Abstract
Modern use of reproductive technologies has revolutionized the treatment of infertile couples. Strategies to improve ovarian function in cases of diminished ovarian reserve are perhaps the least understood area in this field and will be the chief focus of this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Infertility
- Oocytes
- Primary follicles
- Gonadotropin
- Functional ovarian reserve
- Antral follicle count (AFC)
- Ovulation Induction
- Egg retrieval
1 Introduction
We often take our biological functions for granted. When a natural function ceases to occur normally, we feel cheated, betrayed by our own bodies. This is never truer than when a couple finds themselves dealing with the problem of infertility.
Infertility has been defined as the failure to achieve an ongoing pregnancy within the course of 1 year of trying to conceive. But in fact, for couples who have decided to build a family, each month without an established pregnancy can feel like a little death. Since reproduction is commonly dependent on sexual intercourse between a man and woman, in the past, it was relegated to a very private sphere of discussion. For decades couples suffered privately with this problem, too embarrassed to discuss it with their families or even with their physicians.
A deeper understanding of reproductive processes has evolved in our lifetime to encompass greater control of reproduction, both in contraception and in family building, and has gradually brought this conversation into the open and provided new tools to help couples faced with these problems.
Reproductive problems that, in the past, were often surgically treated can now be approached with less intervention. Men and women who, in the past, would be left childless can now find hope to build their families. This chapter will describe some of the tools we now use to help couples achieve their family goals, especially those dealing with diminished ovarian reserve, and will look at how we expect these opportunities to grow in the future.
2 Causes of Infertility
In simplest terms there are three major components in evaluating the infertile couple: (1) evaluation of normal sperm production and function, (2) evaluation of normal ovarian production of oocytes, and (3) evaluation of anatomical factors that might prevent the joining of these gametes.
2.1 Male Factor
Semen analysis should be one of the first evaluations undertaken. Male factor infertility is present in half of all infertile couples [1]. Evaluation by means of a semen analysis is inexpensive and noninvasive. Today, most cases of male factor infertility can be addressed using assisted reproductive technologies; however, male factor will not be a major focus of this chapter.
2.2 Tubal Factor
Today, instead of undergoing tubal surgery, it is more likely for a patient to choose in-vitro fertilization to achieve pregnancy. Today’s reproductive surgeons are not likely to experience many tubal ligation reversals or even lysis of adhesions as part of their training. Surgical correction of tubal infertility has become a thing of the past as the generation of surgeons trained in those procedures is aging out of practice and IVF techniques and laboratories continue to improve. Instead, the surgeons in training are more likely to find themselves performing a salpingectomy or proximal tubal ligation in order to improve pregnancy rates with IVF [2, 3]. While such surgery can improve the odds of a successful IVF cycle, benefit of this improvement must be weighed against the risks of surgery. Women undergoing IVF after salpingectomy may not respond as well to ovulation induction [4], though this change in response may have little clinical importance for a woman beginning with normal ovarian reserve, it may well have clinical importance for those who already have evidence of diminished ovarian reserve. In our practice, where most of our patients have diminished functional ovarian reserve, we rarely recommend tubal surgery before undergoing IVF.
2.3 Ovarian Reserve
A thorough understanding of ovarian physiology is needed to understand and treat problems of ovarian reserve. Women are born with all the oocytes they will ever have. Primordial follicles form during the first 5 months of fetal development. At birth a woman may have up to four million primordial follicles; however, by puberty these numbers will have diminished to only 200,000–400,000. This cohort is further depleted each month, and, in a woman’s late 30s, when the total cohort has fallen below 25,000, loss of follicles continues to fall even more rapidly eventually reaching menopausal levels when there are fewer than 1000 follicles at around age 51 [5].
Throughout life primordial follicles (the oocyte surrounded by a thin layer of follicular epithelial cells) will represent most follicles in the ovary. As follicles transition from primordial to primary, secondary, tertiary, and ultimately graafian follicle stages, most will be lost. Over a women’s reproductive lifetime only 400–600 of these follicles will ever achieve ovulation. Thus, for every follicle that achieves ovulation, thousands will have degenerated into atresia. The process of selection as follicles transition toward maturity can take several months and may be thought of in three basic stages: gonadotropin-independent pre-antral follicles, gonadotropin-dependent antral follicles, and growing graafian follicles. Each of these stages has specific characteristics and opportunities for clinical manipulation, although in general only the last (graafian follicle) stage has been subject to treatment in the past.
The clinical index of the ability of the ovary to produce oocytes is known as functional ovarian reserve (FOR) which is generally dependent on the antral follicle pool. Age is the primary marker of ovarian reserve; however, in any given age group, there may be a wide range of FOR that can be estimated by other predictors in addition to age [6]. Some predictors of FOR, other than a woman’s age, are antral follicle count (AFC), anti-Mullerian hormone (AMH), and cycle basal follicle-stimulating hormone (b-FSH) level.
2.3.1 Antral Follicle Count (AFC)
Antral follicles are those follicles variously defined as being between 4 and 10 mm in diameter. The antral follicle is characterized by having a fluid-filled antrum. Antral follicles are gonadotropin dependent, and, under influence of LH, antral thecal cells secrete androgens that, together with circulating androgen from the adrenal cortex, can then, under influence of FSH, be aromatized to estrogen by the neighboring granulosa cells.
Modern sonography allows the recognition of the small developing antral follicles in the ovary. Antral follicle count (AFC) may be used as an index of a woman’s ovarian reserve. A normal AFC is between 4 and 24 follicles between 2 and 10 mm in diameter [7]. Both AFC and ovarian volume decrease with age; however, AFC has been shown to be a better predictor of poor ovarian response than ovarian volume [8]. An AFC of less than four was associated with an almost nine times lower chance of achieving a pregnancy with IVF [9]. Having a higher AFC is associated with an increased risk of ovarian hyperstimulation syndrome [10]; this risk increases continuously with increased AFC, though there is no established consistent cutoff for risk of hyperstimulation.
2.3.2 Basal Follicle-Stimulating Hormone (b-FSH)
For more than 30 years, basal follicle-stimulating hormone has been used to estimate ovarian reserve and a woman’s potential to achieve pregnancy [11,12,13]. Basal FSH (b-FSH) is an indirect measure of ovarian reserve since it is measuring the hypothalamic-pituitary response to feedback from developing ovarian antral follicles which secrete activins, inhibins, and follistatins in addition sex steroids [14]. The testing is done on day 2 or 3 of the menstrual cycle since the ovarian sex steroids from developing follicles are at their lowest level at that time. Because of this the b-FSH level reflects the feedback of the other peptides more than that of the sex steroids. When the b-FSH testing is done, it is necessary to also measure estradiol simultaneously to confirm that estradiol is at a basal level, less than 60 pg/mL. As women age there are fewer antral follicles producing substances to inhibit FSH, and as a result, basal FSH will steadily rise. In general women with levels of b-FSH less than 10–12 mIU/mL are considered to have “normal” ovarian reserve, and those with higher levels are considered to be potential “poor responders” [13, 15]. The highest b-FSH a woman has had is a better predictor than a current b-FSH of her response in any treatment cycle [16, 17], and waiting for a cycle with more favorable lower b-FSH does not improve IVF outcomes.
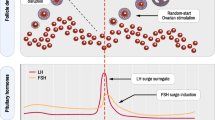
The concept of age-specific testing considers that there is a specific normal range for b-FSH for each age group (Fig. 1). Age-specific b-FSH levels can be a useful guide in measuring a woman’s ovarian reserve at any age [18,19,20]. Age-specific testing allows the adjustment of planned ovulation induction protocols appropriate for a woman’s specific ovarian reserve [21]. Too often an b-FSH greater than 12 is used to recommend the use of donor eggs rather than a women’s own [22]. In our practice we have found that women with b-FSH greater than 20 mIU/mL can still achieve live birth rates of up to 6% per initiated IVF cycle [23]. While such rates are still far lower than those that could be anticipated using eggs of a younger donor, we believe that couples should have a right to choose what they feel is the best option for them.
Cycle day-3 baseline follicle-stimulating hormone mean 95% confidence interval by age. Shaded area superimposed on figure to illustrate patients identified with premature ovarian aging (diagonal lines) or diminished ovarian reserve (black). (From Barad. Age-Specific Ovarian Function Testing. Obstet Gynecol 2007)
2.3.3 Anti-Mullerian Hormone (AMH)
Anti-Mullerian hormone (AMH) is a glycoprotein hormone in the transforming growth factor family and is a product of pre-antral and small antral follicles. Since AMH is produced by each pre-antral and small antral follicle, the serum AMH levels can be used as an index of the functional ovarian reserve, not reflecting the entire ovarian cohort of follicles but only those in the antral follicle stage [24,25,26,27]. These follicles are, indeed, those that are about to enter competition for graafian follicle dominance.
As follicles approach the antral stage, they gain the ability to produce anti-Mullerian hormone (AMH). AMH plays an important role in regulating follicular development. High levels of AMH will inhibit the transition of early follicle stages to the antral stage and will inhibit FSH stimulation of antral follicle transition to graafian follicles [28]. Once follicles transition to the graafian follicle stage, they no longer have the capacity to produce AMH. Any factor that will influence the antral follicle cohort will be reflected in changes in AMH serum levels. Both recent pregnancy and use of strong hormonal contraception can lower AMH levels [29, 30]. Serum levels of AMH decrease as a woman ages, and the number of antral follicles and functional ovarian reserve is decreased. Consequently age-specific levels of AMH can be used to judge a woman’s current state of FOR relative to her peers [25, 31, 32].
3 Ovulation Induction
More than 40 years ago, the first successful IVF cycle was conducted without ovulation induction [33]. It soon became clear that success in IVF could be improved by recruiting as many oocytes as possible [34]. Thereafter, therapeutic strategies originally used to treat an ovulatory women were transitioned to the treatment of ovulatory women. In nature the human ovulatory cycle allows the promotion of only one, or rarely two, graafian follicle to reach maturity and ovulate. Exogenous administration of gonadotropins to an ovulatory woman was used to raise the level of follicle-stimulating hormone high enough that the full cohort of gonadotropin-dependent follicles was allowed to grow and mature leading to the production of multiple oocytes [35]. This type of controlled ovarian stimulation (COS) created a greater chance of conception and a parallel consequent increase in the risk of multiple pregnancy if conception was allowed in vivo.
It soon became clear that the cumulative likelihood of live birth with IVF increases with the number of oocytes retrieved [36,37,38]. Increased rates of live birth were even more apparent when taking cumulative pregnancy rate (fresh plus subsequent transfer of cryopreserved embryos) into account [37, 39].
When using COS for IVF, dosing of FSH generally ranges from 150 to 450 IU with the dose often adjusted according to estimated ovarian reserve based on age, antral follicle count (AFC), anti-Mullerian hormone (AMH), and day-3 FSH. The intent of such individualized COS is to produce an effective number of mature oocytes with minimal risk of hyperstimulation or other complications. Among women with good ovarian reserve, increased gonadotropin dosage will lead to increased recovery of oocytes [40] and a subsequent improved live birth rate, as noted above.
However, past experience has shown that for woman with evidence of decreased ovarian reserve, the use of increased gonadotropin doses alone to achieve greater oocyte recovery is often futile [41, 42]. One study of indicators of ovarian reserve found that there is great individual variation in response to COS that appears to be independent of these predictors [43]. Another found that while women with diminished FOR had greater risk of cancelation of their IVF cycles, those who reached transfer had only a small, though significant, decreased adjusted relative risk of live birth [44]. A meta-analysis found that IVF live birth rates with individualized COS did not differ significantly from live birth rates when 150 units of FSH was administered no matter what the assessed ovarian reserve [45]. Thus, the use of individualized COS remains controversial. In our practice we believe that response to ovulation induction is most dependent on the functional ovarian reserve (FOR) which is in turn dependent on maintenance of the antral follicle pool.
4 Maintenance of the Antral Follicle Pool
A good antral follicle pool is essential for providing best chance of reproductive success [46,47,48]. Antral follicles are the resource from which graafian follicles will develop. Past approaches to ovulation induction focused on promotion of antral follicles into graafian follicle growth by provision of excess gonadotropin either by exogenous injection of gonadotropins or by inducing production of endogenous gonadotropin by inhibiting normal feedback to the pituitary of hypothalamus [49].
Women lose ovarian reserve as they age through the depletion of their remaining follicles and consequent diminished replenishment of the antral follicle pool. With fewer antral follicles, fewer graafian follicles can develop. As the population of developing follicles is diminished, so too is the endocrine milieu of the ovary. This leads to a variety of other consequences for the reproductive system in general including progressive symptoms of sex hormone deprivation such as vaginal dryness, loss of libido, hot flashes, and sleep disturbance.
Over the past decade, our group has explored various ways of helping women to get the most function out of their remaining follicle pool. The underlying philosophy of this approach is to foster the preservation and growth of pre-antral and antral follicles to provide maintenance of the antral follicle pool.
4.1 Factors Promoting Growth of Pre-antral and Antral Follicles
4.1.1 Androgens
In women circulating androgens are derived from both the adrenal glands and the ovaries [50]. The major adrenal androgens are dehydroepiandrosterone (DHEA) and its sulfate DHEAS which are produced in the zona reticularis of the human adrenal cortex [51]. With age the number of cells in the zona reticularis is known to decrease leading to decreased production of DHEA and DHEAS [51,52,53]. Androgens are precursors required for normal ovarian steroidogenesis [54, 55]. Thus, with loss of adrenal androgen production, there is a consequent loss of ovarian function.
Androgens are important growth factors for early follicle development. Support for the concept that androgens are necessary for normal early follicular development comes from experiments using androgen receptor knockout mouse models [56, 57]. In these experiments granulosa cell-specific androgen receptor knockout (ARKO) mice were used to examine the role of androgens in normal follicular development. GC-specific ARKO mice were more likely than wild type to have ovarian failure and longer estrous cycles. In addition, ovaries from the GC-specific ARKO mice had a greater proportion of pre-antral and atretic follicles with evidence of fewer antral follicles or corpora lutea [57]. In later studies androgens were found to decrease follicular atresia by suppression of proapoptotic protein expression and enhancement of FSH receptor expression, independent of transcription [58]. Similar non-genomic modulation of androgen action has been reported in other species [59].
In the setting of excess androgens, more follicles can develop to the antral follicle stage. One consequence of this androgen excess is the typical picture of polycystic ovary syndrome in which excess androgens lead to an excess number of antral follicles and excessively high AMH. The high AMH levels interfere with the action of follicle-stimulating hormone and may contribute to anovulation.
Recognition of this interaction of androgens and antral follicle growth has led to the use of androgens to promote greater numbers of follicles to the antral follicle stage in women who have diminished ovarian reserve. This approach presumes that there are still pre-antral follicles that could be promoted.
We first became aware of this phenomenon while treating a patient who was almost 43 years old and was undergoing back-to-back oocyte banking cycles. In her first few cycles, she produced only one or two oocytes, but then, after a few more cycles, her oocyte production increased markedly ultimately producing 17 oocytes in her eighth consecutive treatment cycle [60]. When we observed the remarkable increase in production of oocytes, we asked what she might be doing that was promoting this response. She told us that she had begun using dehydroepiandrosterone (DHEA) after her early cycles had failed to produce many oocytes. She became aware of the potential for DHEA to augment ovarian response to ovulation induction by reading an earlier series of case reports on the internet [61].
Following this experience, we began offering DHEA to other patients with evidence of diminished ovarian reserve and found that in a remarkable number of cases we were able to make a significant difference in response and in pregnancy rates [62, 63]. Recent meta-analyses have concluded that in women reported to be poor responders, pre-treatment with DHEA or testosterone may be associated with improved IVF live birth rates [64, 65].
There are a few important caveats in using DHEA among women with poor ovarian reserve. It is important not to heavily suppress gonadotropins in the preparatory phase of treatment. Effectiveness of androgen treatment appears to be partly dependent on the interaction of androgens and the endogenous gonadotropins. This may be a possible explanation why some trials of DHEA in which long agonist IVF protocol was used have been unable to show a significant benefit of DHEA treatment [66].
We do not use oral contraceptives to schedule IVF cycles. The use of oral contraceptives to help schedule cycles may lead to a 20% reduction in live birth rate [67]. The use of oral contraceptives has been associated with reduction in the antral follicle pool [68] and can reduce IVF oocyte yields [29].
In our practice the current preferred approach is for patients with diminished ovarian reserve to use micronized DHEA 25 mg three times a day for up to 2 months prior to initiation of ovulation induction. We prefer our patients to use divided doses because DHEA is rapidly absorbed and rapidly cleared and we would like to provide a steady blood level. We test for serum androgens and ovarian reserve parameters before starting DHEA and at baseline for each menstrual cycle. In general, in the presence of SHBG around 50–60 nmol/L, our target for total testosterone is between 28 and 56 ng/dL (1.0–2.0 nmol/L). We found that most of our patients with diminished ovarian reserve had baseline testosterone of less than 20 ng/dL and that only those who were able to raise testosterone to approach a minimal level of 30 ng/dL had favorable results to treatment [69].
4.1.2 Growth Hormone
Growth hormone is a hormone secreted by the somatotroph cells of the anterior pituitary peptide hormone important for cell growth, normal development, and metabolism. Growth hormone acts on the liver to produce IGF-1, which is responsible for growth hormone’s metabolic effects. IGF-1 receptors are present both in human oocytes and cumulus granulosa cells [70,71,72]. IGF-1 has been shown to have a role in murine granulosa cell differentiation [73], follicle recruitment [74], oocyte maturation and FSH receptor development [75], and inhibition of apoptosis [76]. IGF-1 increases the estradiol secretory response of granulosa cells to follicle-stimulating hormone [77]. In women undergoing IVF, the level of follicular fluid IGF-1 was found to be proportional to the number of oocytes retrieved and inversely proportional to the amount of gonadotropin needed for successful ovulation induction [78]. Co-treatment with growth hormone was found to be associated with increased density of FSH receptors, LH receptors, bone morphogenetic hormone receptors, and growth hormone receptors of granulosa cells of older women with a history of poor ovarian reserve [79].
Growth hormone supplementation is potentially useful in ovulation induction. Over the last decades as recombinant growth hormone has become commercially available, there have been many studies looking at the effects of growth hormone on ovulation induction [80,81,82,83,84,85,86]. Almost all these studies administered growth hormone along with routine fertility medication during the ovulation induction cycle. Most studies used GH doses between 4 and 12 units per day. A few studies started GH on day 21 of the previous cycle. Evidence for meta-analysis has suggested that growth hormone has the greatest benefit when used to treat women classed as poor responders, those with a history of fewer than four oocytes retrieved in a previous cycle.
Recent randomized trials in poor responders have confirmed a growth hormone benefit in increased collected oocytes; however, growth hormone had no effect on the primary outcome of live birth [87, 88].
A recent Cochrane review found that while GH did not improve results in routine IVF cycles there is “some evidence of increased pregnancy and birth rates in women who are considered ‘poor responders’ to in vitro fertilization.”
Growth hormone is reported to modulate the action of FSH on follicles by upregulating local synthesis of IGF-1. Interestingly a similar effect was noted by Casson [61, 89] in early experiments using DHEA with treated patients having increased IGF-1. Much of the focus on gonadotropin/IGF-1 interaction has revolved around the effects on granulosa cell cultures to increase aromatase activity , estradiol production, progesterone production, and LH receptor formation. However, IGF-1 also has a proposed role in stimulating early follicle development and oocyte maturation [90, 91].
Synthetic human growth hormone was developed in 1985 and approved by the FDA for specific uses in children and adults. Synthetic growth hormone use as a supplement for ovulation induction has not been FDA approved.
We believe the greatest potential for GH would be during preparation for an ovulation induction cycle. Theoretically administration of GH during the 6 weeks before starting a cycle will influence developing antral follicles to present a better cohort of follicles when ovulation induction is begun.
4.1.3 Platelet-Rich Plasma
As women age oocytes are gradually depleted with a consequent progressive loss of ovarian function and fertility. When a woman’s follicle cohort falls below a critical level, she enters a transitional time of diminished ovarian reserve known as ovarian insufficiency. For most women this phase begins in the mid to late 30s and may last over 10–15 years before the onset of actual menopause. During this transition fertility is continuously reduced as is the production of ovarian sex steroids leading to increased symptoms of estrogen deficiency. These changes naturally occur at a time in their lives when contemporary women may only just be beginning to think about having a family.
Various strategies have been applied to help women restore ovarian function or to maximize the utility of what function may remain. Most past approaches to treatment of ovarian insufficiency have focused on maximizing induction of the cohort of antral follicles which constitute a women’s functional ovarian reserve. These are the follicles which have survived the several months of development from primordial follicle to the antral follicle stage and most likely represent only a fraction of that original cohort.
Recently the use of platelet-rich plasma (PRP) has been proposed as an additional strategy for improving ovarian function [89]. PRP has been used in other medical fields to regenerate skin [90] and cartilage [91]. The rational for the use of PRP in these settings is that it contains growth factors which stimulate cellular anabolism, inflammatory modulators that create an anti-inflammatory effect, and fibrinogen which acts as a scaffold for regenerating tissue [92,93,94].
One current hypothesis regarding the possible effect of PRP in the ovary is that the growth factors released by activated PRP may induce the transformation of germline stem cells (GSCs) into primordial follicles, thus replenishing a diminished follicle pool [95]. Evidence in support of this hypothesis is limited [89, 96,97,98]. A few case reports of pregnancies occurring in women said to have premature ovarian failure have recently been reported [89, 99].
Women with POI may still have occasional irregular periods and may even occasionally achieve a pregnancy. In our practice we are presently recruiting women with POI into a clinical trial (NCT03542708) in which one randomly selected ovary is treated with PRP and the other ovary remains as a control. The endpoint of this study is to see if there is a differential response in follicle development between the treated and untreated ovary.
4.1.4 Estrogen Priming
We use estrogen priming for 7–10 days before beginning ovulation induction. Once ovulation induction is begun, we switch the estrogen prime to ethinylestradiol, which does not interfere with assay reading of estradiol coming from the patient’s ovaries.
5 Optimizing Oocyte Production
5.1 Individualized Egg Retrieval
Over the past 2 years, we rarely used any cycle control at all. Instead we time our retrievals earlier than spontaneous ovulation would be expected to occur in the protocol we have called highly individualized egg retrieval [103].
As women age, oocyte quantity [5] and quality [104] are significantly diminished. Among older women undergoing ovulation induction for in vitro fertilization, a large percentage of oocytes retrieved are atretic [105]. The bidirectional communication in the cumulus granulosa cells and the oocyte is critical for the oocytes growth and differentiation [106,107,108]. With aging, the number of cumulus granulosa cells per oocyte [109] and the competence of those cells to maintain oocyte health become compromised [110].
We found that granulosa cell molecular function was diminished among older women. FSH receptor (FSHR), aromatase (CYP19A1), and 17b-hydroxysteroid dehydrogenase (HSD17B) expression were downregulated, while LH receptor (LHCGR), P450scc (CYP11A1), and progesterone receptor (PGR) were upregulated in granulosa cells collected from follicular fluid of women undergoing in vitro fertilization cycles. Together these findings revealed age-related changes consistent with premature luteinization [105] (Fig. 2).
mRNA expression of genes from granulosa cells collected from follicular fluid of women undergoing in vitro fertilization cycles determined by real-time PCR. Values with same letters or without letters above the columns within each unit figure were not different significantly (PO0.05). White columns: group 1 (oocyte donors), n = 7; gray columns: group 2 (middle-aged infertile patients), n = 10; black columns: group 3 (older infertile patients), n = 10. (FSH receptor (FSHR), aromatase (CYP19A1), 17b-hydroxysteroid dehydrogenase (HSD17B) LH receptor (LHCGR), P450scc (CYP11A1), and progesterone receptor (PGR)). (Adapted from Wu, Barad et al. J Endocrinol. 2015 Sep;226(3):167–80)
We reasoned that loss of cumulus granulosa cell support could be a direct cause of the increasing incidence of oocyte atresia observed among our older patients. Based on this observation, we began timing retrieval at earlier stages of follicular development among older women [105]. This change in timing of oocyte retrieval resulted in a lower percentage of atretic eggs and a significantly improved pregnancy rate [103] (Table 1).
5.2 In Vitro Maturation of Immature Oocytes (IVM)
One consequence of moving the timing of the oocyte retrieval to earlier stages of follicular development is that a greater percentage of oocytes are MI or GV. We chose to retrieve immature oocytes rather than atretic oocytes that had no possibility of salvage. As expected, many of the MI oocytes progressed to MII once the cumulus granulosa cells were stripped away. Although these oocytes achieve nuclear maturity, they may not have achieved cytoplasmic maturity, and thus embryos produced from these in vitro oocytes do not have the same potential to achieve pregnancy as those oocytes that were already MII at collection. Clearly, this is an area that will need more exploration.
5.3 Rebound
When caring for patients with significant POI/POA with several previous failed cycles of ovulation induction, it is common to find women who do not respond at all to ovulation induction; they have no evidence of follicle growth and no estradiol rise. Paradoxically, we found that when we stop all medications and recheck in 3 days about half of such patients would show evidence of follicle growth and rising estradiol. We have termed this paradoxical phenomenon “rebound.” We now routinely ask patients to return for a “rebound check” 3–4 days after stopping ovulation induction. When we see evidence of such response, ovulation induction is restarted, and many of these patients reach retrieval.
Through August 2019, 49 women with maximal levels FSH greater than 20 mIU/mL, AMH less than 0.01 ng/mL, and failure to exhibit a rise in estradiol greater than 60 after more than 8 days of ovulation induction have been treated in “rebound” cycles in our practice. Of these 24 responded and ovulation induction was restarted. Twenty-two patients reached retrieval and 15 patients reached embryo transfer. Until now, managing the “rebound” has not resulted in a pregnancy.
6 Embryo Factors
6.1 Fertilization and Implantation
In vivo fertilization occurs in the fallopian tube within 24 h of ovulation. The fertilized zygote then travels down the fallopian tube over 3–4 days to the uterus, arriving there around the fifth day after ovulation. Implantation may not occur until 6–8 days following fertilization.
6.1.1 Fertilization
Fertilization is a complex process dependent on competent sperm being transported to the distal fallopian tube. Tubal disease that could hinder the ability of ovum pickup by the distal fimbria or hinder transport of either the oocyte, sperm, or fertilized zygote may all give rise to infertility. In the past tubal disease was surgically treated with various procedures designed to restore tubal patency. However, even in the best of hands, the live birth rate was often less than 25% within a year of surgery [111, 112]. Furthermore, after surgery to repair a damaged fallopian tube, the odds of ectopic pregnancy increase fourfold [113]. For these reasons, surgical approaches to tubal disease have fallen out of favor.
6.1.2 Ectopic Pregnancy
The incidence of ectopic pregnancy following IVF embryo transfer (ET) ranges from 1 to 2% [113,114,115]. Ectopic pregnancy following ET is more common among women with a history of tubal disease and less common following day-5 ET [115]. No one knows what happens to an embryo that has been transferred to the uterine cavity days before endometrial receptivity has been achieved. It may be that embryos move often back into the proximal fallopian tube, retracing the normal path of development. Since ectopic pregnancy is known to occur after ET, at least some embryos must migrate back into the fallopian tubes, but perhaps most do, and only a few unlucky embryos result in a tubal implantation. As noted above implantation will not begin for a few days after ET, so a day-3 ET will spend more time preimplantation than a day-5 embryo, increasing the risk of ectopic implantation for women with a history of tubal disease [114]. Women who used the contraceptive device known as Essure©, which creates proximal tubal occlusion, experienced lower pregnancy rates after IVF embryo transfer compared to those who were treated by laparoscopic salpingectomy before IVF [116]; perhaps the opportunity to migrate back into the fallopian tube improves the chance of a successful cycle? Thus, one may speculate that passage back to a normal tube may give embryos an advantage, while transit in a damaged tube creates a risk.
6.2 Embryo Selection
A typical cycle of in vitro fertilization will produce multiple oocytes and many embryos. As already noted above, the major rationale for using ovulation induction was to produce multiple oocytes and allow formation of multiple embryos. One goal of producing multiple embryos was to allow selection of the most favorable embryo or set of embryos for transfer. Over the years, various strategies have been used to select embryos and decide how many embryos to transfer.
6.2.1 Embryo Morphology
Once an egg becomes fertilized, the resulting zygote will begin to divide. Each cell division is an independent event based on each cell’s individual metabolic response to its immediate environment. After 3 days in culture, we expect that a zygote will have divided three times, each time doubling the number of blastomeres. For this reason, we expect an embryo that has undergone three normal divisions to have eight blastomeres. Embryos with greater or fewer numbers of blastomeres might represent abnormal cell division and are considered less favorably. Embryos are also graded based on appearance color, texture, symmetry, and on the percent of small cytoplasmic fragments surrounding the blastomeres [117]. The best graded embryos are symmetrical and have few or no fragments (Table 2).
Embryos allowed to remain in culture for 5 days will, under normal conditions, progress to the blastocyst stage. The typical blastocyst will have a spherical trophectoderm made up of 200–300 cells and a much smaller inner cell mass. The trophectoderm will develop into the placenta, and the inner cell mass will go on to form the embryo proper. Day-5 embryos are scored based on their stage of development and on the characteristics of the cells in the inner cell mass and in the trophectoderm [117] (Table 2).
Since the process of embryo implantation does not occur until several days after fertilization, transfers could in theory occur anytime in the first 6 days after fertilization. When embryo culture methods had improved enough to allow culture of embryos to blastocyst, it soon became apparent that embryos which had survived to the blastocyst stage had a greater chance of implanting and establishing a pregnancy that could result in a live birth. Although many assumed that culture to blastocyst resulted in a more successful embryo, this was not the case. Embryos that could survive in the laboratory to the blastocyst stage simply proved that they were stronger, not as a result of extended culture but because they had always been the most fit of their cohort. One of the costs of using extended culture to identify highly successful embryos is the loss of embryos that could not survive in extended culture. Current best evidence suggests that while blastocyst culture allows selection of a successful embryo for a fresh embryo transfer, because fewer embryos are cryopreserved, there is no evidence of difference in cumulative pregnancy rates using embryos produced from a single oocyte retrieval [118].
6.2.2 Preimplantation Genetic Testing
In the 1980s preimplantation genetic testing (PGT) was developed with the goal of identifying genetic disease when both parents were known to be carriers for conditions like cystic fibrosis, Huntington’s disease, or hemophilia. Techniques were soon extended to try to rule out balanced translocations and aneuploidies in embryos before embryo transfer.
This technique, first called preimplantation genetic diagnosis (PGD), used fluorescence in situ hybridization (FISH) to identify aneuploidy in polar bodies or single blastomere biopsies from day-3 embryos [119,120,121]. As evidence accumulated over time, it became clear that damage to the embryo from blastomere biopsy and the inaccuracy of the FISH technique [122] actually decreased the chance of a successful pregnancy [123,124,125].
In response to these observations , new techniques were developed to test embryos at a later stage of development when more cells could be sampled recognizing that at this later stage of development these cells would represent a smaller percentage of the resulting embryo and hopefully lead to less embryo damage [126, 127]. The resulting technique of trophectoderm biopsy would sample 4 to 6 cells from the trophectoderm and would use new methods of genetic analysis of these cells that would allow reporting of the ploidy of all 24 chromosomes [128,129,130].
For young women less than 35 years old, selection of a blastocyst with a euploid biopsy promised an excellent chance of a successful pregnancy. However, by the age 40 years, almost 60% of embryos had aneuploid biopsy results, while by 44 years the percentage of aneuploid biopsies increased to 88% and more than 40% of couples had no embryos with euploid biopsy for transfer [131].
Recognizing that no test is 100% accurate, we and others began offering to transfer embryos that had been called “aneuploid” based on their trophectoderm biopsy. We realized that the only way to lose all chance of a successful pregnancy was to never offer an embryo transfer. When these embryos were transferred, it turned out that a substantial number were able to achieve pregnancy and normal live birth [132, 133]. A recent survey found more than 400 live births after transfer of embryos determined by PGT to be abnormal [134]. It turned out that the accuracy of preimplantation testing was limited by the mosaic nature of early human embryos [135] and by the ability of some embryos to self-correct mitotically derived aneuploidy [136, 137]. Today, preimplantation genetic testing (PGT) is widely practiced, though many still consider it to be controversial [138]. In general, we do not recommend PGT to our patients with diminished ovarian reserve as we believe our role is to promote the best chance of pregnancy and not to try to guarantee a so-called perfect embryo.
References
Brugh MV, Lipshultz L. Male factor infertility: evaluation and management. Med Clin North Am. 2004;88(2):367–85.
Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–6.
Iliodromiti S, Nelson SM. Biomarkers of ovarian reserve. Biomark Med. 2013;7(1):147–58.
Coelho Neto MA, Ludwin A, Borrell A, Benacerraf B, Dewailly D, da Silva Costa F, Condous G, Alcazar JL, Jokubkiene L, Guerriero S, Van den Bosch T, Martins WP. Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet Gynecol. 2018;51(1):10–20.
Hendriks DJ, Kwee J, Mol BW, te Velde ER, Broekmans FJ. Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril. 2007;87(4):764–75.
Gibreel A, Maheshwari A, Bhattacharya S, Johnson NP. Ultrasound tests of ovarian reserve; a systematic review of accuracy in predicting fertility outcomes. Hum Fertil (Camb). 2009;12(2):95–106.
Broer SL, Dolleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17(1):46–54.
Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51(4):651–4.
Toner JP. The significance of elevated FSH for reproductive function. Baillieres Clin Obstet Gynaecol. 1993;7(2):283–95.
Toner JP, Philput CB, Jones GS, Muasher SJ. Basal follicle-stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertil Steril. 1991;55(4):784–91.
Ling N, DePaolo LV, Bicsak TA, Shimasaki S. Novel ovarian regulatory peptides: inhibin, activin, and follistatin. Clin Obstet Gynecol. 1990;33(3):690–702.
Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril. 2001;76(6):1185–90.
Gingold JA, Lee JA, Whitehouse MC, Rodriguez-Purata J, Sandler B, Grunfeld L, Mukherjee T, Copperman AB. Maximum basal FSH predicts reproductive outcome better than cycle-specific basal FSH levels: waiting for a “better” month conveys limited retrieval benefits. Reprod Biol Endocrinol. 2015;13:91.
Lass A, Gerrard A, Abusheikha N, Akagbosu F, Brinsden P. IVF performance of women who have fluctuating early follicular FSH levels. J Assist Reprod Genet. 2000;17(10):566–73.
Barad DH, Weghofer A, Gleicher N. Age-specific levels for basal follicle-stimulating hormone assessment of ovarian function. Obstet Gynecol. 2007b;109(6):1404–10.
Fang T, Su Z, Wang L, Yuan P, Li R, Ouyang N, Zheng L, Wang W. Predictive value of age-specific FSH levels for IVF-ET outcome in women with normal ovarian function. Reprod Biol Endocrinol. 2015;13:63.
Fasouliotis SJ, Simon A, Laufer N. Evaluation and treatment of low responders in assisted reproductive technology: a challenge to meet. J Assist Reprod Genet. 2000;17(7):357–73.
Gleicher N, Barad D. “Ovarian age-based” stimulation of young women with diminished ovarian reserve results in excellent pregnancy rates with in vitro fertilization. Fertil Steril. 2006;86(6):1621–5.
Toner JP, Flood JT. Fertility after the age of 40. Obstet Gynecol Clin N Am. 1993;20(2):261–72.
Kushnir VA, Safdie M, Darmon SK, Albertini DF, Barad DH, Gleicher N. Age-specific IVF outcomes in infertile women with baseline FSH levels >/=20 mIU/mL. Reprod Sci. 2018;25(6):893–8.
Barad DH, Weghofer A, Gleicher N. Comparing anti-Mullerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril. 2009;91(4 Suppl):1553–5.
Barad DH, Weghofer A, Gleicher N. Utility of age-specific serum anti-Mullerian hormone concentrations. Reprod Biomed Online. 2011;22(3):284–91.
Gleicher N, Darmon SK, Kushnir VA, Weghofer A, Wang Q, Zhang L, Albertini DF, Barad DH. How FSH and AMH reflect probabilities of oocyte numbers in poor prognosis patients with small oocyte yields. Endocrine. 2016a;54(2):476–83.
Gleicher N, Weghofer A, Barad DH. Anti-Mullerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril. 2010;94(7):2824–7.
Kushnir VA, Seifer DB, Barad DH, Sen A, Gleicher N. Potential therapeutic applications of human anti-Mullerian hormone (AMH) analogues in reproductive medicine. J Assist Reprod Genet. 2017;34(9):1105–13.
Barad DH, Kim A, Kubba H, Weghofer A, Gleicher N. Does hormonal contraception prior to in vitro fertilization (IVF) negatively affect oocyte yields? A pilot study. Reprod Biol Endocrinol. 2013;11:28.
Weghofer A, Dietrich W, Ortner I, Bieglmayer C, Barad D, Gleicher N. Anti-Mullerian hormone levels decline under hormonal suppression: a prospective analysis in fertile women after delivery. Reprod Biol Endocrinol. 2011;9:98.
Hiedar Z, Bakhtiyari M, Foroozanfard F, Mirzamoradi M. Age-specific reference values and cut-off points for anti-mullerian hormone in infertile women following a long agonist treatment protocol for IVF. J Endocrinol Investig. 2018;41(7):773–80.
Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–50.
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366.
Fleming R, Coutts JR. Induction of multiple follicular development for IVF. Br Med Bull. 1990;46(3):596–615.
Fauser BC, Devroey P, Macklon NS. Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet. 2005;365(9473):1807–16.
Hamoda H, Sunkara S, Khalaf Y, Braude P, El-Toukhy T. Outcome of fresh IVF/ICSI cycles in relation to the number of oocytes collected: a review of 4,701 treatment cycles. Hum Reprod. 2010;25.
Magnusson Å, Källen K, Thurin-Kjellberg A, Bergh C. The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod. 2017;33(1):58–64.
van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, Fauser BC, Macklon NS. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online. 2006;13(4):476–80.
Stanger JD, Yovich JL. Follicle recruitment determines IVF productivity rate via the number of embryos frozen and subsequent transfers. Reprod Biomed Online. 2013;27(3):286–96.
Arce JC, Andersen AN, Fernandez-Sanchez M, Visnova H, Bosch E, Garcia-Velasco JA, Barri P, de Sutter P, Klein BM, Fauser BC. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimullerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014;102(6):1633–40.e1635.
Lekamge DN, Lane M, Gilchrist RB, Tremellen KP. Increased gonadotrophin stimulation does not improve IVF outcomes in patients with predicted poor ovarian reserve. J Assist Reprod Genet. 2008;25(11–12):515–21.
Out HJ, Braat DD, Lintsen BM, Gurgan T, Bukulmez O, Gokmen O, Keles G, Caballero P, Gonzalez JM, Fabregues F, Balasch J, Roulier R. Increasing the daily dose of recombinant follicle stimulating hormone (Puregon) does not compensate for the age-related decline in retrievable oocytes after ovarian stimulation. Hum Reprod. 2000;15(1):29–35.
Rustamov O, Wilkinson J, La Marca A, Fitzgerald C, Roberts S. How much variation in oocyte yield after controlled ovarian stimulation can be explained? A multilevel modelling study. Human Reproduction Open. 2017;2017:hox018.
Kawwass JF, Hipp HS, Session DR, Kissin DM, Jamieson DJ, A. R. T. S. S. G. National. Severity of diminished ovarian reserve and chance of success with assisted reproductive technology. J Reprod Med. 2017;62(3–4):153–60.
Lensen SF, Wilkinson J, Leijdekkers JA, La Marca A, Mol BWJ, Marjoribanks J, Torrance H, Broekmans FJ. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane Database Syst Rev. 2018;2:CD012693.
Almog B, Shehata F, Shalom-Paz E, Tan SL, Tulandi T. Age-related normogram for antral follicle count: McGill reference guide. Fertil Steril. 2011;95(2):663–6.
Holte J, Brodin T, Berglund L, Hadziosmanovic N, Olovsson M, Bergh T. Antral follicle counts are strongly associated with live-birth rates after assisted reproduction, with superior treatment outcome in women with polycystic ovaries. Fertil Steril. 2011;96(3):594–9.
Hsu A, Arny M, Knee AB, Bell C, Cook E, Novak AL, Grow DR. Antral follicle count in clinical practice: analyzing clinical relevance. Fertil Steril. 2011;95(2):474–9.
Muasher SJ, Abdallah RT, Hubayter ZR. Optimal stimulation protocols for in vitro fertilization. Fertil Steril. 2006;86(2):267–73.
Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. 1986;15(2):213–28.
Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81(10):3558–65.
Hornsby PJ. Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann N Y Acad Sci. 1995;774:29–46.
Hornsby PJ. Aging of the human adrenal cortex. Sci Aging Knowl Environ. 2004;2004(35):re6.
McNatty KP, Makris A, De Grazia C, Osathanondh R, Ryan KJ. The production of progesterone, androgens and oestrogens by human granulosa cells in vitro and in vivo. J Steroid Biochem. 1979;11(1C):775–9.
Ryan KJ. Granulosa-thecal cell interaction in ovarian steroidogenesis. J Steroid Biochem. 1979;11(1C):799–800.
Prizant H, Gleicher N, Sen A. Androgen actions in the ovary: balance is key. J Endocrinol. 2014;222(3):R141–51.
Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24(7):1393–403.
Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci U S A. 2014;111(8):3008–13.
Sen A, Prizant H, Hammes SR. Understanding extranuclear (nongenomic) androgen signaling: what a frog oocyte can tell us about human biology. Steroids. 2011;76(9):822–8.
Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84(3):756.
Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–32.
Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007a;24(12):629–34.
Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21(11):2845–9.
Liu Y, Hu L, Fan L, Wang F. Efficacy of dehydroepiandrosterone (DHEA) supplementation for in vitro fertilization and embryo transfer cycles: a systematic review and meta-analysis. Gynecol Endocrinol. 2018;34(3):178–83.
Nagels HE, Rishworth JR, Siristatidis CS, Kroon B. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst Rev. 2015;11:CD009749.
Narkwichean A, Maalouf W, Baumgarten M, Polanski L, Raine-Fenning N, Campbell B, Jayaprakasan K. Efficacy of dehydroepiandrosterone (DHEA) to overcome the effect of ovarian ageing (DITTO): a proof of principle double blinded randomized placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2017;218:39–48.
Griesinger G, Venetis CA, Tarlatzis B, Kolibianakis EM. To pill or not to pill in GnRH-antagonist cycles: the answer is in the data already! Reprod Biomed Online. 2015;31(1):6–8.
Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Mullerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39(5):574–80.
Gleicher N, Kim A, Weghofer A, Shohat-Tal A, Lazzaroni E, Lee HJ, Barad DH. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). J Assist Reprod Genet. 2013;30(1):49–62.
Abir R, Garor R, Felz C, Nitke S, Krissi H, Fisch B. Growth hormone and its receptor in human ovaries from fetuses and adults. Fertil Steril. 2008;90(4 Suppl):1333–9.
Buyalos RP. Insulin-like growth factors: clinical experience in ovarian function. Am J Med. 1995;98(1A):55S–66S.
Menezo YJ, el Mouatassim S, Chavrier M, Servy EJ, Nicolet B. Human oocytes and preimplantation embryos express mRNA for growth hormone receptor. Zygote. 2003;11(4):293–7.
Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27(3):511–23.
Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10(7):903–18.
Zhou J, Kumar TR, Matzuk MM, Bondy C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol. 1997;11(13):1924–33.
Chun SY, Billig H, Tilly JL, Furuta I, Tsafriri A, Hsueh AJ. Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology. 1994;135(5):1845–53.
Adashi EY, Resnick CE, D'Ercole AJ, Svoboda ME, van Wyk JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function∗. Endocr Rev. 1985;6(3):400–20.
Oosterhuis GJ, Vermes I, Lambalk CB, Michgelsen HW, Schoemaker J. Insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations in fluid from human stimulated follicles. Hum Reprod. 1998;13(2):285–9.
Regan SLP, Knight PG, Yovich JL, Arfuso F, Dharmarajan A. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells, with improved pregnancy outcomes. Fertil Steril. 2018;110(7):1298–310.
Bergh C, Hillensjo T, Wikland M, Nilsson L, Borg G, Hamberger L. Adjuvant growth hormone treatment during in vitro fertilization: a randomized, placebo-controlled study. Fertil Steril. 1994;62(1):113–20.
Hazout A, Junca A, Menezo Y, Demouzon J, Cohen-Bacrie P. Effect of growth hormone on oocyte competence in patients with multiple IVF failures. Reprod Biomed Online. 2009;18(5):664–70.
Kucuk T, Kozinoglu H, Kaba A. Growth hormone co-treatment within a GnRH agonist long protocol in patients with poor ovarian response: a prospective, randomized, clinical trial. J Assist Reprod Genet. 2008;25(4):123–7.
Owen EJ, West C, Mason BA, Jacobs HS. Co-treatment with growth hormone of sub-optimal responders in IVF-ET. Hum Reprod. 1991;6(4):524–8.
Suikkari A, MacLachlan V, Koistinen R, Seppala M, Healy D. Double-blind placebo controlled study: human biosynthetic growth hormone for assisted reproductive technology. Fertil Steril. 1996;65(4):800–5.
Tesarik J, Hazout A, Mendoza C. Improvement of delivery and live birth rates after ICSI in women aged >40 years by ovarian co-stimulation with growth hormone. Hum Reprod. 2005;20(9):2536–41.
Zhuang GL, Wong SX, Zhou CQ. [The effect of co-administration of low dosage growth hormone and gonadotropin for ovarian hyperstimulation in vitro fertilization and embryo transfer]. Zhonghua Fu Chan Ke Za Zhi. 1994;29(8):471–74, 510.
Dakhly DMR, Bassiouny YA, Bayoumi YA, Hassan MA, Gouda HM, Hassan AA. The addition of growth hormone adjuvant therapy to the long down regulation protocol in poor responders undergoing in vitro fertilization: randomized control trial. Eur J Obstet Gynecol Reprod Biol. 2018;228:161–5.
Norman RJ, Alvino H, Hull LM, Mol BW, Hart RJ, Kelly TL, Rombauts L, investigators L. Human growth hormone for poor responders: a randomized placebo-controlled trial provides no evidence for improved live birth rate. Reprod Biomed Online. 2019;38(6):908–15.
Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, Buster JE. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70(1):107–10.
Yoshimura Y, Ando M, Nagamatsu S, Iwashita M, Adachi T, Sueoka K, Miyazaki T, Kuji N, Tanaka M. Effects of insulin-like growth factor-I on follicle growth, oocyte maturation, and ovarian steroidogenesis and plasminogen activator activity in the rabbit. Biol Reprod. 1996a;55(1):152–60.
Yoshimura Y, Aoki N, Sueoka K, Miyazaki T, Kuji N, Tanaka M, Kobayashi T. Interactions between insulin-like growth factor-I (IGF-I) and the renin-angiotensin system in follicular growth and ovulation. J Clin Invest. 1996b;98(2):308–16.
Pantos K, Nitsos N, Kokkali G, Vaxevanoglou T, Markomichali C, Pantou A, Grammatis M, Lazaros L, Sfakianoudis K. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment ESHRE 32nd Annual Meeting. Helsinki, Finland. Hum Reprod: i301; 2016.
Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30(02):157–71.
Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16(1):204.
Macaulay IC, Carr P, Gusnanto A, Ouwehand WH, Fitzgerald D, Watkins NA. Platelet genomics and proteomics in human health and disease. J Clin Invest. 2005;115:3370.
McRedmond JP, Park SD, Reilly DF, Coppinger JA, Maguire PB, Shields DC, Fitzgerald DJ. Integration of proteomics and genomics in platelets: a profile of platelet proteins and platelet-specific genes. Mol Cell Proteomics. 2004;3:133.
Watson SP, Bahou WF, Fitzgerald D, Ouwehand W, Rao AK, Leavitt AD. ISTH platelet physiology subcommittee: mapping the platelet proteome: a report of the ISTH platelet physiology subcommittee. J Thromb Haemost. 2005;3:2098.
Sills ES, Wood SH. Autologous activated platelet-rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response. Biosci Rep, 2019;39(6).
Farimani M, Heshmati S, Poorolajal J, Bahmanzadeh M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol Biol Rep. 2019;46(2):1611–6.
Hosseini L, Shirazi A, Naderi MM, Shams-Esfandabadi N, Borjian Boroujeni S, Sarvari A, Sadeghnia S, Behzadi B, Akhondi MM. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod Biomed Online. 2017;35(4):343–50.
Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol. 2018;34(9):756–60.
Pantos K, Simopoulou M, Pantou A, Rapani A, Tsioulou P, Nitsos N, Syrkos S, Pappas A, Koutsilieris M, Sfakianoudis K. A case series on natural conceptions resulting in ongoing pregnancies in menopausal and prematurely menopausal women following platelet-rich plasma treatment. Cell Transplant. 2019;28(9–10):1333–40. https://doi.org/10.1177/0963689719859539.
Wu YG, Barad DH, Kushnir VA, Wang Q, Zhang L, Darmon SK, Albertini DF, Gleicher N. With low ovarian reserve, highly individualized egg retrieval (HIER) improves IVF results by avoiding premature luteinization. J Ovarian Res. 2018;11(1):23.
Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337(8754):1375–7.
Wu YG, Barad DH, Kushnir VA, Lazzaroni E, Wang Q, Albertini DF, Gleicher N. Aging-related premature luteinization of granulosa cells is avoided by early oocyte retrieval. J Endocrinol. 2015;226(3):167–80.
Da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J Assist Reprod Genet. 2018;35(5):735–51.
Herlands RL, Schultz RM. Regulation of mouse oocyte growth: probable nutritional role for intercellular communication between follicle cells and oocytes in oocyte growth. J Exp Zool. 1984;229(2):317–25.
Viveiros MM, De La Fuente R. Chapter 11—regulation of mammalian oocyte maturation. In: Leung PCK, Adashi EY, editors. The ovary. 3rd ed: Academic Press; 2019. p. 165–80.
Seifer DB, Charland C, Berlinsky D, Penzias AS, Haning RV Jr, Naftolin F, Barker BE. Proliferative index of human luteinized granulosa cells varies as a function of ovarian reserve. Am J Obstet Gynecol. 1993;169(6):1531–5.
Seifer DB, Gardiner AC, Ferreira KA, Peluso JJ. Apoptosis as a function of ovarian reserve in women undergoing in vitro fertilization. Fertil Steril. 1996;66(4):593–8.
Barad DH. Infertility surgery by laparotomy. Curr Opin Obstet Gynecol. 1991;3(3):398–403.
Teoh TG, Kondaveeti U, Darling MR. The management of female infertility by tubal microsurgical reconstruction: a ten year review. Ir J Med Sci. 1995;164(3):212–4.
Farquhar CM. Ectopic pregnancy. Lancet. 2005;366(9485):583–91.
Johnson N, van Voorst S, Sowter MC, Strandell A, Mol BW. Surgical treatment for tubal disease in women due to undergo in vitro fertilisation. Cochrane Database Syst Rev. 2010;(1):CD002125.
Tsiami A, Chaimani A, Mavridis D, Siskou M, Assimakopoulos E, Sotiriadis A. Surgical treatment for hydrosalpinx prior to in-vitro fertilization embryo transfer: a network meta-analysis. Ultrasound Obstet Gynecol. 2016;48(4):434–45.
Pereira N, Pryor KP, Voskuilen-Gonzalez A, Lekovich JP, Elias RT, Spandorfer SD, Rosenwaks Z. Ovarian response and in vitro fertilization outcomes after salpingectomy: does salpingectomy indication matter? J Minim Invasive Gynecol. 2017;24(3):446–54.e441.
Li Z, Sullivan EA, Chapman M, Farquhar C, Wang YA. Risk of ectopic pregnancy lowest with transfer of single frozen blastocyst. Hum Reprod. 2015;30(9):2048–54.
Santos-Ribeiro S, Tournaye H, Polyzos NP. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Hum Reprod. 2016;31(2):393–402.
Xu B, Zhang Q, Zhao J, Wang Y, Xu D, Li Y. Pregnancy outcome of in vitro fertilization after Essure and laparoscopic management of hydrosalpinx: a systematic review and meta-analysis. Fertil Steril. 2017;108(1):84–95.e85.
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, Wininger D, Gibbons W, Conaghan J, Stern JE. Standardization of grading embryo morphology. J Assist Reprod Genet. 2010;27(8):437–9.
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;6:CD002118.
Kuliev A, Verlinsky Y. Place of preimplantation diagnosis in genetic practice. Am J Med Genet A. 2005;134A(1):105–10.
Márquez C, Sandalinas M, Bahçe M, Al ikani M, Munné S. Chromosome abnormalities in 1255 cleavage-stage human embryos. Reprod Biomed Online. 2000;1(1):17–26.
Munné S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities∗∗Presented at the 50th Annual Meeting of The American Fertility Society, San Antonio, Texas, November 4 to 9, 1994, where it was awarded the prize paper of the Society for Assisted Reproductive Technology. Fertil Steril. 1995;64(2):382–91.
Northrop LE, Treff NR, Levy B, Scott RT Jr. SNP microarray-based 24 chromosome aneuploidy screening demonstrates that cleavage-stage FISH poorly predicts aneuploidy in embryos that develop to morphologically normal blastocysts. Mol Hum Reprod. 2010;16(8):590–600.
Gleicher N, Kushnir VA, Barad DH. Preimplantation genetic screening (PGS) still in search of a clinical application: a systematic review. Reprod Biol Endocrinol. 2014;12(1):22.
Hardarson T, Hanson C, Lundin K, Hillensjo T, Nilsson L, Stevic J, Reismer E, Borg K, Wikland M, Bergh C. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23(12):2806–12.
Mastenbroek S, Twisk M, van der Veen F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. 2011;17(4):454–66.
de Boer KA, Catt JW, Jansen RP, Leigh D, McArthur S. Moving to blastocyst biopsy for preimplantation genetic diagnosis and single embryo transfer at Sydney IVF. Fertil Steril. 2004;82(2):295–8.
McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84(6):1628–36.
Marin D, Zimmerman R, Tao X, Zhan Y, Scott RT Jr, Treff NR. Validation of a targeted next generation sequencing-based comprehensive chromosome screening platform for detection of triploidy in human blastocysts. Reprod Biomed Online. 2018;36(4):388–95.
Scott RT Jr, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril. 2012;97(4):870–5.
Treff NR, Forman EJ, Scott RT Jr. Next-generation sequencing for preimplantation genetic diagnosis. Fertil Steril. 2013;99(6):e17–8.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT Jr. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–63.. e651
Gleicher N, Vidali A, Braverman J, Kushnir VA, Barad DH, Hudson C, Wu YG, Wang Q, Zhang L, Albertini DF, P. G. S. C. S. G. International. Accuracy of preimplantation genetic screening (PGS) is compromised by degree of mosaicism of human embryos. Reprod Biol Endocrinol. 2016b;14(1):54.
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089–90.
Patrizio P, Shoham G, Shoham Z, Leong M, Barad DH, Gleicher N. Worldwide live births following the transfer of chromosomally “abnormal” embryos after PGT/A: results of a worldwide web-based survey. J Assist Reprod Genet. 2019;36:1599.
Tiegs AW, Hodes-Wertz B, McCulloh DH, Munne S, Grifo JA. Discrepant diagnosis rate of array comparative genomic hybridization in thawed euploid blastocysts. J Assist Reprod Genet. 2016;33(7):893–7.
Bolton H, Graham SJL, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, Voet T, Zernicka-Goetz M. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016;7:11165.
Santos MA, Teklenburg G, Macklon NS, Van Opstal D, Schuring-Blom GH, Krijtenburg PJ, de Vreeden-Elbertse J, Fauser BC, Baart EB. The fate of the mosaic embryo: chromosomal constitution and development of day 4, 5 and 8 human embryos. Hum Reprod. 2010;25(8):1916–26.
Takahashi S, Patrizio P. The impact of mosaic embryos on procreative liberty and procreative responsibility: time to put innovative technology on “pause”. Curr Stem Cell Rep. 2019;5:125.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barad, D. (2020). Hormonal Effects in Reproductive Technology with Focus on Diminished Ovarian Reserve. In: Deligdisch-Schor, L., Mareş Miceli, A. (eds) Hormonal Pathology of the Uterus . Advances in Experimental Medicine and Biology, vol 1242. Springer, Cham. https://doi.org/10.1007/978-3-030-38474-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-38474-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38473-9
Online ISBN: 978-3-030-38474-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)