Abstract
Morpho-functional traits of Antarctic seaweeds are modeled by different physical and biological factors. Due to the extreme seasonality, which imposes light limitation for extended periods, Antarctic seaweeds are shade-adapted organisms that are physiologically able to thrive at considerable depths down to 40 m. This vertical distribution is defined by a suite of bio-optical and morphological features that allow algae occupying habitats with different environmental conditions in the water column. However, various species can also colonize the highly perturbed intertidal zone where environmental setting, e.g. ice scouring, high solar radiation, extremely variable temperature, limit growth, and reproduction. In the maritime Antarctic region, large endemic brown algae attaining a massive (leathery) morphology and perennial life history dominate at depths below 10 m or less. Here, they coexist with perennial highly shade-adapted coarsely branched rhodophytes, which show understory characteristics. At shallower locations, various annual species with very rapid growth can be found. The intertidal zone, characterized by a depauperate diversity, is populated mostly by ephemeral and delicate green algae. In the present chapter, form and function of seaweeds is revisited in the context of a changing Antarctic environment. Here, the functional groups display different acclimation mechanisms, which can operate at different temporal scales and consequently with variable impact on the biogeochemical coastal processes. The role of canopy-forming algae, whose “bioengineering” processes alleviate the impact of environmental variability, is fundamental in determining the fate of the benthic communities in the coastal system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Brief Overview of Form and Function in Seaweeds
In contrast to unicellular algae, organisms bearing multiple cells (from simple pluricellular colonies to more advanced forms attaining, e.g. parenchyma) have to synchronize a more complex structural organization, characterized by different function-specific elements that follow a morpho-genetic-based program. Thus, multicellular algae adjust form and function through an intricate molecular network that allows them to interact with their physical, chemical, and biotic environment (Grosberg and Strathmann 2007). A suite of morphological models found in benthic organisms (e.g. seaweeds, sponges, corals, bryozoa) coexist in a physical environment, which raises a question of how similar convergent forms have evolved even in species phylogenetically very distant or, alternatively, how related organisms display completely different growth patterns and shape (Kaandorp and Kübler 2001).
A striking characteristic of almost all seaweeds is their morphological plasticity, i.e. although the basic thallus plan is based on a determined morphogenetic design, body shape can change through the life span of an organism or within the life history sequence. These variations in the morphological traits within a genotype can be subtle or drastic depending on the intensity of the endogenous and environmentally driven shifts (Innes 1984; Taylor and Hay 1984). Especially in sites where the physical perturbations are extreme, seaweeds display complex mechanisms to adjust form and function to the prevailing environmental condition (Hay 1986). Phenotypic plasticity, one of the most well-known types of intrinsic morphological variability, is normally prompted by environmental conditions and thereby complicates efforts to identify the routes of morpho-functional responses within a multi-specific assemblage: intrinsic properties at an organismal level mask the morpho-functional differences at community scale (Steneck and Dethier 1994). Changes in morphology due to ontogenetic development and heteromorphic phase expression within of life cycle are also important to characterize form and function in seaweeds. For example, in the brown alga Himantothallus grandifolius, the largest Antarctic seaweed, the thallus undergoes considerable changes with development: while juvenile individuals are characterized by partial cortication and coarsely branched morphology, adult plants are characterized by a thick leathery strap-like anatomical structure, where lateral branches are absent (Moe and Silva 1981; Wiencke and Clayton 1990).
Traditionally, functional groups of seaweeds (which could also be applied to other groups of benthic marine organisms) are defined by their thallus architecture (also called life form). This concept implies intuitively a series of intrinsic properties of an organism, which can or not be shared by other unrelated organisms. Clearly this gives a high value to the anatomical features (form) and less emphasis on the function. Such conceptual framework represented the basis on, which Littler and Littler (1980) and Steneck and Watling (1982) developed their general functional form models, where functionality of algae, e.g. resistance to biotic disturbance, was inferred from gross morphology. Although the general applicability of these models has been questioned since similar morphologies often show different functional responses to, e.g. disturbance gradients (Phillips et al. 1997; Ingólfsson 2005; Padilla and Allen 2000), functionality, which depends on extrinsic factors (e.g. resource utilization, disturbance, biotic interactions, etc.), has an unavoidable expression in the morphology. The rationale to understand in what ways form and function is modeled by the environment, known as “the holy grail framework”, considers necessarily different areas of knowledge, e.g. genomics, physiology, ecology, demography, etc., and has been used with different emphasis to explain the structure of different types of vegetation, both in terrestrial and aquatic realms (Littler and Littler 1980; Grime 1981; Steneck and Dethier 1994; Lavorel and Garnier 2002). Thus, if one assumes that different habitats have a different environmental setting, it is possible to argue that they host assemblages of organisms with similar morphology but different functional attributes. For example, in littoral stress, tolerance of macroalgae depends strongly on fine photochemical adjustments, which are related to their position on the shore and less with functional form groups (Aguilera et al. 1999; Gómez and Huovinen 2011; Balata et al. 2011). This has been commonly found in different types of terrestrial vegetation where environmental tolerance, and not gross morphology, defines functional groups (Grime 1981; Ackerly and Reich 1999; Poorter and Bongers 2006).
2 Functional Groups of Seaweeds in the Antarctic
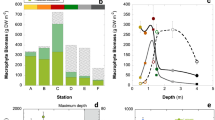
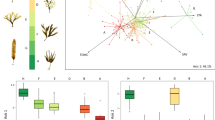
The coastal systems around Antarctica may be regarded as highly inhospitable for life, where physical disturbance associated with ice scouring, extreme light limitation, and low temperatures imposes severe restrictions for marine organisms. However, benthic algae thrive in these habitats displaying different functional strategies and morphologies (Fig. 11.1). Based on various surveys, 131 species of seaweeds (Fig. 11.2) are distributed among different types of functional groups: filamentous and finely branched (45); foliose (9); coarsely branched, including corticated species (48); thick leathery, including terete forms (11); and postrate species (18) (Fig. 11.2). However, when the different functional forms are grouped according to the major phylogenetic categories, it is possible to observe that 64% of green algae are filamentous, while practically the totality of thick leathery forms belong to brown algae. In red algae, 58% of the known species can be recognized as finely and coarsely branched morphs. In the case of Chlorophyta, most of the species attain delicate filamentous or sheet-like morphs, and with the exception of the endemic Lambia antarctica and Monostoma hariotii (Wiencke and Clayton 2002; see also Chap. 2 by Oliveira et al. and Chap. 5 by Pellizzari et al.), all are restricted to intertidal zones. However, it should be emphasized that many species cannot be easily assigned to these major functional categories. For example, the brown algae Adenocystis utricularis and Utriculidium durvillaei are the only species with a saccate morphology (and thus were not included in this analysis). Moreover, the number of postrate species, which may include crustose, calcareous, and endophytic life forms, is largely underrepresented. In fact, these algae have been very little studied, due to mostly that they are not ubiquitous (e.g. encrusting morphs) or can inhabit deeper locations (e.g. calcified coralline algae) (Alongi et al. 2002).
Diversity of gross morphologies in Antarctic seaweeds. (a) Crustose Rhodophyta; (b) Saccate morphology (Adenocystis utricularis); (c) Filamentous tubular (Ulva intestinalis); (d) finely branched Plocamium cartilagineum; (e) coarsely branched (Trematocarpus antarcticus); (f) Thick leathery (Ascoseira mirabilis). (Photos a, d, e and f by Ignacio Garrido; b and c by Iván Gómez)
The absence of a marked dominance of a given seaweed gross morphology in the Antarctic can only be explained in terms of the distribution of these different functional forms in the mosaic of benthic habitats. In fact, the arrangement of species across different environmental gradients implies also an ordination of organismal traits that can be classified in different functional entities (e.g. gross morphology, size, life forms, physiological responses). Thus, it is possible to understand why similar morpho-functional “solutions” are exploited by different taxa, many of them phylogenetically unrelated. For example, of the 25 species of green algae recorded in the Antarctic, 56% correspond to widely distributed taxa, which normally display filamentous or sheet-like forms (Fig. 11.3). According to Gómez et al. (2019), form and function and biogeographic affinity are highly correlated in Antarctic green algae. In contrast, only 12% of Rhodophyta can be regarded as widely distributed, contrasting with the high prevalence of endemic (42%) and Antarctic/sub-Antarctic taxa (46%). Similar pattern can be observed in brown algae where endemic and Antarctic/sub-Antarctic species (characterized by thick leathery and saccate morphs) account by 88% of the total numbers of recorded taxa (Fig. 11.2).
Antarctic seaweeds and their organization in relation to form and function, size, geographic affinity, and taxonomy. Number of taxa extracted from Fig. 11.2
3 The Vertical Zonation of Antarctic Seaweeds: A Paradigm of Spatial Distribution of Different Morpho-functional Traits
Knowledge on zonation and in general the structure of the submarine landscape in the shallow sublittoral in the Antarctic began to increase in the 1960s and 1970s, along with scuba diving–based surveys (Neushul 1965; Delépine et al. 1966; Zaneveld 1966; DeLaca and Lipps 1976; Lamb and Zimmerman 1977). These studies across different geographical zones demonstrated that the vertical distribution of macroalgae could be defined in terms of functional groups, which apparently do not follow uniform patterns, principally due to differences in latitude, substrate, influence of ice, associated fauna, etc. The vertical distribution of Antarctic seaweeds has been much more studied in the Western Antarctic Peninsula and adjacent islands, an eco-region known as the Maritime Antarctic. Due to the relatively milder climatic conditions, seaweed assemblages reach their maximal development in terms of abundance and diversity in the north-western part of the Antarctic Peninsula, decreasing the macroalgal biodiversity towards the southern regions (Wiencke et al. 2014; Mystikou et al. 2014). The zonation in the Maritime Antarctic can be characterized by a dominance of large canopy-forming endemic species of the order Desmarestiales (Desmarestia menziesii, D. anceps, and Himantothallus grandifolius) between 10 and 40 m or greater depth (Fig. 11.4a). These three species have thick leathery and terete gross morphology and can alternate their dominance depending on the substrate characteristics, whose consolidation can vary considerably depending on closeness to glaciers, slope of the vertical profile, terrestrial runoff, etc. (Klöser et al. 1994). Coexisting at this level, it is possible to found delicate understory red algae, e.g. Myriogramme, Gymnogongrus, and Georgiella (Amsler et al. 1995). Between 0 and 5 m depth, a zone marked by ice abrasion and waves, the substrate is colonized by fast-growing species, algae with an ability for re-sprout from basal shoots and crustose forms. In contrast, the intertidal rocky shores are dominated by ephemeral, turf species, mainly filamentous Chlorophyceans (e.g. Urospora, Ulva, Ulothrix) and the saccate brown alga Adenocystis utricularis (Huovinen and Gómez 2013; Marcías et al. 2017).
In areas outside the Western Antarctic Peninsula, e.g. around the Ross Sea and some sites along the East Antarctica, the diversity and abundance of seaweeds decreases and their vertical distribution is much more constrained by available substrata and the longer permanence of sea ice cover (Zaneveld 1966; Miller and Pearse 1991; Gambi et al. 1994; Johnston et al. 2007; Clark et al. 2011). In these sites, although some large Desmarestiales (e.g. H. grandifolius and D. menziesii) can be found at deeper locations, in general the coarsely branched red algae Iridaea cordata and Phyllophora antarctica are the dominant assemblages, especially at intermediate depths (between 2 and 20 m) (Cormaci et al. 2000) (Fig. 11.4b). Another particular feature of these ecosystems is the massive presence of crustose coralline red algae at deeper locations, especially of Phymatolithon foecundum (Hommersand et al. 2009), which can cover >70% of the available substrate under the canopy of red and brown algae (Irving et al. 2005). Remarkably, algae have to adapt to very low light conditions for primary productivity, irrespective of their functional form architecture. In fact, due to their extreme shade adaptation, these species can reach considerable depths and live with <2% of surface irradiances (Schwarz et al. 2003, 2005; see also Chap. 7 by Huovinen and Gómez).
4 Light Use Characteristics as a Major Factor Delineating Physiological Thallus Anatomy of Seaweeds
The arrangement of different functional forms along the depth gradient is strongly determined by different physico-chemical and biological factors. However, spatial and temporal availability of light is probably the most relevant factor by which zonation of Antarctic seaweeds can be explained. Because light governs the primary processes of photosynthesis, and, hence, primary productivity and biomass formation, Antarctic seaweeds, irrespective of their morphological organization, display efficient mechanisms of light harvesting. In fact, in the Antarctic, the marked seasonality in light availability defines strongly an intrinsic shade adaptation of macroalgae. Here the whole phenology of algae is closely tuned with the Antarctic light regime, which exposes organisms to darkness in winter (Wiencke et al. 2009). However, although in summer algae can be exposed to very high doses of solar radiation due to extended daylengths of up to 24 h at the highest latitudes, they do not acclimate and retain the capacity for very low light requirements for metabolism (Gómez and Huovinen 2015). This ability to use very low irradiances for photosynthesis and an intrinsic positive metabolic carbon balance (an indicator of compensation of carbon losses due to respiration) (Gómez et al. 1997; Deregibus et al. 2016) has important implications for the spatial dimension of the algal zonation: it allows Antarctic algae to colonize shaded locations, especially deeper sites. As a consequence, many Antarctic species can occupy extended ranges of depth and hence different light fields (Gómez et al. 1997). This situation contrasts with zonation patterns of various cold and temperate coasts, where the different algal groups are arranged in well-defined “belts” (Lüning 1990). Light trapping, especially under very limited conditions of irradiance, requires not only a specific pigment configuration but also morphological features such as thickness and thallus translucency, which are important in terms of absorptance of the different wavelengths (Gómez and Huovinen 2011). Algae increase light trapping through their thallus architecture, which can result in different in vivo spectral absorptance (Lüning and Dring 1985; Gómez et al. 2019). In Fig. 11.5, the spectral characteristics of several Antarctic macroalgae with different functional form and thickness are exemplified. In the case of thick leathery and coarsely branched morphs (e.g. Himantothallus, Desmarestia, Iridaea), attaining thallus thickness >500 μm, show high absorptance practically along the whole spectrum. In contrast, delicate morphs with thickness <100 μm, mostly foliose and finely branched such as Monostroma, Pantoneura, Myriogramme, and Pyropia, show decreased absorptance between 550 and 650 nm. Interestingly, some thin filamentous algae, e.g. Ulva intestinalis, Acrosiphonia, and Urospora, can exhibit high absorptance at these wavelengths, which is related to their turf arrangement, i.e. the overlapping of different filaments equals the several cell layers of thicker algae. Overall, these patterns are related to algal taxonomy and distribution in the zonation profile. In fact, thick leathery forms commonly belong to the brown algae, and their efficient light absorption over an extended range of wavelengths allows using the impoverished light field at higher depths (Gómez and Huovinen 2015). Similarly, understory coarsely branched and finely branched red algae, inhabiting deep sites with high absorption between 400 and 500 nm, are well suited to live at these depths (Gómez et al. 2019). Considering that, irrespective of their thallus shape and taxonomical affinity, Antarctic seaweeds are shade-adapted organisms, whose morphological and optical traits (e.g. thickness), pigment composition, and intrinsic photochemical capacities are superimposed in their vertical distribution (Huovinen and Gómez 2013; Gómez et al. 2019).
5 Form and Function in the Context of Life Strategies and Stress Tolerance
Since the publication of MacArthur and Wilson in 1967 on r and k selection, which put into a context the evolutionary divergences of organisms in relation to the production and care of offsprings, many studies have tried to expand these concepts to other adaptive traits (concept discussed in Pianka 1970). Because r and k strategies involve normally differences in energy allocation and, hence, body size, the form and function concept could be easily integrated in the theory (Grime 1981). For the case of marine macroalgae, in the 1980s, Joanna Kain used the terms “season responder” and “season anticipator” to describe the different phenological responses of seaweeds to seasonal changes in the environment (Kain 1987). Accordingly, “season responders” correspond to organisms that grow and reproduce under favourable environmental conditions, which could be analogous to r strategists, while “season anticipators” are organisms whose development is triggered by environmental factors at which they anticipate. The latter classification resembles k-selection strategy. Although not a strict rule, most of the season responders identified in the literature seem to correspond to delicate, small-sized forms, which normally exhibit an opportunistic strategy. In contrast a number of season anticipators can be associated with long-lived (perennial) seaweeds attaining normally large thalli (Kain 1989). However, although intuitively one may argue that differences in thallus complexity (including size) are correlated with differential responses to season, in general they are complex and depend on different environmental factors, type of biological indicator (growth, photosynthesis, reproduction, etc.), age and life history phase, and endogenous rhythms, all which can show complementary or divergent patterns (Kain 1986; Lüning and Kadel 1993; see also Chap. 10 by Navarro et al.).
In the Antarctic benthos, seaweeds are exposed to a marked seasonality, and thus, the concepts of “responders” and “anticipators” could explain well the different phenological patterns found in Antarctic seaweeds. In fact, various Antarctic species, e.g. Iridaea cordata, Ulva intestinalis, Acrosiphonia arcta, and Adenocystis utricularis, have been regarded as “season anticipators”, while Antarctic Desmarestiales, Ascoseira mirabilis and Palmaria decipiens, can be considered as “season anticipators” (Wiencke 1990a, b). Although these classifications were based mostly on growth responses to the Antarctic light regime, it has been shown that photosynthetic light use characteristics can respond in the same seasonal manner. For example, the brown alga Adenocystis utricularis and the red alga Iridaea cordata, two species regarded as responders, maintain high photosynthetic functionality still in winter, when light is very limited (Gutkowski and Maleszewski 1989; Weykam et al. 1997). This strategy is completely different in large Desmarestiales and Ascoseira mirabilis, as well as the red alga Palmaria decipiens, which activate their photosynthetic apparatus during early spring to optimize the available irradiance after the ice break-up (Gómez et al. 1995a, b; Wiencke et al. 2009). For large brown algae whose thalli can have length of various meters, these responses have important morpho-functional implications: Firstly, photosynthesis and growth during early spring are strongly synchronized to potentiate the use of newly fixed and stored carbon. Secondly, there is spatial separation between carbon production and sink zones with different metabolic activity, which can also be exposed to very contrasting light fields. Because these massive thick leathery species require compensation for the enhanced carbon burning due to high rates of dark respiration during the rapid biomass formation, the lamina elongation is powered by carbon stored in the previous season (Gómez and Wiencke 1998), similarly as in high-latitude kelps (e.g. Laminaria, Saccharina) (Dunton and Schell 1986). A well-studied case is Ascoseira mirabilis, which grows through the action of an intercalary meristem and presents “conducting channels” in medullary cell regions (Clayton and Ashburner 1990; Gómez et al. 1995b). In this species, during the growth phase, carbon stored as laminarin in distal parts is remobilized through the conducting cells (normally as mannitol and some amino acids) towards the meristem to replenish carbon substrates in the so-called light-independent carbon fixation (LICF) reactions (Kremer 1981; Gómez and Huovinen 2012). Such morpho-functional strategies have not only been demonstrated in large brown algae: in the perennial, coarsely branched red alga Palmaria decipiens, LICF reactions accounting 9% of the total fixed carbon have been reported (Weykam et al. 1997), suggesting that this type of mechanisms are operating in algae with complex thallus anatomy and season anticipation phenology, which allow them thriving at high depths and under extreme seasonality in the Antarctic.
Form and function in the context of stress tolerance have been revisited in the last years. Interestingly, several anatomical traits related to resistance to physical disturbance, e.g. multilayered architecture, thickness, and large size, are also functional to increase light trapping, e.g. efficient absorptance (Gómez and Huovinen 2011). Recently it was claimed that populations of three species of Desmarestiales (D. anceps, D. menziesii, and H. grandifolius) and Ascoseira mirabilis extending between 5 and 30 m depth show similar photosynthetic characteristics along the depth profile (Gómez and Huovinen 2015). However, not only the efficient and highly conserved light use across different irradiances but also an intrinsic capacity for UV stress tolerance was shown in these algae (see Chap. 7 by Huovinen and Gómez). Although all these traits conferring UV shielding show a strong overlapping with other factors, e.g. competence for space and overgrowth, scape from herbivores, there appears to exist a trade-off between photoprotection against enhanced solar UV radiation, mostly due to an increased thallus cross section (low area/weight ratio) and ultrastructural compounds, and highly efficient shade adaptation (Gómez and Huovinen 2015). A key element explaining this feature in algae rarely exposed to UV radiation is their constitutively high levels of phenolics (phlorotannins) (Flores-Molina et al. 2016). These secondary metabolites in Antarctic brown algae represent multifunctional compounds with putative roles in, e.g. resistance to grazing, temperature, and UV radiation (Amsler et al. 2005; Iken et al. 2009; Huovinen and Gómez 2013; Rautenberger et al. 2015) (for a description of functional roles of phlorotannins in Antarctic seaweeds, see Chap. 17 by Amsler et al. and Chap. 18 by Gómez and Huovinen).
In the case of delicate morphs, mostly filamentous and finely branched green algae, the opportunistic life strategy of these organisms allows them to respond rapidly to environmental stressors and, in virtue of their high metabolic rates per weight, to restore the homeostasis at short term (Holzinger and Karsten 2013). Albeit stress tolerance of seaweeds living in the intertidal zone would rely on highly efficient metabolic adjustments (Holzinger and Lutz 2006; Karsten et al. 2009; Gómez and Huovinen 2011), some structural adaptations have been described. For example, in Urospora penicilliformis a dense cell wall, presence of mucilage and external mineral deposition provide efficient shielding from high solar radiation and desiccation (Roleda et al. 2010). In many cases, filamentous green algae can form mats or turf-like structures that are effective to minimize the harmful effects of changing environment (Bischof et al. 2006). In all, in terms of photosynthetic characteristics and physiological responses to stress, form and function of some Antarctic seaweed assemblages have been related to biogeographic affinity and depth. Based on 31 species from King George Island, three major groups of species were defined: (a) coarsely branched Rhodophyta are mostly found at shallow subtidal sites and have an Antarctic-sub-Antarctic origin; (b) endemic Antarctic brown algae are dominant at depths between 10 and 30 m and practically all attain thick leathery morphology; and (c) filamentous and sheet-like green algae, mostly intertidal species, normally can be categorized as algae with wide geographic distribution (Gómez et al. 2019).
6 Functional Traits of Seaweeds and Properties of Benthic Communities
Seaweeds in practically all cold-temperate and polar coastal ecosystems represent foundational organisms, whose processes and fate determine key community indicators, such as structure and functional and taxonomic richness (Chapman 1987; Lüning 1990). In King George Island, the distribution and composition of different functional groups in both intertidal and subtidal sites are regulated by different factors, which are defined by some species that account by 90% of the dissimilarities between depth strata (Valdivia et al. 2014). When representative taxa are analysed, effects are scale dependent: variance components increase at the finer scale of variation (from centimeters to meters) compared to shore level (hundreds of meters) (Valdivia et al. 2014). In the intertidal system dominated by filamentous and finely branched morphs, the grazing by the limpet Nacella concinna is probably one of the most important biological interactions (Kim 2001; Segovia-Rivera and Valdivia 2016). Apart from green algae, N. concinna exerts control on periphyton, thus determining far-reaching ecological processes, e.g. the fate of re-colonization and succession in these systems (Campana et al. 2009; Valdivia et al. 2019; see also Chap. 12 by Campana et al. and Chap. 13 by Valdivia).
At the subtidal zone, facilitative interactions held by large brown algae through bioengineering seem relevant for the structure and maintenance of the benthic communities (Valdivia et al. 2015). These canopy-forming seaweeds are important as they shelter other species of algae and invertebrates from harmful environmental conditions and thus have an important effect on the community biomass of the whole ecosystem (Valdivia et al. 2015; Ortiz et al. 2016; see also Chap. 15 by Momo et al. and Chap. 16 by Ortiz et al.). However, in locations exposed to severe impact of physical disturbance, small organisms can be favoured while canopy-forming algae would be more sensitive (Smale 2007). For example, in eastern Antarctica where ice cover can be considerably extended through spring, canopy-forming macroalgae were only abundant at sites where sea-ice cover break-up occurs during spring, but absent at sites that retained ice cover until summer (Johnston et al. 2007). Thus, these organisms appear to respond slowly to the changing environment due, for example, to enhanced warming. For example, in new ice-free areas originated from glacier retreat where enhanced sediment input limits light penetration, establishment of large brown algae is highly constrained (Quartino et al. 2013). In these highly perturbed sites, ice scouring and unconsolidated substrate affect considerably the presence of canopy-forming algae and hence the taxonomic richness (Klöser et al. 1994; Smale 2007; Smale et al. 2008; Valdivia et al. 2015). On the other hand, environmental shifts driven by climate change can affect the morpho-functional responses of Antarctic species. For example, physiology of canopy-forming algae (e.g. Desmarestia spp.) may have consequences for the whole benthic community (Schoenrock et al. 2015). In the case of crustose species, fleshy encrusting forms (Hildenbrandia) could be favoured in scenarios of changing pH and temperature compared to calcified Coralline species (Clathromorphum) (Schoenrock et al. 2016). In general, morpho-functional and anti-stress mechanisms of macroalgae to cope with sharp physical gradients percolate towards upper hierarchies through insurance of functional richness in the community, which set high degree of resilience to physical perturbation (Ortiz et al. 2016, 2017) or to minimize the impact of alien species (Arenas et al. 2006; see Chap. 16 by Ortiz et al.).
Considering some functional form models for marine seaweeds, similitudes and analogies with terrestrial vegetation strategies can be identified. For example, according to the functional groups described by Grime (1981) for terrestrial vegetation, opportunistic green algae growing at the intertidal zone could correspond to the “ruderal” species, permanently subjected to strongly physical perturbation. In contrast, large endemic brown algae, which thrive in sites with lower physical perturbation, can be analogue to “competitive species” in virtue of their exuberant canopy and perennial characteristics. For many temperate ecosystems, the life history traits conferring advantages under high levels of disturbance are convergent in different types of algal assemblages, suggesting that some patterns could be generalized (Steneck and Dethier 1994). However, in Antarctic communities some factors associated with disturbance and stress require adjustment to the extreme Antarctic conditions. In the conceptual framework in Fig. 11.6, three major functional groups of Antarctic seaweeds (turf algae, dominated by intertidal green algae; canopy-forming algae, especially large brown algae; and corticated red algae, grouping many understory species) can be oriented through the three axes following a Grime’s CSR triangle schema. Here, the extreme action of ice (perturbation), light limitation (stress), and biomass (competition) dimensions determine the separation among algal groups. Corticate red algae in virtue of their extreme shade adaptation represent the stress tolerant group. Here, many crustose species growing at very low light conditions in the eastern Antarctic can also be added to this group. In the perturbation axis, filamentous and finely branched green algae and some little saccate brown algae (Adenocystis utricularis) exemplify the colonizers, well adapted to occupy sites highly perturbed by ice, terrestrial run-off, and high solar radiation. Under these conditions abundance and species richness are less influenced by biological interactions (Valdivia et al. 2014; Segovia-Rivera and Valdivia 2016). Finally, the canopy-forming algae, represented by species of the order Desmarestiales, and Ascoseira and Cystosphaera that exhibit high biomass production, are dominating at sites with lower physical perturbation. However, they show competitive abilities for light and substrate (Gómez et al. 1997; Valdivia et al. 2015).
7 Concluding Remarks
The main ecological expression of the morpho-functional adaptation of Antarctic seaweeds is the macroalgal zonation, which is not only a vertical arrangement of species but also represents an ordination of organismal traits that can be classified in different functional entities (e.g. gross morphology, life forms, physiological responses). These attributes can be scaled up to community structure and ecosystem functioning. The concept, well studied in plants, has been revitalized in the last decade in the context of the contemporary climate change.
Due to the seasonally changing light conditions, characterized in the highest latitudes by several months of very dim light, Antarctic seaweeds are adapted to very low light levels. In contrast, after the ice break-up in spring, they suddenly can be exposed to strong solar radiation. Thus the adaptations of Antarctic algae are finely tuned with the daylength, changes in water turbidity, and ice perturbations. This environmental variability is fully exploited by seaweeds in virtue of their efficient morpho-functional adaptations. However, due to climate change, the environmental settings in which Antarctic seaweeds have evolved for millions of years are changing. In these new scenarios, the adaptive capacities of these organisms as well as the ecosystem functions they provide will be challenged (Constable et al. 2014; Gutt et al. 2015). Although one can recognize that polar seaweeds are particularly susceptible to these changes with unpredictable consequences for the whole coastal ecosystem, we have still a limited understanding on how physiological and morphological traits respond and how they will be integrated in, for example, molecular mechanisms of environmental tolerance and stress resilience.
References
Ackerly DD, Reich PB (1999) Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. Am J Bot 86:1272–1128
Aguilera J, Karsten U, Lippert H, Vögele B, Philipp E, Hanelt D, Wiencke C (1999) Effects of solar radiation on growth, photosynthesis and respiration of marine macroalgae from the Arctic. Mar Ecol Prog Ser 191:109–119
Alongi G, Cormaci M, Furnari G (2002) The Corallinaceae (Rhodophyta) from the Ross Sea (Antarctica): a taxonomic revision rejects all records except Phymatolithon foecundum. Phycologia 41:140–146
Amsler CD, Rowley RJ, Laur DR, Quetin LB, Ross RM (1995) Vertical distribution of Antarctic peninsular macroalgae: cover, biomass and species composition. Phycologia 34:424–430
Amsler CD, Iken K, McClintock JB, Amsler MO, Peters KJ, Hubbard JM, Furrow FB, Baker JB (2005) Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Mar Ecol Prog Ser 294:141–159
Arenas F, Sánchez I, Hawkins SJ, Jenkins SRJ (2006) The invasibility of marine algal assemblages: role of functional diversity and identity. Ecology 87(11):2851–2861
Balata D, Piazzi L, Rindi F (2011) Testing a new classification of morphological functional groups of marine macroalgae for the detection of responses to stress. Mar Biol 158:2459–2469
Bischof K, Rautenberger R, Brey L, Pérez-Lloréns JL (2006) Physiological acclimation to gradients of solar irradiance within mats of the filamentous green macroalga Chaetomorpha linum from southern Spain. Mar Ecol Prog Ser 306:165–175
Campana GL, Zacher K, Fricke A, Molis M, Wulff A, Quartino ML, Wiencke C (2009) Drivers of colonization and succession in polar benthic macro- and microalgal communities. Bot Mar 52:655–667
Chapman ARO (1987) Population and community ecology of seaweeds. Adv Mar Biol 23:1–161
Clark GF, Stark JS, Perrett LA, Hill NA, Johnston EL (2011) Algal canopy as a proxy for the disturbance history of understorey communities in East Antarctica. Polar Biol 34:781–790
Clayton MN, Ashburner CM (1990) The anatomy and ultrastructure of “conducting channels” in Ascoseira mirabilis (Ascoseirales, Phaeophyceae). Bot Mar 33:63–70
Clayton M, Wiencke C, Klöser H (1997) New records of temperate and sub Antarctic marine benthic macroalgae from Antarctica. Polar Biol 17:141–179
Constable AJ, Melbourne-Thomas J, Corney SP, Arrigo KR, Barbraud C, Barnes DK, Bindoff NL et al (2014) Climate change and Southern Ocean ecosystems. I: how changes in physical habitats directly affect marine biota. Glob Chang Biol 20:3004–3025. https://doi.org/10.1111/gcb.12623
Cormaci M, Furnari G, Scammacca B (2000) The macrophytobenthos of Terra Nova Bay. In: Faranda FM et al (eds) Ross Sea ecology. Italian Antarctic expeditions (1987–1995). Springer, Berlin, pp 493–502
DeLaca TE, Lipps JH (1976) Shallow-water marine associations, Antarctic Peninsula. Antarct J US 11:12–20
Delépine R, Lamb JM, Zimmermann MH (1966) Preliminary report on the vegetation of the Antarctic Peninsula. Proc Int Seaweed Symp 5:107–116
Deregibus D, Quartino ML, Campana GL, Momo FR, Wiencke C, Zacher K (2016) Photosynthetic light requirements and vertical distribution of macroalgae in newly ice-free areas in Potter Cove, South Shetland Islands, Antarctica. Polar Biol 39:153–166
Dunton KH, Schell DM (1986) Seasonal carbon budget and growth of Laminaria solidungula in the Alaskan high Arctic. Mar Ecol Prog Ser 31:57–66
Flores-Molina MR, Muñoz P, Rautenberger R, Huovinen P, Gómez I (2016) Stress tolerance to UV radiation and temperature of the endemic Antarctic brown alga Desmarestia anceps is mediated by high concentrations of phlorotannins. Photochem Photobiol 92:455–466
Gambi M, Lorent IM, Russo G, Scipione M (1994) Benthic associations of the shallow hard bottoms off Terra Nova Bay, Ross Sea: zonation, biomass and population structure. Antarct Sci 6:449–462
Gómez I, Huovinen P (2011) Morpho-functional patterns and zonation of south Chilean seaweeds: the importance of photosynthetic and bio-optical traits. Mar Ecol Prog Ser 422:77–91
Gómez I, Huovinen P (2012) Morpho-functionality of carbon metabolism in seaweeds. In: Wiencke C, Bischof K (eds) Seaweed biology: novel insights into ecophysiology, ecology and utilization. Springer, Berlin, pp 25–46
Gómez I, Huovinen P (2015) Lack of physiological depth patterns in conspecifics of endemic Antarctic brown algae: a trade-off between UV stress tolerance and shade adaptation? PLoS One 10(8):e0134440
Gómez I, Wiencke C (1998) Seasonal changes in C, N and major organic compounds, and their significance to morpho-functional processes in the endemic Antarctic brown alga Ascoseira mirabilis. Polar Biol 19:115–124
Gómez I, Thomas DN, Wiencke C (1995a) Longitudinal profiles of growth, photosynthesis and light independent carbon fixation in the Antarctic brown alga Ascoseira mirabilis. Bot Mar 38:157–164
Gómez I, Wiencke C, Weykam G (1995b) Seasonal photosynthetic characteristics of Ascoseira mirabilis (Ascoseirales, Phaeophyceae) from King George Island, Antarctica. Mar Biol 123:167–172
Gómez I, Weykam G, Klöser H, Wiencke C (1997) Photosynthetic light requirements, daily carbon balance and zonation of sublittoral macroalgae from King George Island (Antarctica). Mar Ecol Prog Ser 148:281–293
Gómez I, Navarro NP, Huovinen P (2019) Bio-optical and physiological patterns in Antarctic seaweeds: a functional trait based approach to characterize vertical zonation. Prog Oceanogr 174:17–27
Grime JP (ed) (1981) Plant strategies and vegetation processes. Wiley, Chichester, p 222
Grosberg RK, Strathmann RR (2007) The evolution of multicellularity: a minor major transition? AREES 38:621–654. https://doi.org/10.1146/annurev.ecolsys.36.102403.114735
Gutkowski R, Maleszewski S (1989) Seasonal changes of the photosynthetic capacity of the Antarctic macroalga Adenocystis utricularis (Bory) Skottsberg. Polar Biol 10:145–148
Gutt J, Bertler N, Bracegirdle TJ, Buschmann A, Comiso J, Hosie G, Isla E, Schloss IR et al (2015) The Southern Ocean ecosystem under multiple climate change stresses – an integrated circumpolar assessment. Glob Chang Biol 21:1434–1453. https://doi.org/10.1111/gcb.12794
Hay ME (1986) Functional geometry of seaweeds: ecological consequences of thallus layering and shape in contrasting light environments. In: Givnish TJ (ed) On the economy of plant form and function. Proceedings of the Sixth Maria Moors Cabot Symposium, “Evolutionary Constraints on Primary Productivity: Adaptive Patterns of Energy Capture in Plants”, Harvard Forest, August 1983. Cambridge University Press, Cambridge
Holzinger A, Karsten U (2013) Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological, and molecular mechanisms. Plant Sci 22:327. https://doi.org/10.3389/fpls.2013.00327
Holzinger A, Lütz C (2006) Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron 37:190–207. https://doi.org/10.1016/j.micron.2005.10.015
Hommersand MH, Moe RL, Amsler CD, Fredericq S (2009) Notes on the systematics and biogeographical relationships of Antarctic and sub-Antarctic Rhodophyta with descriptions of four new genera and five new species. Bot Mar 52:509–534
Huovinen P, Gómez I (2013) Photosynthetic characteristics and UV stress tolerance of Antarctic seaweeds along the depth gradient. Polar Biol 36:1319–1332
Iken K, Amsler CD, Amsler MO, McClintock JB, Baker BJ (2009) Field studies on deterrent roles of phlorotannins in Antarctic brown algae. Bot Mar 52:547–557
Ingólfsson A (2005) Community structure and zonation patterns of rocky shores at high latitudes: an interocean comparison. J Biogeogr 32:169–182
Innes DJ (1984) Genetic differentiation among populations of marine algae. Helgoländer Meeresunters 38:401–417
Irving A, Connell S, Johnston E, Pile A, Gillanders B (2005) The response of encrusting coralline algae to canopy loss: an independent test of predictions on an Antarctic coast. Mar Biol 147:1075–1083
Johnston EL, Connell SD, Irving AD, Pile AJ, Gillanders BM (2007) Antarctic patterns of shallow subtidal habitat and inhabitants in Wilke’s land. Polar Biol 30:781–788
Kaandorp JA, Kübler JE (eds) (2001) The algorithmic beauty of seaweeds, sponges and corals. Springer, Heidelberg, p 194
Kain JM (1986) Plant size and reproductive phenology of six species of Rhodophyta in subtidal Isle of Man. Br Phycol J 21:129–138
Kain JM (1987) Seasonal growth and photoinhibition in Plocamium cartilagineum (Rhodophyta) off the Isle of Man. Phycologia 26:88–99
Kain JM (1989) The seasons in the subtidal. Br Phycol J 24:203–215
Karsten U, Wulff A, Roleda MY, Müller R, Steinhoff FS, Fredersdorf J, Wiencke C (2009) Physiological responses of polar benthic algae to ultraviolet radiation. Bot Mar 52:639–654
Kim D (2001) Seasonality of marine algae and grazers of an Antarctic rocky intertidal, with emphasis on the role of the limpet Nacella concinna Strebel (Gastropoda: Patellidae). Fachbereich Biologie/Chemie, Ph.D. thesis, University of Bremen, Germany
Klöser H, Mercuri G, Laturnus F, Quartino ML, Wiencke C (1994) On the competitive balance of macroalgae at Potter Cove (King George Island, South Shetlands). Polar Biol 14:11–16
Kremer BP (1981) Aspects of carbon metabolism in marine macroalgae. Oceanogr Mar Biol Annu Rev 19:41–94
Lamb IM, Zimmermann MH (1977) Benthic marine algae of the Antarctic Peninsula. Antarct Res Ser 23:130–229
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the holy grail. Funct Ecol 16:545–556
Littler MM, Littler DS (1980) The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. Am Nat 116:25–44
Lüning K (ed) (1990) Seaweeds: their environment, biogeography and ecophysiology. Wiley, New York, p 527
Luning K, Dring MJ (1985) Action spectra and spectral quantum yield of photosynthesis in marine macroalgal with thin and thick thalli. Mar Biol 87:119–129
Lüning K, Kadel P (1993) Daylength range for circannual rhythmicity in Pterygophora californica (Alariaceae, Phaeophyta) and synchronization of seasonal growth by daylength cycles in several other brown algae. Phycologia 32:379–387
Marcías ML, Deregibus D, Saravia LA, Campana GL, Quartino ML (2017) Life between tides: spatial and temporal variations of an intertidal macroalgal community at Potter Peninsula, South Shetland Islands, Antarctica. Estuar Coast Shelf Sci 187:193–203
Miller KA, Pearse JS (1991) Ecological studies of seaweeds in McMurdo Sound, Antarctica. Am Zool 31:35–48
Moe RL, Silva PC (1981) Morphology and taxonomy of Himantothallus (including Phaeoglossum and Phyllogigas), an Antarctic member of the Desmarestiales (Phaeophyceae). J Phycol 17:15–29. https://doi.org/10.1111/j.1529-8817.1981.tb00814.x
Mystikou A, Peters AF, Asensi AO, Fletcher KF, Brickle P, van West P, Convey P, Küpper FC (2014) Seaweed biodiversity in the south-western Antarctic Peninsula: surveying macroalgal community composition in the Adelaide Island/Marguerite Bay region over a 35-year time span. Polar Biol 37(11):1607–1619
Neushul M (1965) Diving observations of subtidal Antarctic marine vegetation. Bot Mar 8:234–243
Ortiz M, Berríos F, González J, Rodríguez-Zaragoza FF, Gómez I (2016) Macroscopic network properties and short-term dynamic simulations in coastal ecological systems at Fildes Bay (King George Island, Antarctica). Ecol Complex 28:145–157. https://doi.org/10.1016/j.ecocom.2016.06.003
Ortiz M, Hermosillo-Nuñez B, González J, Rodríguez-Zaragoza F, Gómez I, Jordán F (2017) Quantifying keystone species complexes: ecosystem-based conservation management in the King George Island (Antarctic Peninsula). Ecol Indic 81:453–460. https://doi.org/10.1016/j.ecolind.2017.06.016
Padilla DK, Allen BJ (2000) Paradigm lost: reconsidering functional form and group hypotheses in marine ecology. J Exp Mar Biol Ecol 250:207–221
Phillips JC, Kendrick GA, Lavery PS (1997) A test of a functional group approach to detecting shifts in macroalgal communities along a disturbance gradient. Mar Ecol Prog Ser 153:125–138
Pianka ER (1970) On r and K selection. Am Nat 104(940):592–597
Poorter L, Bongers F (2006) Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743
Quartino ML, Deregibus D, Campana GL, Edgar G, Latorre J, Momo FR et al (2013) Evidence of macroalgal colonization on newly ice-free areas following glacial retreat in Potter Cove (South Shetland Islands), Antarctica. PLoS One 8:e58223
Ramírez ME (2010) Flora marina bentónica de la región austral de Sudamérica y la Antártica. An Inst Patagon 38(1):57–71
Rautenberger R, Huovinen P, Gómez I (2015) Effects of increased seawater temperature on UV-tolerance of Antarctic marine macroalgae. Mar Biol 162:1087–1109
Roleda MY, Lütz-Meindl U, Wiencke C, Lütz C (2010) Physiological, biochemical, and ultrastructural responses of the green macroalga Urospora penicilliformis from Arctic Spitsbergen to UV radiation. Protoplasma 243:105–116
Schoenrock KM, Schram JB, Amsler CD, McClintock JB, Angus RA (2015) Climate change impacts on overstory Desmarestia spp. from the western Antarctic Peninsula. Mar Biol 162:377–389. https://doi.org/10.1007/s00227-014-2582-8
Schoenrock KM, Schram JB, Amsler CD, McClintock JB, Angus RA, Vohra YK (2016) Climate change confers a potential advantage to fleshy Antarctic crustose macroalgae over calcified species. J Exp Mar Biol Ecol 474:58–66
Schwarz A, Hawes I, Andrew N, Norkko A, Cummings V, Thrush S (2003) Macroalgal photosynthesis near the southern global limit for growth, Cape Evans, Ross Sea, Antarctica. Polar Biol 26:789–799
Schwarz A-M, Hawes I, Andrew N, Mercer S, Cummings V, Thrush S (2005) Primary production potential of non-geniculate coralline algae at Cape Evans, Ross Sea, Antarctica. Mar Ecol Prog Ser 294:131–140
Segovia-Rivera V, Valdivia N (2016) Independent effects of grazing and tide pool habitats on the early colonisation of an intertidal community on western Antarctic Peninsula. Rev Chil Hist Nat 89:3
Smale DA (2007) Ice disturbance intensity structures benthic communities in nearshore Antarctic waters. Mar Ecol Prog Ser 349:89–102
Smale DA, Brown KM, Barnes DKA, Fraser KPP, Clarke A (2008) Ice scour disturbance in Antarctic waters. Science 321:371
Steneck RS, Dethier MN (1994) A functional group approach to the structure of algal-dominated communities. Oikos 69:476–498
Steneck RS, Watling L (1982) Feeding capabilities and limitations of herbivorous molluscs: a functional group approach. Mar Biol 68:299–319
Taylor PR, Hay ME (1984) Functional morphology of intertidal seaweeds: adaptive significance of aggregate vs. solitary forms. Mar Ecol Prog Ser 18:295–302
Valdivia N, Díaz MJ, Holtheuer J, Garrido I, Huovinen P, Gómez I (2014) Up, down, and all around: scale-dependent spatial variation in rocky-shore communities of Fildes Peninsula, King George Island, Antarctica. PLoS One 9(6):e100714. https://doi.org/10.1371/journal.pone.0100714
Valdivia N, Díaz MJ, Garrido I, Gómez I (2015) Consistent richness-biomass relationship across environmental stress gradients in a marine macroalgal-dominated subtidal community on the Western Antarctic Peninsula. PLoS One 10(9):e0138582
Valdivia N, Huovinen P, Gómez I, Macaya E, Pardo LM (2019) Different ecological mechanisms lead to similar grazer controls on the functioning of periphyton Antarctic and sub-Antarctic communities. Prog Oceanogr 174:7–16. https://doi.org/10.1016/j.pocean.2018.01.008
Weykam G, Thomas DN, Wiencke C (1997) Growth and photosynthesis of the Antarctic red algae Palmaria decipiens (Palmariales) and Iridaea cordata (Gigartinales) during and following extended periods of darkness. Phycologia 36:395–405
Wiencke C (1990a) Seasonality of brown macroalgae from Antarctica – a long-term culture study under fluctuating Antarctic daylengths. Polar Biol 10:589–600
Wiencke C (1990b) Seasonality of red and green macroalgae from Antarctica – a long-term culture study under fluctuating Antarctic daylengths. Polar Biol 10:601–607
Wiencke C, Clayton MN (1990) Sexual reproduction, life history, and early development in culture of the Antarctic brown alga Himantothallus grandifolius (Desmarestiales, Phaeophyceae). Phycologia 29:9–18
Wiencke C, Clayton MN (2002) Antarctic seaweeds. In: Wägele JW (ed) Synopses of the Antarctic benthos, vol 9. A.R.G Gantner Verlag KG, Ruggell, p 239
Wiencke C, Gómez I, Dunton K (2009) Phenology and seasonal physiological performance of polar seaweeds. Bot Mar 52:585–592
Wiencke C, Amsler CD, Clayton MN (2014) Chapter 5.1. Macroalgae. In: De Broyer C, Koubbi P, Griffiths H, Raymond B, Udekem d’Acoz C et al (eds) Biogeographic atlas of the Southern Ocean, vol 8. Scientific Committee on Antarctic Research, Cambridge, pp 66–73
Zaneveld JS (1966) Vertical zonation of Antarctic and subantarctic benthic marine algae. Antarct J US 1(5):211–213
Acknowledgements
The authors acknowledge the financial support from Conicyt Chile (FONDAP 15150003, Anillo-PIA ART1101, Fondecyt 1161129) and INACH T-20-09 from the Instituto Antártico Chileno. The helpful assistance and collaboration of the members of our laboratories at Universidad Austral de Chile as well as the staff of the Instituto Antártico Chileno for during various Antarctic expeditions are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gómez, I., Huovinen, P. (2020). Form and Function in Antarctic Seaweeds: Photobiological Adaptations, Zonation Patterns, and Ecosystem Feedbacks. In: Gómez, I., Huovinen, P. (eds) Antarctic Seaweeds. Springer, Cham. https://doi.org/10.1007/978-3-030-39448-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-39448-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39447-9
Online ISBN: 978-3-030-39448-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)