Abstract
Liver fibrosis is a wound-healing response to chronic liver injury such as alcoholic/nonalcoholic fatty liver disease, and viral hepatitis with no FDA-approved treatments. Liver fibrosis results in a continual accumulation of extracellular matrix (ECM) proteins and paves the way for replacement of parenchyma with non-functional scar tissue. During liver fibrosis, alterations in hepatocytes phenotype including apoptosis, oxidative stress, and loss of metabolic function have been shown to precede fibrosis and promote hepatic stellate cell activation. Specifically, hepatocyte death, as part of the original injury, triggers a cascade of events, including pathological accumulation of ECM leading to the increased tissue stiffness during liver injury. This chapter provides an overview of the interplay of hepatocytes with stiffness using in vitro models mimicking physiological and pathological matrix rigidity to provide insight into the pivotal changes in hepatocytes physiology and the extent to which it mediates the progression of liver fibrosis. Establishing the molecular aspects of hepatocytes in the light of fibrotic liver stiffness is valuable towards development of novel therapeutic and diagnostic targets of liver fibrosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Liver stiffness
- Hepatocytes
- Fibrosis
- Metabolism
- Matrix
- Bio-mimetic models
- Mechanotransduction
- Mechanosensors
- Matrix
- Collagen
- Polydimethylsiloxane
Introduction
Liver damage as a consequence of liver injury or disease (e.g., chronic hepatitis C virus (HCV) infection, alcohol abuse, and nonalcoholic steatohepatitis (NASH)) is extremely prevalent worldwide and results in a huge economic burden on patients [1, 2]. Several liver diseases can lead to fibrosis, which results from an imbalance between production and resorption of extracellular matrix (ECM) and restructuring of the liver microenvironment (LME). The earliest changes in LME as a result of liver disease occur in response to ECM remodeling, resulting in accumulation of ECM proteins and an increase in liver stiffness. Furthermore, the balance of matrix production and degradation is compromised, leading to deleterious effects on the liver function. Clinically, stiffness measurement is considered as the best read-out to monitor, stage and diagnosis, clinical outcomes of new drugs, and survival correlation in liver diseases. Furthermore, clinical studies have shown that liver stiffening provides a permissive milieu for the development of cellular dysplasia and is a key feature of liver dysfunction that leads to cirrhosis and hepatocellular carcinoma (HCC) [3,4,5].
Noninvasive elastography techniques and direct rheometry measurements of the whole liver have established that the liver stiffness increases as fibrosis progresses [6,7,8,9]. Clinical research assessing liver stiffness by transient elastography has revealed that the stiffness of normal, early, and late stage liver fibrosis are <6 kPa, 6–12.5 kPa, and 12.5–75 kPa, respectively (Fig. 55.1) [10, 11]. For more details, see also other book parts, namely, book Part IV “Important (Patho)physiological Confounders of LS.” Studies of both humans and rats suggest that increased liver stiffness is associated with progression of fibrosis [12,13,14]. In patients with chronic hepatitis C infection, magnetic resonance elastography studies have shown that livers at stage F0 (with no detectable fibrosis) are stiffer than the livers of uninfected patients; similarly, in rats with carbon tetrachloride-mediated injury, increased liver stiffness preceded fibrosis [8]. Despite these data, there is lack in the complete understanding of the role of the mechanical cues elicited by the varying stiffness on the fate of liver cells, including hepatocytes.
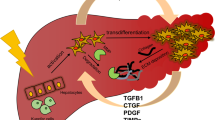
Etiologic factors and changes in liver stiffness in the pathogenic development of hepatic diseases. Increase in liver stiffness is the major pathogenic event occurring in several liver disorders. Chronic liver injury due to HBV and HCV infection, inadequate alcohol consumption, and metabolic disorders results in a gradual and dramatic increase in liver stiffness and corresponds to higher hepatocytes damage, necrosis, apoptosis, and proliferation. Instauration of hepatocyte oxidative stress condition results in liver fibrosis and cirrhosis, which may lead to hepatocellular carcinoma
Hepatocytes as Key Players of Liver Parenchyma and Function
The liver is the largest solid organ in the body comprising about 3% of the adult body weight and can be considered the metabolic center that performs hundreds of vital functions necessary for maintaining homeostasis [15]. The different functional roles of the liver can be grouped into categories of synthesis, storage, detoxification, and metabolism [16]. The highly metabolic nature of the organ demands approximately 30% of the total oxygen consumption. Some of the major functions carried out by the liver are bile synthesis, regulation of blood glucose, detoxification of xenobiotic substances, maintenance of serum oncotic pressure, nitrogen disposal, lipid synthesis and breakdown, and regulation of blood clotting [17]. The liver is a complex organ built by at least seven different resident cell types—hepatocytes, liver sinusoidal endothelial cells, dendritic cells, Kupffer cells, stellate cells, biliary epithelial cells, and lymphocytes of multiple types that are organized in a precise manner for maximal functional stability. Hepatocytes comprise the majority (~85%) of tissue mass in the liver as the liver parenchyma that performs several key liver functions. Hepatocytes perform the bulk of metabolic and synthetic functions of the organ. They synthesize the majority of circulating proteins in the plasma such as albumin, protease inhibitors, clotting factors, and inflammatory complexes [18]. They metabolize biomolecules such as cholesterol, heme, triglycerides, vitamins, glucose, and bilirubin for homeostasis [18]. Hepatocytes are large polygonal, multinucleated cells of about 20 microns in size and are connected to adjacent hepatocytes through adhesion complexes such as tight junctions, and desmosomes [15]. These cells have distinct polarity; along with a distinct signature of cell surface receptors, carrier proteins and pumps, the cell-layer surface that faces the space of Disse has microvilli extensions, allowing for maximum surface area for transport of molecules from the lumen. Due to their high metabolic nature, they contain a high density of intracellular machinery such as mitochondria, peroxisomes, lysosomes, and endoplasmic reticulum. In a healthy liver, hepatocytes possess a superior capacity for proliferation, thereby allowing for regeneration of the organ under manageable stress. Along with the complex functional profile, maintenance of the replicative capacity of hepatocytes is highly dependent upon the upkeep of the intricate elements of the liver microenvironment such as mechanical stresses, cell–cell interactions, and cell–ECM interactions. Consequently, primary hepatocytes isolated from the liver and disconnected from their natural environment experience a drastic loss in functions and a complete loss in proliferative capacity.
Liver Microenvironment in Normal and Diseased Livers
The liver microenvironment (LME) is essential for the maintenance of tissue functionality. The various components that constitute the LME are the parenchymal cells, non-parenchymal cells, spatial organization of the heterogeneous cell population, liver-specific ECM, soluble factors, oxygen gradient, and several mechanical cues [19,20,21,22]. Numerous studies have investigated the various aspects of LME and incorporated the findings towards creating in vitro liver models. Uygun et al. employed derivative of decellularized liver matrix to demonstrate the importance of the chemical composition of the ECM in the maintenance of hepatocyte function for a prolonged duration [23]. Kidambi et al. established that high oxygen regulates the stability of hepatocyte function in vitro. Similarly, a study by Wong et al. showed that the autocrine signaling between hepatocytes forming tight cell–cell junctions is essential towards maintaining their synthetic functions in vitro [24]. Paracrine signaling is equally important in prolonging the hepatocyte health, as shown through multiple co-culture studies of hepatocytes with the stellate cells, fibroblasts, and endothelial cells [21, 25,26,27]. Interestingly, the LME changes drastically in the event of liver pathological conditions such as fibrosis.
Liver Fibrosis
Liver fibrosis is a sustained wound-healing response in the organ resultant of chronic stressors such as viral infections, autoimmune disorders, metabolic disorders, or alcohol abuse [28]. During liver fibrosis, stellate cells and other hepatic cell types acquire a pro-fibrogenic phenotype that primarily results in (1) excessive production of ECM molecules forming scar tissue (2) increased inflammatory response and (3) loss of parenchymal function [29]. The reversibility of liver fibrosis depends on the nature and severity of the stressor, and irreversible fibrosis can result in fatal conditions such as cirrhosis, kidney failure, and hepatocellular carcinoma [30]. The most critical challenges in liver fibrosis intervention are the following: (1) absence of noninvasive biomarkers, (2) lack of mechanistic studies on the reversibility of liver fibrosis, and (3) absence of effective anti-fibrosis therapies.
Functional maintenance of the liver in a healthy state and the rate of progression of liver fibrosis are both regulated by the complex factors of the LME. Researchers anticipate that restoration of the healthy liver milieu will determine the success of anti-fibrosis therapies. Establishing the role of these individual liver-specific cues such as mechanical stiffness on different hepatic cell types is crucial for a systematic bottom-up approach towards (1) functional tissue engineering and (2) creating physiologically relevant disease models. A gaping hole in the literature of LME is that a majority of these studies focused primarily on maintaining the hepatocyte functions but the correlation of stiffness on hepatocytes dysfunction is limited.
Role of Mechanical Environment in Liver Function and Fibrosis
Tissue development and function are driven by several mechanical elements of the microenvironment such as shear stress, compression forces, surface tension, traction, and osmotic pressure [31]. In the context of the liver, matrix elasticity (stiffness) has been a particularly important aspect correlating to the healthy and the diseased state of the organ. Over the last decade, clinicians have routinely been using direct and noninvasive elastography techniques to determine the stiffness of the liver as a diagnostic measure for establishing the occurrence/severity of liver fibrosis [9, 32,33,34]. The cells that build tissues are viscoelastic in nature, and their anchorage dependence with adjacent cells and matrix is essential for the regulation of events such as proliferation, apoptosis, differentiation, and stress response [35,36,37]. The short range forces that the cells experience as a result of this adhesion are sensed by the cells through focal adhesion and cytoskeleton-mediated pathways [38]. Studies demonstrate that integrins, the heterodimeric receptors on the cell surface that mediate anchorage with the ECM, are the principle mechanosensors of the cells. The bi-directional signaling between integrins and the cytoskeletal molecules regulates changes in the cellular phenotype [39, 40].
In the event of pathological conditions in the liver such as liver fibrosis, the stiffness of the organ can increase dramatically [28]. The occurrence of liver fibrosis is considered synonymous with a malfunctioning ECM production and maintenance. The increase in the stiffness of the organ can be attributed to the ECM changes, both through the sheer amount of ECM components such as collagen 1 and proteoglycans that are deposited and by the modification of the existing components through posttranslational modification and cross-linking [41, 42]. Research shows that adherent cells can sense mechanical changes, but the implications of mechanical changes that accompany liver fibrosis on hepatocytes are not well established.
Significance of Mechanobiology for Hepatocyte Function
A majority of the mechanotransduction studies of the liver have focused on hepatic stellate cells due to their importance in liver fibrosis progression [43, 44]. Recent advancement in liver fibrosis research has established that the liver parenchyma and other non-parenchymal cells are also critical in the progression of liver disease. Zeisberg et al. demonstrated that epithelial to mesenchymal transition in hepatocytes results in an accumulation of activated fibroblasts in animal models with CCl4-induced liver fibrosis [45]. Similarly, a study shows that hepatocellular carcinoma cells have shown a difference in resistance towards chemotherapeutic drugs when subjected to varying mechanical stiffness [46]. These studies suggest that it is vital to establish the nature of mechanosensitivity in hepatocytes to provide a better understanding of the various mechanistic triggers that regulate liver fibrosis.
From a different perspective, consideration of the mechanical microenvironment is equally important for improving the functionality of in vitro liver tissue model. By mimicking the mechanical properties of the healthy liver, we could achieve superior hepatotoxicity screening, bio-artificial livers and potential to expand cellular population for cell-based therapies [47, 48]. The conventional in vitro model for these purposes is hepatocytes cultured on polystyrene dishes that are a few gigapascals in elastic modulus and cells exposed to such a physiologically irrelevant stiffness demonstrate a functional compromise. An in vitro model that recapitulates the mechanical stiffness of the liver as seen in physiological and pathological conditions will prove to be valuable towards (1) advancing the field of mechanobiology of the liver, (2) modeling fibrotic phenotype in vitro, and (3) elucidating the role of stiffness on the phenotypic regulation of hepatocytes.
Need for Bioengineered In Vitro Liver Models
Despite the accuracy of animal models in capturing several vital physiological parameters, it is challenging to capture the dynamic changes in physiological and pathological liver stiffness at various stages of disease progression. Functional in vitro liver models are alternative and simplistic research tools towards establishing a fundamental understanding of the microenvironmental regulation of the liver. Additionally, in vitro models provide the opportunity to utilize human-derived cells/tissues, which tremendously improves their physiological relevance. A vast section of novel drugs fail in the clinical trial phase due to rodent/human biological difference in the preclinical stage, and this could potentially be reduced by employing in vitro liver models as preclinical screening platforms [49]. Most popular in vitro model for the liver utilized in pharmaceutical industry and research setting is the simple monoculture of primary hepatocytes or hepatic cell lines (HepG2 or Huh7) [50, 51]. These models are typically used to study liver metabolism or drug screening, and they suffer from critical limitations in the form of altered phenotypic drift and loss in functions [52]. Advanced engineering techniques that can mimic the vital microenvironment elements of the liver will be required to create functional in vitro models of the liver.
In Vitro Substrates for Recreating Liver Stiffness
In vitro tools have been instrumental in the advancement of mechanobiology research. A significant portion of these in vitro studies utilizes protein-based substrates for creating platforms of tunable stiffness [53, 54]. The impact of extracellular matrix on the differentiated functions of hepatocytes has been widely studied. In general, the efficiency of hepatocyte attachment is enhanced by coating substrates with simple extracellular matrix proteins (typically collagen); however, in most cases, a concomitant increase in hepatocyte spreading leads to a loss of liver-specific functions [55]. Presentation of extracellular matrices of different compositions and topologies can stabilize hepatocyte morphology and a limited set of phenotypic functions. For instance, hepatocyte culture on biomatrix, a complex mixture of extracellular matrix components extracted from liver, has been shown to improve hepatocyte function compared with monolayers on collagen [55, 56]. When sandwiched between two layers of gelled collagen whose stiffness parallels physiological liver stiffness, hepatocytes from a variety of species maintain a cuboidal shape, secrete albumin, and synthesize urea (marker of nitrogen metabolism) [57]. Rat hepatocytes, in particular, secrete albumin at a high rate for 40 days in sandwich cultures, exhibit improved cytochrome P450 induction, and form a contiguous, anastomosing network of bile canaliculi indicative of polarity [52]. The disadvantages of using biological substrates to create tunable stiffness are their lack of reproducibility in the physical characteristics, cost-ineffectiveness and, most importantly, unwanted variability in chemical and topographic cues [27]. Recent study demonstrates that the liver during fibrosis experiences shear strain softening and compression stiffening, whereas collagen gels display the opposite phenomenon with respect to shear stress and compression [58].
Among the synthetic materials that are at our disposal for stiffness modeling, polyacrylamide gels have been a popular choice due to the tunability of stiffness in the physiologically relevant stiffness range [59,60,61]. The limitation of polyacrylamide gels lies in the possible toxicity of unpolymerized acrylamide and difficulty in uniform surface functionalization [62]. Hyaluronic acid (HA) gels were used for the stiffness study by altering the gel concentration through a cross-linking process. HA hydrogels contained liver extracellular matrix (ECM), which were used to study the cells morphology of human hepatocytes due to the tremendous medical applications of HA used [54, 63]. Just like HA hydrogels, polyvinyl alcohol (PVA) hydrogels were also developed to study the effect of stiffness on hepatocytes [64]. Polydimethylsiloxane (PDMS) has emerged as a novel alternative synthetic substrate to study mechanical properties of biological tissues. PDMS is a bio-inert, versatile inorganic silicone material widely used in micro/nano fabrication techniques [65, 66]. Conventionally, stiffness in PDMS substrates has been modified by varying the ratio of cross-linker to elastomer in Sylgard 184, but the drawback here is that the cellular toxicity due to non-crosslinked PDMS has not been established [67]. We have developed an attractive alternative to this stiffness tunability as well as developing a protein-free matrix for primary hepatocytes attachment to tease out the effect of stiffness as a sole parameter on hepatocytes function. Here we utilized varying weight ratios of Sylgard 184 and Sylgard 527 to create resultant substrates of different stiffness and integrated a polymer-based interface on PDMS to overcome its hydrophobic and cell-resisting nature to facilitate cell-based studies (Fig. 55.2) [51, 68,69,70].
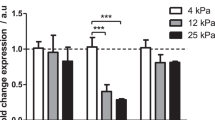
(a) Design of PDMS-based substrate coated with polymer films for tunable substrates for mechanical stimulation for primary hepatocytes. (b) Phase contrast images of primary hepatocytes on soft, stiff and TCPS substrates; Quantification of urea synthesis by primary hepatocytes on soft, stiff and TCPS substrates; Quantification of albumin synthesis by primary hepatocytes cultured on soft, stiff and TCPS substrates using ELISA
Stiffness and Hepatocytes In Vitro
The in vitro culture of hepatocytes can exhibit many hepatic functions for a finite period. Studying the loss of hepatic functional markers such as urea and albumin, supplementing study of the non-specific end points, can be utilized as the tool to evaluate the effect of an external stimulus on the cellular behavior. Studies investigating the role of matrix stiffness on hepatocyte biology have observed that hepatocytes remain differentiated (functional) on soft supports and dedifferentiate (lose their functions) on stiff supports [71,72,73]. Studies have also demonstrated that when cultured on stiff, thin films of monomeric collagen, hepatocytes spread, proliferate, and otherwise adopt a dedifferentiated phenotype, whereas on soft gels of fibrillar collagen or matrigel, they remain differentiated and growth arrested [74, 75]. The primary goal of these studies was to extend the differentiated function of hepatocytes in order to use these as platforms for drug screening and toxicity studies and the effect of stiffness was not investigated in detail. Furthermore, it is inherently difficult to utilize bio-responsive materials to study the isolated effect of mechanical cues, independent of the ligand density. Accumulating evidence demonstrates the differential effect of matrix components on cultured hepatocytes. When isolated mature hepatocytes are cultured on type I collagen-coated dishes, the cells appear as a flattened monolayer and express low levels of liver function-specific mRNA and proteins. In dramatic contrast, when hepatocytes are cultured on a model basement membrane Engelbreth–Holm–Swarm (EHS) gel, hepatocytes retain their normal polarity and structure, and the products of liver-specific genes continue to be secreted for prolonged periods of culture [76, 77]. Cell–matrix interaction influences the determination of the differentiated phenotype of hepatocytes in cell culture, and maintains liver-specific functions for long-term culture, which effects have been associated with upregulation of liver-enriched transcription factors, including hepatocyte nuclear factor (HNF). Upregulation of liver-specific genes induced by ECM is mediated via upregulation of HNF-4α and HNF-1 induced by ECM. A collagen gel matrix increased the levels of HNF-3α in the hepatocyte-derived cell line H2.35, but not those of HNF-3β and -3γ, responsible for the transcription of liver-specific genes. ECM regulates HNF-4 and tissue-specific gene expression in fetal hepatocytes as well as adult hepatocytes [78, 79].
Recently, there has been more study of the relationship between substrate stiffness and cellular functions, such as adhesion, migration, cell differentiation, and proliferation [13, 51, 61, 80,81,82]. Studies have explored the use of synthetic substrates of varying mechanical properties to examine hepatic phenotype expression. Chen and co-workers demonstrated that primary hepatocytes cultured on varying elastic modulus of polyelectrolyte multilayers had decreasing albumin production with increasing film stiffness [83]. Semler and co-workers investigated the effects of graded mechanical compliance on the function of primary hepatocytes using modified polyacrylamide gels with cell adhesive ligands and demonstrated that increasing hydrogel compliance resulted in increased albumin secretion [84]. You and co-workers utilized heparin-based hydrogels to investigate the effect of varying stiffness on primary hepatocytes function [54]. This study demonstrated that hepatocytes cultured on a softer heparin gel (10 kPa) were synthesizing five times higher levels of albumin compared to those on a stiffer heparin gel (110 kPa) after 5 days. Also, the study confirmed that softer gels promoted better maintenance of the hepatic phenotype as determined by hepatic markers (albumin and E-cadherin) demonstrating the importance of substrate mechanical properties on hepatocyte function. Xia and co-workers used RNA-Seq technology to study the transcriptome of hepatocytes cultured on soft, moderate, stiff, and plastic substrates [64]. Compared to soft substrate, their RNA-Seq results revealed 1131 genes that were upregulated and 2534 that were downregulated on moderate substrate, 1370 genes that were upregulated and 2677 downregulated genes on stiff substrate. Further analysis indicated that differentially expressed genes were primarily associated with the regulation of actin cytoskeleton, focal adhesion, tight junction, adherens junction as well as antigen processing and presentation. In another study, three levels of stiffness were used that corresponded to the stiffness levels found in normal liver tissue (4.5 kPa), the early (19 kPa) and late stages (37 kPa) of fibrotic liver tissues [85]. This study showed that cytoskeleton of hepatocyte was influenced by substrate stiffness and soft substrates promoted the cellular migration and directionality. Integrin-β1 and β-catenin expression on cytomembrane were upregulated and downregulated with the increase of substrate stiffness, respectively. This study suggests that hepatocytes were sensitive to substrate stiffness and potential relationship among substrate stiffness, cellular Young’s modulus and the dynamic balance of integrin-β1 and β-catenin pathways. Chang and co-workers demonstrated that fibrotic levels of matrix stiffness significantly inhibit hepatocyte-specific functions in part by inhibiting the HNF4α transcriptional network mediated through the Rho/Rho-associated protein kinase pathway [80]. Fibrotic levels of matrix stiffness activated mechanotransduction in primary hepatocytes through focal adhesion kinase. In addition, blockade of the Rho/Rho-associated protein kinase pathway rescued HNF4α expression from hepatocytes cultured on stiff matrix. However, these experiments were carried out using polyacrylamide gels which has few limitations: (1) covalently crosslink proteins using harsh chemicals is necessary for cell adhesion, (2) protein structure is regulated in varying stiffness [86,87,88], and (3) elastic creasing instability of the softer polyacrylamide gel that may lead to surface artifacts capable of contributing to non-specific cell behavior beyond stiffness [89].
Kidambi and co-workers demonstrated that stiffness impedes hepatic urea and albumin production, expression of drug transporter gene and epithelial cell phenotype marker, hepatocyte nuclear factor 4 alpha (HNF4a) (Fig. 55.2) [26, 51]. It was observed that hepatocytes cultured on soft substrates displayed a more differentiated and functional phenotype for a longer duration as compared to stiff substrates and TCPS. It was also demonstrated that hepatocytes on soft substrates exhibited higher urea and albumin synthesis. Cytochrome P450 (CYP) activity, another critical marker of hepatocytes, displayed a strong dependence on substrate stiffness, wherein hepatocytes on soft substrates retained 2.7-folds higher CYP activity on day 7 in culture, as compared to TCPS. Recently, Kidambi and co-workers further observed that increase in stiffness induces downregulation of key drug transporter genes (NTCP, UGT1A1, and GSTM-2). In addition, they observed that the epithelial cell phenotype was better maintained on soft substrates as indicated by higher expression of hepatocyte nuclear factor 4α, cytokeratin18, and connexin 32. It was also demonstrated that hepatocytes cultured on NAFLD-like stiffness showed an induction of lipogenic genes, and lowered-oxidation genes expression, mitochondrial respiration, and glycolytic capacity, (2) increased ROS production, and (3) disruption of the mitochondrial fusion process and dynamics. Furthermore, significant increase in oxidized glutathione (GSSG) and reduced glutathione (GSH) in hepatocytes cultured on NAFLD-like stiffness compared to healthy liver stiffness was observed (Fig. 55.3) [90]. Similar effect was observed in hepatocytes isolated from fatty liver rat models indicating correlation to physiological conditions. Ganesan and co-workers demonstrated that levels of HIV and HCV mono- and co-infections were more prominent in primary hepatocytes cultured on substrates mimicking fibrotic stiffness (25 kPa-stiff) compared to substrates mimicking healthy liver (2.5 kPa-soft). Also the hepatocytes apoptosis due to viral infection was significantly higher in stiffer matrix compared to softer matrix. This study concluded that the increased matrix stiffness is not only a consequence of liver inflammation/fibrosis, but the condition that further accelerates liver fibrosis development. These studies suggest a plausible mechanism that increased stiffness modulates hepatocyte function causing liver functional failure. These results indicate that the substrate stiffness plays a significant role in modulating hepatocyte behavior. Understanding the impact of stiffness on hepatocytes biology will provide significantly more nuanced data to aid drug development for liver diseases.
Conclusions and Future Directions
Although many challenges remain for the improvement of in vitro models to study the effect of stiffness on liver function, substantial progress has been made towards a thorough understanding of the necessary components. The parallel development of highly functional in vitro systems mimicking physiological and pathological liver stiffness is based on contributions from diverse disciplines, including regenerative medicine, developmental biology, transplant medicine, and bioengineering. In particular, novel technologies such as scaffold chemistries, high-throughput platforms, and micro/nano technologies represent enabling tools for investigating the critical role of the liver microenvironment including stiffness in liver function and, subsequently, the development of structurally complex and clinically effective engineered liver systems. Despite these developments, the following things are still required to address this gap in knowledge; (1) the need to develop mechanically tunable technology capable of pressure-mimicking conditions with varying stiffness without any biochemical or protein intervention, (2) a better understanding of the effect of stiffness on hepatocyte metabolic changes during various stages of cirrhosis, (3) dissect the effect of stiffness in driving hepatocytes-mediated stellate cell activation, and (4) a high-throughput method to investigate the impact of stiffness on hepatocytes-non-parenchymal cell communication. Understanding the effect of increased matrix stiffness during the course of liver fibrosis on hepatocyte function will provide more insight in the role of matrix rigidity as a contributor to the disease progression and hepatic functional failure. Given that change in stiffness regulates cell function independent of the biochemical signals, in vitro study of stiffness and understanding its impact on hepatocytes function is critical for new therapeutic interventions for liver fibrosis and liver failure. Together, all these studies demonstrate the plausible role of stiffness in regulating hepatocytes function and contribute to metabolic dysregulation. Understanding the impact of stiffness on hepatocytes biology will provide significantly more nuanced data to aid drug development for liver diseases.
References
Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375–82.e1–2.
Liver disease in Europe. Lancet. 2013;381(9866):508.
Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: biochemistry and regulation. Biochim Biophys Acta. 2013;1832(7):876–83.
Mederacke I. Liver fibrosis—mouse models and relevance in human liver diseases. Z Gastroenterol. 2013;51(1):55–62.
Ramachandran P, Iredale JP. Liver fibrosis: a bidirectional model of fibrogenesis and resolution. QJM. 2012;105(9):813–7.
Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55(3):403–8.
Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1147–54.
Yin M, Kolipaka A, Woodrum DA, Glaser KJ, Romano AJ, Manduca A, et al. Hepatic and splenic stiffness augmentation assessed with MR elastography in an in vivo porcine portal hypertension model. J Magn Reson Imaging. 2013;38(4):809–15.
Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5(10):1207–13.e2.
Takeda T, Yasuda T, Nakayama Y, Nakaya M, Kimura M, Yamashita M, et al. Usefulness of noninvasive transient elastography for assessment of liver fibrosis stage in chronic hepatitis C. World J Gastroenterol. 2006;12(48):7768–73.
Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med. 2010;2:49–67.
Henderson NC, Forbes SJ. Hepatic fibrogenesis: from within and outwith. Toxicology. 2008;254(3):130–5.
Lozoya OA, Wauthier E, Turner RA, Barbier C, Prestwich GD, Guilak F, et al. Regulation of hepatic stem/progenitor phenotype by microenvironment stiffness in hydrogel models of the human liver stem cell niche. Biomaterials. 2011;32(30):7389–402.
Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47(4):1394–400.
Arias I, Wolkoff A, Boyer J, Shafritz D, Fausto N, Alter H, et al. The liver: biology and pathobiology. Chichester: Wiley; 2011.
Arias IM, Boyer J, Shafritz D, Fausto N, Alter H, Cohen DE, Wolkoff A. The liver: biology and pathology. Hoboken: Wiley Blackwell; 2010. 1216 p.
Rouiller C. The liver: morphology, biochemistry, physiology. New York: Academic; 2013.
Zakim D, Boyer T, Hepatology A. Textbook of liver disease. Philadelphia: WB Saunders Company; 1996.
Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18(1):2–13.
Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Høyer-Hansen G, et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73(7):2031–43.
Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci. 2009;106(37):15714–9.
Bhatia S, Balis U, Yarmush M, Toner M. Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–900.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis M-L, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–20.
Wong SF, Choi YY, Kim DS, Chung BG, Lee S-H. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials. 2011;32(32):8087–96.
Bhandari RN, Riccalton LA, Lewis AL, Fry JR, Hammond AH, Tendler SJ, et al. Liver tissue engineering: a role for co-culture systems in modifying hepatocyte function and viability. Tissue Eng. 2001;7(3):345–57.
Kidambi S, Sheng LF, Yarmush ML, Toner M, Lee I, Chan C. Patterned co-culture of primary hepatocytes and fibroblasts using polyelectrolyte multilayer templates. Macromol Biosci. 2007;7(3):344–53.
Kidambi S, Lee I, Chan C. Controlling primary hepatocyte adhesion and spreading on protein-free polyelectrolyte multilayer films. J Am Chem Soc. 2004;126(50):16286–7.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–18.
Friedman SL. Liver fibrosis–from bench to bedside. J Hepatol. 2003;38:38–53.
Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–56.
Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137(9):1407–20.
Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56(6):2125–33.
Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960–74.e8.
Yin M, Woollard J, Wang X, Torres VE, Harris PC, Ward CJ, et al. Quantitative assessment of hepatic fibrosis in an animal model with magnetic resonance elastography. Magn Reson Med. 2007;58(2):346–53.
Wang H-B, Dembo M, Wang Y-L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279(5):C1345–C50.
Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85(1):145–56.
Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011;32(16):3921–30.
Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–43.
Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20(7):811–27.
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–95.
Desmoulière A, Darby I, Costa A, Raccurt M, Tuchweber B, Sommer P, et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest. 1997;76(6):765–78.
Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, et al. New aspects of hepatic fibrosis. J Hepatol. 2000;32:32–8.
Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46(4):1246–56.
Sakata R, Ueno T, Nakamura T, Ueno H, Sata M. Mechanical stretch induces TGF-β synthesis in hepatic stellate cells. Eur J Clin Investig. 2004;34(2):129–36.
Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282(32):23337–47.
Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53(4):1192–205.
Tateno C, Yoshizato K. Long-term cultivation of adult rat hepatocytes that undergo multiple cell divisions and express normal parenchymal phenotypes. Am J Pathol. 1996;148(2):383.
Clayton DF, Darnell J. Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983;3(9):1552–61.
Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–24.
Sawada H, Takami K, Asahi S. A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 2005;83(2):282–92.
Natarajan V, Berglund EJ, Chen DX, Kidambi S. Substrate stiffness regulates primary hepatocyte functions. RSC Adv. 2015;5(99):80956–66.
Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3(2):174–7.
Youssef J, Chen P, Shenoy VB, Morgan JR. Mechanotransduction is enhanced by the synergistic action of heterotypic cell interactions and TGF-β1. FASEB J. 2012;26(6):2522–30.
You J, Park SA, Shin DS, Patel D, Raghunathan VK, Kim M, et al. Characterizing the effects of heparin gel stiffness on function of primary hepatocytes. Tissue Eng Part A. 2013;19(23–24):2655–63.
LeCluyse E, Bullock P, Madan A, Carroll K, Parkinson A. Influence of extracellular matrix overlay and medium formulation on the induction of cytochrome P-450 2B enzymes in primary cultures of rat hepatocytes. Drug Metab Dispos. 1999;27(8):909–15.
Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10(7–8):1046–53.
Guillouzo A. Liver cell models in in vitro toxicology. Environ Health Perspect. 1998;106(Suppl 2):511–32.
Perepelyuk M, Chin L, Cao X, van Oosten A, Shenoy VB, Janmey PA, et al. Normal and fibrotic rat livers demonstrate shear strain softening and compression stiffening: a model for soft tissue mechanics. PLoS One. 2016;11(1):e0146588.
Zustiak S, Nossal R, Sackett DL. Multiwell stiffness assay for the study of cell responsiveness to cytotoxic drugs. Biotechnol Bioeng. 2014;111(2):396–403.
Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60(1):24–34.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89.
Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 2009;9(15):2132–9.
Deegan DB, Zimmerman C, Skardal A, Atala A, Shupe TD. Stiffness of hyaluronic acid gels containing liver extracellular matrix supports human hepatocyte function and alters cell morphology. J Mech Behav Biomed Mater. 2015;55:87–103.
Xia T, Zhao R, Liu W, Huang Q, Chen P, Waju YN, et al. Effect of substrate stiffness on hepatocyte migration and cellular Young’s modulus. J Cell Physiol. 2018;233(9):6996–7006.
Mata A, Fleischman AJ, Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed Microdevices. 2005;7(4):281–93.
Dario P, Carrozza MC, Benvenuto A, Menciassi A. Micro-systems in biomedical applications. J Micromech Microeng. 2000;10(2):235.
Tzvetkova-Chevolleau T, Stéphanou A, Fuard D, Ohayon J, Schiavone P, Tracqui P. The motility of normal and cancer cells in response to the combined influence of the substrate rigidity and anisotropic microstructure. Biomaterials. 2008;29(10):1541–51.
Natarajan V, Moeller M, Casey CA, Harris EN, Kidambi S. Matrix Stiffness Regulates Liver Sinusoidal Endothelial Cell Function Mimicking Responses in Fatty Liver Disease. bioRXiv, 2020, https://doi.org/10.1101/2020.01.27.921353.
Daverey A, Mytty A, Kidambi S. Topography mediated regulation of HER-2 expression in breast cancer cells. Nano LIFE. 2012;2(3):1241009.
Kidambi S, Udpa N, Schroeder SA, Findlan R, Lee I, Chan C. Cell adhesion on polyelectrolyte multilayer coated polydimethylsiloxane surfaces with varying topographies. Tissue Eng. 2007;13(8):2105–17.
Huang X, Hang R, Wang X, Lin N, Zhang X, Tang B. Matrix stiffness in three-dimensional systems effects on the behavior of C3A cells. Artif Organs. 2013;37(2):166–74.
Ben-Ze’ev A, Robinson GS, Bucher N, Farmer SR. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci. 1988;85(7):2161–5.
Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151(3):497–505.
Hansen LK, Wilhelm J, Fassett JT. Regulation of hepatocyte cell cycle progression and differentiation by type I collagen structure. Curr Top Dev Biol. 2006;72:205–36, 1 plate.
Fassett J, Tobolt D, Hansen LK. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell. 2005;17(1):345–56.
Nagaki M, Sugiyama A, Naiki T, Ohsawa Y, Moriwaki H. Control of cyclins, cyclin-dependent kinase inhibitors, p21 and p27, and cell cycle progression in rat hepatocytes by extracellular matrix. J Hepatol. 2000;32(3):488–96.
Nagaki M, Shidoji Y, Yamada Y, Sugiyama A, Tanaka M, Akaike T, et al. Regulation of hepatic genes and liver transcription factors in rat hepatocytes by extracellular matrix. Biochem Biophys Res Commun. 1995;210(1):38–43.
DiPersio CM, Jackson DA, Zaret KS. The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol Cell Biol. 1991;11(9):4405–14.
Brill S, Zvibel I, Halpern Z, Oren R. The role of fetal and adult hepatocyte extracellular matrix in the regulation of tissue-specific gene expression in fetal and adult hepatocytes. Eur J Cell Biol. 2002;81(1):43–50.
Desai SS, Tung JC, Zhou VX, Grenert JP, Malato Y, Rezvani M, et al. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology. 2016;64(1):261–75.
Wilson CL, Hayward SL, Kidambi S. Astrogliosis in a dish: substrate stiffness induces astrogliosis in primary rat astrocytes. RSC Adv. 2016;6(41):34447–57.
Cozzolino AM, Noce V, Battistelli C, Marchetti A, Grassi G, Cicchini C, et al. Modulating the substrate stiffness to manipulate differentiation of resident liver stem cells and to improve the differentiation state of hepatocytes. Stem Cells Int. 2016;2016:5481493.
Chen AA, Khetani SR, Lee S, Bhatia SN, Van Vliet KJ. Modulation of hepatocyte phenotype in vitro via chemomechanical tuning of polyelectrolyte multilayers. Biomaterials. 2009;30(6):1113–20.
Semler EJ, Lancin PA, Dasgupta A, Moghe PV. Engineering hepatocellular morphogenesis and function via ligand-presenting hydrogels with graded mechanical compliance. Biotechnol Bioeng. 2005;89(3):296–307.
Xia T, Zhao R, Feng F, Song Y, Zhang Y, Dong L, et al. Gene expression profiling of human hepatocytes grown on differing substrate stiffness. Biotechnol Lett. 2018;40(5):809–18.
Bowler BE. Thermodynamics of protein denatured states. Mol BioSyst. 2007;3(2):88–99.
Battle AR, Ridone P, Bavi N, Nakayama Y, Nikolaev YA, Martinac B. Lipid-protein interactions: lessons learned from stress. Biochim Biophys Acta. 2015;1848(9):1744–56.
Bordeleau F, Califano JP, Negron Abril YL, Mason BN, LaValley DJ, Shin SJ, et al. Tissue stiffness regulates serine/arginine-rich protein-mediated splicing of the extra domain B-fibronectin isoform in tumors. Proc Natl Acad Sci U S A. 2015;112(27):8314–9.
Saha K, Kim J, Irwin E, Yoon J, Momin F, Trujillo V, et al. Surface creasing instability of soft polyacrylamide cell culture substrates. Biophys J. 2010;99(12):L94–6.
Moeller M, Thulasingam S, Narasimhan M, Kidambi S. Stiffness Induces NAFLD-Like Metabolic Dysfunction in Primary Hepatocytes, Hepatology. 2019;70:119A.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kidambi, S. (2020). Stiffness and Hepatocytes Function In Vitro. In: Mueller, S. (eds) Liver Elastography. Springer, Cham. https://doi.org/10.1007/978-3-030-40542-7_55
Download citation
DOI: https://doi.org/10.1007/978-3-030-40542-7_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40541-0
Online ISBN: 978-3-030-40542-7
eBook Packages: MedicineMedicine (R0)