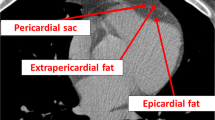
Abstract
Epicardial adipose tissue (EAT) is a measurable risk factor and marker of visceral adipose tissue. Magnetic resonance imaging (MRI) is considered the gold standard technique to measure the adipose tissue, also because it does not require ionizing radiations. Dedicated cardiac magnetic resonance (CMR) imaging protocols have been developed to assess EAT volume. CMR provides a precise and noninvasive assessment of both EAT thickness and volume. As EAT is not equally distributed through the heart and when abundant may fill in folding spaces, steady-state free precession (SSFP) sequences of CMR should be used to more accurately measure the different and deeper EAT locations. Proton magnetic resonance spectroscopy (1H-MRS) is the gold standard technique to measure myocardial triglyceride content that is independently correlated with ultrasound measured EAT thickness.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Epicardial fat
- Epicardial adipose tissue
- Cardiac magnetic resonance imaging
- Proton magnetic resonance spectroscopy
-
Cardiac magnetic resonance (CMR) imaging provides a precise and noninvasive assessment of both epicardial adipose tissue (EAT) thickness and volume.
-
Steady-state free precession (SSFP) CMR can measure EAT filling in different and deeper heart areas.
-
Proton magnetic resonance spectroscopy (1H-MRS) is the gold standard technique to measure myocardial triglyceride content that is independently correlated with EAT thickness
Introduction
Epicardial fat (EAT) is a measurable risk factor and marker of visceral adiposity. It can be easily and not invasively measured with echocardiography, as proposed by Iacobellis et al. [1, 2]. However this method has some limitations, such as the operator dependency and the linear measurement. The echocardiographic measurement of EAT has been validated with magnetic resonance imaging (MRI) and actually showed a good agreement. MRI is considered the gold standard technique to measure the adipose tissue [3]. MRI is noninvasive and does not use ionizing radiation, and therefore it is well suitable to measure EAT in both research and clinical setting.
Cardiac MRI of the Epicardial Fat
A dedicated cardiac magnetic resonance (CMR) imaging has been developed to assess EAT volume. Flüchter et al. were the first to develop a protocol to measure EAT using CMR [4]. EAT was measured using a dark blood prepared T1-weighted multislice turbo spin-echo pulse sequence with a water suppression prepulse to obtain a transversal four-chamber view and short-axis images in the same orientations used for the cine short-axis images. Both EAT thickness and volume were measured using CMR by Flüchter and colleagues. Following the anatomical findings and the method previously described by Iacobellis et al. [1, 2], EAT thickness was measured on the right ventricular free wall. Maximum epicardial fat thickness at the right ventricular free wall was measured in different views: in a transversal four-chamber view (Fig. 7.1a) and in consecutive short-axis views (EFT-SAX) covering the whole ventricle (Fig. 7.1b) [4]. Epicardial fat in and near the atrioventricular and interventricular grooves was not considered. While EAT thickness measured with CMR well correlated with autopsy [5], results were different from the EAT thickness values, measured according to the ultrasound methodology proposed by Iacobellis [1, 2]. This discrepancy could be attributed to the measurement of EAT thickness from the four-chamber view with the CMR rather than from the long-axis view as described in the echocardiographic method . The population studies by Flüchter and Iacobellis were also quite different, as some of the patients assessed with CMR presented with congestive heart failure. The left ventricular remodeling could have therefore influenced EAT measurements from the four-chamber view.
Epicardial fat thickness of the right ventricular free wall measured in the four-chamber view (a) and in a representative short-axis view (b). (From Flüchter et al. [4], with permission from John Wiley & Sons)
In the CMR study, EAT volume was calculated by using the modified Simpson’s rule with integration over the image slices: EAT contours were outlined at end-diastole in the short-axis views covering the entire left and right ventricle [4]. The area subtended by the manual tracings was determined in each slice and multiplied by the slice thickness to yield the fat volume. Total EAT volume was obtained after the summation of data of all slices (Fig. 7.2). CMR-measured EAT volume showed excellent agreements with autopsy results [6].
Volumetric measurement of epicardial fat mass. The contours of epicardial adipose tissue were outlined in end-diastolic images of short-axis views covering the whole left and right ventricle. (From Flüchter et al. [4], with permission from John Wiley & Sons)
Overall, CMR provides a precise and noninvasive assessment of both EAT thickness and volume. However, the method used by Flüchter may have some limitations. A recent study raised up the concern and need of detecting the diverse EAT anatomy and distribution [7]. EAT is not equally distributed through the heart. EAT is located at the interatrial groove, the atrioventricular septum, and the inferior pyramidal space, the left lateral ridge, the atrioventricular grooves, and the transverse pericardial sinus. Leo et al. recently proposed to use balanced steady-state free precession (SSFP) sequences of CMR to measure the different EAT locations [7]. SSFP sequences have the ability to distinguish the fat from the blood and the muscle, so providing a great quality imaging of EAT distribution. The authors nicely underline that EAT, particularly when abundant, should not be considered as accumulation of layers covering the surface of the heart, but rather fat deeply penetrating into the heart and filling any possible folding. SSFP CMR can catch and detect the folding appearance of human EAT.
Regional fat distribution of EAT plays an important role in the development and potentially stratification of different conditions. For example, peri-atrial EAT has shown to be strongly correlated with atrial fibrillation. Hence, clinical assessment of peri-atrial EAT could be instrumental in the prognosis of patients with atrial fibrillation. One study validated the use of a semiautomated three-dimensional atrial PAT model utilizing standard (clinical) CMR sequences for accurate and reproducible assessment of atrial PAT [8].
Nelson et al. also criticized the method proposed by Flüchter [9]. They used an ovine model, to validate a CMR-derived paracardial adipose tissue volume assessment [9]. The measurement was not limited to the adipose thickness over the RV free wall, but included epicardial, paracardial, and pericardial adipose depots. Starting at the mitral annulus, consecutive end-diastolic ventricular images were used to determine the area and volume of epicardial, paracardial, and pericardial adipose tissue included paracardial adipose tissue mass. Unquestionably this method allows the measurement of the entire cardiac adiposity. Nevertheless, this approach is argumentative, as EAT, pericardial, and paracardial adipose tissue are embryologically, anatomically, and functionally very different. The inclusion of the three fat depots as one single entity may not reflect the separate functions and clinical implications of each single adipose tissue.
CMR has been used in several studies to evaluate EAT in patients with different cardiac diseases, such as CAD, HF, and dilated cardiomyopathy [10,11,12]. CMR imaging can not only measure EAT but also detect myocardial fat infiltration [13,14,15]. In patients with aortic stenosis, CMR imaging myocardial steatosis correlated with the degree of left ventricular strain impairment [14]. CMR study using variable projection water/fat separation showed a relation between left ventricular function and fat accumulation within the septal myocardium in patients with dilated cardiomyopathy [15]. Additionally, multiparametric CMR provides imaging of cardiac function and myocardial fibrosis by late gadolinium enhancement.
Advantages of CMR Imaging of Epicardial Fat
CMR allows a clear visualization of EAT. CMR imaging using SSFP allows a clear distinction between muscular and adipose tissue. This sequence provides a high signal/noise ratio and an optimal blood/myocardial contrast, which allows a precise definition of the endocardial borders. When using SSFP CMR, the strength of the signal comes from different tissues and depends on T1/T2 ratio. Water (blood) and fat have the same high T1/T2 ratio; then both tissues produce a very high signal. On the contrary, the muscular tissue has a low T1/T2 ratio and produces weak signal. SSFP CMR can distinguish the different EAT locations better than any other noninvasive imaging technique.
Limitations of CMR Imaging of Epicardial Fat
When abundant, EAT not only covers the entire epicardial surface but also invades spaces and folds that usually are almost virtual [7]. Some CMR protocols may be not able and reliable in depicting the exact and deeper EAT distribution. CMR images side by side with corresponding anatomic specimens should be used. CMR is more expensive than echocardiography and requires different skills and labor in measuring EAT. CMR may not be readily available in nonacademic hospitals or non-research centers.
Proton Magnetic Resonance Spectroscopy and Epicardial Fat
Myocardial fat accumulation has gained growing attention as independent and direct cause of cardiac diseases. The presence of myocardial fat deposition, otherwise called cardiac steatosis, could represent an additional CMR imaging feature of prognostic importance in high-risk patients. The significance of cardiac steatosis in normal hearts remains to be determined. Proton magnetic resonance spectroscopy (1H-MRS) is the gold standard technique to measure myocardial triglyceride content [16, 17]. 1H-MRS is used as a noninvasive, non-radiation exposure technique for the clinical assessment of myocardial lipid content. The recent use of high-field scanners (3.0 T) has improved the chemical shift resolution and signal-to-noise ratio of cardiac 1H-MRS. This feature allows the discrimination between fatty acid (FA) and unsaturated fatty acid (UFA) deposition in the myocardium. Myocardial TG signals and the water signal are acquired from spectra with water suppression and without water suppression, respectively (Fig. 7.3). The spectroscopic signals are acquired at end-systole and end-expiration with electrocardiographic triggering and respiratory navigator gating.
(Left) Proton MR spectroscopic image of septal myocardial fat. (Right) Example of nonwater-suppressed proton MR spectrum of interventricular septal fat in an obese study patient. Amplitude is expressed in arbitrary units and with two different scales (left of images). (Center) Two-chamber plane (top), short-axis plane (middle), and four-chamber plane (bottom) MR images show voxel positioning in the septum. (From Malazavos et al. [20], with permission from Elsevier)
In clinical studies with 1H-MRS, myocardial triglyceride content is correlated with left ventricular mass and systolic function in patients with heart failure and left ventricular hypertrophy [18, 19]. Ultrasound measured EAT thickness has shown to independently correlate with 1H-MRS myocardial triglyceride content (R = 0.79, p < 0.01) in subjects with a wide range of obesity and body fat distribution [20]. EAT, as measured by standard cardiac ultrasound, may serve a predictor of triglyceride fat content better than waist circumference and other more traditional biochemical risk factors. This statistical correlation is based on the lack of barrier separating EAT from the underlying myocardium. Hence, EAT excessive free fatty acid influx can reach directly the myocardium via paracrine or vasocrine pathways. It is plausible to speculate that the consensual increase in EAT and myocardial fat might affect the heart morphology and function.
References
Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–9.
Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–10.
Gronemeyer SA, Steen RG, Kauffman WM, Reddick WE, Glass JO. Fast adipose tissue (FAT) assessment by MRI. Magn Reson Imaging. 2000;18:815–8.
Flüchter S, Haghi D, Dinter D, Heberlein W, Kühl HP, Neff W, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity (Silver Spring). 2007;15:870–8.
Schejbal V. Epicardial fatty tissue of the right ventricle--morphology, morphometry and functional significance. Pneumologie. 1989;43:490–9.
Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–43.
Leo LA, Paiocchi VL, Schlossbauer SA, Ho SY, Faletra FF. The intrusive nature of epicardial adipose tissue as revealed by cardiac magnetic resonance. J Cardiovasc Echogr. 2019;29:45.
Mahajan R, Kuklik P, Grover S, Brooks AG, Wong CX, Sanders P, et al. Cardiovascular magnetic resonance of total and atrial pericardial adipose tissue: a validation study and development of a 3-dimensional pericardial adipose tissue model. J Cardiovasc Magn Reson. 2013;29(15):73.
Nelson AJ, Worthley MI, Psaltis PJ, Carbone A, Dundon BK, Duncan RF, et al. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J Cardiovasc Magn Reson. 2009;11:15.
Doesch C, Haghi D, Flüchter S, Suselbeck T, Schoenberg SO, Michaely H, et al. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12:40.
Doesch C, Haghi D, Suselbeck T, Schoenberg SO, Borggrefe M, Papavassiliu T. Impact of functional, morphological and clinical parameters on epicardial adipose tissue in patients with coronary artery disease. Circ J. 2012;76:2426–34.
Doesch C, Streitner F, Bellm S, Suselbeck T, Haghi D, Heggemann F, et al. Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity (Silver Spring). 2013;21:E253–61.
Ng A, Strudwick M, van der Geest RJ, Ng ACC, Gillinder L, Goo SY, et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content and interstitial fibrosis on myocardial contractile function. Circ Cardiovasc Imaging. 2018;11:e007372.
Mahmod M, Bull S, Suttie JJ, Pal N, Holloway C, Dass S, et al. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ Cardiovasc Imaging. 2013;6:808–16.
Lu M, Zhao S, Jiang S, Yin G, Wang C, Zhang Y, et al. Fat deposition in dilated cardiomyopathy assessed by CMR. JACC Cardiovasc Imaging. 2013;6:889–98.
Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, JD MG, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol Endocrinol Metab. 1999;276:E977–89.
Reingold JS, McGavock JM, Kaka S, Tillery T, Victor RG, Szczepaniak LS. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab. 2005;289:E935–9.
Liao PA, Lin G, Tsai SY, Wang CH, Juan YH, Lin YC, et al. Myocardial triglyceride content at 3 T cardiovascular magnetic resonance and left ventricular systolic function: a cross-sectional study in patients hospitalized with acute heart failure. J Cardiovasc Magn Reson. 2016;18:9.
Sai E, Shimada K, Yokoyama T, Hiki M, Sato S, Hamasaki N, et al. Myocardial triglyceride content in patients with left ventricular hypertrophy: comparison between hypertensive heart disease and hypertrophic cardiomyopathy. Heart Vessel. 2017;32:166–74.
Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol. 2010;105:1831–5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Iacobellis, G. (2020). Magnetic Resonance Imaging of the Epicardial Adipose Tissue. In: Iacobellis, G. (eds) Epicardial Adipose Tissue. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-40570-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-40570-0_7
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-40569-4
Online ISBN: 978-3-030-40570-0
eBook Packages: MedicineMedicine (R0)