on the acutely involved orbit with signs of congestion and inflammation is progressively gaining wider acceptance, and the decision rests with the surgeon and patient based on a personalized, customized approach to the rehabilitation process.
TED with imminent visual threat due to dysthyroid compressive optic neuropathy (CON) occurs in approximately 6% of patients [55] and represents a unique, more urgent situation. These orbits will typically require decompression surgery. However, the urgency of surgery is not clear. Many surgeons may choose to “buy time” using pulsed intravenous corticosteroids [56] and/or low-dose orbital radiation. These options are particularly important when patients are medically unstable or not fully optimized for surgical anesthesia. Importantly, unlike other causes of optic neuropathy, such as microvascular disease, visual field loss from CON is often reversible [57], revealing a unique resilience in retinal ganglion cells and axons that may have to do with axonal stasis. Still, that resilience should not be tested without important medical reasons, and orbital decompression is often required at the earliest opportunity for optimal outcomes.
In patients whose vision is not acutely threatened, the decision regarding when to perform orbital decompression is more nuanced. A single pulse of intravenous corticosteroids may serve as a therapeutic trial that can differentiate the inflammatory and congestive processes in the acute phase: inflammatory orbitopathy improves following corticosteroid administration, while congestive orbitopathy is usually unresponsive [58]. Early orbital decompression may have beneficial effects on the overall course of the orbitopathy by relieving the orbital congestion and improving blood flow to orbital tissues in patients with symptomatic orbital congestion [53, 54]. Hence, orbital decompression surgery is no longer relegated to a rehabilitative stage once the disease has become quiescent, but is rather considered a key stage of a multistep approach to control TED and avoid its more significant consequences.
Although early decompression may be beneficial, orbital decompression surgery should not be undertaken prior to the stabilization of endocrine disease. Conventional wisdom is that the thyroid status is not directly related to the orbit. Our clinical experience and an extensive nuanced reading of the literature [59,60,61] have revealed that this is not necessarily true. In particular, we have found that in patients who have successfully ceased smoking and whose thyroid function tests are stable prior to (and following) decompression, a primary decompression is frequently successful, with rare need for additional decompressions at a later date. Thus, to obtain the best surgical result, the patient’s endocrine status needs to be normal and stable. Furthermore, the presence of TED is a contraindication for RAI therapy, which may provoke a recurrence or worsening of the orbitopathy.
Preoperative Planning
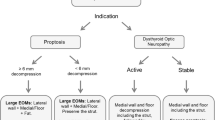
Selection of the surgical procedure is customized through an analysis of the clinical examination, orbital imaging studies, and premorbid facial photographs. The clinical examination focuses on the exophthalmometry measurements, lid retraction, ocular motility, the amount of lagophthalmos, and degree of corneal exposure. By reviewing the premorbid photos in conjunction with clinical measurements, an assessment of the amount of proptosis reduction that would be required to return to the normal state can be made.
CT or MR imaging in the axial and coronal planes is analyzed to determine the relative contribution of the enlargement of the extraocular muscles to the observed proptosis [19, 39]. TED can be divided into three categories based on the radiographic appearance: fat-predominant disease, muscle-predominant disease, and disease with mixed fat and muscle expansion (Figs. 58.1 and 58.2).
Image analysis should also consider the size of the paraorbital sinuses and thickness of the diploic spaces of the sphenoid, lateral frontal, and zygomatic bones, which can vary considerably and impact the success of orbital decompression. Additionally, the surgeon should review the radiographic images for potential coexisting orbital or sinus pathology.
In fat-predominant TED, fat decompression alone may be beneficial. In these patients, an average reduction of proptosis of as much as 3–4 mm can be expected. The greatest mean proptosis reduction, 4.7 mm, is expected in patients with preoperative Hertel measurements over 25 mm, whereas the smallest mean proptosis reduction, 1.5 mm, is achieved in patients whose preoperative Hertel measurements are less than 20 mm [62]. Fat decompression in these patients is most effective when the fat is thick and fibrotic, as opposed to soft, supple fat lobules that characterize the normal orbit [63].
In contrast, patients with predominately enlarged EOMs typically benefit most from bone decompression. The amount of proptosis reduction achieved by a given decompression is highly variable and depends on both individual patient anatomy and the surgeon’s technique. Based on published data, one can expect an average of 3.5–6.3 mm of proptosis reduction from a lateral wall decompression [33, 64,65,66], 2.5–4.5 mm from an extended medial wall decompression with removal of the posterior floor [29, 64, 67,68,69], and a somewhat smaller effect from an anterior floor decompression [70]. Importantly, significant decompression can be achieved with minimal reduction in proptosis secondary to the stiff EOMs resisting the posterior repositioning of the globe within a decompressed orbit. Hence, proptosis reduction is only one variable when assessing outcomes of orbital decompression surgery, with other variables that include conjunctival chemosis and orbital pressure pain, both of which assess improvement in orbital congestion. Both lateral and medial decompression can achieve a good decompression of the orbital apex. Because the optic foramen is located within the lesser wing of the sphenoid at the posterior edge of the medial wall, a posterior medial decompression has historically been thought to be the most effective approach to treating CON [71]. However, it has been demonstrated that a full lateral decompression with deep bone removal can be just as effective in treating CON as a medial decompression [64] (Tables 58.1 and 58.2).
Surgical Techniques
In all orbital decompression, we recommend preoperative administration of intravenous steroids, e.g., 8–10 mg of dexamethasone. Consideration should also be given to preoperative antibiotics. To reduce bleeding during decompression into the sinus cavities, patients can be given preoperative oxymetazoline spray (1–2 puff into the nostrils 3 times over 15 minutes), and their nasal passages are packed with neurosurgical patties soaked in a vasoconstrictor such as oxymetazoline, epinephrine, or cocaine.
Orbital Fat Decompression
The main surgical approach for orbital fat decompression is through the inferior fornix and usually does not require a canthotomy/cantholysis [6, 7, 38, 72, 73]. This approach allows good access to both intra- and extraconal fat, particularly inferolaterally, with reduced risk of collateral injury to important periocular structures. It is important to point out that removal of the preaponeurotic fat through an upper lid blepharoplasty approach does not qualify as an orbital fat decompression. While it can provide cosmetic rehabilitation (important as that might be), it does not reduce proptosis or orbital congestion. In general, extended dissection through the soft tissues of the superior orbit carries significant risk for collateral damage, including to the lacrimal gland and levator muscle. Fat decompression can usually be performed under conscious sedation and local anesthesia, with rapid recovery, and has relatively low risk of inducing new-onset strabismus.
The surgical approach to the inferolateral orbital fat compartments involves a conjunctival incision at least 4 mm inferior to the inferior edge of the tarsal plate, in a manner similar to the surgical approach to the orbital floor for fracture repair, or to the anterior fat pads for lower eyelid blepharoplasty. The anatomic boundaries for fat removal are the lateral orbital wall (consisting of the zygoma and greater wing of the sphenoid), lateral orbital floor (zygoma and maxillary bones), the lateral rectus muscle superiorly, the inferior rectus muscle medially, and the globe superonasally. Blunt dissection is used the release fibrotic clumps of fat from surrounding tissue followed by piecemeal excision while directly observing the rectus muscles to avoid injury. Some surgeons use a hemostat to clamp fat prior to excision, but care must be taken because muscle tissue can inadvertently get caught within the clamp. Usually, 2–3 mL of fibrotic fat can be excised, and we routinely measure this volume using a 3 or 5 mL syringe. Hemostasis is critical and can be obtained with bipolar cautery or other hemostatic agents such as thrombin-soaked surgical foam or fibrin sealant. Once hemostasis is obtained, the surgical incision is closed with suture.
Lateral Wall Decompression
Decompression of the lateral wall may be approached through a lateral upper eyelid crease incision or through a canthotomy/cantholysis. The authors have utilized both approaches and find the laterally extended upper eyelid crease approach to be superior regarding with the postoperative healing and avoiding unnecessary injury to key structures of the lateral commissure and canthal tendon.
Following infiltration with local anesthetic mix of the surgeon’s choice, dissection is carried down to the lateral orbital rim. Once the lateral orbital rim periosteum is exposed, it is incised with monopolar cautery and elevated off of the bony rim. A periosteal elevator is used to elevate the lateral periorbita to expose the perforating zygomaticotemporal and zygomaticofacial neurovascular bundles. These can be cauterized and divided with bipolar and monopolar cautery. With a malleable ribbon retractor in place, the lateral wall periorbita is elevated to expose the zygoma, sphenoid, and lateral frontal bones.
If desired, additional exposure can be obtained to the external lateral wall through the temporalis fossa. This is achieved by dissecting the periosteum from the lateral orbital rim to the insertion of the superficial and deep layers of the temporalis fascia and then sharply separating the fascia from the bone with monopolar cautery to expose the temporalis muscle. The fascia is then opened horizontally in a T-fashion with monopolar cautery at the level of the lateral commissure, taking care to stay less than 25 mm from the lateral rim to avoid the temporal branch of the facial nerve. The muscle is then carefully dissected from the lateral surface of the zygomatic bone with a sharp periosteal elevator, taking care to avoid excessive damage to the muscle fibers. This temporalis fossa dissection can greatly aid in maximizing exposure to the deep lateral orbit and provides another angle of approach for deep bone removal. It is particularly helpful in cases where the lateral orbital rim is kept in place intraoperatively.
The lateral orbital rim can be removed to enhance exposure, although we do not find this maneuver to be necessary in most cases. If removed, the lateral orbital rim itself should always be preserved and repositioned at the end of the surgery, as it serves an important protective and structural role for the globe and periocular tissues. Discarding the lateral rim does not provide any significant decompressive effect but only contributes to facial disfigurement and can aggravate eyelid malpositioning. Rim-sparing orbital decompression has been shown to provide efficacious outcomes while preserving the integrity of the lateral vertical buttress, and greater improvements in patient quality of life are reported compared to the rim-removal technique [74].
Removal of bone can be achieved with a combination of osteotomes and bone rongeurs for the thinner anterior portion of the lateral wall, and a high-speed drill or ultrasonic bone aspirator (e.g., Sonopet, Stryker Corp.) for deep bone removal [75] (Fig. 58.3). Several tips are available for both the drill and the ultrasonic aspirator, and surgeons should be sure to develop a good understanding of different tips prior to surgical use. In general terms, more aggressive tips such as the cutting burr or the ultrasonic Payner 360° tip remove bone faster but are more likely to damage soft tissue, whereas the reverse is true for less aggressive tips such as the diamond burr or ultrasonic Spetzler claw. The order of bone removal is dictated by surgeon preference, but most surgeons well start with the posterior zygoma, which is the thinnest portion of the lateral wall and is amenable to removal with osteotomes and Kerrison rongeurs. When using the osteotome, care must be taken to create the osteotomies anterior and inferior enough to avoid direct or indirect damage to the temporal and frontal lobes of the brain (Fig. 58.4). Complete removal of the posterior zygomatic bone provides excellent access to the sphenoid and frontal trigones. An alternative strategy, as described by Chang et al. [76], is to start the bone removal with the drill or ultrasound through the temporalis fossa approach posteriorly at the base of the sphenoid trigone and immediately expose the dura over the temporal lobe. This gives the surgeon a definitive posterior limit very early in the case, and bone removal proceeds only anteriorly from that point. However, exposure of dura can be unnecessarily risky, and there are other safer methods for assessing the extent of needed bone removal.
One may argue that dura should never be in contact with orbital muscle and connective tissues since dura is highly vascular and bioactive, releasing multiple neuropeptides that can lead to postoperative muscle enlargement, orbital inflammation, and swelling [77,78,79,80]. Indeed, the dura mater is highly biologically active tissue that in nature is never in contact with muscle or orbital connective tissue. Not only is the external dura highly vascular compared to internal dura – the reason why dural switch works for treating Moyamoya disease [81] – but the external dura also interacts robustly with the cranial bones and cranial sutures during development, to drive bone growth and suture closure timing [82, 83]. It does that by releasing TGF-beta, collagen I and III, osteocalcin, FGF, and other osteogenic cytokines. Furthermore, the dura is highly sensitive to mechanical tension changes [77], such as what would happen when you suddenly remove bone and leave a large surface area abutting orbital fat and muscle. Such tension changes can cause release of bioactive cytokines [78, 79]. Lastly, the dura is highly innervated [79, 84, 85], particularly with C fibers expressing CGRP intertwined with noradrenergic sympathetic fibers and non-peptidergic unmyelinated “C-like” A-delta fibers expressing NF200. These dural nerves respond to CGRP as well as substance P, neuropeptide Y, and serotonin, which regulate blood flow – an important potential regulator of tissue swelling. Substance P in particular is known to cause tissue swelling through a histamine-independent mechanism [86].
Once the trigone is exposed, bone removal is performed through the anterior cortical bone to expose the diploic space, which is filled with cancellous bone (Figs. 58.5 and 58.6). Bone removal continues through the diploe, during which time brisk bleeding is typically encountered and can be controlled intermittently with bone wax. An effective technique for application of bone wax is to place a small pea-sized sphere of wax on the wooden end of a cotton-tipped applicator (CTA) and use the CTA to loosely apply the wax to the bone surface. The CTA is then turned over and the cotton end used to press the wax firmly into the bleeding crevices of bone. Once the cancellous bone is removed and the cortical bone covering the temporal and frontal lobes is reached, the bleeding typically abates. The diploic space of the sphenoid is continuous with the diploe of the lateral frontal bone. To achieve a maximal decompression, the diploic space should be removed entirely to expose the inner cortical table of bone covering the temporal lobe posteriorly and the frontal lobe superiorly. Depending on the surgeon’s comfort level, the inner cortical table can be focally thinned to expose dura of the frontal and temporal lobes, thus ensuring that the limits of bone removal have been reached. However, exposure of large areas of dura is unnecessary and increases the risk of CSF leakage as well as the potential for altering orbital tissue biology through non-physiologic contact with dura mater, as described earlier [77,78,79]. Alternatively, intraoperative navigation can be used to confirm that maximal bone removal has been achieved without the need to expose dura. In addition to the sphenoid and frontal trigones, significant bone removal can be accomplished along the inferior orbital fissure and extend to the inferolateral orbital floor. Occasionally, the roof of the maxillary sinus may be exposed. Superolaterally, the bone above the lacrimal gland fossa can also be removed.
Decompression of the orbital floor, whether directly or through inferior extension of a lateral decompression, can lead to inferior globe displacement. The combination of posterior and inferior displacement of the globe has the secondary effect of reducing lower eyelid retraction while increasing upper eyelid retraction and can be customized to patients’ needs and overall surgical plan.
At this point, the periorbita is opened sharply to allow for herniation of soft tissue into the newly created space (Figs. 58.7 and 58.8). Adjunctive fat excision can now be performed, with excellent access to both inferolateral and superolateral fat compartments. Because of the increased risk of bleeding and injury to the lacrimal gland, superolateral fat debulking is usually reserved for the most severe cases of orbitopathy. However, the authors routinely debulk inferolateral orbital fat, with good effect on both proptosis and lower eyelid contour/fullness.
If the rim was removed, it can now be repositioned with either titanium plates or screws or sutured through pre-drilled holes. The periosteum is closed over the rim with absorbable sutures, and the surgical incision is closed using sutures of the surgeon’s choice.
Medial Transconjunctival Approach to the Orbit
The medial transconjunctival approach to orbital decompression was developed by Shorr et al. in the 1990s [27]. While the incision was originally described as being made through the lateral third of the caruncle (“transcaruncular”), most surgeons now place the incision immediately lateral to the caruncle, for which some use the term “retrocaruncular .” While the terms “transcaruncular ” and “retrocaruncular ” refer to slightly different locations of incision placement, they are often used interchangeably. In this chapter, we will refer to both of these approaches jointly as the medial transconjunctival approach to the orbit. Prior to the introduction of the medial transconjunctival approach, the transcutaneous Lynch incision was the most common approach to the medial orbit, but its use has largely been abandoned by orbital surgeons.
Following infiltration of local anesthetic of the surgeon’s choice, the caruncle and plica semilunaris are grasped and retracted away from one another. Westcott scissors are used to make the conjunctival incision between these structures (i.e., retrocaruncular), curving the incision supero- and inferolaterally to follow the contour of the plica. Curved Stevens scissors are placed through the incision in a posteromedial direction at a 45° angle in the axial plane and used to bluntly dissect tissue down to the posterior lacrimal crest, while the globe is retracted with a small malleable ribbon retractor and the caruncle with a small retractor or skin hook. This dissection should be tangential to the globe to prevent damage of the (frequently enlarged) medial rectus muscle (Fig. 58.9). Once exposed, the periosteum over the posterior lacrimal crest is incised vertically and carefully elevated to expose the medial orbital wall (Fig. 58.10). Subperiosteal dissection is then carried along the lamina papyracea with periosteal elevators and malleable ribbon retractors. Every effort should be made to keep the periorbita intact during this dissection, as this can improve visualization and decrease the risk of damage to intraorbital structures. A critical aspect of this surgical approach is the identification of the anterior and posterior ethmoidal foramina, which typically demarcate the frontoethmoidal suture and with it the superior boundary of decompression (Fig. 58.11). Any bone removal above this suture line may result in entry into the anterior cranial fossa. The anterior ethmoidal neurovascular bundle is cauterized and may be divided with bipolar and monopolar cautery. The posterior ethmoidal neurovascular bundle is located only 4–7 mm away from the optic foramen, and may or may not be divided, depending on surgeon preference. This important landmark usually demarcates the posterior superior edge of the bone removal. It should be noted that variations exist in the anatomic position of the ethmoidal vessels, so the frequently quoted 24-12-6 rule, as well as their location at the frontoethmoidal suture, cannot be considered absolute [87]. Careful study of a preoperative CT scan, and possibly use of stereotactic navigation, can greatly assist with identifying and utilizing appropriate landmarks.
Medial transconjunctival wall decompression. (Reproduced with permission from [27])
Decompression begins by fracturing the lamina papyracea with an elevator or suction tip and removing the thin medial wall with nasal forceps (e.g., Takahashi or Blakesley). As the bone becomes thicker near the frontoethmoidal suture or inferomedial strut, rongeurs or an ultrasonic bone aspirator can be used if necessary. A useful surgical approach is to remove the posterior part of the medial orbital wall first, since the sinus mucosa on the other side can bleed and anterior bleeding will accumulate posteriorly, obscuring good visualization of the orbital apex. By extension, the best time to visualize the orbital apex and to plan out the deep part of the surgery is prior to any bone removal. Once the lamina papyracea is removed to the extent planned, a complete ethmoidectomy can be performed with nasal forceps to reduce bleeding, improve the space enhancement, and prevent postoperative sinusitis. The sphenoid sinus will be encountered at the posterior end of the ethmoid, and this defines the posterior limit of bone removal. Venturing further into the sphenoid sinus does not add appreciably to the decompressive effect and carries a high risk of damage to the optic nerve and internal carotid artery. Once the ethmoidectomy is complete, the orbital process of the palatine bone and the inferomedial strut can be visualized and removed with an osteotome or ultrasonic aspirator. If using an osteotome, care must be taken to direct energy away from the optic foramen and fovea ethmoidalis. If floor decompression is planned in conjunction with the medial wall, the posterior half to two-thirds of the floor can be removed with nasal forceps up to the infraorbital canal. Leaving the anterior third of the floor intact is thought to decrease the risk of postoperative diplopia and globe dystopia.
An important consideration of medial orbital decompression is strabismus, as this technique is known to be associated with greater degree of induced strabismus [88, 89]. The authors have noted that leaving 10–12 mm of anterior medial wall posterior to the posterior lacrimal crest reduces significantly the risk of induced severe strabismus. Our current approach in most patients is to set the anterior boundary of the medial decompression at the level of the anterior ethmoid foramen (Fig. 58.12).
Once the bony decompression is completed, the periorbita is opened sharply with scissors or a sickle blade to allow for soft tissue herniation into the newly created space. The medial transconjunctival incision may be left to heal by secondary intention or closed with a buried absorbable suture [90].
Orbital Floor Decompression
The orbital floor can be accessed via an inferior fornix approach, similar to repair of an orbital blowout fracture repair. Because this is a very familiar approach to most orbital surgeons, it is a commonly used technique for decompression. And because the bone is thin, bone removal can be easily achieved with minimal instrumentation while avoiding injury to the infraorbital nerve. However, it is more likely to cause worsening strabismus and globe dystopia, carries a high risk of infraorbital hypoesthesia, and does not provide good access to the orbital apex. As a result, most specialists in thyroid eye disease prefer to decompress the lateral and medial walls, in what has been referred to as a “balanced” decompression [20, 44, 91,92,93]. The floor decompression has now become more of an adjunctive procedure for patients in whom a balanced decompression provides insufficient proptosis reduction or in whom the compartment syndrome is so severe that maximal decompression is required.
Endonasal Endoscopic Orbital Decompression (Medial Wall and Floor)
The endonasal approach to orbital decompression is frequently performed by otolaryngologists, often with some involvement by an ophthalmologist. It is a good approach that offers good visualization and access to the medial orbital wall, orbital floor, and the orbital apex [10, 32]. When an orbital surgeon is not easily available, this approach provides a good alternative for many patients. Once the nasal passages are vasoconstricted, the endoscope is introduced into the nose, the middle turbinate is endoscopically medialized, and an uncinectomy performed. A complete endoscopic ethmoidectomy is then performed with sinus forceps and/or a microdebrider. The anatomic limits of the dissection are the frontal sinus ostium anteriorly, sphenoid sinus posteriorly, fovea ethmoidalis superiorly, lamina papyracea laterally, and middle turbinate medially. A frontal sinusotomy can be done to ensure patency of the ostium. The sphenoid sinus may be entered using a sphenoid punch to create a 4 × 4 mm opening, although entering the sphenoid sinus is usually not necessary for an orbital decompression. Nasal antral windows are fashioned by cannulating and expanding the natural ostium of the maxillary sinus. Endonasal access is now complete, allowing for removal of bone from the medial orbital wall and floor. The periorbita is then opened to allow herniation of soft tissue into the newly created space. Extreme care must be taken to avoid injury to the extraocular muscles. Additional care must be taken to avoid removal of the anterior aspect of the inferomedial strut, in order to reduce the risk of worsening strabismus and globe dystopia [94, 95].
Although endoscopic decompression with inferomedial strut preservation was once considered technically difficult due to concerns of potential limited orbital floor access [96], advances in endoscopic surgery have demonstrated the feasibility of accessing the orbital floor with this technique [97]. A modification of this method, with selective endoscopic removal of the posterior inferomedial strut and preservation of the anterior aspect of the strut, can provide deeper decompression and reduce diplopia [98]. However, the level of expertise required is higher. In general, a direct orbital approach to decompression is usually favored unless not available to the patient.
Postoperative Considerations
Following orbital decompression surgery, patients are prescribed oral analgesic medication and cold compresses for 3–5 days, along with the usual regimen of incisional care including an antibiotic ointment. They should be instructed to avoid exertion, bending, or lifting for at least 2–3 days postoperatively – the key time period for postoperative bleeding. Following surgery in the sinuses, nasal saline spray can be prescribed to facilitate healing and reduce postoperative congestive symptoms.
Overall, the list of potential complications of orbital decompression is quite long [65, 99, 100] and may vary based on surgical technique [101]. The most important and common risks include infection, worsened strabismus/diplopia, and postoperative hemorrhage causing an acute compartment syndrome. Temporary postoperative hypoesthesia has been reported in up to 29% of lateral decompressions and 17% of other bony decompressions [65]. CSF leak is a rare, potentially morbid, complication; it may be more common in lateral than medial approaches [102, 103], but because the lateral exposure is greater and orbital tissues can tamponade a leak, the medial CSF leak is usually considered more consequential. Additionally, temporal wasting may occur, particularly in patients undergoing a coronal approach [102]. While the risk of postoperative new-onset strabismus is present for any decompression surgery, this risk appears to be highest with medial and floor decompression and lowest with lateral and fat decompression surgery [88, 104, 105]. Diligent follow-up of patients in the postoperative period is critical in order to detect potential complications and monitor healing.
Strabismus Surgery
Extraocular muscle surgery for TED is routinely performed by a pediatric ophthalmologist or neuro-ophthalmologist to address ocular misalignment and any associated diplopia. Ocular misalignment may be affected by orbital decompression surgery – positively or, more commonly, negatively [35, 80, 88, 104, 105]. Hence, patients who need orbital decompression should undergo that procedure prior to any strabismus surgery. Ideally, the orbital and strabismus surgery teams work closely together to optimize outcomes through proper sequencing and timing of surgeries. When available, orthoptic examinations before and after decompression surgery can provide useful information for planning eventual strabismus surgery.
In the past, conventional wisdom dictated that strabismus surgery should be delayed at least 6 months after decompression surgery. However, with improved coordination between orbital and strabismus surgeons, availability of more frequent orthoptic measurements, and the more modern surgical techniques for orbital decompression (as outlined above), strabismus surgery may be performed as early as 6–8 weeks after decompression surgery, with good results as long as measurements were stable postoperatively.
Once a patient is ready for strabismus surgery, muscle recession is usually performed in TED to weaken the action of fibrotic muscles. The most commonly affected muscles are the inferior and medial rectus muscles, and these are often the targets of muscle recession surgery. Restrictive EOM disease can be challenging to treat since the altered muscles may not respond to surgery in a predictable fashion [106]. Additionally, recessing fibrotic muscles may worsen proptosis [1] and potentially even induce optic nerve stretching, which is a risk for vision loss. More frequently, it may worsen lower eyelid retraction [107, 108].
Optimal outcomes for strabismus surgery necessitate stability in ocular alignment measurements as documented over time. Since patients who smoke often have a less stable disease course, achieving optimal alignment in smokers with TED may be impossible. Patients should therefore be strongly encouraged to cease smoking prior to initiation of surgical rehabilitation.
A full discussion of the techniques involved in strabismus surgery for TED is beyond the scope of this chapter. There are many variations in use of absorbable versus nonabsorbable and adjustable versus nonadjustable sutures, but overall the success rate of allowing patients to fuse without prisms or with less than 4 prism diopter correction is quite high in experienced hands.
An important consideration is the effect of strabismus surgery on eyelid position. In patients with a restricted inferior rectus muscle, attempting to bring the eye to primary position results in secondary upper eyelid retraction. Such retraction is improved following inferior rectus recession. However, recessing the inferior rectus can worsen lower eyelid retraction, which can be partially mitigated by carefully separating the inferior rectus muscle from the lower eyelid retractors during the recession surgery.
Eyelid Surgery
Etiology of Eyelid Changes in TED
Eyelid retraction is the most common feature of TED. The etiology of upper eyelid retraction is multifactorial, including fibrosis of the upper eyelid retractors (similar to EOMs), increased sympathetic tone, and extreme effort to supraduct the globe against a restricted inferior rectus muscle (i.e., pseudoretraction) [109,110,111]. Lower eyelid retraction is also multifactorial, including fibrosis of the lower eyelid retractors, increased sympathetic tone, or globe malposition/proptosis. It may be exacerbated by malar hypoplasia, and assessment of photos from prior to disease onset can be very helpful in assessing the patient’s natural lower eyelid position.
Whenever ptosis is noted in a patient with TED, the diagnosis of myasthenia gravis must be considered, since it occurs in approximately 5% of patients with TED [112]. Other eyelid findings that may be encountered in TED include dermatochalasis, anterior herniation of the orbital fat pads, and periorbital soft tissue expansion (i.e., thyroid periorbitopathy [113]) (Fig. 58.13).
While many surgical techniques for eyelid reconstruction can be employed, the role of a good decompression in improving eyelid position is important [114]. An effective decompression can reduce axial proptosis and sometimes reduce or even eliminate lower lid retraction without any additional lower lid surgery [115, 116].
Alternatives to surgery, such as the historic use of the topical sympatholytic guanethidine [117, 118] and local injection of botulinum toxin or hyaluronic acid filler [119,120,121], are limited by additional corneal surface toxicity and temporary effect, respectively. Additionally, botulinum toxin has the known side effects of ptosis and diplopia from iatrogenic extraocular muscle palsy.
Eyelid retraction surgery is required when medical therapy, including topical lubricant drops and ointments, patching, and moisture chambers, is unsuccessful in addressing corneal compromise in TED [122]. In addition, the psychosocial effects of disfigurement secondary to eyelid retraction should not be underestimated [123].
The simplest surgical approach to eyelid retraction is a tarsorrhaphy – lateral, medial, or both. However, this is a disfiguring surgery that should only be used when patients are not good candidates for more definitive and physiological surgery.
The standard approach to eyelid retraction surgery is a lid-lengthening procedure. In the upper eyelids, this involves recession of the upper lid retractors. There is rarely (if ever) a need to place a spacer graft in the upper eyelid. In the lower eyelids, surgical repair also includes retractor and conjunctival recession. However, a spacer graft is sometimes required.
Blepharoplasty , brow lift, and sub-brow fat debulking are all excellent adjunctive procedures that can be performed as part of the surgical rehabilitation of patients with TED. These techniques are not TED-specific and have been described well elsewhere [124,125,126,127,128]. Importantly, they should not be combined with eyelid retraction surgery since the additional scarring may undermine the surgical effort to release scar tissue. Eyelid retraction repair should be performed first, and adjunctive procedures can be performed once the patient has healed and stability achieved.
Surgical Techniques
Upper Eyelid Recession
There are two general approaches to upper eyelid retraction repair: percutaneous and transconjunctival. Both approaches can be successful, and each technique has its advantages and disadvantages. The percutaneous approach (e.g., full-thickness blepharotomy) is more likely to raise the lid crease – an acceptable result in many women but potentially unacceptable in men. It also injures the orbicularis muscle, which functions to close the eyelid, and hence its preservation can be important. The septum may be opened in the percutaneous technique, which can cause additional scarring as the eyelid heals. Nevertheless, this approach offers excellent exposure, ease of hemostasis, and full control of eyelid contour. The posterior approach is technically more demanding, with reduced exposure and more challenging hemostasis. However, it avoids a cutaneous scar and injury to the orbicularis or septum. For both techniques, the ability to sit the patient intraoperatively and make final adjustments with the patient awake and alert can be extremely helpful.
Anterior Approach (Full-Thickness Blepharotomy)
The incision is made at the desired position of the eyelid crease, although final length of the incision can vary significantly. In the standard approach, each tissue layer is incised in turn until the conjunctiva is encountered, with retention of a bridge of tissue that would form the peak of the eyelid and with careful consideration of eyelid margin contour. In the variant introduced by Leo Koornneef and popularized by Elner et al. [129], a protective plate is introduced under the eyelid, and a #15 blade is used to make a full-thickness incision medial to lateral through the skin, muscle, septum, levator, Müller, and conjunctiva while still leaving a small bridge at the desired point of an eyelid peak. Both approaches offer excellent control. Overcorrection can be repaired by suturing conjunctiva and/or retractors with 6-0 Vicryl suture. Adjustments should be made with the patient in a sitting position. Wound closure can be layered (orbicularis and skin) or just skin.
Posterior Approach
The posterior approach involves a palpebral conjunctival incision. The fornix is infiltrated with a small amount of local anesthetic, and the presence or absence of epinephrine depends on surgeon preference. Presence of epinephrine can alter eyelid height, which needs to be compensated for based on experience. Additional anesthesia can be injected along the frontal nerve, as a nerve block that will only minimally affect eyelid position.
A traction suture is placed through the central upper eyelid, and the lid everted over a baby Desmarres retractor (in a manner similar to performing a Mullerectomy surgery for ptosis repair). Westcott scissors are used to incise conjunctiva 4–5 mm superior to the superior tarsal border on either side of the centrally placed traction suture. Westcott scissors are then used to carefully incise eyelid retractors in a graded fashion, with frequent assessment of eyelid height and contour with patient cooperation. Bipolar cautery is used for hemostasis. Once eyelid height and contour are close to ideal, the patient is brought to a sitting position and final adjustments made. Overcorrection can be repaired with 6-0 Vicryl suture to the conjunctiva and/or retractors. Once ideal eyelid height, contour, and symmetry are achieved, the traction suture is cut, and the operation is over – there is no need to close the surgical incision.
Lower Eyelid Retraction Repair
As in upper eyelid retraction repair, the lower eyelid can be lengthened either through an anterior or posterior approach. It is technically easier to perform the posterior, transconjunctival approach for the lower eyelid. Spacer graft materials are more frequently needed in the lower lid and can provide 3–4+ mm of elevation. Examples of lower eyelid donor materials include autologous cartilage (ear or nasal septum), autologous hard palate, donor sclera, acellular dermis (human, bovine or porcine), or an alloplastic material such as porous polyethylene [130,131,132,133,134,135,136,137].
Regardless of the spacer material used, a similar surgical approach is employed. First, the lower eyelid is injected with lidocaine +/− epinephrine. An incision is then made in the conjunctiva 2–4 mm inferior to the inferior border of the tarsus (Fig. 58.14). This may be combined with a lateral canthotomy and inferior cantholysis if lateral eyelid tightening is required. The lower eyelid retractors are then identified and disinserted from the tarsus. A spacer graft is placed into the newly created pocket between the retractors and tarsal border and sutured into position with an absorbable suture such as 6-0 Vicryl, keeping the knots away from the ocular surface. A double-armed bolster suture is placed through the spacer graft, exiting on skin and tied over a bolster of the surgeon’s choosing. If a lateral tarsal suspension is required, a sufficiently long lateral tarsal strip is then created to allow for appropriate tightening of the lower lid margin. The lateral tarsal strip is fixed to the periosteum of the lateral orbital rim in the usual fashion.
(a) Lower eyelid retraction secondary to adhesions in and around the capsulopalpebral fascia. Lower eyelid retraction (b) is approached internally. A skin hook is placed beneath the inferior tarsal border, and the lower eyelid is everted. A conjunctival incision is made beneath the inferior tarsal border (dotted line) (c). A sagittal view demonstrates the severance of the capsulopalpebral fascia from the inferior tarsal border (d). A spacer graft is inserted beneath the inferior border of the tarsus and the advancing recessed edge of the retractors. The graft does not need to be sutured in its entirety but stabilized with a percutaneous through-and-through mattress suture (e). Two mattress sutures are used to stabilize the graft nasally and temporally and tied over bolsters (f)
Spacer Graft Materials
A variety of spacer graft materials are available for use in eyelid retraction repair. The choice of graft material varies by surgeon, though the most frequently utilized grafts in modern practice are autologous cartilage, hard palate, and acellular dermis. Dermis fat graft (DFG) is a reasonable option for some patients, but because the fat compartment of TED patients is already expanded, DFG is usually not the first option in most patients with lower eyelid retraction.
Autologous Auricular Cartilage
Autologous auricular cartilage has the benefit of producing predictable postoperative results due to minimal graft shrinkage and postoperative inflammation. Auricular cartilage is harvested from the superior aspect of the ear, using the flattest area between the helix and antihelix (Fig. 58.15). Either anterior or posterior approaches can be used for the harvest. After administration of local anesthesia, a curved 15–20 mm skin incision is made just behind the helix, and the cartilage is exposed with blunt dissection. The graft is then harvested with a no. 15 Bard-Parker blade or razor blade knife. For graft sizing, a 1:1 vertical graft height to lower eyelid elevation ratio is typically used. Minimal cautery is used for hemostasis, with care to avoid cautery to the cartilage, which can lead to significant postoperative pain. Primary closure of the ear skin incision is then performed.
Auricular cartilage is much thicker than other spacers and certainly much thicker than an eyelid that has undergone only levator recession. The flattened portion of the posterior ear at the junction between the ear and skull is used (a–c). The graft is difficult to thin, because it is brittle and friable. It is possible to thin the graft by stabilizing it to a cutting block with sterile cyanoacrylate glue or even Mastisol (d). The graft is then shaved with mucotome or dermatome to thin it without breakage (e). The graft is elevated off the cutting block with a Freer elevator (f) to break the temporary adhesive connection. (g) Preoperative retraction. (h) Postoperative view at 2 months after auricular cartilage spacer utilization
Because ear cartilage has a curve to it, the cartilage needs to be treated to reduce its rigidity and curvature. This can be done with a Septal Morselizer (available from numerous manufacturers) or by scoring with a #15 blade. The cartilage is then placed into the posterior lamellar pocket of the treated eyelid and sutured into position using buried interrupted 6-0 Vicryl sutures. One edge of the graft should be sutured to retractors and the other to the edge of tarsus. Since auricular cartilage is not epithelialized, the graft should be covered posteriorly with conjunctiva. If two eyelids need to be treated simultaneously, the graft can be divided to provide two right triangles. A key maneuver in the grafting of ear cartilage to the eyelid is the placement of a bolster suture in a horizontal mattress fashion through the center of the graft, exiting on skin where it is tied over a bolster. A 5-0 Prolene is usually used for this purpose, to make sure that the surface of the cartilage graft is tightly adherent to the septum and will not be displaced toward the eye during the acute healing process.
Hard Palate Mucosa
When used as a lower eyelid spacer, hard palate mucosa offers the advantages of an epithelial lining, minimal postoperative graft shrinkage, and ready availability [131]. Hard palate mucosa is similar in structure to native tarsus. The graft is taken from the hard palate between the gingival processes and the midline raphe (Fig. 58.16). The prospective site is marked and submucosally injected with local anesthetic. During dissection, care is taken to avoid the incisive and greater palatine foramina, which transmit the nasopalatine and greater palatine neurovascular bundles, respectively.
Hard palate mucosal grafting . (a) A bite plate or silicone block is used to stabilize the mouth. (b1) The prospective donor site is marked and injected with local anesthetic with epinephrine. The nasopalatine neurovascular bundle is avoided, and the greater palatine vascular bundle is also avoided. (b2) Demonstrates the ballooning of the hard palate mucosal graft with local anesthesia with adrenaline. It is preferred to allow 2–5 min for vasoconstrictive effect. (c) The graft is harvested by outlining the mucosal site with a cutting Bovie on a sharp-pointed tip such as a Colorado needle. (d) The graft is harvested using a right-angle sharp blade such as a super blade or crescent knife or #57 Beaver blade. The graft is undermined, but an attempt is made to keep it thin. (e) Hemostasis is obtained with cautery or a bone wax pledget. (f) Immediate appearance of the hard palate mucosal grafting site. (g), Appearance at 1 month after surgery
The desired graft size is harvested with a #57 Beaver blade and dissected from the underlying submucosa with a crescent-shaped blade (Fig. 58.17). Electrocautery should be avoided as much as possible, and gel foam may be placed in the donor bed to control bleeding. The graft is then thinned with a pair of curved Stevens scissors and sewn into position in the recipient bed with 6-0 chromic or Vicryl sutures (Fig. 58.18). The lower eyelid is then placed on upward stretch with a Frost suture for 1 week. The donor site heals in the postoperative state by reepithelialization (Fig. 58.19). A pre-made dental splint can be used postoperatively to reduce discomfort while eating.
(a) The graft must be debulked by thinning with a curved Stevens scissors and removing all subcutaneous tissue. (b) This spacer can be used in either upper or lower eyelids, but sutures are avoided on the posterior surface. The graft is located in its interpositional space, and a double-armed 6-0 suture is led through the graft to be tied externally over bolsters or up through the upper eyelid to keep the grafted lid on the stretch and then secured over a bolster (c). Preoperative appearance of a patient with thyroid eye disease and slight lower eyelid retraction. This was objectionable to the patient aesthetically, and he underwent a hard palate mucosal graft (d) tied over bolsters (e). His appearance at 1 month after surgery demonstrates an elevation of both lower eyelids of approximately 2 mm (f). His lids are now above the inferior limbus compared with 1–2 mm of lower eyelid retraction preoperatively (g)
Acellular Dermis
Acellular human, porcine, and bovine dermal matrices are available as viable alternatives for autogenous upper [136, 138] and lower eyelid [137, 139] spacer grafts. These materials are derived from cadaveric human, porcine, or bovine dermis and are immunologically inert. They offer enough structural rigidity to replace tarsus while allowing for conjunctival epithelial migration and graft surface repopulation [133, 137, 140, 141]. Unlike autogenous human tissue, acellular human dermis avoids the need for tissue harvesting at time of surgery. Although this material has excellent handling properties and is associated with minimal inflammation, its long-term effects may not be as long lasting as seen with hard-palate grafting due to graft shrinkage [141]. Porcine acellular dermis is available ready for use as a prehydrated dermal matrix. Bovine acellular dermal matrix is also available, though this material needs to be hydrated prior to use. These materials are both relatively straightforward to use surgically since neither has a particular orientation [137].
Free Tarsal Grafts
In TED, use of free tarsal grafts for lower lid retraction requires that the upper eyelid from which the graft is taken is not retracted, as this would worsen the retraction by shortening the posterior lamella. Therefore, this approach is typically avoided in TED due to the potential for contralateral eye involvement.
A free tarsal graft is prepared by everting the donor upper eyelid on a Desmarres retractor. Then, a straight incision is made parallel to the lid margin 3 mm superior to the margin at the same horizontal length as the corresponding lower eyelid conjunctival incision. At least 3 mm of vertical tarsal height must remain to maintain the structural integrity of the upper lid. A second incision is made 4–5 mm superior to the first to form an ellipse of tarsus, which is dissected from the upper lid. The tarsal graft is then sutured into the recipient bed in the lower eyelid with interrupted or running 6-0 suture (usually gut or Vicryl) (Fig. 58.20).
Free tarsal grafts can be used to elevate a retracted lower eyelid if the patient happens to have unilateral ptosis (a). A free tarsal graft can be harvested, similar to a Fasanella-Servat specimen (b). This is transferred to a similar incision as produced in Fig. 58.14, to recess the retractors and space the gap (c). The conjunctival incision does not need to be closed because this is a composite graft with conjunctiva intact. (d) This graft also is stabilized nasally and temporally and tied over external bolsters with a mattress suture of 6-0 silk. The conjunctiva is not sutured closed but is left well opposed to the surrounding conjunctival edges
Alloplastic Material
Alloplastic material, such as porous polyethylene, can effectively raise the lower lid height and has been shown to bio-integrate on a histopathologic level [142, 143]. However, there is a high incidence of complications with this material, including revision and exposure. Therefore, porous polyethylene spacers are best used in refractory cases not responding to other techniques [142, 143].
Banked Sclera
Sclera was historically used for spacer grafts, though it has fallen out of favor in modern practice as the more desirable aforementioned options have become available.
Results
Limited studies are currently available that analyze the surgical results of these various spacer graft materials. With regard to surgical outcomes comparing autogenous auricular cartilage, bovine acellular matrix, and porcine acellular matrices for lower lid retraction repair, a recent prospective, randomized study by Barmettler and Heo found no significant difference among the three groups [137].
Postoperative Considerations
Following lid retraction surgery, patients are discharged the same day and instructed to follow up in a clinic for re-evaluation within 1 week. An antibiotic or a combination of antibiotic-steroid ointment is prescribed topically for the surgical wounds. If an underlying corneal epithelial disease is present, the patient should continue ocular surface treatment as per the managing ophthalmologist.
In the event that initial eyelid retraction surgery results in postoperative ptosis or residual retraction, additional surgery may be needed depending on the level of patient satisfaction and functional outcome. Among the surgical options available, repeat posterior lamellar grafting [144] for persistent retraction and “hang-back” suture repair of secondary ptosis [145] may be considered. In general, it is always easier to address an undercorrected upper eyelid than an eyelid that becomes ptotic following retraction surgery. The fibrosis of the retractors makes ptosis surgery more challenging. Hence, we recommend that surgeons err on the side of undercorrecting vs. overcorrecting upper eyelid retraction. With spacer grafts, lower eyelids typically remain where the surgeons placed them, with good consistency of outcomes.
Summary
Surgical management of the TED begins with careful assessment of the patient and thoughtful planning regarding the timing and approach to surgical rehabilitation. A variety of surgical techniques have been developed to address the functional and cosmetic complications of TED, and these approaches can be tailored to the needs of the individual patient. Traditionally, surgery is performed in a stepwise fashion, first with orbital decompression, followed by strabismus surgery, then lastly, eyelid repositioning. Careful selection of patients for surgery and clear explanation of the limitations and possible complications of surgery will maintain patient satisfaction and maximize successful surgical results.
References
Gomi CF, Yang SW, Granet DB, et al. Change in proptosis following extraocular muscle surgery: effects of muscle recession in thyroid-associated orbitopathy. J AAPOS. 2007;11(4):377–80.
Lund VJ, Larkin G, Fells P, Adams G. Orbital decompression for thyroid eye disease: a comparison of external and endoscopic techniques. J Laryngol Otol. 1997;111(11):1051–5.
van der Wal KG, de Visscher JG, Boukes RJ, Smeding B. Surgical treatment of Graves orbitopathy: a modified balanced technique. Int J Oral Maxillofac Surg. 2001;30(4):254–8.
Rizk SS, Papageorge A, Liberatore LA, Sacks EH. Bilateral simultaneous orbital decompression for Graves’ orbitopathy with a combined endoscopic and Caldwell-Luc approach. Otolaryngol Head Neck Surg. 2000;122(2):216–21.
Weisman RA, Osguthorpe JD. Orbital decompression in Graves’ disease. Arch Otolaryngol Head Neck Surg. 1994;120(8):831–4.
Lyons CJ, Rootman J. Orbital decompression for disfiguring exophthalmos in thyroid orbitopathy. Ophthalmology. 1994;101(2):223–30.
McCord CD. Current trends in orbital decompression. Ophthalmology. 1985;92(1):21–33.
Kennerdell JS, Maroon JC. An orbital decompression for severe dysthyroid exophthalmos. Ophthalmology. 1982;89(5):467–72.
Paridaens DA, Verhoeff K, Bouwens D, van Den Bosch WA. Transconjunctival orbital decompression in Graves’ ophthalmopathy: lateral wall approach ab interno. Br J Ophthalmol. 2000;84(7):775–81.
Tyler MA, Zhang CC, Saini AT, Yao WC. Cutting-edge endonasal surgical approaches to thyroid ophthalmopathy. Laryngoscope Investig Otolaryngol. 2018;3(2):100–4.
Zhang-Nunes SX, Dang S, Garneau HC, et al. Characterization and outcomes of repeat orbital decompression for thyroid-associated orbitopathy. Orbit. 2015;34(2):57–65.
Korkmaz S, Konuk O. Surgical treatment of dysthyroid optic neuropathy: long-term visual outcomes with comparison of 2-wall versus 3-wall orbital decompression. Curr Eye Res. 2016;41(2):159–64.
Bernardini FP, Nerad J, Fay A, Zambelli A, Cruz AA. The revised direct transconjunctival approach to the orbital floor. Ophthalmic Plast Reconstr Surg. 2017;33(2):93–100.
Cruz AA, Leme VR. Orbital decompression: a comparison between trans-fornix/transcaruncular inferomedial and coronal inferomedial plus lateral approaches. Ophthalmic Plast Reconstr Surg. 2003;19(6):440–5; discussion 445
Abuzayed B, Tanriover N, Gazioglu N, Eraslan BS, Akar Z. Endoscopic endonasal approach to the orbital apex and medial orbital wall: anatomic study and clinical applications. J Craniofac Surg. 2009;20(5):1594–600.
White WA, White WL, Shapiro PE. Combined endoscopic medial and inferior orbital decompression with transcutaneous lateral orbital decompression in Graves’ orbitopathy. Ophthalmology. 2003;110(9):1827–32.
Ference EH, Sindwani R, Tan BK, et al. Open versus endoscopic medial orbital decompression: utilization, cost, and operating room time. Am J Rhinol Allergy. 2016;30(5):360–6.
Mueller SK, Freitag SK, Bleier BS. Morphometric analysis of the orbital process of the palatine bone and its relationship to endoscopic orbital apex surgery. Ophthalmic Plast Reconstr Surg. 2018;34(3):254–7.
Leone CR, Piest KL, Newman RJ. Medial and lateral wall decompression for thyroid ophthalmopathy. Am J Ophthalmol. 1989;108(2):160–6.
Shepard KG, Levin PS, Terris DJ. Balanced orbital decompression for Graves’ ophthalmopathy. Laryngoscope. 1998;108(11 Pt 1):1648–53.
Kikkawa DO, Pornpanich K, Cruz RC Jr, Levi L, Granet DB. Graded orbital decompression based on severity of proptosis. Ophthalmology. 2002;109(7):1219–24.
Richter DF, Stoff A, Olivari N. Transpalpebral decompression of endocrine ophthalmopathy by intraorbital fat removal (Olivari technique): experience and progression after more than 3000 operations over 20 years. Plast Reconstr Surg. 2007;120(1):109–23.
Kazim M, Trokel SL, Acaroglu G, Elliott A. Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol. 2000;84(6):600–5.
Goldberg RA. Advances in surgical rehabilitation in thyroid eye disease. Thyroid. 2008;18(9):989–95.
Graham SM, Thomas RD, Carter KD, Nerad JA. The transcaruncular approach to the medial orbital wall. Laryngoscope. 2002;112(6):986–9.
Gockeln R, Winter R, Sistani F, Kretschmann U, Hussein S. Minimal invasive decompression of the orbit in Graves’ orbitopathy. Strabismus. 2000;8(4):251–9.
Shorr N, Baylis HI, Goldberg RA, Perry JD. Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology. 2000;107(8):1459–63.
Schaefer SD, Soliemanzadeh P, Della Rocca DA, et al. Endoscopic and transconjunctival orbital decompression for thyroid-related orbital apex compression. Laryngoscope. 2003;113(3):508–13.
Wee DT, Carney AS, Thorpe M, Wormald PJ. Endoscopic orbital decompression for Graves’ ophthalmopathy. J Laryngol Otol. 2002;116(1):6–9.
Tooley AA, Godfrey KJ, Kazim M. Evolution of thyroid eye disease decompression-dysthyroid optic neuropathy. Eye (Lond). 2019;33(2):206–11.
Cubuk MO, Konuk O, Unal M. Orbital decompression surgery for the treatment of Graves’ ophthalmopathy: comparison of different techniques and long-term results. Int J Ophthalmol. 2018;11(8):1363–70.
Kingdom TT, Davies BW, Durairaj VD. Orbital decompression for the management of thyroid eye disease: an analysis of outcomes and complications. Laryngoscope. 2015;125(9):2034–40.
Mehta P, Durrani OM. Outcome of deep lateral wall rim-sparing orbital decompression in thyroid-associated orbitopathy: a new technique and results of a case series. Orbit. 2011;30(6):265–8.
Boboridis KG, Uddin J, Mikropoulos DG, et al. Critical appraisal on orbital decompression for thyroid eye disease: a systematic review and literature search. Adv Ther. 2015;32(7):595–611.
Wu CY, Niziol LM, Musch DC, Kahana A. Thyroid-related orbital decompression surgery: a multivariate analysis of risk factors and outcomes. Ophthal Plast Reconstr Surg. 2017;33(3):189–95.
Wu CY, Stacey AW, Kahana A. Simultaneous versus staged balanced decompression for thyroid-related compressive optic neuropathy. Ophthal Plast Reconstr Surg. 2016;32(6):462–7.
Prat MC, Braunstein AL, Dagi Glass LR, Kazim M. Orbital fat decompression for thyroid eye disease: retrospective case review and criteria for optimal case selection. Ophthal Plast Reconstr Surg. 2015;31(3):215–8.
Braun TL, Bhadkamkar MA, Jubbal KT, Weber AC, Marx DP. Orbital decompression for thyroid eye disease. Semin Plast Surg. 2017;31(1):40–5.
Trokel SL, Jakobiec FA. Correlation of CT scanning and pathologic features of ophthalmic Graves’ disease. Ophthalmology. 1981;88(6):553–64.
Peyster RG, Ginsberg F, Silber JH, Adler LP. Exophthalmos caused by excessive fat: CT volumetric analysis and differential diagnosis. AJR Am J Roentgenol. 1986;146(3):459–64.
Goldberg RA, Kim AJ, Kerivan KM. The lacrimal keyhole, orbital door jamb, and basin of the inferior orbital fissure. Three areas of deep bone in the lateral orbit. Arch Ophthalmol. 1998;116(12):1618–24.
Spalthoff S, Jehn P, Zimmerer R, Rana M, Gellrich NC, Dittmann J. Modified lateral orbital wall decompression in Graves’ orbitopathy using computer-assisted planning. Int J Oral Maxillofac Surg. 2018;47(2):167–74.
Dubin MR, Tabaee A, Scruggs JT, Kazim M, Close LG. Image-guided endoscopic orbital decompression for Graves’ orbitopathy. Ann Otol Rhinol Laryngol. 2008;117(3):177–85.
Kacker A, Kazim M, Murphy M, Trokel S, Close LG. "Balanced" orbital decompression for severe Graves’ orbitopathy: technique with treatment algorithm. Otolaryngol Head Neck Surg. 2003;128(2):228–35.
Wu CY, Kahana A. Stereotactic navigation with a registration mask in orbital decompression surgery: preliminary results. Ophthalmic Plast Reconstr Surg. 2015;31(6):440–4.
Servat JJ, Elia MD, Gong D, Manes RP, Black EH, Levin F. Electromagnetic image-guided orbital decompression: technique, principles, and preliminary experience with 6 consecutive cases. Orbit. 2014;33(6):433–6.
Lee KY, Ang BT, Ng I, Looi A. Stereotaxy for surgical navigation in orbital surgery. Ophthalmic Plast Reconstr Surg. 2009;25(4):300–2.
Naik MN, Nema A, Ali MH, Ali MJ. Piezoelectric surgery versus mechanical drilling for orbital floor decompression: effect on infraorbital hypoaesthesia. Orbit. 2019;38(3):184–6.
Siracuse-Lee DE, Kazim M. Orbital decompression: current concepts. Curr Opin Ophthalmol. 2002;13(5):310–6.
Sisler H, Jakobiec F, Trokel S. Ocular abnormalities and orbital changes of Graves’ disease. In: Tasman W, Jaeger E, editors. Duane’s clinical ophthalmology, rev. ed, vol. 2. Philadelphia: JB Lippincott; 1992.
Prat MC, Braunstein AL, Dagi Glass LR, Kazim M. Orbital fat decompression for thyroid eye disease: retrospective case review and criteria for optimal case selection. Ophthalmic Plast Reconstr Surg. 2015;31(3):215–8.
Bhatti MT, Dutton JJ. Thyroid eye disease: therapy in the active phase. J Neuroophthalmol. 2014;34(2):186–97.
Onaran Z, Konuk O, Oktar SO, Yucel C, Unal M. Intraocular pressure lowering effect of orbital decompression is related to increased venous outflow in Graves orbitopathy. Curr Eye Res. 2014;39(7):666–72.
Perez-Lopez M, Sales-Sanz M, Rebolleda G, et al. Retrobulbar ocular blood flow changes after orbital decompression in Graves’ ophthalmopathy measured by color Doppler imaging. Invest Ophthalmol Vis Sci. 2011;52(8):5612–7.
Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988;95(11):1515–21.
Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90(9):5234–40.
Kauh CY, Gupta S, Douglas RS, et al. Compressive optic neuropathy and repeat orbital decompression: a case series. Ophthalmic Plast Reconstr Surg. 2015;31(5):385–90.
MacAndie K, Harnett AN, Kyle P. Restoration of vision by radiotherapy for severe, acute, steroid-refractory, congestive thyroid orbitopathy. Clin Oncol (R Coll Radiol). 2000;12(3):188–91.
Kotwal A, Stan M. Current and future treatments for Graves’ disease and Graves’ ophthalmopathy. Horm Metab Res. 2018;50(12):871–86.
Genere N, Stan MN. Current and emerging treatment strategies for Graves’ orbitopathy. Drugs. 2019;79(2):109–24.
Soeters MR, van Zeijl CJ, Boelen A, et al. Optimal management of Graves orbitopathy: a multidisciplinary approach. Neth J Med. 2011;69(7):302–8.
Trokel S, Kazim M, Moore S. Orbital fat removal. Decompression for Graves orbitopathy. Ophthalmology. 1993;100(5):674–82.
Rajabi MT, Papageorgiou K, Taban M, et al. Ultrasonographic motion analysis of lower eyelid compartments in patients with chronic thyroid associated ophthalmopathy. J Curr Ophthalmol. 2017;29(4):310–7.
Choe CH, Cho RI, Elner VM. Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2011;27(1):4–11.
Jefferis JM, Jones RK, Currie ZI, Tan JH, Salvi SM. Orbital decompression for thyroid eye disease: methods, outcomes, and complications. Eye (Lond). 2018;32(3):626–36.
Choi SU, Kim KW, Lee JK. Surgical outcomes of balanced deep lateral and medial orbital wall decompression in Korean population: clinical and computed tomography-based analysis. Korean J Ophthalmol. 2016;30(2):85–91.
Liao SL, Chang TC, Lin LL. Transcaruncular orbital decompression: an alternate procedure for Graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol. 2006;141(5):810–8.
Woo KI, Kim YD. Isolated caruncular approach for orbital decompression. Jpn J Ophthalmol. 2004;48(4):397–403.
Borumandi F, Hammer B, Kamer L, von Arx G. How predictable is exophthalmos reduction in Graves’ orbitopathy? A review of the literature. Br J Ophthalmol. 2011;95(12):1625–30.
Ben Simon GJ, Schwarcz RM, Mansury AM, Wang L, McCann JD, Goldberg RA. Minimally invasive orbital decompression: local anesthesia and hand-carved bone. Arch Ophthalmol. 2005;123(12):1671–5.
Blandford AD, Zhang D, Chundury RV, Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol. 2017;12(2):111–21.
Olivari N. Transpalpebral decompression of endocrine ophthalmopathy (Graves’ disease) by removal of intraorbital fat: experience with 147 operations over 5 years. Plast Reconstr Surg. 1991;87(4):627–41; discussion 642–623
Wu CH, Chang TC, Liao SL. Results and predictability of fat-removal orbital decompression for disfiguring graves exophthalmos in an Asian patient population. Am J Ophthalmol. 2008;145(4):755–9.
Zhang S, Li Y, Wang Y, et al. Comparison of rim-sparing versus rim-removal techniques in deep lateral wall orbital decompression for Graves’ orbitopathy. Int J Oral Maxillofac Surg. 2019;48(4):461–7.
Cho RI, Choe CH, Elner VM. Ultrasonic bone removal versus high-speed burring for lateral orbital decompression: comparison of surgical outcomes for the treatment of thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2010;26(2):83–7.
Chang EL, Piva AP. Temporal fossa orbital decompression for treatment of disfiguring thyroid-related orbitopathy. Ophthalmology. 2008;115(9):1613–9.
Fong KD, Warren SM, Loboa EG, et al. Mechanical strain affects dura mater biological processes: implications for immature calvarial healing. Plast Reconstr Surg. 2003;112(5):1312–27.
Valdivia-Gandur I, Engelke W, Beltrán V, Borie E, Fuentes R, Manzanares-Céspedes MC. Novel use of cranial epidural space in rabbits as an animal model to investigate bone volume augmentation potential of different bone graft substitutes. Head Face Med. 2016;12(1):35.
Keller JT, Marfurt CF. Peptidergic and serotoninergic innervation of the rat dura mater. J Comp Neurol. 1991;309(4):515–34.
Gupta A, Nobori A, Wang Y, Rootman D, Goldberg R. Lateral rectus muscle expands more than medial rectus following maximal deep balanced orbital decompression. Ophthal Plast Reconstr Surg. 2017;34(2):140–2.
Dauser RC, Tuite GF, McCluggage CW. Dural inversion procedure for moyamoya disease. Technical note. J Neurosurg. 1997;86(4):719–23.
Greenwald JA, Mehrara BJ, Spector JA, et al. Regional differentiation of cranial suture-associated dura mater in vivo and in vitro: implications for suture fusion and patency. J Bone Miner Res. 2000;15(12):2413–30.
Greenwald JA, Mehrara BJ, Spector JA, et al. Immature versus mature dura mater: II. Differential expression of genes important to calvarial reossification. Plast Reconstr Surg. 2000;106(3):630–8; discussion 639
Rice FL, Xie JY, Albrecht PJ, et al. Anatomy and immunochemical characterization of the non-arterial peptidergic diffuse dural innervation of the rat and Rhesus monkey: Implications for functional regulation and treatment in migraine. Cephalalgia. 2017;37(14):1350–72.
Baillie LD, Ahn AH, Mulligan SJ. Sumatriptan inhibition of N-type calcium channel mediated signaling in dural CGRP terminal fibres. Neuropharmacology. 2012;63(3):362–7.
Cappugi P, Tsampau D, Lotti T. Substance P provokes cutaneous erythema and edema through a histamine-independent pathway. Int J Dermatol. 1992;31(3):206–9.
Abed SF, Shams P, Shen S, Adds PJ, Uddin JM. A cadaveric study of ethmoidal foramina variation and its surgical significance in Caucasians. Br J Ophthalmol. 2012;96(1):118–21.
Fabian ID, Rosen N, Ben Simon GJ. Strabismus after inferior-medial wall orbital decompression in thyroid-related orbitopathy. Curr Eye Res. 2013;38(1):204–9.
Zloto O, Ben Simon G, Didi Fabian I, et al. Association of orbital decompression and the characteristics of subsequent strabismus surgery in thyroid eye disease. Can J Ophthalmol. 2017;52(3):264–8.
Verity DH, Rose GE. Acute thyroid eye disease (TED): principles of medical and surgical management. Eye (Lond). 2013;27(3):308–19.
Alsuhaibani AH, Carter KD, Policeni B, Nerad JA. Orbital volume and eye position changes after balanced orbital decompression. Ophthalmic Plast Reconstr Surg. 2011;27(3):158–63.
Unal M, Leri F, Konuk O, Hasanreisoğlu B. Balanced orbital decompression combined with fat removal in Graves ophthalmopathy: do we really need to remove the third wall? Ophthalmic Plast Reconstr Surg. 2003;19(2):112–8.
Unal M, Ileri F, Konuk O, Hasanreisogğlu B. Balanced orbital decompression in Graves’ orbitopathy: Upper eyelid crease incision for extended lateral wall decompression. Orbit. 2000;19(2):109–17.
Wright ED, Davidson J, Codere F, Desrosiers M. Endoscopic orbital decompression with preservation of an inferomedial bony strut: minimization of postoperative diplopia. J Otolaryngol. 1999;28(5):252–6.
Finn AP, Bleier B, Cestari DM, et al. A retrospective review of orbital decompression for thyroid orbitopathy with endoscopic preservation of the inferomedial orbital bone strut. Ophthalmic Plast Reconstr Surg. 2017;33(5):334–9.
Metson R, Samaha M. Reduction of diplopia following endoscopic orbital decompression: the orbital sling technique. Laryngoscope. 2002;112(10):1753–7.
Bleier BS, Lefebvre DR, Freitag SK. Endoscopic orbital floor decompression with preservation of the inferomedial strut. Int Forum Allergy Rhinol. 2014;4(1):82–4.
Yao WC, Sedaghat AR, Yadav P, Fay A, Metson R. Orbital decompression in the endoscopic age: the modified inferomedial orbital strut. Otolaryngol Head Neck Surg. 2016;154(5):963–9.
Goldberg RA, Perry JD, Hortaleza V, Tong JT. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthalmic Plast Reconstr Surg. 2000;16(4):271–7.
Ben Simon GJ, Wang L, McCann JD, Goldberg RA. Primary-gaze diplopia in patients with thyroid-related orbitopathy undergoing deep lateral orbital decompression with intraconal fat debulking: a retrospective analysis of treatment outcome. Thyroid. 2004;14(5):379–83.
Boboridis KG, Bunce C. Surgical orbital decompression for thyroid eye disease. Cochrane Database Syst Rev. 2011(12):CD007630.
Sellari-Franceschini S, Dallan I, Bajraktari A, et al. Surgical complications in orbital decompression for Graves’ orbitopathy. Acta Otorhinolaryngol Ital. 2016;36(4):265–74.
Massoud VA, Fay A, Yoon MK. Cerebrospinal fluid leak as a complication of oculoplastic surgery. Semin Ophthalmol. 2014;29(5–6):440–9.
Rootman DB, Golan S, Pavlovich P, Rootman J. Postoperative changes in strabismus, ductions, exophthalmometry, and eyelid retraction after orbital decompression for thyroid orbitopathy. Ophthal Plast Reconstr Surg. 2017;33(4):289–93.
Wu CY, Kahana A. Geriatric patients are predisposed to strabismus following thyroid-related orbital decompression surgery: a multivariate analysis. Orbit. 2017;36(2):95–101.
Thomas SM, Cruz OA. Comparison of two different surgical techniques for the treatment of strabismus in dysthyroid ophthalmopathy. J AAPOS. 2007;11(3):258–61.
Pacheco EM, Guyton DL, Repka MX. Changes in eyelid position accompanying vertical rectus muscle surgery and prevention of lower lid retraction with adjustable surgery. J Pediatr Ophthalmol Strabismus. 1992;29(5):265–72.
Flanders M, Hastings M. Diagnosis and surgical management of strabismus associated with thyroid-related orbitopathy. J Pediatr Ophthalmol Strabismus. 1997;34(6):333–40.
Bartley GB, Fatourechi V, Kadrmas EF, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121(3):284–90.
Bartley GB. The differential diagnosis and classification of eyelid retraction. Ophthalmology. 1996;103(1):168–76.
Byun JS, Lee JK. Relationships between eyelid position and levator-superior rectus complex and inferior rectus muscle in patients with Graves’ orbitopathy with unilateral upper eyelid retraction. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):2001–8.
Marinó M, Ricciardi R, Pinchera A, et al. Mild clinical expression of myasthenia gravis associated with autoimmune thyroid diseases. J Clin Endocrinol Metab. 1997;82(2):438–43.
Papageorgiou KI, Hwang CJ, Chang SH, et al. Thyroid-associated periorbitopathy: eyebrow fat and soft tissue expansion in patients with thyroid-associated orbitopathy. Arch Ophthalmol. 2012;130(3):319–28.
Rajabi MT, Jafari H, Mazloumi M, et al. Lower lid retraction in thyroid orbitopathy: lamellar shortening or proptosis? Int Ophthalmol. 2014;34(4):801–4.
Pieroni Goncalves AC, Gupta S, Monteiro MLR, Douglas RS. Customized minimally invasive orbital decompression surgery improves lower eyelid retraction and contour in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2017;33(6):446–51.
Cho RI, Elner VM, Nelson CC, Frueh BR. The effect of orbital decompression surgery on lid retraction in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2011;27(6):436–8.
Gay AJ, Wolkstein MA. Topical guanethidine therapy for endocrine lid retraction. Arch Ophthalmol. 1966;76(3):364–7.
Cant JS, Lewis DR. Unwanted pharmacological effects of local guanethidine in the treatment of dysthyroid upper lid retraction. Br J Ophthalmol. 1969;53(4):239–45.
Morgenstern KE, Evanchan J, Foster JA, et al. Botulinum toxin type a for dysthyroid upper eyelid retraction. Ophthalmic Plast Reconstr Surg. 2004;20(3):181–5.
Salour H, Bagheri B, Aletaha M, et al. Transcutaneous dysport injection for treatment of upper eyelid retraction associated with thyroid eye disease. Orbit. 2010;29(2):114–8.
Uddin JM, Davies PD. Treatment of upper eyelid retraction associated with thyroid eye disease with subconjunctival botulinum toxin injection. Ophthalmology. 2002;109(6):1183–7.
MacCarthy CF, Hollenhorst RW. Protective moist-chamber eye dressing. Am J Ophthalmol. 1971;71(6):1333–4.
Terwee CB, Gerding MN, Dekker FW, Prummel MF, Wiersinga WM. Development of a disease specific quality of life questionnaire for patients with Graves’ ophthalmopathy: the GO-QOL. Br J Ophthalmol. 1998;82(7):773–9.
Yang P, Ko AC, Kikkawa DO, Korn BS. Upper eyelid blepharoplasty: evaluation, treatment, and complication minimization. Semin Plast Surg. 2017;31(1):51–7.
Codner MA, Kikkawa DO, Korn BS, Pacella SJ. Blepharoplasty and brow lift. Plast Reconstr Surg. 2010;126(1):1e–17e.
Georgescu D, Anderson RL, McCann JD. Brow ptosis correction: a comparison of five techniques. Facial Plast Surg. 2010;26(3):186–92.
Arneja JS, Larson DL, Gosain AK. Aesthetic and reconstructive brow lift: current techniques, indications, and applications. Ophthalmic Plast Reconstr Surg. 2005;21(6):405–11.
McCord CD, Doxanas MT. Browplasty and browpexy: an adjunct to blepharoplasty. Plast Reconstr Surg. 1990;86(2):248–54.
Elner VM, Hassan AS, Frueh BR. Graded full-thickness anterior blepharotomy for upper eyelid retraction. Arch Ophthalmol. 2004;122(1):55–60.
Doxanas MT, Dryden RM. The use of sclera in the treatment of dysthyroid eyelid retraction. Ophthalmology. 1981;88(9):887–94.
Oestreicher JH, Pang NK, Liao W. Treatment of lower eyelid retraction by retractor release and posterior lamellar grafting: an analysis of 659 eyelids in 400 patients. Ophthalmic Plast Reconstr Surg. 2008;24(3):207–12.
Cohen MS, Shorr N. Eyelid reconstruction with hard palate mucosa grafts. Ophthalmic Plast Reconstr Surg. 1992;8(3):183–95.
Chang EL, Rubin PA. Upper and lower eyelid retraction. Int Ophthalmol Clin. 2002;42(2):45–59.
Rubin PA, Fay AM, Remulla HD, Maus M. Ophthalmic plastic applications of acellular dermal allografts. Ophthalmology. 1999;106(11):2091–7.
Baylis HI, Perman KI, Fett DR, Sutcliffe RT. Autogenous auricular cartilage grafting for lower eyelid retraction. Ophthalmic Plast Reconstr Surg. 1985;1(1):23–7.
McCord C, Nahai FR, Codner MA, Nahai F, Hester TR. Use of porcine acellular dermal matrix (Enduragen) grafts in eyelids: a review of 69 patients and 129 eyelids. Plast Reconstr Surg. 2008;122(4):1206–13.
Barmettler A, Heo M. A prospective, randomized comparison of lower eyelid retraction repair with autologous auricular cartilage, bovine acellular dermal matrix (surgimend), and porcine acellular dermal matrix (enduragen) spacer grafts. Ophthalmic Plast Reconstr Surg. 2018;34(3):266–73.
Sun J, Liu X, Zhang Y, et al. Bovine acellular dermal matrix for levator lengthening in thyroid-related upper-eyelid retraction. Med Sci Monit. 2018;24:2728–34.
Lee EW, Berbos Z, Zaldivar RA, Lee MS, Harrison AR. Use of DermaMatrix graft in oculoplastic surgery. Ophthalmic Plast Reconstr Surg. 2010;26(3):153–4.
Li TG, Shorr N, Goldberg RA. Comparison of the efficacy of hard palate grafts with acellular human dermis grafts in lower eyelid surgery. Plast Reconstr Surg. 2005;116(3):873–8; discussion 879–880
Sullivan SA, Dailey RA. Graft contraction: a comparison of acellular dermis versus hard palate mucosa in lower eyelid surgery. Ophthalmic Plast Reconstr Surg. 2003;19(1):14–24.
Tan J, Olver J, Wright M, Maini R, Neoh C, Dickinson AJ. The use of porous polyethylene (Medpor) lower eyelid spacers in lid heightening and stabilisation. Br J Ophthalmol. 2004;88(9):1197–200.
Mavrikakis I, Francis N, Poitelea C, Parkin B, Brittain P, Olver J. Medpor lower eyelid spacer: does it biointegrate? Orbit. 2009;28(1):58–62.
McAlister CN, Oestreicher JH. Repeat posterior lamellar grafting for recalcitrant lower eyelid retraction is effective. Orbit. 2012;31(5):307–12.
Shah-Desai S, Azarbod P, Szamocki S, Rose GE. Nylon Hang back sutures in the repair of secondary ptosis following overcorrected dysthyroid upper eyelid retraction. Ophthalmic Plast Reconstr Surg. 2016;32(1):61–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Carniciu, A.L., Cho, R.I., Kahana, A. (2021). Surgical Management of Thyroid Eye Disease. In: Servat, J.J., Black, E.H., Nesi, F.A., Gladstone, G.J., Calvano, C.J. (eds) Smith and Nesi’s Ophthalmic Plastic and Reconstructive Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-41720-8_58
Download citation
DOI: https://doi.org/10.1007/978-3-030-41720-8_58
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41719-2
Online ISBN: 978-3-030-41720-8
eBook Packages: MedicineMedicine (R0)