Abstract
Nonalcoholic fatty liver disease (NAFLD) is emerging as the most common chronic liver disorder, affecting approximately 25% of the population globally. The association of NAFLD with metabolic morbidity and cardiovascular disease (CVD), as well as its association with significant liver-related morbidity and mortality, create important challenges for primary care physicians in relation to the prevention, diagnosis and treatment of NAFLD and its associated risks for CVD and liver-related morbidity [1]. The importance of establishing a policy in regards to early detection of NAFLD and advanced fibrosis in primary care settings or diabetes clinics, among people with obesity, diabetes or the metabolic syndrome, who represent high-risk populations for the more advanced forms of NAFLD, is increasingly recognized. As most NAFLD patients in primary care settings have simple steatosis (NAFL), and are not at increased risk for liver-related morbidity, it is extremely important to provide physicians who see NAFLD patients in primary care settings and diabetes clinics with tools to identify patients at high liver-related risk, who will benefit from specialist care. The implementation of predictive models for risk stratification may change the landscape of early detection in non-specialist clinical settings. From a public health perspective, primary prevention policies and actions may be implemented for subjects categorized as being at high risk according to these models. Some of the tools incur minimal additional costs, being based on readily available lab tests, and can be calculated automatically in computerized medical systems. Their availability can be harnessed as a tool to increase awareness among general practitioners, diabetologists and the public to promote early diagnosis and appropriate management. Although, as for now, there are no approved drugs for NASH, and treatment focuses mainly on lifestyle modification, awareness may increase motivation among patients to improve their lifestyle, and intensify monitoring for liver-related (e.g. occult cirrhosis, hepatocellular carcinoma) and cardiovascular risks by physicians following the patient. With the advent of pharmaceutical treatments for NASH, which are expected in the near future, low cost, readily available prediction models will assist in identifying suitable patients for treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is emerging as the most common chronic liver disorder, affecting approximately 25% of the population globally. The association of NAFLD with metabolic morbidity and cardiovascular disease (CVD), as well as its association with significant liver-related morbidity and mortality, create important challenges for primary care physicians in relation to the prevention, diagnosis and treatment of NAFLD and its associated risks for CVD and liver-related morbidity [1]. The importance of establishing a policy in regards to early detection of NAFLD and advanced fibrosis in primary care settings or diabetes clinics, among people with obesity, diabetes or the metabolic syndrome, who represent high-risk populations for the more advanced forms of NAFLD, is increasingly recognized. As most NAFLD patients in primary care settings have simple steatosis (NAFL), and are not at increased risk for liver-related morbidity, it is extremely important to provide physicians who see NAFLD patients in primary care settings and diabetes clinics with tools to identify patients at high liver-related risk, who will benefit from specialist care. The implementation of predictive models for risk stratification may change the landscape of early detection in non-specialist clinical settings. From a public health perspective, primary prevention policies and actions may be implemented for subjects categorized as being at high risk according to these models. Some of the tools incur minimal additional costs, being based on readily available lab tests, and can be calculated automatically in computerized medical systems. Their availability can be harnessed as a tool to increase awareness among general practitioners, diabetologists and the public to promote early diagnosis and appropriate management. Although, as for now, there are no approved drugs for NASH, and treatment focuses mainly on lifestyle modification, awareness may increase motivation among patients to improve their lifestyle, and intensify monitoring for liver-related (e.g. occult cirrhosis, hepatocellular carcinoma) and cardiovascular risks by physicians following the patient. With the advent of pharmaceutical treatments for NASH, which are expected in the near future, low cost, readily available prediction models will assist in identifying suitable patients for treatment.
The Epidemiology of NAFLD
NAFLD encompasses two pathologically distinct conditions with different prognoses: non-alcoholic fatty liver (NAFL) which is pure steatosis with or without mild lobular inflammation, and non-alcoholic steatohepatitis (NASH), that can progress to liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC). Approximately 20% of NAFL patients progress to NASH with an average progression rate of 11% during a 15-year period; the fibrosis progression rate is highly variable and influenced, to a large extent, by metabolic risk factors. A meta-analytic assessment of the global epidemiology of NAFLD estimates that 40% of NASH patients progress to fibrosis with an annual fibrosis progression rate of 0.09%. The proportion of NASH among the NAFLD population is estimated to increase in the coming decades due to an aging population and the rising prevalence of type-2 diabetes mellitus (T2DM) and obesity [1,2,3]. NASH has been recognized as one of the leading causes of cirrhosis in adults in the United States, and NASH-related cirrhosis is currently the second indication for liver transplants in the United States [4].
Within the NAFLD spectrum, the prognosis of NAFL differs significantly from that of NASH, with no increase in liver-related mortality and minimal risk for disease progression. However, NASH with fibrosis progresses at a faster rate than NASH without fibrosis, with high risk of developing HCC, liver failure, and death [5, 6]. Although evidence clearly supports the development of HCC in patients with NASH-cirrhosis, data now suggest that HCC can also occur in NASH patients without advanced fibrosis. It is estimated that the yearly incidence of HCC among NASH-cirrhotic patients is 2–3% and that the annual incidence of HCC in NAFLD patients is 0.44 per 1000 person-years [1, 2].
The prevalence of NAFLD is increasing worldwide. A meta-analytic assessment of the global epidemiology of NAFLD estimates that the global prevalence of NAFLD is ~25% with the highest prevalence in the Middle East and South America, and the lowest prevalence in Africa. It is the most common liver disease in Western countries, affecting 17–46% of adults, with differences in prevalence values stemming from variability related to diagnostic method, age, sex and ethnicity [7].
The prevalence of NAFLD is influenced by the diagnostic modality. Use of elevated liver enzymes as the primary diagnostic tool significantly underestimates the prevalence of NAFLD compared to abdominal ultrasonography (AUS) and liver biopsy. It has been shown that aminotransferase levels may be only mildly elevated in NAFLD, and that more than 50% of NAFLD patients may have normal liver enzymes. In a prospective cohort study, in which NAFLD diagnosis was based on ultrasound and liver biopsy, the prevalence of NAFLD and NASH in asymptomatic middle-aged patients in the United States was found to be 46% and 12.2%, respectively, compared to 13% for an elevated aminotransferase-based NAFLD diagnosis [8].
The age and gender distributions of NAFLD vary. It is assumed that the overall prevalence of NAFLD is approximately 30–40% in men and 15–20% in women, with prevalence rates increasing with age and the presence of T2DM [9]. Furthermore in regards to age, Kohler et al. found that advancing age is associated with clinically relevant liver fibrosis in patients with NASH, in the presence or absence of T2DM [10].
An ethnic variation in the distribution of NAFLD has also been suggested; in the US, Hispanics have the highest prevalence (45–58%), followed by whites (33–44%) and blacks (24–35%). These variations are probably secondary to lifestyle and genetic predisposition, as Hispanics with NASH tend to be younger, less active, and with unhealthy dietary habits [2, 11]. There are distinct phenotypes of NAFLD in different regions of the world, secondary to complex interactions between genetics, diet, the microbiome, and other environmental influences on the development of NAFLD. Previous studies showed that patients with NAFLD from East Asia have lower BMI and higher T2DM rates than patients in the West. This “Asian paradox” may be secondary to distinct genetic or environmental susceptibility to NAFLD, that differs from that of individuals in the West [12].
Obesity, T2DM and the metabolic syndrome are consistently identified as the most important risk factors for NAFLD, fibrosis and CVD. The prevalence of NAFLD in patients with T2DM is 40–70%. Kohler et al. have found a strong association between increased liver stiffness and presence of T2DM and/or insulin resistance, suggesting that having T2DM, especially in the presence of NASH, may result in an increased risk of clinically relevant fibrosis, cirrhosis, and mortality [10].
The prevalence of NAFLD in obese or morbidly obese patients is 75–92%, and over 90% of morbidly obese patients undergoing bariatric surgery have NAFLD [13]. According to a meta-analytic assessment of the global epidemiology of NAFLD, the pooled overall obesity prevalence among NAFLD and NASH patients is ~ 50% and ~80% respectively, with the highest rates in North America (86%) [1].
NAFLD has been referred to as the hepatic manifestation of the metabolic syndrome. It is increasingly viewed as an independent contributor to cardiovascular risk, via insulin resistance, oxidative stress, worsening inflammatory state and endothelial dysfunction [1], that accelerate the development and progression of atherosclerosis and arterial stiffness. Multiple epidemiological studies have linked NAFLD to increased CVD, concluding that, although the primary liver pathology in NAFLD involves morbidity and mortality from cirrhosis, liver failure and hepatocellular carcinoma, the majority of deaths among NAFLD patients are attributable to CVD [9]. In fact, death from cardiovascular causes in patients with NAFLD is twofolds higher than death related to liver disease [14].
To summarize, ~25% of the adults in the developed world have NAFLD, with a large proportion of these patients having T2DM and obesity, that increase the risk for progression to NASH, cirrhosis and HCC, and liver-related mortality. Alongside the liver-related risk, NAFLD is increasingly recognized as an independent risk factor for cardiovascular disease, and most patients with NAFLD die from CVD. The increasing prevalence of T2DM and obesity, in conjunction with aging of the population, calls for screening and early treatment strategies to prevent potentially life-threatening hepatic and cardiovascular complications.
The Approach to Diagnosing NAFLD in Primary Care Settings and Risk Stratification
In primary care settings, patients with NAFLD are usually diagnosed for one or more of the following reasons: (1) in the framework of an investigation for elevation of liver enzymes (2) due to evidence of a fatty liver in AUS undertaken for another reason (3) as part of case finding. Patients in the last two categories may not have elevated liver enzymes.
Clinical assessment involves three practical steps:
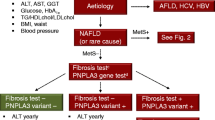
The first step is to identify patients who should be screened for NAFLD; the second step is to diagnose NAFLD, and to rule out etiologies other than NAFLD for liver fat accumulation or elevated liver enzymes; the third step, risk stratification, involves identification of patients who are at risk of fibrosis and liver related outcomes, as advanced liver fibrosis is associated with increased overall mortality and liver related events [15], and referral of these higher-risk patients to specialist care in hepatology clinics.
Step 1: Who Should Be Screened for NAFLD?
There is no universal directive for systematic screening of the general population for NAFLD for the following reasons: (1) Although NAFLD is a common disease, the prevalence of severe complications in the general population is low; (2) There are currently no approved drug therapies. (3) Lack of large scale cost effectiveness analysis. Compared to the low prevalence of severe complications in the general population, the prevalence/incidence of fibrosis and HCC increases in diabetic and obese persons. Thus, there is a consensus among some of the international professional associations to screen obese and T2DM patients for NAFLD [16,17,18,19]. The European Association for the Study of the Liver (EASL) also recommends screening patients with features of the metabolic syndrome by liver enzymes and or AUS. It is emphasized that the presence of NAFLD should be assessed in these patients irrespective of the liver enzymes level, since T2DM patients are at high risk of disease progression [16]. In contrast, the American Association for the Study of Liver Diseases (AASLD) and the Italian Association for the Study of the Liver (AISF) do not recommend screening for fatty liver [20, 21]. Nonetheless, the AASLD recommends a high index of suspicion for NAFLD and NASH in patients with T2DM (Table 2.1).
Step 2: Diagnosing NAFLD
NAFLD is usually detected either by investigation of abnormal liver enzymes or following incidental detection of hepatic steatosis on AUS. Detection of these patients may also occur in the framework of screening programs for NAFLD in high risk patients. In all cases, it is important to rule out other liver diseases, including alcoholic liver disease (ALD), viral hepatitis, drug related, autoimmune or metabolic disease, that may cause steatosis or elevation of liver enzymes.
Standard Blood Tests
Elevated liver enzymes are the most common blood test abnormality to trigger an investigation for NAFLD, but have important drawbacks. It is estimated that liver enzymes may be normal in up to 80% of NAFLD patients [11, 23]. When liver enzymes are elevated, the aberration is commonly a slight to modest elevation of alanine transaminase (ALT) and aspartate transaminase (AST). AST and ALT levels are usually between ×2–5 the upper limit of normal (levels above 300 IU/L are rare), with an AST:ALT ratio <1 [24]. An AST: ALT ratio >2 increases the likelihood of alcoholic liver disease, and this likelihood increases further if the ratio exceeds 3. Gamma glutamyl transferase (GGT) may represent a complementary test to identify patterns of alcoholism or alcohol abuse, but GGT by itself is not helpful in establishing a diagnosis of alcoholic liver disease [24]. Elevated carbohydrate deficient transferrin (CDT) and high MCV may also imply chronic alcohol consumption. The sensitivity for detection of daily ethanol consumption of more than 50 g is 69% for CDT and 73% for GGT. The specificity is 92% for CDT and 75% for GGT, respectively [25]. An AST/ALT ratio >1 is also characteristic of cirrhosis (of any etiology) [24].
Although many individuals with NAFLD, suggested by hepatic steatosis on imaging, may have normal liver enzymes, the presence of abnormal liver enzymes signals a higher likelihood for NASH with or without fibrosis, and warrants further clinical evaluation [24]. It is important to note that the degree of aminotransferase elevation does not predict the extent of hepatic injury, and having normal liver enzymes is not synonymous to the absence of steatosis or fibrosis in adults as well as in pediatric NAFLD [23, 26,27,28]. On the other hand, NASH resolution following weight reduction by lifestyle intervention was demonstrated to be strongly related to normalization of ALT (≤19 in females or ≤30 in males) [29]; leading to the development of a NASH resolution calculator (http://www.aeeh.es/calculadora-nashres/), in which normalization of ALT is an item [30].
Another marker of liver damage is serum ferritin. Elevation of serum ferritin levels is common in NAFLD patients, and usually indicates disease progression. There is evidence that serum ferritin greater than 1.5 times the upper normal limit is associated with the diagnosis of NASH and advanced hepatic fibrosis in both males and females [31,32,33,34]. Notably, in patients with high serum ferritin and increased iron saturation, the AASLD recommends exclusion of hemochromatosis [20].
Diagnostic Modalities for Steatosis and NASH
A number of diagnostic radiological imaging modalities can confirm the presence of hepatic steatosis—AUS, CT, MRI, and FibroScan Controlled Attenuation Parameter (CAP). Use of AUS is the most commonly used first-line imaging modality to assess for suspected NAFLD. Its main advantages are low cost and broad availability, but it has limited sensitivity in morbidly obese patients, and in the presence of less than 20% steatosis (assessed by liver biopsy) [16, 20].
Some serum markers can also detect steatosis, but with limited validity. Such markers are usually used for large scale screening studies and not in the setting of primary care clinics. Better validated steatosis scores include the Fatty Liver Index (FLI) [35], the lipid accumulation product (LAP) [36] and the Steatotest [37] (costly and not available in clinical practice). The FLI takes into account BMI, waist circumference, triglycerides and GGT levels (a free web-based calculator is available). A score ≤ 30 has a sensitivity of 87%; and a score ≥ 60 has a specificity of 86% for detection of hepatic steatosis [35].
Steatohepatitis is a histological diagnosis, defined as the combined presence of steatosis (>5% of hepatocytes), inflammation and hepatocyte injury (ballooning). At present, there are no well-established biomarkers to distinguish NASH from NAFL. Circulating levels of keratin 18 fragments (CK-18), which are released from apoptotic or dead cells, have been extensively investigated. However, CK18 has limited validity as a screening test for NASH and is currently not available in clinical care settings [38, 39].
Step 3: Risk Stratification of NAFLD Patients:
Liver fibrosis is the only parameter that was found to be correlated with liver-related morbidity, liver transplantation and liver-related mortality in patients with NAFLD. Therefore, risk stratification, based on the presence or absence of advanced fibrosis, is recommended in this patient population [40,41,42]. Patients with advanced fibrosis or cirrhosis (Metavir stages F3 or F4, respectively) are at risk for clinically significant liver outcomes (i.e. complications of cirrhosis, need for liver transplantation or liver-related death), and should be referred to specialist care for early detection and management of cirrhosis and its complications. Patients with no fibrosis or minimal fibrosis (Metavir stages F0 or F1, respectively) are considered to be at low risk for liver-related outcomes, and can be followed in primary care settings, with periodic reassessments [16, 42].
A number of clinical factors may suggest an increased risk for advanced liver fibrosis, including age ≥50, male gender, alcohol consumption, severe obesity, and presence of the metabolic syndrome (the risk for advanced fibrosis increases with increasing metabolic burden), elevated transaminases (≥×2 upper limit of normal), and an elevated ferritin level [20, 31, 43,44,45,46,47]. T2DM is also associated with more severe manifestations of NAFLD, including advanced fibrosis, cirrhosis and HCC [48]. Thus, the pre-test probability of advanced fibrosis in an obese 55 year-old patient with T2DM and other features of the metabolic syndrome is significantly higher than that in a young, overweight patient without these comorbidities. This should be taken into account in risk assessment, as NAFLD patients with multiple clinical risk factors may benefit from early referral to specialist care [49]. Finally, patients in whom there is suspicion for cirrhosis (e.g. compatible physical examination findings, AST/ALT >1 in the absence of alcohol consumption, splenomegaly, thrombocytopenia) should be referred promptly for further evaluation and management in a liver clinic.
While liver biopsy remains the gold standard for diagnosing NASH and fibrosis in patients with NAFLD [20], it is not feasible or justified in all NAFLD patients. Consequently, a number of non-invasive measures have been developed, that aid in classification of patients with NAFLD into those who are, or are not, at increased risk for advanced fibrosis [50,51,52,53,54]. The use of non-invasive tools to assess liver fibrosis for initial risk stratification in clinical settings is endorsed by professional societies [16] and is becoming widespread, while liver biopsies are increasingly reserved for situations in which: (a) a diagnostic question remains as to whether the patient has NAFLD or another liver disorder—for example, in patients with significant liver enzyme elevations or high titres of autoimmune antibodies, patients without metabolic syndrome, etc. Notably, given the high prevalence of NAFLD, it is not uncommon for patients with other liver disorders to also have NAFLD. (b) To accurately establish or confirm the degree of histological damage to the liver, particularly in subjects who are suspected to have advanced fibrosis or cirrhosis based on high-risk clinical features [49], or suggestion of advanced fibrosis by non-invasive tests.
Currently available, commonly used non-invasive tools to classify NAFLD patients into those who are at high versus low risk for advanced fibrosis include laboratory test-based risk scores (e.g. Fibrosis 4 (FIB4); NAFLD fibrosis score (NFS) or the Enhanced Liver Fibrosis (ELF) panel), and imaging modalities (e.g. Fibroscan—Vibration Controlled Transient Elastography (VCTE)—the most widespread and studied of these methods; Acoustic Radiation Force Impulse elastography, and Magnetic resonance elastography (MRE)). It is notable that all of these methods have been validated in comparison to liver biopsy as a gold standard, which has imperfect accuracy by itself (due to sampling error and intra- and inter-observer reliability), leading to an inherent bias in the performance accuracy of these non-invasive tests.
Non-invasive Assessment of Fibrosis
Laboratory Test-Based Risk Scores
A number of risk scores have been developed based on readily available clinical and laboratory parameters that are simple to use at point of care with the help of web-based calculators, and can be implemented into computerized medical systems. These include, among others, the APRI (AST to platelet ratio index) [55], BARD (BMI; AST/ALT ratio; diabetes) score [56], FIB4 (Age; AST; ALT; platelets) and NFS (Age; BMI; AST; ALT; albumin; impaired fasting glucose/diabetes) scores. Of these, FIB4 and NFS have been most extensively studied and validated in diverse populations, and shown to predict overall mortality, cardiovascular mortality and liver-related mortality in patients with NAFLD [16]. FIB4 and the NFS are currently recommended for the initial assessment of subjects with NAFLD and metabolic risk factors [16, 20, 42], who are older than 35 years of age (alternative modalities for fibrosis assessment are recommended in younger patients) [57]. FIB4, which is calculated as Age × AST (IU/L)/platelet count (×109/L) × √ALT (IU/L), has an area under the receiver operating characteristic curve (AUROC) >0.8 for detection of stage F3 or F4 fibrosis. The NFS is calculated as: −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glycemia (IFG) or diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet (×109/l) − 0.66 × albumin (g/dL); an AUROC 0.85 for detection of advanced fibrosis was reported for the NFS in a meta-analysis of 13 studies, that included 3064 patients with NAFLD [14]. Commercial laboratory test-based risk scores for fibrosis include such tests as the proprietary ELF® panel, Fibrotest®, FibroMeter® and Hepascore® [52, 58]. In contrast to the FIB4 and NFS, that incur minimal additional costs, being based on routine lab tests that have often already been done in the patient, these tests are proprietary algorithms that include non-routinely tested parameters (e.g. for Fibrotest®: alpha 2 macroglobulin, haptoglobin and apolipoprotein A1 [59]; for ELF®: procollagen III amino terminal peptide, hyaluronic acid and tissue inhibitor of matrix metalloproteinase-1, which are direct markers of fibrosis [60, 61]), carry additional costs, and depend on local availability. For Fibrotest®, which has been validated in NAFLD as well as other common liver diseases, a mean standardized AUROC for advanced fibrosis of 0.84 (95% CI 0.76–0.92) has been reported. The AUROC, sensitivity and specificity of the ELF panel have been reported to be 0.90, 0.80 and 0.90, respectively, for identifying advanced fibrosis [62]. The test has been approved for commercial use in Europe, and has been recommended as a test of choice for ruling out advanced fibrosis in NAFLD patients in a 2016 National Institute for Health and Care Excellence (NICE) guideline [17].
For the FIB4 and the NAFLD fibrosis score, two cut-off values are defined. Patients who score lower than the lower cut-off value (FIB4 score ≤ 1.3; NFS ≤ −1.455) [42, 57] can be regarded as having a low risk for advanced fibrosis, and do not need to be referred to a hepatology clinic at that point in time (in NAFLD patients ≥65 years of age, recommended lower risk cut-offs are <2.0 and <0.12 for the FIB4 and NAFLD fibrosis scores, respectively [57]). According to the current EASL recommendations, such patients should be non-invasively re-assessed after 2 years. In patients whose score is higher than the higher cut-off value (FIB4 score > 2.67; NFS >0.675), there is suspicion of advanced fibrosis or cirrhosis, and these patients should be referred to a hepatology clinic for further assessment. Thirty to fifty percent of patients have an indeterminate score, and in these cases, additional testing is needed (e.g. by the ELF panel or VCTE). Although 2 cut-off values have also been defined for the ELF panel, the NICE guideline refers to a single cut-off value (10.51) [In recently published guidelines from the British Society of Gastroenterology, the recommended cut-off is 9.5 [42].] for prediction of advanced fibrosis. According to the guideline, adult subjects with an ELF panel score below this cut-off can be reassured that they do not have advanced fibrosis, and should be followed up by an additional ELF test after 3 years [17].
Generalizability to Primary Care Settings
Most of the clinical and laboratory parameter-based risk scores have been developed and validated in specific patient populations attending liver clinics; this should be kept in mind when considering their widespread use in different patient populations (e.g. patients with diabetes, elderly patients, patients attending primary care clinics [63]). In line with this, questions have been raised regarding the generalizability of current cut-offs for all NAFLD patients. For example, it has been shown that the performance of FIB4 and NFS may differ with age [57], that the performance of APRI, BARD, FIB4 and NFS may differ with the degree of steatosis [64], that the performance of ELF may differ with age [65, 66] or gender [65] and that biomarker panels for the diagnosis of NAFLD, NASH and advanced fibrosis (SteatoTest, ActiTest, NashTest and FibroTest) may underperform in patients with T2DM [67]. As the prevalence of NAFLD among patients with T2DM is high, and diabetes has been repeatedly shown to be a key predictor for advanced fibrosis, it is very important that non-invasive risk scores would be applicable to this patient population [10, 68, 69]. It was recently suggested that the frequency of indeterminate or high scores of fibrosis is higher in patients with T2DM. In a cross-sectional analysis of a study involving higher-risk patients with obesity, metabolic syndrome or diabetes, more than 84% of patients had indeterminate or high NFS or FIB4 scores, requiring further assessment [66]. A clinical model based on routinely available clinical and metabolic biochemical factors has been developed specifically for patients with T2DM, to determine the likelihood of NASH (AUROC 0.8) and advanced fibrosis (AUROC 0.8). The sensitivity and specificity for both NASH and advanced fibrosis was 57% and 90%, respectively. However, the main limitation of this tool was high percent of gray zone; 44% of patients could not be classified for NASH and 87% could not be classified for advanced fibrosis [70].
Liver Elastography-Based Assessment
Several liver elastography devices have been evaluated in cohorts of patients with NAFLD: vibration-controlled transient elastography (VCTE), shear-wave elastography (SWE), acoustic radiation force impulse (ARFI) imaging and magnetic resonance elastography (MRE). Fibrosis assessment is expressed as liver stiffness measure (LSM), measured in kilo-pascals (KPa); notably, the LSM ranges and cut-offs are different for the different modalities, and cannot be directly compared. Choice depends largely on availability and cost considerations. Advantages of SWE and ARFI include the combination of conventional ultrasound with liver stiffness measurements, allowing focus on an anatomic region of interest [71], and the capability to obtain liver stiffness values in patients with ascites [72, 73]. MRE may be more accurate than ultrasound-based modalities, has a lower risk of failure in patients with severe obesity, and measures a larger area of the liver, which may reduce sampling variability secondary to heterogeneity of fibrosis [74]. In a cross-sectional study of more than 100 patients, MRE was found to be more accurate than VCTE in identification of liver fibrosis (stage 1 or more), using biopsy analysis as the standard [75]. However, at present, MRE is expensive and not widely available in most geographies, and is used mostly in the setting of clinical trials. Currently, VCTE is the most common and widely clinically available diagnostic modality [76,77,78,79]. VCTE is performed using a Fibroscan® device (Echosens. Paris, France). It has been validated and found to be accurate across a wide spectrum of chronic liver disorders, and has important advantages, including: (1) It can be done at the point of care; (2) It is simple to learn; (3) It is well tolerated by patients; (4) Exam duration is short; (5) It assesses a liver volume that is ×100–200 greater than that assessed by a liver biopsy; and (6) There are standardized quality criteria [an adequate VCTE examination includes ten valid shots (>60% success rate), with an interquartile range (IQR)-to-median LSM ratio of ≤0.3). In a meta-analysis of nine studies on VCTE, that included 1047 NAFLD patients, accuracy was moderate for F ≥ 2 (sensitivity and specificity 79% and 75%, respectively), and very good for F3 and F4 [sensitivity and specificity both 85% for F3; sensitivity and specificity both 92% for F4 [79]; AUROC 0.83 the prediction of F3/F4 fibrosis] [53]. Another advantage of VCTE is a simultaneous measurement of the controlled attenuation parameter (CAP) score that provides a quantitative assessment of hepatic steatosis [77]. Limitations of VCTE include high failure rates in patients with a narrow intercostal space or ascites, interference of liver stiffness measurements by extrahepatic cholestasis, elevated central venous pressure, post-prandial hepatic hyperaemia (patients should fast for 2–3 h) or acute liver injury, and reduced reproducibility in early stages of fibrosis and in the presence of steatosis [75, 80,81,82]. Notably, a relatively high failure rate that was reported for this modality in the past, especially in obese patients, has largely been overcome with the introduction of an additional probe (the XL probe), for use in subjects with BMI ≥30 kg/m2 [76].
Approach to the Use of Non-invasive Fibrosis Assessment in Patients with NAFLD in Primary Care Settings
Recent guidelines recommend that calculation of FIB4 or NFS be the first step in risk assessment of NAFLD patients, to be done in primary care settings in which subjects with NAFLD are routinely followed. In fact, recent British Society for Gastroenterology guidelines recommend incorporation of calculation of FIB4 and NFS in all primary care computer systems [42]. Of interest in this regard, in health-care records for 17.7 million adults from four large European primary-health-care databases, in which the FIB-4 could be calculated in 40.6% of patients, 1/3 had intermediate or high-risk scores [83]. According to current EASL guidelines, for subjects without liver enzyme elevation and with FIB4 or NFS scores consistent with a low risk for advanced fibrosis, follow-up should be by repetition of liver enzyme tests and FIB4 or NFS scores after 2 years [16]. Recent guidelines from the UK recommend repeat assessment after 2–5 years, depending on clinical risk [42]. Subjects whose liver enzymes are above the upper limit of normal or FIB4 or NFS scores above the higher cut-off should be referred to a hepatology clinic for further assessment. Subjects with indeterminate NFS or FIB4 scores can be referred to a second tier non-invasive assessment, such as VCTE or ELF; this approach is supported by studies that showed that combinations of non-invasive tests increase accuracy of prediction [16, 42, 74, 84, 85].
In subjects in whom the second non-invasive test indicates a low risk for advanced fibrosis, recommended follow-up is similar to that in subjects who were assessed as having a low risk for advanced fibrosis in the initial non-invasive test (FIB4 or NFS). The approach to NAFLD patients in whom a non-invasive test reveals advanced fibrosis is individualized, and adjusted to the subject’s clinical features. When the initial test indicating a risk for advanced fibrosis is the FIB4 or NFS score, a second tier non-invasive test (e.g. VCTE) is sometimes done. However, in view of suboptimal specificity and positive predictive value (PPV) of all non-invasive modalities, and taking into account the significance of cirrhosis diagnosis for the individual patient, the impact of this diagnosis on the use of healthcare resources and additional information that can be obtained from a liver biopsy, a permissive approach to referring such patients to a liver biopsy is usually practiced [42, 85].
Future Perspectives
There is an unmet need for additional non-invasive tools, that is likely to increase in the foreseeable future, with the advent of new therapies for non-alcoholic steatohepatitis [86]. The target population for such interventions, as reflected by recent guidance documents from the US Food and Drug Administration (FDA) [87] and European Medicines Agency (EMA) [88], is of patients who are at risk of cirrhosis, defined histologically as NAFLD activity score (NAS) ≥4 and F ≥ 2. Current non-invasive measures are not useful for detection of this population, and research is ongoing to develop non-invasive tools that would enable identification of relevant patients without a liver biopsy. The NIS4 algorithm, that is being commercially developed by Genfit and is based on four parameters (Alpha 2 macroglobulin, miR-34a, YKL-40 and Hemoglobin A1c) [89], and the FS3 [90] algorithm, that is based on fibroscan assessment (CAP and LSM scores) combined with AST, hold promise to meet this end. Another important area of unmet need pertains to accurate non-invasive follow-up of fibrosis in NAFLD patients over time. Current guidelines recommend periodic re-assessment of liver fibrosis by the available non-invasive tools (FIB4, NFS [16, 20, 42] or ELF). Re-assessments may indicate progression of fibrosis, as suggested by a recent study in which APRI, FIB4 and NFS could detect progression to severe fibrosis with a C statistic of 0.82, 0.81 and 0.80 respectively [91]. Non-invasive tools are sought that can more reliably differentiate between fibrosis stages as a continuum; in addition to indicating disease progression, such tools may be useful to monitor the therapeutic benefit of new treatments for NASH.
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–58.
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904.
Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55.
Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54. e1–9; quiz e39–40.
Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, Gill PS, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234–40.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85.
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31.
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–64.
Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, Leebeek FW, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63:138–47.
Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95.
Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90.
Singh S, Osna NA, Kharbanda KK. Treatment options for alcoholic and non-alcoholic fatty liver disease: a review. World J Gastroenterol: WJG. 2017;23:6549–70.
Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49.
Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65.
European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
National Institute for Health and Care Excellence (NICE) UK Guideline. Non-alcoholic fatty liver disease (NAFLD): assessment and management 2016. ISBN-13: 978-1-4731-1996-3.
European Association for Study of L, Asociacion Latinoamericana para el Estudio del H. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64.
Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, Fan J, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57.
Italian Association for the Study of the L. AISF position paper on nonalcoholic fatty liver disease (NAFLD): updates and future directions. Dig Liver Dis. 2017;49:471–83.
Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroenterol: WJG. 2018;24:3361–73.
Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26:856–63.
Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69:154–81.
Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–92.
Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33:1398–405.
Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100:2231–8.
Vilar-Gomez E, Yasells-Garcia A, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Development and validation of a noninvasive prediction model for nonalcoholic steatohepatitis resolution after lifestyle intervention. Hepatology. 2016;63:1875–87.
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90.
Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85.
Du SX, Lu LL, Geng N, Victor DW, Chen LZ, Wang C, Yue HY, et al. Association of serum ferritin with non-alcoholic fatty liver disease: a meta-analysis. Lipids Health Dis. 2017;16:228.
Buzzetti E, Petta S, Manuguerra R, Luong TV, Cabibi D, Corradini E, Craxi A, et al. Evaluating the association of serum ferritin and hepatic iron with disease severity in non-alcoholic fatty liver disease. Liver Int. 2019;39:1325–34.
Yoneda M, Nozaki Y, Endo H, Mawatari H, Iida H, Fujita K, Yoneda K, et al. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig Dis Sci. 2010;55:808–14.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010;10:98.
Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10.
Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–8.
Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, Ortiz-Lopez C, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167–74.
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97. e10.
Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54.
Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, Hall R, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6–19.
Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–57. e17.
Patel YA, Gifford EJ, Glass LM, McNeil R, Turner MJ, Han B, Provenzale D, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47:268–78.
Ekstedt M, Franzen LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, Kechagias S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366–74.
Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, Merriman R, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644–54.
Harris R, Harman DJ, Card TR, Aithal GP, Guha IN. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol. 2017;2:288–97.
Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224–9, 29 e1–2.
Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196–205.
Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–501.
Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349–60.
Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570–8.
Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri BA, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–63. e2.
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305–15.
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26.
Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–7.
McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–51.
Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, Tahiri M, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6.
Poynard T, Munteanu M, Charlotte F, Perazzo H, Ngo Y, Deckmyn O, Pais R, et al. Diagnostic performance of a new noninvasive test for nonalcoholic steatohepatitis using a simplified histological reference. Eur J Gastroenterol Hepatol. 2018;30:569–77.
Day JW, Rosenberg WM. The enhanced liver fibrosis (ELF) test in diagnosis and management of liver fibrosis. Br J Hosp Med (Lond). 2018;79:694–9.
Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–13.
Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, Kaye P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–60.
Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–81. e4.
Joo SK, Kim W, Kim D, Kim JH, Oh S, Lee KL, Chang MS, et al. Steatosis severity affects the diagnostic performances of noninvasive fibrosis tests in nonalcoholic fatty liver disease. Liver Int. 2018;38:331–41.
Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236–42.
Patel P, Hossain F, Horsfall LU, Banh X, Hayward KL, Williams S, Johnson T, et al. A pragmatic approach identifies a high rate of nonalcoholic fatty liver disease with advanced fibrosis in diabetes clinics and at-risk populations in primary care. Hepatol Commun. 2018;2:893–905.
Bril F, McPhaul MJ, Caulfield MP, Castille JM, Poynard T, Soldevila-Pico C, Clark VC, et al. Performance of the SteatoTest, ActiTest, NashTest and FibroTest in a multiethnic cohort of patients with type 2 diabetes mellitus. J Investig Med. 2019;67:303–11.
Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, Rhee EJ. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J. 2019;43:31–45.
Roulot D, Roudot-Thoraval F, NKontchou G, Kouacou N, Costes JL, Elourimi G, Le Clesiau H, et al. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int. 2017;37:1897–906.
Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care. 2015;38:1347–55.
Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138–47.
Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–9.
Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–27.
Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15:274–82.
Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152:598–607. e2.
Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, Neuschwander-Tetri BA, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–44.
Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–68.
Tapper EB, Castera L, Afdhal NH. FibroScan (vibration-controlled transient elastography): where does it stand in the United States practice. Clin Gastroenterol Hepatol. 2015;13:27–36.
Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, Chan HL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease—the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–69.
Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Buchler MW, Seitz HK, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–23.
Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–9.
Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206–10.
Alexander M, Loomis AK, Fairburn-Beech J, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16:130.
Petta S, Vanni E, Bugianesi E, Di Marco V, Camma C, Cabibi D, Mezzabotta L, et al. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2015;35:1566–73.
Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. 2018;3:509–17.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22.
U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER). Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment: Guidance for Industry. 2018
European Medicines Agency (EMA). Reflection paper on regulatory requirements for the development of medicinal products for chronic non-infectious liver diseases (PBC, PSC, NASH). EMA/CHMP/299976/2018.
Hanf R, Pierre C, Zouher M, Cordonnier G, Brozek J, Praca E, Sudrick FB, Bedossa P, Anstee Q, Francque S, Harrison S, Ratziu V, Megnien S, Roudot A, Hum D, Sanyal A. Validation of NIS4 algorithm for detection of NASH at risk of cirrhosis in 467 NAFLD patients prospectively screened for inclusion in the RESOLVE-IT trial. J Hepatol. 2018;68:S115–S16.
Sasso M, Allison M, Tsochatzis E, Anstee Q, Sheridan D, Guha IN, Cobbold J, Paradis V, Bedossa P, Miette V, Fournier C, Sandrin L, Newsome P. P01-08 Comparison of FS3 with different biomarkers to identify patients with active NASH (NAS≥4) and advandced fibrosis (F≥2). NAFLD Summit Abstract Book; 2018:18.
Siddiqui MS, Yamada G, Vuppalanchi R, Van Natta M, Loomba R, Guy C, Brandman D, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17(9):1877–85.e5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Margalit, M., Yeshua, H., Gotlieb, N., Zelber-Sagi, S. (2020). Detection of NAFLD/NASH in the General Population and in Primary Care Clinics. In: Romero-Gomez, M. (eds) NAFLD and NASH. Springer, Cham. https://doi.org/10.1007/978-3-030-37173-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-37173-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-37172-2
Online ISBN: 978-3-030-37173-9
eBook Packages: MedicineMedicine (R0)