Abstract
Interventional radiology is widely applied in hepatic pathology, both with diagnostic and therapeutic purposes. In this chapter its main applications are analyzed, and technical details of some procedures are explained with particular emphasis to transjugular intrahepatic portosystemic shunt (TIPS) and post transplant vascular complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Interventional radiology plays a double role in the imaging of the vascular diseases of the liver: diagnostic and interventional, each with individual claims but often closely related.
Ultrasonography (US) and contrast-enhanced cross-sectional imaging (mainly CT angiography and less frequently MR angiography) represent important noninvasive diagnostic tools in the study of hepatic vascular diseases.
Nowadays US with color Doppler mode is a safe technology mostly used in the follow-up and early detection of abnormal hepatic vascular flow. Although in the literature there are many useful criteria in the diagnostic process, they are not properly easy to study in a substantial number of patients. In this context contrast-enhanced ultrasonography (CEUS) is spreading as an alternative tool in assessing hepatic vessel patency (for example in portal thrombosis study).
CT angiography is a high-performance noninvasive imaging that, despite its static nature, is often essential before any therapeutic approach because it provides a panoramic anatomy of hepatic vessels.
On the other hand, interventional radiology holds a central position in the diagnostic process of hepatic vascular disease, in particular through the opportunity to perform highly sensitive dynamic exams, by making angiography the diagnostic gold standard in many vascular abnormalities.
While the utility of diagnostic angiography could be questioned given the number of noninvasive imaging techniques, its interventional role continues to grow. Many vascular interventional procedures are increasingly gaining ground: percutaneous transluminal angioplasty, stent placement, arterial embolization, transjugular intrahepatic portosystemic shunt, and portal embolization.
It is important to underline that interventional radiology is not in opposition to traditional surgical approaches, but it is complementary to it. This is especially true with regard to vascular complications following liver transplantation, where critical patients have to be handled by a multidisciplinary team, in particular during the first 30 days after surgery characterized by a greater number of complications, usually with high mortality rate.
1.1 Hepatic Artery Angiography
Diagnostic hepatic artery angiography, even including celiac and superior mesenteric arteries, is performed under conscious sedation to determine liver arterial supply and patency of the portal vein in a later phase. Variant hepatic artery anatomy is present almost in half of the population. This evaluation is usually done using the femoral route; brachial or radial approaches are also feasible, especially in cases with bilateral femoro-iliac occlusion, extremely tortuous iliac axes, or femoral surgical grafts.
The technique includes the infiltration of the skin and subcutaneous tissue with a small amount of local anesthetic (lidocaine 1%) before puncturing the common femoral, brachial, or radial artery; single-wall puncture is preferable. Therefore a short 0.035″ guidewire is advanced to introduce a 4–5 French sheath. Especially in the presence of atheromatous or calcified iliac arteries, a 0.035″ hydrophilic coated guidewire is advanced in the abdominal aorta, in order to move a diagnostic catheter (with a Rosch Celiac, Cobra 1, or Sidewinder configuration) towards the celiac artery. After the catheterization of the celiac artery, which is approximately at the level of T12, a selective angiography is performed injecting 30 mL of iodinated nonionic contrast, preferably at high concentration (320/370 mgI/mL), at 5 mL/s. When the diagnostic problem is focused on the arterial supply, arterial and parenchymal phases are usually performed, with a frame rate of acquisition of at least 3 frames/s in the first phase and of 2 frames/s in the parenchymal one. Long acquisition is used to visualize the portal system.
1.2 Hepatic and Portal Vein Technique
Venous access is necessary to perform several diagnostic or therapeutic hepatic interventional radiology procedures, via jugular vein, like hepatic vein pressure gradient (HVPG) measurement or transjugular intrahepatic portosystemic shunt (TIPS), and percutaneously for the portal access, like portal embolization.
Local anesthesia (i.e., lidocaine) on the access site is performed; subsequently venous puncture is done under ultrasound guidance with the Seldinger technique.
An access system is then positioned, appropriately as per type, caliber, and length for the materials necessary for the procedure.
Further details are provided in the dedicated sections of this chapter.
2 Hepatic Vein Pressure Gradient (HVPG)
2.1 Introduction
Portal hypertension (PH) is a frequent clinical syndrome defined as a pathological increase in portal pressure gradient (PPG) which is the difference in pressure between the portal vein and the inferior vena cava and represents the perfusion pressure of the liver with portal blood.
The normal range of the PPG is 1–5 mmHg; values between 5 and 9 mmHg represent subclinical portal hypertension.
When the PPG increases to ≥10 mmHg, complications of portal hypertension can arise; these complications incorporate formation of portosystemic collaterals, varices (e.g., esophageal, gastric, and hemorrhoids), congestive gastropathy, hypersplenism, disturbance in the metabolism of drugs or endogenous substances that are normally eliminated by the liver, and severe ones, such as upper gastrointestinal bleeding resulting from ruptured gastroesophageal varices, ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, porto-pulmonary hypertension, and hepatic encephalopathy.
The causes of portal hypertension can be classified according to their anatomical location as prehepatic (involving the splenic, mesenteric, or portal veins, e.g., portal vein thrombosis), intrahepatic (parenchymal liver disease), and posthepatic (diseases involving the hepatic venous outflow, e.g., Budd-Chiari syndrome).
Hepatic cirrhosis is the main cause of this syndrome in Western countries; it is the 14th most common cause of death worldwide but 4th in central Europe (Tsochatzis et al. 2014).
The measurement of the portal vein pressure was first attempted by Hallion and Francois-Frank but was invasive and impractical in terms of clinical practice (Hallion and Francois-Frank 1896).
Myers and Taylor first described the measurement of wedged hepatic venous pressure (WHVP), which reflected sinusoidal pressure, an indirect measure of PVP (Myers and Taylor 1951).
The currently preferred technique for determining portal venous pressure involves, through catheterization of the hepatic vein, measurement of the hepatic venous pressure gradient (HVPG) which is the difference between the WHVP and the free hepatic venous pressure (FHVP):
This method has almost totally replaced direct measurement of portal pressure by more invasive techniques, such as splenic pulp puncture and percutaneous transhepatic or transvenous catheterization of the portal vein. These last direct techniques for determining the portal pressure gradient require the simultaneous puncture of a hepatic vein and are used only in specific cases, almost entirely to determine presinusoidal portal hypertension.
The WHVP is measured by occluding the hepatic vein; stopping the blood flow causes the static column of blood to equalize in pressure with the proximal vascular territory, in this case, the hepatic sinusoids. So WHVP is a measure of hepatic sinusoidal pressure, not of portal pressure. In the normal liver WHVP is slightly lower (approximately 1 mmHg) than portal pressure; this fact is due to the low-resistant sinusoidal system that dissipates most of the pressure (Groszmann and Wongcharatrawee 2004). In liver cirrhosis the connections between sinusoids are disrupted by the presence of fibrous septa and nodule formation and consequently the static column of blood created by occluding the hepatic vein cannot be dispersed (Bosch et al. 2006). So in this case WHVP gives an accurate estimate of portal pressure gradient (PPG) (Perello et al. 1999).
FHVP is a measure of the pressure of the unoccluded hepatic vein.
There are two techniques for measuring WHVP: catheter advancement technique and balloon occlusion technique. In the former, the catheter is pushed down in the hepatic vein until it cannot be advanced further; this results in a complete obstruction of the venous flow and the pressure recorded in this occluded position is the WHVP.
The second one, the balloon occlusion technique, was validated by Groszmann et al. (1979). It requires the use of a balloon-tipped catheter; inflation and deflation of the balloon within the hepatic vein allow measurement of wedged and free pressures without the need to advance and retract the catheter for each WHVP and FHVP determination.
Using the catheter advancement technique the WHVP is measured in a small hepatic venule. Keiding and Vilstrup showed different values of the WHVP when the catheter is advanced in different hepatic veins and the heterogeneity of sinusoidal involvement in diseases like liver cirrhosis is probably the cause of these differences (Keiding and Vilstrup 2002; Maharaj et al. 1986).
In contrast, the balloon occlusion technique is preferred because it allows measurement in the hepatic veins at the lobar and sublobar levels. The obtained pressure is an average of pressures in several segments of the liver and thus represents more accurately the true portal venous pressure (Groszmann and Wongcharatrawee 2004).
2.2 Technique
Patient should be informed about the technique and its risks; he/she must have fasted for at least 6 h and a written informed consent should be provided.
Clotting studies, serum creatinine, and an adequate management of anticoagulant therapy are required before the exam. A peripheral venous access should be placed.
The procedure is performed in an interventional radiology room, under strictly aseptic conditions, and patient’s vital signs (blood pressure, digital oxygen saturation, electrocardiographic parameters, and heart rate) are monitored during the procedure.
Conscious sedation with low-dose midazolam (0.02 mg/kg intravenously) increases patient comfort and relieves anxiety without modifying hepatic pressures (Steinlauf et al. 1999).
Previous US evaluation gives precise information of topographic location of the right internal jugular vein and confirms its permeability; if this access is not feasible, left internal jugular, antecubital vein, or femoral vein can be used.
Doppler US should be used to facilitate venous localization and puncture and to avoid complications.
After skin’s disinfection, positioning of a sterile drop, and subcutaneous local anesthetic infiltration, under US guidance, the right internal jugular vein is punctured using an 18-gauge needle connected to a saline-filled syringe.
Under fluoroscopic control, a 0.035 in. J-tipped guidewire is inserted into the vein and the introducer is passed through according to Seldinger technique over the guidewire.
A J-tipped 0.035 in. flexible hydrophilic guide and an end-hole catheter or a balloon-tipped catheter are inserted through the introducer via the superior vena cava, right atrium, inferior vena cava, and right hepatic vein or an appropriate alternative hepatic vein.
FHVP is measured by maintaining the tip of the catheter “free” in the hepatic vein, at 2–4 cm from its opening into the inferior vena cava (Fig. 1). The FHVP should be similar in value to the inferior vena cava (IVC) pressure; IVC pressure should be measured at the level of the hepatic vein ostium. A difference of >2 mmHg signifies that the catheter is probably inadequately placed or that a hepatic vein obstruction exists.
WHVP is measured by occluding the hepatic vein, either by “wedging” the catheter into a small branch of a hepatic vein or by inflating a balloon at the tip of the catheter.
The slow injection of 2–5 mL of contrast dye confirms the accurate occlusion of the hepatic vein; this method should be in a typical “wedged” pattern (sinusoidogram) without observing any reflux of the contrast or its washout through shunts with other hepatic veins (Figs. 2 and 3). If adequate occlusion is not achieved, the reading should not be considered and a new reading is taken and occlusion reconfirmed.
It is important that the WHVP readings should always be taken before injecting the contrast medium; otherwise the value would be falsely high and the catheter should be carefully washed with heparinized saline solutions before taking each set of readings.
As mentioned above the use of a balloon-tipped catheter, the preferred technique, reduces measurement variability.
FHVP and WHVP should be measured until the value remains stable and all measurements should be taken at least in duplicate (Groszmann and Wongcharatrawee 2004; Kumar et al. 2008).
2.3 Complications
There are no absolute contraindications to HVPG measurement.
If the patient is allergic to iodine contrast medium it can be avoided and CO2 can be used instead.
In the presence of known episodes of cardiac arrhythmia the catheter in the right cardiac atrium must be moved carefully.
Although coagulation disorders are common in patients with cirrhosis, only cases of severe thrombocytopenia (platelet levels <20,000/dL) or a low prothrombin ratio (below 30%) call for the replacement of platelets or transfusion of fresh frozen plasma.
The procedure of measuring the HVPG has proved to be extremely safe and usually carries only a modest discomfort (Bosch et al. 2009). Complications are infrequent (<1% of cases); most of them are related to local injury at the venous access site (e.g., leakage, hematoma, arteriovenous fistulae) and with the use of US guidance for performing the venous puncture this risk is greatly reduced.
Passage of the catheter through the right atrium might cause supraventricular arrhythmias (most commonly ectopic beats), but these are self-limited in most cases.
In the medical literature there are no reports of serious complications.
In Berzigotti et al. (2013) experience no fatalities have occurred in over 12,000 procedures in 30 years. In addition, hepatic vein catheterization offers the possibility to perform liver biopsies in patients with poor coagulation and contraindications for transcutaneous liver biopsies (Huet and Pomier-Layrargues 2004).
2.4 Indications
Normal portal pressure (determined by the HVPG) ranges from 1 to 5 mmHg. Pressure above this limit defines the presence of portal hypertension, regardless of clinical evidence (D’Amico and Garcia-Tsao 2001; Groszmann et al. 2003).
An HVPG value of 6–9 mmHg corresponds to preclinical sinusoidal portal hypertension, whereas clinically significant portal hypertension is diagnosed when HVPG is ≥10 mmHg, at which point clinical manifestations of portal hypertensive syndrome, such as varices, bleeding, gastropathy, and ascites, might appear (Garcia-Tsao et al. 1985; Groszmann et al. 1990, 2005; Ripoll et al. 2007).
HVPG measurement is the gold standard method to assess the presence of clinically significant portal hypertension (CSPH) CSPH, which is defined as HVPG ≥10 mmHg, in patients with compensated advanced chronic liver disease (cACLD) (De Franchis 2015).
In addition to diagnosing portal hypertension by pressure criteria, the patterns of the HVPG, WHVP, and FHVP obtained during portal pressure measurement can be used to delineate the types of portal hypertension and its possible causes.
Any condition that interferes with the blood flow from the spleno-mesenteric-portal axis to the inferior vena cava can cause portal hypertension so the latter is classified according to the site of obstruction as prehepatic, intrahepatic, and posthepatic.
Intrahepatic portal hypertension can be further subclassified into presinusoidal portal hypertension (e.g., schistosomiasis, sarcoidosis, tuberculosis), sinusoidal portal hypertension (e.g., cirrhosis), and postsinusoidal portal hypertension.
In patients with portal hypertension of unknown causes a normal HVPG with normal WHVP and FHVP is typical of prehepatic and presinusoidal intrahepatic portal hypertension. In these cases the catheter is not in continuity with the actual area of increased resistance, so the recorded pressure will be that of the normal sinusoids. The finding of an increased HVPG owing to an increase in WHVP indicates an intrahepatic sinusoidal hypertension, which is most frequently due to cirrhosis. In postsinusoidal intrahepatic portal hypertension and in posthepatic portal hypertension (e.g., Budd-Chiari syndrome) an increased FHVP and WHVP are found, while the HVPG remains normal.
The measurement of the HVPG moreover manages the clinical evolution of liver disease and the pharmacological therapy.
The main therapeutic goal for portal hypertension should be preventing its complications, such as varices hemorrhage, ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome.
It has been demonstrated that there are threshold HVPGs necessary for the development of ascites and gastroesophageal varices, respectively, 8–10 mmHg for ascites and 10–12 mmHg for varices (Bosch et al. 1986; Rector 1986).
It was demonstrated that varices never bleed when the HVPG is less than 12 mmHg (Groszmann et al. 1990). A reduction in the HVPG to less than 12 mmHg is considered the single most useful prognostic indicator of portal hypertension complications and is the most important goal in pharmacologic therapy of portal hypertension.
In addition Feu and colleagues demonstrated that a 20% or greater reduction in HVPG from baseline after the initiation of beta-blocker therapy is associated with a significant reduction in the risk of variceal bleeding, even if the absolute HVPG of less than 12 mmHg is not reached (Feu et al. 1995).
It has been well demonstrated by many studies that the HVPG is a reliable parameter for predicting survival in cirrhotic patients (Vorobioff et al. 1996; Gluud et al. 1988; Merkel et al. 1992).
Transition from a compensated to a decompensated stage of cirrhosis is marked by the development of the complications of portal hypertension and the risk of developing these complications can be reduced by decreasing the portal pressure.
Albrades et al. (2014) showed that in cirrhotic patients treated pharmacologically for the prevention of variceal rebleeding, the long-term probability of survival was significantly higher for those who had an HVPG reduction of 20% or more from baseline or to less than 12 mmHg (defined as HVPG responders) than for nonresponders.
HVPG also is linked with the hepatocellular carcinoma (HCC).
Ripoll and colleagues showed that in patients with cirrhosis the risk of developing HCC is considerably higher in patients with clinically significant portal hypertension (HVPG ≥10 mmHg) than who have HVPG values <10 mmHg (Ripoll et al. 2009).
Moreover, in patients with well-compensated cirrhosis and resectable HCC, the presence of clinically significant portal hypertension markedly increases the risk of unresolved hepatic decompensation occurring within 3 months of hepatic resection (Llovet et al. 1999; Forner and Bruix 2009).
Thus according to Barcellona Clinic Liver Cancer (BCLC) staging surgical resection for HCC should be restricted to patients without clinically significant portal hypertension.
3 Transjugular Intrahepatic Portosystemic Shunt (TIPS)
3.1 Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) is a direct communication between the portal system and the systemic venous circulation (Fig. 4) and allows the correction of portal hypertension in order to obtain a decrease of the hepatic vein pressure gradient (HVPG) at a value <12 mmHg or a reduction of at least 20% thanks to the portal decompression through a low-resistance vascular pathway (Reiberger et al. 2017).
3.1.1 TIPS History
TIPS discovery occurred in the 1960s, thanks to an accidental portal access during the first transjugular cholangiographic investigations; subsequently, in 1969, Rösch et al. advanced the hypotheses of a “radiologic portocaval shunt” (Rösch et al. 1969). Thirteen years later Colapinto et al. performed the first human balloon-dilated transjugular portosystemic shunt, but only the application of a metallic stent by Richter et al. guaranteed a longer term TIPS patency revealing TIPS as a valid alternative to the surgical portosystemic shunts (Colapinto et al. 1983; Richter et al. 1989).
With expanded polytetrafluoroethylene (e-PTFE)-covered stents TIPS procedure gained more and more acceptance as a treatment for the complications of portal hypertension until in 2004 Gore Viatorr® endoprostheses were designed for a longer TIPS patency and to date represents a well-accepted minimally invasive nonsurgical method for the establishment of a bypass in a congested hepatic vascular bed (Saad 2014).
3.2 Indications
TIPS represents the treatment for some portal hypertension complications, mainly variceal hemorrhage and refractory ascites (Copelan et al. 2014; Fagiuoli et al. 2017).
3.2.1 Variceal Hemorrhage
In portal hypertension a hepatofugal portal circulation may be established, due to a diffuse parenchymal obstacle or a vascular obstruction, and the blood flow gathers its way back to the right heart reaching the vena cava system through anatomical venous shunts, the portosystemic anastomosis (Wachsberg et al. 2002).
When portal hypertension is such that HVPG >10 mmHg, the flow in the esophageal and paraesophageal varices gets prominent and the higher the gradient the higher the likelihood of variceal hemorrhage, with 12 mmHg considered as gradient threshold for bleeding (Garcia-Tsao et al. 1985). Risk factors for variceal bleeding are the stage of liver disease (i.e., Child-Pugh class A, B, or C) and the superficial aspect and dimensions of the varices (F1, F2, or F3 according to JRSPH classification) (Beppu et al. 1981). Gold standard for esophageal varix diagnosis is esophago-gastro-duodenoscopy (EGDS), which must be performed at the moment of cirrhosis diagnosis and then repeated for periodical follow-up (Garcia-Tsao et al. 2007).
About 50% of cirrhotic patients develop esophageal varices and since variceal hemorrhage is a life-threatening complication associated with higher morbidity and mortality rate (10–20% of patients die within 6 weeks), its prevention is of primary interest to consent the improvement of survival rates (Garcia-Tsao et al. 2007; Triantos and Kalafateli 2014; Garcia-Tsao and Bosch 2010).
Nowadays preventive therapy of esophageal varices mainly employs endoscopic procedures (i.e., endoscopic variceal band ligation, EVL; endoscopic injection sclerotherapy, EIS) (Kim 2014). When the rupture of varices occurs, there is a severe gastrointestinal hemorrhage that manifests itself through hematemesis and, sometimes, melena or hematochezia; in these cases, once the hemodynamic stabilization is achieved, the therapeutic protocol (Baveno V) foresees the combination of pharmacologic, antibiotic, and endoscopic treatment, and the latter should be performed ideally between 6 and 12 h from admission, especially when cirrhosis is suspected (De Franchis 2010).
The insertion of the Sengstaken-Blakemore tube in the esophagus is effective in most cases (up to 80%) in which conventional medical and endoscopic treatments fail; however the bleeding relapses are constant after the decompression of the balloons (esophageal and gastric); therefore this procedure should be considered as a bridge therapy to a definitive therapeutic intervention (Kim 2014).
TIPS positioning is strongly advised as secondary prevention of esophageal variceal rebleeding, to treat uncontrollable variceal hemorrhage and portal hypertension gastropathy, or when ascites is concomitant to variceal hemorrhage (Copelan et al. 2014).
Furthermore, recent meta-analyses confirmed the superiority of TIPS over EVL in preventing rebleeding of esophageal varices and high-risk patients (i.e., Child-Pugh class C10-13 patients, Child-Pugh class B patients with active variceal bleeding, patients with HVPG >20 mmHg) treated with early TIPS intervention (<72 h) are more likely to survive or to not show significant bleeding than after an endoscopic treatment (Zheng et al. 2008; Deltenre et al. 2015).
3.2.2 Refractory Ascites
Ascites is associated with cirrhosis in 75% of cases and develops in 50% of cirrhotic patients, representing the most common manifestation of decompensation (Moore and Aithal 2006).
It is defined as an accumulation of fluid in the abdominal cavity in amounts above 250 mL and up to 10 L or more, due to two main pathogenetic mechanisms: portal hypertension that induces extravasation of fluids from the congested hepatic sinusoids and splanchnic capillaries, and renal sodium retention. This mechanism perpetuates in a vicious cycle, leading towards a progressive deterioration of the patient’s conditions (Salerno et al. 2010).
At early stage of ascites-complicated cirrhosis, a therapeutic strategy that maintains a negative sodium balance, reducing its intake (that should be between 5 and 5.2 g NaCl/die, to avoid malnutrition) and increasing its renal excretion by diuretics administration, is sufficient (Runyon 2004; Wong 2012).
The evaluation of diuretic therapy effectiveness foresees patient’s weight daily monitoring; up to 10% of patients do not obtain satisfactory results, due to the refractoriness of ascites at maximal doses of diuretics or due to their side effects (i.e., dysionemia, renal failure, encephalopathy), and therefore requires the execution of evacuative paracentesis (Moore and Aithal 2006).
Due to the poor prognosis of patients with refractory ascites, liver transplantation should be considered, but TIPS seems to improve the transplant-free survival of these patients and is preferable to repeated paracentesis of large volumes (Salerno et al. 2007; Garcia-Tsao 2005). Indeed natriuresis improves within a month after TIPS positioning; however, in order to obtain ascites clearance, patients should follow a sodium-restricted diet for a while, or continue diuretic therapy and within 12 months from TIPS procedure 80% of them achieve a complete resolution of ascites.
3.2.3 Other Portal Hypertension-Related Conditions
In changes of the gastric mucosa (i.e., portal hypertensive gastropathy, PHG) endoscopically described as “snakeskin” lesions, TIPS positioning may improve gastric perfusion (Copelan et al. 2014).
Hydrothorax can be found in cirrhotic patients (5%) with refractory ascites because of the migration of ascitic fluid through the diaphragm into the pleural cavity (Strauss and Boyer 1997). At its first diagnosis, an investigative thoracentesis for the differential diagnosis should be performed; evacuative thoracenteses may be necessary in addition to salt restriction and use of diuretic drugs. In this situation TIPS can help in relieving hydrothorax-related respiratory discomfort (Dhanasekaran et al. 2010).
Budd-Chiari syndrome is a rare condition caused by an occlusion of the hepatic veins (2 out of 3), due to thrombosis or ab extrinsic compression, in which TIPS represents the most common interventional measure, recommended in case of thrombolytic therapy failure (pharmacological and interventional), low functional liver reserve, or HVPG >10 mmHg, or is considered as a bridge therapy to liver transplantation (Ryu et al. 1999).
In case of advanced cirrhosis and clinically significant portal hypertension, systemic and splanchnic vasodilatation compromises renal perfusion causing acute kidney injury (AKI) and hepatorenal syndrome (HRS). Patients with HRS-AKI have more than one indication for TIPS placement, which may improve renal function (Rossle and Gerbes 2010).
Hepatopulmonary syndrome (HPS) occurs when a hepatopathic patient experiences dyspnea and hypoxemia due to an abnormal gas exchange, caused by vasodilatation of pulmonary capillaries. There is no evidence for TIPS effectiveness on this, but neither for TIPS to be unsafe if placed in these patients in order to treat other concomitant complications of portal hypertension (Martínez-Pallí et al. 2005).
Other particular conditions for TIPS positioning could be to maintain or achieve eligibility for liver transplantation with mild portal vein thrombosis, to reduce morbidity and mortality prior to extrahepatic major surgery, as access for portal or mesenteric endovascular intervention (i.e., thrombolysis, thromboaspiration) or as palliative measure in oncologic patients (Wallace et al. 2004).
On the contrary, TIPS should never be established in patients with no proven portal hypertension or in clinical conditions that may increase the risk of post-intervention complications (Krajina et al. 2012; Dariushnia et al. 2016), such as elevated right or left heart pressure, severe pulmonary hypertension, heart failure or sever cardiac valvular insufficiency, rapidly progressive liver failure, severe uncontrolled hepatic encephalopathy, uncontrolled systemic infections or sepsis, polycystic liver disease, extensive primary or metastatic hepatic malignancy, and severe uncorrectable coagulopathy.
Finally, TIPS should never be performed as primary prophylaxis of gastroesophageal variceal hemorrhage, with the exception of selected high-risk patients.
3.3 Preoperative Assessment
3.3.1 Anatomical References
For liver anatomy, refer to Chap. 2; here are some considerations related to the specific procedure.
Liver’s vascularization is composed of two different types of afferents, the hepatic artery proper and the portal vein. The hepatic artery proper is the only liver arterial blood provider, and accounts only for the 25% of the hepatic blood needs; the remaining 75% of liver blood supply is granted by the portal vein that divides into two lobar veins, the right and left portal vein (RPV, LPV): this bifurcation may be extrahepatic (48%) and intrahepatic (26%), or in correspondence of the hepatic hilum (26%) (Schultz et al. 1994). The short but capacious RPV (see Chap. 2 for anatomical details) continues on the same direction of the portal trunk, with just a slight change of the axial angle and divide in the right anterior branch of portal vein (RAPV) and right posterior branch of portal vein (RPPV) which subdivide into superior and inferior segmental branches to supply the right lobe of the liver; the LPV is long twice as much as the RPV, but has half of its caliber and arises from the portal vein’s trunk with an acute angle. The LPV turns medially toward the ligamentum teres, supplying the lateral segments (II and III) of the left lobe and describes a wide and anteriorly concave curve and ends in the superior and inferior segmental branches of segment IV. The cystic vein drains into the RPV or, sometimes, into the PV’s trunk, while the ligamentum venosum (or umbilical vein, normally nonfunctional) drains into the LPV.
Despite the relative infrequency of portal vein anatomic variants (15%), four different variants of portal vein branching have been categorized according to Cheng et al. (1996):
-
Type I represents the classical anatomy mentioned above (65–75%).
-
Type II consists of the trifurcation of the portal trunk: RAPV, RPPV, and LPV (9–11%).
-
Type III occurs when the RPPV is the first collateral of the portal trunk which ends with RAPV and LPV (5–13%).
-
Type IV is characterized by the emergence on the RAPV from the distal tract of the LPV.
Even if the portal bifurcation absence is rare (1%), when unrecognized it can seriously compromise TIPS procedure’s success.
The venous drainage of the liver occurs through three hepatic veins, which converge into the inferior vena cava (IVC): the right hepatic vein (RHV); the middle hepatic vein (MHV) which may drain into the IVC with a common trunk with the left hepatic vein (LHV) in 60% of population; and the LHV. These three venous trunks receive blood from smaller veins, the collector canals, which originate from the merging of the sublobular veins, the very first venous structure draining the hepatic functional unit. Anatomical variants of this district, such as accessory hepatic veins, are not infrequent, as the presence of supernumerary hepatic veins; the absence of one or more hepatic veins might be observed, too.
Normal and variant anatomy of the portal branching and hepatic veins should be accurately acknowledged and identified on preoperative computed tomography (CT) scan, considering that the vessels involved in a desired TIPS should be the right hepatic vein and the right portal vein (Saad et al. 2008).
Portal vein variants of interest when creating a TIPS are type II and III. Furthermore, since the portal vein puncture may cause bleeding when accomplished externally to the hepatic parenchyma, the location of the portal vein bifurcation should be considered prior to TIPS positioning.
Abdominal ultrasound and CT are employed also for the assessment of portal vein patency and the presence of primary or metastatic hepatic malignancy (Fig. 5).
3.3.2 Medical Evaluation
The evaluation of cardiac performance status, hepatic functional reserve, renal function and coagulation capacity are mandatory prior to a TIPS intervention (Chana et al. 2016).
An abnormal thrombocytic count and coagulopathy, not infrequent in cirrhotic patients, should be adjusted prior to the intervention; although there are controversies on the cutoffs to be obtained before the procedure, a platelet count >50,000/mm3 and an INR <1.5 are advisable. Platelet infusion as well as fresh frozen plasma may be used for the correction of INR and thrombocytic count, respectively.
It should be considered also that TIPS intervention foresees the use of generous amounts of contrast agent that may furtherly deteriorate an already compromised renal function.
Since portosystemic hepatic encephalopathy (PHE) is a possible complication of TIPS creation, the presence of hepatic encephalopathy (HE) must be evaluated before the intervention.
Such a comprehensive medical workup may not be feasible in emergency scenarios; nevertheless a baseline hemato-chemical and bio-humoral screening should always be performed and a strict control for the maintenance of hemodynamic stability is required (Krajina et al. 2012).
MELD score allows the stratification of patients’ survival prognosis in accordance to the risk of post-TIPS hepatic decompensation (Farsad and Kolbeck 2014): when MELD score is >20 (or CTP score >C13) TIPS intervention is avoided, except for patients with hepatorenal syndrome who present a particularly high level of creatinine value.
Twenty-four hours prior to the intervention, prophylactic broad-spectrum antibiotic therapy (i.e., ceftriaxone intravenous administration) has begun and in case of known allergy to iodinated contrast medium, premedication with corticosteroids is recommended at least 12 h before the procedure.
A paracentesis (accompanied by volume replacement) can be performed the day before the procedure if needed: this allows the reduction of the angle between the hepatic veins and the inferior vena cava, and therefore an easier access to the hepatic venous system, in addition to better fluoroscopic images. If the presence of hydrothorax severely compromises the patient’s respiratory performance, drainage should be considered.
Finally, fasting is required at least within 6 h before the procedure and informed consent must not be forgotten.
3.4 Procedure
For the TIPS positioning procedure, as well as for other interventional radiology procedures, a multidisciplinary team composed of interventional radiologist(s), anesthetist, radiology technician, and specialized nurse is required.
The patient, lying upon an angiographic table, is positioned with the head slightly turned to the left. Factors related to the patient’s status will affect the choice between deep sedation and general anesthesia (Chana et al. 2016). Conscious sedation induced by sedative agents with a short action (e.g., midazolam, propofol, and fentanyl) may be employed together with supplemental oxygen supply. Many patients complain of great discomfort due to prolonged time in obliged supine position and balloon dilatation of the intrahepatic tracts; furthermore there is no guarantee of airway protection and ventilation may be compromised, so the feasibility of a prompt shift to general anesthesia should always be ensured. General anesthesia on the other hand is the preferred choice of many operators, especially when procedural complications occur. To permit a quick post-procedural recovery, the most appropriate dosage of short-acting agents should be aimed. Tracheal intubation is the safest option as it prevents the occurrence of chemical pneumonia due to gastric reflux during the procedure. Furthermore, controlled ventilation permits breath holds whenever the radiologist needs the patient to be motionless, like during the most delicate phases of the shunt creation. In case of emergency TIPS positioning, general anesthesia and airway protection with tracheal intubation are mandatory.
Continuous cardiac activity monitoring with ECG is required and extreme caution should be paid while maneuvering the guidewire through the right atrium, because arrhythmic events may follow accidental cardiac inner wall stimulation.
At least two cross-matched blood units should be available during the procedure. It should be kept in mind that patients who experienced variceal bleeding are likely to have undergone multiple transfusions in the past: extended crossmatching is required by the possible presence of atypical antibodies in the blood.
3.4.1 Materials
3.4.1.1 TIPS Set and Stent
To date, five different sets to perform a transjugular venous hepatic access are available: the Ring, the Rösch-Uchida and the Haskal set provided by Cook Medical (Bloomington, IN, USA), the AngioDynamics set (Albany, NY, USA), and the Gore set (W. L. Gore & Associates, Inc., Newark, DE, USA).
The shunt can be created with bare metal stents (Wallstent™ Boston Scientific, Marlborough, MA, USA) or graft stents covered in expanded polytetrafluoroethylene (Gore Viatorr® ePTFE-coated stent grafts); the latter, nearly exclusively used for TIPS creation, is constituted by auto-expandable Nitinol covered in its last 4/5 by a thin layer of ePTFE and its employment results to be safe and effective (Vignali et al. 2005). Because of its low permeability to mucin and bile, the outer surface of this device inhibits the hyperplastic growth of the nearby liver parenchyma. While the ePTFE-covered portion (4–8 cm) is designed to be placed in the intrahepatic and hepatic venous tracts, the bare part (2 cm) of the stent is planned to be positioned in the portal vein.
The stent’s diameter (8, 10, or 12 mm) is chosen taking into account the HVPG, the patient’s age, his/her general clinical conditions, his/her hepatic encephalopathy grade, and his/her cardiac performance status (Fanelli et al. 2006; Schepis et al. 2018).
3.4.1.2 Balloons
Before and after TIPS placement, angiographic balloons are used for pre-dilatation of the intrahepatic tract and for dilatation of the stent after being completely released. The two types of balloon most used nowadays are Mustang balloon dilatation catheter (Boston Scientific, Marlborough, MA, USA) and ATB PTA dilatation catheter (Cook Medical, Bloomington, IN, USA).
3.4.2 Technique
TIPS creation can be considered a multistep procedure divided into four main phases (Keller et al. 2016) (Fig. 6).
After catheter-blocked transhepatic portography through a sheath placed in the right hepatic vein and transhepatic puncture (not shown), the portal system is achieved (a) and direct portography is performed using a marked pigtail catheter used for choosing the right length of stent (b); (c) dilatation of the transhepatic tract with angiographic balloon; (d) post-dilatation of the Gore Viatorr® stent
3.4.2.1 Jugular Vein Puncture
The midportion of the right internal jugular vein is the preferred access point because the apex of the lung is usually lower and on this side it is possible to establish a more direct path for the achievement of the hepatic vein district. Since the common carotid artery is located next to this vein, the puncture should be performed under ultrasound guidance with an 18 G needle at the apex of the triangle drawn from the two ends of the sternocleidomastoid muscle. Successful access occurs in 75–99% of cases, depending on the experience of the operators. If the right jugular vein should not be available (e.g., agenesis, occlusion, or surgical ligation), the right external jugular vein, the left internal jugular vein, or the subclavian vein may be chosen.
For the technique of performing jugular venous access under ultrasound guidance, see the introductory part of the chapter.
3.4.2.2 Hepatic Vein Cannulation
Once in the jugular vein, a 0.035″ guidewire is driven down to the inferior vena cava. Passing through the atrial chamber caution is required for the avoidance of extrasystole. Then, a 12 F introducer sheath is advanced in the right atrium. Once the path to the inferior vena cava is gained, a curved catheter is used for the catheterization of the hepatic vein and the venous district’s anatomy is eventually studied with a venogram (iodinated contrast or CO2 may be used).
3.4.2.3 Portal Vein Access
Subsequently, the metal cannula of the TIPS set is used to direct the stylet, anteriorly when aiming the RPV or posteriorly when aiming the LPV, through the hepatic parenchyma for 4–5 cm. The stylet’s access point to the portal vein should ideally be 1–2 cm from the portal bifurcation; this allows the Gore Viatorr® TIPS stent graft to assume a gentle curve that optimizes the shunt flow, reducing the risk of excessive turbulence and consequent obstruction.
After the removal of the stylet and a slow needle retraction together with a gentle syringe aspiration, once blood withdrawal is observed, contrast medium is injected to check whether the landing site is in the targeted portal vein or not.
Several attempts may be required for the attainment of a successful puncture, especially in cirrhotic patients who usually present a distorted intrahepatic vascular anatomy. Ultrasound guidance may be employed to facilitate the intrahepatic puncture and minimize the risk of hemorrhage. This is the most critical step of TIPS procedure, as it may be complicated by hepatic capsule perforation, hepatic artery puncture, biliary duct puncture, or extrahepatic portal tract puncture.
The degree of portal hypertension is then determined by measuring the HVPG, as discussed before in this chapter.
A portography is finally performed for an appropriate portal system anatomy study, the measurements of the shunt’s length, and the definition of varices. Very curved shunt courses require to take into account a 1–2 cm longer stent graft compared to the measured shunt’s length.
3.4.2.4 Stent Graft Deployment
Dilatation by an angiographic balloon of the intraparenchymal tract of the shunt precedes the placement of the stent that is subdivided into two steps: the release of the uncovered portion of the stent and then of the covered portion. A 12F introducer sheath is advanced in the portal system for at least 3 cm. Once the stent graft has been introduced in it, this can gently be unsheathed to permit the endoprosthesis’ uncovered portion expansion in the portal vein. This is a delicate phase as a wrong positioning of this stent graft’s portion cannot be corrected; moreover, in liver-transplant candidates, the positioning of both TIPS ends is particularly critical and has to be as precise as possible (Krajina et al. 2012). The introducer sheath is then retracted until a higher resistance, due to the transition from the portal vein tract to the intrahepatic tract, is felt; continuing in the retraction of the introducer into the inferior vena cava or the right atrium, the stent is allowed to be completely released with the proximal end at the junction of the chosen hepatic vein with the inferior vena cava. Keeping the system firmly still, the PTFE-covered portion is then deployed by pulling its constraining cord.
Stent dilatation by angiographic balloon is then performed; under-dilatation may be chosen to target a precise HVPG value or prevent excessive shunt.
To prevent thrombus formation inside the TIPS endoprosthesis 5000 IU of heparin could be administered immediately after its positioning.
Finally, a portogram is performed to check the shunt’s functioning and another HVPG measurement is obtained to calculate the ΔHVPG: the procedure’s hemodynamic success is achieved if HVPG is reduced to a value <12 mmHg or at least 20%.
3.4.3 Challenges
Compared to surgical shunts, TIPS procedure has lower mortality and morbidity rates: a fatal periprocedural complication occurs in 1.7% of patients (range 0.6–4.3%) (Krajina et al. 2012). Some procedural related events that may cause the patient’s death, like extrahepatic portal vein puncture (2%), hepatic arterial vessel laceration (1%), or transcapsular puncture with transjugular needle (<1%) should be avoided. Other nonfatal periprocedural complications include neck hematoma due to accidental carotid puncture (1%), pneumothorax due to an overly low attempt of jugular puncture (<1%), and biliary duct lesion with consequent hemobilia (10%).
3.4.3.1 Portal Vein Thrombosis
In the case of cirrhosis complicated by PVT, the TIPS positioning requires, in addition to the venous transjugular access, portal access via transhepatic or trans-splenic track. Portal recanalization attempts can also be undertaken (Lombardo et al. 2018) (Fig. 7).
3.5 Follow-Up
In patients that after the intervention present normal coagulative parameters, prophylactic anticoagulation therapy should be initiated (12,500 IU of heparin per 500 mL of physiological solution for the first 24 h, and subsequently 0.4 mg of low-molecular-weight heparin—LMWH—twice a day for at least 1 week). Further anticoagulation is not recommended, except for patients who underwent TIPS positioning with Budd-Chiari syndrome indication (i.e., massive hepatic vein thrombosis) or who experience PVT: in these cases INR target is >2 (Krajina et al. 2012).
The prophylactic antibiotic therapy initiated before the intervention has to be prolonged for at least 48 h after the procedure.
Post-procedure hospitalization foresees the evaluation of the liver’s functional status. Whenever clinical abnormal findings should be encountered, the recovery is prolonged for further investigations and management.
TIPS patency should be evaluated with US examination within 1–5 days from the procedure.
After TIPS creation, the worsening or outbreak of HE may be detected by the evaluation of sleep pattern behaviors, working capacity, and changes of personality and of speech abilities. Psychometric tests are useful tools for the detection of subclinical HE (Campagna et al. 2017).
Impaired liver function may follow TIPS intervention: liver sufferance is presented with abnormal increase of serum bilirubin concentration.
Patients should be signed off only when their clinical status is sufficiently good and no laboratory examinations are out of range. At discharge, patients are provided with detailed dietary instructions as well as with prophylactic therapy for PHE.
Follow-up in patients with TIPS foresees a periodic evaluation of nutritional status, functional status, grade of encephalopathy, liver and renal functions, as well as status of the pathology for which TIPS was indicated. This allows to understand if the clinical endpoints of each patient have been reached or not and to perform further examinations or modify the therapy appropriately.
3.5.1 Ultrasonographic Examination
Due to its noninvasive nature, US is the first-line examination method for patients with TIPS. In patients who received a Wallstent™ (bare metal stent), the first investigation should be performed 24 h after the procedure, aiming the ruling out of the immediate onset of complications such as stent occlusion or insufficient shunt flow (if these are encountered, a prompt TIPS angiographic revision is required) (ŽiŽka et al. 2000). On the contrary, patients who underwent the positioning of a Gore Viatorr® TIPS endoprosthesis have no recommendation for such early examination. This is due to the fact that a thin air layer may be trapped between the stent’s ePTFE sheets, impeding a proper TIPS insonation and resulting in a false-positive evaluation for occlusion (Ferral et al. 2016). Therefore, the first radiological evaluation of these patients should be carried out 5–10 days after the intervention. From then on, follow-up should be performed every 6 months even if shorter intervals (i.e., 3 months) between one and another examination may be required in case of critical patients (Darcy 2012). Furthermore, the success of radiological follow-up also depends on the patients’ compliance to attend such clinical investigations. US is employed for the assessment of the liver’s anatomy as well as for the ruling out of nodular lesions, but it is a valid method for the detection of shunt malfunctioning and occlusion too (85–100% sensitivity; 96–100% specificity) (Kanterman et al. 1997).
The caliber of the portal vein and of the stent graft should be measured for the evaluation, respectively, of possible excessive ectasia or stenoses. Ultimately, the TIPS diameter, measured on its proximal, middle, and distal tracts, is compared to the nominal caliber of the endoprosthesis’ design. Color Doppler US could give information about the intra-TIPS blood flow and the assessment of TIPS patency, in addition to portal blood flow direction (Feldstein et al. 1996).
The baseline portal vein blood flow speed (Vmax 10–20 cm/s) rises two- to fourfold after the insertion of TIPS in the hepatic parenchyma. Therefore, in patients with TIPS, a portal vein blood flow slower than 30 cm/s is suspicious of TIPS malfunctioning, and a blood flow speed lower than 20 cm/s is most likely indicative of endoprosthesis stenosis (ŽiŽka et al. 2000).
For ePTFE-covered stents, mean intra-TIPS flow rates <90 cm/s or >250 cm/s have to be considered suggestive of TIPS malfunctioning and hence worthy of further investigation (i.e., angiographic revision), even if there are no cutoff values in literature.
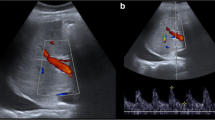
Contrast-enhanced ultrasound (CEUS) allows a direct evaluation of the TIPS patency and may serve as a complementary tool to the otherwise insufficient ultrasonographic investigation by showing enhancement defects or even a total absence of enhancement (Micol et al. 2012). Thus, in the follow-up of patients with TIPS, CEUS could play a bridge role between Doppler ultrasound examination and angiographic TIPS revision (Figs. 8 and 9).
The contrast agent used for CEUS consists of sulfur hexafluoride microbubbles stabilized by a shell of phospholipids that allows a notable increase of the ultrasound backscatter with or without contrast medium flowing movement and has the capability to entirely remain in the bloodstream and not permeate into the extravascular space.
The use of CEUS contrast agents has been demonstrated to be safe with a very rare incidence of side effects (Piscaglia and Bolondi 2006). There is no need for a laboratory workup prior to the examination and it is enough to keep the patient monitored for at least 15 min after the contrast agent injection. Ultrasound contrast agent administration is forbidden in patients affected by allergy to sulfur hexafluoride or any other component, cardiac right-to-left shunt, severe pulmonary hypertension, uncontrollable hypertension, acute respiratory distress syndrome, and severe cardiac disease that contraindicates the use of dobutamine.
Informed consent should always be obtained before the use of ultrasound contrast agent as well.
3.5.2 Complications
The complications can be technical (i.e., related to the procedure), or resulting from the successful realization of the shunt, or directly related to the TIPS; the main conditions are listed in Table 1 (Gaba et al. 2011).
3.5.2.1 Arterial Injury
The accidental puncture of an arterial branch during the procedure may lead to the development of a fistula, which must be corrected by embolization or covered stents to treat hemorrhage (Fig. 10).
3.5.2.2 Acute Hepatic Failure
Hepatic parenchymal ischemia can occur due to reduced sinusoidal flow and can be treated by reducing the caliber of the stent (Fig. 11).
3.5.2.3 Portosystemic Hepatic Encephalopathy (PHE)
The most common medical complication of TIPS positioning is a higher incidence of PHE, a medical condition caused by an excess of toxins in the central nervous system because of bypass of bloodstream hepatic filter and characterized by confusion, disorientation, obtundation, anomalous sleep patterns, and a general compromised quality of life (Riggio et al. 2008; Madoff et al. 2004).
Clinical classification of PHE, that may be classified as episodic, recurrent, or persistent according to its periodicity, is based on the West-Haven criteria or Glasgow coma scale (GCS). The main risk factors for the reoccurrence or new outbreak of PHE are history of PHE, older age, bigger shunt caliber, creatinine blood levels, hyponatremia, and liver dysfunction. Such TIPS complication may be prevented by aiming a lower HVPG reduction, reaching a compromise between portal hypertension decrease and control of excessive blood shunting. Furthermore, treatment of precipitating factors prior to TIPS positioning assures a lower risk of PHE outbreak. Medical prophylaxis foresees the administration of lactulose, which causes ammonia to transform into ammonium, excreted in stool.
PHE has been encountered in up to 35% of patients who underwent TIPS intervention, but among these only 8% are refractory to medical management; in this case a re-intervention to reduce the flow of shunting blood could be necessary and liver transplantation should be considered.
4 Portal Venous Embolization (PVE)
4.1 Introduction
Surgery is nowadays the gold standard for radical treatment in patients with primitive neoplastic or metastatic liver disease. The available options are liver transplant or resection. Because of the limited number of organs available, liver resection is the most common procedure in the treatment of neoplastic liver disease. In more than 45% of patients extended liver surgery is needed to achieve clear margins and liver ability to regenerate has allowed larger and larger resection. However, the wider the resections the higher the risk for the patient of liver insufficiency. This is more pronounced in the early postoperative period, with a mortality rate ranging from 3.2% to 7% after major liver resections that reaches 32% in patients with cirrhosis (Broering et al. 2002).
In order to support a rapid growth of the future liver remnant (FLR) in 1965 portal vein ligation was initially reported in humans as part of a two-stage extended hepatectomy. In 1982 preoperative portal vein embolization (PVE) was performed in patients with cholangiocarcinoma, while the first use of preoperative PVE for patients with hepatocellular carcinoma was reported in 1986 (Makuuchi et al. 1990). The main aim of PVE is the complete occlusion of the portal branches feeding the future resected liver segments in order to induce hypertrophy of the FLR and atrophy of the embolized liver parenchyma (Denys et al. 2010).
A further procedure is liver venous deprivation (LVD). It consists in the simultaneous embolization of right portal vein (RPV) and the right and/or intermediate hepatic veins, in order to increase the damage to the embolized liver leading to increased hypertrophy of the contralateral parenchyma (Panaro et al. 2019).
Since its original description indications for PVE have been expanded and now include any primary or metastatic liver cancer requiring better FLR prior to hepatectomy.
4.2 PVE and Surgical Portal Ligature
The effectiveness of right portal vein occlusion in large hepatic resections is well known but it is still unsure whether surgical portal ligation has to be preferred over the interventional PVE, or vice versa.
In literature there are studies that proved that PVE can reach a FLR growth between 10% and 46% after 2–8 weeks (Liu and Zhu 2009). Similarly other studies found a growth of 38% in 8 weeks after surgical portal ligation (Aussilhou et al. 2008). In conclusion there is still no evidence that can help choose between interventional and surgical treatment (Pandanaboyana et al. 2015).
4.3 Technical Considerations
There are no absolute contraindications to PVE.
Relative contraindications are uncorrectable coagulopathy, tumor invasion of the portal vein, tumor precluding transhepatic access, biliary dilatation (pre-procedural positioning of a biliary drainage is recommended in these cases), portal hypertension, and renal failure.
Intravenous broad-spectrum antibiotic prophylaxis is given on the day of the procedure to minimize the possibilities of biliary sepsis.
Local anesthetic (1% lidocaine hydrochloride) and intravenous sedatives are administered.
Ultrasonography is used to find the best route to the portal venous system; it is recommended not to pass through the tumor during the access in order to avoid neoplastic seeding.
Under sterile conditions a 21-gauge needle is used to enter the portal system by ultrasonic or fluoroscopic guidance (Fig. 12).
The percutaneous procedure can be performed using an ipsilateral or contralateral percutaneous access.
The ipsilateral approach has the advantage that it does not damage the FLR, but it is more technically difficult because of the sharp angulation encountered in cannulating portal branches. It consists of the puncture of a peripheral right portal vein and a 180° reverse-curved catheter to embolize ipsilateral portal branches (Fig. 13).
The contralateral approach has the advantage of cannulation without pronounced angulation and allows a prograde delivering of embolic agents and contrast; nonetheless it may injure the FLR. In the contralateral approach the portal vein of segment three is usually punctured as it is the most anterior branch (Fig. 14).
In both cases at least 1 cm proximal to the main portal vein should be left untouched, in order to allow surgical control during the resection. Five-French materials are usually recommended.
It has also been described a trans-ileocolic approach that is now rarely used. It requires general anesthesia and a surgical incision to extract a portion of the ileum in order to cannulate an ileocolic vein.
A final portography must be performed to verify the correctness of the procedure, the complete occlusion of targeted liver segments, and redistribution of flow to the FLR branches only. Embolization has to involve the entire portal branch with its distal ramification to prevent porto-portal shunts (Denys et al. 2010; Liu and Zhu 2009; Narula and Aloia 2017).
There are various embolic agents that can be used but any large randomized study has ever been performed to compare their efficacy; up-to-date information is derived from small retrospective studies and expert opinion.
Two products are not recommended in PVE: gelfoam because of the high rate of recanalization and alcohol because of a significant post-procedural morbidity (parenchymal necrosis and venous thrombosis).
Recommended embolic agents are cyanoacrylate and microspheres. N-butyl-cyanoacrylate (NBCA) has been used mixed to lipiodol showing good results and low morbidity. Spherical microparticles are mostly used in North America and are associated with coil embolization at the end of the procedure; most teams start with 300–500 μm particles followed by 700–900 μm spheres (Denys et al. 2010).
4.4 Complications
CIRSE guidelines indicate a minor and major post-procedural complication rate of less than 20–25% and 5%, respectively (Denys et al. 2010).
Puncture-related complications include mechanical injuries to vessel, biliary structure, and pleura (Narula and Aloia 2017). They are the majority of complications, so that many authors advice for the ipsilateral approach.
The most common complication of percutaneous transhepatic procedures is hemorrhage; after PVE it occurs in 2–4% of patients. Bleeding can present as subcapsular hematoma or hemoperitoneum and it can be immediate or delayed. Bleeding sources include intercostal artery, portal vein, hepatic vein, and hepatic artery. Transarterial embolization can be an effective treatment.
Biliary injuries are usually less common because biliary puncture is rarer and it becomes symptomatic less frequently. The main manifestations are bile leak, which necessitate biliary drainage, and hemobilia treated with embolization of the underlying artero-biliary fistula.
Pneumothorax and hemothorax can also happen but they are rare.
Embolization-related injuries are non-targeted embolization, portal vein thrombosis, liver infarction, portal hypertension, post-embolization syndrome, and recanalization (Narula and Aloia 2017).
Non-targeted embolization strongly depends on the embolic materials. Especially for inexperienced operators, liquid embolic materials can flow distally and inadvertently embolize areas of the FLR, forcing the surgeon to enlarge the resection (Fig. 15).
PVT is one of the most dangerous complications because it can cause acute liver failure and jeopardize post-PVE surgery; immediate treatment is mandatory (Yeom and Shin 2015).
Post-embolization syndrome includes minor symptoms such as abdominal pain, fever, nausea, and vomiting. It happens rarer than in arterial embolization probably because PVE mainly activates apoptosis mechanism rather than ischemic necrosis so inflammatory mediator release is limited.
Half of the patients after PVE show little liver enzyme alteration 3 days after the embolization which returns to baseline after 7–10 days (Liu and Zhu 2009).
Complications are more frequent in patients with chronic liver disease overall.
4.5 Outcome
The degree of hypertrophy and the time interval through which it manifests after PVE have a great variability among patients. In patients with a normal functioning liver the regeneration time is about 2 weeks and it grows 12–21 cm3/day. Otherwise in patients with cirrhosis the growth is limited to 9 cm3/day (Madoff et al. 2002).
There are several factors that inhibit FLR growth, such as pre-procedural chemotherapy, high bilirubin levels, and diabetes mellitus.
The risk of neoplastic progression after PVE is still under debate. Several studies report a risk of neoplastic progression of 25% during the period the FLR growth is expected (Hoekstra et al. 2012). Some patients with liver metastasis from colon-rectal cancer had a pre-procedural chemotherapy, because literature reports that the risk of disease progression is low if the time between the end of chemotherapy and PVE is short (Simoneau et al. 2015).
Regarding the two-stage hepatectomy (resection of the lesions in the FLR followed by PVE and subsequently by the resection of the contralateral neoplastic lobe) the survival rate at 5 years is between 51% and 32% with a median survival time of 39.6 months (Brouquet et al. 2011; Narita et al. 2011).
5 Posttransplant Vascular Complications
5.1 Introduction
Vascular complications (VCs) following orthotopic liver transplantation (OLTx) are linked to a high incidence of both graft loss and mortality.
After the transplant the hepatic artery becomes the primary blood supply to the graft and the only one to the biliary tree; although liver parenchyma is partially supplied by the portal vein, the absence of hepatic arterial flow (possible native collaterals are lost) can lead to acute graft ischemia and biliary tree complications.
For this reason it is really important to have a good timing of diagnosis and to perform the best therapeutic management in order to improve the outcomes of liver allograft recipients.
In a recent review the overall incidence of VCs in adult patients was quite different among different centers (Piardi et al. 2016). However, it is around 7% in deceased donor liver transplantation and around 13% in case of living donor liver transplant.
In order to detect complications correlated with OLTx, physicians should perform careful surveillance with US and, in particular to detect VCs, using color and Doppler mode.
Once a suspected VC is recognized, it must be evaluated with second-level imaging examinations, as CT angiography or angiography. In case of confirmation of VCs it has to be managed promptly.
While in the past the surgical treatment was considered the first approach towards these complications, nowadays the advances in endovascular intervention have increased and made it a viable therapeutic option.
With regard to the type and entity of VCs, we can perform surgical revascularization, retransplantation, percutaneous transluminal angioplasty with or without stenting, intra-arterial thrombolysis, embolization, or conservative approach (Chen et al. 2014).
In this text, for the sake of simplicity, we will distinguish the VCs that regard the blood inflow from the ones that regard the blood outflow. Kinking or sudden bleeding, stenosis, and thrombosis can arise at any of the vascular anastomoses, although with a different rate: arterial complications are the most common (overall incidence 5–10%), accounting for more than 50%, VCs after OLTx, while both portal and caval venous complications are less frequent (in both cases overall incidence about 2%) (Piardi et al. 2016).
The knowledge of postoperative anatomy is the key to assess the best management.
OLTx involves four anastomotic sites, each with specific VCs:
-
Hepatic artery anastomosis: conduits or jump grafts or end-to-end anastomosis, typically end-to-end hepatic artery anastomosis
-
Portal vein anastomosis: commonly end-to-end recipient portal vein to donor portal vein anastomosis
-
Hepatic veins/inferior vena cava anastomosis: piggyback reconstruction, interposition; caval anastomosis for whole grafts; end-to-end anastomosis between the graft hepatic venous outflow and the recipient hepatic veins (split graft)
-
Common bile duct anastomosis
5.2 Arterial Complications
A transplanted liver maintains a dual-inflow blood supply as a native one, portal and arterial, but after OLTx, the arterial blood gives a major contribution to the irroration of the hepatic graft, perfusing both liver parenchyma and biliary tree.
It is important to remember that in an OLTx recipient there are no arterial collateral vessels (which could prevent liver parenchymal ischemia during a hepatic artery occlusion) due to total hepatectomy, and if arterial inflow is reduced, the allograft may survive only if new arterial collaterals have developed due to insufficient portal inflow (Panaro et al. 2011). Arterial collaterals can develop as early as within 2 weeks.
The hepatic artery complications following OLTx are:
-
Thrombosis
-
Stenosis
-
Pseudoaneurysm and rupture
-
Splenic steal syndrome
Furthermore, although the definition of early and late complications is a problem, in this text complications will be classified in this way: early, with maximum onset within 1 month of transplant, and late, with onset more than 1 month after transplant.
We would like to underline the importance of early complications, because they are associated with higher graft loss and mortality rates.
5.2.1 Hepatic Artery Thrombosis (HAT)
HAT is defined as a complete thrombotic occlusion of the hepatic artery and it represents the most frequent and severe VCs following OLTx.
HAT is the second leading cause of graft loss after primary nonfunction (Meek et al. 2018).
In a systematic review a HAT overall incidence of 4.4% was reported after OLTx. In adults, the incidence of HAT was 2.9% (Bekker et al. 2009). Late HAT is less prevalent, counting for less than 2% of cases.
HAT can be classified in early HAT and late HAT, each one characterized by different clinical expressions, depending on the timing of the onset and on the existence of arterial collateral vessels.
While early HAT has an acute presentation with variable but severe clinical course (from an elevation in liver enzyme to ischemic biliary necrosis, primary dysfunction, and graft loss), late HAT, due to existence of collaterals, is usually less serious with 15–23% mortality rate and the majority of patients are asymptomatic or can present biliary complications (Nikeghbalian et al. 2007; Stange et al. 2003; Gunsar et al. 2003).
Most patients with early HAT presented acute fulminant hepatic failure (30%). In most cases, they undergo a retransplant (81%) (Pareja et al. 2010).
Up to 20% of HAT cases are probably due to surgical technical problems in the arterial anastomosis (such as technical imperfections, kinking, stenotic anastomosis). There are many other causes, often unacknowledged, as the median arcuate ligament celiac artery compression, which discussion is beyond the purpose of this chapter.
5.2.1.1 Diagnosis
Early diagnosis is pivotal to treat the complication and to try to prevent graft loss.
During allograft recipient follow-up it is mandatory to recognize patients with abnormal biological findings and/or morphological (ultrasonography) exams suggestive of HAT.
Doppler US is the gold standard for screening protocols (Vaidya et al. 2007).
Abdominal contrast-enhanced computed tomography (CT) angiography or DSA usually allows to confirm the diagnosis: in particular, DSA may detect predisposing anatomical anomalies and allow therapeutic management at the same time.
US diagnosis of hepatic artery thrombosis is based on the absence of Doppler arterial signal at the hilus as well as in the intrahepatic arterial branches.
In 2010 Pareja et al. established a screening protocol for early HAT: Doppler US within 48 h after OLTx and 7 days later. If this was not conclusive, they performed CEUS or CT. There are many other ultrasound protocols suggested in the literature (Murata et al. 2016).
Once diagnosis of HAT has been confirmed, arteriography or a retransplantation, depending on the degree of liver graft damage, must be performed.
In HAT follow-up, collaterals can be identified during angiography examination as early as 2 weeks after OLTx.
After the first month, considering that intimal hyperplasia can induce progressive hepatic artery stenosis and secondary late HAT, a yearly Doppler US assessment should be performed.
5.2.1.2 Therapeutic Management and Prognosis
In general, the therapeutic options for management of HAT are revascularization (surgical or endovascular), retransplantation, and observation (20% of cases).
Traditional percutaneous endovascular revascularization includes intra-arterial thrombolysis (IAT), percutaneous transluminal angioplasty (PTA), and stent placement.
Endoluminal success is defined as the complete resolution of the thrombus without residual thrombus or arterial anatomic defects that reduce the arterial diameter lumen more than 50% (Saad et al. 2007) (Fig. 16).
Direct anastomosis of the donor hepatic artery to the supraceliac aorta with extension graft. Supraceliac arterial graft occlusion. (a) Contrast-enhanced CT, axial scan, arterial phase. Extensive peri-anastomotic stenosis of arterial inflow; intraparenchymal arterial branches were patent. (b) DSA confirmed graft occlusion; hepatic artery and its intraparenchymal branches are not displayed. (c) Intra-arterial thrombolysis was performed and partial patency of arterial graft was restored (not complete endoluminal success). There is a residual discrepancy between donor and recipient hepatic arteries with persistent filling defects
Murata et al. reported an overall technical success with endovascular treatment of 77.8% (Murata et al. 2016).
Even if efficacy (around 50% in literature) and safety of thrombolytic treatment are proven, also with different drugs (urokinase, streptokinase, alteplase) and doses, there are no currently specific guidelines for thrombolytic therapy. Furthermore, considering that anatomic defects can lead to rethrombosis, IAT should be associated with underlying anatomic defect treatment if present: association of IAT with PTA and/or stenting showed better efficacy and survival rates when compared to IAT alone (Zhang et al. 2017) (Fig. 17).
(a) Digital subtraction angiography (DSA) shows celiac trunk stenosis and complete occlusion of left hepatic artery; right hepatic artery is patent but of narrow caliber. (b) After balloon angioplasty (PTA) and stent placement in celiac trunk and intra-arterial thrombolysis and PTA in hepatic artery main branches, DSA shows optimal results (good caliber of celiac trunk, left hepatic artery patent, and minimal residual stenosis of right hepatic artery) with improved hepatic arterial inflow
In Zhou et al.’s experience, early diagnosis and treatment of HAT are very important for successful revascularization by thrombolysis because urokinase therapy is more effective when the clots are fresh. In the same study the quantity of urokinase used in patients with HAT was as much as 950,000 units to nine million units (Zhou et al. 2005).
Dose and timing of IAT may vary; Zhou et al. recommend the administration of a 100,000–250,000 IU bolus, followed by a second infusion of 250,000–750,000 IU 30 min later in case of unsatisfactory result. After this, a continuous perfusion of 50,000–100,000 UI/h is administered for 12–24 h. During the treatment, at least every 12 h, a DSA imaging is performed.
The catheter sheath should be maintained for 2–3 days after initial recanalization of the hepatic artery so that thrombus recurrence can be detected and rethrombolysis can be performed immediately.
Thrombolysis is stopped if no significant differences during monitoring or if bleeding complications occur.
Finally, some patients could survive without revascularization or retransplantation. Fouzas et al. (2012) described how these patients with hepatic artery thrombosis can develop arterial collaterals, which maintain adequate blood inflow and allow conservative treatment (Fig. 18).
(a) Celiac axis DSA shows complete occlusion of the proper hepatic artery; left gastric artery is patent and hypertrophic, with hepatic collaterals. (b) Same patient, superior mesenteric artery (SMA) angiogram: hypertrophic peripancreatic arcades provide collaterals from the SMA to the intraparenchymal hepatic artery
Based on the relative lack of utility of revascularization of late HAT and the contraindication to early postoperative thrombolysis, Saad et al. proposed that the clinical utility window of IAT should be from 1–3 weeks to 1–3 months posttransplantation, unless there are contraindications (Saad et al. 2007).
Despite encouraging results of endovascular interventions, the efficacy and risk of complications (mainly represented by hemorrhage risk) make this therapeutic option still controversial. Moreover, in some cases, endovascular approach is not conclusive and anastomotic revision and retransplantation are necessary.
In a meta-analysis of 2009 HAT was a major cause of graft loss (53.1%) and mortality (33.3%) in the early postoperative period (Bekker et al. 2009).
The main complication of thrombolysis is anastomotic and intra-abdominal bleeding (about 20% of cases in literature). More safer and effective therapy can be obtained if the infusion catheter is placed inside the thrombus (Figueras et al. 1995). Furthermore selective thrombolysis has several advantages, such as a smaller thrombolytic dose and a highly localized concentration with a little influence on systemic coagulation.
The complications of PTA include thrombosis, vascular dissection, pseudoaneurysm, and arterial rupture with arterial bleeding (up to 5% of cases).
In recent years the use of Penumbra System (PS; Penumbra, Alameda, Calif) has been described to perform thromboaspiration of thrombi in the hepatic artery of patients with high risk of thrombus fragmentation-distal embolization and bleeding (Gandini et al. 2016).
Meek et al. tried to treat HAT using a mechanical endovascular approach, a stent retriever device for revascularization (Meek et al. 2018).
5.2.2 Hepatic Artery Stenosis (HAS)
HAS following OLTx can be defined as a narrowing of the hepatic artery diameter, more or less extended along the vessel; significant HAS is defined on angiography as a narrowing of the hepatic artery diameter greater than 50% (Saad et al. 2005).
HAS occurs from 2% to 13% of transplants (Piardi et al. 2016). It was assumed that HAS can progress to HAT considering that HAS and HAT are part of the same contiguous ischemic spectrum.
Similar to HAT, HAS may be divided into two groups: early HAS and late HAS. Chen et al. reported an overall HAS incidence of 2.8%, with an early HAS incidence of 40% vs. a late HAS incidence of 60% (Chen et al. 2009).
In several studies, up to 60% of the cases HAS occur at the level of the hepatic artery anastomosis (Fig. 19).
Patients with HAT can show a variable symptomatology, ranging from normal liver function to transplant failure secondary to ischemia or necrosis; most commonly, they only present with abnormal liver function tests; for this reason, most HAS are detected during routine Doppler US screening.
5.2.2.1 Diagnosis
Doppler US efficiency in the early diagnosis of HAS has been reported in several studies.
Dodd III et al. criteria for HAS diagnosis consist of a tardus parvus waveform: resistive index (RI) less than 0.5, systolic acceleration time (SAT) greater than 0.08 s, and peak systolic velocity (PSV) greater than 200 cm/s (Dodd III et al. 1994). Intrahepatic arterial branches have to be visualized.
To exclude HAT with the development of collateral vessels, that shows a dampening of the systolic peak with normal RI, similar to tardus parvus waveform, it is necessary to detect a focal poststenotic systolic peak velocity greater than 200 cm/s, diagnostic for hepatic artery stenosis (Zheng et al. 2017; Hom et al. 2006).
With regard to the many questionable cases and considering that measuring the acceleration time is hard and imprecise, arterial RI should be used solely as a screening method for detecting abnormality of arterial flow and further imaging methods such as contrast-enhancement CT angiography and standard angiography are used to confirm the diagnosis. Conventional angiography is the gold standard for HAS diagnosis (Frongillo et al. 2013).
5.2.2.2 Therapeutic Management and Prognosis
The therapeutic management of HAS includes surgical revision, retransplant, or percutaneous endovascular interventions (PTA with or without stent placement).
Endovascular treatment success has been defined by luminal restoration with 30% residual stenosis and without consequences such as dissection (rate limiting or not) or arterial leaks/rupture (Saad et al. 2005).
It is demonstrated that early HAS management with PTA has 6-month HAT rate of 19%, compared to HAT rate of 65% in untreated patients with HAS.
Similar to Abbasoglu et al. (1997), Saad et al. (2005) reported 81% successful PTA treatment of significant HAS, with incidence of immediate complications (dissection and arterial rupture) around 7% and of delayed complications (HAT) within 30 days of PTA occurring in 5% of cases.
Different rates of restenosis have been reported in literature, from no restenosis to rates as high as 75%. It is been proved that repeated endovascular treatment of recurring HAS improves the rate of success (Sommacale et al. 2013).
Ueno et al. (2006) documented that hepatic artery stent placement is feasible and shows low complication rate.
Stent placement has been employed when there was >30% residual stenosis or when a flow-limiting dissection was present.
Saad et al. (2005 and 2007) reported that restenosis after a stent placement occurred later than after angioplasty alone (Fig. 20).
(a) Celiac axis DSA shows patent arterial anastomosis between the recipient’s celiac trunk and donor’s superior mesenteric artery (SMA); the right hepatic artery arises from SMA in donor and after OLTx, in recipient, its caliber is narrow in the origin site. (b) Post-balloon angioplasty (PTA) and stent placement DSA shows suboptimal results with residual <30% stenosis and improved arterial hepatic inflow
Prompt early diagnosis of HAS and percutaneous endovascular revascularization are usually successful with long-term graft and patient survival, in particular if stenosis is not associated with biliary complications.
The reported risk of procedural complications after endovascular treatment of HAS is quite variable, ranging from 0% to 23% in the literature.
In pediatric patients stent placement is recommended only if angioplasty fails or if other procedure-related VCs occur (hepatic artery dissection or rupture) because its long-term patency is not known and a possible retransplantation has to be taken into account considering the patient’s age (Miraglia et al. 2009).
5.2.3 Hepatic Artery Pseudoaneurysm (HAP)
HAP is defined as a pulsating, encapsulated hematoma in communication with the lumen of a ruptured vessel (Sueyoshi et al. 2005): blood leaks through the artery wall and it pools outside of it.
There is a persistent communication between the hepatic artery, patent, and resultant perfused sac; it is contained by the media or adventitia or simply by soft-tissue structures surrounding the injured vessel (Saad et al. 2005).
Post-OLTx HAPs are classified as intra- or extrahepatic; while intrahepatic ones are uncommon and secondary to percutaneous transhepatic interventions, extrahepatic HAPs are more common (69–100% of post-OLTx HAP) and usually due to local infection, from both bacterial and fungal organisms, in anastomotic site.
Additionally, HAPs have been described secondary to salvage procedures related to thrombolysis and angioplasty for hepatic artery (Fistouris et al. 2006).
The clinical presentation is not specific: from asymptomatic state to abdominal pain with fever, and from self-limiting hemobilia to hemorrhagic shock.
5.2.3.1 Diagnosis
The diagnosis of HAP is made by Doppler US, contrast-enhanced CT angiography, or DSA, but almost 50% of HAP is not recognized before rupture (Volpin et al. 2014).
5.2.3.2 Therapeutic Management and Prognosis
The management of HAP can be carried out by a surgical team (mainly hepatic artery ligation) or an interventional radiologist.
Hepatic artery ligation mortality rate is quite variable: from high mortality rate of 60% to 35%.
There are only few case reports of patients treated with endovascular approach because of the low incidence of this VC. Different techniques are available: intentional occlusive embolization (in order to produce an intentional thrombosis of the hepatic artery to stop bleeding without preserving arterial inflow) and bare stents and covered stents. Selective embolization (in order to perform an intentional thrombosis of the pseudoaneurysm with preservation of the arterial inflow) is usually reserved for intrahepatic HAP.
The use of coil has been reported to embolize the hepatic artery and the HAP exclusion with a covered stent inserted into the hepatic artery.
Embolization can lead to subsequent graft ischemia. Furthermore, a large percentage of these pseudoaneurysms are mycotic, and a stent graft may become an infectious nidus, reducing the survival of the graft.
Detecting HAP before rupture should improve outcome, with 100% successful therapy; from this perspective, it seems legit in the early management of HAP to perform prompt percutaneous endovascular approach, to take time and stabilize patients. Surgical intervention is an option if endovascular management has failed or once patients are stabilized (Piardi et al. 2016).
5.2.4 Hepatic Artery Rupture (HAR)
HAR is defined as a severe hemorrhage from the trunk or from a main branch of the hepatic artery, resulting in the absence of the arterial blood supply of the graft.
Clinical presentation is always sudden hemorrhage: hemoperitoneum, gastrointestinal bleeding, hematoma, and hemobilia.
5.2.4.1 Therapeutic Management and Prognosis
It is usually a complication of HAP or of its treatment; it requires an aggressive treatment, usually emergency major surgery: anastomotic revision, aorto-hepatic grafting, hepatic artery ligation, or emergency/elective retransplantation.
However, some endovascular possibilities are available. Goldsmith et al. (2017) placed a bare-metal self-expanding stent and did a prolonged balloon tamponade; if this treatment was not successful they used a covered balloon-expandable coronary stents (2.5–3.5 mm) off-label.
Other options for treating active extravasation include embolization of the hepatic artery when low-profile covered stents are not available or cannot be delivered to the site of vessel injury.
Anyway, the reported mortality rate for endovascular management is high.
Kim et al. (2004) underlined that endovascular treatment of hepatic artery rupture is not always feasible, listing some critical points in this kind of approach, in particular the difficulty of superselective catheterization of bleeding arteries.
5.2.5 Arterial Steal Syndromes
The arterial steal syndromes, currently diagnosed on DSA, are characterized by low arterial flow towards the graft caused by a shift of flow into an enlarged splenic artery (splenic artery steal syndrome, the most frequent) or into the gastroduodenal artery (gastroduodenal artery steal syndrome). Overall incidence is 4.7%.
On Doppler US, there is a high RI of the hepatic artery with absent diastolic flow.
DSA is required for the diagnosis: slow hepatic artery flow as opposed to splenic artery flow in the absence of significant hepatic artery anatomical defects, often associated with large caliber of the splenic artery with earlier preferential splenic parenchymal perfusion compared to the liver (Nüssler et al. 2003).
Endovascular proximal splenic artery embolization is currently considered the best approach towards this syndrome; it can reverse flow abnormalities and improve liver function tests in most cases. Coil embolization of the splenic artery has been reported to be safe and effective.
Common complications of splenic artery embolization include splenic infarction, abscess formation, and sepsis (Zhu et al. 2011).
5.2.6 Non-hepatic Arterial Bleeding
OLTx is often associated with abdominal bleeding, especially during early postoperative period. A study reported a 9% overall incidence of postoperative abdominal bleeding within 1 month (Jung et al. 2012).
The most frequent non-hepatic arterial bleeding sites are the inferior phrenic arteries (the right ones are especially important because they can act as extrahepatic collateral vessels), the right and left epigastric arteries, and the intercostal arteries.
The treatment of the rupture of these vessels is performed mainly by transarterial embolization with microcoils.
5.3 Venous Complications
Venous VC linked to OLTx overall incidence is less than 3%, quite lower than arterial VCs (Pawlak et al. 2003; Pérez-Saborido et al. 2011).
Like arterial VCs, they represent an important cause of morbidity and mortality after OLTx, especially if they occur in the early postoperative period (Woo et al. 2007).
Furthermore, the dramatic nature of these complications lies in its higher incidence in pediatric transplants than in adult transplants (Orlandini et al. 2014).
Venous VCs after OLTx shall include portal (thrombosis and stenosis) and caval (thrombosis, stenosis, and kinking) problems.
As arterial VCs, they can be distinguished in early or late complications.
Currently, a vast majority of authors agree on endovascular intervention management of venous complications considering the good outcomes.
5.4 Portal Vein Complications
The incidence of portal vein complications after liver transplantation is low, ranging from 1% to 3% of patients. These complications are more common with split liver and also in pediatric transplantation.
5.4.1 Portal Vein Thrombosis (PVT)
The incidence of PVT following OLTx is between 0.3% and 2.6% (Piardi et al. 2016), occurring more frequently within 3 months after transplant (Kyoden et al. 2008).
Similar to HAT, the clinical presentation depends on the timing of thrombosis and portal hypertension development: when it occurs early, there is a severe acute liver insufficiency with graft failure; meanwhile if it occurs later, portocaval collateral circulation could exist and the clinical course will be mild (abdominal pain and/or elevated liver enzymes).
The most common causes of PVT are technical surgical errors related to venous redundancy and kinking and/or stenosis of the anastomosis. Other reported risk factors exist, such as early PVT caused by coronary vein steal after OLTx, but their discussion is beyond the aims of this chapter (Koo et al. 2008).
PVT has been associated with technical surgical problems, discrepancy between donor and recipient calibers, and hypercoagulability state; furthermore it can occur also in the case of elevated downstream flow resistance, such as in inferior vena cava stricture, or low portal inflow, related to arterial steal syndromes (see below) (Girometti et al. 2014).
5.4.1.1 Diagnosis
Portal VCs are usually detected by Doppler US or CEUS.
Doppler US is the most used examination: it should document the absence of flow in the main extrahepatic trunk, with or without definite delineation of an intraluminal echogenic thrombus on B-mode. There is not a specific protocol, but it is recommended to perform a daily evaluation of the main liver vessels.
Recently the use of CEUS has been proposed to avoid frequent false-positive results after preliminary Doppler US (Lee et al. 2013).
Furthermore, contrast-enhanced CT or contrast-enhanced MR should be used in patients who have been diagnosed with PVT by US to achieve a detailed characterization (collateral mapping, detection of local factors, and complications) (Rodrigues et al. 2017).
The invasive angiographic approach is reserved for the cases with uncertain diagnosis on noninvasive imaging and it is used when an endovascular treatment could be performed. On portography, stenosis is considered hemodynamically significant when the prestenotic/poststenotic pressure gradient is >5 mmHg.
5.4.1.2 Therapeutic Management and Prognosis
The Yerdel classification system of PVT is based on partial or complete obstruction of the lumen and extension into the splenic vein or superior mesenteric vein (SMV) (Yerdel et al. 2000). The approach to surgical management and portal vein reconstruction is dictated by the grade of PVT.
Conservative management with anticoagulation (vitamin K antagonists or heparins) is the current mainstay of treatment of PVT, getting thrombosis and secondary complications under control.
Kaneko et al. (2003) described a case of an OLTx recipient treated conservatively with anticoagulation after an early diagnosis of PVT.
However PVT remains a life-threatening event associated with a high rate of graft loss or death and in patients not responding to anticoagulation (with thrombus extension or worsening symptoms) an alternative approach should be considered.
If in the past the first choice of treatment of PVT was the surgical approach, through thrombectomy, anastomosis revision, or retransplantation, currently, except early PVT, percutaneous endovascular procedures are the first-line treatment for PVT following OLTx, and the portal VCs in general.
These include percutaneous thrombolytic therapy, transhepatic portal vein angioplasty with or without stent placement, and thrombectomy (Chamarthy et al. 2016).
Percutaneous thrombolysis involves administration of low-dose thrombolytic agents close to the clot; it can be direct using transhepatic, trans-splenic, and transjugular intrahepatic portal venous accesses or indirect when thrombolytic drugs are administered into the superior mesenteric artery.
Transjugular intrahepatic puncture is the most used approach in PVT and it is usually followed by TIPS to enhance venous outflow (Saad 2012).
Another advantage of the TIPS approach is that it may reduce the risk of intraperitoneal bleeding.
The main complications of endovascular thrombolytic therapy are risk of vessel injury, re-thrombosis, and pulmonary embolism and bradycardia if performed after creation of TIPS.
While the disadvantages of TIPS approach are that it takes longer, has more complications, and may need anesthesia, the percutaneous direct approach may have an increased bleeding risk.
Endovascular thrombectomy requires mechanical thrombus fragmentation by means of pigtail catheter, balloons, or dedicated devices (balloon thrombectomy, rheolytic thrombectomy, and suction thrombectomy) (Seedial et al. 2018).
The fragmented thrombus should be aspired using an aspiration catheter or a thrombectomy device (Uflacker 2003).
This method is mainly used in patients with contraindications to thrombolytic agents, but it may be associated with thrombolysis: the main reason of thrombolysis failure is the size of the thrombus and fragmentation facilitates the action of thrombolytic agents. In addition, thrombolysis dissolves the smaller thrombi not targetable by mechanical actions.
Percutaneous thrombectomy can also be performed through the TIPS by pulling a Fogarty catheter from the portal vein and into the IVC (Saad 2012).
Balloon angioplasty with or without stenting is meaningful in case of residual or refractory thrombosis of previous thrombolysis/thrombectomy because it can treat the underlying causes of PVT; it increases luminal gain, helps to decrease the risk of recurrent thrombosis, and removes any residual thrombus (Baccarani et al. 2001).
Moreover, initial angioplasty and/or stenting can help to restore the patency of the portal vein without prolonged thrombolysis and it reduces the risk of intrahepatic embolism that might occur during a thrombectomy or thrombolytic procedure (Adani et al. 2007).
The risks of this management are the suture dehiscence during angioplasty, the long-term patency rate being not excellent, and the interference of the stent with future surgical treatment.
To summarize, according to the time of onset of thrombosis, the management is different:
-
Complete PVT within the first 48 h post-OLTx: surgical approach is mandatory (revision of the anastomosis or retransplantation).
-
PVT at 48 h and within 30 days post-OLTx (early PVT): primarily endovascular approach, regardless of complete or partial PVT.
-
Later than 30 days (late PVT): management depends on clinical course; if liver function tests are normal, observation may be justified (there is the development of hepatoportal collaterals), while if PVT is symptomatic it should be treated with percutaneous or surgical approach. In particular, if liver function exams are stable and the intrahepatic portal vein is patent due to cavernous transformation, a conservative management is to be considered (Shibata et al. 2005).
PVT is associated with poor survival without treatment, whereas the success rate with different endovascular methods ranges from 68% to 100% with mortality and morbidity rates of 0% and 11%, respectively (Cavallari et al. 2001).
Durham et al. (1994) reported that technical success is probably around 55–70% and the mid- to long-term patency is probably as high as 50–60%.
5.4.2 Portal Vein Stenosis (PVS)
PVS is a rare vascular complication, with incidence rate reported to be less than 3% (Woo et al. 2007).
If treated late, it could lead to portal hypertension and its corollary signs, up to graft dysfunction.
In the past, surgical treatments have been considered the standard for PVS; recently percutaneous endovascular management has become established due to its minimal invasiveness and low complication and high success rates (Vignali et al. 2004).
Early PVS evolves into an early thrombosis if not treated promptly.
Most patients with PVS are asymptomatic; when symptomatic they may show signs of portal hypertension (gastroesophageal varices, ascites, and splenomegaly). Abnormal liver function tests are not constant.
Similar to PVT, the main risk factors of PVS are surgical technical complications: in children with split OLTx and living-donor liver graft there are a relatively short donor portal vein segment and usually a mismatched diameter between native portal vein segment and donors’ one.
Other predisposing factors for portal complications are preexisting thrombosis and large portocaval collaterals.
Late PVS may have different etiologies and be secondary to fibrosis or intimal hyperplasia.
5.4.2.1 Diagnosis
Doppler US is the first examination to screen for VCs, but definite and objective criteria for PVS do not exist (Piardi et al. 2016).
The PVS criteria for diagnosis include narrowing portal caliber, abnormal velocities at the anastomotic site (PSV greater than 125 cm/s; anastomotic to preanastomotic segment PSV rate greater than 3:1), and scarcity of flow of the intrahepatic portal vein. If findings are suggestive of PVS, contrast-enhanced CT should be performed to confirm the diagnosis (more than 50% narrowing of the main portal venous diameter with or without poststenotic dilatation).
Contrast-enhanced CT or MRI angiography could also be used to detect a focal stenosis because it is hard to investigate by Doppler US.
When a patient is asymptomatic, indirect portography is an option to detect a PVS; percutaneous transhepatic portography is performed when the measurement of portal pressure gradients is required. Criteria for diagnosing PVS are stenosis more than 50% of the main portal venous diameter and a pressure gradient across the stenosis >5 mmHg.
Although a pre-post-anastomotic pressure gradient >5 mmHg has been considered diagnostic of PVS, there are no standard criteria to define a pressure gradient as significant. Park et al. showed that trans-stenotic pressure gradient does not appear to be directly related to the clinical and therapeutic results (Park et al. 2005).
If PVS is suspected, operative therapeutic management is necessary to prevent PVT and other complications. Some authors recommend the use of anticoagulant therapy for the prevention of recurrent PVT (Sanada et al. 2010).
5.4.2.2 Therapeutic Management and Prognosis
As in arterial VCs, classically and especially in very early portal VCs, surgical treatment (anastomotic revision or retransplantation) was usually performed, while nowadays interventional radiology is the first-line treatment for PVS following OLTx.
The approach can be transhepatic or transjugular, and unlike in PVT the most used is the first one (Glanemann et al. 2001).
In case of asymptomatic patients with PVS diagnosis and normal hepatic function test, management may be conservative and an appropriate follow-up is normally sufficient (Kaneko et al. 2003).
Percutaneous treatment includes balloon angioplasty with or without stent placement, or primary stent placement (Ko et al. 2007).
Technical success of the procedure was defined as <30% residual stenosis at portography with the absence of varices or collateral circulation (Park et al. 2005).
Shibata et al. (2005) reported that a single-balloon dilatation was sufficient to maintain portal vein patency with relative low recurrence rate (28.6%); similar results have been experienced by Park and colleagues.
Stents have usually been used to treat recurrent and elastic portal venous stenosis following balloon angioplasty. Majority early PVS is secondary to technical factors (tight suture line, discrepancy of the PV size) that does not benefit from balloon angioplasty alone.
Ko et al. (2007) performed primary stent placement rather than balloon angioplasty with positive technical and clinical outcome in almost 78% of patients.
Wei et al. (2009) performed first balloon angioplasty and then placed a balloon-expandable metallic stent in order to reduce the incidence “jump forward” of the stent (the most common displacement during stent deployment), achieving promising results although PVT or stent-edge stenosis may occur.
It seems reasonable that angioplasty with stenting can restore the normal portal flow once and for all, reducing the number of procedure-related complications, especially in PVS with fibrosis or intimal hyperplasia etiology (most late PVS).
5.5 Caval Complications
Hepatic blood outflow obstruction following OLTx is mainly related to VCs in caval anastomosis site, i.e., kinking, stenosis, or thrombosis of inferior vena cava or hepatic veins.
These VCs are relatively uncommon, with a reported incidence of less than 2% (Zhang et al. 2017).
Hepatic vein stenosis is more likely to occur after living-related transplants because the preservation of the recipient IVC with the piggyback technique has been associated with an increased risk of thrombosis or stenosis.
Clinical course of hepatic vein occlusion may vary from mildly abnormal liver function tests to an acute Budd-Chiari syndrome with abdominal pain, ascites, and hepatomegaly, or from lower limb edema and pericardial and pleural effusion to hypotension leading to allograft loss and multiorgan failure.
Almost 20% of patients are asymptomatic because chronic hepatic venous obstruction is associated with intrahepatic and portosystemic collateral vessels.
Usually, technical factors lead to venous obstruction in the early postoperative period, whereas the delayed presentation may be related to intimal hyperplasia, perivascular fibrosis, or extrinsic caval compression (Darcy 2007).
5.5.1 Diagnosis
Diagnosis should be achieved by Doppler US, contrast-enhanced CT, and RM, and finally, if findings are suggestive of venous outflow impairment, by cavography.
Although Doppler US is useful, venography and pressure measurements are still considered to be the gold standard.
Usually the gradient across the hepatic venous anastomosis is assessed to prove the diagnosis: a gradient higher than 10 mmHg is one commonly used threshold, but a gradient ranging from 3 to 20 mmHg has been considered to be the threshold of abnormality in different studies.
For this reason, stenoses have to be validated considering symptoms and response to treatments.
5.5.2 Therapeutic Management and Prognosis
Modified piggyback consists of a complete resection of the recipient inferior caval vein and interposition of the donor intrahepatic part of the vena cava with two end-to-end anastomoses; it is reported a three-vein technique for anastomosis with a low rate of these VCs.
Except for severe allograft dysfunction and multiorgan failure requiring retransplantation, percutaneous radiological intervention is the first-line treatment for liver blood outflow VCs in order to attempt to rescue the outflow patency, reserving surgery for a lower number of cases.
Endovascular management can be performed by transjugular approach or percutaneous transhepatic access.
Technical success is defined as morphologic improvement or as elimination or significant reduction of the trans-stenotic pressure gradient; venous outflow VCs are often treated with 100% success rate (Wang et al. 2005).
Thrombolysis has been described to treat anastomotic stenosis with superimposed thrombosis (Borsa et al. 1999). Mechanical thrombolytic devices may also be used to avoid bleeding.
Balloon angioplasty can restore anastomotic patency in almost 100% of cases, but the recurrence rate is high; however it is possible to repeat balloon dilatations (Cheng et al. 2005).
PTA associated with stent placement seems to have a higher rate of success than PTA alone, ranging from 73% to 100% in the literature (Ko et al. 2002).
While thrombolysis and angioplasty may cause anastomotic bleeding, stent migration is an infrequent but reported complication, not only during the procedure but also later, related to changes in the size of the IVC.
During PTA, a close monitoring is required since prolonged balloon inflation reduces blood return to the heart.
Considering the high incidence of restenosis, stenting may be preferable in adults, while in pediatric patients stenting may not be the best option: considering the potential growth of children repeated PTA is probably a better strategy.
References
Abbasoglu O, Levy MF, Vodapally MS, Goldstein RM, Husberg BS, Gonwa TA, Klintmalm GB (1997) Hepatic artery stenosis after liver transplantation—incidence, presentation, treatment, and long-term outcome. Transplantation 63:250–255
Abraldes JG, Tarantino I, Turnes J et al (2003) Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 37:902–908
Abraldes JG, Tandon P, Yap J (2014) The adult survivor with variceal bleeding. Clin Liver Dis 4(4):89–92
Adani GL, Baccarani U, Risaliti A (2007) Percutaneous transhepatic portography for the treatment of early portal vein thrombosis after surgery. Cardiovasc Intervent Radiol 30:1222–1226
Aussilhou B, Lesurtel M, Sauvanet A, Farges O, Dokmak S, Goasguen N, Silbert A, Vilgrain V, Belghiti J (2008) Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg 12:297–303
Baccarani U, Gasparini D, Risaliti A, Vianello V, Adani GL, Sainz M, Sponza M, Bresadola F (2001) Percutaneous mechanical fragmentation and stent placement for the treatment of early posttransplantation portal vein thrombosis. Transplantation 72:1572–1582
Bekker J, Ploem S, de Jong KP (2009) Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant 9:746–757
Beppu K, Inokuchi K, Koyanagi N, Nakayama S, Sakata H, Kitano S, Kobayashi M (1981) Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc 27(4):213–218
Berzigotti A, Seijo S, Reverter E et al (2013) Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol 7(2):141–155
Borsa JJ, Daly CP, Fontaine AB et al (1999) Treatment of inferior vena cava anastomotic stenoses with the Wallstent endoprosthesis after orthotopic liver transplantation. J Vasc Interv Radiol 10(1):17–22
Bosch J, Mastai R, Kravetz D et al (1986) Hemodynamic evaluation of patients with portal hypertension. Semin Liver Dis 6(4):309–317
Bosch J, Garcia-Pagan JC, Berzigotti A et al (2006) Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis 26:348–362
Bosch J, Abraldes JG, Berzigotti A (2009) The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 6(10):573–582
Broering DC, Hillert C, Krupski G et al (2002) Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg 6:905–913
Brouquet A, Abdalla E, Kopetz S, Garrett C, Overman M, Eng C et al (2011) High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 29(8):1083–1090
Campagna F, Montagnese S, Ridola L, Senzolo M, Schiff S, De Rui M et al (2017) The animal naming test: an easy tool for the assessment of hepatic encephalopathy: Campagna et al. Hepatology 66:198–208
Cavallari A, Vivarelli M, Bellusci R, Jovine E, Mazziotti A, Rossi C (2001) Treatment of vascular complications following liver transplantation: multidisciplinary approach. Hepato-Gastroenterology 48:179–183
Chamarthy MR, Anderson ME, Pillai AK, Kalva SP (2016) Thrombolysis and transjugular intrahepatic portosystemic shunt creation for acute and subacute portal vein thrombosis. Tech Vasc Interv Radiol 19:42–51
Chana A, James M, Veale P (2016) Anaesthesia for transjugular intrahepatic portosystemic shunt insertion. BJA Educ 16:405–409
Chen GH, Wang GY, Yang Y, Li H, Lu MQ, Cai CJ, Wang GS, Xu C, Yi SH, Zhang JF, Fu BS (2009) Single-center experience of therapeutic management of hepatic artery stenosis after orthotopic liver transplantation. Report of 20 cases. Eur Surg Res 42:21–27
Chen J, Weinstein J, Black S, Spain J, Brady PS, Dowell JD (2014) Surgical and endovascular treatment of hepatic arterial complications following liver transplant. Clin Transpl 28:1305–1312
Cheng YF, Huang TL, Lee TY, Chen TY, Chen CL (1996) Variation of the intrahepatic portal vein; angiographic demonstration and application in living-related hepatic transplantation. Transplant Proc 28:1667–1668
Cheng YF, Chen CL, Huang TL et al (2005) Angioplasty treatment of hepatic vein stenosis in pediatric liver transplants: long-term results. Transpl Int 18:556–561
Colapinto RF, Stronell RD, Gildiner M, Ritchie AC, Langer B, Taylor BR, Blendis LM (1983) Formation of intrahepatic portosystemic shunts using a balloon dilatation catheter: preliminary clinical experience. AJR Am J Roentgenol 140:709–714
Copelan A, Kapoor B, Sands M (2014) Transjugular intrahepatic portosystemic shunt: indications, contraindications, and patient work-up. Semin Interv Radiol 31:235–242
D’Amico G, Garcia-Tsao G (2001) Diagnosis of portal hypertension. How and when? In: de Franchis R (ed) Portal hypertension. In: Proceedings of the third Baveno international consensus workshop on definitions, methodology and therapeutic strategies. Blackwell Science, Oxford, UK, pp 36–64
Darcy M (2007) Management of venous outflow complications after liver transplantation. Tech Vas Intervent Radiol 10(3):240–245
Darcy M (2012) Evaluation and management of transjugular intrahepatic portosystemic shunts. Am J Roentgenol 199:730–736
Dariushnia SR, Haskal ZJ, Midia M, Martin LG, Walker TG, Kalva SP et al (2016) Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 27:1–7
De Franchis R (2010) Baveno V faculty: revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 53:762–768
De Franchis R (2015) Expanding consensus in portal hypertension Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 63:743–752
Deltenre P, Trépo E, Rudler M, Monescillo A, Fraga M, Denys A et al (2015) Early transjugular intrahepatic portosystemic shunt in cirrhotic patients with acute variceal bleeding: a systematic review and meta-analysis of controlled trials. Eur J Gastroenterol Hepatol 27:e1–e9
Denys A, Bize P, Demartines N, Deschamps F, De Baere T (2010) Quality improvement for portal vein embolization. Cardiovasc Intervent Radiol 33(3):452–456
Dhanasekaran R, West JK, Gonzales PC, Subramanian R, Parekh S, Spivey JR et al (2010) Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol 105:635–641
Dodd GD III, Memel DS, Zajko AB, Baron RL, Santaguida LA (1994) Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology 192(3):657–661
Durham JD, LaBerge JM, Altman S, Kam I, Everson GT, Gordon RL, Kumpe DA (1994) Portal vein thrombolysis and closure of competitive shunts following liver transplantation. J Vasc Interv Radiol 5:611–615; discussion 616–618
Fagiuoli S, Bruno R, Debernardi Venon W, Schepis F, Vizzutti F et al (2017) Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis 49:121–137
Fanelli F, Salvatori FM, Corona M, Bruni A, Pucci A, Boatta E et al (2006) Stent graft in TIPS: technical and procedural aspects. Radiol Med (Torino) 111:709–723
Farsad K, Kolbeck KJ (2014) Clinical and radiologic evaluation of patients before TIPS creation. Am J Roentgenol 203:739–745
Feldstein VA, Patel MD, LaBerge JM (1996) Transjugular intrahepatic portosystemic shunts: accuracy of Doppler US in determination of patency and detection of stenoses. Radiology 201:141–147
Ferral H, Gomez-Reyes E, Fimmel CJ (2016) Post-transjugular intrahepatic portosystemic shunt follow-up and management in the VIATORR era. Tech Vasc Interv Radiol 19:82–88
Feu F, Garcia-Pagan JC, Bosch J et al (1995) Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal hemorrhage in patients with cirrhosis. Lancet 346:1056–1059
Figueras J, Busquets J, Diminguez J et al (1995) Intra-arterial thrombosis in the treatment of acute hepatic artery thrombosis after liver transplantation. Transplantation 59:1356
Fistouris J, Herlenius G, Beackman L et al (2006) Pseudoaneurysm of the hepatic artery following liver transplantation. Transplant Proc 38:2679
Forner A, Bruix J (2009) East meets the west: portal pressure predicts outcome of surgical resection for hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol 6:14–15
Fouzas I, Sklavos A, Bismpa K, Paxiadakis I, Antoniadis N, Giakoustidis D, Katsiki E, Tatsou N, Mouloudi E, Karapanagiotou A, Tsitlakidis A, Karakatsanis A, Patsiaoura K, Petridis A, Gakis D, Imvrios D, Papanikolaou V (2012) Hepatic artery thrombosis after orthotopic liver transplantation: 3 patients with collateral formation and conservative treatment. Transplant Proc 44:2741–2744
Frongillo F, Grossi U, Lirosi MC, Nure E, Sganga G, Avolio AW, Inchingolo R, Di Stasi C, Rinaldi P, Agnes S (2013) Incidence, management, and results of hepatic artery stenosis after liver transplantation in the era of donor to recipient match. Transplant Proc 45:2722–2725
Gaba RC, Khiatani VL, Knuttinen MG, Omene BO, Carrillo TC, Bui JT, Owens CA (2011) Comprehensive review of TIPS technical complications and how to avoid them. Am J Roentgenol 196:675–685
Gandini R, Konda D, Toti L, Abrignani S, Merolla S, Tisone G, Floris R (2016) Endovascular mechanical thromboaspiration of right hepatic arterial thrombosis after liver transplantation. Cardio Vascular Intervent Radiol 40(4):621–624
Garcia-Tsao G (2005) Transjugular intrahepatic portosystemic shunt in the management of refractory ascites. Semin Interv Radiol 22:278–286
Garcia-Tsao G, Bosch J (2010) Management of varices and variceal hemorrhage in cirrhosis. New Engl J Med 362(9):823–832
Garcia-Tsao G, Groszmann RJ, Fisher RL et al (1985) Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 5:419–424
Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W (2007) Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 46:922–938
Girometti R, Como G, Bazzocchi M, Zuiani C (2014) Post-operative imaging in liver transplantation: state-of-the-art and future perspectives. World J Gastroenterol 20(20):6180–6200
Glanemann M, Settmacher U, Langrehr JM, Kling N, Hidajat N, Stange B, Staffa G, Bechstein WO, Neuhaus P (2001) Portal vein angioplasty using a transjugular, intrahepatic approach for treatment of extrahepatic portal vein stenosis after liver transplantation. Transpl Int 14:48–51
Gluud C, Henriksen JH, Nielsen G (1988) Prognostic indicators in alcoholic cirrhotic men. Hepatology 8:222–227
Goldsmith LE, Wiebke K, Seal J, Brinster C, Smith TA, Bazan HA, Sternbergh WC (2017) Complications after endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg 66(5):1488–1496
Groszmann RJ, Wongcharatrawee S (2004) The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology 39:280–282
Groszmann RJ, Glickman M, Blei AT et al (1979) Wedged and free hepatic venous pressure measured with a balloon catheter. Gastroenterology 76:253–258
Groszmann RJ, Bosch J, Grace ND et al (1990) Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 99:1401–1407
Groszmann RJ, Garcia-Tsao G, Makuch R et al (2003) Multicenter randomized placebo-controlled trial of non-selective b-blockers in the prevention of the complications of portal hypertension: final results and identification of a predictive factor. Hepatology 38:206
Groszmann RJ, Garcia-Tsao G, Bosch J et al (2005) Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 353:2254–2261
Gunsar F, Rolando N, Pastacaldi S, Patch D, Raimondo ML, Davidson B, Rolles K, Burroughs AK (2003) Late hepatic artery thrombosis after orthotopic liver transplantation. Liver Transpl 9(6):605–611
Hallion L, Francois-Frank CA (1896) Recherches experimentales executees a l’aide d’um novel appareil volumetrique sur l’innervation vaso-motrice de l’intestin. Arch Physiol Norm Pathol 8:493–508
Hoekstra LT, Van Lienden KP et al (2012) Tumor progression after preoperative portal vein embolization. Ann Surg 256(5):812–817
Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, Wells JK, Grant EG (2006) Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology 241:267–274
Huet PM, Pomier-Layrargues G (2004) The hepatic venous pressure gradient: “remixed and revisited”. Hepatology 39(2):295–298
Jung JW, Hwang S, Namgoong JM, Yoon SY, Park CS, Park YH, Lee HJ, Park HW, Park GC, Jung DH, Song GW, Ha TY, Ahn CS, Kim KH, Moon DB, Ko GY, Sung KB, Lee SG (2012) Incidence and management of postoperative abdominal bleeding after liver transplantation. Transplant Proc 44:765–768
Kaneko J, Sugawara Y, Ohkubo T, Matsui Y, Kokudo N, Makuuchi M (2003) Successful conservative therapy for portal vein thrombosis after living donor liver transplantation. Abdom Imaging 28:58–59
Kanterman RY, Darcy MD, Middleton WD, Sterling KM, Teefey SA, Pilgram TK (1997) Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. Am J Roentgenol 168:467–472
Keiding S, Vilstrup H (2002) Intrahepatic heterogeneity of hepatic venous pressure gradient in human cirrhosis. Scand J Gastroenterol 37:960–964
Keller FS, Farsad K, Rösch J (2016) The transjugular intrahepatic portosystemic shunt: technique and instruments. Tech Vasc Interv Radiol 19:2–9
Kim YD (2014) Management of acute variceal bleeding. Clin Endosc 47:308–314
Kim JH, Ko GY, Yoon HK et al (2004) Causes of arterial bleeding after living donor liver transplantation and the results of transcatheter arterial embolization. Korean J Radiol 5:164
Ko GY, Sung KB, Yoon HK et al (2002) Endovascular treatment of hepatic venous outflow obstruction after living-donor liver transplantation. J Vasc Interv Radiol 13:591–599
Ko GY, Sung KB, Yoon HK, Lee S (2007) Early posttransplantation portal vein stenosis following living donor liver transplantation: percutaneous transhepatic primary stent placement. Liver Transpl 13:530–536
Koo BY, Yu HC, So MC, Jin GY, Kwak HS, Cho BH (2008) Endovascular management of early portal vein thrombosis caused by coronary vein steal after liver transplantation and its outcome. Clin Transpl 22:668–671
Krajina A, Hulek P, Fejfar T, Valek V (2012) Quality improvement guidelines for transjugular intrahepatic portosystemic shunt (TIPS). Cardiovasc Intervent Radiol 35:1295–1300
Kumar A, Sharma P, Sarin SK (2008) Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol 27:74–80
Kyoden Y, Tamura S, Sugawara Y, Matsui Y, Togashi J, Kaneko J, Kokudo N, Makuuchi M (2008) Portal vein complications after adult-to-adult living donor liver transplantation. Transpl Int 21:1136–1144
Lee SJ, Kim KW, Kim SY, Park YS, Lee J, Kim HJ, Lee JS, Song GW, Hwang S, Lee SG (2013) Contrast-enhanced sonography for screening of vascular complication in recipients following living donor liver transplantation. J Clin Ultrasound 41:305–312
Liu H, Zhu S (2009) Present status and future perspectives of preoperative portal vein embolization. Am J Surg 197:686–690
Llovet JM, Fuster J, Bruix J (1999) Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 30:1434–1440
Lombardo S, Espejo JJ, Pérez-Montilla ME, Zurera LJ, Gonzalez-Galilea A (2018) The keys for successful TIPS in patients with portal vein thrombosis and cavernous transformation. Australas Radiol 60:94–104
Madoff DC, Marshall EH et al (2002) Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics 22(5):1063–1076
Madoff DC, Wallace MJ, Ahrar K, Saxon RR (2004) TIPS-related hepatic encephalopathy: management options with novel endovascular techniques. Radiographics 24:21–36
Maharaj B, Maharaj RJ, Leary WP et al (1986) Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet 1:523–525
Makuuchi M, Thai BL et al (1990) Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 107(5):521–527
Martínez-Pallí G, Drake BB, García-Pagán J-C, Barberà J-A, Arguedas MR, Rodriguez-Roisin R et al (2005) Effect of transjugular intrahepatic portosystemic shunt on pulmonary gas exchange in patients with portal hypertension and hepatopulmonary syndrome. World J Gastroenterol 11:6858–6862
Meek JC, McDougal Jonathan S, Borja-Cacho D, Meek ME (2018) Use of a mechanical thrombectomy device to treat early hepatic artery thrombosis after orthotopic liver transplant. Radiol Case Rep 13(2):522–526
Merkel C, Bolognesi M, Bellon S et al (1992) Prognostic usefulness of hepatic vein catheterization in patients with cirrhosis and esophageal varices. Gastroenterology 102:973–979
Micol C, Marsot J, Boublay N, Pilleul F, Berthezene Y, Rode A (2012) Contrast-enhanced ultrasound: a new method for TIPS follow-up. Abdom Imaging 37:252–260
Miraglia R, Maruzzelli L, Caruso S, Milazzo M, Marrone G, Mamone G, Carollo V, Gruttadauria S, Luca A, Gridelli B (2009) Interventional radiology procedures in adult patients who underwent liver transplantation. World J Gastroenterol 15:684–693
Moore KP, Aithal GP (2006) Guidelines on the management of ascites in cirrhosis. Gut 55:vi1–vi12
Murata Y, Mizuno S, Kato H, Tanemura A, Kuriyama N, Azumi Y, Kishiwada M, Usui M, Sakurai H, Fujimori M, Yamanaka T, Nakatsuka A, Yamakado K, Isaji S (2016) Technical feasibility and clinical outcomes of interventional endovascular treatment for hepatic artery thrombosis after living-donor liver transplantation. Transplant Proc 48:1142–1148
Myers JD, Taylor WJ (1951) An estimation of portal venous pressure by occlusive catheterization of a hepatic venule. J Clin Invest 30:662–663
Narita M, Oussoultzoglou E et al (2011) Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg 98(10):1463–1475
Narula N, Aloia TA (2017) Portal vein embolization in extended liver resection. Langenbeck’s Arch Surg 402(5):727–735
Nikeghbalian S, Kazemi K, Davari HR, Salahi H, Bahador A, Jalaeian H, Khosravi MB, Ghaffari S, Lahsaee M, Alizadeh M, Rasekhi AR, Nejatollahi SM, Malek-Hosseini SA (2007) Early hepatic artery thrombosis after liver transplantation: diagnosis and treatment. Transplant Proc 39(4):1195–1196
Nüssler N, Settmacher U, Haase R, Stange B, Heise M, Neuhaus P (2003) Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl 9:596–602
Orlandini M, Feier FH, Jaeger B, Kieling C, Vieira SG, Zanotelli ML (2014) Frequency of and factors associated with vascular complications after pediatric liver transplantation. J Pediatr 90:169–175
Panaro F, Gallix B, Bouyabrine H, Ramos J, Addeo P, Testa G, Carabalona JP, Pageaux G, Domergue J, Navarro F (2011) Liver transplantation and spontaneous neovascularization after arterial thrombosis: “the neovascularized liver”. Transpl Int 24:949–957
Panaro F, Giannone F, Riviere B, Sgarbura O, Cusumano C, Deshayes E, Navarro F, Guiu B, Quenet F (2019) Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center. Hepatobiliary Surg Nutr 8(4):329–337
Pandanaboyana S, Bell R et al (2015) A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery 157(4):690–698
Pareja E, Cortes M, Navarro R et al (2010) Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc 42:2970–2972
Park KB, Choo SW, Do YS, Shin SW, Cho SG, Choo IW (2005) Percutaneous angioplasty of portal vein stenosis that complicates liver transplantation: the mid-term therapeutic results. Korean J Radiol 6:161–166
Pawlak J, Grodzicki M, Leowska E, Małkowski P, Michałowicz B, Nyckowski P, Rowiński O, Pacho R, Zieniewicz K, Andrzejewska M, Ołdakowska U, Grzelak I, Patkowski W, Alsharabi A, Remiszewski P, Dudek K, Krawczyk M (2003) Vascular complications after liver transplantation. Transplant Proc 35:2313–2315
Perello A, Escorsell A, Bru C et al (1999) Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology 30:1393–1397
Pérez-Saborido B, Pacheco-Sánchez D, Barrera-Rebollo A, Asensio-Díaz E, Pinto-Fuentes P, Sarmentero-Prieto JC, Rodríguez-Vielba P, Martínez-Díaz R, Gonzalo-Martín M, Rodríguez M, Calero-Aguilar H, Pintado-Garrido R, García-Pajares F, Anta-Román A (2011) Incidence, management, and results of vascular complications after liver transplantation. Transplant Proc 43:749–750
Piardi T, Lhuaire M, Bruno O, Memeo R, Pessaux P, Kianmanesh R, Sommacale D (2016) Vascular complications following liver transplantation: a literature review of advances in 2015. World J Hepatol 8(1):36–57
Piscaglia F, Bolondi L (2006) The safety of Sonovue® in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 32:1369–1375
Rector WG (1986) Portal hypertension: a permissive factor only in the development of ascites and variceal bleeding. Liver 6:221–226
Reiberger T, Püspök A, Schoder M, Baumann-Durchschein F, Bucsics T, Datz C et al (2017) Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr 129:135–158. https://doi.org/10.1007/s00508-017-1262
Richter GM, Palmaz JC, Noldge G, Rossle M, Siegerstetter V, Franke M, Wenz W (1989) The transjugular intrahepatic portosystemic stent-shunt. A new nonsurgical percutaneous method. Radiologe 29:406–411
Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A et al (2008) Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol 103:2738–2746
Ripoll C, Groszmann RJ, Garcia-Tsao G et al (2007) Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 133:481–488
Ripoll C, Groszmann RJ, Garcia-Tsao G et al (2009) Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 50:923–928
Rodrigues SG, Maurer Martin H, Baumgartner I, De Gottardi A, Berzigotti A. (2017) Imaging and minimally invasive endovascular therapy in the management of portal vein thrombosis. Abdom Radiol.
Rösch J, Hanafee WN, Snow H (1969) Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology 92:1112–1114
Rossle M, Gerbes AL (2010) TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut 59:988–1000
Runyon BA (2004) Management of adult patients with ascites due to cirrhosis. Hepatology 39:841–856
Ryu RK, Durham JD, Krysl J, Shrestha R, Shrestha R, Everson GT et al (1999) Role of TIPS as a bridge to hepatic transplantation in Budd-Chiari syndrome. J Vasc Interv Radiol 10:799–805
Saad WEA (2012) Liver transplant-related vascular disease. In: Dake M, Geshwind J-F, eds. Abrams angiography: interventional radiology 3rd ed.
Saad WEA (2014) The history and future of transjugular intrahepatic portosystemic shunt: food for thought. Semin Interv Radiol 31:258–261
Saad NEA, Saad WEA, Davies MG, Waldman DL, Fultz PJ, Rubens DJ (2005) Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics 25:S173–S189
Saad WEA, Davies MG, Saad NEA, Westesson KE, Patel NC, Sahler LG, Lee DE, Kitanosono T, Sasson T, Waldman DL (2007) Catheter thrombolysis of thrombosed hepatic arteries in liver transplant recipients: predictors of success and role of thrombolysis. Vasc Endovasc Surg 41:19–26
Saad NEA, Darcy M, Saad WEA (2008) Portal anatomic variants relevant to transjugular intrahepatic portosystemic shunt. Tech Vasc Interv Radiol 11:203–207
Salerno F, Cammà C, Enea M, Rössle M, Wong F (2007) Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology 133:825–834
Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P et al (2010) Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int 30:937–947
Sanada Y, Kawano Y, Mizuta K, Egami S, Hayashida M, Wakiya T, Fujiwara T, Sakuma Y, Hydo M, Nakata M, Yasuda Y, Kawarasaki H (2010) Strategy to prevent recurrent portal vein stenosis following interventional radiology in pediatric liver transplantation. Liver Transpl 16:332–339
Schepis F, Vizzutti F, Garcia-Tsao G, Marzocchi G, Rega L, De Maria N et al (2018) Under-dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non-randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol 16(7):1153.e7–1162.e7
Schultz SR, LaBerge JM, Gordon RL, Warren RS (1994) Anatomy of the portal vein bifurcation: intra- versus extrahepatic location—implications for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 5:457–459
Seedial S, Mouli S, Desai K (2018) Acute portal vein thrombosis: current trends in medical and endovascular management. Semin Interv Radiol 35(3):198–202
Shibata T, Itoh K, Kubo T, Maetani Y, Shibata T, Togashi K, Tanaka K (2005) Percutaneous transhepatic balloon dilation of portal venous stenosis in patients with living donor liver transplantation. Radiology 235:1078–1083
Simoneau E, Hassanain M et al (2015) Portal vein embolization and its effect on tumour progression for colorectal cancer liver metastases. Br J Surg 102(10):1240–1249
Sommacale D, Aoyagi T, Dondero F, Sibert A, Bruno O, Fteriche S, Francoz C, Durand F, Belghiti J (2013) Repeat endovascular treatment of recurring hepatic artery stenoses in orthotopic liver transplantation. Transpl Int 26:608–615
Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmuller T, Neuhaus P (2003) Hepatic artery thrombosis after adult liver transplantation. Liver Transpl 9:612–620
Steinlauf AF, Garcia-Tsao G, Zakko MF et al (1999) Low-dose midazolam sedation: an option for patients undergoing serial hepatic venous pressure measurements. Hepatology 29:1070–1073
Strauss RM, Boyer TD (1997) Hepatic hydrothorax. Semin Liver Dis 17:227–232
Sueyoshi E, Sakamoto I, Nakashima K, Minami K, Hayashi K (2005) Visceral and peripheral arterial pseudoaneurysms. AJR 185:741–749
Triantos C, Kalafateli M (2014) Endoscopic treatment of esophageal varices in patients with liver cirrhosis. World J Gastroenterol 20:13015–13026
Tsochatzis EA, Bosch J, Burroughs AK (2014) Liver cirrhosis. Lancet 383:1749–1761
Ueno T, Jones G, Martin A, Ikegami T, Sanchez EQ, Chinnakotla S, Randall HB, Levy MF, Goldstein RM, Klintmalm GB (2006) Clinical outcomes from hepatic artery stenting in liver transplantation. Liver Transpl 12:422–427
Uflacker R (2003) Applications of percutaneous mechanical thrombectomy in transjugular intrahepatic portosystemic shunt and portal vein thrombosis. Tech Vasc Interv Radiol 6:59–69
Vaidya S, Dighe M, Kolokythas O et al (2007) Liver transplantation: vascular complications. Ultrasound Q 23:239
Vignali C, Cioni R, Petruzzi P, Cicorelli A, Bargellini I, Perri M, Urbani L, Filipponi F, Bartolozzi C (2004) Role of interventional radiology in the management of vascular complications after liver transplantation. Transplant Proc 36:552–554
Vignali C, Bargellini I, Grosso M, Passalacqua G, Maglione F, Pedrazzini F et al (2005) TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. Am J Roentgenol 185:472–480
Volpin E, Pessaux P, Sauvanet A, Sibert A, Kianmanesh R, Durand F, Belghiti J, Sommacale D (2014) Preservation of the arterial vascularisation after hepatic artery pseudoaneurysm following orthotopic liver transplantation: long-term results. Ann Transplant 19:346–352
Vorobioff J, Groszmann RJ, Picabea E et al (1996) Prognostic value of hepatic venous pressure measurement in alcoholic cirrhosis: a 10-year prospective study. Gastroenterology 111:701–709
Wachsberg RH, Bahramipour P, Sophocleous CT et al (2002) Hepatofugal flow in the portal venous system: pathophysiology, imaging findings, and diagnostic pitfalls. Radiographics 22:123–140
Wallace MJ, Madoff DC, Ahrar K, Warneke CL (2004) Transjugular intrahepatic portosystemic shunts: experience in the oncology setting. Cancer 101:337–345
Wang SL, Sze DY, Busque S et al (2005) Treatment of hepatic venous outflow obstruction after piggyback liver transplantation. Radiology 236:352–359
Wei BJ, Zhai RY, Wang JF, Dai DK, Yu P (2009) Percutaneous portal venoplasty and stenting for anastomotic stenosis after liver transplantation. World J Gastroenterol 15:1880–1885
Wong F (2012) Management of ascites in cirrhosis. J Gastroenterol Hepatol 27:11–20
Woo DH, Laberge JM, Gordon RL, Wilson MW, Kerlan RK (2007) Management of portal venous complications after liver transplantation. Tech Vasc Interv Radiol 10:233–239
Yeom YK, Shin JH (2015) Complications of portal vein embolization: evaluation on cross sectional imaging. Korean J Radiol 16(5):1079–1085
Yerdel MA, Gunson B, Mirza D et al (2000) Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 69(9):1873–1881
Zhang ZY, Jin L, Chen G, Su TH, Zhu ZJ, Sun LY, Wang ZC, Xiao GW (2017) Balloon dilatation for treatment of hepatic venous outflow obstruction following pediatric liver transplantation. World J Gastroenterol 23(46):8227–8234
Zheng M, Chen Y, Bai J, Zeng Q, You J, Jin R et al (2008) Transjugular intrahepatic portosystemic shunt versus endoscopic therapy in the secondary prophylaxis of variceal rebleeding in cirrhotic patients: meta-analysis update. J Clin Gastroenterol 42:507–516
Zheng BW, Tan YY, Fu BS, Tong G, Wu T, Wu LL, Meng XC, Zheng RQ, Yi SH, Ren J (2017) Tardus parvus waveforms in Doppler ultrasonography for hepatic artery stenosis after liver transplantation: can a new cut-off value guide the next step? Abdom Radiol 43(7):1634–1641
Zhou J, Fan J, Wang JH, Wu ZQ, Qiu SJ, Shen YH, Shi YH, Huang XW, Wang Z, Tang ZY, Wang YQ (2005) Continuous transcatheter arterial thrombolysis for early hepatic artery thrombosis after liver transplantation. Transplant Proc 37:4426–4429
Zhu X, Tam MD, Pierce G et al (2011) Utility of the Amplatzer vascular plug in splenic artery embolization: a comparison study with conventional coil technique. Cardiovasc Intervent Radiol 34:522–531
Žižka J, Eliáš P, Krajina A, Michl A, Lojík M, Ryška P et al (2000) Value of doppler sonography in revealing transjugular intrahepatic portosystemic shunt malfunction: a 5-year experience in 216 patients. Am J Roentgenol 175:141–148
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pauro, A., Lupi, A., Mattolin, C., Lazzarin, M., Quaia, E. (2021). Hepatic Angiography and Vascular Interventional Radiology. In: Quaia, E. (eds) Imaging of the Liver and Intra-hepatic Biliary Tract. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-030-38983-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-38983-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38982-6
Online ISBN: 978-3-030-38983-3
eBook Packages: MedicineMedicine (R0)