Abstract
In this chapter, we first provide an overview of the general context given by screening mammography in the general female population, including the hot debate about its harm-to-benefit balance and the wide range of estimated overdiagnosis, associated with overtreatment. In particular, the limitations of the principle one size fits all underlying population-based screening programs is discussed, with the perspectives for more personalized approaches, based on risk stratification. We then describe the main results obtained by intra-individual prospective studies comparing breast magnetic resonance (MRI) with clinical breast examination and conventional imaging, i.e., mammography and ultrasound (US), for screening women at high risk of breast (BRCA mutation carriers and women with strong family history of breast/ovarian cancers). In particular, we distinguish the initial studies, published from 2000 to 2005, on whose results the American Cancer Society based its 2007 guidelines in favor of MRI as an adjunct to mammography for high-risk screening, from the subsequent studies published between 2007 and 2015. The latter expanded the existing body of knowledge showing not only that MRI is more accurate than mammography and/or US for high-risk screening but also that, when MRI is performed, the added value of conventional imaging is low, leading to the reverted principle of mammography as an adjunct to MRI, only if necessary. In addition, the ten key points suggested in 2010 by the multidisciplinary panel of EUSOMA for MRI screening of high-risk women are here reported. Finally, some relevant retrospective studies specifically focusing on the contribution of mammography and on MRI false-negative cases in high-risk screening are considered.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Evidence-based medicine (EBM) [1] is generally adopted as a method for guiding clinicians as well as governmental bodies so that we should be able to define the best evidence-based medical practices. The EBM principles are increasingly applied to radiology [2], where a specific safety criterion regards the reduction of radiation exposure to a level defined as low as reasonably achievable (ALARA) [3].
The Oxford center for EBM [4] clearly distinguishes between diagnostic tests and screening tests.Footnote 1 For example, the definition of the disease size (or extent, at large) is a diagnostic task for which tests can be validated by cohort studies with reference standards independent of the test and applied blindly or objectively to all patients. This means that non-randomized prospective (especially intra-individual) studies enable us to choose the test with the best sensitivity/specificity, without needing randomized controlled trials (RCTs). Conversely, screening tests should be demonstrated to be effective in terms of patient outcome (i.e., overall or disease-specific survival, disease-free or metastasis-free survival, etc.) by RCTs before being implemented in practice [4], a rule also affirmed in 2002 by a European Guideline [5].
As already outlined in Chap. 1, during the 1990s, the availability of both BRCA genetic testing and contrast-enhanced (CE) breast MRI determined the conditions for studies aimed at comparing MRI with conventional imaging, i.e., mammography and ultrasound (US), for the detection of breast cancers (BCs) in BRCA mutation carriers, in their first-degree relatives as well as in women with family history implying a high risk of hereditary BC predisposition. Thus, an intra-individual design was adopted to firstly demonstrate the diagnostic performance of MRI, characterized by a superior sensitivity coupled with an acceptable specificity. This was the aim of the studies that initially reported on MRI versus mammography/US for screening women at high BC risk [6,7,8,9,10,11,12].
The gap in sensitivity between MRI and mammography, the standard BC screening tool, was so high that the ideal second phase, i.e., RCTs, to demonstrate that high-risk women screened with MRI have an advantage in terms of patient outcome became ethically unfeasible. This unfeasibility was due to the combination of the high MRI sensitivity with the high probability of BC in a high-risk population: the BC diagnosis anticipated by MRI was considered as more likely positively impacting survival than determining a negative effect in terms of overdiagnosis and overtreatment. The known effect of mortality reduction by early BC detection through screening mammography in the general population was translated to MRI in the high-risk population.
In this chapter, after an overview of the general context given by screening mammography in the general female population, we describe the main results obtained by intra-individual studies comparing MRI with conventional imaging for screening women at high BC risk.
9.2 The Context: Secondary Prevention of BC by Screening Mammography
In the last 50 years, the context of secondary prevention of BC in the general female population has been the kingdom of screening mammography, notwithstanding its intrinsic limitations in terms of sensitivity and specificity. Mammography evolved from the screen-film to the digital technique, demonstrated to be more sensitive in women under 50, those with dense breasts, or in premenopausal or perimenopausal age [13]. Although substantial differences do exist in terms of organizational matters and testing performance between organized population-based mass screening in most European countries and spontaneous screening in the United States (mainly consisting in a higher false-positive recall rate in the latter modality [14]), the general issue characterizing the debate on screening mammography in the last two decades is the effectiveness of screening mammography in reducing the BC mortality and the harm-to-benefit balance.
This debate has been dominated by a never-ending discussion on overdiagnosis , i.e., the screening diagnosis of a cancer that would not become clinically evident during the woman’s lifetime in the absence of the screening participation. The harm of overdiagnosis is not only the psychological effect of the diagnosis but mainly the overtreatment that follows the overdiagnosis. It is clear that the final judgment on the harm-to-benefit balance of screening mammography is dependent on the extent of this phenomenon in relation to the mortality reduction. A review [15] recently highlighted the huge variability in the ratio between the estimated overdiagnosis and the estimated mortality reduction in eight studies. A 25-fold variation (from 0.4 to 10) was found to strongly correlate with the “attitude” of the corresponding authors to the screening, which could mean that being either in favor or against screening mammography influences the results. We do not enter here in the highly complex statistical issues regarding the estimation of overdiagnosis. We only note that the discussion is hot and probably will continue in the next years.
What is more relevant is the other side of the dilemma, i.e., the general question about the role of early (preclinical) detection in determining a mortality reduction. A basic argument against screening mammography is the following: the more effective the treatments, the less favorable is the harm-to-benefit balance of screening mammography [16]. Considering that therapies (especially adjuvant treatment and radiation therapy) strongly improved in the last 20 years, a fundamental question is: did we reach the break-even point where the T stage of the tumor is no longer impacting patients’ outcome? If yes, there would be no reason to organize any screening, independently of the preferred estimation of overdiagnosis. If no, to screen for detecting smaller cancers than those we would encounter waiting for their clinical appearance should remain a major goal of preventive medicine.
In 2005, Donald A. Berry and coworkers [17] estimated the changes in the rate of deaths from BC (the number of deaths/100,000 women) from the 1970s to 2000, showing that only the combination of screening and adjuvant therapy explained the reduction of this rate from a peak near to 50 BC deaths/100,000 women to about 35 in 2000. The proportion in this reduction attributed to screening mammography varied from 28% to 65% in seven models considered (median 46%), the remaining proportion being attributed to adjuvant therapy. Thus at that time, the authors described a near 50%-to-50% contribution of screening mammography and adjuvant therapy in determining the decline of BC mortality.
Today, the crucial question is the following: is early detection still relevant for BC patient outcome in the era of modern powerful systemic therapies including targeted biological treatments? The answer is yes. This has been demonstrated by a population-based study from the Netherlands Cancer Registry [18], evaluating more than 170,000 patients: although the rate of those receiving neoadjuvant/adjuvant therapy from 1995–2005 to 2006–2012 increased from 53% to 60%, the mortality in 2006–2012 still increased with progressing tumor stage, significantly for T1c versus T1a (hazard ratio [HR] 1.54), and independently of the nodal status. Moreover, we must consider that screening mammography has a relevant role in making neoadjuvant treatment more effective, as shown by its ability to downscale the clinico-pathological features of invasive BCs and reducing the need for loco-regional and adjuvant treatments [19,20,21,22].
In 2015, the International Agency for Research on Cancer (IARC) summarized the evidence for screening mammography [23], contributing to clarify a so hotly discussed matter [24]. The estimated reduction in BC mortality has been estimated to be 23% for all women aged 50–69 invited to be screened (i.e., also including those not accepting the invitation) and 40% for women aged 50–69 who are screened. A limited evidence was reported for mortality reduction in women aged 40–49 (less pronounced mortality reduction) and 70–74 (substantial mortality reduction). The IARC working group also reported the overdiagnosis rate to be from 1% to 10% or from 4% to 11%, according to different estimation methods, substantially confirming the estimates provided in 2012 by the EUROSCREEN working group [25].
The EUROSCREEN working group [26] also presented their estimate of the harm-to-benefit balance of screening mammography using natural frequencies, a method that allows for a better understanding by the public. They say that for every 1,000 women that have biennial mammography in a European population-based screening program from 50 to 69 years of age and are followed up to 79 years of age, we observe:
-
8 women with a screen-detected BC, treated for the disease, who survived thanks to the screening
-
Other 47 women diagnosed with a BC, treated and survived
-
4 women with BC overdiagnosis (and overtreatment)
-
12 women who died for BC
-
30 women who underwent image-guided needle biopsy for benign findings
-
170 women who underwent further imaging (during the recall session) for benign findings
-
729 women, never recalled, reassured on the absence of cancer in their breasts
This means that the risk for a false-positive recall is limited to 20% for women aged 50–69 who have ten screens in 20 years; 15% of recalled women have an invasive procedure, which results in a probability during the 20 years of 3%. The probability of overdiagnosis is half the probability to have the life saved. Notably, overdetection , a radiological issue, should be considered as a quite different topic from overdiagnosis [27], which implies also an essential role of pathologists, with their suboptimal reproducibility, especially in the case of differential diagnosis between atypical ductal hyperplasia and ductal carcinoma in situ [DCIS] [28, 29], where a second opinion may be beneficial [29, 30], while more efforts should be directly dedicated to the reduction of overtreatment .
However, one weak point of population-based screening programs is the one size fits all general principle: in Europe, mammography every 2 years (every 3 years in the United Kingdom) from 49 to 69 years. Some changes mainly regarded the invitation of women from 40 or, more frequently, from 45 to 49 to get a mammogram every year. All in all, organizational issues and other factors worked against the idea to stratify the screening strategy according to the risk level and breast density. The latter factor is relevant: even though density as an independent risk factor is commonly overestimated [31], its masking effect results in a relevant reduction in mammography sensitivity [32], as also discussed in Chap. 20 of this book. An organized screening strategy tailored for the woman’s individual risk, also considering breast density, is a hope for the future.
Coming to the crucial point, in the late 1990s and the early 2000s, the current recommendations for BRCA mutation carriers were to undergo breast surveillance from age 25 years onward with annual mammography and clinical breast examination (CBE) every 6 months [33, 34]. It was clear that screening mammography in high-risk women was inadequate. Its sensitivity ranged from 29% to 50%, interval cancer rate from 35% to 50%, and metastatic nodal involvement at diagnosis from 20% to 56% [35].
A new strategy to be implemented had to consider three crucial needs:
-
1.
To start very early in the life of high-risk women, accounting for the high probability of an early onset of BC
-
2.
To perform screening events every year or closer, accounting for the fast BC growth in these women
-
3.
To warrant independence of the screening tool from breast density, accounting for the woman’s young age and for the higher breast density in high-risk women
In addition, the possibility of avoiding ionizing radiation exposure is an important issue, accounting for the higher susceptibility to radiation of BRCA mutation carriers, as extensively discussed in Chap. 12 of this book.
This was the context when the first MRI-including screening studies were reported, during the first decade of 2000. As mentioned above, mammography had moved from screen-film to digital but no impact from this transition was expected for high-risk women.
9.3 High-Risk Screening with MRI: From a Mission Impossible to the First Evidence (2000–2006)
To explore the diagnostic power of breast MRI in a screening setting was initially considered as a mission impossible. The typical criticism, especially from epidemiologists, was: MRI specificity is too low, and you will be flooded by a deluge of false positives . The reasons for this view are extensively explained in Chap. 2 of this book.
Several breast imaging research groups started to verify the hypothesis that CE-MRI could be useful for BC screening in women at increased BC risk, especially those with hereditary predisposition. This was also a way to begin to discuss, from the side of high risk, the one size fits all principle. Breast radiologists had to get at least basic knowledge about familial/genetic predisposition to BC. In 2010, we summarized this knowledge as follows [36]:
-
Autosomal dominant inherited BCs are only 5% of all BCs (one third of all familial BCs).
-
BRCA1/2 mutations account for only about 40% of autosomal dominant inherited BCs and other known genes explain about 10%, while the remaining 50% has no gene mutation clearly identified. BRCA1/2 deleterious mutations confer to the carrier an over 40–50% of lifetime risk (LTR).Footnote 2
-
Most BCs in very young women are associated with a BRCA1 mutation, a condition that may also show association with ovarian cancer.
-
In women carrying a BRCA2 mutation, the risk profile is shifted to a slightly more advanced age, while BCs in males are commonly associated with this type of mutation.
More detailed information on this topic can be found in Chap. 3 of this book.
This basic knowledge allowed radiologists to identify those women whose family history indicates the possibility of an inherited BC predisposition. Since 2004–2005, software could be used for a preliminary risk evaluation, such as that based on the Tyrer-Cuzick model [37, 38] (BC risk modeling is extensively treated in Chap. 20 of this book). However, radiologists (and other professionals who suspected a BC genetic predisposition) had to refer the woman suspected to be at high-risk to a specialized department/center for genetic and psycho-oncology counseling to define the possibility of genetic testing. Importantly, radiologists learned that in the case of strong family history of BC and/or ovarian cancer without identification of known gene mutations in the family, genetic testing had to be defined as inconclusive and the case had to be labeled as BRCAX [39]. Finally, it was important to know that for different reasons, including unsuitable psycho-oncologic condition, women with strong family history often prefer not to perform any genetic testing.
The first pilot study was reported by Christiane K. Kuhl in 2000 [6]. In 192 asymptomatic women proven or suspected to be carriers of a BC susceptibility gene mutation included in this report (which also included 6 symptomatic cases, here not considered), 9 BCs were detected at the University of Bonn Medical Center in 293 screening events. Sensitivity was 33% for mammography, 33% for US (44% for mammography and US combined), and 100% for MRI; the positive predictive value (PPV) was 30% for mammography, 14% for US, and 64% for MRI. The authors concluded that the accuracy of MRI was significantly higher than that of conventional imaging in screening high-risk women. These data were later included in the final report published in 2005 [12].
Thereafter, several cohort prospective single- or multi-center studies on asymptomatic high-risk women followed, building a robust body of evidence in favor of breast MRI screening in this population. We will now focus on these studies for which reports were published up to 2017. To present the historical pathway that led to the acceptance of MRI in this setting, we firstly describe the results of the studies on which the American Cancer Society (ACS) based the 2007 guidelines [40] in favor of MRI screening for women at high risk, which represented a turning point in this story. In the next section we will describe the results of the prospective studies published after the publication of the ACS guideline.
In 2002, we reported [7] the preliminary results of the first phase (21 months) of the High Breast Cancer Risk Italian (HIBCRIT-1) study. At that time, 105 asymptomatic women (mean/median age 46/51 years; range 25–77 years) had been enrolled in 12 centers in Italy, under the coordination of the Istituto Superiore di Sanità, Roma. They either were proven BRCA1 or BRCA2 mutation carriers, or had a 1:2 probability of being BRCA mutation carriers, or had a high record of first- and/or second-degree relatives at very high incidence of breast cancer. Importantly, 40 of 105 (38%) had a previous personal history of BC. The study protocol included yearly mammography, US, and MRI, independently interpreted.Footnote 3 During this first phase of the study (119 screening events), 8 BCs were detected (2 invasive ductal; 2 invasive lobular; 1 invasive mixed ductal/lobular; 2 multifocal DCIS; 1 DCIS associated with lobular carcinoma in situ). All study-detected BCs (8/8) were identified by MRI, while mammography and US correctly classified only one. MRI had one false-positive case, mammography and US none. Of 7 BCs detected on MRI-only (4 invasive, 3 DCIS), 2 occurred in premenopausal women, 5 in postmenopausal women. Despite the still preliminary nature of these data, we confirmed that MRI is a very useful tool to screen subjects at high genetic risk for breast carcinoma, not only in premenopausal but also in postmenopausal age, with a low probability of false-positive cases. We also estimated that the cost per MRI-only detected BC in the high-risk setting was substantially lower than that of a screen-detected cancer in the general female population undergoing screening mammography.
The general trends were already clear:
-
1.
High BC prevalence due to the eligibility criteria
-
2.
An overall very large gap in sensitivity between MRI and conventional imaging, i.e., not only mammography but also US
-
3.
Lower sensitivity of mammography also in postmenopausal high-risk women
-
4.
Absence of data suggesting high frequency of false positives, low specificity, and low PPV
In 2004, Mieke Kriege and coworkers [8] reported the results of the Magnetic Resonance Imaging Screening (MRISC) study carried out in six centers in the Netherlands comparing clinical breast examination (CBE), performed every 6 months, MRI and mammography (both of them performed yearly) in women with a cumulative LTR for BC ≥15%. They screened 1,909 women, including 358 carriers of germ-line mutations. A total of 51 malignant lesions (44 invasive cancers, 6 DCIS, 1 lymphoma) and 1 lobular carcinoma in situ were diagnosed in a total of 5,249 woman-years at risk. The sensitivity for detecting invasive BCs was 18% for CBE, 33% for mammography, and 80% for MRI; specificity was 98%, 95%, and 90%, respectively. The reported sensitivity values for al BCs (invasive or DCIS) were 18% for CBE, 40% for mammography, and 71% for MRI. The overall diagnostic power of MRI (area under the curve [AUC] at receiver operator characteristics [ROC] analysis 0.83) was significantly higher than that of mammography (AUC 0.69).
The authors also compared their results with those obtained in two control groups external to the study, matched for age with the patients in the study group. The first control group was derived from all women diagnosed with BC in 1998 in the Netherlands (data from the National Cancer Registry). The second control group consisted of patients diagnosed with primary BC in Leiden or Rotterdam from 1996 to 2002, participating in a prospective study of the prevalence of gene mutations. The second control group included all the unscreened patients with 25–60 years of age and cumulative LTR for BC higher than 15% on the basis of the family history. The proportion of invasive tumors ≤10 mm in diameter was significantly greater in the study group (43%) than in either control group (14% and 13%, respectively). In the study, 21% invasive cancers had positive axillary nodes or micrometastases, while this rate was significantly higher in the two control groups (52% and 56%, respectively). The straightforward conclusion was: MRI appears to be more sensitive than mammography in detecting tumors in women with an inherited susceptibility to BC [8].
In 2004, Ellen Warner and coworkers [9] compared the sensitivity and specificity of CBE, mammography, US, and MRI for screening in high-risk women. A total of 236 Canadian women aged 25 to 65 years being BRCA1 or BRCA2 mutation carriers underwent 1–3 annual screening events (for a total of 457 screening events) at the Sunnybrook and Women’s College Health Sciences Centre and University of Toronto. CBE was performed on the day of imaging examinations and at 6-month intervals. Twenty-two cancers were detected (16 invasive and 6 DCIS). The sensitivity and specificity (based on biopsy rates) were 77% and 95.4% for MRI, 36% and 99.8% for mammography, 33% and 96% for US, and 9.1% and 99.3% for CBE, respectively. All screening modalities combined had a sensitivity of 95% (1 interval cancer) to be compared with 45% for mammography and CBE combined. The authors concluded that in BRCA mutation carriers, MRI is more sensitive for detecting breast cancers than mammography, US, or CBE alone, and noted that the possibility of MRI to reduce BC mortality in high-risk women required further investigation.
The year after, in 2005, Martin O. Leach and coworkers [10] published the results of a prospective cohort study (Magnetic Resonance Imaging Breast Screening, MARIBS) performed in 22 centers in the United Kingdom. A total of 649 women aged 35–49 years with a strong family history of BC or a high probability of a BRCA1, BRCA2, or TP53 mutation underwent annual screening with CE MRI and mammography for 2–7 years. Thirty-five BCs were diagnosed during 1,881 screening events, 19 by CE-MRI only, 6 by mammography only, and 8 by both, with two interval cancers. The sensitivity of MRI (77%) was significantly higher than that of mammography (40%), reaching 94% when combining both of them. The specificity of mammography (93%) was significantly higher than that of MRI (81%), and 77% when combining both modalities. The authors noted that the difference in sensitivity between MRI and mammography was very high in BRCA1 mutation carriers (92% versus 23%, respectively, on a total of 13 cancers). Again, the authors concluded that in this population, MRI was more sensitive than mammography for cancer detection and that specificity for both procedures was acceptable, also noting that, despite a high proportion of grade 3 cancers, tumors were small, with few cases of nodal involvement. They suggested the combined use of MRI and mammography for screening this high-risk group.
In the same year (2005), Constance D. Lehman and coworkers of the International Breast MRI Consortium Working Group [11] compared the performance of mammography versus MRI for screening genetically high-risk women through a prospective study carried out in 13 centers in the United States and Canada. They were eligible from the age of 25 years, even if they had a personal BC history (contralateral screening when they had been diagnosed within 5 years; bilateral screening if they had been diagnosed more than 5 years previously). A total of 367 women completed (only once) all examinations in 13 centers, under the coordination of the University of Washington, Seattle Cancer Care Alliance, Seattle, United States. Imaging evaluations recommended 38 biopsies, 27 of them being performed, resulting in 4 cancers diagnosed; MRI detected all cancers, mammography only one. The biopsy recommendation rate was 8.5% for MRI and 2.2% for mammography. The conclusion, based on a lower BC incidence if compared to the other studies, was that screening MRI in high-risk women was capable of detecting mammographically and clinically occult BC with a tradeoff in terms of false positives causing a 5% rate of benign biopsy.
Finally, still in 2005, Christiane K. Kuhl and coworkers [12] reported on the final results of the single-center study whose preliminary results we mentioned earlier [6]. They compared mammography, US, and MRI for screening women with a lifetime risk ≥20%. The surveillance cohort study, carried out at the University of Bonn, enrolled 529 asymptomatic women suspected or proven to be BRCA mutation carriers. A total of 1,542 annual rounds were completed. A total of 43 BCs cancers were identified during the study (34 invasive, 9 DCIS). The sensitivity of mammography (33%) and ultrasound (40%) or the combination of both (49%) was significantly lower than that of MRI (91%). The overall node-positive rate was 16%. The specificity of MRI (97.2%) was equivalent to that of mammography (96.8%). The authors concluded that mammography, even when combined with US, was insufficient for early BC diagnosis in women at increased familial risk and that screening MRI allowed for BC diagnosis in this population with a significantly higher sensitivity and at a more favorable stage.
Thus, by 2005, 7 prospective studies on a total of 3,794 women undergoing multimodality screening and 172 cancers diagnosed in a total of 9,614 annual screening events showed that MRI emerged as a breast imaging modality with a sensitivity ranging from 77% to 100%, always by far superior to that of mammography or US (not over 50% even when combined), with a variable but substantially acceptable specificity, as also judged by the group from the United Kingdom [10], where a long tradition of BC screening with mammography should be considered a reliable testing bench for evaluating a new screening modality.
9.4 The American Cancer Society 2007 Guidelines
What we have described was the basis of evidence available to the panel of experts of the ACS Breast Cancer Advisory Group who, in 2007, published the new guidelines for breast screening with MRI as an adjunct to mammography [40]. Their conclusions were as follows:
Screening MRI is recommended for women with an approximately 20–25% or greater lifetime risk of breast cancer, including women with a strong family history of breast or ovarian cancer and women who were treated for Hodgkin disease. There are several risk subgroups for which the available data are insufficient to recommend for or against screening, including women with a personal history of breast cancer, carcinoma in situ, atypical hyperplasia, and extremely dense breasts on mammography. Diagnostic uses of MRI were not considered to be within the scope of this review. [40]
The panel recommended MRI screening (as an adjunct to mammography) on the basis of evidence from nonrandomized screening trials and observational studies (those we have described above) in:
-
BRCA mutation carriers
-
First-degree relative of BRCA mutation carriers, but untested
-
All women with a modeled cumulative LTR of ~20% to 25% or greater
Conversely, the panel also recommended MRI screening (as an adjunct to mammography) on the basis of expert consensus opinion taking into consideration only the evidence for LTR for BC in the case of:
-
Radiation to chest between age 10 and 30 years
-
Li-Fraumeni syndrome (TP53 mutation carriers) and first-degree relatives
-
Cowden and Bannayan-Riley-Ruvalcaba syndromes and first-degree relatives
As we will see, the evidence subsequently accumulated reinforced the indication of MRI screening for women at hereditary high risk (see the following paragraphs of this Chapter and also Chaps. 10 and 11) and offered a new basis of evidence for the indication to MRI screening for women with previous chest radiation therapy (see Chap. 14). As outlined in Chap. 16, the thresholds for LTR to recommend MRI was already a matter for discussion, as demonstrated by the choice of the ACS Breast Cancer Advisory Group that defined a threshold as a range of 20–25% of LTR, which implies to offer (when the cutoff is 20%) or not to offer screening MRI (when the cutoff is 25%) to thousands and thousands of women in Europe or North America. Recent reviews highlighted the role of MRI surveillance for TP53 mutation carriers [41] and more generally in the era of next-generation sequencing and moderate-risk genetic mutations, anyway defined as associated with a LTR of 20% or higher, such as ATM, CHEK2, and PALB2 [42].Footnote 4
The new paradigm launched by the ACS was MRI as an adjunct to mammography. The subsequent body of evidence will work for reverting this scheme opening the discussion about whether and when mammography should be used as an adjunct to MRI.
9.5 High-Risk Screening with MRI: More Evidence from Prospective Studies (2007–2017)
A number of studies followed and the body of evidence have grown up in the 10 years after the ACS 2007 guidelines publication. The general trend for a huge difference in diagnostic power, especially in sensitivity, between MRI and conventional imaging modalities was largely confirmed. The list of all the studies published in the period from 2000 to 2015, with their main results, is reported in Table 9.1, grouping together the results of subsequent phases of individual projects [6,7,8,9,10,11,12, 43,44,45,46,47,48,49,50
].
In 2007, Anne I. Hagen and coworkers [43,44,45,46,47,48,49,] described their results obtained offering breast MRI screening besides conventional imaging (mammography ± US) to 445 BRCA1 and 46 BRCA2 mutation carriers at five centers in Norway (total of 867 screening events). They observed a total of 25 BCs (including 21 invasive and 4 DCIS), 5 of them (20%) as interval cancers. At the time of diagnosis, sensitivity was 19/22 (86%) for MRI and 12/24 (50%) for mammography. Among 21 cancers that were examined by both methods (in 19/21 BRCA mutation carriers), the sensitivity of mammography was 10/21 (48%) and that of MRI was 18/21 (86%). Furthermore, the authors noted that MRI had a higher sensitivity than mammography to diagnose all BCs staged less than pT2, which was a major conclusion of their study.
In the same year (2007), Christopher C. Riedl and coworkers [44] reported preliminary results obtained at the Medical University of Vienna by multimodality BC screening in 327 high-risk women (BRCA mutation carriers and women with a familial LTR higher than 20%) who underwent 672 complete annual rounds. Of a total of 28 BCs diagnosed, sensitivities were 50% for mammography, 43% for US, and 86% for MRI (the sensitivity of MRI was higher than that of conventional imaging also for the DCIS subgroup), specificities 98%, 98%, and 92%, respectively. Of 101 false-positive findings, 35 (35%) were atypical ductal hyperplasias, 9 (26%) detected by mammography, 2 (6%) by US, and 32 (91%) by MRI. They concluded that MRI improves the detection of invasive and pre-invasive BCs as well as premalignant lesions in a high-risk population.
The results of this study were updated in 2015 [45] for 559 women (including 156 BRCA1/BRCA2 mutation cariers) with 1,365 complete rounds. The sensitivity of MRI (90%) was significantly higher than that of mammography (38%) and ultrasound (38%). Of 40 cancers, 18 (45.0%) were detected by MRI alone, 2 cancers were found by mammography alone (a DCIS with microinvasion and a DCIS with less than 10-mm invasive areas), without a significant increase in sensitivity compared to MRI alone. No BCs were detected by US alone. Of 14 DCIS, all were detected by MRI, whereas mammography and US each detected 5 DCIS (36%). The authors also noted that age, mutation status, and breast density did not influence MRI sensitivity, confirming the MRI superiority over mammography and US under these different conditions. They concluded that MRI allows early detection of familial breast cancer regardless of patient age, breast density, or risk status. In addition, they noted that in this setting US provides no additional value, mammography only a limited one.
Still in 2007, we published the mid-term results of the HIBCRIT Italian study [46] for 278 BRCA1 or BRCA2 mutation carriers, first-degree relatives of BRCA1 or BRCA2 mutation carriers, or women enrolled because of a strong family history of breast or ovarian cancer for a total of 377 rounds: the criteria for enrolling women on the only basis of family history were: three or more events in first- or second-degree relatives in either maternal or paternal line; these included breast cancer in women younger than 60 years, ovarian cancer at any age, and male breast cancer at any age. Of 18 BCs diagnosed, 6 (33%) were detected only with MRI. Sensitivity was 50% for CBE, 59% for mammography, 65% for US, 94% for MRI; PPV3 (i.e., based on performed biopsy) was 82%, 77%, 65%, and 63%, respectively.
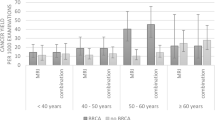
We updated these data as final results in 2011 [47] for 501 high-risk women enrolled in 18 centers in Italy. Considering a total of 1,592 rounds (3.2 rounds/woman), 49 screen-detected and 3 interval BCs were diagnosed: 8 DCIS and 44 invasive; 4 pT2 stage and 32 G3 grade. Twenty-eight of 39 patients explored for nodal status (72%) were negative. The incidence per year-woman resulted 3.3% overall, significantly lower (2.1%) under 50 years of age than over 50 (5.4%), significantly higher (4.3%) in women with previous personal BC than in those without (2.5%). MRI was significantly more sensitive (91%) than CBE (18%), mammography (50%), US (52%), or mammography plus US (63%). Specificity ranged from 97% to 99%, PPV from 56% to 71%, positive likelihood ratio from 25 to 50, without significant differences. MRI showed a significantly better negative predictive value (99.6%) and negative likelihood ratio (0.09) than those of the other modalities. At ROC analysis, the AUC of MRI (0.97) was significantly higher than that of mammography (0.83) or US (0.82) and not significantly increased when MRI was combined with mammography and/or US (examples in Fig. 9.1; Table 9.2). Of 52 BCs, 16 (31%) were diagnosed only by MRI, 8 of 21 (38%) in women <50, and 8 of 31 (26%) in women ≥50 years of age. A subanalysis distinguishing screen-film from digital mammography did not find any increase in sensitivity. We concluded that MRI largely outperformed mammography, US, and their combination for screening high-risk women below and over 50.
A case from the HIBCRIT study. A 53-year-old BRCA1 mutation carrier, already treated for an invasive ductal cancer of the left breast at 33 years of age, underwent multimodal screening including clinical breast examination (CBE), mammography, US, and MRI. The left breast only showed minimal signs of the previous treatment at each screening modality (not shown). Mammography of the right breast showed a negative dense breast (a and b). Also CBE and US (not shown) were negative; at MRI the unenhanced T2-weighted axial short-tau inversion recovery sequence (c) showed a small hyperintense mass, confirmed at the subtracted (contrast-enhanced minus unenhanced T1-weighted gradient echo) coronal image (d). Final diagnosis: node-negative invasive ductal carcinoma (6 mm in diameter). From Podo et al. (2016) Clin Cancer Res 22:895–904
In 2010, Christiane K. Kuhl and coworkers [48] published the results of the EVA observational cohort study, conducted at four academic centers in Germany. They enrolled 687 asymptomatic women with familial LTR ≥ 20% who underwent 1,679 annual rounds with CBE, mammography, US, and MRI; 371 women had additional half-yearly US and CBE during 869 rounds. A total of 27 BCs were diagnosed: 11 DCIS (41%) and 16 invasive BCs (59%); 3/27 (11%) with positive nodal status. No interval cancers; no cancers detected with half-yearly US. The BC yield of US (6.0/1,000) and mammography (5.4/1,000) was equivalent, not significantly increased when mammography and US were combined (7.7/1,000). BC yield by MRI alone (14.9/1,000) was significantly higher than that of mammography, US, or their combination and was not significantly improved by adding mammography or US; PPV was 39% for mammography, 36% for US, and 48% for MRI. The authors concluded that in women at elevated familial risk, MRI screening shifts the distribution of screen-detected BCs toward the pre-invasive stage, while neither mammography, nor annual or half-yearly ultrasound or CBE significantly increase BC detection over MRI alone.
In the same year (2010), Adriana J. Rijnsburger and coworkers [49] updated the results of the Dutch MRISC study, which had enrolled women with LTR for BC ≥ 15%, screened with biannual CBE and annual mammography and MRI [8]. Considering 2,157 eligible women, 599 of them being mutation carriers, 97 primary BCs were diagnosed. The MRI overall sensitivity was significantly higher than that of mammography for invasive cancer (77% versus 36%), but not for DCIS. Mammography sensitivity was only 25.0% in the BRCA1 group, 62% in the BRCA2 group, 46% in the high-risk group (with a 30–50% LTR), and 47% in the moderate-risk groups (with a 15–30% LTR). Results in the BRCA1 group were also worse compared with the BRCA2 group, high- and moderate-risk group regarding tumor size ≤1 cm at diagnosis (21%, 62%, 41%, and 64%, respectively); proportion of DCIS (7%, 19%, 15%, and 31.3%); and interval cancers (32%, 6%, 4%, and 6%). The authors also reported on cumulative distant metastasis-free and overall survival at 6 years for invasive BCs, which were 84% and 93%, respectively, in 42 BRCA mutation carriers with invasive BC and 100% in 43 women of familial groups. They concluded that screening results were somewhat worse in BRCA1 mutation carriers, but the 6-year survival was high in all groups.
Still in 2010, Isabelle Trop and coworkers [50] reported results obtained at the Université de Montréal, Canada. They enrolled 184 asymptomatic women being BRCA1/2 mutation carriers or with >30% probability of being BRCA1/2 mutation carriers as estimated by BRCAPRO. During 387 rounds, 12 BCs were detected (9 invasive, 3 DCIS), for an overall yield of 6.5%; 7/9 invasive cancers were smaller than 2 cm in diameter; only 1 case of positive nodal status was observed; all BCs were negative to the human epidermal growth factor receptor 2 (HER2). Sensitivity was 10/12 for MRI (83%), 7/12 (58%) for mammography; US did not detect any additional cancers. The recall rate was 22% for MRI, 16% for mammography, and 11% for US. Importantly, the authors noted that recall rates declined with successive screening rounds. In total, 45 biopsies were performed: 21 due to US, 17 due to MRI, and 7 due to mammography. The authors concluded that MRI offers to high-risk women the best sensitivity for BC screening and that the combination of yearly MRI and mammography reached a negative predictive value of 100%.
In 2012, Wendy D. Berg and coworkers [51] reported on the results of a subproject of ACRIN 6666 multicenter study to determine supplemental cancer detection yield of US and MRI in women at elevated BC risk. Women were eligible if being asymptomatic, having heterogeneously dense or extremely dense breast tissue, and also having at least one of other risk factors. A total of 2,809 women at 21 sites had annual independent screens with mammography and US in randomized order; after three rounds of both screenings, 612 women underwent MRI and had complete data. A total of 2,662 women underwent 7,473 mammogram and US screenings, 110 of whom had 111 BCs diagnosed: 33 detected by mammography only, 32 by US only, 26 by both, and 9 by MRI after mammography + US; 11 were not detected by any imaging modality. Supplemental US identified additional BCs in 3.7/1,000 screens. Sensitivity for mammography + US was 76%, specificity 84%, and PPV3 (i.e., based on performed biopsy) 16%. For mammography alone, sensitivity was 52%, specificity 91%, and PPV3 38%. Of the MRI participants, 16 women (2.6%) had a BC diagnosed. The supplemental yield of MRI was 14.7/1,000. Sensitivity for MRI and mammography plus US was 100%, specificity was 65%, and PPV3 19%. For mammography and US, sensitivity was 44%, specificity 84%, and PPV3 18%. The number of screens needed to detect one cancer was 127 for mammography, 234 for supplemental US, and 68 for MRI after negative mammography and US. The authors concluded that the addition of screening US or MRI to mammography in women at increased risk of breast cancer resulted in a higher cancer detection yield, but also an increase in false-positive findings. The study has a particular interest: it shows the additional diagnostic power of each breast imaging technique when applied sequentially, with MRI associated with the lowest number of screens needed for detecting one cancer (68) as third examination versus mammography (127) at the beginning of the sequence, and US (234) in between. However, the study design does not allow an intra-individual comparative analysis. Data are not comparable with those of the other prospective studies. For this reason, we did not include this study in Table 9.1.
Finally, in 2014, Anna M. Chiarelli and coworkers [52] reported on the results obtained by the Ontario Breast Screening Program which in July 2011 started to screen women at high BC risk from 30 to 69 years of age with annual MRI and digital mammography in 28 centers. Eligibility was based on the following criteria: known BRCA1 or BRCA2 mutation or other gene mutations associated with high BC risk; untested first-degree relative of a mutation carrier; family history consistent with hereditary BC syndrome and estimated personal LTR ≥ 25%; or chest radiation therapy (before age 30 and ≥8 years previously). These results have a particular relevance, for being the first screening program for high-risk women organized on a regional base. Thirty-five BCs were diagnosed (16.3/1,000), none of them by mammography alone, 23 (66%) by MRI alone (10.7/1,000); 25/35 BCs (71%) were detected among mutation carriers (30.8/1,000). The recall rate was significantly higher in the cases of positive MRI alone (15.1%) than with mammography alone (6.4%); PPV was highest for detection based on both mammography and MRI (12.4%). The authors concluded that screening with annual MRI and mammography has the potential to be implemented into an organized breast screening program for women at high risk for breast cancer.
To summarize, in 10 years after the ACS guidelines, different prospective studies performed in Europe and in North America increased the body of knowledge on BC screening in high-risk women (see Table 9.1), showing that:
-
1.
The higher sensitivity of MRI versus mammography (combined with acceptable MRI specificity and PPV values) was confirmed on a larger basis.
-
2.
The transition from screen-film to digital technique did not provide an increase in BC detection by mammography.
-
3.
When performed, the additional value of US appeared very low, if any, also with a 6-month interval.
-
4.
The additional value of mammography also appeared open for debate, due to the low number of cases diagnosed by mammography only, mostly of them being DCIS.
-
5.
A higher diagnostic power of MRI was also reported in postmenopausal women.
-
6.
The value of MRI screening was also shown in high-risk women already treated for BC.
Points 1, 2, and 3 above were reinforced by the ROC analysis curves from the HIBCRIT-1 study [47, 53] (Fig. 9.2); also the EVA trial [48] gave similar results.
ROC analysis of diagnostic performance of annual mammography (XM), US, MRI, and their combinations for screening high-risk women in the HIBCRIT-1 study. The MRI AUC was significantly higher than that of mammography, US, or their combination, without a significant increase in diagnostic power when mammography and/or US were combined with MRI. With permission, from Sardanelli F, Podo F [53]
Lastly, we wish to mention the multicenter study by Tomasz Huzarski and coworkers [54] from the Polish Hereditary Breast Cancer Study Group, investigating the role of MRI for screening women at average or intermediate risk, hence being outside our focus on high risk. However, their results can be useful to a general reasoning. They enrolled 2,995 women aged 40–65, without previous BC history: 356 (12%) with a CHEK2 mutation, 370 (12%) with a first-degree relative with BC but without CHEK2 mutation, and 2,269 (76%) without any risk factor. These women underwent two rounds of MRI, US, and mammography, 1 year apart and were followed for 3 years. During the 4-year time frame, 27 invasive cancers, 6 DCIS, and 1 angiosarcoma were diagnosed. Of the 27 cancers, 20 were screen-detected, 2 interval, and five during follow-up. For invasive cancers, sensitivity was 86% for MRI, 59% for US, and 50% for mammography; of the 19 invasive cancers detected by MRI, 17 (89%) were also detected by US or mammography. MRI prompted 156 biopsies, US 57, mammography 35. The authors concluded that MRI sensitivity was only slightly better than that of mammography/US and that, also considering costs, MRI screening is probably not warranted outside of high-risk populations. In Chaps. 21 and 22, the reader can find an extensive explanation of the limited evidence for using MRI in intermediate-risk population. Anyway, this study shows how the application of MRI screening to a mixed population composed of average-risk women for over three quarters does not seem to provide relevant results in terms of additional cancer yield.
Of note, after 2007, studies also offered a basis of evidence in favor of MRI screening in women who underwent chest radiation therapy, even though with lower sensitivity than for women with hereditary BC predisposition. Mammography as adjunct to MRI has been suggested for women of this BC risk category, in consideration of the relatively higher probability of DCIS with microcalcifications and low angiogenesis [55]. This topic is extensively treated in Chap. 14.
9.6 Other Guidelines and the Ten Key Points from EUSOMA Recommendations
After 2007, many other national and international bodies issued guidelines and recommendations for MRI screening of women at high BC risk, among them, the American College of Radiology [56], the European Society of Breast Imaging [57, 58], or the multidisciplinary European Society of Breast Cancer Specialists (EUSOMA) [59], but also governmental bodies such as the National Comprehensive Cancer Network [60] in the United States and the National Institute for Health and Care Excellence [61] in the United Kingdom. Differences exist among guidelines, especially for the threshold of LTR to define the indication to MRI, lower (20–25%) in guidelines from the United States (where the ACR recently recommended screening MRI also in lower risk categories [62]), higher (30% or more) in some European guidelines. However, in all guidelines MRI is proposed for screening high-risk women. In Chap. 16, the reader can find an extensive review of these and other guidelines.
In this paragraph, we only wish to reserve a special mention to the EUSOMA recommendations published in 2010 [59] for their characteristic of having been provided by a multisciplinary panel, with a list of ten key points for breast MRI screening in high-risk women that we still consider useful today (Table 9.3).
9.7 Rethinking of the Relative Role of Mammography versus MRI for Screening High-Risk Women
During the last two decades, also retrospective studies on breast MRI screening of high-risk women were published. We did not mention them earlier because of the lower value that a retrospective study design implies in this context. However, some of them, recently published, deserve in our opinion a particular consideration.
In particular, three retrospective studies provided further contribution to rethinking the role of mammography for screening high-risk women.
In 2014, Inge-Marie Obdeijn and coworkers [63] reported specifically on 93 cases of BC in BRCA1 mutation carriers who underwent screening with MRI and digital mammography at the Erasmus Medical Center in Rotterdam, the Antoni van Leeuwenhoek Hospital in Amsterdam, and at the University Medical Center in Nijmegen: 82 invasive cancers and 12 DCIS. Screening sensitivity was 90/94 (96%) overall, significantly higher for MRI (88/94, 94%) than for mammography (48/94, 51%). While 42/94 malignancies (45%) were detected only by MRI, only 2 DCIS (2/94, 2%) were detected only with mammography (one G3 DCIS in a 50-year-old patient and one G2 in a 67-year-old patient). All the 4 interval cancers (4/94, 4%) were G3 triple-negative invasive ductal carcinomas. The authors concluded that digital mammography added only 2% to the breast cancer detection in BRCA1 patients, without any benefit of additional mammography under 40 years of age. They proposed that, given the potential risk of radiation-induced breast cancer in young mutation carriers, BRCA1 mutation carriers could be screened yearly with MRI from age 25 onward and with mammography not earlier than age 40.
In 2017, Lo and coworkers [64] reviewed the prospective database of 3,934 screening studies (1,977 MRI and 1,957 mammography examinations) performed on 1,249 high-risk women at three academic hospitals in Canada. A total of 45 cancers (33 invasive and 12 DCIS) were diagnosed, 43 of them seen with MRI and 14 with both mammography and MRI. Additional tests (further imaging and/or biopsy) were recommended in 461 screening MRI (recall rate, 23%) while mammography recalled 217 (recall rate, 11%). The detection rate was significantly higher for MRI (21.8/1,000) than for mammography (7.2/1,000). The sensitivity of MRI (96%) was significantly higher than that of mammography (31%); the specificity of MRI (78%) was significantly lower than that of mammography (89%); the PPV1 (i.e., for recalls) of MRI (9.3%) was higher, but not significantly, than that of mammography (6.5%). The authors concluded that mammography did not have an added value for BC detection in high-risk women undergoing MRI screening. As a consequence, they said that routine mammography in women undergoing screening MRI imaging warrants reconsideration.
Lastly, Suzan Vreeman and coworkers [65] from Radboud University Medical Center, Nijmegen, investigated the added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI, based on 6,553 rounds in 2,026 women at increased BC risk of breast cancer (1 January 2003–1 January 2014). Of a total of 125 screen-detected cancers, 112 were detected by MRI and 66 by mammography: 13 cancers were detected only by mammography, 8 of them being DCIS. Cancer detected only by mammography were 3/61 (5%) in BRCA mutation carriers, and 10/64 (16%) in non-BRCA mutation carriers. While 77% of mammography-only cancers were detected in women ≥50 years of age, mammography also added more to the false-positive recalls in these women. Below 50 years of age, the number of mammographic examinations needed to find an MRI-occult cancer was 1,427. The authors concluded that the benefit of mammography appears slightly larger in women over 50 years of age without BRCA mutation, associated with a substantial increase in false-positive recalls.
Conversely, two recent retrospective reports focused on missed BCs in high-risk screening, in particular on MRI false negative cases.
Antony J Maxwell and coworkers [66] from Nightingale Centre, University Hospital of South Manchester, Manchester, reported on 32 high-risk women who had undergone screening MRI and had been diagnosed with breast cancer within 2 years after a negative MRI. For 23 cases, MRI images were available for review. Fourteen were diagnosed at MRI, 4 at interim mammography, two symptomatically, one incidentally on US, and two at risk-reducing mastectomy. Ten of the 23 women (43%) had a potentially avoidable delayed diagnosis. The preceding MRIs were classified as false-negative screens in five women (one prevalent, four incident), false-negative assessment in seven, and minimal signs in three (three women were assigned dual classifications). Reasons for the diagnostic delay mostly were small overlooked enhancing masses, areas of non-mass enhancement showing little, if any, change between screens, false reassurance from normal conventional imaging at assessment, and overreliance on repeat MRI at short-interval. The authors concluded recommending double reading of both screening and assessment examinations, ready access to MRI biopsy, and limited use of short-interval repeat MRI only for areas likely to be benign glandular enhancement. They also recommend annual mammography in these women.
Suzan Vreemann and coworkers by the Nijmegen group [67] investigated the same issue for a larger case series of 131 missed BCs for which negative prior MRI was available. Overall, visible findings on prior negative MRI were observed in 31% of cases, minimal signs in 34%, no signs in 35%. These visible findings were significantly less frequent in BRCA mutation carriers (19%) than in non-carriers (46%). Less than perfect image quality significantly increased the probability of visible findings and minimal signs in the negative prior MRI. The author concluded that almost one-third of cancers detected in a high-risk screening program are already visible at the last negative MRI scan, and even more so in women without BRCA mutations, so that regular auditing and double reading for breast MRI are warranted.
Finally, the same group from Nijmegen [68] reported on real-life performance of a large screening program for women with different categories of increased risk in their academic hospital. They analyzed 8,818 MRI and 6,245 mammography examinations performed in 2,463 women. On a total of 170 cancers, 129 were screen-detected cancers, 16 interval, and 25 found at prophylactic mastectomy. Overall sensitivity was 76% including cancers from prophylactic mastectomy and 90% excluding them. Sensitivity was lowest for carriers of the BRCA1 mutation (66 and 81%, respectively). Specificity was higher at follow-up (96%) than in first rounds (85%) and was high for both MRI (97%) and mammography (99%); PPV of recall and of biopsy were lowest in women with only family BC history. The authors’ conclusions were that screening performance was dependent on risk category, with lowest sensitivity in BRCA1 mutation carriers, and that specificity improved at follow-up rounds.
9.8 Conclusions and Open Issues
As the readers can understand, a general agreement for recommending breast MRI annual screening in women at high risk does exist on the basis of a large body of evidence provided by a dozen of prospective studies including 6,360 women, about 18,900 rounds, and 357 BCs diagnosed. However, a number of issues deserve attention and, for them, we refer the reader to other Chapters in this book.
First, systematic reviews and meta-analyses have explored interesting aspects, especially allowing for subgroup analyses that the power of original studies would not have permitted. The reader can find these results in Chap. 11.
Second, the possibility of using MRI alone for screening at least certain categories at high-risk women should be considered, not only for the low contribution of mammography and US to the screening sensitivity, but also for their increase in false-positive recalls rate, a topic extensively treated in Chap. 10. In addition, also radioprotection considerations may play in favor of avoiding mammography [69] (and other radiation exposure of the chest, including computed tomography!), especially in BRCA mutation carriers, a topic extensively treated in Chap. 12.
Third, the top sensitivity of breast MRI in high-risk women should determine positive effects in terms of patient outcome, i.e., at least disease-specific and disease-free survival. The reader can find the illustration of the results already available in the absence of randomized controlled trials in Chap. 13.
At any rate, due to the very low, if any, contribution of US and the low contribution of mammography when compared to MRI for screening a high-risk population, we can propose the following simple recommendations [53]:
-
1.
MRI alone up to 35 years of age for all high-risk women
-
2.
MRI alone for BRCA1 and TP53 mutation carriers without age limitations
-
3.
Mammography as an adjunct to MRI for BRCA2 mutation carriers after 35 years of age and to women who had previous chest radiation therapy
Thus, the paradigm “MRI as an adjunct to mammography” has been reverted into its contrary. When “mammography as an adjunct to MRI” is under consideration for high-risk women, a good conservative approach has been suggested, consisting of performing only one projection, the mediolateral oblique one [70].
About two decades after the start of the first prospective studies on breast MRI screening in high-risk women, the efforts of several research groups in Europe and North America have opened an efficient way of surveillance as an alternative to prophylactic mastectomy to be offered to these women. Much work still needs to be done but one important step forward has been done.
Abbreviations
- ACS:
-
American Cancer Society
- AUC:
-
Area under the curve
- BC:
-
Breast cancer
- CBE:
-
Clinical breast examination
- CE-MRI:
-
Contrast-enhanced magnetic resonance imaging
- DBT:
-
Digital breast tomosynthesis
- DCIS:
-
Ductal carcinoma in situ
- EBM:
-
Evidence-based medicine
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- LTR:
-
Lifetime risk
- MRI:
-
Magnetic resonance imaging
- PPV:
-
Positive predictive value
- RCT:
-
Randomized controlled trial
- ROC:
-
Receiver operating characteristic
- US:
-
Ultrasound
References
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS (1996) Evidence based medicine: what it is and what it isn’t. BMJ 312:71–72
Sardanelli F, Hunink MG, Gilbert FJ, Di Leo G, Krestin GP (2010) Evidence-based radiology: why and how? Eur Radiol 20:1–15
Prasad KN, Cole WC, Haase GM (2004) Radiation protection in humans: extending the concept of as low as reasonably achievable (ALARA) from dose to biological damage. Br J Radiol 77:97–99
Oxford Centre for Evidence-based Medicine (2009) Levels of Evidence. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed 30 Jun 2020
The Council of the European Union (2003) Council recommendation of 2 December 2003 on cancer screening (2003/878/EC). https://ec.europa.eu/jrc/sites/jrcsh/files/2_December_2003%20cancer%20screening.pdf. Accessed 30 Jun 2020
Kuhl CK, Schmutzler RK, Leutner CC et al (2000) Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology 215:267–279
Podo F, Sardanelli F, Canese R et al (2002) The Italian multi-centre project on evaluation of MRI and other imaging modalities in early detection of breast cancer in subjects at high genetic risk. J Exp Clin Cancer Res 21(3 Suppl):115–124
Kriege M, Brekelmans CT, Boetes C et al; Magnetic Resonance Imaging Screening Study Group (2004) Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 351:427–437
Warner E, Plewes DB, Hill KA et al (2004) Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 292:1317–1325
Leach MO, Boggis CR, Dixon AK et al (2005) Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 365:1769–1778
Lehman CD, Blume JD, Weatherall P et al; International Breast MRI Consortium Working Group (2005) Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer 103:1898–1905
Kuhl CK, Schrading S, Leutner CC et al (2005) Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 23:8469–8476
Pisano ED, Gatsonis C, Hendrick E et al; Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group (2005) Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 353:1773–1783
Kemp Jacobsen K, Abraham L, Buist DS et al (2015) Comparison of cumulative false-positive risk of screening mammography in the United States and Denmark. Cancer Epidemiol 39:656–663
Hofmann B (2018) Fake facts and alternative truths in medical research. BMC Med Ethics 19:4
Autier P, Boniol M (2018) Mammography screening: a major issue in medicine. Eur J Cancer 90:34–62
Berry DA, Cronin KA, Plevritis SK et al; Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353:1784–1792
Saadatmand S, Bretveld R, Siesling S, Tilanus-Linthorst MM (2015) Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. BMJ 351:h4901
Hofvind S, Sørum R, Thoresen S (2008) Incidence and tumor characteristics of breast cancer diagnosed before and after implementation of a population-based screening-program. Acta Oncol 47:225–231
Cutuli B, Dalenc F, Cottu PH et al (2015) Impact of screening on clinicopathological features and treatment for invasive breast cancer: results of two national surveys. Cancer Radiother 19:295–302
Dong W, Berry DA, Bevers TB et al (2008) Prognostic role of detection method and its relationship with tumor biomarkers in breast cancer: the university of Texas M.D. Anderson Cancer Center experience. Cancer Epidemiol Biomark Prev 17:1096–1103
Nagtegaal ID, Allgood PC, Duffy SW et al (2011) Prognosis and pathology of screen-detected carcinomas: how different are they? Cancer 117:1360–1368
Lauby-Secretan B, Scoccianti C, Loomis D et al; International Agency for Research on Cancer Handbook Working Group (2015) Breast cancer screening—viewpoint of the IARC working group. N Engl J Med 372:2353–2358
Sardanelli F (2015) Screening mammography: a clear statement by the IARC handbook. Epidemiol Prev 39:149–150
Puliti D, Duffy SW, Miccinesi G, de Koning H, Lynge E, Zappa M, Paci E; EUROSCREEN Working Group (2012) Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen 19(Suppl 1):42–56
Paci E; EUROSCREEN Working Group (2012) Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J Med Screen 19(Suppl 1):5–13
Colin C, Devouassoux-Shisheboran M, Sardanelli F (2014) Is breast cancer overdiagnosis also nested in pathologic misclassification? Radiology 273:652–655
Elmore JG, Longton GM, Carney PA et al (2015) Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 313:1122–1132
Tosteson ANA, Yang Q, Nelson HD et al (2018) Second opinion strategies in breast pathology: a decision analysis addressing over-treatment, under-treatment, and care costs. Breast Cancer Res Treat 167:195–203
Sardanelli F, Trimboli RM, Tot T (2018) Expert review of breast pathology in borderline lesions: a chance to reduce overdiagnosis and overtreatment? JAMA Oncol 4:1325–1326
Colin C, Schott AM, Valette PJ (2014) Mammographic density is not a worthwhile examination to distinguish high cancer risk women in screening. Eur Radiol 24:2412–2416
Freer PE (2015) Mammographic breast density: impact on breast cancer risk and implications for screening. Radiographics 35:302–315
Burke W, Daly M, Garber J et al (1997) Recommendations for follow-up care of individuals with an inherited predisposition to cancer II BRCA1 and BRCA2: Cancer genetics studies consortium. JAMA 277:997–1003
Daly MB and coworkers (2003) The NCCN 2003 genetic/familial high-risk assessment clinical practice guidelines in oncology, version 1. https://www2.trikobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed 30 Jun 2020
Dent R, Warner E (2007) Screening for hereditary breast cancer. Semin Oncol 34:392–400
Sardanelli F, Carbonaro LA, Santoro F, Podo F (2010) Sorveglianza RM nelle donne ad alto rischio di carcinoma mammario. In: Ragozzino A (ed) Imaging RM nella donna. Idelson-Gnocchi, Napoli, pp 47–72. isbn:978-88-7947-521-1
Tyrer J, Duffy SW, Cuzick J (2004) A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111–1130
International Breast Cancer Intervention Study (IBIS). https://www.fairfaxradiology.com/services/exams/IBIS-Tool.php. Accessed 30 Jun 2020
Hedenfalk I, Ringner M, Ben-Dor A et al (2003) Molecular classification of familial non-BRCA1/BRCA2 breast cancer. Proc Natl Acad Sci USA 100:2532–2537
Saslow D, Boetes C, Burke W et al (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57:75–89
Schon K, Tischkowitz M Clinical implications of germline mutations in breast cancer: TP53. Breast Cancer Res Treat 167:417–423
Macklin S, Gass J, Mitri G, Atwal PS, Hines S (2018) The role of screening MRI in the era of next generation sequencing and moderate-risk genetic mutations. Familial Cancer 17:167–173
Hagen AI, Kvistad KA, Maehle L et al (2007) Sensitivity of MRI versus conventional screening in the diagnosis of BRCA-associated breast cancer in a national prospective series. Breast 16:367–374
Riedl CC, Ponhold L, Flöry D et al (2007) Magnetic resonance imaging of the breast improves detection of invasive cancer, preinvasive cancer, and premalignant lesions during surveillance of women at high risk for breast cancer. Clin Cancer Res 13:6144–6152
Riedl CC, Luft N, Bernhart C, et al (2015) Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 33:1128–1135
Sardanelli F, Podo F, D’Agnolo G et al (2007) Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology 242:698–715
Sardanelli F, Podo F, Santoro F, et al for the High Breast Cancer Risk Italian 1 (HIBCRIT-1) Study (2011) Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the high breast cancer risk italian 1 study): final results. Investig Radiol 46:94–105
Kuhl C, Weigel S, Schrading S et al (2010) Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 28:1450–1457
Rijnsburger AJ, Obdeijn IM, Kaas R et al (2010) BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC screening study. J Clin Oncol 28:5265–5273
Trop I, Lalonde L, Mayrand MH, David J, Larouche N, Provencher D (2010) Multimodality breast cancer screening in women with a familial or genetic predisposition. Curr Oncol 17:28–36
Berg WA1, Zhang Z, Lehrer D et al; ACRIN 6666 Investigators (2012) Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307:1394–1404
Chiarelli AM, Prummel MV, Muradali D et al (2014) Effectiveness of screening with annual magnetic resonance imaging and mammography: results of the initial screen from the Ontario high risk breast screening program. J Clin Oncol 32:2224–2230
Sardanelli F, Podo F (2017) Radiological screening of breast cancer: evolution. High-risk population. In: Veronesi U, Goldhirsch A (eds) Breast cancer. Innovations in research and management. Springer, Cham, pp 189–203
Huzarski T, Górecka-Szyld B, Huzarska J et al (2017) Screening with magnetic resonance imaging, mammography and ultrasound in women at average and intermediate risk of breast cancer. Hered Cancer Clin Pract 15:4
Mariscotti G, Belli P, Bernardi D et al (2016) Mammography and MRI for screening women who underwent chest radiation therapy (lymphoma survivors). Recommendations for surveillance from the Italian College of Breast Radiologists by SIRM. Radiol Med 121:834–837
American College of Radiology practice parameter for the performance of contrast-enhanced MRI of the breast. http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/MRI_Breast.pdf. Accessed 30 Jun 2020
Mann RM, Kuhl CK, Kinkel K, Boetes C (2008) Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 18:1307–1318
Mann RM, Balleyguier C, Baltzer PA, et al; European Society of Breast Imaging (EUSOBI), with language review by Europa Donna–The European Breast Cancer Coalition (2015) Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol 25:3669–3678
Sardanelli F, Boetes C, Borisch B et al (2010) Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer 46:1296–1316
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Breast Cancer Screening and Diagnosis. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
National Institute for Health and Care Excellence (NICE). Protocols for the surveillance of women at higher risk of developing breast cancer. Version 4. Updated NICE guidance on women with a familial history of breast cancer. NHSBSP Publication no. 74—June 2013
Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA (2018) Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 15(3 Pt A):408–414
Obdeijn IM, Winter-Warnars GA, Mann RM, Hooning MJ, Hunink MG (2014) Tilanus-Linthorst MM. Should we screen BRCA1 mutation carriers only with MRI? A multicenter study. Breast Cancer Res Treat 144:577–582
Lo G, Scaranelo AM, Aboras H et al (2017) Evaluation of the utility of screening mammography for high-risk women undergoing screening breast MR imaging. Radiology 285:36–43
Vreemann S, van Zelst JCM, Schlooz-Vries M et al (2018) The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res 20:84
Maxwell AJ, Lim YY, Hurley E, Evans DG, Howell A, Gadde S (2017) False-negative MRI breast screening in high-risk women. Clin Radiol 72:207–216
Vreemann S, Gubern-Merida A, Lardenoije S et al (2018) The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat 169:323–331
Vreemann S, Gubern-Mérida A, Schlooz-Vries MS et al (2018) Influence of risk category and screening round on the performance of an MR imaging and mammography screening program in carriers of the BRCA mutation and other women at increased risk. Radiology 286:443–451
Sardanelli F, Podo F (2007) Management of an inherited predisposition to breast cancer. N Engl J Med 357:1663
Colin C, Foray N (2012) DNA damage induced by mammography in high family risk patients: only one single view in screening. Breast 21:409–410
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sardanelli, F., Podo, F. (2020). Primary Studies on Breast MRI Screening of High-Risk Women. In: Sardanelli, F., Podo, F. (eds) Breast MRI for High-risk Screening. Springer, Cham. https://doi.org/10.1007/978-3-030-41207-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-41207-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41206-7
Online ISBN: 978-3-030-41207-4
eBook Packages: MedicineMedicine (R0)