Abstract
In a fuel cell technology, the membrane will function as an ion-exchange membrane where it will act as a semipermeable membrane that enables the passage of selectively dissolved ions while blocking the others. This chapter describes the recent progress on the research of solid electrolyte membranes in fuel cells. It focuses on the utilization of ceramic and polymeric membranes in solid oxide fuel cell, proton exchange membrane fuel cell, and direct methanol fuel cell, respectively. The chapter then discusses the membrane configurations that have been widely used in fuel cell as well as their fabrication technique. Recent performance evaluation was also discussed for each type of membrane fuel cell. A brief discussion on the applications, potential, and future direction of this membrane fuel cell is also included. Finally, this chapter concludes the challenges of membrane electrolyte utilization in fuel cell.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Membrane is a selective barrier that consists of thin sheets of layer of the cell, where it allows selective things to pass through but stop the others. Basically, membrane depends on its materials sources, either ceramic membrane or polymeric membrane. Ceramic membrane is an artificial membrane that made from inorganic materials like alumina, zirconia oxide, silicon carbide, or some glassy materials. While polymeric membrane is made of different kinds of polymer that usually soluble in organic solvents, membrane technology has been explored widely which covers all engineering approaches for substance transportation from one fraction to another fraction such as water treatment industry, food technology industry, and pharmaceutical industry. Fuel cell technology is also not excluded from using membrane as its main component’s base.
In a fuel cell technology, the membrane will act as ion-exchange membrane where it will behave as semipermeable membrane that allows selectively dissolved ions to pass through, while blocking the others. This ions exchange membrane will serve as electrolyte that lies between two electrodes: anode and cathode. There are several main types of fuel cell ion-exchange membrane consisting of cation-exchange membrane (CEM), anion-exchange membrane (AEM), and bipolar membrane. CEM, known as proton-exchange membrane, usually will transport H+ while AEM usually used in certain alkaline fuel cells to transport OH− anions from one electrode to another electrode, whereas a bipolar membrane is a combination of anionic and cationic membrane that laminated together (Tanaka 2007).
Commonly, fuel cell technology uses a membrane that allows the transportation of ions from one electrode to another electrode where ions will meet at one point and generate the electrons through an electrochemical reaction. Ions exchange membrane is made up from organic or inorganic materials with charger. For instance, CEM contains fixed anionic groups with abundantly mobile cationic groups; thus, most of the conductivity comes from cation transport. The basic operations for AEM are vice versa with CEM. Membrane structure could be divided into two main structures of heterogeneous and homogeneous. This classification depends on the degree of heterogeneity of the membrane (Ariono et al. 2017). Homogeneous membrane formed from a polymer, while heterogeneous membrane formed from two different polymers. Heterogeneous membranes are less expensive compared to homogeneous membrane, but the composition of these membranes is thicker with rough surface. Thus, heterogeneous membrane is having higher resistance than homogeneous membrane.
Essentially, for example, proton-exchange membrane fuel cell (PEMFC) consists of porous composite of polymer electrolyte binder and supported nanoparticle catalyst on carbon particles (Litster and McLean 2004). Function of polymer electrolyte binder is to provide ionic conductivity, whereas electrical conductivity maintains by the carbon support catalyst. Its electrode consists of carbon support which acts as an electrical conductor; Pt particles as the reaction site; Nafion ionomer which provides pathway for proton conduction and Teflon binder which increases hydrophobicity of the cell. Apart from that, gas diffusion layer also important for PEM cell where it provides electrical connection between the current collector and catalyst. This layer must be thin and porous as well as electrically conductive.
Direct methanol fuel cell (DMFC) usually will have a thin membrane that is covered with sparse layer of platinum-base catalyst on its both sides, which sandwiched between two electrodes. A methanol solution introduces to the electrode with negative charges. Typically, anode structure of DMFC membrane composed of supported/unsupported catalyst layers bonded with Nafion resin, Teflon-bonded carbon black diffusion layer (GDL), and a carbon cloth or paper diffusion layer (Allen et al. 2005). This type of fuel cell will setup according to its Standard Newcastle flow bed-design (Allen et al. 2005).
In contrast, solid oxide fuel cell (SOFC) membrane usually comprises of thin and dense electrolyte, porous asymmetric anode, and porous cathode. Thin electrolyte is significant to transport the oxide ions from cathode to anode while the dense structure is a must in order to ensure there is no gas leaking or crossover between the fuel and the oxidizing agent. A porous asymmetric anode is meant by two different structures that composed of anode layer that consists of finger-like void and sponge-like void. Finger-like void is essential in providing a pathway for fuel to enter the cell; sponge-like void gives a support to the whole cell and also being sites for chemical reaction to take place. Porous cathode will allow the oxidizing agent like oxygen or air to pass through before entering the electrolyte.
2 Membranes Applications in Fuel Cells
Since all fuel cells involve the transfer or movement of ion (O2− in SOFC, H+ in PEM and DMFC) in electrolyte between anode and cathode; it is thus important to make the electrolyte layer to be thin to reduce the distance needed for the ion to travel but thick enough to separate the anode and cathode to prevent spillage. A general schematic of a fuel cell is provided in Fig. 1. Fabrication of the electrode and electrolyte layer in the form of thin membrane has become one of the challenges in fuel cell technology nowadays.
2.1 Solid Oxide Fuel Cell
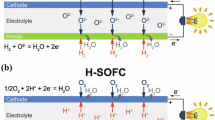
Solid oxide fuel cell (SOFC) involves solid electrode and electrolyte, whereby the oxide ion (O2−) moves from anode to cathode through the electrolyte layer. The oxygen from atmosphere is reduced at the cathode to form O2− where it travels to anode through the electrolyte layer. The fuel, for example, H2 gas will combine with the O2− ion at the anode releasing the electron which will flow through external circuit to the cathode layer. The electrolyte layer consists of ceramic metal oxide material, usually having lattice structure of fluorite or perovskite and doped with metal with different valencies or atomic radii to introduce defect into the lattice. This defect will create oxygen vacancy in the lattice structure that will allow O2− ion to hop from atom to atom when the material is heated to a certain temperature (operational temperature). Examples of the material are zirconia doped with 8 mol% yttria called yttria-stabilized zirconia (YSZ) and ceria doped with 10 mol% gadolinium called gadolinium-doped ceria (GDC).
Fabrication of SOFC using membrane generally involves the mixture of the ceramic electrolyte or electrode material with polymer to form the structure of the initial thin layer membrane followed by the removal of the polymer membrane leaving the ceramic material in the desired structure. This removal can be achieved by heating the membrane at high temperature to burn off the polymer material, or simply by heating to the sintering temperature of the ceramic material. The resulting material will then further heat to sinter temperature to strengthen the structure of the ceramic.
In terms of configuration, the membrane is being produced either in planar or tubular or micro-tubular design as shown in Fig. 2. Screen printing and tape casting techniques are common fabrication techniques in producing a planar membrane. Besides, compressing or dried pressing technique also being a most famous technique in fabricating a membrane with button cell design or disk design (Horri et al. 2012; Yoo and Lim 2013). In contrast, extrusion technique usually used to fabricate membrane with tubular configuration (Jamil et al. 2015). Conventionally, dry-jet wet and plastic mass ram are methods that employed to produce a tubular membrane via the extrusion technique. Both techniques are used to fabricate a single support layer of the membrane either anode-supported, electrolyte-supported, or cathode-supported. Until then, an advanced dry-jet wet extrusion technique known as phase inversion-based extrusion technique had been introduced which successfully produce a smaller size of tubular membrane which called as micro-tubular solid oxide fuel cell.
2.1.1 Planar SOFC
Planar form of SOFC (P-SOFC) involves layering flat sheet of electrode and electrode material. Electrolyte in planar membrane will lie and sandwich between anode and cathode. One of the advantages of the planar membrane is its configuration shorter the path lengths between anode to cathode for the electron movement which offers a production of high power output. However, the larger area of this configuration is being a factor to the gas sealing problem during the high-temperature operation, making the thermal stability of the cell reduces. Conventional technique of fabricating planar SOFC usually involves pressing the ceramic material at high temperature followed by sintering.
However, fabrication of planar SOFC via membrane route is also widely found in literatures. Example of this is by using polymer material like polyvinyl butyral as the binder. Through this method, the ceramic material is mixed into polymer polyvinyl butyral that was dissolved in methyl ethyl ketone solvent together with other additives such as pore former, plasticizer, and dispersant to form suspension. The suspension then can be transformed into thin layer by tape casting using doctor blade and consequently dried to remove the solvent. Thickness of the membrane layer can be controlled by the application of several layers. Two layers of electrolyte and electrode can be fabricated by tape casting the anode onto the electrolyte layer. The membrane then heated to remove the other material leaving the ceramic material and then to sinter temperature and to sinter the ceramic anode and electrolyte layer. Finally, the cathode layer is then deposited onto the electrolyte layer using painting and heated to sinter temperature (Kaur and Basu 2015).
2.1.2 Tubular and Micro-tubular SOFC
Tubular configuration that having a cylindrical shape offers more advantages and ultimately solving the problem facing by the planar configuration. Same like planar membrane, tubular membrane configuration can be differentiated according to its support. For example, anode-supported tubular membrane will produce a thicker anode layer among the electrolyte and cathode layer; it applied to the cathode- and electrolyte-supported tubular membranes. Anyhow, tubular configuration often increasing the ohmic loss of the cell due to its longer current pathways. The enhancement of this configuration had led to the introduction of micro-tubular membrane. This micro-tubular membrane means by the tubular membrane is fabricated in a hollow fiber design with a smaller diameter of 2–3 mm. The reduction of the diameter possesses various potential benefits including higher volumetric output, quicker start-up capability, good thermal cycling as well as portable characteristics (Meng et al. 2013).
Fabrication of SOFC offers additional advantages which is the ability for two or three layers of electrode or electrolyte to be co-fabricated together, reducing the time and step of fabrications. This is usually can be achieved by fabrication of anode and electrolyte layer together, or the recent technique: cathode–electrolyte–anode triple layer. Currently SOFC is investigated to be used not only with H2 fuel, but also the hydrocarbons such as methane, butane, and alcohol such as ethanol and butanol as well as biogas. The variation of the fuel source is made possible due to the fact that high operating temperature of SOFC actually encompasses the working temperature of steam reforming process together with some modification in anode layer. The modification can be either (1) allowed anode to directly catalyze oxidation of fuel or (2) by adding material that performs reforming process in the anode, or (3) by joining the reforming layer onto the anode. This modification was achieved by using metal or metal layer alloy or usage of other ceramic material with fluorite and perovskite structure.
A tubular SOFC had been developed since the late 1950s by the Westinghouse Electric Corporation (Stambouli and Traversa 2002). Commonly, plastic mass-ram extrusion technique via a die with desired dimensions will be applied in the fabrication of tubular SOFC. This technique will involve the mixing of support materials with the binder and solvent in order to form a viscous paste. The paste is then extruded through the die forming with the support tube, and it then followed up by drying and firing that tubular membrane. As in 2004, a tubular anode-supported SOFC manages to be produced via the plastic mass extrusion method assisted with vacuum dip-coating and painting (Du and Sammes 2004). 300 MPa of Ni-YSZ tube with 10–50 µm gastight YSZ layer was obtained in this work. Apart of that, thermal spraying technique is also being employed in the development of tubular SOFC. For example, Ni–Al2O3 cermet-supported tubular SOFC had been produced by using this technique in the study conducted by Li et al. (2006). 800 µm of porous Ni–Al2O3 supporting tube was successfully developed together with 25 µm of NiO-4.5YSZ, anode layer deposited on supporting tube via an atmospheric plasma spraying method.
Micro-tubular SOFC (MT-SOFC) can be fabricated using ram extrusion or co-extrusion coupled with phase inversion process, whereby the electrode or electrolyte is mixed with polymer material together with its solvent to form suspension. For co-extrusion phase inversion process, the suspension then can be extruded through tubular opening (called spinnerets) into non-solvent at which the polymer will form hollow fiber (HF) with the electrode or electrolyte material in it. MT-SOFC fabricated using this method usually requires one layer to act of support at which the layer will hold the highest mechanical strength or the layer will be fabricated to have the highest thickness to provide the required mechanical strength. The layer may consist of cathode-, electrolyte-, or anode-supported solid oxide fuel cell. The support layer may be extruded first followed by sintering at which other layer will be deposited onto the support, or the support layer will be extruded together to form dual-layer or even triple-layer HF.
Example of anode-supported MT-SOFC was done by Azzolini et al. (2015) using ram extrusion process. The initial anode materials of GDC, CuO or Cu2O, and LiNO3 were mixed with hydroxypropyl methylcellulose and water to form paste which were later extruded and dried. The electrolyte GDC later was deposited onto the electrolyte using dip-coating method followed by sintering and deposition of cathode LSCF using the dip-coating method as well. However, there is no mention of thickness of the anode layer obtained in this study. Sumi et al. (2015, 2017) also investigated anode-supported MT-SOFC by fabricating the anode layer using ram extrusion. Mixture of 60% NiO and 40% GDC with binder acrylic resin, water, and cellulose was extruded using piston cylinder to form the micro-tube followed by air drying. The YSZ electrolyte layer was later dip-coated onto the anode, followed by brush painting of LSCF onto the electrolyte layer. Using this method, anode, electrolyte, and cathode thickness of 640, 10, and 20 μm were each obtained.
Recent research on extrusion of electrolyte layer for electrolyte-supported SOFC using phase inversion was carried by Rabuni et al. (2018). The initial YSZ material was mixed with N-methyl-2-pyrrolidone (NMP), dispersant and polyethersulfone (PESf), and extruded into microtubular form. The process produces electrolyte with two different layers, dense thick outer layer of approximately 10 µm and micro-channel/porous layer. The anodic material of Cu and CeO2 was deposited onto the porous layer using wet impregnation technique where precursor aqueous metal nitrate was deposited followed by sintering at 1450 °C and reduction by H2. Cathode layer of LSM was brush painted onto the electrolyte using mixture of LSM and ethylene glycol followed by sintering at 1200 °C to form complete cell. In other study, Meng et al. (2014) fabricated dual-layer HF to produce NiO and YSZ layer for electrolyte-supported SOFC. The precursor ceramic layer of NiO and YSZ was mixed with PESf, dispersant and NMP and co-extruded together, followed by sintering at 1450 °C to obtain dense structure with thickness of 32 and 210 μm each for anode and electrolyte layer.
Cathode-supported MT-SOFC is being a preference to the researchers in this area due to the reason of good stability control during redox cycles. This reliable stability comes from the cathode-supported configuration itself where thinner anode would reduce the detrimental effect due to the expansion and contraction of Ni particles within the anode layer that may lead to the re-oxidation of Ni back to NiO. Dual-layer YSZ/YSZ-LSM of cathode-supported MT-SOFC well-developed via co-spinning/so-sintering technique with 5-µm-dip-coated Ni-YSZ layer (Meng et al. 2013). This asymmetric dual-layer hollow fiber is comprised of dense YSZ electrolyte layer supported on the porous cathode layer of YSZ-LSM. Besides, an effort in broadening the three-phase boundary length had been achieved through the dual-layer MT-SOFC fabrication of cathode functional layer LSM-YSZ sandwiched with LSM cathode layer (Meng et al. 2015). The main porous cathode layer usually will act as current-collecting layer as well as site for oxygen reduction process. Afterward, Panthi et al. (2017a, b) had carried out an investigation with the purpose of lowering down the co-sintering temperature as an attempt to avoid the chemical reaction between cathode and electrolyte layer during the co-sintering process. Co-sintering temperature can be lowering down till 1250–1300 °C with the help of sintering additive, NiO and Fe2O3, and microcrystalline cellulose pore former, adding into electrolyte and cathode, respectively (Panthi et al. 2017a).
In many studies, the anode layer is made as the support layer due to the fact that anode layer is the layer where the oxidation of fuel takes place; thus, thicker anode layer may be anticipated to give more area for the reaction to take place yielding better performance. However due to the limitation of material thermal expansion coefficient (TEC), the anode layer is usually made with composition consisting of mixture of electrolyte material and the anode material to ensure stability of the layers during co-sintering of the dual layer. This made the TEC of the anode and electrolyte layer to be closed to each other, resulting in the opportunity of the two layers to be fabricated together. Thus in co-extrusion of dual-layer MT-SOFC, the electrolyte layer and anode layer are usually co-extruded, co-sintered and coated with the cathode layer. Omar et al. (2018) and Jamil et al. (2019) fabricated dual-layer HF using almost similar method. Using anode/electrolyte material of NiO-GDC/GDC, the ceramic material was initially mixed with PESf polymer, dispersant, and DMSO or NMP as solvent and were extruded through spinnerets with water as the non-solvent phase to produce dual-layer HF. The anode/electrolyte layer obtained was found to range from 160 to 200 µm for anode and 30 to 60 µm for electrolyte.
Co-extrusion of triple-layer SOFC consisting of cathode, electrolyte, and anode has garnered interest in recent years. This method will further ease and reduce the fabrication step compared to the co-extrusion of two layers. Jamil et al. (2018) fabricated triple-layer HF in one single step consisting of anode, electrolyte, and cathode layers. The anode/electrolyte/cathode that were made up of NiO-GDC/GDC/LSCF-GDC employed similar phase inversion technique where the ceramic materials were mixed with solvent NMP, polymer PESf, and dispersant to form initial suspension. The triple-layer HF was later heat-treated at 400 °C, 800 °C, and later sintered at 1450 °C for 8 h. By varying the extrusion rate of anode/electrolyte/cathode to each 7/2/2 ml min−1 is producing thickness of 234/13.5/40 µm after co-sintering.
2.2 Proton Exchange Membrane Fuel Cells
Fuel cells generally create their names by electrolyte type and responding substances. PEMFC is a type of fuel cell that uses hydrogen as the fuel, oxygen as the oxidant and a type of membrane that is only permissible to hydrogen ion or proton. During PEMFC operation, the H2 gas flows into the fuel cell through the anode and is oxidized by the reaction at catalyst site to form hydrogen ion. The hydrogen ion will move from anode to the cathode layer through the electrolyte membrane that is only permeable to hydrogen ion but not to electron. Upon reaching the cathode, the hydrogen ion will react with oxygen from air and electron to form water. The electron flow externally from anode to cathode layer through external circuit attached to the cell to generate electricity. Chemical equation for the reaction is shown in equation below.
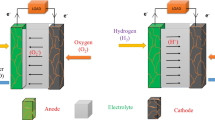
The electrodes layer in PEMFC (anode and cathode) consists of catalyst layer (CL) that catalyzes the oxidation of hydrogen or reduction of oxygen to produce water and gas diffusion layer (GDL) that permit the diffusion of hydrogen or oxygen into or water out of the CL and conduct electron to complete the circuit. Illustration of PEMFC is shown in Fig. 3 (Mehta and Cooper 2003; Wang et al. 2011).

Adapted from Majlan et al. (2018)
Schematic diagram of polymer electrolyte fuel cell system.
The electrolyte membrane in the center of PEMFC is considered to be the most significant element. Generally, the electrolyte membrane should have strong affinity for proton while being insulator to electron in order to be regarded as material for membrane. Other criteria include durability, resistance to chemical attack, and the state which needs to be solid. The range of operating temperatures is an important factor to consider when selecting membrane materials, where common operation temperature of PEMFC ranges from 30 to 200 °C.
Many distinct membranes exist and that are made from distinct material types. The selection of materials used as membrane depends on the physical and chemical properties required to ensure effective membrane efficiency (Awang et al. 2015; Mehta and Cooper 2003; Omar et al. 2018; Wang et al. 2011). PEMFC membranes are categorized into three primary classifications. These membranes are perfluorinated compound, partially fluorinated compound, and non-fluorinated. Besides these, however, we have other membranes obtained from these main classifications or using extra materials such as acid–base mixtures and composite membrane supported (Wang et al. 2011). The material used for the membrane therefore accounts for the majority of cost-effective components and components would go a long way in decreasing the overall manufacturing cost. Nafion produced by DuPont USA, Aciplex and Flemion produced by Asahi Japan, and the composites are currently the raw materials used. These above components are costly, and it is becoming very crucial to need a material that is inexpensive with better results. Composite membranes were produced from, among others, materials such as hydrocarbons, ceramics, and graphene. Current study attempts are aimed at producing fully composite catalytic membranes that can replace Nafion (Mehta and Cooper 2003).
The membrane can be prepared using various methods. There are five popular techniques that have been widely researched such as polymerization technique for irradiation grafting, crosslinking technique, polymerization process for plasma grafting, sol–gel technique, and direct monomer polymerization (Ogungbemi et al. 2019). Usually, the technique used relies on the type of membrane to be produced and the materials and facilities available. In order to choose the right preparation technique, a thorough understanding of materials and characteristics is required. On the other side, the preparation technique determines the final product and the quality thereof. PEM fuel cell technology is indeed the future of the renewable power industry, but less expensive and efficient material is needed to decrease the general price of fuel cells without limiting their efficiency.
2.3 Direct Methanol Fuel Cell
Direct methanol fuel cells (DMFC) operate on similar basis as PEMFC but with methanol as fuel to supply the hydrogen ion. The oxidation of methanol by reaction with water at the catalyst site produces carbon dioxide, electron, and hydrogen as shown in equation below.
Due to their reduced weight and quantity compared to indirect fuel cells, DMFC is appealing for several applications. Solid polymers were shown as an appealing alternative to traditional liquid electrolytes in this type of fuel cells. Polymers of Nafion perfluorosulfonic acid are the most frequently used in membranes fuel cells. The DMFC system is illustrated in Fig. 4. The DMFC has the ability to substitute rechargeable lithium-ion batteries in mobile electronic systems, but is presently experiencing important power density and effectiveness losses owing to elevated methanol crossover via polymer electrolyte membranes (PEMs) (Heinzel and Barragán 1999). Although spontaneously oxidizing methanol at the cathode would be appropriate, a transportation of methanol across the membrane has been noted. It creates losses of depolarization in terms of lost energy in the cathode and conversion losses. To enhance the DMFC’s efficiency, it is essential to eliminate or, at least reducing fuel loss across the cell which generally referred to as “methanol crossover.” The membrane technology is one of the options in this sense to try to fix this issue (Heinzel and Barragán 1999).

Adapted from Radenahmad et al. (2016)
Schematic diagram of direct methanol fuel cell system.
Significant progress in the growth of polymer electrolyte membranes for DMFCs has been produced in the latest years in terms of cost reduction and functionality enhancement along with other related technological advances. Common requirements for a polymer electrolyte membrane in DMFC application include: (1) elevated heat operation; (2) low methanol crossover; (3) high ionic conductivity; (4) high chemical and mechanical stability; (5) low ruthenium crossover; and (6) low-cost operation. There are currently four major membrane types used in DMFCs. These included membranes of nafion and non-nafion, flouronated composite membranes and non-flouronated composite membranes. Among these, composite fluorinated and non-fluorinated (hydrocarbon) membranes with low cost, methanol and ruthenium crossover (for Pt–Ru anodes), wider temperature range (80–180 °C), and higher ionic conductivity compared to Nafion® membranes have been recorded (Teresa and Gámez 2007).
Not all DMFC requirements are met by the traditional Nafion® membranes for DMFC applications. Unlike DMFCs, thinner membrane materials are preferred in hydrogen PEMFC application because they decreased ionic strength and enhanced MEA efficiency. However, in DMFCs, thin Nafion®112 membranes lead to a strong crossover of methanol. These disadvantages exceed the advantage of low ionic resistance, and therefore, thicker membranes such as Nafion®117 are typically used. However, this membrane give a very small cell voltage in a DMFC (Thomas et al. 2002).
The main candidates for replacing the expensive Nafion® membranes are hydrocarbon membranes. Improving Nafion®-based membranes by adding inorganic compounds (SiO2, silans, Zr, MoPh-a, etc.) and acid-based composites (polyaryl) reduces methanol crossover but does not reduce costs. Hydrocarbon membranes are cheaper for DMFC than Nafion® membranes and are more technically efficient. They have reduced methanol crossover and greater conductivity and stability. Hydrocarbon and composite fluorinated membranes presently exhibit the greatest potential for low-cost membranes with low permeability of methanol and high durability (Jörissen et al. 2002). Some of these membranes are already starting to impact the market for mobile fuel cells.
3 Performance Evaluation of Fuel Cells Involving Membrane Applications
3.1 Performance of Solid Oxide Fuel Cell
Performance evaluation of fuel cells is done in-situ under operating condition or ex-situ which is not under operating condition. Common in-situ measurement involves measuring the open-circuit voltage (OCV) and power density under operating condition and ex-situ by measuring the cell impedance using electrochemical impedance spectroscopy (EIS). Measurement of OCV for SOFC is usually accompanied by the measurement of current generated shown in I–V or I–P diagram. Since the recent interest of utilizing SOFC with hydrocarbon fueled has gained interest, performance of SOFC operating under hydrocarbon fueled is usually done in comparison with performance using H2 fuel. However, the performance of SOFC running on H2 fuel is still generally better compared to cell running on hydrocarbon fuel such as CH4 as given in Table 1.
From the latest literature finding, there are not much differences in cell performance between planar and tubular membrane SOFC. However as described earlier, micro-tubular form offers advantages in terms of the fabrication step and the void of need to design for interconnect. The electrolyte layer which is the most important layer functions optimally at different temperature depending on the material. Current use of YSZ which is considered as high-temperature SOFC (HT-SOFC) with operating temperature ranging from, 800 to 1000 °C received the same interest as GDC electrolyte, which considered as intermediate temperature SOFC (IT-SOFC) with operating temperature ranging from 500 to 800 °C. IT-SOFC hs higher ionic conductivity at lower working temperature compared to HT-SOFC but the tendency for cerium oxide to form Ce4+/Ce3+ species in reducing environment resulted in undesired electronic conductivity that cause current leakage, hence lowering the performance of the cell.
Electrochemical impedance spectroscopy (EIS) has become major tool to characterize and test fuel cells. Electrical impedance (usually denoted by Z) similar to electrical resistance (R) is a measurement of the circuit resistance to electrical flow when electrical potential is applied across the circuit. However unlike resistance, impedance measurement also concerns the phase shift of the current flowing when AC potential is applied. This phase shift is the characteristics of resistor–capacitor circuit (RC circuit) which is be used to model the layer of the fuel cell in the form of equivalent electrical circuit. Electrochemical impedance is measure by applying AC potential across the membrane at various frequencies, and the resultant impedance is measured and expressed in complex form consisting of real and imaginary part called Nyquist plot as illustrated in Fig. 5. The data from Nyquist plot then will be fitted into mathematical model to find equivalent electrical circuit consisting of electrical elements of resistor, inductor, and capacitor. EIS interpretation correlates to the cell characterization due to the fact that certain process works like electrical components (Pivac and Barbir 2016).
From literatures, most of studies on SOFC performance also include EIS as part of result reporting to not only show the resistance value of the cell, but also investigated the effect of modification of electrode or electrolyte on the EIS and the postulated microscopic change associated with the change. Effect of metal and metal alloy layer on the ohmic and polarization resistance change has been investigated by Meng et al. (2014), Yan et al. (2016), Wu et al. (2016), Jamil et al. (2019), Lee et al. (2016) and Harris et al. (2017). EIS is also used to investigate degradation at the anode by carbon deposition associated with the utilization of hydrocarbon fuel by Sarruf et al. (2017), Panthi et al. (2017b), Omar et al. (2018) and Akdeniz et al. (2016) where the utilization of methane fuel was shown to cause increase in ohmic resistance of the cell.
Modification of traditional anode layer of Ni-YSZ to enable SOFC to be able to be used with varieties of fuel seems to be the current direction of recent research. Since Ni-YSZ suffers from performance degradation due to carbon deposition when used with hydrocarbon fuel, anode modification through the insertion of ceria, metal alloy or used of fluorite or perovskite material to act as oxidation catalyst has been extensively studied. Insertion of ceria and copper metal in anode-supported MT-SOFC using YSZ electrolyte was studied by Meng et al. (2014) and Rabuni et al. (2018). The former anode composition containing YSZ, Ni, CeO2, and Cu obtained increased the performance from H2 and CH4 fuel (0 0.15 and 0.25 W cm−2, respectively) while later study, lacking Ni managed to obtain 0.55 and 0.16 W cm−2 each for H2 and CH4 fueled. The results contradicted each other but may also indicate the importance of Ni even in hydrocarbon fueled SOFC. This is supported by Azzolini et al. (2015) wherein tubular SOFC with anode contains copper-GDC and GDC electrolyte system, maximum of 0.008 W cm−2 power density was achieved which is far lower than common value. This is further clarified by study from Sumi et al. (2015) with anode containing Nickel-GDC and GDC electrolyte where maximum power density 0.25 W cm−2 was obtained using H2 fuel.
Co-extrusion of dual-layer hollow fiber to produce SOFC by Omar et al. (2018) and Jamil et al. (2019) shown that even though the SOFC was produced with the same composition of anode/electrolyte/cathode of Ni-GDC/GDC/LSCF-GDC, different performance SOFC can be obtained when tested on the same fuel H2. The difference in result of 0.67 and 0.29 W cm−2 by Omar et al. (2018) and Jamil et al. (2019) may be attributed to the higher porosity (between 31.5 and 52.5%) in later compared to the former (25.6%). Omar et al. (2018) also tested the SOFC on CH4 gas, obtaining a lower performance of 0.22 W cm−2 compared to running on H2. This suggests that not only the material is important but also the microstructure is similar importance for the performance of SOFC.
Co-extrusion of triple-layer HF containing complete anode/electrolyte/cathode layer in single step by Jamil et al. (2018) produced roughly the same power density as the SOFC from dual-layer HF. With anode/electrolyte/cathode consisting of Ni-GDC/GDC/LSCF-GDC each, maximum power density was obtained at 0.48 W cm−2 running on H2, similar to the average result of the same setting. Thus, it is evident that there are significant challenges remaining in the development of SOFC in a single step. The increase in TPB density by controlling the anode morphology (e.g. by adjusting the length of finger-like voids and enhancing the porosity) might lead to improved MT-SOFC performance. Under those circumstances, a comprehensive study on the fabrication of SOFC is important in order to produce defect-free hollow fibers with the desired morphologies that maintain high mechanical strength, decent tightness properties, and sufficient anode porosity to produce high-performance MT-SOFCs.
3.2 Performance of Proton Exchange Membrane Fuel Cell
Improvement of PEMFC performance is mainly targeted to be achieved by solving the main issues faced by the development of PEMFC which are (1) lowering cost, (2) water management system, (3) proton conductivity at high temperature, (4) lower gas crossover, and (5) improved thermal stability and mechanical and chemical strength (Peighambardoust et al. 2010; Majlan et al. 2018). Effort on lowering the cost is targeted to be achieved by developing lower cost material compared to the current traditional fluorinated membrane and by developing lower cost catalyst other than Pt and Rh while other developments are targeted at improvement of the cathode layer. Table 2 summarizes few literature that address the development of new electrolyte membrane and the resulted maximum power density achieved by the studies.
All of the studies highlighted in Table 2 involved doping or adding functional layer to the existing membrane to enhance the properties. This in effect highlighted the rise in the usage of composite membrane in PEMFC as opposed to single membrane. In addition, analysis of EIS in PEM allows the identification of (1) layers in fuel cell that occurs due to the different processes such as mass transport process and polarization effect, (2) effect of different layer to the total resistance and impedance of the cell, and (3) microscopic detail such as change in granule size that affects the impedance and performance of the cell (Pivac and Barbir 2016). Zhiani et al. (2016) studied the effect of thermal and pressure stress on PEM and found that PEM running with membrane electrode assembly (MEA) conditioned under low stress resulted in higher performance, peaking at 1.6 W cm−2 compared to MEA conditioned under high stress. From the EIS equivalent circuit, it was postulated that the higher performance may be the result of extension of the triple-phase boundary in MEA that increases the reactive area for reaction.
For PEM, inductive phenomenon in EIS at high frequency was known to be caused simply by the effect of the wire and cable setup of the cell (Pivac and Barbir 2016). However, inductive effect at low frequency might suggest the possibilities of side reactions occurring in the cell between intermediate species, carbon monoxide poisoning of the cell or water movement across the layer. From equivalent circuit of EIS in PEM, the total resistance, RT of the cell can be determined together with the contribution resistance by charge transfer, RCT and ionic resistance Rion inside the cell (Moghaddam and Easton 2018). Low RCT and high Rion may significantly impact performance of MEA and RT value may be used as basic reference to assess the performance of Nafion-based PEMFC.
EIS has also been investigated to function as online measurement tool to study and monitor the effect of load current, air humidification rate, and hydrogen flow rate effect on the online performance of PEM to be used in electric vehicle (Depernet et al. 2016). In that study, the DC bus from the vehicle is connected to inverter and to DC/AC/DC power converter and the EIS spectra was obtained at discrete and few frequency (as opposed to cycling from low- to high-frequency AC) and the resulting equivalent circuit was generated and compared with ex-situ equivalent circuit. The study found that online EIS is comparable with ex-situ offline EIS and hence can be used as reliable tool to monitor the performance of PEM online.
3.3 Performance of Direct Methanol Fuel Cell
The focus of DMFC researches is mainly targeted to solve the issue in Sect. 2.3. Similar to PEMFC, the improvement of the electrolyte layer is also one of the main focuses of studies in DMFC. However, since DMFC also faced the problem with methanol crossover, this issue added another complexity in DMFC research as compared to PEMFC. Few research that are presented with maximum power density obtained in the studies are given in Table 3.
The studies are mainly done to resolve the issues by focusing on high temperature use of DMFC, methanol crossover and by managing water and gas flow in the cell and the catalyst to improve the performance of the DMFC (Kim et al. 2015; Radenahmad et al. 2016; Zainoodin et al. 2010; Li et al. 2013; Ong et al. 2017). Overall output of DMFCs relies on several variables, the most significant of which are: (i) anode’s electrocatalytic activity, (ii) ionic conductivity and methanol crossover strength of the proton conductive membrane, and (iii) water management on the cell’s cathode side (flow-field and back design function). As mentioned earlier, optimization of various DMFC parts can lead to a significant increase in power density and fuel utilization.
4 Fuel Cell Applications and Technology
SOFC covers wide range of application from small portable system until power generation system. Normally, portable SOFC systems are needed primarily for emergency, transportation utilization and even it is used for certain military appliance. Seeing that SOFC system having a superior fuel flexibility characteristic; fuel others than hydrogen could be feed into the system like JP-8 military fuel, ethanol, gasoline, kerosene, and others hydrocarbon fuels (Irvine and Connor 2013). Such portable SOFC system generally consumes about milliwatts to hundred watts of power. USA company such as Adaptive Materials Inc. had been developed such portable system called Amie25 and Amie150 (Narayan and Valdez 2008). This technology base on a lightweight micro-tubular SOFC and propane utilized as a fuel. 1700 Wh of energy can be generated from this portable system that weighed about 1.5 kg, which required 1 kg of propane. Besides, Protonex Technology Corporation also offers a portable generator-based SOFC, Valta P75 with an output of 75 W which fuelled by propane.
Japan is one of the countries that lead in the application of small SOFC systems for residential combined heat and power (CHP) units. Generally, the implementation of this system for residential uses required 1–5 kW power to supply. A company from Japan Kyocera had introduced anode-supported flat tubular cells. This technology was coupled together with the hot water tank. In 2005–2006, a 1 kW of this system was operated for the first time and successfully sustain for 2000 h with 44.1% of electric efficiency and 34% hot water heat recovery efficiency (Irvine and Connor 2013). In 2017, the same company had launched the first 3 kW of SOFC cogeneration system for institutional use. The overall electrical efficiency of this system achieves nearly 90% with exhaust heat recovery. Other company such as Toto Ltd. (Japan) also had introduced such SOFC-CHP units which using cathode-supported tubular cell. After all, numerous organizations such as Hexis (Switzerland), Ceres Power (UK), Ceramic Fuel Cell Ltd. (Australia), and Siemen (USA) had commercialized this SOFC-CHP technology practical for residential use either in planar- or tubular-based SOFC configurations.
SOFC technology proved its high level of energy efficiency and acquisition when it could be used as a power generation system. SOFC power generation system firstly designs and builts in early 1984 (Singhal 2000). By referring to Singhal (2000), this system had been installed and worked at the Southern California Edison Company’s Highgrove Generating Station in Grand Terrace (near San Bernardino), California, which successfully run for almost 5582 with five times thermal cycles endurance. Apart from that, Mitsubishi Hitachi Power system had brought out a hybrid SOFC-micro gas turbine system. For the record, in September 2013, model 10 hybrid power generation system can operate for 4100 h during tested at Tokyo Gas Co., Ltd. Senju Techno Station; while model 15 hybrid power generation system can achieve 10,000 h operation when demonstrate at the Kyushu University Ito Campus on October 2016.
On the other hands, PEMFC system usually applied to the vehicle application, low power generation of the portable unit along with combined heat and power unit. Mostly, PEMFC technology would enforce on the transportation field or fuel cell vehicle (FVC). However, there are still having extensive focus on stationary and portable applications. PEMFCs promise divers advantages, including low operating temperature, long stack life, quick start-up, and compactness which lead to the launched on numerous applications, technologies along with prototype by different companies with different backgrounds such as fuel cell technology (Ballard, Plug Power, Smart fuel cell, Toshiba, NovArs, and Hydrogenics), (Ford, Nissan, Hyundai, BMW, Toyota, and, Renault), and electricity-based company (IBM, Samsung, and NTT) (Wee 2007).
In Taiwan and China, electric-powered bicycle is being the daily basic transportation. 20–250 kW is needed to power up the electric car, electric bus as well as utility vehicle, while 1–50 mW are required for stationary application and 100–1 kW generally for small-scale application (Shamim et al. 2015). According to Wee (2007), stack composed of 40 units of PEMFC could generate 378 W of power for electric bicycle with 35% of fuel cell efficiency. Mercedes-Benz Citaro fuel cell buses had been launched in Stockholm city which operate for about 200 km of the journey which produces the total power of 25 kW. By referring to Clean Urban Transportation for Europe (CUTE) program, half of 33 unit buses were run in Europe, 15% in North America and quarter in Asia regions.
In the meantime, PEMFC portable application being another focus in fulfilling the high power demands. Instead of indoor applications like mobile phone, computers, and digital camcorder; PEMFC portable is also widely utilized for outdoor conditions. It can be categorized into three PEMFC stack which are for medium power applications (forerunner stacks Ballard Mk5); automotive usage (Ballard Mk902); and OutdoorFC stack (Oszcipok et al. 2006). In addition, PEMFC could be seen as a good candidate for stationary power plant applications. PEMFC also being a great alternative technology in coping with environmental pollution and energy crises came from the combustion of fossil fuel for energy sources (Shamim et al. 2015). Altergy (USA), Ebara Ballard (Japan), P21 (Germany), and ClearEdge (USA) are among of the companies that contribute to the development and commercialization of small stationary PEMFC power plants.
Meanwhile, DMFC applications are mostly focused on the small vehicles system since it can only generate a small amount of power which make it not ideal for powering large scale of applications. Anyhow, this DMFC system could produce power for long period of time, which can supply up to 25–5 W for 100 h of operations as long as having the fuel supply. Like PEMFC, DMFC also mainly assists in the transportation or vehicle industry technology. This technology has huge potential in competing with internal combustion engine vehicles (ICEVs) fuelled by fossil fuel in terms of cost and performance as well as reducing global warming issues (Shukla et al. 1998). Besides, it also can be implemented in military applications, man-portable tactical equipment and battery chargers.
A company from Germany, Smart Fuel Cell Inc. had developed a small unit of the DMFC system with power supply of 15–150 W. Furthermore, electronic companies like Toshiba Corporation, Sony, Samsung, and MTI had stated their small development of DMFC system that applied in particular portable electronics application and some exhibits as prototypes (Narayan and Valdez 2008). In the same papers, it also stated that 1 kW of the DMFC power source had been designed and manufactured by Oorja Protonics Inc. called ad OorjaPacTM. This system works as a battery charger for vehicles and grants about 20 kWh of energy per six gallons of methanol, which provides sufficient energy for a day operation. For all that, it yields about 20% of fuel to electric efficiency. In fact, various of DMFC system had been introduced such as SFC Jenny (480 Wh), EFOY 1600-M5 (4500 Wh), and EFOY 1600-M28 (25,200 Wh). Although the energy efficiency of the DMFC system is relatively low, progress in the development of the mobile DMFC at either the research or commercialization scale has been continued to this day.
5 Potential and Future Direction
Due to the high operating temperature of SOFC that is in the region of hydrocarbon reforming, common direction of research now is aimed at utilizing readily available hydrocarbon fuel such as methane in natural gas and biogas, propane, butane from butane canister and heavier hydrocarbon such as octane and kerosene from vehicle fuel. The effort is made in finding reforming catalyst and fabricating reforming layer on top of the anode to be used as internal reformer or fixing external reformer to preprocess the fuel (Sengodan et al. 2018). Metal oxide catalyst such as Ni-Al2O3 and Ru-CeO2 with various doping is actively research as internal reformer for SOFC. Other than that metal–cermet also shows promising catalytic behavior for direct oxidation of hydrocarbon fuel in the anode layer. This enables the hydrocarbon to be used directly in the anode layer with reforming layer (Mahato et al. 2015).
Another direction for SOFC research is into lowering the operating temperature of the SOFC. As the function of SOFC is mainly dictated by the ceramic electrolyte membrane that control the passing of O2-ion, finding O2-ceramic material that operates at low temperature is actively done. The current GDC operating at 500–800 °C classed as intermediate temperature SOFC (IT-SOFC) was used electrolyte compared to YSZ operating at 800–1000 °C. Nonetheless, GDC is also known to undergo reduction reaction under reducing hydrogen environment, causing the Ce(IV) to be reduced to Ce(III) which creates electron hole in the GDC structure due to the mixture of Ce(IV)/Ce(III) species. This made the GDC to obtain electronic conductivities, turning the material to MIEC which have electronic current leakage in the electrolyte reducing the OCV and power densities. This problem is currently researched to be resolved by adding intermediate layer between the electrolyte and cathode to avoid contact with reducing gas and is subject to under research. Apart from that other material such from perovskite class such as La0.8Sr0.2Ga0.8Mg0.2O3−δ (LSGM), and bismuth vanadate, BiVO3 is also actively research for application as IT-SOFC (Mahato et al. 2015). The prospect of lowering the temperature of SOFC to 500 °C is attractive due to the main challenge of finding material compatible with high temperature. Lowering the temperature of SOFC will allow better mechanical strength and compatibility among material such as anode, electrolyte, cathode, and the interconnect.
Meanwhile, the development of electrolyte membrane layer in PEMFC has managed to show remarkable improvement over recent years. While the membrane layer in PEMFC originally used commercial and high-cost Nafion membrane, the development of other membrane as alternative had produced similar or better result. The practice of doping of low-cost membrane such as PVA, sPAES, sPEEK, and PBI with acid such as phosphoric acid has shown increase in proton conductivities as well as hydration of the membrane (Kim et al. 2015; Lade et al. 2017). Composite membrane consisting of the mixture of the said polymer has also been the further researched. Compositing membrane will enable the combination of the best properties such as mechanical and chemical strength, thermal stability and gas tightness as well as lowering the cost. To enable this compositing, new fabrication method such as sputtering and electrospunning should be further researched as well as this enables the creation of layered electrolyte membrane.
High-temperature PEMFC (HT-PEMFC) that enables the operation of PEMFC at higher than 120 °C has received attention due to the fact the higher temperature will favor better kinetics for the hydrogenation oxidation reaction, allowing for lesser use of the expensive Pt catalyst, thus reducing the cost (Rosli et al. 2017). Lower thermal resistance Nafion membrane (<100 °C) can be avoided by using acid-doped PBI membrane or composite membrane consisting of organic/inorganic component such as fluorinated polymer/SiO2, PWA/PVA, and polyalkoxysilane/phosphotungstic acid composite. The composite membrane by far is the most promising candidate for the HT-PEMFC.
Generally, the DMFC may use the same membrane electrolyte as PEMFC however with added catalyst layer at the electrode. Since the inception DMFC traditionally uses diluted methanol at concentration <1 M, effort now has focused on using higher concentration methanol with concentration up to 4 M. The higher concentration methanol enables higher energy density fuel storage, thus increasing the prospect of DMFC as mobile power source. The challenge associated with the effort such as methanol crossover is being researched actively. Methanol crossover from the anode to the cathode reduces fuel efficiency and caused mixed potential at the cathode (Li and Faghri 2013). This problem may be controlled by using hydrophobic PTFE coating at the electrode side, by employing gas diffusion layer or by using vapor fed methanol fuel system. Vapor fed DMFC has gained interest in recent years. In vapor fed DMFC, the methanol fuel is vaporized first by external heater or by pervaporation membrane and fed into the anode of the SOFC in vapor phase. At the anode, the principle operation of oxidation is the same as the liquid DMFC (Mallick et al. 2016).
In both PEMFC and DMFC, control of the carbon dioxide and water produced from the operation has been identified to play crucial role in controlling the performance of the fuel cell. Thus, research now targeted at improving the management of the water at the electrode by improving the GDL and the back layer (Majlan et al. 2018).
6 Conclusion
The implementation of membrane electrolyte in the fuel cell has been extensively researched. Membrane technology places an enormous benefit over prevalent products in terms of efficiency. The focus has already been well-founded on producing PEM for PEMFC and DMFC as well as ceramic membrane for SOFC. All the accomplishments reported in the fuel cell technology so far have been due to ongoing studies on the membrane base fuel cell and generally renewable energy. Improvement on catalysts, MEA elements, and bipolar plates is notably essential for PEMFCs and DMFCs to overcome the two significant obstacles to marketing (i.e., durability and price). While it is crucial for SOFCs to improve the anode side in order to overcome the phenomenon of carbon deposition before using hydrocarbon such as methane in MT-SOFC, thus, further study on the utilization of membrane electrolyte in fuel cell is still needed to improve the overall fuel cell efficiency.
References
Ahmad, M. M., Kamarudin, S. K., Daud, W. R. W., & Yaakub, Z. (2010). High power passive μDMFC with low catalyst loading for small power generation. Energy Conversion and Management, 51, 821–825. https://doi.org/10.1016/j.enconman.2009.11.017.
Akdeniz, Y., Timurkutluk, B., & Timurkutluk, C. (2016). Development of anodes for direct oxidation of methane fuel in solid oxide fuel cells. International Journal of Hydrogen Energy, 41, 10021–10029. https://doi.org/10.1016/j.ijhydene.2016.03.169.
Allen, R. G., Lim, C., Yang, L. X., Scott, K., & Roy, S. (2005). Novel anode structure for the direct methanol fuel cell. Journal of Power Sources, 143, 142–149. https://doi.org/10.1016/j.jpowsour.2004.11.038.
Amirinejad, M., Madaeni, S. S., Lee, K.-S., Ko, U., Rafiee, E., & Lee, J.-S. (2012). Sulfonated poly(arylene ether)/heteropolyacids nanocomposite membranes for proton exchange membrane fuel cells. Electrochimica Acta, 62, 227–233. https://doi.org/10.1016/j.electacta.2011.12.025.
Amjadi, M., Rowshanzamir, S., Peighambardoust, S. J., Hosseini, M. G., & Eikani, M. H. (2010). Investigation of physical properties and cell performance of Nafion/TiO2 nanocomposite membranes for high temperature PEM fuel cells. International Journal of Hydrogen Energy, 35, 9252–9260. https://doi.org/10.1016/j.ijhydene.2010.01.005.
Ariono, D., Khoiruddin, S., & Wenten, I. G. (2017). Heterogeneous structure and its effect on properties and electrochemical behavior of ion-exchange membrane. Materials Research Express, 4, 024006. https://doi.org/10.1088/2053-1591/aa5cd4.
Awang, N., Ismail, A. F., Jaafar, J., Matsuura, T., Junoh, H., Othman, M. H. D., & Rahman, M. A. (2015). Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. Reactive & Functional Polymers, 86, 248–258. https://doi.org/10.1016/j.reactfunctpolym.2014.09.019.
Azzolini, A., Sglavo, V. M., & Downs, J. A. (2015). Production and performance of copper-based anode-supported SOFCs. Journal of the Electrochemical Society, 68, 2583–2596.
Bochentyn, B., Chlipała, M., Gazda, M., Wang, S. F., & Jasiński, P. (2019). Copper and cobalt co-doped ceria as an anode catalyst for DIR-SOFCs fueled by biogas. Solid State Ionics, 330, 47–53. https://doi.org/10.1016/j.ssi.2018.12.007.
Brandão, L., Boaventura, M., & Ribeirinha, P. (2012). Single wall nanohorns as electrocatalyst support for vapour phase high temperature DMFC. International Journal of Hydrogen Energy, 37, 19073–19081. https://doi.org/10.1016/j.ijhydene.2012.09.133.
Cho, E.-B., Luu, D. X., & Kim, D. (2010). Enhanced transport performance of sulfonated mesoporous benzene-silica incorporated poly(ether ether ketone) composite membranes for fuel cell application. Journal of Membrane Science, 351, 58–64. https://doi.org/10.1016/j.memsci.2010.01.028.
Chun, J. H., Kim, S. G., Lee, J. Y., Hyeon, D. H., Chun, B.-H., Kim, S. H., & Park, K. T. (2013). Crosslinked sulfonated poly(arylene ether sulfone)/silica hybrid membranes for high temperature proton exchange membrane fuel cells. Renewable Energy, 51, 22–28. https://doi.org/10.1016/j.renene.2012.09.005.
Depernet, D., Narjiss, A., Gustin, F., Hissel, D., & Péra, M. C. (2016). Integration of electrochemical impedance spectroscopy functionality in proton exchange membrane fuel cell power converter. International Journal of Hydrogen Energy, 41, 5378–5388. https://doi.org/10.1016/j.ijhydene.2016.02.010.
Devrim, Y., Erkan, S., Baç, N., & Eroğlu, I. (2009). Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 34, 3467–3475. https://doi.org/10.1016/j.ijhydene.2009.02.019.
Du, Y., & Sammes, N. M. (2004). Fabrication and properties of anode-supported tubular solid oxide fuel cells. Journal of Power Sources, 136, 66–71. https://doi.org/10.1016/j.jpowsour.2004.05.028.
Fatyeyeva, K., Bigarré, J., Blondel, B., Galiano, H., Gaud, D., Lecardeur, M., & Poncin-Epaillard, F. (2011). Grafting of p-styrene sulfonate and 1,3-propane sultone onto Laponite for proton exchange membrane fuel cell application. Journal of Membrane Science, 366, 33–42. https://doi.org/10.1016/j.memsci.2010.09.023.
Harris, J., Lay-Grindler, E., Metcalfe, C., & Kesler, O. (2017). Degradation of metal-supported cells with Ni-YSZ or Ni-Ni3Sn-YSZ anodes operated with methane-based fuels. ECS Transactions, 78, 1293–1304. https://doi.org/10.1149/07801.1293ecst.
He, Y.-L., Miao, Z., & Yang, W.-W. (2012). Characteristics of heat and mass transport in a passive direct methanol fuel cell operated with concentrated methanol. Journal of Power Sources, 208, 180–186. https://doi.org/10.1016/j.jpowsour.2012.02.033.
Heinzel, A., & Barragán, V. M. (1999). A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. Journal of Power Sources, 84, 70–74. https://doi.org/10.1016/S0378-7753(99)00302-X.
Horri, B. A., Selomulya, C., & Wang, H. (2012). Characteristics of Ni/YSZ ceramic anode prepared using carbon microspheres as a pore former. International Journal of Hydrogen Energy, 37, 15311–15319. https://doi.org/10.1016/j.ijhydene.2012.07.108.
Hua, D., Li, G., Lu, H., Zhang, X., & Fan, P. (2018). Investigation of carbon formation on Ni/YSZ anode of solid oxide fuel cell from CO disproportionation reaction. International Communications in Heat and Mass Transfer, 91, 23–29. https://doi.org/10.1016/j.icheatmasstransfer.2017.11.014.
Ilbeygi, H., Ismail, A. F., Mayahi, A., Nasef, M. M., Jaafar, J., & Jalalvandi, E. (2013). Transport properties and direct methanol fuel cell performance of sulfonated poly (ether ether ketone)/Cloisite/triaminopyrimidine nanocomposite polymer electrolyte membrane at moderate temperature. Separation and Purification Technology, 118, 567–575. https://doi.org/10.1016/j.seppur.2013.07.044.
Irvine, J. T. S., & Connor, P. (2013). Solid oxide fuels cells: Facts and figures. London: Springer.
Jamil, S. M., Othman, M. H. D., Mohamed, M. H., Adam, M. R., Rahman, M. A., Jaafar, J., & Ismail, A. F. (2018). A novel single-step fabrication anode/electrolyte/cathode triple-layer hollow fiber micro-tubular SOFC. International Journal of Hydrogen Energy, 43, 18509–18515. https://doi.org/10.1016/j.ijhydene.2018.08.010.
Jamil, S. M., Othman, M. H. D., Rahman, M. A., Jaafar, J., Ismail, A. F., Honda, S., & Iwamoto, Y. (2019). Properties and performance evaluation of dual-layer ceramic hollow fiber with modified electrolyte for MT-SOFC. Renew Energy, 134, 1423–1433. https://doi.org/10.1016/j.renene.2018.09.071.
Jamil, S. M., Othman, M. H. D., Rahman, M. A., Jaafar, J., Ismail, A. F., & Li, K. (2015). Recent fabrication techniques for micro-tubular solid oxide fuel cell support: A review. Journal of the European Ceramic Society, 35, 1–22. https://doi.org/10.1016/j.jeurceramsoc.2014.08.034.
Jheng, L., Huang, C., & Hsu, S. L. (2013). Sulfonated MWNT and imidazole functionalized MWNT/polybenzimidazole composite membranes for high-temperature proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 38, 1524–1534. https://doi.org/10.1016/j.ijhydene.2012.10.111.
Jin, Y. C., Nishida, M., Kanematsu, W., & Hibino, T. (2011). An H3PO4-doped polybenzimidazole/Sn0.95Al0.05P2O7 composite membrane for high-temperature proton exchange membrane fuel cells. Journal of Power Sources, 196, 6042–6047. https://doi.org/10.1016/j.jpowsour.2011.03.094.
Jörissen, L., Gogel, V., Kerres, J., & Garche, J. (2002). New membranes for direct methanol fuel cells. Journal of Power Sources, 105, 267–273. https://doi.org/10.1016/S0378-7753(01)00952-1.
Kannan, R., Aher, P. P., Palaniselvam, T., Kurungot, S., Kharul, U. K., & Pillai, V. K. (2010). Artificially designed membranes using phosphonated multiwall carbon nanotube−polybenzimidazole composites for polymer electrolyte fuel cells. The Journal of Physical Chemistry Letters, 1, 2109–2113. https://doi.org/10.1021/jz1007005.
Kaur, G., & Basu, S. (2015). Physical characterization and electrochemical performance of copper-iron-ceria-YSZ anode-based SOFCs in H2 and methane fuels. International Journal of Energy Research, 39, 1345–1354. https://doi.org/10.1002/er.3332.
Kim, D. J., Jo, M. J., & Nam, S. Y. (2015). A review of polymer–nanocomposite electrolyte membranes for fuel cell application. Journal of Industrial and Engineering Chemistry, 21, 36–52. https://doi.org/10.1016/j.jiec.2014.04.030.
Lade, H., Kumar, V., Arthanareeswaran, G., & Ismail, A. F. (2017). Sulfonated poly(arylene ether sulfone) nanocomposite electrolyte membrane for fuel cell applications: A review. International Journal of Hydrogen Energy, 42, 1063–1074. https://doi.org/10.1016/j.ijhydene.2016.10.038.
Lee, D. C., Yang, H. N., Park, S. H., & Kim, W. J. (2014). Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. Journal of Membrane Science, 452, 20–28. https://doi.org/10.1016/j.memsci.2013.10.018.
Lee, J. G., Jeon, O. S., Hwang, H. J., Jang, J., Lee, Y., Hyun, S. H., & Shul, Y. G. (2016). Durable and high-performance direct-methane fuel cells with coke-tolerant ceria-coated Ni catalysts at reduced temperatures. Electrochimica Acta, 191, 677–686. https://doi.org/10.1016/j.electacta.2016.01.091.
Li, C., Li, C., Xing, Y., Gao, M., & Yang, G. (2006). Influence of YSZ electrolyte thickness on the characteristics of plasma-sprayed cermet supported tubular SOFC. Solid State Ionics, 177, 2065–2069. https://doi.org/10.1016/j.ssi.2006.03.004.
Li, P., Wang, Z., Yao, X., Hou, N., Fan, L., Gan, T., et al. (2019). Effect of Sn addition on improving the stability of Ni-Ce0.8Sm0.2O1.9 anode material for solid oxide fuel cells fed with dry CH4. Catalysis Today, 330, 209–216. https://doi.org/10.1016/j.cattod.2018.04.030.
Li, X., & Faghri, A. (2011). Effect of the cathode open ratios on the water management of a passive vapor-feed direct methanol fuel cell fed with neat methanol. Journal of Power Sources, 196, 6318–6324. https://doi.org/10.1016/j.jpowsour.2011.03.047.
Li, X., & Faghri, A. (2013). Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions. Journal of Power Sources, 226, 223–240. https://doi.org/10.1016/j.jpowsour.2012.10.061.
Li, X., Faghri, A., & Xu, C. (2010). Water management of the DMFC passively fed with a high-concentration methanol solution. International Journal of Hydrogen Energy, 35, 8690–8698. https://doi.org/10.1016/j.ijhydene.2010.05.033.
Litster, S., & McLean, G. (2004). PEM fuel cell electrodes. Journal of Power Sources, 130, 61–76. https://doi.org/10.1016/j.jpowsour.2003.12.055.
Lobato, J., Cañizares, P., Rodrigo, M. A., Úbeda, D., & Pinar, F. J. (2011a). A novel titanium PBI-based composite membrane for high temperature PEMFCs. Journal of Membrane Science, 369, 105–111. https://doi.org/10.1016/j.memsci.2010.11.051.
Lobato, J., Cañizares, P., Rodrigo, M. A., Úbeda, D., & Pinar, F. J. (2011b). Enhancement of the fuel cell performance of a high temperature proton exchange membrane fuel cell running with titanium composite polybenzimidazole-based membranes. Journal of Power Sources, 196, 8265–8271. https://doi.org/10.1016/j.jpowsour.2011.06.011.
Mahato, N., Banerjee, A., Gupta, A., Omar, S., & Balani, K. (2015). Progress in material selection for solid oxide fuel cell technology: A review. Progress in Materials Science, 72, 141–337. https://doi.org/10.1016/j.pmatsci.2015.01.001.
Majlan, E. H., Rohendi, D., Daud, W. R. W., Husaini, T., & Haque, M. A. (2018). Electrode for proton exchange membrane fuel cells: A review. Renewable and Sustainable Energy Reviews, 89, 117–134. https://doi.org/10.1016/j.rser.2018.03.007.
Mallick, R. K., Thombre, S. B., & Shrivastava, N. K. (2016). Vapor feed direct methanol fuel cells (DMFCs): A review. Renewable and Sustainable Energy Reviews, 56, 51–74. https://doi.org/10.1016/j.rser.2015.11.039.
Mamlouk, M., Scott, K., & Hidayati, N. (2011). High temperature direct methanol fuel cell based on phosphoric acid PBI membrane. Journal of Fuel Cell Science and Technology, 8, 061009. https://doi.org/10.1115/1.4004557.
Mehta, V., & Cooper, J. S. (2003). Review and analysis of PEM fuel cell design and manufacturing. Journal of Power Sources, 114, 32–53. https://doi.org/10.1016/S0378-7753(02)00542-6.
Meng, X., Gong, X., Yang, N., Tan, X., Yin, Y., & Ma, Z. F. (2013). Fabrication of Y2O3-stabilized-ZrO2(YSZ)/La0.8Sr0.2MnO3-α-YSZ dual-layer hollow fibers for the cathode-supported micro-tubular solid oxide fuel cells by a co-spinning/co-sintering technique. Journal of Power Sources, 237, 277–284. https://doi.org/10.1016/j.jpowsour.2013.03.026.
Meng, X., Gong, X., Yang, N., Yin, Y., & Tan, X. (2014). Carbon-resistant Ni-YSZ/Cu–CeO2-YSZ dual-layer hollow fiber anode for micro tubular solid oxide fuel cell. International Journal of Hydrogen Energy, 39, 3879–3886. https://doi.org/10.1016/j.ijhydene.2013.12.168.
Meng, X., Yang, N., Gong, X., Yin, Y., Ma, Z.-F.F., Tan, X., et al. (2015). Novel cathode-supported hollow fibers for light weight micro-tubular solid oxide fuel cells with an active cathode functional layer. Journal of Materials Chemistry A, 3, 1017–1022. https://doi.org/10.1039/C4TA04635H.
Moghaddam, R. B., & Easton, E. B. (2018). Impedance spectroscopy assessment of catalyst coated Nafion assemblies for proton exchange membrane fuel cells. Electrochimica Acta, 292, 292–298. https://doi.org/10.1016/j.electacta.2018.09.163.
Narayan, S. R., & Valdez, T. I. (2008). High-energy portable fuel cell power sources. Electrochemical Society Interface, 17, 40–45.
Ogungbemi, E., Ijaodola, O., Khatib, F. N., Wilberforce, T., El Hassan, Z., Thompson, J., et al. (2019). Fuel cell membranes—Pros and cons. Energy, 172, 155–172. https://doi.org/10.1016/j.energy.2019.01.034.
Omar, A. F., Othman, M. H. D., Gunaedi, C. N., Jamil, S. M., Mohamed, M. H., Jaafar, J., et al. (2018). Performance analysis of hollow fibre-based micro-tubular solid oxide fuel cell utilising methane fuel. International Journal of Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2018.03.107.
Ong, B. C., Kamarudin, S. K., & Basri, S. (2017). Direct liquid fuel cells: A review. International Journal of Hydrogen Energy, 42, 10142–10157. https://doi.org/10.1016/j.ijhydene.2017.01.117.
Oszcipok, M., Zedda, M., Hesselmann, J., Huppmann, M., Wodrich, M., Junghardt, M., & Hebling, C. (2006). Portable proton exchange membrane fuel-cell systems for outdoor applications. Journal of Power Sources, 157, 666–673. https://doi.org/10.1016/j.jpowsour.2006.01.005.
Panthi, D., Choi, B., Du, Y., & Tsutsumi, A. (2017a). Lowering the co-sintering temperature of cathode–electrolyte bilayers for micro-tubular solid oxide fuel cells. Ceramics International, 43, 10698–10707. https://doi.org/10.1016/j.ceramint.2017.05.003.
Panthi, D., Choi, B., & Tsutsumi, A. (2017b). Direct methane operation of a micro-tubular solid oxide fuel cell with a porous zirconia support. Journal of Solid State Electrochemistry, 21(1), 255–262. https://doi.org/10.1007/s10008-016-3366-5.
Park, K. T., Kim, S. G., Chun, J. H., Jo, D. H., Chun, B.-H., Jang, W. I., et al. (2011). Composite membranes based on a sulfonated poly(arylene ether sulfone) and proton-conducting hybrid silica particles for high temperature PEMFCs. International Journal of Hydrogen Energy, 36, 10891–10900. https://doi.org/10.1016/j.ijhydene.2011.05.151.
Peighambardoust, S. J., Rowshanzamir, S., & Amjadi, M. (2010). Review of the proton exchange membranes for fuel cell applications. International Journal of Hydrogen Energy, 35, 9349–9384. https://doi.org/10.1016/j.ijhydene.2010.05.017.
Pivac, I., & Barbir, F. (2016). Inductive phenomena at low frequencies in impedance spectra of proton exchange membrane fuel cells—A review. Journal of Power Sources, 326, 112–119. https://doi.org/10.1016/j.jpowsour.2016.06.119.
Rabuni, M. F., Li, T., Punmeechao, P., & Li, K. (2018). Electrode design for direct-methane micro-tubular solid oxide fuel cell (MT-SOFC). Journal of Power Sources, 384, 287–294. https://doi.org/10.1016/j.jpowsour.2018.03.002.
Radenahmad, N., Afif, A., Petra, P. I., Rahman, S. M. H., Eriksson, S.-G., & Azad, A. K. (2016). Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renewable and Sustainable Energy Reviews, 57, 1347–1358. https://doi.org/10.1016/j.rser.2015.12.103.
Rosli, R. E., Sulong, A. B., Daud, W. R. W., Zulkifley, M. A., Husaini, T., Rosli, M. I., et al. (2017). A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. International Journal of Hydrogen Energy, 42, 9293–9314. https://doi.org/10.1016/j.ijhydene.2016.06.211.
Santasalo-Aarnio, A., Borghei, M., Anoshkin, I. V., Nasibulin, A. G., Kauppinen, E. I., Ruiz, V., & Kallio, T. (2012). Durability of different carbon nanomaterial supports with PtRu catalyst in a direct methanol fuel cell. International Journal of Hydrogen Energy, 37, 3415–3424. https://doi.org/10.1016/j.ijhydene.2011.11.009.
Sarruf, B. J. M., Hong, J.-E., Steinberger-Wilckens, R., & de Miranda, P. E. V. (2017). Double layered CeO2-Co3O4-CuO based anode for direct utilisation of methane or ethanol in SOFC. ECS Transactions, 78, 1343–1351. https://doi.org/10.1149/07801.1343ecst.
Sarruf, B. J. M., Hong, J. E., Steinberger-Wilckens, R., & de Miranda, P. E. V. (2018). CeO2–Co3O4–CuO anode for direct utilisation of methane or ethanol in solid oxide fuel cells. International Journal of Hydrogen Energy, 43, 6340–6351. https://doi.org/10.1016/j.ijhydene.2018.01.192.
Sengodan, S., Lan, R., Humphreys, J., Du, D., Xu, W., Wang, H., & Tao, S. (2018). Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renewable and Sustainable Energy Reviews, 82, 761–780. https://doi.org/10.1016/j.rser.2017.09.071.
Shamim, S., Sudhakar, K., Choudhary, B., & Anwar, J. (2015). A review on recent advances in proton exchange membrane fuel cells: Materials, technology and applications. Advances in Applied Science Research, 6, 89–100.
Shukla, A. K., Ravikumar, M. K., & Gandhi, K. S. (1998). Direct methanol fuel cells for vehicular applications. Journal of Solid State Electrochemistry, 2, 117–122. https://doi.org/10.1007/s100080050075.
Singhal, S. (2000). Advances in solid oxide fuel cell technology. Solid State Ionics, 135, 305–313. https://doi.org/10.1016/S0167-2738(00)00452-5.
Stambouli, A. B., & Traversa, E. (2002). Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renewable and Sustainable Energy Reviews, 6, 433–455. https://doi.org/10.1016/S1364-0321(02)00014-X.
Sumi, H., Yamaguchi, T., Shimada, H., Hamamoto, K., Suzuki, T., & Barnett, S. A. (2017). Direct butane utilization on Ni-(Y2O3)0.08(ZrO2)0.92-(Ce0.9Gd0.1)O1.95 composite anode-supported microtubular solid oxide fuel cells. Electrocatalysis, 8, 288–293. https://doi.org/10.1007/s12678-017-0369-7.
Sumi, H., Yamaguchi, T., Suzuki, T., & Shimada, H. (2015). Direct hydrocarbon utilization in microtubular solid oxide fuel cells. Journal of the Ceramic Society of Japan, 08, 213–216. https://doi.org/10.2109/jcersj2.123.213.
Sung, I. H., Yu, D. M., Yoon, Y. J., Kim, T.-H., Lee, J. Y., Hong, S. K., & Hong, Y. T. (2013). Preparation and properties of sulfonated poly(arylene ether sulfone)/hydrophilic oligomer-g-CNT composite membranes for PEMFC. Macromolecular Research, 21, 1138–1144. https://doi.org/10.1007/s13233-013-1136-0.
Tanaka, Y. (2007). Bipolar membrane electrodialysis. In Membrane science and technology (Chap. 3, pp. 405–436).
Teresa, L., & Gámez, M. (2007). Polymer electrolyte membranes for the direct methanol fuel cell: A review. Journal of Polymer Science, 45, 2007–2009. https://doi.org/10.1002/POLB.
Thanganathan, U., & Nogami, M. (2014). Proton conductivity and structural properties of precursors mixed PVA/PWA-based hybrid composite membranes. Journal of Solid State Electrochemistry, 18, 97–104. https://doi.org/10.1007/s10008-013-2235-8.
Thomas, S. C., Ren, X., Gottesfeld, S., & Zelenay, P. (2002). Direct methanol fuel cells: Progress in cell performance and cathode research. Electrochimica Acta, 47, 3741–3748.
Wang, Y., Chen, K. S., Mishler, J., Cho, S. C., & Adroher, X. C. (2011). A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Applied Energy, 88, 981–1007. https://doi.org/10.1016/j.apenergy.2010.09.030.
Wee, J.-H. (2007). Applications of proton exchange membrane fuel cell systems. Renewable and Sustainable Energy Reviews, 11, 1720–1738. https://doi.org/10.1016/j.rser.2006.01.005.
Wu, Q. X., Zhao, T. S., Chen, R., & An, L. (2013). A sandwich structured membrane for direct methanol fuel cells operating with neat methanol. Applied Energy, 106, 301–306. https://doi.org/10.1016/j.apenergy.2013.01.016.
Wu, Q. X., Zhao, T. S., Chen, R., & Yang, W. W. (2010). Enhancement of water retention in the membrane electrode assembly for direct methanol fuel cells operating with neat methanol. International Journal of Hydrogen Energy, 35, 10547–10555. https://doi.org/10.1016/j.ijhydene.2010.07.178.
Wu, Q. X., Zhao, T. S., & Yang, W. W. (2011). Effect of the cathode gas diffusion layer on the water transport behavior and the performance of passive direct methanol fuel cells operating with neat methanol. International Journal of Heat and Mass Transfer, 54, 1132–1143. https://doi.org/10.1016/j.ijheatmasstransfer.2010.11.009.
Wu, X., Tian, Y., Zhang, J., Zuo, W., Kong, X., Wang, J., et al. (2016). Enhanced electrochemical performance and carbon anti-coking ability of solid oxide fuel cells with silver modified nickel-yttrium stabilized zirconia anode by electroless plating. Journal of Power Sources, 301. https://doi.org/10.1016/j.jpowsour.2015.10.006.
Xu, C., & Faghri, A. (2010). Mass transport analysis of a passive vapor-feed direct methanol fuel cell. Journal of Power Sources, 195, 7011–7024. https://doi.org/10.1016/j.jpowsour.2010.05.003.
Xu, C., Faghri, A., & Li, X. (2011a). Improving the water management and cell performance for the passive vapor-feed DMFC fed with neat methanol. International Journal of Hydrogen Energy, 36, 8468–8477. https://doi.org/10.1016/j.ijhydene.2011.03.115.
Xu, Q., Zhao, T. S., Yang, W. W., & Chen, R. (2011b). A flow field enabling operating direct methanol fuel cells with highly concentrated methanol. International Journal of Hydrogen Energy, 36, 830–838. https://doi.org/10.1016/j.ijhydene.2010.09.026.
Yan, N., Pandey, J., Zeng, Y., Amirkhiz, B. S., Hua, B., Geels, N. J., et al. (2016). Developing a thermal- and coking-resistant cobalt-tungsten bimetallic anode catalyst for solid oxide fuel cells. ACS Catalysis, 6, 4630–4634. https://doi.org/10.1021/acscatal.6b01197.
Yan, X. H., Zhao, T. S., Zhao, G., An, L., & Zhou, X. L. (2015). A hydrophilic-hydrophobic dual-layer microporous layer enabling the improved water management of direct methanol fuel cells operating with neat methanol. Journal of Power Sources, 294, 232–238. https://doi.org/10.1016/j.jpowsour.2015.06.058.
Yoo, Y., & Lim, N. (2013). Performance and stability of proton conducting solid oxide fuel cells based on yttrium-doped barium cerate-zirconate thin-film electrolyte. Journal of Power Sources, 229, 48–57. https://doi.org/10.1016/j.jpowsour.2012.11.094.
Yu, D. M., Yoon, Y. J., Kim, T.-H., Lee, J. Y., & Hong, Y. T. (2013). Sulfonated poly(arylene ether sulfone)/sulfonated zeolite composite membrane for high temperature proton exchange membrane fuel cells. Solid State Ionics, 233, 55–61. https://doi.org/10.1016/j.ssi.2012.12.006.
Zainoodin, A. M., Kamarudin, S. K., & Daud, W. R. W. (2010). Electrode in direct methanol fuel cells. International Journal of Hydrogen Energy, 35, 4606–4621. https://doi.org/10.1016/j.ijhydene.2010.02.036.
Zhang, H., Liu, Z., Gao, S., & Wang, C. (2014). A new cathode structure for air-breathing DMFCs operated with pure methanol. International Journal of Hydrogen Energy, 39, 13751–13756. https://doi.org/10.1016/j.ijhydene.2014.02.145.
Zhang, Z., Yuan, W., Wang, A., Yan, Z., Tang, Y., & Tang, K. (2015). Moisturized anode and water management in a passive vapor-feed direct methanol fuel cell operated with neat methanol. Journal of Power Sources, 297, 33–44. https://doi.org/10.1016/j.jpowsour.2015.07.097.
Zhao, X., Yuan, W., Wu, Q., Sun, H., Luo, Z., & Fu, H. (2015). High-temperature passive direct methanol fuel cells operating with concentrated fuels. Journal of Power Sources, 273, 517–521. https://doi.org/10.1016/j.jpowsour.2014.09.128.
Zhiani, M., Majidi, S., Silva, V. B., & Gharibi, H. (2016). Comparison of the performance and EIS (electrochemical impedance spectroscopy) response of an activated PEMFC (proton exchange membrane fuel cell) under low and high thermal and pressure stresses. Energy, 97, 560–567. https://doi.org/10.1016/j.energy.2015.12.058.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jamil, S.M., Rahman, M.A., Shabri, H.A., Othman, M.H.D. (2021). Solid Electrolyte Membranes for Low- and High-Temperature Fuel Cells. In: Zhang, Z., Zhang, W., Chehimi, M.M. (eds) Membrane Technology Enhancement for Environmental Protection and Sustainable Industrial Growth . Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-41295-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-41295-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41294-4
Online ISBN: 978-3-030-41295-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)