Abstract
The present chapter revises the current knowledge about the use of salivary biomarkers for metabolic and endocrine disease diagnosis and monitoring in humans and different animal species. Currently, most information available comes from human medicine and studies performed with experimental animals such as mouse or rat, and less from other veterinary species. Furthermore, since obesity and its-related pathologies are currently recognised as the biggest worldwide public health crisis and socioeconomic problem in the twenty-first century, this chapter will focus most on this disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara ObjectivesThe present chapter revises the current knowledge about the use of salivary biomarkers for metabolic and endocrine disease diagnosis and monitoring in humans and different animal species. Currently, most information available comes from human medicine and studies performed with experimental animals such as mouse or rat, and less from other veterinary species. Furthermore, since obesity and its-related pathologies are currently recognised as the biggest worldwide public health crisis and socioeconomic problem in the twenty-first century, this chapter will focus most on this disease.
1 Introduction
Endocrine and metabolic diseases are among the most common diseases that can affect humans and animals. In recent decades there has been an increase in the prevalence and incidence of many of them, especially those related to nutrition and metabolism. Metabolic disease is considered a disease or disorder that disrupt normal metabolism on a cellular level. Currently, the most often occurring metabolic disease is obesity and diseases associated with it, among which endocrine disease such as diabetes mellitus (DM). Endocrine diseases comprise disorders that result in altered production of endocrine hormone(s) (also referred as hormone imbalance) or development of lesions (such as nodules or tumors) in the endocrine system, which may or may not affect hormone levels. Close relationship between metabolic and endocrine disease exists since metabolic alterations can finally result in endocrine system disruption and vice versa. For instance, patients suffering from some endocrine diseases such as DM, hyperadrenocortisism or hypothyroidism present obesity.
Obesity is a multicausal disease that due to increased insulin resistance and chronic inflammatory status leads to increased risk of developing DM, cardiovascular diseases, cancer, and renal diseases among others. However, despite human obesity was recognised as a disease in 2008, and a number of studies were undertaken in order to increase knowledge about its pathogenesis, treatment options and prevention, its prevalence is keeping rising and it is considered to be one of the largest contributors to poor health in most countries (NCD Risk Factor Collaboration (NCD-RisC) 2016; Swinburn et al. 2019). Therefore, obesity was stated to be a worldwide public health crisis and socioeconomic problem in the twenty-first century (Hartman et al. 2016; Canfora et al. 2019). In as similar manner, obesity rates are increasing among veterinary species including dogs, cats, horses, resulting not only in worsened health and quality of life of these animals, but also decreased life span (Kealy et al. 2002). In cats and dogs, obesity was recognized to be a disease just in 2018 (https://bit.ly/2vbTTyO), endorsed by a total of 23 veterinary organisations.
In humans, obesity is usually diagnosed by calculating the body mass index (BMI) following the formula: BMI = BWkg/(heightm)2, where BW refers to body weight. People having BMI equal or above 30 are considered obese (Table 8.1). In animals, the obesity is considered as BW increase in 30% when compared with the ideal BW of the species. However, in many cases, it is very hard or even impossible to know the accurate ideal BW, thus body charts were developed in order to score body condition of an animal. The body condition scores (BCS) are based on the visual evaluation and palpation of the animals (Table 8.1). However, when the possible obesity-related risks have to be evaluated, the serum biochemistry is performed. Traditionally, triglycerides, total cholesterol, high density lipoprotein (HDL) cholesterol, glucose and insulin are determined in serum, since alterations in these analytes were related with the increased risk to develop cardiovascular diseases and diabetes mellitus among others. However, recently new biomarkers such as adipokines, oxidative stress markers, or acute phase proteins have emerged contributing to the pathogenesis of the disease.
Endocrine disease diagnosis is traditionally performed by determination of the concentrations of the hormone of interest in serum and, frequently, dynamic function test, either stimulating or suppressing a particular hormonal axis and observing the appropriate hormonal response, are undertaken. However, general biochemistry analysis can permit to suspect ongoing endocrine disease, since alterations in lipids, acute phase proteins, or glucose among others occur in these diseases.
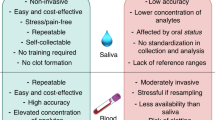
Nevertheless, in both metabolic and endocrine diseases, the use of saliva is getting attention mainly due to its non-invasive nature (see Chap. 2). Furthermore, saliva analysis is leaded not only to evaluate the possible utility of this biofluid as a source of traditional biomarkers, but also for new biomarker identification with the aim to clarify and contribute to increased knowledge about pathophysiology of these diseases.
In the following sections we will review state-of-the-art of data obtained via sialochemistry, proteomics, metabolomics and microbiome analyses in metabolic and endocrine diseases.
2 Sialochemistry
2.1 Glucose
Humans
It was hypothesized that glucose into saliva could enter due to leakage from blood across the basement membrane of salivary glands (Puttaswamy et al. 2017) and due to the capacity to cross the salivary gland epithelium in proportion to its concentration in blood (Abikshyeet et al. 2012). Microvascular alterations in the blood vessels due to DM could also contribute to enter glucose into saliva (Puttaswamy et al. 2017). Different authors have studied salivary glucose concentrations in healthy controls and patients with DM (Smriti et al. 2016; Carramolino-Cuéllar et al. 2017). And, although contradiction exists, saliva was suggested to be an useful biofluid for glucose monitoring, since moderate to positive correlation between salivary and serum glucose concentrations and between salivary glucose and serum glycated hemoglobin (HbA1c) were observed (Abikshyeet et al. 2012; Panchbhai 2012; Gupta et al. 2017).
However, cautions should be taken when analyzing glucose metabolism-related biomarkers due to their stability in saliva samples. It was observed that salivary glucose is rapidly decomposed by oral bacteria and enzymes of saliva (Sandham and Kleinberg 1969a, b), thus samples should be refrigerated at all the moments and analyzed or stored at −80 °C as soon as possible.
Animals
One study in dogs indicate that glucose passes to saliva by a simply passive process from plasma (Langley et al. 1963). Intravenous glucose administration revealed delay in peak of around 25 min of salivary glucose with respect to serum, which could be responsible for the lack of correlation between serum and salivary levels (Muñoz-Prieto et al. 2019b). No studies have been reported evaluating salivary glucose concentrations in dogs with diabetes mellitus or other metabolic/endocrine diseases.
2.2 Fructosamine
Fructosamine is considered as an index of average blood glucose levels up to 2 weeks that is not affected by the diet. For this reason, this analyte is used as a blood glucose control index.
Humans
Nakamoto et al. (2003) described positive correlation between salivary fructosamine glycated protein and serum HbA1c and blood glucose and suggested its possible utility in diabetes diagnosis.
Animals
A method for fructosamine determination in canine saliva was satisfactory validated showing adequate precision and accuracy and, as expected, no significant changes in its concentrations after intravenous glucose administration were observed (Muñoz-Prieto et al. 2019b). Further studies are now needed to evaluate the possible utility of salivary fructosamine in cases of diabetes. Nevertheless, it is important to mention, that fructosamine was described to decrease up to 65% even after being stored at −80 °C, thus, samples should be analyzed in fresh (Muñoz-Prieto et al. 2019b). For this reason, some authors suggested determine glycated proteins, such as fructosamine glycated protein, in saliva since it was hypothesized that glycated proteins were more stable (Nakamoto et al. 2003).
2.3 Insulin
Humans
Insulin-like immunoreactivity in saliva has been reported by different groups of authors (Marchetti et al. 1986; Fekete et al. 1993; Messenger et al. 2003).
There are two main hypotheses regarding the insulin presence in the saliva:
-
1.
Insulin is actively synthesized and secreted by salivary gland. This hypothesis is based mainly on animal studies, in which insulin mRNA have been found in salivary glands and that salivary glands were sensitive to changes in glucose levels (Shubnikova et al. 1984; Kerr et al. 1995; Taouis et al. 1995). Furthermore, synthesis of this hormone was confirmed in humans and animal models through detection of radiolabeled product in salivary gland tissue incubated in vitro with [3H]leucine (Murakami et al. 1982).
-
2.
Insulin enters saliva from blood by ultrafiltration. This hypothesis is supported by studies that detected relation (although delayed) between blood and saliva insulin levels together with the lower salivary insulin concentrations and the observation that salivary insulin was affected by iv glucose administration (Messenger et al. 2003).
In subjects with normal glucose tolerance, both normal weight and obese, the concentrations of insulin in saliva increase after oral glucose tolerance test, although with a delay in the peak of approximately 30–45 min (Marchetti et al. 1986; Pasic and Pickup 1988; Fekete et al. 1993). In healthy school-age girls, salivary insulin levels were correlated with serum insulin and BMI, while this correlation was not detected in boys (Tvarijonaviciute et al. 2019). Nevertheless, insulin handling by salivary glands of patients with type I and II diabetes seem to differ (Marchetti et al. 1986; Pasic and Pickup 1988). Interestingly, strong positive correlation between salivary and serum insulin was observed when pooled data of patients with type I diabetes were studied, while the correlation did not exist when comparing salivary and serum insulin concentrations of each individual separately (Pasic and Pickup 1988). For this reason, some authors indicate that the use of salivary insulin levels for diabetes control would not be reasonable (Pasic and Pickup 1988).
Animals
Rat salivary glands and saliva were shown to present biologically active insulin-like immunoreactivity that can participate in metabolic regulations via amino acid uptake and lipogenesis (Taouis et al. 1995). Furthermore, as stated above, it was demonstrated the active secretion of insulin by salivary glands in rats and mice (Kerr et al. 1995). Kerr et al. (1995) observed that the behavior of salivary insulin in healthy normal mice differs from the mice with induced diabetes, being much lower in those with diabetes and suggesting that salivary glands can act as extrapancreatic source of insulin in cases of diabetes.
In dogs, intravenous glucose administration resulted in a delayed increase of insulin in saliva with respect to serum of about 10 min (Muñoz-Prieto et al. 2019b). The significant increase was noted in saliva 15 min after injection remaining high 45 min after. This delay, could be the reason of the lack of correlation between serum and saliva insulin concentrations. Unfortunately, no data exist about salivary insulin levels in dogs with metabolic diseases such as obesity or diabetes mellitus.
2.4 Lipids
Dysregulation of lipid metabolism is related to development of a number of pathologies including metabolic diseases obesity and diabetes mellitus among others (Gianfrancesco et al. 2018; Hou et al. 2019). In order to evaluate, diagnose or monitor lipid metabolism, in human medicine, triglycerides, cholesterol, low density lipoproteins (LDC) and high density lipoproteins (HDL) are determined in serum. Both HDL and LDL are predominantly involved in cholesterol metabolism. HDL is considered the “good” lipoprotein with protective role on vascular system, while increases LDL were associated with arteriosclerosis. It is important to notice, that the lipid and lipoprotein distribution in serum of humans and different animal species differ, for this reason, caution should be taken when selecting appropriate animal model for human dyslipidemia studies (Table 8.2) (Yin et al. 2012).
Humans
Very few information about salivary lipids is available (Matczuk et al. 2017). From eight main groups of lipids, five were identified in saliva (Table 8.3). Salivary lipid profile in different major salivary glands is similar, while considerable differences were observed in minor glands (Rabinowitz and Shannon 1975). Although, this is not of big importance when total saliva is used, it should be taken in consideration in patients with salivary gland pathologies. Just for instance, alterations in salivary lipid profile was reported in patients with cystic fibrosis, a disease that involves impaired secretion by the endocrine glands, including salivary glands (Matczuk et al. 2017). Nevertheless, no studies exist evaluating possible salivary lipid profile changes in presence of systemic metabolic diseases.
Animals
In animals, only experimental studies in rats were reported describing salivary lipid profiles and their possible alterations in pathologies. For instance, experimental model consisting in diabetes induction in rats with streptozotocin resulted in increased lipids, and in particular stearic and linoleic acids that were normalized after successful treatment with insulin (Anderson and Garrett 1986; Morris et al. 1992; Mahay et al. 2004). However, no studies exist about salivary lipid profiles in naturally occurring pathologies in animals.
2.5 Adipokines
Adipokines are proteins mainly synthesized in the adipose tissue, although in the last years their synthesis by other tissues such as salivary gland was confirmed by different researchers (Katsiougiannis et al. 2006). Adiponectin and leptin are two of the most studied adipokines in metabolic diseases due to their close relationship with insulin resistance and inflammation among others (Bastard et al. 2006). The presence of both adiponectin and leptin in saliva has been described by different groups of authors.
Humans
Salivary adiponectin concentrations correlate positively with serum and, thus, saliva was indicated as appropriate biofluid for this adipokine measurement in different clinical situations such as insulin resistance and obesity (Desai and Mathews 2014; Nigro et al. 2015; Teke et al. 2019) and could serve as convenient adjunct method in predicting cardio-metabolic risks in the population (Attlee et al. 2019). Salivary leptin concentrations were shown to be higher in overweight individuals and patients with diabetes mellitus as compared with normal weight individuals (Jayachandran et al. 2017; Tvarijonaviciute et al. 2017). However, no association between salivary adiponectin or leptin was detected with metabolic syndrome (Thanakun et al. 2014). Furthermore, cautions must be taken in presence of the gingivitis, since an increase in salivary adiponectin was described (Meriç et al. 2018).
Animals
Salivary adiponectin was described in dogs and its correlation with serum was reported (Tvarijonaviciute et al. 2014). Furthermore, salivary adiponectin concentrations were lower, although not statistically significantly, in obese dogs in comparison to normal weight or overweight dogs (Muñoz-Prieto et al. 2019a). However, as occur in humans, salivary adiponectin was shown to be increased in dogs with gingivitis naturally occurring and due to teeth cleaning procedures (Tvarijonaviciute et al. 2014), thus oral health should be evaluated in the dogs when saliva for adiponectin determination is collected.
In pigs, salivary leptin was related with body weight, food ingestion and inflammation (Schmidt et al. 2016).
2.6 Inflammatory Biomarkers
Some metabolic diseases, such as obesity or diabetes mellitus, are related with the presence of low grade inflammation. For this reason, acute phase proteins, especially C-reactive protein (CRP) and interleukins are usually used to evaluate pro-inflammatory status of patients with metabolic and endocrine diseases (Bastard et al. 2006).
Humans
Utility of CRP and interleukins in saliva were proved to be useful biomarkers in studies of obesity, metabolic syndrome or diabetes mellitus being levels of pro-inflammatory biomarkers elevated in these metabolic pathologies (Naidoo et al. 2012; Dezayee and Al-Nimer 2016; Hartman et al. 2016; Balaji et al. 2017; Janem et al. 2017; Tvarijonaviciute et al. 2019).
Animals
The association of salivary S100, a protein participating in the regulation of the immune homeostasis and inflammation (Bao et al. 2012), with metabolic diseases was suggested, since its increase in submandibular glands of rats, after 2 months of induced-hyperglycemia, was observed and linked to the inflammatory response and impaired metabolic and energy production processes (Alves et al. 2013). Furthermore, S100 was higher in saliva of dogs with Obesity Related Metabolic Disease (ORMD) as compared with overweight obese dogs without ORMD (Lucena et al. 2019).
2.7 Antioxidants
Humans
Presence of pro-oxidant status has been reported in different metabolic diseases. However, studies on salivary oxidative biomarker behavior in metabolic diseases are scarce, although some reported results evidence alterations in levels of these biomarkers including malondialdehyde, uric acid, superoxide dismutase, total oxidant status (TOS) and total antioxidant status (TAS) in saliva of patients with diabetes mellitus, gestational DM, obesity, hepato-metabolic comorbidities (Hartman et al. 2016; Madi et al. 2016; Troisi et al. 2019b; Zygula et al. 2019). In addition, oxidative stress was shown to be a strong inducer of alkaline phosphatase in various tissues (Torino et al. 2016). And its salivary concentrations were higher in patients with chronic periodontitis with type-2 diabetes mellitus than chronic periodontitis without diabetes mellitus and healthy patients (Sridharan et al. 2017; De et al. 2018). The adequate metabolic control and periodontal treatment result in normalisation of levels of oxidative stress biomarkers in saliva (Aral et al. 2017).
Animals
Despite the limited number of studies about salivary antioxidants and metabolic diseases in animals, in dogs, the presence of oxidative stress was suggested in dogs with ORMD, since alterations in salivary glutathione S-transferase, superoxide dismutase and Hsp70 were observed in presence of ORMD (Lucena et al. 2019). Furthermore, a 3-month experimental-weight loss resulted in increased salivary levels of copper chaperone ATOX1 and alkaline phosphatase in purebred Beagles (unpublished data), leading to the hypothesis of a reduction in oxidative stress, resulting in elevation of antioxidant defense markers, as it was reported in serum in humans (Bawahab et al. 2017).
Rats with insulin resistance presented higher values of superoxide dismutase, catalase, peroxidase and total antioxidant status in the parotid glands in comparison with the control rats (Zalewska et al. 2014).
2.8 Stress Markers
Humans
Among stress-related biomarkers, the majority of the studies highlight the disturbance of salivary cortisol, cortisone and salivary alpha amylase levels in metabolic and endocrine diseases. These biomarkers were related with BMI, fasting glucose concentrations and insulin sensitivity among others (Incollingo Rodriguez et al. 2015; Aldossari et al. 2019; Liu et al. 2019).
In endocrine diseases, although varying results have been reported, salivary cortisol and cortisone have been suggested to reflect inappropriate production of cortisol in the organism – both excess (e.g., Cushing’s syndrome with a sensitivity of 92–100% and a specificity of 93–100%) and insufficiency (e.g., Addison’s disease) and to monitor in a non-invasive manner the response to treatment (Gilbert and Lim 2008; Blair et al. 2017). It is important to notice, that saliva collection for cortisol determination when its inappropriate production is suspected should be performed at midnight to eliminate bias due to circadian rhythm (Viardot et al. 2005; Gilbert and Lim 2008).
For the reasons described above and because of their high stability in saliva (i.e. cortisol is stable in saliva up to 6 weeks at room temperature when preserved with citric acid 10 g/L) salivary stress-related biomarkers were reported as promising biomarkers in studies of metabolic and endocrine diseases (Chen et al. 1992; Hartman et al. 2016; Blair et al. 2017).
Animals
In dogs, salivary cortisol usefulness to diagnose hiperadrenocortisims was studied (Wenger-Riggenbach et al. 2010). The authors observed that although salivary cortisol in dogs with diagnosed hiperadrenocortisims was higher, overlap between healthy (n = 21) and diseased dogs (n = 6) were detected (2 healthy dogs showed elevated results and 2 dogs with hiperadrenocortisims showed low results) (Wenger-Riggenbach et al. 2010). Nevertheless, low number of animals was used, thus future studies are required in order to clarify the clinical utility of salivary cortisol determination in dogs. Nevertheless, it is important to highlight that when salivary cortisol is up to be determined, sex and neuter status, age, regular living environment, time in environment before testing, testing environment, owner presence during testing, and collection media should be taken into consideration (Cobb et al. 2016). In addition, methods for sample collection, storage and analysis should be acknowledged when comparing results from different studies (Cobb et al. 2016; Damián et al. 2018).
Furthermore, some of the stress-related biomarkers, such as salivary alpha amylase, were observed while using proteomic approach to be altered in metabolic/endocrine diseases in animals (for more information see section Proteomics).
2.9 Thyroid Hormones
Humans
There is a controversy about the possible use of thyroid hormone measurements in saliva for the diagnosis of hypothyroidism. A report that used a RIA, indicated a good correlation between saliva and serum concentrations of thyroxine (T4) (r = 0.74) and a good agreement between saliva T4 values and the functional state of the thyroid (Putz et al. 2009), being this high correlation later confirmed (Gotovtseva and Korot’ko 2002). Meanwhile, Al-Hindawi et al. (2017) did not observe significant differences in free T4 and TSH in saliva between patients with hypothyroidism and healthy individuals, although the values of saliva were parallels to their values in serum. It is of interest to note that in this later study ELISA kits that usually are less sensitive that RIA were used. Furthermore, samples were stored at −20 °C during a not defined time, fact that could have influenced concentrations of the hormones. The sensitivity of the assays has been indicated as one of the main limitations for the measurement of T4 in saliva since concentrations of T4 in this fluid is much lower than in serum (1/100) (Vining et al. 1983).
Animals
Till the date, thyroid hormones were not evaluated in saliva of animals in relation to metabolic and/or endocrine diseases.
2.10 Trace Elements
Humans
Trace elements, such as magnesium, zinc, and calcium levels in saliva were suggested to be useful biomarkers for differentiating patients with type 2 diabetes mellitus from non-diabetics being higher as in controls (Marín Martínez et al. 2018). Furthermore, the salivary magnesium could serve as a marker of high cardiovascular risk since its levels were associated with abdominal obesity in men (Marín Martínez et al. 2018).
3 Proteomics
Proteomics means the analysis of the total protein set expressed by a cell or an organism. Different approaches can be used for proteomic analysis, namely expression proteomics, functional proteomics and structural proteomics. In this part of the chapter, we will focus mainly in expression proteomics studies that have been performed in the context of metabolic diseases. Expression proteomics is mainly used to generate a large qualitative data set with the expression levels of the proteins present in the simple. This provides a global analysis of protein composition, posttranslational modifications, and the dynamic nature of protein expression.
Salivary proteomics has been shown particularly valuable, since it allows to have a source of information about proteins from a fluid that is collected under a non-invasive way. This opened new doors for disease biomarker discovery, which can be particularly interesting also in the case of endocrine and metabolic diseases. Among these last, diabetes was the one receiving greater attention in terms of saliva proteome. Although fewer, some studies did also report changes in salivary proteome induced by obesity. A review about what is known in humans and animals will be subsequently presented.
Humans
Studies in adults and children showed existing differences in salivary proteome of obese individuals in comparison with normal-weight controls. Just for instance, Rangé et al. (2012) observed higher levels of albumin, α- and β- haemoglobin chains, as well as α-defensins 1,2 and 3 in obese individuals, comparatively to normal weight ones. These authors emphasized the interest of saliva as a biological fluid to monitor inflammatory status in obesity, which is considered a low-grade inflammatory condition. Furthermore, differences between obese and non-obese women were observed at the level of proteins such as α-amylase, zinc-α2 glycoprotein and cystatins, among others (Lamy et al. 2015).
Salivary proteome of obese patients was also studied evaluating the effect that body weight loss could have. Bariatric surgery appeared to have effect at the level of salivary proteins like salivary amylase, cystatins and carbonic anhydrase VI (Lamy et al. 2015), proteins referred as potentially involved in oral food perception (Rodrigues et al. 2017). A different study, where weight loss was obtained after weeks of continuous physical activity and caloric restriction, showed also changes in salivary proteome, namely amylase, carbonic anhydrase VI and cystatins, but in opposite direction as changes detected in the study about the effect of bariatric surgery, suggesting that the weight-loss procedures can differently affect physiological changes, reflected in saliva (Simões and Lamy, not published).
Also in children, salivary proteome of overweight individuals present differences from normal-weight ones (Rodrigues et al. 2019). When these differences were accessed by in-gel based methods, namely two-dimensional electrophoresis (2-DE), protein spots identified as zinc-α2 glycoprotein, α-amylase and S-type cystatins were observed increased in obese children (Rodrigues et al. 2019).
Concerning salivary α-amylase, different studies report different associations with obesity: some authors refer decreased copy number of the gene that codifies for this protein (Mejía-Benítez et al. 2015; Pinho et al. 2018) and decreased enzymatic activity (Lasisi et al. 2019); however, some other authors observed increased expression levels of spots identified as containing this salivary enzyme (Lamy et al. 2015). At the same time that these different results may seem contradictory, if we look to the proteoforms of the protein that are increased, is possible to see that are mainly forms with molecular masses lower than the one from the native form of the protein (Lamy et al. 2015). It is possible that these are non-active forms, probably resultant from higher proteolysis in the mouth of obese individuals. But this needs further elucidation. Finally, a recent study points that this relationship between obesity and salivary amylase is not simple and that starch intake can modify it (Rukh et al. 2017). Nevertheless, some authors failed to observe a relationship between salivary proteome and BMI (Mosca et al. 2019).
Several studies about saliva proteome in metabolic diseases were performed in the context of diabetes. By using a gel-free based approach, namely multidimensional liquid chromatography/tandem mass spectrometry (2D-LC-MS/MS), whole saliva from type-2 diabetic individuals was compared with non-diabetic ones, with the identification of 65 proteins with an increased greater than twofold, in diabetic, comparatively to control (Rao et al. 2009). The majority of these proteins are involved in metabolism or immune responses. The usefulness of saliva proteome assessment in diabetes were reinforced by the existence of changes in it according to glycemic control, both in adults (Bencharit et al. 2013) and children (Pappa et al. 2018).
Animals
Comparatively to humans, there are less studies about salivary proteomics in animals. In the case of metabolic diseases, it is mainly concerning diabetes, using rodent models and, only more recently, studies in dogs concerned with metabolic dysfunctions and obesity were published.
Among the different salivary proteins observed to be related with obesity and metabolic diseases, in animals, salivary α-amylase secretion has been suggested of interest to predict susceptibility for weight gain (Rodrigues et al. 2015). In the mentioned study, rats with susceptibility to obesity presented higher α-amylase levels prior to weight gain experimentally-induced with high-fat diet. But this relationship between this salivary protein and obesity needs to be further elucidated, since some studies observed decreased levels of this salivary protein after diet-induced obesity (Lasisi et al. 2019). A potential role of leptin in salivary α-amylase secretion has been recently suggested (Lamy et al. 2018), leading to the hypothesis that the changes in this salivary protein, associated with obesity, can be due to leptin action. In fact, decreased levels of expression levels and enzymatic activity of salivary α-amylase were observed when hyperleptinemia was induced in an animal model (Lamy et al. 2018), leading to the hypothesis that obese, by having higher circulating leptin levels could also have lower α-amylase levels in their saliva.
Recently, through a proteomic approach, the presence of salivary amylase in dog saliva samples has been reported (de Sousa-Pereira et al. 2015), being this presence supported by the measurement of its enzymatic activity in saliva from these animals (Contreras-Aguilar et al. 2017). Nevertheless, until the moment, a potential association between its levels and dog obesity was not reported.
Potential changes in salivary proteome of dogs submitted to weight loss were recently studied. An increase of BPIFA1 salivary levels was found in purebred Beagles dogs after a 3-month experimentally-weight loss (unpublished data), which goes in line with studies of obesity and insulin resistance in humans (Guo et al. 2017). This protein has been positively correlated with insulin action and associated with immune system and inflammatory pathways (Gubern et al. 2006). As such, the aforementioned study (unpublished data) goes in line with a possible improvement in insulin sensitivity after weight loss. Besides this, other salivary proteins, whose abundance was changed after weight loss, were proteins related with immune system/inflammation, oxidative stress and glucose metabolism. These were the cases of copper chaperone ATOX1 and alkaline phosphatase, for example, whose increased levels lead to the hypothesis of a reduction in oxidative stress, after weight loss (unpublished data).
Also in the case of experimentally-weight loss in Beagle dogs, the salivary levels of angiopoietin like 5 protein was increased and strongly positively correlated with the percentage of weight loss (data not shown). This protein is involved in glucose metabolism and the observed relationship with body weight loss suggests a positive effect of this process in glucose regulation. These results are in accordance with a reported negative association between the levels of serum angiopoietin like 3 and 4 proteins and body weight, diabetes status, and parameters of glucose control across a wide range of BMI (Cinkajzlová et al. 2018). To the best of the authors’ knowledge there were no reports of an association between angiopoietin like 5 and obesity.
The relationship between salivary proteome and metabolic diseases was recently demonstrated in a study performed with Obesity Related Metabolic Disease (ORMD) dogs (Lucena et al. 2019). Among the different proteins observed to be different in ORMD dogs, comparatively to control ones, salivary levels of Glucose-6-phosphate dehydrogenase were found to be increased. The levels of the enzyme glucose-6-phosphate dehydrogenase were already reported to be increased in diabetes (Hamzah et al. 2018). This enzyme is involved in the pentose phosphate pathway and its activity is related with the production of NADPH. It was suggested that, at an initial phase of diabetes, increases levels of this enzyme prevent the oxidative stress known to be associate to the development of diabetes and metabolic syndrome. Furthermore, other authors reported a decreased levels of glucose-6-phosphate dehydrogenase in rats with chronic hyperglycemia, resulting in increased oxidative stress (Xu et al. 2005). Other salivary proteins, referred as antioxidant and/or inflammatory biomarkers were also observed to be changed in dog ORMD, by this proteomic approach, and were referred in previous sections of this chapter.
The salivary proteins kallikreins were referred as associated with diabetes, in rodent model. A significant decrease of Kallikrein proteins after 2 months of induced-hyperglycemia in rats was observed, reinforcing results of the effect of chronic hyperglycemia on the proteome of submandibular glands (Alves et al. 2013). Kallikrein proteins are extracellular matrix protein constituents, which had already been reported having salivary levels decreased in type 2 diabetes human subjects (Rao et al. 2009).
4 Metabolomics
Being part of the “omic” sciences, metabolomics is the global assessment and validation of endogenous small-molecule metabolites within a biologic system. Salivary metabolomics gained interest in the last few years, becoming of interest to monitor biological status and for monitoring diseases.
Humans
Salivary metabolomics is an emerging area, with a limited number of studies, at the moment. However, recently this approach has been used to study metabolic syndrome and fatty liver in obese children (Troisi et al. 2019a) and diabetes and diabetes-related periodontal disease in adults (Barnes et al. 2014). Distinct salivary metabolic signatures for pediatric obesity and its related fatty liver and metabolic syndrome were observed as defined mainly by energy, amino and organic acid metabolism, as well as in intestinal bacteria metabolism (Troisi et al. 2019a). These metabolic processes were associated with the diet, fatty acid synthase pathways, microbiota and intestinal mucins (Troisi et al. 2019a). In the same line, 69 out of 475 detected metabolites (14%) in saliva were over or under-expressed in saliva of patients with diabetes as compared to healthy controls (Barnes et al. 2014). Oxidative stress and anti-oxidadtive capacity through increased purin degradation signature and decreased redox balance and lipid metabolism through altered ω-3/ω-6 fatty acid profiles were reported to be the main processes associated with diabetes and periodontal disease (Barnes et al. 2014). These data suggest about potentials that have salivary metabolomics studies to identify early occurring metabolic alterations in a non-invasive way, although future research is necessary to confirm these results and to further study the complex biological pathways, their interactions and their possible changes in metabolic and endocrine diseases.
Animals
To date, metabolomics was not performed in saliva of animals to evaluate possible alterations in metabolic diseases. Future studies are needed to fill this knowledge gap.
5 Microbiome
Microbiome refers to the entire habitat, including microorganisms (bacteria, archaea, lower and upper eukaryotes, and viruses), their genomes (ie, genes), and environmental conditions (Marchesi and Ravel 2015). However some authors limit the definition of microbiome to the collection of genes and genomes of members of a microbiota (Marchesi and Ravel 2015) and in some literature these terms are often used interchangeably (Ursell et al. 2012).
Interest in the study of microbiome has increased in recent years in humans (Maguire and Maguire 2017; Thomas et al. 2017; Mohajeri et al. 2018) and animals (Deng and Swanson 2015; Trinh et al. 2018), since the evidence is growing that associated microorganisms make essential contributions to health and well-being, and changes in their amounts/proportions are associated with a number of different diseases. In the case of the oral microbiota, it has a significant impact on both the oral and systemic health (Dewhirst et al. 2010; Wade 2013; Verma et al. 2018) and is also a potential diagnostic indicator of several oral and systemic diseases (Gao et al. 2018).
Inter-individual variations were significantly larger than intra-individual variations for most of the dominant genera in the oral microbiota (Monteiro-Da-Silva et al. 2014; Barroso et al. 2015; Sato et al. 2015). Multiple factors and their interactions modulate oral microbiome. These factors are intrinsic or extrinsic to the individual (host) and include:
-
(i)
the genetic composition of the host (ex: ethnicity, gender, circadian rhythm);
-
(ii)
oral environment (ex: saliva composition; (Marsh et al. 2016; Lynge Pedersen and Belstrøm 2019));
-
(iii)
lifestyle, behaviour and diet;
-
(iv)
socioeconomic status (Cornejo Ulloa et al. 2019);
-
(v)
climatic conditions (Li et al. 2014) and geographical location (Shaw et al. 2017) although some authors report that the influence of geographical location on the oral microbiome is not significant (Nasidze et al. 2009).
It should also be mentioned, in order to understand the complexity and the dynamics of oral microbiota, composed of hundreds of taxa interacting across multiple spatial scales, that the different structures and tissues of the oral cavity present distinct microbial populations and there are scientific evidences that most oral microbes are site specialists (Aas et al. 2005; Mark Welch et al. 2016; Welch et al. 2019). Distinct microbial communities appear to be observed between dental, tongue, and salivary samples, with high levels of similarity observed between the tongue and salivary communities (Hall et al. 2017). Furthermore, bacterial communities vary along an ecological gradient from the front to the back of the mouth (Proctor et al. 2018).
Humans
Scientific evidences exist that oral/salivary microbiome have a link with the presence of obesity and other metabolic diseases. Si et al. (2017) compared the oral microbiome from subgingival plaque with the gut microbiome and their results support the notion that metabolic disease can influence the non-gut human microbiome. In the same manner, other studies also observed differences in oral microbiota between overweight and normal-weight persons (Goodson et al. 2009; Wu et al. 2018; Mervish et al. 2019; Raju et al. 2019). Furthermore, the overweight was related to the greater diversity of microbial species, although lower total amounts (Mervish et al. 2019). Microbiota diversity and composition were significantly associated with body size and gender in school-age children (Raju et al. 2019). Overall, these studies emphasize the utility of local oral bacteria as potential biomarkers for systemic metabolic disease.
Some studies have confirmed that the oral diseases and diabetes mellitus are closely related (Negrato et al. 2013). According to Ebersole et al. (2008) the increased severity of periodontal disease associated with type 2 diabetes may reflect an alteration in the pathogenic potential of periodontal bacteria and/or a modification of the characteristics of the host’s inflammatory response. Furthermore, oral microbiota is an important factor in the development of diabetes, affecting oral bone development and increasing the risk and severity of tooth loss (Xiao et al. 2017), what in periodontitis can compromise the chewing process and the sensory function of periodontal tissues (Borges et al. 2012). Saeb et al. (2019) observed a reduction of the biological and phylogenetic diversity in the diabetes and prediabetes oral microbiota in comparison with that in the normoglycemic oral microbiota and this was associated with an increase in the pathogenic content of the hyperglycemic microbiota.
The results of Tam and colleagues suggested that obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus, but the impact of glycemic control on oral microbiota, however, remains to be elucidated (Tam et al. 2018). Detailed information on the different taxa and species can be find in literature (Gao et al. 2018; Lu et al. 2019).
Animals
To date there are only a few reports that assessed the characterization of dogs (Dewhirst et al. 2012; Sivakami et al. 2015) and cats (Sturgeon et al. 2014; Dewhirst et al. 2015; Adler et al. 2016) oral microbiomes using modern sequencing technology. Equine subgingival plaque microbiota shares many similarities with the human, canine and feline oral microbiomes (Gao et al. 2016). Nevertheless, contrary to what happens in humans, the evaluation of the relationship between the oral microbiome and metabolic diseases is not yet documented.
There is a growing number of families having pets, mainly dogs and cats. Since many of these animals are treated as family members and are in direct contact with the people, it is expected that the exchange of microorganisms between humans and animals occur, through various routes, including saliva (Nishiyama et al. 2007; Song et al. 2013; Misic et al. 2015). However, dogs and their owners presented appreciably differences in oral microbiome, being their oral microbiotas not correlated with residing in the same household (Oh et al. 2015). Further studies are needed to study the oral microbiome relation with metabolic and endocrine disease in animals and the possible connection with changes in animal-owner microbiota.
6 Conclusion
Strong scientific evidences exist suggesting saliva to constitute a promising tool to evaluate metabolic diseases and endocrinopathies-related alterations.
References
Aas JA et al (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43:5721–5732. https://doi.org/10.1128/JCM.43.11.5721-5732.2005
Abikshyeet P, Ramesh V, Oza N (2012) Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetol Metab Syndr Obes Target Ther Dove Press 5:149–154. https://doi.org/10.2147/DMSO.S32112
Adler CJ et al (2016) Diet may influence the oral microbiome composition in cats. Microbiome 4:23. https://doi.org/10.1186/s40168-016-0169-y
Aldossari NM, El Gabry EE, Gawish GEH (2019) Association between salivary amylase enzyme activity and obesity in Saudi Arabia. Medicine 98(23):e15878. https://doi.org/10.1097/MD.0000000000015878
Al-Hindawi S, Luaibi N, Al-Ghurabei B (2017) Possible use of saliva as a diagnostic tool in hypothyroidism. Glob J BioSci Biotechnol 6(3):539–542. Available at: http://scienceandnature.org/GJBB_Vol6(3)2017.php. Accessed 11 Aug 2019
Alves RMP et al (2013) iTRAQ-based quantitative proteomic analysis of submandibular glands from rats with STZ-induced hyperglycemia. J Biochem 153:209–220. https://doi.org/10.1093/jb/mvs142
Anderson LC, Garrett JR (1986) Lipid accumulation in the major salivary glands of streptozotocin-diabetic rats. Arch Oral Biol 31(7):469–475
Aral CA et al (2017) Metabolic control and periodontal treatment decreases elevated oxidative stress in the early phases of type 1 diabetes onset. Arch Oral Biol 82:115–120. https://doi.org/10.1016/j.archoralbio.2017.06.009
Attlee A et al (2019) Relationship of salivary adipocytokines, diet quality, physical activity, and nutrition status in adult Emirati females in United Arab Emirates. Diabetes Metab Syndr Clin Res Rev 13(1):40–46. https://doi.org/10.1016/J.DSX.2018.08.006
Balaji A et al (2017) Salivary Interleukin-6 – a pioneering marker for correlating diabetes and chronic periodontitis: a comparative study. Indian J Dent Res 28(2):133. https://doi.org/10.4103/ijdr.IJDR_167_14
Bao L, Odell AF, Stephen SL, Wheatcroft SB, Walker JH, Ponnambalam S (2012) The S100A6 calcium-binding protein regulates endothelial cell-cycle progression and senescence. FEBS J 279(24):4576–4588
Barnes VM et al (2014) Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS One. Edited by Ö. Yilmaz. Public Library of Science 9(8):e105181. https://doi.org/10.1371/journal.pone.0105181
Barroso E et al (2015) Stability of saliva microbiota during moderate consumption of red wine. Arch Oral Biol 60:1763–1768. https://doi.org/10.1016/j.archoralbio.2015.09.015
Bastard JP et al (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17(1):4–12. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16613757. Accessed 4 Aug 2019
Bawahab MA, Assiri AS, Maksoud WA, Patel A, Kadoumi O, Zaman GS, Alessih RMK, Haider SS (2017) Effects of weight reduction after sleeve gastrectomy on metabolic variables in Saudi obese subjects in Aseer province of kingdom of Saudi Arabia. Obes Surg 27(8):2005–2014
Bencharit S et al (2013) Salivary proteins associated with hyperglycemia in diabetes: a proteomic analysis. Mol BioSyst NIH Public Access 9(11):2785–2797. https://doi.org/10.1039/c3mb70196d
Blair J et al (2017) Salivary cortisol and cortisone in the clinical setting. Curr Opin Endocrinol Diabetes Obes 24(3):161–168. https://doi.org/10.1097/MED.0000000000000328
Borges Tde F et al (2012) Changes in masticatory performance and quality of life in individuals with chronic periodontitis. J Periodontol 84:325–331. https://doi.org/10.1902/jop.2012.120069
Canfora EE et al (2019) Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol Nat Publ Group 15(5):261–273. https://doi.org/10.1038/s41574-019-0156-z
Carramolino-Cuéllar E et al (2017) Salivary glucose as a metabolic control marker in patients with type 2 diabetes. J Biol Regul Homeost Agents 31(2 Suppl 1):181–187. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28691471. Accessed 4 July 2019
Chen YM, Cintrón NM, Whitson PA (1992) Long-term storage of salivary cortisol samples at room temperature. Clin Chem 38(2):304. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1541015. Accessed 11 Aug 2019
Cinkajzlová A et al (2018) Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: the effect of weight reduction and realimentation. Nutr Diabetes 8(1):21. https://doi.org/10.1038/s41387-018-0032-2
Cobb ML et al (2016) A systematic review and meta-analysis of salivary cortisol measurement in domestic canines. Domest Anim Endocrinol Elsevier 57:31–42. https://doi.org/10.1016/J.DOMANIEND.2016.04.003
Contreras-Aguilar MD et al (2017) Detection and measurement of alpha-amylase in canine saliva and changes after an experimentally induced sympathetic activation. BMC Vet Res 13:266. https://doi.org/10.1186/s12917-017-1191-4
Cornejo Ulloa P, van der Veen MH, Krom BP (2019) Review: modulation of the oral microbiome by the host to promote ecological balance. Odontology 107:437–448. https://doi.org/10.1007/s10266-019-00413-x
Damián JP et al (2018) Serial collection method of dog saliva: effects of different chemical stimulants on behaviour, volume and saliva composition. Open Vet J 8(3):229. https://doi.org/10.4314/ovj.v8i3.1
de Sousa-Pereira P et al (2015) Cross species comparison of mammalian saliva using a LC-MALDI-based proteomic approach. Proteomics 15:1598–1607. https://doi.org/10.1002/pmic.201400083
De A et al (2018) Estimation of salivary and serum alkaline phosphatase level as a diagnostic marker in type-2 diabetes mellitus with periodontal health and disease: a clinico-biochemical study. J Oral maxillofac Pathol JOMFP Wolters Kluwer Medknow Publ 22(3):445. https://doi.org/10.4103/jomfp.JOMFP_212_18
Deng P, Swanson KS (2015) Gut microbiota of humans, dogs and cats: current knowledge and future opportunities and challenges. Br J Nutr 113:S6–S17. https://doi.org/10.1017/S0007114514002943
Desai GS, Mathews ST (2014) Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J Diabetes Baishideng Publ Group Inc 5(6):730–738. https://doi.org/10.4239/wjd.v5.i6.730
Dewhirst FE et al (2010) The human oral microbiome. J Bacteriol 192:5002–5017. https://doi.org/10.1128/JB.00542-10
Dewhirst FE et al (2012) The canine oral microbiome. PLoS One 7:e36067. https://doi.org/10.1371/journal.pone.0036067
Dewhirst FE et al (2015) The feline oral microbiome: a provisional 16S rRNA gene based taxonomy with full-length reference sequences. Vet Microbiol 175:294–303. https://doi.org/10.1016/j.vetmic.2014.11.019
Dezayee ZI, Al-Nimer MM (2016) Saliva C-reactive protein as a biomarker of metabolic syndrome in diabetic patients. Indian J Dent Res 27(4):388. https://doi.org/10.4103/0970-9290.191887
Ebersole JL et al (2008) Microbiologic and immunologic characteristics of periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol 79:637–646. https://doi.org/10.1902/jop.2008.070455
Fekete Z et al (1993) Salivary and plasma insulin levels in man. Biochem Mol Biol Int 30(4):623–629. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8401319. Accessed 1 Aug 2019
Gao W et al (2016) In-depth snapshot of the equine subgingival microbiome. Microb Pathog 94:76–89. https://doi.org/10.1016/j.micpath.2015.11.002
Gao L et al (2018) Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell 9:488–500. https://doi.org/10.1007/s13238-018-0548-1
Gianfrancesco MA et al (2018) Lipid bilayer stress in obesity-linked inflammatory and metabolic disorders. Biochem Pharmacol 153:168–183. https://doi.org/10.1016/j.bcp.2018.02.022
Gilbert R, Lim EM (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. Clin Biochem Rev Aust Assoc Clin Biochem 29(3):103–106. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19107223. Accessed 11 Aug 2019
Goodson JM et al (2009) Is obesity an oral bacterial disease? J Dent Res Int Assoc Dent Res 88(6):519–523. https://doi.org/10.1177/0022034509338353
Gotovtseva LP, Korot’ko GF (2002) Salivary thyroid hormones in evaluation of the functional state of the hypophyseal-thyroid system. Klin Lab Diagn 7:9–11. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12187594. Accessed 11 Aug 2019
Gubern C et al (2006) Natural antibiotics and insulin sensitivity: the role of bactericidal/permeability-increasing protein. Diabetes 55:216–224. https://doi.org/10.2337/diabetes.55.01.06.db05-1108
Guo Y et al (2017) Associations of salivary BPIFA1 protein in chronic periodontitis patients with type 2 diabetes mellitus. Int J Endocrinol 2017:1087017. https://doi.org/10.1155/2017/1087017
Gupta S et al (2017) Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J Oral Maxillofac Pathology JOMFP Wolters Kluwer Medknow Publ 21(3):334–339. https://doi.org/10.4103/jomfp.JOMFP_222_15
Hall MW et al (2017) Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. npj Biofilms Microbiomes 3:2. https://doi.org/10.1038/s41522-016-0011-0
Hamzah SA, Hamza LA, Rahman HS (2018) Impact of diabetes mellitus type 2 in the activity of glucose-6-phosphate dehydrogenase in human erythrocyte. Kurdistan Journal of Applied Research 3(1):58–62
Hartman ML et al (2016) Salivary biomarkers in pediatric metabolic disease research. Pediatr Endocrinol Rev PER 13(3):602–611. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27116847. Accessed 27 Feb 2018
Hou C et al (2019) Beneficial effects of pomegranate on lipid metabolism in metabolic disorders. Mol Nutr Food Res 63:1800773. https://doi.org/10.1002/mnfr.201800773
Incollingo Rodriguez AC et al (2015) Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology 62:301–318. https://doi.org/10.1016/j.psyneuen.2015.08.014
Janem WF et al (2017) Salivary inflammatory markers and microbiome in normoglycemic lean and obese children compared to obese children with type 2 diabetes. PLoS One. Edited by Ö. Yilmaz 12(3):e0172647. https://doi.org/10.1371/journal.pone.0172647
Jayachandran T, Srinivasan B, Padmanabhan S (2017) Salivary leptin levels in normal weight and overweight individuals and their correlation with orthodontic tooth movement. Angle Orthod 87(5):739–744. https://doi.org/10.2319/120216-869.1
Katsiougiannis S et al (2006) Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum Wiley-Blackwell 54(7):2295–2299. https://doi.org/10.1002/art.21944
Kealy RD et al (2002) Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc Am Vet Med Assoc 220(9):1315–1320. https://doi.org/10.2460/javma.2002.220.1315
Kerr M et al (1995) Detection of insulin and insulin-like growth factors I and II in saliva and potential synthesis in the salivary glands of mice: effects of type 1 diabetes mellitus. Biochem Pharmacol Elsevier 49(10):1521–1531. https://doi.org/10.1016/0006-2952(95)00017-T
Lamy E et al (2015) Changes in the salivary protein profile of morbidly obese women either previously subjected to bariatric surgery or not. J Physiol Biochem Springer Neth 71(4):691–702. https://doi.org/10.1007/s13105-015-0434-8
Lamy E et al (2018) Effects of hyperleptinemia in rat saliva composition, histology and ultrastructure of the major salivary glands. Arch Oral Biol Pergamon 96:1–12. https://doi.org/10.1016/J.ARCHORALBIO.2018.08.005
Langley LL et al (1963) Secretion of glucose-C14 by dog parotid gland. Arch Oral Biol Pergamon 8(2):127–133. https://doi.org/10.1016/0003-9969(63)90050-5
Lasisi TJ, Shittu STT, Alada AR (2019) Re-establishing normal diet following high fat-diet-induced obesity reverses the altered salivary composition in Wistar rats. J Basic Clin Physiol Pharmacol 30:111–120. https://doi.org/10.1515/jbcpp-2018-0006
Li J et al (2014) Comparative analysis of the human saliva microbiome from different climate zones: Alaska, Germany, and Africa. BMC Microbiol 14:316. https://doi.org/10.1186/s12866-014-0316-1
Liu X et al (2019) Gender-specific independent and combined effects of the cortisol-to-cortisone ratio and 11-deoxycortisol on prediabetes and type 2 diabetes mellitus: from the Henan rural cohort study. J Diabetes Res 2019:1–8. https://doi.org/10.1155/2019/4693817
Lu M, Xuan S, Wang Z (2019) Oral microbiota: a new view of body health. Food Sci Human Wellness 8:8–15. https://doi.org/10.1016/j.fshw.2018.12.001
Lucena S et al (2019) Comparative proteomic analysis of saliva from dogs with and without obesity-related metabolic dysfuntion. J Proteome 201:65–72. https://doi.org/10.1016/j.jprot.2019.04.010
Lynge Pedersen AM, Belstrøm D (2019) The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent 80:S3–S12. https://doi.org/10.1016/j.jdent.2018.08.010
Madi M et al (2016) Status of serum and salivary levels of superoxide dismutase in type 2 diabetes mellitus with oral manifestations: a case control study. Ethiop J Health Sci 26(6):523. https://doi.org/10.4314/ejhs.v26i6.4
Maguire M, Maguire G (2017) The role of microbiota, and probiotics and prebiotics in skin health. Arch Dermatol Res 309:411–421. https://doi.org/10.1007/s00403-017-1750-3
Mahay S, Adeghate E, Lindley MZ, Rolph CE, Singh J (2004) Streptozotocin-induced type 1 diabetes mellitus alters the morphology, secretory function and acyl lipid contents in the isolated rat parotid salivary gland. Mol Cell Biochem 261(1):175–181
Marchesi JR, Ravel J (2015) The vocabulary of microbiome research: a proposal. Microbiome 3:31. https://doi.org/10.1186/s40168-015-0094-5
Marchetti P et al (1986) Salivary insulin concentrations in type 2 (non-insulin-dependent) diabetic patients and obese non-diabetic subjects: relationship to changes in plasma insulin levels after an oral glucose load. Diabetologia 29(10):695–698. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3542670. Accessed 25 June 2019
Marín Martínez L, Molino Pagán D, López Jornet P (2018) Trace elements in saliva as markers of type 2 diabetes mellitus. Biol Trace Elem Res 186(2):354–360. https://doi.org/10.1007/s12011-018-1326-x
Mark Welch JL et al (2016) Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci 113:E791–E800. https://doi.org/10.1073/pnas.1522149113
Marsh PD et al (2016) Influence of saliva on the oral microbiota. Periodontology 2000:80–92. https://doi.org/10.1111/prd.12098
Matczuk J et al (2017) Salivary lipids: a review. Adv Clin Exp Med 26(6):1023–1031. https://doi.org/10.17219/acem/63030
Mejía-Benítez MA et al (2015) Beneficial effect of a high number of copies of salivary amylase AMY1 gene on obesity risk in Mexican children. Diabetologia 58:290–294. https://doi.org/10.1007/s00125-014-3441-3
Meriç P et al (2018) Salivary adiponectin and leptin levels are increased in women with gestational diabetes mellitus and gingival inflammation. Oral Health Prev Dent 16(6):541–547. https://doi.org/10.3290/j.ohpd.a41658
Mervish NA et al (2019) Associations of the oral microbiota with obesity and menarche in inner city girls. J Child Obes iMedPub 4(1):2–10.21767/2572-5394.100068
Messenger B, Clifford MN, Morgan LM (2003) Glucose-dependent insulinotropic polypeptide and insulin-like immunoreactivity in saliva following sham-fed and swallowed meals. J Endocrinol 177(3):407–412. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12773121. Accessed 24 June 2019
Misic AM et al (2015) The shared microbiota of humans and companion animals as evaluated from Staphylococcus carriage sites. Microbiome 3:2. https://doi.org/10.1186/s40168-014-0052-7
Mohajeri MH et al (2018) The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr 57:1–14. https://doi.org/10.1007/s00394-018-1703-4
Monteiro-Da-Silva F, Araujo R, Sampaio-Maia B (2014) Interindividual variability and intraindividual stability of oral fungal microbiota over time. Med Mycol 52:498–505. https://doi.org/10.1093/mmy/myu027
Morris PA, Prout RES, Proctor GB, Garrett JR, Anderson LC (1992) Lipid analysis of the major salivary glands in streptozotocin-diabetic rats and the effects of insulin treatment. Arch Oral Biol 37(6):489–494
Mosca AC et al (2019) How are macronutrient intake, BMI, ethnicity, age, and gender related to the composition of unstimulated saliva? A case study. J Texture Stud 50:53–61. https://doi.org/10.1111/jtxs.12362
Muñoz-Prieto A, Martínez-Subiela S et al (2019a) A new highly sensitive immunoassay for the detection of adiponectin in serum and saliva of dogs and its application in obesity and canine leishmaniosis. Res Vet Sci WB Saunders 125:374–381. https://doi.org/10.1016/J.RVSC.2019.07.019
Muñoz-Prieto A, Escribano D et al (2019b) Glucose, fructosamine, and insulin measurements in saliva of dogs: variations after an experimental glucose administration. Domest Anim Endocrinol Elsevier 66:64–71. https://doi.org/10.1016/J.DOMANIEND.2018.10.002
Murakami K, Taniguchi H, Baba S (1982) Presence of insulin-like immunoreactivity and its biosynthesis in rat and human parotid gland. Diabetologia Springer-Verlag 22(5):358–361. https://doi.org/10.1007/BF00253582
Naidoo T et al (2012) Elevated salivary C-reactive protein predicted by low cardio-respiratory fitness and being overweight in African children. Cardiovasc J Afr Clinics Cardive Publ (Pty) Ltd 23(9):501–506. https://doi.org/10.5830/CVJA-2012-058
Nakamoto I et al (2003) Correlation between saliva glycated and blood glycated proteins. Environ Health Prev Med Springer Verlag 8(3):95–99. https://doi.org/10.1007/BF02897922
Nasidze I et al (2009) Global diversity in the human salivary microbiome. Genome Res 19:636–643. https://doi.org/10.1101/gr.084616.108
NCD Risk Factor Collaboration (NCD-RisC) (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387(10026):1377–1396. https://doi.org/10.1016/S0140-6736(16)30054-X
Negrato CA et al (2013) Periodontal disease and diabetes mellitus. J Appl Oral Sci Rev FOB 21(1):1–12. https://doi.org/10.1590/1678-7757201302106
Nigro E et al (2015) Evaluation of salivary adiponectin profile in obese patients. Peptides 63:150–155. https://doi.org/10.1016/j.peptides.2014.11.007
Nishiyama SAB et al (2007) Detection of putative periodontal pathogens in subgingival specimens of dogs. Braz J Microbiol 38:23–28. https://doi.org/10.1590/S1517-83822007000100006
Oh C et al (2015) Comparison of the oral microbiomes of canines and their owners using next- generation sequencing. PLoS One 10:e0131468. https://doi.org/10.1371/journal.pone.0131468
Panchbhai AS (2012) Correlation of salivary glucose level with blood glucose level in diabetes mellitus. J Oral Maxillofac Res 3(3):e3. https://doi.org/10.5037/jomr.2012.3303
Pappa A-M et al (2018) Organic electronics for point-of-care metabolite monitoring. Trends Biotechnol Elsevier Curr Trends 36(1):45–59. https://doi.org/10.1016/J.TIBTECH.2017.10.022
Pasic J, Pickup JC (1988) Salivary insulin in normal and type I diabetic subjects. Diabetes Care 11(6):489–494. https://doi.org/10.2337/diacare.11.6.489
Pinho S, Padez C, Manco L (2018) High AMY1 copy number protects against obesity in Portuguese young adults. Ann Hum Biol 45:435–439. https://doi.org/10.1080/03014460.2018.1490452
Proctor DM et al (2018) A spatial gradient of bacterial diversity in the human oral cavity shaped by salivary flow. Nat Commun 9:681. https://doi.org/10.1038/s41467-018-02900-1
Puttaswamy KA, Puttabudhi JH, Raju S (2017) Correlation between salivary glucose and blood glucose and the implications of salivary factors on the oral health status in type 2 diabetes mellitus patients. J Int Soc Prev Community Dent Wolters Kluwer Medknow Publ 7(1):28–33. https://doi.org/10.4103/2231-0762.200703
Putz Z, Vaňuga A, Velemínský J (2009) Radioimmunoassay of thyroxine in saliva. Exp Clin Endocrinol Diabetes 85(02):199–203. https://doi.org/10.1055/s-0029-1210436
Rabinowitz JL, Shannon IL (1975) Lipid changes in human male parotid saliva by stimulation. Arch Oral Biol Pergamon 20(7):403–406. https://doi.org/10.1016/0003-9969(75)90223-X
Raju SC et al (2019) Gender-specific associations between saliva microbiota and body size. Front Microbiol 10:767. https://doi.org/10.3389/fmicb.2019.00767
Rangé H et al (2012) Salivary proteome modifications associated with periodontitis in obese patients. J Clin Periodontol 39(9):799–806. https://doi.org/10.1111/j.1600-051X.2012.01913.x
Rao PV et al (2009) Proteomic identification of salivary biomarkers of type-2 diabetes. J Proteome Res 8:239–245. https://doi.org/10.1021/pr8003776
Rodrigues L et al (2015) Effects of high fat diet on salivary α-amylase, serum parameters and food consumption in rats. Arch Oral Biol 60:854–862. https://doi.org/10.1016/j.archoralbio.2015.02.015
Rodrigues L et al (2017) Association between salivary leptin levels and taste perception in children. J Nutr Metab Hindawi 2017:1–7. https://doi.org/10.1155/2017/7260169
Rodrigues L et al (2019) Comparison of salivary proteome of children with different sensitivities for bitter and sweet tastes: association with body mass index. Int J Obes Nat Publ Group 43(4):701–712. https://doi.org/10.1038/s41366-018-0289-5
Rukh G et al (2017) Dietary starch intake modifies the relation between copy number variation in the salivary amylase gene and BMI. Am J Clin Nutr 106:256–262. https://doi.org/10.3945/ajcn.116.149831
Saeb ATM et al (2019) Relative reduction of biological and phylogenetic diversity of the oral microbiota of diabetes and pre-diabetes patients. Microb Pathog 128:215–229. https://doi.org/10.1016/j.micpath.2019.01.009
Sandham HJ, Kleinberg I (1969a) The effect of glucose concentration on the interrelation between glucose utilization, pH and carbohydrate storage in a salivary system. Arch Oral Biol 14(6):603–618. https://doi.org/10.1016/0003-9969(69)90184-8
Sandham HJ, Kleinberg I (1969b) Utilization of glucose and lactic acid by salivary sediment. Arch Oral Biol 14(6):597–602. https://doi.org/10.1016/0003-9969(69)90183-6
Sato Y et al (2015) Inter-individual differences in the oral bacteriome are greater than intra-day fluctuations in individuals. PLoS One 10:e0131607. https://doi.org/10.1371/journal.pone.0131607
Schmidt EMS et al (2016) Development and validation of an assay for measurement of leptin in pig saliva. BMC Vet Res BioMed Cent 12(1):242. https://doi.org/10.1186/s12917-016-0871-9
Shaw L et al (2017) The human salivary microbiome is shaped by shared environment rather than genetics: evidence from a large family of closely related individuals. MBio 8(5):pii: e01237-17. https://doi.org/10.1128/mBio.01237-17
Shubnikova EA, Volkova EF, Printseva OYA (1984) Submandibular glands as organs of synthesis and accumulation of insulin-like protein. Acta histochemica 74(2):157–171. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6431732. Accessed 24 June 2019
Si J, Lee C, Ko G (2017) Oral microbiota: microbial biomarkers of metabolic syndrome independent of host genetic factors. Front Cell Infect Microbiol 7. https://doi.org/10.3389/fcimb.2017.00516
Sivakami B, Ambarin Farizah B, Aishwarya T (2015) Vaishnavi, Viveka Devi R, Sakthivel K. Isolation and characterisation of salivary microbiota of street dogs. Journal of Biological and Information Sciences. 4(1):1–5. Available from: http://archive.biolim.org/jbis/read/BOJ001A0029
Smriti K et al (2016) Salivary glucose as a diagnostic marker for diabetes mellitus. J Diabetes Sci Technol Diabetes Technol Soc 10(4):991–992. https://doi.org/10.1177/1932296816637619
Song SJ et al (2013) Cohabiting family members share microbiota with one another and with their dogs. elife 2:e00458. https://doi.org/10.7554/eLife.00458
Sridharan S et al (2017) Salivary alkaline phosphatase as a noninvasive marker for periodontal disease in children with uncontrolled type 1 diabetes mellitus. J Clin Pediatr Dent 41(1):70–74. https://doi.org/10.17796/1053-4628-41.1.70
Sturgeon A et al (2014) Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet J 201:223–229. https://doi.org/10.1016/j.tvjl.2014.01.024
Swinburn BA et al (2019) The global syndemic of obesity, undernutrition, and climate change: the Lancet Commission report. Lancet (Lond Engl) Elsevier 393(10173):791–846. https://doi.org/10.1016/S0140-6736(18)32822-8
Tam J et al (2018) Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLoS One 13:e0204724. https://doi.org/10.1371/journal.pone.0204724
Taouis M et al (1995) Biological activity of immunoreactive insulin-like activity extracted from rat submandibular gland. Am J Physiol Am Physiol Soc 269(2 Pt 1):E277–E282. https://doi.org/10.1152/ajpendo.1995.269.2.E277
Teke E et al (2019) Does metabolic control affect salivary adipokines in type 2 diabetes mellitus? Dent Med Prob 56(1):11–20. https://doi.org/10.17219/dmp/103417
Thanakun S et al (2014) Comparison of salivary and plasma adiponectin and leptin in patients with metabolic syndrome. Diabetol Metab Syndr BioMed Cent 6(1):19. https://doi.org/10.1186/1758-5996-6-19
Thomas S et al (2017) The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res 77:1783–1812. https://doi.org/10.1158/0008-5472.CAN-16-2929
Torino C et al (2016) Oxidative stress as estimated by gamma-glutamyl transferase levels amplifies the alkaline phosphatase-dependent risk for mortality in ESKD patients on dialysis. Oxidative Med Cell Longev Hindawi Ltd 2016:8490643. https://doi.org/10.1155/2016/8490643
Trinh P et al (2018) One health relationships between human, animal, and environmental microbiomes: a mini-review. Front Public Health 6:235. https://doi.org/10.3389/fpubh.2018.00235
Troisi J, Belmonte F, Bisogno A, Pierri L et al (2019a) Metabolomic salivary signature of pediatric obesity related liver disease and metabolic syndrome. Nutrients 11:pii: E274. https://doi.org/10.3390/nu11020274
Troisi J, Belmonte F, Bisogno A, Lausi O et al (2019b) Salivary markers of hepato-metabolic comorbidities in pediatric obesity. Dig Liver Dis WB Saunders 51(4):516–523. https://doi.org/10.1016/J.DLD.2018.11.009
Tvarijonaviciute A et al (2014) Measurement of salivary adiponectin concentrations in dogs. Vet Clin Pathol 43(3):416–421. https://doi.org/10.1111/vcp.12169
Tvarijonaviciute A et al (2017) Leptin and NGF in saliva of patients with diabetes mellitus type 2: a pilot study. J Oral Pathol Med 46(9):853–855. https://doi.org/10.1111/jop.12587
Tvarijonaviciute A et al (2019) Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clin Nutr. https://doi.org/10.1016/j.clnu.2019.10.034
Ursell LK et al (2012) Defining the human microbiome. Nutr Rev 70:S38–S44. https://doi.org/10.1111/j.1753-4887.2012.00493.x
Verma D, Garg PK, Dubey AK (2018) Insights into the human oral microbiome. Arch Microbiol 200:525–540. https://doi.org/10.1007/s00203-018-1505-3
Viardot A et al (2005) Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinary free cortisol and overnight dexamethasone suppression test. J Clin Endocrinol Metab Narnia 90(10):5730–5736. https://doi.org/10.1210/jc.2004-2264
Vining RF, McGinley RA, Symons RG (1983) Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem 29(10). Available at: http://clinchem.aaccjnls.org/content/29/10/1752.long. Accessed 11 Aug 2019
Wade WG (2013) The oral microbiome in health and disease. Pharmacol Res Acad 69(1):137–143. https://doi.org/10.1016/J.PHRS.2012.11.006
Welch JLM, Dewhirst FE, Borisy GG (2019) Biogeography of the oral microbiome: the site-specialist hypothesis. Annu Rev Microbiol 73:335–358. https://doi.org/10.1146/annurev-micro-090817-062503
Wenger-Riggenbach B et al (2010) Salivary cortisol concentrations in healthy dogs and dogs with hypercortisolism. J Vet Int Med Wiley 24(3):551–556. https://doi.org/10.1111/j.1939-1676.2010.0494.x
Wu Y et al (2018) Characterization of the salivary microbiome in people with obesity. PeerJ 6:e4458. https://doi.org/10.7717/peerj.4458
Xiao E et al (2017) Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe 22:120–128.e4. https://doi.org/10.1016/j.chom.2017.06.014
Xu Y, Osborne BW, Stanton RC (2005) Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Ren Physiol 289:F1040–F1047. https://doi.org/10.1152/ajprenal.00076.2005
Yin W et al (2012) Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J Lipid Res Am Soc Biochem Mol Biol 53(1):51–65. https://doi.org/10.1194/jlr.M019927
Zalewska A et al (2014) Antioxidant profile of salivary glands in high fat diet-induced insulin resistance rats. Oral Dis 20(6):560–566. https://doi.org/10.1111/odi.12173
Zygula A et al (2019) Oxidative stress markers in saliva and plasma differ between diet-controlled and insulin-controlled gestational diabetes mellitus. Diabetes Res Clin Pract Elsevier 148:72–80. https://doi.org/10.1016/J.DIABRES.2018.11.021
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tvarijonaviciute, A., Lucena, S., Capela e Silva, F., Lamy, E. (2020). Salivary Biomarkers in the Diagnosis and Monitoring of Metabolic and Endocrine Diseases. In: Tvarijonaviciute, A., Martínez-Subiela, S., López-Jornet, P., Lamy, E. (eds) Saliva in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-37681-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-37681-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-37680-2
Online ISBN: 978-3-030-37681-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)