Abstract
The birth of a child may be one of the most significant events in a women’s life, and it is associated with significant emotional, physical, and painful events during the labor and delivery process. It is critical to give the patient control in decision-making for the labor and delivery process including methods of pain relief. Studies reveal the extent to which a parturient pain is controlled during and after delivery has implications for short- and long-term psychological consequences including but not limited to depression, post-traumatic stress disorder, and negative thoughts about sexual relationships.
There are many available methods of pain relief during labor including nonpharmacologic, pharmacologic, regional, and neuraxial techniques. The goal of this book chapter is to discuss the various pain management modalities available during labor, operative deliveries, and the immediate postpartum period.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Natural childbirth
- Labor analgesia
- Cesarean analgesia
- Postpartum analgesia
- Paracervical block
- Pudendal block
Introduction
The process of labor is the onset of regular contractions leading to progressive dilation and effacement of the cervix and descent of the fetus from the uterus to the birth canal. Labor is also referred to as parturition and is defined by three stages: first, second, and third.
The International Society for the Study of Pain describes pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” [1]. During the perinatal and labor and delivery process, each woman will experience pain in a unique way. Pain is not only a sensory experience but also has emotional consequences. A sense of personal control over decision-making processes in labor has consistently been shown to correlate with overall maternal satisfaction with childbirth [2, 3]. As an example, a study of 100 women undergoing vaginal delivery reported that satisfaction with pain relief was associated with a feeling of being in control and having input in the decision-making process [3].
Bajaj et al. studied women who underwent experimental cervical dilation and compared them to women who were in labor, who were undergoing spontaneous abortion, or who had dysmenorrhea as to the sensory and affective qualities to their pain [4]. There was a vast array of descriptors for each category, but the women experiencing dysmenorrhea reported descriptors that indicated suffering versus the women in labor who did not describe similar severity. To some women, the effects are almost a rite of passage and expected, but to others it can be psychologically damaging including but not limited to depression, post-traumatic stress disorder, and negative thoughts about their sexual relationships if the pain is not treated appropriately.
Pain control options for labor and delivery can be classified into nonpharmacologic and pharmacologic modalities. Nonpharmacological options are offered to increase the patient’s comfort and to allow the parturient to confront the pain without suffering. Pharmacological options are offered to prevent or greatly decrease the pain of the labor and delivery process.
Over 40 years ago, Melzack and Wall described the gate control theory for pain which has revolutionized the understanding of the mechanisms responsible for pain and analgesia [5]. The gate control theory has been updated to a concept of a neuromatrix which a dynamic system which has the capability to undergo rapid change [6]. The gate theory and neuromatrix have led to greater understanding of the mechanisms and treatment for chronic pain, but studies are lacking outlining the neurophysiologic mechanisms for the pain of labor and delivery.
This chapter will describe the stages of labor and delivery and explore nonpharmacological as well as pharmacological options for pain control.
Knowledge about the stages of labor and delivery and its effects on the mother is important when deciding on the appropriate pain control modality that would be the most beneficial for patients.
Stages of Labor Pain
During the labor and delivery process, the pain experienced by parturients is dynamic and changes as multiple different neurologic sites become effected. Figure 4.1 shows the location of thoracic innervation associated with labor.
First Stage of Labor
Labor pain during the first stage is a visceral or cramp-like and is primarily due to distention of uterine and cervical distention mechanoreceptors and by ischemia of uterine and cervical [7]. The first stage concludes when cervical dilation is complete at 10 cm. The period of time during which the cervix dilates from 7 to 10 cm is referred to as transition and may cause increased pain secondary to vaginal distension and somatic pain. Primary nerves involved in the first stage include T10, T11, T12, and L1 (Fig. 4.1). Labor pain can cause referred pain to the abdominal wall, lumbosacral region, iliac crests, as well as gluteal and thigh areas.
Second Stage of Labor
The pain associated with this stage is a combination of somatic pain from the vagina, perineum, and pelvic floor and stretching of the pelvic ligaments and visceral pain from uterine contractions and cervical stretching. Pain is transmitted to the spinal cord through nerves, S2, S3, and S4. The second stage begins when the cervix is completely dilated and is complete when the fetus is delivered. The parturient will additionally experience rectal pressure as the fetus descends into the pelvic outlet [7].
Third Stage of Labor
The third stage of labor begins when the infant is delivered and continues through delivery of the placental tissue [7].
Maternal Effects of Labor Pain
The stress and pain associated with labor and delivery can cause many physiologic changes within the mother and potentially affect the fetus. Studies in primates reveal that pain and stress can lead to decreased fetal oxygenation, acidosis, and a slow fetal heart rate [8].
Hyperventilation/increased oxygen consumption
The intermittent pain of uterine contraction causes hyperventilation with resultant hypocarbia and respiratory alkalosis. Profound hypocarbia may inhibit the patient’s respiratory drive between contractions which may lead to maternal and fetal hypoxemia, lightheadedness, and rarely loss of consciousness [9]. Respiratory alkalosis may impair maternal to fetal oxygen transfer. Additionally, alkalosis causes shifting of the oxygen hemoglobin dissociation curve to the left which results in increased affinity of oxygen for maternal hemoglobin and may also cause uteroplacental vasoconstriction leading to decreased uterine blood flow [9]. The respiratory effects of labor are generally well tolerated by healthy parturients [10].
Increased peripheral vascular resistance and cardiac output
Labor causes increases in circulating catecholamines which result in an increase in maternal peripheral vascular resistance and cardiac output. With severe labor pain, plasma epinephrine levels can reach equivalent levels of an epinephrine bolus of 15. Increased norepinephrine and epinephrine levels have been associated with decreased uterine blood flow [11]. Increased levels of catecholamines can lead to uterine artery vasoconstriction with resultant decreased placental blood flow to the fetus. The cardiovascular effects of labor are generally well tolerated by healthy parturients.
Gastric inhibition /delay gastric emptying
The combination of the pain associated with labor along with the anxiety and emotional distress results in an increase in gastrin release and inhibits the segmental and suprasegmental reflexes of gastrointestinal and urinary motility [12]. There is also an increase in gastric acidity. These may lead to an increased risk for aspiration if emergent airway manipulation occurs as in induction of general anesthesia for emergent cesarean section.
Methods for Managing Labor Pain
The pain, stress, and emotional factors associated with the process of labor and delivery bring unique challenges with each patient. The ultimate goal for these patients is as pleasant of an experience as possible for what some women is the most memorable joyous occasion of their lives. Each patient will present with a different set of expectations for this experience which include a variety of methods for pain control and methods for relieving the suffering. Suffering may be defined by several psychological elements including a perceived threat to the body and/or psyche, helplessness and loss of control, distress, insufficient strategies for coping with the distressing situation, or fear of death of the mother or baby [10].
Many parturients are choosing birth plans to reduce conflicts and misunderstandings between women and the healthcare providers surrounding the birth. Plans discussed prior to the birth should also include normal, complicated, and emergency scenarios and the possible options for pain control in each scenario. The plan may change as the process progresses, but this agreement serves as documentation of agreement between the patient and provider emphasizing issues most important to them.
The majority of women use some form of nonpharmacologic control and may in addition use pharmacologic forms of pain control. If a patient opts for nonpharmacologic forms of pain control, these methods do not take away the pain but rather help women cope with the intense pain of labor and maintain a sense of personal control over the birth process, thus reducing suffering [2]. During the prenatal process, women need to be presented with the risks and benefits of both methods of pain control in order to make informed decisions when it comes to their care in the labor and delivery process. There should be discussions with their partner, support team, and healthcare provider to understand the issues important to the mother, so she feels safely supported and confident in labor [13].
Some scientific thought leaders still consider labor pain to be minor. Melzack developed a questionnaire to study the intensity and emotional impact of pain and reported that nulliparous women with no prepared childbirth training rated labor pain to be as painful as a digit amputation without anesthesia [14]. Nulliparous women report labor pain as more severe than multiparous women although the difference is not significant. Women who experience unrelieved pain during childbirth may be more likely to develop postpartum depression [13] and a study of 1288 patients who either had cesarean section or vaginal delivery, developed postpartum depression not based on delivery method but on the severity of postpartum pain [15]. Post-traumatic stress disorder has also noted to occur in women who are postpartum with a reported range from 1.7% to 6.9% [16].
Nonpharmacological Methods of Pain Control
Lamaze proposed psychoprophylaxis as a method to prepare for birth, and his philosophy has shaped natural childbirth methods. Individuals have stated that labor pain is minor and there is no need for medication alternatives [17]. Table 4.1 illustrates several nonpharmacological methods of pain control with natural childbirth and comfort measures.
Pharmacological Methods of Pain Control
Analgesic medications decrease the sensation of pain without complete loss of sensation or loss of consciousness. In contrast, anesthetic medications block partial or complete sensation of pain with or without loss of consciousness. Pharmacologic approaches to pain control for labor include systemic, local, and regional methods.
Systemic Analgesics
Systemic analgesics provide relief to the entire body rather than a localized area as regional analgesia provides. Some parturients prefer to have less invasive methods of pain control and opt for systemic analgesics which are known to be less effective than regional anesthesia and can produce side effects that are discussed below.
There are several reasons parturients either choose not to or are unable to receive neuraxial analgesia. There still remain hospitals worldwide that do not have the resources including specialized staff and equipment to provide safe neuraxial analgesia to patients. Additionally, some women may have contraindications to neuraxial analgesia such as coagulopathy, complicated anatomy, and previous complicated back surgery. In 2011 the United Kingdom reported less than one third of parturients received a neuraxial analgesic technique during labor and vaginal delivery [18].
Systemic parenteral opioids are very commonly used due to the fact that they are easy to administer, have lower costs, and are readily available. They also negate the need for specialized equipment and specialized care providers. Systemic opioids are frequently administered during early labor but should be discontinued as the patient advances toward delivery due to potential effects of respiratory depression and level of consciousness in the infant and potential sedative effects on the mother that could prevent her from actively and effectively participating in the delivery process.
Parenteral opioids have many side effects including but not limited to respiratory depression, sedation, dizziness, nausea/vomiting, delayed gastric emptying, constipation, pruritus, urinary retention, and dysphoria.
Opioids can be administered via intravenous (IV) bolus, intramuscular (IM), subcutaneous (SQ), and patient-controlled analgesia (PCA) routes.
Following SQ or IM administration of opioids, the onset, quality, and duration are variable and dependent on absorption at the injection site and local and regional blood flow. Subcutaneous and IM administration can also be painful depending on the drug administered in contrast to intravenous administration which is generally not painful, is easy to administer, and has predictable effects. Patient-controlled administration of opioids is also an effective method of pain control during labor and delivery and provides rapid onset of analgesia and better control in contrast to side effects than parenteral opioid injection and also provides the patient with a sense of control [19].
A review by Smith [20] et al. demonstrated that parenteral opioid administration by either IM, IV, or PCA routes provided some pain relief and moderate satisfaction with analgesia, although up to two thirds of patients who received opioids reported moderate or severe pain and/or poor or moderate pain relief 1–2 hours after administration, and maternal side effects included maternal nausea, vomiting, and drowsiness. The sedation and somnolence may contribute to the relief without great strides in pain scores.
Opioids are highly lipophilic with a low molecular weight which leads to rapid crossing of the placenta. The consequences of these properties add risk of respiratory depression and neurobehavioral changes in the neonate. The effects are dose dependent and associated with the timing of last administration prior to birth. Opioids have been shown to decrease fetal heart rate variability, although this change usually does not reflect a worsening of fetal oxygenation or acid-base balance [21]. In utero, the fetus can be affected as well. Metabolism and elimination of these drugs is prolonged as well as a less developed blood-brain barrier which may lead to greater effects centrally.
Halpern et al. [22] reported in a multicenter randomized study comparing patient-controlled epidural anesthesia with local anesthetic and opioid vs systemic parenteral opioids and found there was an increased requirement for active neonatal resuscitation in the parenteral opioid group (52% vs 31%).
Labor analgesia reduces circulating catecholamines and therefore reduces the beta-adrenergic effects on the myometrium. It has been noted that once analgesia, including epidural, paravertebral, or systemic meperidine, is administered, labor patterns may go from dysfunctional to normal [23]. The rapid onset of decreased catecholamine levels with intrathecal opioids has led to a transient period of uterine hyperstimulation with decreased beta-adrenergic tocolysis which may lead to fetal stress and heart rate abnormalities [24, 25].
Opioid Analgesics
There are a number of opioid analgesics used to relieve pain during labor.
Meperidine
In 1947, meperidine (pethidine) became the first synthetic opioid used for intrapartum analgesia [26] and still remains the most commonly administered opioid for labor analgesia worldwide. Meperidine can be administered via an IV or IM route with intermittent bolus dosing. Dosages range from 25 to 50 mg IV and can be repeated every 4 hours, with onset within 5 minutes and duration of action of 2–3 hours. Meperidine can be given IM in doses between 50 and 100 mgs with a time to peak effect of 45 minutes. Meperidine is metabolized in the liver to normeperidine which is an active metabolite. Unfortunately, the effects of normeperidine cannot be reversed by naloxone [27]. Both compounds rapidly cross the placenta, and neonatal effects are related to accumulation of normeperidine especially if the parturient received multiple doses. Normeperidine has a long half-life ranging from 14 to 21 hours and may affect newborn neuroadaptive scores and breastfeeding behaviors [28].
Meperidine should be administered within 1 hour or more than 4 hours of delivery as maximum fetal concentration occurs 2–3 hours after the drug is administered [29, 30].
Maternal side effects include nausea, vomiting, and sedation [31]. Other potential side effects include serotonergic crises, seizures, and normeperidine neurotoxicity and possible drug interactions with MAOIs [32]. Meperidine has also been associated with temporary decreased fetal heart rate variability [33, 34].
The analgesic effect of meperidine is variable with some reports stating less than 20% of laboring women receiving satisfactory pain control. Elbohoty et al. [35] compared meperidine 50 mg IV with acetaminophen 1000 mgs IV and found the analgesic effects comparable and the meperidine group having 64% incidence of side effects compared with none for the acetaminophen group.
Morphine
Morphine has been administered since the late 1800s for analgesia and was used in the past in combination with scopolamine for “twilight sleep.” Morphine has fallen out of common use due to excessive maternal sedation and neonatal respiratory depression [36]. Morphine 2–5 mgs IV is the standard dose administered and can be given every 4 hours with a peak onset in 3–5 minutes and duration of action of 3–4 hours. Morphine can also be administered IM with dosing of 0.1–0.2 mg/kg [36]. The peak effect occurs in 10–30 minutes with duration of action of 3–4 hours.
Morphine is primarily metabolized in the liver with up to 70% being transformed into the largely inactive metabolite morphine-3-glucuronide and the other 30% to an active metabolite morphine-6-glucuronide which is 13 times more potent than morphine [36]. Morphine crosses the placenta and has been detected in the fetal circulation within 5 minutes of administration.
Maternal side effects include sedation, respiratory depression, nausea and vomiting, dysphoria, and histamine release with possible rash. Respiratory depression is the biggest concern for the neonate. Olofsson et al. [37] compared IV morphine (up to 1.5 mg/kg) with meperidine (up to 1.5 mg/kg) and found both groups to have high pain scores and high levels of maternal sedation.
Fentanyl
Fentanyl is a highly lipophilic and protein-bound short-acting synthetic opioid which crosses the placenta and is commonly used for labor analgesia. Fentanyl is a potent opioid that is 100 times more potent than morphine and 800 times more potent than meperidine [36]. Fentanyl has properties that make this drug attractive for use during labor including peak onset after administration of 2–4 minutes with a duration of action of 3–60 minutes and no active metabolites [36]. After maternal administration in sheep, fetal levels can be detected within 1 minute of administration with peak levels at 5 minutes post-maternal administration [38]. Standard dosing for fentanyl is 50–100 mcg loading dose and PCA dosing of 10–25 mcg every 5–10 minutes [39]. Fentanyl can also be given in intermittent IV boluses. Fentanyl is metabolized in the liver by CYP34A to inactive metabolites hydroxyfentanyl, norfentanyl, and despropionyl fentanyl [40].
Side effects of fentanyl include respiratory depression, sedation, nausea, and vomiting. Fentanyls can cause neonatal depression as reported by Morley-Forster et al. who found a 44% incidence of 1-minute APGAR <6 in 32 parturients who received fentanyl during labor [41].
Remifentanil
Remifentanil is an ultra-short-acting synthetic potent opioid that is a mu-receptor agonist which is 2 times more potent than fentanyl and 100–200 times more potent than morphine and is used for sedation or general anesthesia during procedures requiring anesthesia and analgesia. Some key features of remifentanil that make it an excellent choice for analgesia during labor include its quick onset and offset due to its short half-life. Unlike other opioids that are hepatically metabolized, remifentanil undergoes metabolism by nonspecific plasma esterases. Remifentanil is administered by PCA and may have a basal infusion along with the PCA function. The recommended dosing is PCA bolus of 15–50 mcg IV. An infusion of remifentanil will have an onset of action in 1 minute and rapidly achieves steady-state levels in the plasma. Remifentanil’s effects resolve within 3–10 minutes after discontinuation. The context-sensitive half-time for remifentanil is 3–4 minutes and is not dependent on the length of the infusion [42,43,44]. There must be careful attention paid to the time interval between dosing. Lockout intervals of 1–5 minutes has been suggested [45]. An interval of 3 minutes will avoid additional doses before the peak analgesic effect has occurred as well as enough time for the side effects has occurred [46]. The primary metabolite is remifentanil acid which has minimal pharmacologic activity. Remifentanil has been shown to rapidly cross the placenta with rapid fetal metabolism and/or redistribution [47]. The initial analgesic affect decreases as labor progresses. Abrupt discontinuation of a long-term infusion of remifentanil can cause withdrawal-like symptoms.
Remifentanil is a Category C drug for use in pregnancy. It is unknown if Category C agents cause fetal harm when administered to parturients. The manufacturer states “the safety of remifentanil during labor has not been demonstrated” and “the drug should be given to a pregnant woman only if clearly needed and the benefit justifies the potential risk to the fetus” [48].
Remifentanil is an effective analgesic and has fewer opioid-related side effects on the neonate compared with other opioids for labor but is inferior to neuraxial analgesia. Comparison studies have been performed with remifentanil and nitrous oxide with results revealing remifentanil provides more effective analgesia than nitrous oxide when evaluating pain scores and patient preference for nitrous oxide vs remifentanil [49].
In order for remifentanil to be effective in labor, the patient would have to predict the onset of a contraction in order to administer the bolus in time to be effective with the peak of the contraction. This would be ideal for patients that have begun a regular contraction pattern and could anticipate when to administer the medication. The standard contraction time is between 60 and 80 seconds.
Remifentanil is known to be a potent respiratory depressant and has been associated with four case reports of respiratory and/or cardiac arrest [46]. Patients may experience hypoventilation, desaturation, and apnea. Van de Velde and Carvalho performed a literature search which included 36 original studies and concluded that remifentanil patient-controlled intravenous anesthesia should not be routinely administered during labor due to the safety concerns [50]. These patients require one-to-one nursing and require continuous monitoring of respiratory rate and oxygenation [50,51,52].
Additional side effects of remifentanil include bradycardia, hypotension, nausea, and skeletal muscle rigidity. Skeletal muscle rigidity occurs more frequently when remifentanil is administered in bolus form.
The rapid elimination of remifentanil also reduces the risk of neonatal respiratory depression compared with other long-acting opioids [53]. Hill et al. stated maternal administration of remifentanil PCA during labor appears to have minimal effect on fetal heart rate abnormalities, umbilical cord blood gas measurements, and APGAR scores [45].
Nalbuphine
Nalbuphine is a synthetic mixed opioid agonist-antagonist with agonist binding at kappa, mu, and delta receptors. It is primarily a kappa-agonist providing analgesia and a partial agonist of the mu receptor. A partial agonist at the mu receptor correlates to less respiratory depression than a full agonist drug profile. Nalbuphine has a ceiling effect on respiratory depression due to its mixed receptor activity in doses up to 0.5 mg/kg [54]. Additionally, nalbuphine can cause maternal sedation but is associated with less maternal nausea and vomiting, drowsiness, and dizziness [55]. Fetal heart rate variability may be decreased, and pseudosinusoidal fetal heart rate patterns have been reported [55, 56]. If respiratory depression does occur, the effects can be reversed with naloxone [56].
Nalbuphine has equal analgesic potency to morphine in equivalent doses. Nalbuphine can be administered via the IV, IM, or SQ route with dosing of 10–20 mg every 4–6 hours. The onset is 2–3 minutes through IV administration and usually within 15 minutes of IM or SQ administration. Metabolism occurs in the liver and produces inactive elements.
Butorphanol
Butorphanol is a synthetic opioid agonist-antagonist with agonist activity at the kappa opioid receptor and an antagonist at the mu opioid receptor [57]. The standard dosing is 1–2 mg IV or IM with a peak onset of 2–3 minutes with IV administration and 10–20 minutes with IM administration and a duration of action of 4–6 hours with both routes of administration [58]. This drug is metabolized in the liver to hydroxybutorphanol, an inactive metabolite [59]. Butorphanol is five times as potent as morphine and 40 times more potent than meperidine [60].
Butorphanol has a ceiling effect on both respiratory depression and analgesic effect; therefore, increased doses do not provide additional analgesic effect or increase respiratory depression but will increase the side effects of the medications.
Non-opioid Analgesics
Non-opioid analgesics are not as effective as IV opioids but do provide some relief in labor.
Acetaminophen is an alternative analgesic for labor as it has minimal side effects on the mother and neonate. It’s analgesic activity is exerted by inhibiting the synthesis of prostaglandins in the central nervous system and has peripheral effects by blocking pain control generation [61, 62]. Acetaminophen also has a serotonergic mechanism and cannabinoid agonist mechanism in providing pain relief [63]. Acetaminophen is also a powerful antipyretic.
Zutshi et al. studied IV acetaminophen 1000 mgs vs normal saline infusion and noted VAS scores at 15 minutes, 1 hour, 2 hours, 3 hours, and 4 hours after drug administration. The reduction VAS scores were significantly higher in the acetaminophen group at all time points excluding the initial 15-minute score. There were no adverse neonatal or maternal effects [64].
Elbohoty et al. performed a randomized prospective study analyzing IV paracetamol (acetaminophen) vs IV pethidine (meperidine) as an analgesic in the first stage of labor. The VAS scores were lower at 15 min, 1 hour, and 2 hours after treatment in both groups and no reduction after 3 hours. The reduction in pain was significantly greater in the pethidine group only at the 15-minute score [65]. Acetaminophen had fewer adverse maternal effects.
Other non-opioid analgesics include promethazine (phenothiazine) and hydroxyzine (antihistamine). The medications do provide some relief during labor when used alone but are most commonly administered in combination with an opioid. Promethazine may be administered IV or IM, while hydroxyzine is usually administered IM, and both help prevent nausea and vomiting associated with opioids [66].
Ketamine was commonly used when it was first released for labor analgesia but feel out of favor due to its side effects. Ketamine is a phencyclidine derivative medication that is a noncompetitive antagonist at the NMDA receptor and at high doses is a mu receptor agonist [54]. It’s a dissociative anesthetic providing pain relief, sedation, and memory loss and can be administered by IM or IV routes. Ketamine has a rapid onset of action of less than 1 minute of administration and has a duration of action of 5–10 minutes when given IV and when given IM has an onset of 2–8 minutes with a duration of 10–20 minutes [54]. Ketamine is known to maintain airway reflexes and respiratory effort but also increases oral secretions. Ketamine’s sympathomimetic effects may cause an increase in heart rate, systolic blood pressure, and cardiac output and should be used cautiously in parturients with preeclampsia or hypertension. Ketamine is frequently administered after benzodiazepine dosing to prevent its psychological effects which may include agitation, confusion, or hallucinations. Providers must consider the potential of the mother not remembering the birth due to its amnestic effects. Jagatia et al. utilized a low-dose ketamine infusion for labor in 100 parturients and found low-dose ketamine infusion is safe without significant maternal or fetal effects, reduces maternal pain and exhaustion, and does not prolong duration of labor or have increased rate of instrumented delivery or cesarean section [67].
Benzodiazepines including midazolam and diazepam are anxiolytics and have been used for sedation during labor. Benzodiazepines are potent amnesics and may decrease the mother’s memory of the birth. Additionally, they blunt airway reflexes and may place the parturient at risk for aspiration. Diazepam crosses the placenta and accumulates in the fetus and has an elimination half-life of 28–48 hours. The active metabolites may be present for up to 120 hours. Diazepam may cause maternal respiratory depression and neonatal respiratory depression/hypotonicity.
Midazolam has an elimination half-life of 1–4 hours [54] and readily crosses the placenta. Table 4.2 summarizes medications used as co-analgesics.
Nitrous Oxide
Nitrous oxide (NO) has been used in Great Britain, Scandinavia, Australia, New Zealand, Canada, and other countries for several decades, while nitrous oxide is becoming more commonly used in the United States [68]. The Food and Drug Administration has approved delivery room administration equipment. The equipment is required to have a scavenging system to decrease the exposure of healthcare personnel and other individuals in the labor room.
Nitrous oxide delivery systems are self-administered by the parturient using a handheld mask that covers the nose and mouth or a mouthpiece with a mixture of 50% oxygen and 50% nitrous oxide. The patient must be sufficiently awake to take a forceful enough breath to open the demand valve which closes with exhalation. This should prevent the patient from inhaling too much as the drowsiness effect will prevent the patient from inhaling. Patients receiving NO should have continuous pulse oximetry, and consideration should be given to patients with baseline oxygen saturations <95% or in patients with respiratory issues. Special risk should be taken in patients who are also receiving opioids also to decrease the risk of respiratory depression. Nitrous oxide is eliminated through the lungs via exhalation and has no effect on uterine contractions and has not been found to accumulate in the mother/fetus/neonate or cause newborn depression [69]. It is common for NO administration to be delivered by obstetric nurses and certified registered nurse-midwives. Special training and maintenance training need to be in place with protocols for nitrous administration. Bobb et al. reported in 2016 that the rate of neuraxial placement for labor has not changed since nitrous oxide has become more common [70].
The effects of NO on the neonatal brain are not known nor are the effects of low-dose environmental exposure in hospital personnel [71]. Animal studies reveal prolonged exposure to NO inhibits methionine synthetase activity and that exposure to anesthetics and sedatives causes neurodegenerative changes in developing animals [72].
The key to effective use of nitrous oxide depends upon pre-contraction inhalation in preparation for the contraction as the analgesic takes approximately 50 seconds to take effect; therefore if the patient waits to inhale with the contraction, the effect may occur after the contraction which in general last 1 minute. In order to obtain the maximum effect of nitrous oxide, inhalation should begin 30 seconds before the contraction begins and continue until the contraction begins to recede.
Side effects of NO include nausea (5–40%) and vomiting (15%) [73].
Currently there is a paucity of quality studies to report the efficacy of NO [71, 74,75,76]. A systematic review by Likis et al. reported that NO relieves labor pain to a significant degree in most patients but does not provide complete analgesia with some patients having no response at all [73]. Richardson et al. evaluated in 6242 parturients the relationship between analgesic effectiveness and patient satisfaction with analgesia in women who delivered vaginally using NO, neuraxial analgesia with either epidural or combines spinal – epidural, or neuraxial analgesia after a trial of NO [77]. They concluded that patients who received NO alone were as likely to report satisfaction with anesthesia care as those who received neuraxial analgesia, even though they were less likely to report excellent analgesia.
Other Modalities for Pain Control
Two alternative methods of pain control for labor and delivery include local anesthesia blocks including pudendal and paracervical nerve block. These nerve blocks are administered in obstetrics for pain control in patients who request minimal pain control during delivery, patients who decline regional anesthesia, patients with a contraindication to regional anesthesia, or patients with regional anesthesia that is not providing adequate pain relief for labor and delivery. These blocks are most commonly performed by OB/GYNs and were commonly used prior to the initiation of epidural anesthesia for labor and delivery. Both types of blocks can additionally be used for gynecologic procedures and can be performed by single-shot injection or multiple injection methods with local anesthesia.
Pudendal Block
The pudendal nerve includes somatic nerve fibers from the anterior primary divisions of the second, third, and fourth sacral nerves. These nerves represent sensation innervation of the lower vagina, vulva, and perineum as well as motor innervation to the perineal muscles and urethral and external anal sphincter [78]. The nerves blocked by a pudendal do not provide pain relief during uterine contractions or cervical dilation. Indications for pudendal block include pain from introital distension during the second stage of labor, operative vaginal delivery (forceps/vacuum), or perineal repair for complex laceration repairs [79]. This block is ineffective for pain relief associated with manual exploration of the uterine cavity (manual extraction of the placenta) or mid-forceps-assisted births and upper vaginal repairs and may be incomplete for cervical repair and forceps rotation [80].
Pudendal blocks may inhibit the bearing-down reflex; therefore this nerve block is most commonly placed immediately prior to the birth of the infant to avoid increasing the second stage of labor [80]. If the duration of the block is inadequate, the block can be repeated keeping in mind the total safe dose of local anesthetic that the patient can receive.
Pudendal blocks can also be used for gynecologic procedures that require cervical dilation and manipulation, pregnancy termination, hysteroscopy, and cervical ablation or excision [81]. McCulloch et al. reported on successful McDonald cerclage placement under pudendal nerve block [82].
The success of the block is largely associated with the experience, skills, and knowledge of the proper site of placement of the individual performing the block. Block inadequacy or failure may be due to decreased opportunities for education of the correct procedure for pudendal block secondary to the increased use of regional anesthesia for delivery. Case studies reveal a block ineffectiveness rate of 10–50% on one or both sides [83]. Pudendal nerve block can be given via a transperineal approach but most commonly is delivered via a transvaginal approach especially in the United States [84]. Scudamore et al. reported bilateral success rates of 50% with transvaginal approach vs 25% after transperineal approach.
The pudendal nerve crosses posterior to the sacrospinous ligament in proximity to where the ligament attaches to the ischial spine [85]. Figure 4.2 shows the transvaginal technique.
The block is most commonly administered bilaterally but can be placed unilaterally if only one-sided coverage is needed. Pudendal nerve kits are available which include a disposable plastic needle guide and needle. If kits are not available, a 20-gauge 15 cm spinal needle with a non-disposable Iowa trumpet (to prevent damage to the vaginal and fetus) can be used. Total local anesthetic doses on each side range from 7 to 10 mL. The local anesthetic most commonly utilized is lidocaine 1% with a maximum recommended dose of 300 mgs. Mepivacaine 1%, bupivacaine 0.25%, and 2-chloroprocaine 2% can also be used. Chloroprocaine can be used for faster onset but has a short duration of action and may be repeated if the second stage of labor is prolonged (15–30 minutes) [84]. With uncomplicated block placement, relief occurs within 5 minutes and with maximum time set up between 10 and 20 minutes [86].
A pudendal block with lidocaine has an average duration of action of 30–60 minutes [87].
Merkow et al. [88] studied the use of 30 mL of 0.5% bupivacaine, 1% mepivacaine, or 3% 2-chloroprocaine for pudendal block and perineal infiltration and had no significant effects on newborn neurobehavioral indices at 4 and 24 hours with the exception of a better response to pinprick at 4 hours with the mepivacaine. Studies have been performed to evaluate the advantages and disadvantages of adding epinephrine to the local anesthetic solution for pudendal blocks. Langhoff et al. [89] performed a double-blind randomized study of 865 who received pudendal block with either 16 mL of 1% mepivacaine, 1% mepivacaine with epinephrine, or 0.25% bupivacaine. The patients who received mepivacaine with epinephrine had adequate analgesia more often and additionally had a greater “loss of the urge to bear down” than the other anesthetics. There was no significant difference in duration of the second stage of labor and incidence of instrumented vaginal delivery.
Aissaoui et al. [90] looked into using a nerve stimulator during pudendal nerve block and reported that administering a unilateral pudendal nerve block following episiotomy repair significantly decreased the need for additional analgesic agents during the first 48 hours postpartum [91].
Potential Complications of Pudendal Nerve Block
Pudendal nerve blocks are safe with a quick onset of pain relief but also pose risks for complications which include laceration of the vaginal mucosa; vaginal, ischiorectal, or retroperitoneal hematomas; retro-psoas space or subgluteal abscess; nerve damage; local anesthetic toxicity; intravascular injection with systemic toxicity; temporary paresthesia in the ischial region; and sacral neuropathy. Abscesses of the retro-psoas or gluteal region can cause significant morbidity and mortality. Hematomas usually resolve on their own unless the patient is on blood thinning medications or has coagulopathy or other bleeding issues. Although there are known complications as above, the risk associated with this procedure is low.
Neonatal and fetal complications are rare but may occur. There can be direct fetal trauma with inadvertent puncture of the infant’s scalp or other body regions [92]. A case report by Pages et al. described three inadvertent scalp injections which resulted in lidocaine toxicity with complete recovery. Bozynski et al. published a case report of lidocaine toxicity; after them other received a pudendal block with symptoms of postnatal apnea, bradycardia, and a prolong QT interval in a term infant [93].
Contraindications to pudendal block include allergy to local anesthetic, known coagulopathy, and vaginal infection.
Paracervical Nerve Block
Pain associated with the first stage of labor is due to cervical dilation and lower uterine segment distention and distention of the upper vagina. Pain impulses are transmitted from the upper vagina, cervix, and lower uterine segment by viscera afferent nerve fibers that join the sympathetic chain (define) at L2 to L3 and enter the spinal cord at T10 to L1 [94] and sacral nerve roots (s1–4). This technique blocks transmission through the uterovaginal plexus (Frankenhauser’s plexus) [95].
Paracervical block does not block the pain caused by the late first stage or second stage of labor. Per Chestnut, contemporary experience suggests that paracervical block results in satisfactory analgesia during the first stage of labor in 50–70% of parturients. Paracervical block (PCB) is not commonly performed in the United States due to fetal complications and neuraxial anesthesia but remains popular in other countries. This block has the advantage of not affecting the time course of labor.
Paracervical nerve block can be administered for gynecologic procedures that involve uterine intervention and cervical dilation. Two percent lidocaine, 1.5% mepivacaine, and 0.2–0.5% ropivacaine are most commonly used for local anesthesia. It is common to add either vasopressin or epinephrine to reduce intra- and postoperative blood loss [95, 96]. Additionally, other benefits of adding a vasoconstrictor include inhibition of drug redistribution/elimination from the injection site, increased block potency and longer duration of action, and reduced systemic toxicity [95]. The effects were more pronounced when vasoconstrictors lidocaine and mepivacaine were used. Epinephrine can lead to cardio-stimulatory effects as well as tachyarrhythmias which may be detrimental to certain groups of patients.
Paracervical blocks for obstetrical analgesia commonly utilize buffered local anesthesia with sodium bicarbonate and don’t contain epinephrine. Commonly used local anesthetics are 2% 2- chloroprocaine and 1% lidocaine. Bupivacaine is not commonly utilized secondary to the cardiac toxicity effects in adult patients [97]. A double-blind study performed by Weiss et al. [98] compared paracervical block in 60 patients that were randomly assigned to 20 mL of 2% 2-chloroprocaine or 20 mL of 1% lidocaine. In the 2% 2-chloroprocaine group, 1 fetus out of 29 fetuses experienced fetal bradycardia compared with 5 of 31 fetuses in the 1% lidocaine group. The results were not statistically significant with a P value of 0.14.
Paracervical blocks are usually administered during active labor in the first stage during cervical dilation between 4 and 8 cm and can be repeated at regular intervals. Once the parturient reaches 8 cm, the procedure is less desirable as the procedure becomes more technically difficulty, is less effective, and has a higher risk of causing fetal bradycardia [99].
Paracervical blocks may be performed with a PCB block kit which includes a needle guide (i.e., Iowa trumpet) to prevent injury in the vagina, a plastic needle spacer, and a needle for injection. If kits are unavailable, a 22-gauge 15 cm spinal needle with a metal Iowa trumpet as a needle guide may be used.
Vidaeff et al. [100] recommend injection of local anesthetic at the 4 and 8 o’clock position as they are less vascular than the recommended 3, 5, 7, and 9 o’clock positions. Figure 4.3 shows the locations for injections. Vidaeff recommends the two point approach to decrease the number of painful injections with similar analgesia to the patient. It is recommended that the needle insertion depth is no more than 3 mm to avoid risks especially fetal bradycardia [101]. A total of 20 ml of local anesthetic is administered for the nerve block. After completion of the injections, the local anesthetic spreads rapidly to the broad ligament. The onset of pain relief is rapid within 2–5 minutes. The duration of analgesia is based upon the pharmacokinetics of the local anesthetic chosen. The block can be repeated, but it is not recommended to repeat more than once an hour.
Cochrane reported on a study of 109 parturients who received opioids vs paracervical block. The paracervical block group was found to have more effective pain relief with additional studies reporting no increased rate of instrumented deliveries or cesarean section rate [102].
Fetal bradycardia usually occurs within 2–10 minutes of block placement with bradycardia usually resolving within 5–10 minutes but can persist up to 30 minutes [103]. The mechanism of fetal bradycardia is unclear. Palomki et al. in a prospective study reported 3.2% of 440 parturients who received a paracervical block developed fetal bradycardia lasting from 2 to 8 minutes [104] with similar rates from labor epidurals [105].
The mechanism of fetal bradycardia is unknown but there are several theories. The local anesthetic injected for PCB rapidly crosses the placenta and is in close proximity to the uterine circulation which may lead to myocardial depression, fetal central nervous system depression, or umbilical vasoconstriction [106]. The local anesthetic has been shown to cause uterine artery vasoconstriction and decreased uteroplacental blood flow [107]. Rogers et al. proposed manipulation of the fetal head, uterus, and uterine blood vessels may cause a reflex fetal bradycardia [108].
The effects have been attributed to the direct toxic effects of the local anesthetic on the fetal heart [109, 110]. Chestnut reports most investigators believe the fetal bradycardia from PCB is due to reduced uteroplacental and/or fetoplacental perfusion. A reduction in uteroplacental blood flow may occur due to increased uterine activity and/or direct vasoconstrictive effects of local anesthesia, while decreased umbilical cord flow may lead to increased uterine activity and/or umbilical cord vasoconstriction.
Levy et al. reported no association between PCB and low umbilical arterial blood pH at delivery [111].
Maternal complications are not common and include vasovagal syncope (cervical shock); laceration of the vaginal mucosa; systemic local anesthetic activity; hematoma; paracervical, retropsoal, or subgluteal abscess; and postpartum neuropathy.
Regional/neuraxial analgesia methods include spinal, epidural, and combined spinal-epidural and are the recommended form of treatment for intrapartum analgesia in the United States and Canada, largely replacing systemic drug administration [112, 113].
Neuraxial Anesthetics
Neuraxial anesthetics are a group of techniques used to create analgesia or anesthesia by targeting the central nervous system directly. These techniques include spinal, epidural, and combined spinal-epidural (CSE) interventions. Because anesthetic is deposited directly at or very near to the central site of action, total dose of local anesthetic and adjuncts are minimized while creating anesthesia in a wide area of the body. Often a primary concern of patients and physicians alike is avoiding treatment that involves sedating medications which can impair maternal participation in the birth process as well as cross the placenta and have postpartum effects on the newborn. The transition from visceral pain during the first stage of labor to somatic pain during the second stage of labor can be difficult to endure as a patient and to manage as a physician. Neuraxial techniques again are ideal for this type of transition because they provide analgesia effective against both types of pain. The labor process can endure for many hours, and epidural catheters can be used reliably for the entire duration of this process to provide continuous, non-sedating analgesia. Pumps are available that can be programmed to allow safe and secure patient-controlled epidural analgesia (PCEA), which give the patient autonomy in the treatment as well as unburdening healthcare providers.
An enduring concern of parturients is the fear that the risk of a cesarean section is increased when an epidural is used for labor pain control. Patients should be reassured that modern randomized controlled trials show that epidurals do not increase the risk of cesarean section. In studies after 2005, the rate of instrumented delivery is no different with epidural analgesia either [114, 115]. Some studies did, and some studies did not find increased rates of use of oxytocin, and the second stage of labor is increased by 15–30 minutes with epidural analgesia versus none [116]. Studies have also shown that timing of placement of the epidural does not impact rate of cesarean section [117].
A major advantage of epidural placement is the ability to transition quickly from labor analgesia to surgical anesthesia should the need for urgent or emergent cesarean section arise. Especially in situations where this transition is more likely, such as during a trial of labor after cesarean, early placement of epidural is highly beneficial in avoiding the risks of general anesthesia for cesarean. Parturients with high-risk comorbidities such as cardiovascular or pulmonary compromise also may benefit from early placement and careful titration of epidural analgesia.
Neuraxial techniques are not risk-free though. Short-term local discomfort is common, but there is no association with neuraxial anesthetics and long-term back pain [115]. Hypotension from sympathetic blockade is common and frequently requires treatment with low doses of vasopressors [117]. Accidental dural puncture can happen, which in some patients leads to the development of a postural headache, called post-dural puncture headache (PDPH), with varied migraine-like symptoms and a variable presentation. The more frightening complications of neurologic injury, epidural abscess, and epidural hematoma are exceedingly rare. The most common of these three is neurologic injury, occurring at a rate of 1 in 6700 (0.015%). Epidural abscess is even rarer at a rate of 1 in 145,000 (0.0007%). Epidural hematoma is the rarest at a rate of 1 in 240,000 (0.0004%) [117]. Airway management supplies should be readily available to manage unintentional intrathecal injection of local anesthetic intended for epidural dosing. Also, unintentional intravenous injection of local anesthetic can lead to total cardiovascular collapse. Lipid emulsion rescue therapy and functioning ACLS resuscitation equipment and medications should be available on site in any location where epidural anesthetics are administered [117].
Few true contraindications to neuraxial anesthesia exist. They include, but are not limited to, patient refusal, clinically significant coagulopathy, use of thrombolytics, hypovolemia, elevated ICP that could result in herniation with dural puncture, and localized infection to intended site of needle entry [117]. The decision to acquire a platelet count prior to placement should be an individualized decision based on patient history and exam but should not be required prior to epidural placement in healthy parturients [118]. The safety of placement with a platelet count greater than 100,000 per microliter is well established. Recent large retrospective reviews suggest that in the absence of abnormal bleeding or bruising or other signs of a hypocoagulable state, it is likely safe to proceed with epidural or spinal placement with platelet counts as low as 70,000 per microliter [119].
Over the past 30 years, hospitals are increasingly offering 24-hour availability of anesthesia services as well as increasingly utilize combined spinal-epidural technique and patient-controlled epidural analgesia [120]. US birth certificate surveillance has shown that from 2009 to 2015, at least 61% of all mothers utilized some form of neuraxial analgesia or anesthesia for their delivery [121]. In stratum 2 and 3 hospitals where in-house anesthesia services may not be continuously available, ACOG recommends that labor and delivery nursing staff should be trained to manage infusions in already established epidural catheters [122].
Epidural
The epidural space is the space immediately surrounding the spinal dura, the tissue that encases the spinal cord, spinal roots, and cerebrospinal fluid. The epidural space contains blood vessels and fat and is progressively wider moving from cephalad to caudad. The posterior aspect of the epidural space is bounded by the ligamentum flavum. Entering the epidural space can be achieved by passing a needle between vertebral spinous processes through the interspinous ligament and then through the ligamentum flavum. As the clinician advances the needle, injection of either air or saline is tested periodically as the needle is advanced. When the needle tip is located within ligamentous structures, injection is very difficult. Upon entry of the needle tip into the epidural space, a sudden loss of resistance against the injection is found, and the air or saline is easily injected into the epidural space. Once loss is achieved, a catheter is passed through the needle into the epidural space, and the needle is withdrawn over the catheter, and the catheter is then secured in place with an occlusive dressing. An epidural must also be tested for inadvertent vascular or intrathecal entry. Once the catheter is believed to be in the epidural space, it must first be tested. This is done by two methods: aspiration and medication testing. If blood or clear fluid is freely aspirated from the catheter, concern for vascular or intrathecal entry should be raised, and catheter removal should be considered. If aspiration is negative, then a test dose of a volume of 3 mL of 1.5–2% lidocaine with 5 μg of epinephrine per mL is injected. If the catheter is intrathecal, the 3 mL of lidocaine will rapidly induce spinal anesthesia creating sensory and motor block of the lower extremities and abdomen. If the catheter has entered a vessel, the 15 μg of epinephrine will rapidly cause an increased heart rate, an increased blood pressure, and typically a sense of anxiety. If either intravascular or intrathecal placement is confirmed by the test dose, the catheter should be withdrawn, and replacement can be attempted. One option with intrathecal placement is to leave the catheter in place and to alter the planned epidural infusion rate and disallow patient-controlled features of the infusion [123].
Two modalities of administering local anesthetic are typically used, continuous infusion and programmed intermittent bolus (PIB) that can be run either with or without the addition of patient-controlled demand doses. PIB has been shown to provide equivalent analgesia while consuming a lower total volume of local anesthetic. It is theorized that greater spread through the epidural space is achieved with PIB administration because the bolus injection is delivered at a higher pressure resulting in a higher ejection velocity of the medication and thus spreading further in the epidural space [124].
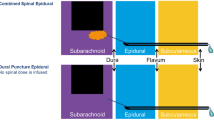
Spinal
Spinal anesthesia , also known as a subarachnoid block, is a viable option that provides both a deep sensory and motor blockade. It is performed by first passing a short introducer needle into the posterior midline space usually between the L2–3, L3–4, and L4–5 interspace. A pencil-point spinal needle is then passed through the introducer and advanced periodically removing the introducer to check for the presence of clear fluid. Once the needle passes through the dura and enters the subarachnoid space, CSF will begin to drain from the needle when the introducer is removed. At this point, the syringe containing the local anesthetic should be attached to the spinal needle after careful removal of all air from the syringe. Aspiration is first performed which serves to verify that after syringe attachment, the needle tip remained within the subarachnoid space. Aspiration is usually easy to note within the syringe as a “swirl” appearance of CSF mixing with local anesthetic. The local is then injected, and the needle and introducer are removed. Patients often experience a fleeting paresthesia when the needle first passes through the dura. However, should that paresthesia persist, the needle should be retracted and injection not performed.
Choice of local depends on desired duration of effect and desired positioning of the patient during the procedure. Hyperbaric 0.75% bupivacaine is the most commonly used medication for cesarean sections. Hyperbaric solutions are denser than CSF and will sink within the CSF when injected. Full coverage of all lumbar, sacral, and lower thoracic levels is desired for cesarean section. Total dose ranges from 7 to 15 mg depending on the addition of opiates and desired longevity of block. Injection should be performed slowly to prevent rapid upward spread within the CSF.
Combined Spinal-Epidural
To perform a combined spinal-epidural , the entire process of placing an epidural is performed up to the point of the loss of resistance to injection. At this point, instead of threading the epidural catheter through the needle, first a pencil-point spinal needle is passed through epidural needle and into the subarachnoid space and opiates; local anesthetic or a combination thereof is injected. The spinal needle is withdrawn and the epidural catheter is placed and secured. Typically the test dose is delayed until just prior to the anticipated use of the epidural catheter, if it will not immediately be connected to an infusion.
Surgical Delivery
For surgical deliveries, it has long been the standard of care to provide surgical anesthesia via either an epidural or spinal anesthetic while maintaining the patient awake. While this enables the mother to be awake and involved during the delivery, it also avoids complications associated with general anesthesia. For this reason, the American Society of Anesthesiologists (ASA), the Society for Obstetric Anesthesia and Perinatology (SOAP) [118], and the American College of Obstetricians and Gynecologists (ACOG) [125] recommend routine use of neuraxial regional techniques for cesarean sections. Workforce survey studies should that regional techniques are used in over 95% of all surgical deliveries in the United States and nearly all elective deliveries unless a contraindication exists [126, 127].
Anesthetic Technique
There are numerous benefits to using a neuraxial technique routinely for cesarean deliveries including avoiding maternal airway management risks, improved neonatal well-being [128], maternal recall of and participation in the birth, and the ability for involvement of family members. In addition to these benefits, the use of spinal, epidural, or combined spinal-epidural procedures also allows for neuraxial administration of opiates. There have been numerous studies comparing spinal anesthesia versus epidural anesthesia. A systematic review [129] of these was performed which included ten such studies and measured outcomes which included adequacy of anesthesia, additional analgesic intervention, patient satisfaction, time from block to starting surgery, hypotension treatment, neonate outcomes, and treatment of side effects. The only advantage spinal offered over epidural was speed, showing an almost 8-minute benefit. However, there was also significantly more hypotension requiring treatment with spinal anesthetics. The ASA practice guidelines for obstetric anesthesia largely agree with these findings, although they cite that epidural vs spinal techniques have equivocal outcomes in all findings including umbilical pH, APGAR scores, and total operating room [118].
A combined spinal-epidural technique has become more commonplace over the last 20 years [127] and offers several benefits to either procedure performed in isolation. Procedurally this can be done by either a needle-through-needle technique or performing the spinal anesthetic and then separately performing the epidural anesthetic. There are fewer studies available comparing CSE to epidural or spinal anesthetics for cesarean section, but those that are available show block failure rates with CSE as low as 0.6% compared to 2–4% failure with spinal or epidural techniques alone [130]. Paradoxically, it was shown that compared to epidural anesthetics, the CSE anesthetics required more frequent additional local via the epidural. This “top-off” rate could be due to a bias toward smaller spinal local anesthetic doses or even due to the fact that the epidural is available, so it is used instead of alternative means such as IV analgesics that could cross the placenta and affect the newborn.
Neonatal outcomes appear to be equivocal when comparing CSE to spinal and epidural techniques as well [131]. The ASA practice guidelines agree with these findings that CSE was equivocal to epidural or spinal anesthetics alone [118]. The most robust comparison and assessment available of the three techniques is the 2009 Audit Project of the Royal College of Anesthetists [132] which surveyed over 700,000 neuraxial blocks. There were no obstetric-specific outcomes such as fetal acid-base status or APGAR scores assessed although it did stratify the assessment based on obstetric versus perioperative use. In obstetric patients, the incidence of permanent harm with CSE was 0–3.9 per 100,000 compared to 0–1.5 per 100,000 with spinal anesthetics and 0.6 per 100,000 with epidural anesthetics.
Neuraxial Opiates
Regardless of neuraxial technique, one of the largest benefits of neuraxial access to pain management of the obstetric patient is the ability to add opiate to the anesthetic. In light of the rising overuse and abuse of opiates among the American population, the ASA, SOAP, and ACOG [118, 125, 133] all have issued practice advisories which recommend inclusion of long-acting neuraxial opiates for women having cesarean sections. This is because after neuraxial administration of opiates, especially hydrophilic ones such as morphine, there is prolonged high-quality analgesia with minimal increased plasma concentrations or euphoric effects. Preservative-free morphine is the most commonly used and studied medication used for this purpose. Several systematic reviews [134, 135] comparing doses have been compiled and show a dose-dependent relationship between the dose of intrathecal morphine and the duration of analgesia it provides. Dosage can be divided into three main categories: ultra-low dose, low dose, and high dose. Ultra-low dose is classified as either ≤50 mcg intrathecal or ≤ 1 mg epidural. Low dose is defined as doses >50 and ≤ 150 mcg intrathecal or > 1 and ≤ 3 mg via epidural. High dose is defined as any dose greater than 150 mcg intrathecal or 3 mg epidural.
Table 4.3 summarizes intrathecal and epidural does ranges for morphine used for caesarian section.
The primary advantage of high-dose morphine is the duration of effect, as defined by duration until first request for postoperative analgesic, which was shown to last from 14 to 39 hours (median 27 hours) across various studies included in systematic reviews. Low-dose morphine on average lasted 4.5 hours less than high dose in these reviews. With either dosing strategy, the prolonged effect is due to morphine’s highly hydrophilic nature. Instead of rapidly crossing into neural tissue or undergoing vascular resorption, it largely remains within the cerebrospinal fluid (CSF) for the duration of its effect [136]. Onset of action is largely dependent on diffusion into the dorsal horn of the spinal cord, where it can act on G-protein-linked opiate receptors. Because this process is reliant on diffusion through neural tissue, the hydrophilic nature of morphine leads to a prolonged time to onset of action up to 2 hours compared to only a few minutes for lipophilic drugs such as fentanyl and sufentanil [137].
A secondary advantage of intrathecal opiates is an improved quality of the anesthesia attained from the local anesthetic. Although the analgesic onset of intrathecal morphine may take up to 2 hours, systematic review shows that the rate of no supplemental intraoperative analgesic increases from 76% without morphine to 96% with both high- and low-dose intrathecal morphine [138]. Statistically this translates to a direct benefit in one out of every five surgical cases (NNT 4.9) which comparatively makes it a highly effective anesthetic adjunct.
However, choice of dose must also be balanced against frequency and severity of side effects. The most feared complications of neuraxial opiates are sedation and respiratory depression . The American Society for Regional Anesthesia (ASRA) with the ASA laid forth updated recommendations in 2016 for routine monitoring for patients who receive neuraxial opiates [139]. These recommendations are not targeted at the obstetrical population, but rather at a broader general surgical population. As their first recommendation, ASRA strongly recommends performing a focused history and physical exam specifically looking for signs or symptoms of sleep apnea and high-risk co-existing disease such as diabetes or obesity and an assessment of current medication regimen for potential additive or synergistic combinations. Other high-risk patient factors also include chronic opiate use or abuse, concomitant use of other sedative or hypnotic medications, other respiratory or cardiac comorbidity, intraoperative respiratory events, or magnesium infusion for the treatment of preeclampsia.
Special attention should be paid to signs and symptoms of sleep apnea. As the American population becomes increasingly more obese, the prevalence of sleep apnea has risen too. In 2010, the prevalence of obstructive sleep apnea (OSA) was estimated to be 8.7% in American women between the ages of 30 and 49, of which 2.7% had severe OSA [140]. However, pregnancy independently increases the risk of OSA. Among all women, the rate of OSA rises to 8.4% during the first trimester and up to 19.7% in the third trimester [141]. In the general population, the STOPBANG screening tool has been validated to identify both patients who are at risk to have any degree of sleep apnea (score of ≥3) and at risk to have severe sleep apnea (score of ≥5) [142]. The STOPBANG questionnaire consists of eight questions which each contribute 1 point to the total score. The criteria are age >50, either treated or untreated hypertension, BMI of 35 or greater, neck circumference of 16 or greater in women or 17 or greater in men, male gender, loud snoring, daytime fatigue, and a third party having observed possible apnea episodes. However, it has been shown that the sensitivity and specificity of the STOPBANG tool is significantly reduced within the pregnant population. Within the pregnant population, the risk factors found to be most highly associated include pre-pregnancy BMI >30, 3rd trimester BMI >35, history of treated or untreated hypertension, and a history of falling asleep while talking to someone [141, 143]. If a patient is identified as at risk for sleep apnea, a sleep study should be performed and treatment initiated.
ASRA continues to further recommend that all patients who receive neuraxial opiate should be monitored for signs of respiratory depression [139]. With regard to hydrophilic opiates such as morphine, they state “(1) monitoring should be performed for a minimum of 24 h after administration and (2) monitoring should be performed at least once per hour for the first 12 h after administration, followed by monitoring at least once every 2 h for the next 12 h (i.e., from 12 to 24 h). The ASA members agree and the consultants strongly agree that after 24 h, the frequency of monitoring should be dictated by the patient’s overall clinical condition and concurrent medications.” For lipophilic medications such as fentanyl, these recommendations are reduced from a total of 24–2 hours of monitoring. With either type of drug, ASRA does recommend maintaining IV access for the duration of monitoring and having ready access to reversal agents and oxygen for immediate treatment should oversedation or respiratory depression develop.
In 2018 Sharawi et al. compiled an extensive systematic review looking for clinically significant respiratory depression after administration of morphine [144]. This review covered 75 studies including over 18,000 women who received neuraxial morphine or diamorphine for either scheduled or urgent cesarean section. Clinically significant respiratory depression (CSRD) was defined as any episode that required oxygen therapy for bradypnea ≤8 breaths per minute or a pulse oximetry oxygen saturation ≤90%, pharmacologic therapy with respiratory stimulants or opioid antagonists, sedation requiring anything beyond verbal stimulation to rouse the patient, or airway intervention including basic maneuvers, adjuncts, or any invasive or noninvasive ventilation. Among the 18,452 study patients, there were only 11 cases of definite morphine-related CSRD and 5 cases of probable morphine-related CSRD. All events happened within the first 16 hours after administration. Only 3 of these 16 cases occurred using low-dose morphine as defined earlier in this chapter as ≤150 μg intrathecal or ≤3 mg epidural. Thus, at all dose ranges, the rate of CSRD was 0.0867% which was further reduced to 0.0163% in the setting of low-dose morphine. The same study also examined ASA Closed Claims Project data looking for any claims involving respiratory events in obstetrical patients following neuraxially administered morphine and found no claims filed.
Given the paucity of respiratory events in the obstetrical population who receive low-dose intrathecal or epidural morphine, there is some debate as to whether obstetrical patients without high-risk features warrant prolonged and close monitoring for a vanishingly rare complication [144]. However, without further clarification or guidance from professional societies, it is difficult to recommend deviating from the guidelines established by ASRA and the ASA.
A standing concern that should be held among any population receiving neuraxial hydrophilic opiates is that order writing for further doses of opiate within the first 24 hours should be limited to a single team with an understanding of the pharmacokinetics and dynamics of both the neuraxial opiate and the additional drug. Systems and workflows should be established so that a nurse always knows to whom to direct requests for additional pain medication. In addition, an evaluation of the patient’s clinical status, including level of pain, opiate side effects, and expected continued effects of the medications already given must be completed prior to administration of additional opiate.
Fentanyl may also be via an epidural or intrathecal route. While morphine doses are approximately tenfold stronger from IV to epidural and again from epidural to intrathecal routes, fentanyl has a much less steep conversion ratio appearing to only increase in potency threefold from IV to epidural and from epidural to intrathecal. This is largely due to fentanyl’s lipophilic nature, which helps it cross membranes and be much more rapidly absorbed and metabolized. It has been shown to significantly reduce the required dose of bupivacaine to achieve an adequate anesthetic for cesarean section [145] from 12 mg bupivacaine without additive to as little as 8 mg bupivacaine when mixed with 10 μg of fentanyl. Reducing the total bupivacaine dose is associated with less motor block, less hypotension, less vasopressor usage, and less intraoperative nausea and vomiting [146].
Addition of fentanyl to bupivacaine also speeds onset of block [147]. A major drawback to fentanyl compared to morphine is its much shorter duration of action. Time to first administration of analgesic after bupivacaine alone is about 2 hours. With additional of fentanyl, median time to first analgesic increases to 4 hours compared to 27 hours with morphine [138].
When considering the faster onset of block with fentanyl, prolonged analgesic action of morphine, and ability of both to improve the quality of anesthesia during surgery, some physicians advocate for adding both opiates to the local anesthetic. Relatively few studies exist comparing the combination of local with morphine and fentanyl to local with a single opiate. The data that does exist is conflicting between studies, and the true risk/benefit ratio is not as clearly defined as it is for either drug in isolation compared to the mixture. Weigl et al. compared 60 patients in a double-blind randomized control trial comparing 100 μg of morphine to a mixture of 100 μg and 25 μg fentanyl. Fewer patients who received the mixture required additional intraoperative analgesia, had non-inferior 24-hour opioid consumption, but did have higher opiate consumption in the first 12 hours and saw more nausea and vomiting than morphine alone [148] In contrast, Thorton et al. found that the addition of 10 μg of fentanyl to 100 μg of morphine resulted in a trend toward faster onset of block with morphine alone achieving T6 block at 6.3 minutes compared to 5.05 minutes when combined with fentanyl. They found no significant contribution of the mixture over morphine without fentanyl but did find a statistically significant higher incidence of pruritus [149]. Finally, Carvalho et al. looked further at postoperative pain scores and opiate consumption and concluded that within the first 24 hours, postop pain scores were higher in women who received the combination than morphine alone. They postulated that fentanyl may “induce a subtle acute opioid tolerance of uncertain significance” [150]. Comparing the various data available, it appears mixing fentanyl with morphine results in an improved intraoperative block at the expense of slightly higher postoperative pain scores for the first 12 hours as well as higher rates of nuisance side effects, although more studies need to be done so meta-analysis can be performed on the topic.
In order to extend the benefits of high-quality analgesia from neuraxial morphine, liposomal morphine (extended-release epidural morphine, DepoDur) has been developed and studied for use in cesarean sections and abdominal surgery [151,152,153]. When delivered into the epidural space, the clinical effects can be appreciated upward of 48 hours. After initial FDA approval in 2004, the package insert has been twice revised. These revisions strengthened the warning on individualized dosing, clarified use only via the epidural route, and cautioned with timing the epidural administration to more than 30 minutes apart from any dose of epidural bupivacaine. When used for treatment of post-cesarean pain, the FDA approval is for administration only after the umbilical cord is clamped. Care should be taken to never confuse preservative-free morphine which is commonly used for intrathecal administration with this liposomal formulation. Case reporting of accidental intrathecal administration does exist and describes successful postoperative management using a low-dose titrated naloxone infusion which was discontinued when the patient first began reporting pain as well as scopolamine and ondansetron for side effect management [154]. In the ASA Task Force guidelines, the only guidance given is that monitoring should be extended to 48 hours instead of only 24 hours as with preservative-free morphine. Monitoring after 24 hours can be reduced from every 2 hours to every 4 hours [139].
In the patient who cannot receive morphine, an option for postoperative pain control is to provide a patient-controlled epidural analgesic with fentanyl. Doing so first requires the presence of an epidural. If the patient is scheduled for an elective cesarean section, this would then dictate that the surgical anesthetic be achieved either via epidural or combined spinal-epidural technique [155, 156]. Various dosing strategies exist although typically the maximum dose per hour is kept at or below 120 μg to be equivalent to a single dose of 3 mg of epidural morphine. This can be balanced between continuous infusion and patient demand doses. The duration of action of epidural fentanyl is short enough that using an all demand dosing strategy has the limitation of requiring the patient to not sleep for several consecutive hours. One distinct disadvantage of this technique is it does require the patient to keep the epidural in place postoperatively. Most women after an uneventful cesarean delivery are encouraged to mobilize early, which is impeded by the presence of an extra pump to mobilize with the patient and an epidural site to be maintained covered with occlusive dressing and kept clean and dry. Patient satisfaction may be impaired by the inability to shower following delivery until removal of the epidural. Should this technique be employed, the ASA again recommends monitoring for the entire duration of the infusion. This monitoring should, at a minimum, be continuous for the first 20 minutes, then at least hourly for the first 12 hours, every 2 hours until 24 has passed, and then every 4 hours until the infusion is stopped [139].
Neuraxial Opiate Side Effect
Aside from the previously discussed risk of sedation and respiratory depression, intrathecal opiates may induce a handful of other side effects. Most common are pruritus, nausea and vomiting, and urinary retention. Rare side effects of neuraxial opiates may include bradycardia, sweating, delayed gastric emptying, constipation, headache, hiccups, hypothermia, or nystagmus [137].
Nausea
Nausea and vomiting during cesarean section are common, happening in as high as 42% of operations. Causation is multifactorial including spinal-/epidural-induced hypotension, manipulation of abdominal and pelvic viscera, administration of opiates, administration of uterotonic medications, and increased vagal activity [157].
Postoperative nausea is much less common and happens much more frequently after intrathecal morphine compared to intrathecal fentanyl. Nearly 1 in every 6 of those who receive intrathecal morphine experience postoperative nausea and/or vomiting compared to only 1 in 22 who receive IT fentanyl [138]. Morphine is thought to directly stimulate the chemotactic zone after rising in the cerebrospinal fluid. Intrathecal morphine may also sensitize the vestibular system, delay gastric emptying, and reduce overall gastrointestinal motility [138, 158].
Because the causative factors are varied, one’s approach to treatment should focus on diagnosis of cause. Hypotension, either spinal anesthetic induced or from acute blood loss hypovolemia, should be ruled out first. Vagal overactivity often will also present with bradycardia and should also be immediately evaluated and treated with a chronotropic agent and/or vasopressor such as ephedrine. Phenylephrine should be avoided with bradycardia due to the reflexive tachycardia it reliable induces. For other causes, a combination therapy or prophylaxis regimen has been found to be most effective. Drugs often used may be a combination of any of ondansetron, dexamethasone, or metoclopramide [159].
Pruritus
Pruritus is the single most common side effect of intrathecal opiates and can be extremely uncomfortable for the patient to the point in rare cases of being unable to tolerate even clothing or linens resting on their skin. One of every 2.6 patients who receive intrathecal morphine for cesarean section will experience pruritus of some degree. Similarly, 1 in every 2.2 who receive intrathecal fentanyl will also develop itching [138]. For morphine, this effect is dose dependent and continues to rise in a linear manner with increasing dosage up to reported rates as high as 100% [160, 161]. Compared to other patients, parturients have higher rates of pruritus than other populations possible due to estrogens interacting with the opioid receptor [162]. The exact mechanism of action leading to this pruritus is not entirely clear, however. Postulated theories include opiate interaction with a cephalad central “itch center” within the spinal trigeminal nucleus, activation of 5-hydroxytryptamine 3 (5HT3) serotonin receptors, prostaglandin activation, interaction with the medullary dorsal horn, or neurotransmitter inhibition [160].
Treatment and prophylaxis against pruritus include several varied options. Nalbuphine, a mixed opiate agonist-antagonist, has been shown to be effective in reversing pruritus without reversing analgesic effect by antagonizing the mu opioid receptor and activating the kappa opioid receptor [163]. Nalbuphine has been shown to be effective at reducing the incidence of opiate-induced pruritus for both fentanyl and morphine delivered by either intrathecal or epidural routes in doses as low as 2–3 mg intravenously.
Ondansetron, a 5HT3 receptor antagonist widely used for treatment and prophylaxis against nausea, has also been shown to effectively reduce the incidence of intrathecal morphine-induced itching. However, it does not appear to have the same potency in reducing fentanyl-induced itching. Mirtazapine inhibits 5HT2 and 5HT3 receptors upstream as a a2-antagonist and can reduce intrathecal morphine-induced pruritus via a similar mechanism [164]. Gabapentin, which is a structural GABA analogue, has also been shown to have some efficacy at mitigating intrathecal morphine-induced pruritus similar to the efficacy of ondansetron and mirtazapine. The mechanism by which it achieves this is not clear [164].
Histamine release is a common causative factor of itching from intravenous morphine. Intrathecal and epidural administered morphine however do not cause the same histamine release thus limiting the efficacy of diphenhydramine in treating morphine induced pruritis when given via a neuraxial route [165, 166].
Urinary Retention
Urinary retention can present in as many as 20–40% of patients within the first 2 hours after intrathecal morphine and up to 10% of patients 24 hours after [167]. Urine output should be followed closely after receiving intrathecal morphine. Low or no output should prompt evaluation for incomplete bladder emptying or complete retention. Untreated retention can lead to the development of neurogenic bladder, so it is important to not allow bladder to become overdistended for prolonged period [137].
Regional Techniques
Wound Infiltration
The wound infiltration technique is performed by the surgeon just below the fascial layer while closing the wound. This can be done either with injection of local anesthetic alone or injection followed by placement of a multi-orifice catheter used to provide a continuous infusion of local anesthetic to the wound postoperatively. Catheter can be placed above the fascia but results in significantly inferior pain control [168]. Choice of local is typically ropivacaine or bupivacaine. Typical dosing of either ropivacaine or bupivacaine infusions is 2–3 mg/mL at a rate of 5 mL/hour. This technique has been shown repeatedly to result in lower postoperative opiate consumption [169].
Transversus Abdominis Plane (TAP) Block
The innervation that provides sensation to the skin and soft tissues that comprise that abdominal wall can be found coursing in the fascial plane superficial to the transversus abdominis muscle and deep to the internal oblique muscle. Depositing local anesthetic within this plane in the lateral abdominal wall immediately superior to the iliac crest results in a field block that anesthetizes many of the sensory nerves that supply the tissues involved in a Pfannenstiel incision when performed bilaterally. This block is often described as done using ultrasound guidance which allows in-plane guidance and identification of the three circumferential muscular layers and viscera. Ropivacaine or bupivacaine is often used dosing to 1.5 mg/kg per side, for a total dose of 3 mg/kg up to a maximum of 300 mg. Use of a blunt tip needle allows for the clinician to appreciate a tactile “pop” as the needle passes through each fascial layer. It is recommended to maintain visualization of the needle tip and inject in a manner that hydro-dissects laterally the fascial plane just superior to the transversus abdominis [170]. TAP blocks have been shown to significantly reduce total postoperative opiate consumption and pain scores for the first 36 hours after surgery [170, 171]. This analgesic effect, however, has not been shown to outperform or even augment the pain relief achieved from intrathecal morphine [172]. TAP block and wound infiltration had no significant difference in outcome, pain relief, or side effects [173].
Post-cesarean Pain Management
Meta-analysis comparing opiates, non-opiates, and combination of opiate/non-opiates shows there is no ideal postoperative pain regimen after cesarean section. All strategies were similar in terms of need for further pain relief and presence of side effects, although opiates had the greatest amount of side effects [174]. Early oral intake should be encouraged which among its many benefits allows for use of oral medication as the mainstay of pain treatment and reserving intravenous options for breakthrough only. Postoperative pain should not be undertreated, however, as poor pain control delays ambulation, functional recovery, and maternal bonding [175]. In addition to synergistic effects with opiates, acetaminophen also has additive effects with NSAIDs. In patients who received intrathecal morphine, postoperative scheduled acetaminophen with a scheduled NSAID and PRN-only opiates provides an excellent and well-tolerated analgesic recovery for most women with minimal impact on breastfeeding [176]. ACOG has issued a committee opinion that directly addresses postpartum pain control, and they recommend neuraxial opiates at the time of cesarean birth followed by multimodal use of acetaminophen and NSAIDs with parenteral opiates reserved for severe breakthrough. In addition, ACOG does not endorse routine administration of gabapentin, which should only be considered for uncontrolled pain or patients with a history of chronic pain [177].
When the parturient intends on breastfeeding her infant, concerns are often raised regarding drug transfer into breastmilk. Ketorolac should be reserved for use only in the immediate first few days after delivery before the onset of copious milk production. After this point, the preferred NSAID of use is ibuprofen as it is well studied and shown to have minimal transference into breastmilk [133]. Opiates can be given to breastfeeding women, but should be done so with caution, specifically drugs containing codeine or tramadol. In 2017 the FDA issued a warning and modified labeling of all drugs containing codeine and tramadol with enhanced warnings against use with breastfeeding mothers. This is because some patients will rapidly metabolize those drugs to their active opiate metabolites and thus will have much higher than typical serum levels and breastmilk levels of these active metabolites. ACOG recommends then that all postpartum mothers who are prescribed an opiate should be counseled regarding the risks and signs of maternal and newborn toxicity. In addition, mothers should also be counseled regarding limiting the use of opiates to as short a time as possible [133]. SOAP has issued a statement as well that encourages multimodal analgesia with minimal opiates which are reserved for breakthrough pain only and use of oral oxycodone or hydrocodone as the preferential first-line rescue agents [178].
Conclusion
Every woman has a unique experience during labor and delivery with most describing the pain as moderate to severe. There are unique analgesic challenges during labor, delivery, and recovery because concern must always be taken for maternal and neonatal well-being. A patient’s satisfaction with their pain control has short- and long-term ramifications, and every effort should be made to educate parturients regarding effective and safe pain control methods. This allows the parturient control in the decision-making process.
Fortunately, there are many methods to provide safe and effective analgesia for labor and delivery. The process of labor and delivery is dynamic which may cause changes in pain control choices as the process progresses. Some parturients choose unmedicated childbirth methods, while others choose systemic or neuraxial techniques.
Neuraxial analgesia is the most effective form of analgesia for labor and delivery but may be contraindicated or not desired by patients. For those patients, other methods of pain control including opioids by IM, IV, and PCA routes, adjuvants such as hydroxyzine and promethazine, sedatives such as midazolam and ketamine, and inhaled nitrous oxide are commonly used. Pudendal and paracervical nerve blocks are also effective choices for these patients.
When cesarean section must be performed, there are many options including neuraxial opiates, wound infiltration, and regional nerve blocks that can provide high-quality analgesia during the initial stages of surgical recovery.
References
International Association for the Study of Pain. Taxonomy. 2017. http://www.iasp-pain.org/Taxonomy#Pain. Accessed 3 Mar 2019.
Lally JE, Murtagh MJ, Macphail S, Thomson R. More in hope than expectation: a systematic review of women’s expectations and experience of pain relief in labor. BMC Med. 2008;6:7.
Raynes-Greenow CH, Roberts CL, McCaffery K, Clark J. Knowledge decision making for labor analgesia of Australian primiparous women. Midwifery. 2007;23:139.
Bajaj P, Drewes AM, Gregersen H, et al. Controlled dilation of the uterine cervix: an experimental visceral pain model. Pain. 2002;99:433–42.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–5.
Melzack R. From the gate to the neuromatrix. Pain. 1999;82(Suppl 6):S121–6.
Chestnut DH, Wong CA, Tsen LC, Ngan Kee WD, Belilin Y, Mhyre JM. Chestnut’s obstetric anesthesia principles and practice. Philadelphia, PA: Elsevier Saunders; 2014.
Schnider SM, Wright RG, Levinson G, et al. Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology. 1979;50:524.
Burden RJ, Janke EL, Brighouse D. Hyperventilation-induced unconsciousness during labor. Br J Anaesth. 1994;73:838.
Lowe NK. The nature of labor pain. Am J Obstet Gynecol. 2002;186:S16–24.
Hood DD, Dewan DM, James FM. Maternal and fetal effects of epinephrine in gravid ewes. Anesthesiology. 1986;64:610–3.
Buchan AS, Sharwood-Smith GH. Physiological changes in pregnancy. The Simpson handbook of obstetric anaesthesia. Edinburgh, Albamedia: Royal College of Surgeons of Edinburgh; 1999.
Lothian JA. Preparation of childbirth. UpToDate 2019.
Melzack R. The myth of painless childbirth. Pain. 1984;19:321–7.
Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87.
Zaers S, Waschke M, Ehlert U. Depressive symptoms and symptoms of post-traumatic stress disorder in women after during childbirth. J Psychosom Obstet Gynaecol. 2008;29:61.
Lamaze F. Painless childbirth. Psychoprophylactic method. London: Burke; 1958.
Information Center for Health and Social Care. NHS Maternity Statistics. 2011. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=815. Accessed 5 Mar 2019.
Schnabel A, Hahn N, Broschiet J, et al. Remifentanil for labor analgesia: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2012;29:177.
Smith LA, Burns E, Cuthbert A. Parenteral opioids for maternal pain management in labour. Cochrane Database Syst Rev. 2018;6(6):CD007396.
Reynolds F, Sharma SK, Seed PT. Analgesia in labor and fetal acid-base balance: a meta-analysis comparing epidural with systemic opioid analgesia. BJOG. 2002;109:1344–53.
Halpern SH, Muir H, Breen TW, et al. A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor. Anesth Analg. 2004;99:1532–8.
Cleland JG. Paravertebral anaesthesia in obstetrics. Surg Gynecol Obstet. 1933;57:51–62.
Abrao KC, Francisco RP, Miyadahira S, et al. Elevation of uterine basal tone and fetal heart rate abnormalities after labor analgesia: a randomized controlled trial. Obstet Gynecol. 2009;113:41–7.
Mardirsoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labor analgesia: a systematic review. BJOG. 2002;109:274–81.
Tuckey JP, Prout RE, Wee MY. Prescribing intramuscular opioids for labor analgesia in consultant-led maternity units: a survey of UK practice. Int J Obstet Anesth. 2008;17:8.
Wittels B, Scott DT, Sinatra RS. Exogenous opioids in human breast milk and acute neonatal neurobehavior: a preliminary study. Anesthesiology. 1990;73(3):864–9.
Anderson D. A review of systemic opioids commonly used for labor pain relief. J Midwifery Womens Health. 2011;56:222–39.
Caldwell J, Wakile LA, Notarianni LJ, et al. Maternal and neonatal disposition of pethidine in childbirth-a study using quantitative gas chromatography-mass spectrometry. Life Sci. 1978;22:589.
Nissen E, Widstrom AM, Lilja G, et al. Effects of routinely given pethidine during labour on infants’ developing breastfeeding behavior. Effects of dose-delivery time interval and various concentrations of pethidine/norepinephrine in cord plasma. Acta Paediatr. 1997;86:201.
Olofsson C, Ekblom A, Ekman-Ordeberg G, Granstrom L, Irestedt L. Lack of analgesic effect of systematically administered morphine or pethidine on labour pain. Br J Obstet Gynaecol. 1996;103(10):968–72.
Fleet K, Belan I, Jones MJ, et al. A comparison of fentayl with pethidine for pain relief during childbirth: a randomised controlled trial. BJOG. 2015;122:983.
Rayburn WF, Smith CV, Parriott JE, Woods RE. Randomized comparison of meperidine and fentanyl during labor. Obstet Gynecol. 1989;74(4):604–6.
Nelson KE, Eisenach JC. Intravenous butorphanol, meperidine, and their combination relieve pain and distress in women in labor. Anesthesiology. 2005;102(5):1008–13.
Elbohoty AE, Abd-Elazek H, Abd-El-Gawad M, Salama F, et al. Intravenous infusion of paracetamol versus intravenous pethidine as an intrapartum analgesic in the first stage of labor. Int J Gynaecol Obstet. 2012;118(1):7–10.
Peck TE, Hill SA, Williams M. Pharmacology for anaesthesia and intensive care. 3rd ed. Cambridge, UK: Cambridge University Press; 2008.
Olofsson C, Ekblom A, Ekman-Ordeberg G, et al. Analgesic efficacy of intravenous morphine in labor pain: a reappraisal. Int J Obstet Anesth. 1996;5:176–80.
Craft JB, Coladrake LA, Bolan JC, et al. Placental passage and uterine effects of fentanyl. Anesth Analg. 1983;62(10):894–8.
Miyakoshi K, Tanaka M, Morisaki H, et al. Perinatal outcomes: intravenous patient-controlled fentanyl versus no analgesia in labor. J Obstet Gynaecol Res. 2013;39:783.
Steer PL, Biddle CJ, Marle WS, Lantz RK, Sulik PL. Concentration of fentanyl in colostrum after an analgesic dose. Can J Anesth. 1992;39(3):231–5.
Morle-Forster PK, Reid DW, Vanderberghe H. A comparison of patient-controlled analgesia fentanyl and alfentanil for labor analgesia. Can J Anesth. 2000;47:113.
Egan TD, Lemmens HJ, Fiset P, et al. The pharmacokinetics of the new short-acting opioid remifentanil (G187084B) in healthy adult male volunteers. Anesthesiology. 1993;79:881–92.
Kapila A, Glass PS, Jacobs JR, et al. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology. 1995;83:968–75.
Westmoreland CL, Hoke JF, Sebel PS, Hug CC Jr, Muir KT. Pharmacokinetics of remifentanil (G187084B) and its major metabolite (G190291) in patients undergoing elective inpatient surgery. Anesthesiology. 1993;79(5):893–903.
Hill D. The use of remifentanil in obstetrics. Anesthesiol Clin. 2008;26:169.
Muchatuta NA, Kinsella SM. Remifentanil for labor analgesia: time to draw breath? Anaesthesia. 2013;68:231.
Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467–74.
Mylan Institutional LLC. Ultiva (remifentanil) package insert. In: LLC MI, editor. Rockford, IL, USA; 2013.
Volmanen P, Akural E, Raudaskoski T, Ohtonen P, Alahuhta S. Comparison of remifentanil and nitrous oxide in labor analgesia. Acta Anaesthesiol Scand. 2005;49:453–8.
Van de Velde M, Carvalho B. Remifentanil for labor analgesia: an evidence-based narrative review. Int J Obstet Anesth. 2016;25:66–74.
Birbach DH, Ranasinghe JS. Is remifentanil a safe and effective alternative to neuraxial labor analgesia? Anesth Analg. 2014;118:491.
Wilson MJA, MacArthur C, Hewitt CA, et al. Intravenous remifentanil patient-controlled analgesia versus intramuscular pethidine for pain relief in labor (RESPITE): an open-label multicentre randomized controlled trial. Lancet. 2018;392:662.
Hanova A, Fernando R. Systemic remifentanil for labor analgesia. Anesth Analg. 2009;109:1925–9.
Sasada M, Smith S. Drugs in anaesthesia & intensive care. 3rd ed. Oxford, UK: Oxford University Press; 2003.
Nicolle E, Devillier P, Delanoy B, Durand C, Bessard G. Therapeutic monitoring of nalbuphine: transplacental transfer and estimated pharmacokinetics in the neonate. Eur J Clin Pharmacol. 1996;49(6):485–9.
Giannina G, Guzman ER, Lai YL, Lake MF, Cernadas M, Vinzileos AM. Comparison of the effects of meperidine and nalbuphine on intrapartum fetal heart rate tracings. Obstet Gynecol. 1995;1995(86):3.
Adtkinson BD, Truitt LJ, Rayburn WF, Turnbull GL, Christinsen HD, Wlodaver A. A double-blind comparison of intravenous butorphanol (Stadol) and fentanyl (Sublimaze) for analgesia during labor. Anaesthesiol Rev. 1991;18(1):31–6.
DiPiro JT, Talbert RL, Yees GC, Matzke GR, Wells BG, Michael PL. Pharmacotherapy: a pathophysiologic approach. New York, NY: McGraw-Hill; 2005.
Trescot AM, Datta S, Lee M, et al. Opioid pharmacology. Pain Physician. 2008;11(Suppl 10):S16–S120.
Nagishima H, Karamanian A, Malovany R, et al. Respiratory and circulatory effects of intravenous butorphanol and morphine. Clin Pharmacol Ther. 1976;10:738–45.
Aronoff DM, Oates JA, Boutaud O, Arbor A. New insights into the mechanism of action of acetaminophen: its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006;89(1):9–19.
Graham GG, Scott KF, Day RO. Tolerability of paracetamol. Drug Saf. 2005;28(3):227–40.
Smith HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;2009(12):1.
Zutshi V, Rani UK, Marwah S, Patel M. Efficacy of intravenous infusion of acetaminophen for intrapartum analgesia. J Clin Diagn Res. 2016;10(8):QC18–21.
Elbohoty AE, Abd-Elrazek H, Abd-El-Gawad M, et al. Intravenous infusion of paracetamol versus intravenous pethidine as an intrapartum analgesic in the first stage of labor. Int J Gynaecol Obstet. 2012;118:7–10.
Othman M, Jones L, Nielson JP. Non-opioid drugs for pain management in labour. Cochrane Database Syst Rev. 2012:CD009223.
Jagatia K, Mehta J, Patel N. Low dose ketamine for painless labour: a comparative study of 100 patients. Int J Med Sci Public Health. 2013;2(3):707–11.
Rooks JP. Nitrous oxide for pain in labor--why not in the United States? Birth. 2007;34(1):3–5.
Rooks JP. Safety and risks of nitrous oxide labor analgesia: a review. J Midwifery Womens Health. 2011;56(6):557–65.
Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an Academic Medical Center. J Clin Anesth. 2016;35:54.
Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 Suppl Nature):S110–26.
Isaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22(3):368–73.
Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118:153.
Kronberg JE, Thompson DEA. Is nitrous oxide an effective analgesic for labor? A qualitative systematic review. In: Evidence-based obstetric anesthesia. Malden, MA: Blackwell; 2006.
Likis FE, Andrews JA, Collins MR, Lewis RM, Seroogy JJ, Starr SA et al. Nitrous oxide for the management of labor pain. comparative effectiveness review no. 67. Vanderbilt Evidence-based Practice Center under Contract No 290-2007-10065-1. Rockville, MD: AHRQ2012 Contract No.: No 12-EHC071-EF.
Klomp T, van Poppel M, Jones L, et al. Inhaled analgesia for pain management in labour. Cochrane Database Syst Rev. 2012;(9):CD009351.
Richardson MG, Lopez BM, Baysinger CL, MS S. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548–53.
Chestnut DH, Wong CA, Tsen LC, Ngan Kee WD, Beilin Y, Mhyre J. Obstetric anesthesia: principles and practice. 5th ed. Philadelphia, PA: Elsevier; 2014.
Bulletins-Obstetrics CoP. Practice bulletin no.177: obstetric analgesia and anesthesia. Obstet Gynecol. 2019;129:73.
Hutchins CJ. Spinal analgesia for instrumental delivery. A comparison with pudendal nerve block. Anaesthesia. 1980;35(4):376–7.
Mody SK, Farala JP, Jimenez B, Nishikawa M, Ngo LL. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132(3):575–82.
McCulloch B, Bergen S, Pielet B, Keller J, Elrad H. McDonald cerclage under pudendal nerve block. Am J Obstet Gynecol. 1993;168(2):499–502.
Schierup L, Schmidt JF, Torp Jensen A, Rye BA. Pudendal block in vaginal deliveries. Mepivacaine with and without epinephrine. Acta Obstet Gynecol Scand. 1988;67(3):195–7.
Chestnut DH, Polley L, Wong CA, Tsen L. Alternative regional anesthetic techniques: paracervical block, lumbar sympathetic block, pudendal nerve block, and perineal infiltration. In: Chestnut’s obstetric anesthesia: principles and practice. 4th ed. Philadelphia, PA: Mosby Elsevier; 2009.
Standring S. Gray’s anatomy: the anatomical basis of clinical practice. 39th ed. Edinburgh: Elsevier Churchill Livingstone; 2005.
Zador G, Lindmark G, Nilsson BA. Pudendal block in normal vaginal deliveries. Clinical efficacy, lidocaine concentrations in maternal and foetal blood, foetal and maternal acid-base values and influence on uterine activity. Acta Obstet Gynecol Scand. 1974;34:51–64.
Anderson D. Pudendal nerve block for vaginal birth. J Midwifery Womens Health. 2014;59(6):651–9.
Merkow AJ, McGuinness GA, Erenberg A, Kennedy RL. The neonatal neurobehavioral effects of bupivacaine, mepivacaine, and 2-chloroprocaine used for pudendal block. Anesthesiology. 1980;52:309.
Kangas-Saarela T, Jouppila R, Poulakka J, Jouppila P, Hollmen A, et al. The effect of bupivacaine paracervical block on the neurobehavioral responses of newborn infants. Obstetric Anesthesia Digest. 1989;9(2):97.
Aissaoui Y, Bruyere R, Mustapha H, Bry D, Kamili ND, et al. A randomized controlled tirla of pudendal nerve block for pain relief after episiotomy. Anesth Analg. 2008;107(2):625–9.
Sullivan JT. What’s new in obstetric anesthesia: the 2009 Gerard W. Ostheimer lecture. Anesth Analg. 2010;110(2):564–9.
Bailard NS, Ortiz J, Flores RA. Additives to local anesthetics for peripheral nerve blocks: evidence, limitations, and recommendations. Am J Health Syst Pharm. 2014;71(5):373–85.
Pages H, de la Gastine B, Quedru-Aboane J, Guillemin MG, Lelong-Boulouard V, et al. Lidocaine intoxication in newborn following maternal pudendal anesthesia: report of three cases. J Gynecol Obstet Biol Reprod (Paris). 2008;37(4):415–8.
Chestnut DPL, Tsen L, Wong C. Alternative regional analgesic techniques for labor and vaginal delivery. In: Chestnut’s obstetric anesthesia: principles and practice. 5th ed. Philadelphia, PA: Elsevier Saunders; 2014.
Vidaeff AC. Pudendal and paracervical block. UpToDate. Wolters Kluwer. USA; 2019. p. 1–23.
Keder LM. Best practices in surgical abortion. Am J Obstet Gynecol. 2003;189(2):418–22.
Neal JM, Bernards CM, Butterworth JF 4th, Di Gregorio G, Drasner K, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35(2):152–61.
Weiss RR, Halevy S, Almonte RO, Gundersen K, Hinsvark ON, et al. Comparison of lidocaine and 2-chloroprocaine in paracervical block: clinical effects and drug concentrations in mother and child. Anesth Analg. 1983;62(2):168–73.
Gad C. Paracervical block. Acta Obstet Gynecol Scand. 1967;46(1):7–18.
Vidadeff A. Pudendal and paracervical block. UpToDate. January 2019 ed. Wolters Kluwer. USA; 2019. p. 1–23.
Jagerhorn M. Paracervical block in obstetrics. An improved injection method. A clinical and radiological study. Acta Obstet Gynecol Scand. 1975;54(1):9–27.
Novikova N, Cluver C. Local anaesthetic nerve block for pain management in labour. Cochrane Database Syst Rev. 2012;18(4):CD009200. https://doi.org/10.1002/14651858.CD009200.pub2.
LeFevre ML. Fetal heart rate pattern and postparacervical fetal bradycardia. Obstet Gynecol. 1984;64(3):343–6.
Palomaki O, Huhtala H, Kirkinen P. A comparative study of the safety of 0.25% levobupivacaine and 0.25% racemic bupivacaine block in the first stage of labor. Acta Obstet Gynecol Scand. 2005;84(10):956–61.
Umstad MP, Ross A, Rushford DD, Permezel M. Epidural analgesia and fetal heart rate abnormalities. Aust N Z J Obstet Gynaecol. 1993;33:269.
Gordon HR. Fetal bradycardia after paracervical block-correlation with fetal and maternal blood levels of local anesthetic (Mepivacaine). N Engl J Med. 1968;279:910–4.
Cibils LA. Response of human uterine arteries to local anesthetics. Am J Obstet Gynecol. 1976;126(2):202–10.
Rogers RE. Fetal bradycardia associated with paracervical block anesthesia in labor. Am J Obstet Gynecol. 1970;106(6):913–6.
Shnider SM, Asling JH, Margolis AJ, et al. High fetal blood levels of mepivacaine and fetal bradycardia (letter). NEJM. 1968;279:947–8.
Asling JH, Shnider SM, Margolis AJ, et al. Paracervical block anesthesia in obstetrics. II. Etiology of fetal bradycardia following paracervical block in anesthesia. Am J Obstet Gynecol. 1970;107:626–34.
Levy BT, Bergus GR, Hartz A, Lofgren M, Goldsborough K. Is paracervical block safe and effective? A prospective study of its association with neonatal umbilical artery pH values. J Fam Pract. 1999;48(10):778–84.
Merkow AJ, McGuinness GA, Ereberg A, Kennedy RL. The neonatal neurobehavioral effects of bupivacaine, mepivacaine, and 2-chloroprocaine used for pudendal block. Anesthesiology. 1980;52(4):309–12.
Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99.
Sharma SKMD, Wiley J, Leveno KJ. Labor analgesia and cesarean delivery: an individual patient meta-analysis of nulliparous women. Anesthesiology. 2004;100(1):142–8.
Anim-Somuah MSR, Cyna A, Cuthbert A. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331.
Birnbach DJHS, Muir H, Breen TW, Campbell DC, Barrett J, Liston R, Blanchard JW. A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor. Anesth Analg. 2004;99(5):1532–8.
Hawkins J. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362:1503–10.
Task Force on Obstetric Anesthesia. Practice guideline for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124:270–300.
Levy NGO, Cattan A, Weiniger CF, Matot I. Neuraxial block for delivery among women with low platelet counts: a retrospective analysis. Int J Obstet Anesth. 2018;35:4–9.
Traynor AJAM, Bucklin BA, et al. Obstetric anesthesia workforce survey: a 30-year update. Anesth Analg. 2016;122(6):1939–46.
Butwick AJWC, Guo N. Maternal body mass index and use of labor neuraxial analgesia: a population-based retrospective cohort study. Anesthesiology. 2018;129(9):448–58.
ACOG. Practice bulletin 209. Obstet Gynecol. 2019;133(3):208–25.
Srivastava UVS, Singh TK, Gupta A, Saxsena A, Jagar KD, Gupta M. Efficacy of trans abdominis plane block for post cesarean delivery analgesia: a double-blind, randomized trial. Saudi J Anaesth. 2015;9(3):298–302.
Wong C, Ratliff JT, Sullivan JT, Scavone BM, Toledo P, McCarthy RJ. A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg. 2006;102(3):904–9.
Society of Obstetric Anesthesia and Perinatology. Comments in response to the ACOG/SMFM Practice Advisory on Codeine and Tramadol for Breastfeeding Women. 2017.
Bucklin BA, Hawkins JL, Anderson JR, Ulrich BA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103(9):645–53.
Traynor AJ, Aragon M, Ghosh D, Choi RS, Dingmann C, Vu Tran Z, et al. Obstetric anesthesia workforce survey: a 30-year update. Anesth Analg. 2016;122(6):1939–46.
Omaygenc DO, Dogu T, Omaygenc MO, Ozmen F, Albayrak MD, Guler GB, et al. Type of anesthesia affects neonatal wellbeing and frequency of transient tachypnea in elective cesarean sections. J Matern Fetal Neonatal Med. 2015;28(5):568–72.
Ng KW, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev. 2004;2. https://doi.org/10.1002/14651858.CD003765.pub2.
Ranasinghe JS. Combined spinal epidural anaesthesia is better than spinal or epidural alone for Caesarean delivery. Br J Anaesth. 2003;91(2):299–300.
Petropoulos G, Sirstaidis C, Salamelekis E, Creatsas G. Spinal and epidural versus general anesthesia for elective Cesarean section at term: effect on the acid-base status of the mother and newborn. J Matern Fetal Neonatal Med. 2003;13(4):260–6.
Cook T, Counsell D, Wildsmith AW. Major complications of central neuraxial block: report of the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102(2):179–90.
American College of Obstetricians and Gynecologists. ACOG committee opinion 742: postpartum pain management. Obstet Gynecol. 2018;132(1):e35–43.
Sultan PHS, Pushpanathan E, Patel S, Carvalho B. The effect of intrathecal morphine dose on outcomes after elective cesarean delivery: a meta-analysis. Anesth Analg. 2016;123(1):154–64.
Dahl JBJI, Jorgensen H, Wetterslev J, Moiniche S. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91(6):1919.
Chaney MA. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42(10):891–903.
DeSousa KA, Chandran R. Intrathecal morphine for postoperative analgesia: current trends. World J Anesthesiol. 2014;3(3):191–202.
Dahl JB, Jeppesen IS, Jorgensen H, Wetterslev J, Moiniche S. Intraoperative and postoperative analgesic efficacy and adverse effects of intrathecal opioids in patients undergoing cesarean section with spinal anesthesia: a qualitative and quantitative systematic review of randomized controlled trials. Anesthesiology. 1999;91(6):1919.
American Society of Anesthesiologists. Practice guidelines for the PRevention, Detection, and Management of Respiratory Depression Associated with Neuraxial Opioid Administration: an updated report by the American Society of Anesthesiologists Task Force on Neuraxial Opiods and the American Soc. Anesthesiology. 2016;124(3):532–52.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, et al. Spinal and epidural versus general anesthesia for elective Cesarean section at term: effect on the acid-base status of the mother and newborn. Am J Epidemiol. 2013;177(9):1006–14.
Pien GW, Al P, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69(4):371–7.
Chung F, Abdullah HR, Liao P. STOP-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631–8.
Lockhart EM, Abdallah A, Tuuli MG, Leighton BL. Obstructive sleep apnea in pregnancy: assessment of current screening tools. Obstet Gynecol. 2015;126(1):93–102.
Sharawi N, Carvalho B, Habib AS, Blak L, Mhyre HM, Sultan P. A systematic review evaluating neuraxial morphine and diamorphine-associated respiratory depression after cesarean delivery. Anesth Analg. 2018;127(6):1385–95.
Choi DH, Ahn HJ, Kim MH. Bupivacaine-sparing effect of fentanyl in spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25(3):240–5.
Ben-David B, Miller G, Gavriel R, Gurevitch A. Low-dose bupivacaine-fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25(3):235–9.
Bano F, Sabbar S, Zafar S, Rafeeq N, Igbal MN, Haider S, et al. Intrathecal fentanyl as adjunct to hyperbaric bupivacaine in spinal anesthesia for caesarean section. J Coll Physicians Surg Pak. 2006;16(2):87–90.
Weigl W, Bierylo A, Wielgus M, Kzemien-Wiczyriska S, Kolacz M, Dabrowski MJ. Perioperative analgesia after intrathecal fentanyl and morphine or morphine alone for cesarean section: a randomized controlled study. Medicine. 2017;96(48):e8892.
Thornton P, Hanumanthaiah D, O’Leary RA, Iohom G. Effects of fentanyl added to a mixture of intrathecal bupivacaine and morphine for spinal anesthesia in elective caesarean section. Rom J Anaesth Intensive Care. 2015;22(2):97–102.
Carvalho B, Drover DR, Ginosar Y, Cohen SE, Riley ET. Intrathecal fentanyl added to bupivacaine and morphine for cesarean delivery may induce a subtle acute opioid tolerance. Int J Obstet Anesth. 2012;21(1):29–34.
Carvalho B, Riley E, Cohen SE, Gambling D, Palmer C, DepoSur Study Group, et al. Single-dose, sustained release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg. 2005;100(4):1150–8.
Gambling D, Hughes T, Martin G, Horton W, Manvelian G. A comparison of Depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth Analg. 2005;100:1065–74.
Carvalho B, Roland LM, Chu LF, Campitelli VA 3rd, Riley ET. Single-dose, extended-release epidural morphine (DepoDur) compared to conventional epidural morphine for post-cesarean pain. Anesth Analg. 2007;105:176–83.
Gerancher JC, Nagle PC. Management of accidental spinal administration of extended-release epidural morphine. Anesthesiology. 2008;108(6):1147–9.
Cooper DW, Saleh U, Taylor M, Whyte S, Ryall D, Kokri MS, et al. Patient-controlled analgesia: epidural fentanyl and iv morphine compared after cesarean section. Br J Anaesth. 1999;82(3):366–70.
Yu PYH, Gambling DR. A comparative study of patient-controlled epidural fentanyl and single dose epidural morphine for post-Caesarean analgesia. Can J Anaesth. 1993;40(5):416–20.
Carpenter RL, Caplan RA, Brown DL, Carol Stephenson RN, Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–16.
Balki M, Carvalho JC. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int J Obstet Anesth. 2005;14(3):230–41.
Jelting Y, Klein C, Harlander T, Eberhart L, Roewer N, Kranke P. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: challenges and solutions. Local Reg Anesth. 2017;10:83–90.
Aly M, Ibrahim A, Farrag W, Adbdelsalam K, Mohamed H, Tawfik A. Pruritus after intrathecal morphine for cesarean delivery: incidence, severity and its relation to serum serotonin level. Int J Obstet Anesth. 2018;35:52–6.
Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90(2):437–44.
Szarvas S, Harmon D, Murphy D. Neuraxial opioid-induced pruritus: a review. J Clin Anesth. 2003;15(3):234–9.
Tubog TD, Harenberg JL, Buszta K, Hestand JD. Prophylactic nalbuphine to prevent neuraxial opioid-induced pruritus: a systematic review and meta-analysis of randomized controlled trials. J Perianesth Nurs. 2018; https://doi.org/10.1016/j/jopan.2018.06.098.
Akhan A, Subasi FD, Bosna G, Ekinci O, Pamuk H, Batan S, et al. Comparison of mirtazapine, gabapentin, and ondansetron to prevent intrathecal morphine-induced pruritus. North Clin Istanb. 2016;3(1):53–9.
Liao CC, Chang CS, Tseng CH, Sheen MJ, Tsai SC, Chang YL, et al. Efficacy of intramuscular nalbuphine versus diphenhydramine for the prevention of epidural morphine-induced pruritus after cesarean delivery. Chang Gung Med J. 2011;34(2):172–8.
Alhashemi JA, Crosby ET, Grodecki W, Duffy PJ, Hull KA, Gallant C. Treatment of intrathecal morphine-induced pruritus following Caserean section. Can J Anaesth. 1997;44:1060.
Raffaeli W, Marconi G, Fanelli G, Taddei S, Borghi GB, Casati A. Opioid-related side-effects after intrathecal morphine: a prospective, randomized, double-blind dose-response study. Eur J Anaesthesiol. 2006;23(7):605–10.
Rackelboom T, Le Strat S, Silvera S, Schmitz T, Bassot A, Goffinet F, et al. Improving continuous wound infusion effectiveness for postoperative analgesia after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2010;116(4):893–900.
Bamigboye AA, Hofmeyr GJ. Local Anesthetic wound infiltration and abdominal nerves block during cesarean section for postoperative pain relief. Cochrane Database Syst Rev. 2009;3. https://doi.org/10.1002/14651858.CD006954.pub2.
McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2008;106(1):186–91.
Srivastava U, Verma S, Singh TK, Gupta A, Saxsena A, Jagar KD. Efficacy of trans abdominis plane block for post cesarean delivery analgesia: a double-blind, randomized trial. Saudi J Anaesth. 2015;9(3):298–302.
McMorrow RC, Ni Mhuircheartaigh RJ, Ahmed KA, Slani A, Ng SC, Conrick-Martin I, et al. Comparison of transversus abdominis plane block vs. spinal morphine for pain relief after Caesarean section. Br J Anaesth. 2011;106(5):706–12.
Tawfik MM, Mohaed YM, Elbadrawi RE, Abdelkhalek M, Mogahed MM, et al. Transversus abdominis plane block versus wound infiltration for analgesia after cesarean delivery: a randomized controlled trial. Anesth Analg. 2017;124(4):1291–7.
Mkontwana N, Novikova N. Oral analgesia for relieving post-cesarean pain. Cochrane Database Syst Rev. 2015;29(3):CD010450. https://doi.org/10.1002/14651858.CD010450.pub2.
Ituk U, Habib AS. Enhanced recovery after cesarean delivery. F1000 Faculty Rev-513. 2018. https://doi.org/10.12688/f1000research.13895.1.
Ituk U, Habib AS. Enhanced recovery after cesarean delivery. F1000 Research. 2018;7(F1000 Faculty Rev-513).
ACOG. Committee opinion 742: postpartum pain management. Obstet Gynecol. 2018;132(1):e35–43.
Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 177: Obstetric Analgesia and Anesthesia. Obstet Gynecol. 2017.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Byerly, S.I., Bryson, T.D. (2020). Pain Management for Obstetrical Patients. In: Noe, C. (eds) Pain Management for Clinicians. Springer, Cham. https://doi.org/10.1007/978-3-030-39982-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-39982-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39981-8
Online ISBN: 978-3-030-39982-5
eBook Packages: MedicineMedicine (R0)