Abstract
The combined coal gasification and flash ironmaking process (CG-FI) was the latest technology practice of the alternative ironmaking process, and it can also be recognized as a multi-generation system for high-quality syngas and reduced iron . The computational fluid dynamics (CFD) model was established to explore the process conditions based on the pilot-scale equipment. The turbulent flow, gas–particle coupling, and kinetic reaction model were considered in the model, which was already validated in the prior researches. In this study, the different material ratio combinations, including oxygen/coal ratio (0.6–0.8) and ore/coal ratio (0.2–1.6), were investigated to illuminate the interrelationship during the gasification–reduction coupling process . The results demonstrated that the qualified reduced iron (Reduction Degree = 52.47%) could be obtained even in the worst case (oxygen/coal ratio = 0.6, ore/coal ratio = 1.6). Moreover, the dispersed hematite particles replaced the role of H2O as the coolant and partial oxidant in the traditional coal gasification process to improve the gasification performance.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Predictive modeling
- Condition optimization
- Gasification–reduction coupling process
- Flash ironmaking
- Coal gasification
- CFD method
- Multi-generation system
Introduction
The long process with traditional blast furnace and converter is the most popular ironmaking process, which occupies more than 80–85% of the production in the world. However, it has been criticized for decades due to more and more serious environmental problems, especially in developing countries [1]. Therefore, the alternative ironmaking processes that do not rely on coke have been developed by both the experimental method and industrial practice [2, 3].
The combined coal gasification and flash ironmaking process (CG-FI) proposed was the innovative scheme to realize the industrialization of flash ironmaking process. The flash ironmaking is actually a kind of gas-based direct reduction process, proposed by Sohn [4]. However, the reduction time was expected to be reduced to several seconds under the extremely high temperature (>1473 K). Benefit from the short reaction time, the production efficiency was significantly improved, and the steep reduction degree of the ore can be easily obtained [5, 6]. Due to the rigorous high-temperature conditions, the traditional fluidized bed cannot be utilized due to the possible sticking phenomenon. Therefore, the industrial flash ironmaking equipment was designed based on the high-temperature flash furnace, which was already applied in the nonferrous metallurgical industry [7, 8].
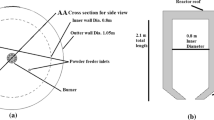
Different from the oxygen–hydrogen combustion in the laboratory-scale researches [9], the more mature coal gasification was adopted in the Outokumpu furnace to generate sufficient heat and provide reducing gas. The hematite concentrate replaced the copper concentrate to be injected into the shaft and expected to be reduced to metallic status rapidly. The qualified syngas and hot metal can be obtained separately in the uptake and settler, as shown in Fig. 1; hence, the process was also recognized as a promising multi-generation system .
However, the designed gasification–reduction coupling process faced the lack of relevant researches or experience. For example, the high-temperature coupling reaction mechanism was unclear; the flow characteristic in the shaft was unknown; the internal temperature distribution was invisible. Since the pilot-scale facility was still under construction, and the relevant questions were worthy of being studied, the numerical simulation was conducted firstly as a pre-design tool in the author’s previous researches. Based on the kinetic theory of the metallurgy and chemical industry, the complex reaction system was described by mathematical equations and coupled into the classical computational fluid dynamics (CFD) solution. The feasibility of basic cases had been established, and the characteristics were investigated. The predictive modeling was conducted in this research to explore the optimum condition (oxygen/coal ratio and ore/coal ratio) of the gasification–reduction coupling process , in which the variation rule of the product quality was our focus point. This application also examines the adaptability and robustness of the model for further improvement through the comparison between different cases.
Equations
Conservation Equations for Gas Flow and Particle Tracking
The continuous phase flow mixed with the sparse discrete particles was the classical CFD problem, which can be solved by the Eulerian–Lagrangian Method (ELM). The solution of continuous phase flow was composed of the mass conservation Eq. (2.1), momentum conservation Eq. (2.2), and energy conservation Eq. (2.3) as shown in Table 1. In addition, the other auxiliary modules, including the species transport model, radiation model, and boundary condition treatment, were coupled into the solution to enrich the details of the model.
For the discrete phase, the Lagrangian method calculates the velocity and position of particles with the time scale according to the force balance Eq. (2.4). The fundamental forces include gravity, buoyancy, and fluid drag in this study.
Theory of Coal Gasification/Flash Ironmaking
As a interdisciplinary product, the gasification–reduction coupling process conforms to the reported researches on the coal gasification and flash ironmaking . The description of the reaction mechanism and determination of kinetic parameters were important to establish the mathematical descriptions of the complex reactions, especially for the reacting flow CFD problem.
Generally, the coal gasification process can be divided into the devolatilization process, gas–solid heterogeneous reactions, and gas–phase homogeneous reactions. The volatile matter was recognized as the reductive gas mixture, including CO, H2, CH4, and C6H6, and the two-competing devolatilization model was adopted in this study. The relevant details were elaborated in the previous research on the coal gasification process, while the included reactions were listed in Table 2.
The flash ironmaking process was the latest result of the metallurgy, which may draw different conclusions from the previous studies. The hematite reduction was recognized undergoing a step-by-step process (Fe2O3–Fe3O4–FeO–Fe) in the gas-based direct reduction studies. However, the research on the flash ironmaking had proved that the multi-stage reduction process was not clear under the high-temperature (1373–1623 K) condition, and the single-step reduction (Fe2O3–Fe) was determined [6, 10]. The kinetic expressions of hematite and magnetite were determined by the experimental method, and the magnetite reduction expression was already coupled into the CFD model. In our research, the hematite reduction by H2 and CO was also investigated by the drop tube reactor (DTR) in the higher temperature range (1523–1823 K). The single-step kinetic expressions were coded as the user-defined function (UDF) into the CFD model to predict the reduction degree of the reduced ore. The experimental and calculated results were compared, and the certain credibility of the programmed kinetic expressions was demonstrated. The verified codes for the flash ironmaking were transplanted to the comprehensive model for the gasification–reduction coupling process
Test Case
Because of the high symmetry of the reaction shaft in the flash furnace, the model can be simplified to the 1/4 cylinder with sudden expansion structure, as shown in Fig. 2. According to the pre-check stage, grid independence had been proved, and the internal flow characteristic can be represented in the 1/4 grid.
Due to the maturity of CFD researches and functional adaptability of the classical algorithm, the commercial CFD software Ansys Fluent 16.0 was used to simulate the gasification –reduction coupling reacting flow. The user-defined function was also adopted to enhance the model. In this study, the different combinations of the material ratios (oxygen/coal ratio, ore/coal ratio) were investigated to figure out the variation rule of the product quality and explore the optimum condition. The part of basic operating conditions and model settings were listed in Table 3.
Results and Discussion
Thermodynamics Analysis of the Gasification–Reduction Coupling Process
The reducing gas components in the coal gas generated during the coal gasification process was mainly carbon monoxide and hydrogen , the ratio of which is about 3:1. Therefore, the gas-based reduction of the gas mixture was a competing/promoting reaction system between the carbon monoxide and hydrogen . The analysis was conducted firstly according to the thermodynamic data. Figure 3 shows that the equilibrium carbon monoxide proportion, in which the ore can be reduced to metallic iron , increased with the rising temperature , and the hydrogen proportion was the opposite. Therefore, the low utilization ratio of coal gas can be predictable during the high-temperature reduction process. However, the residual carbon can further react with the oxidation product during the reduction process to compensate for the decrease of the reducing gas, because the oxygen/coal ratio of CG-FI was relatively lower than coal gasification .
On the other hand, the low-utilized coal gas was also recognized as high-quality syngas that can be used to the synthesis industry after reforming. While the low calorific value blast furnace coal gas can only be used for combustion to recycle the energy. From this point of view, the combined coal gasification and flash ironmaking process is different from the previous gas-based direct reduction process and has the potential to improve the efficiency of the comprehensive utilization of material and energy.
Predicted Gas Components and Temperature
For the further application of the coal gas after the coupling process, the proportion of different components should be obtained for the reforming process. Figure 4 shows the influence of the different oxygen/coal ratios (0.6–0.8) on the composition of syngas under the low-ore/coal-ratio condition (ore/coal ratio = 0.4). With the increasing ore/coal ratio, the carbon monoxide proportion decreased rapidly from 72.67 to 59.94%, while the hydrogen proportion is kept at a specific value of around 25%. In addition to the deterioration of the effective-gas ratio (defined as \( {\varphi }_{\text{CO}} + {\varphi }_{{{\text{H}}_{2} }} \)), the critical index hydrogen /carbon ratio (defined as \( \left( {{\varphi }_{{{\text{H}}_{2} }} - {\varphi }_{{{\text{CO}}_{2} }} } \right)/\left( {{\varphi }_{\text{CO}} + {\varphi }_{{{\text{CO}}_{2} }} } \right) \)) decreased rapidly due to the rising carbon dioxide proportion, which was disadvantageous for the reforming process. Therefore, the relatively low oxygen/coal ratio was recognized to be better for the coupling process. It should be noticed that the hematite ore provides the oxygen element to gasify the residual carbon furtherly, and the carbon conversion rate increased to 99.9% even when the oxygen/coal ratio was 0.6. As a reference, the corresponding carbon conversion ratio in the separate gasification model was predicted to be 71.4%.
The cases with high ore/coal ratio were also compared, as shown in Fig. 5. The carbon monoxide proportion decreased from 64.26 to 33.85% continuously, while the hydrogen proportion shows a significant trend of dropping first and rising latterly. In addition to the fluctuation of hydrogen proportion, the deterioration of the effective-gas ratio and the hydrogen /carbon ratio were still observed in our model.
The conclusions can be safely drawn that the lower oxygen/coal ratio is advantageous for the improvement of the syngas quality. At the same time, the higher ore/coal ratio consumed more reductive components leading to the degraded syngas quality. However, the influence of adding oxidizing ore is significantly weaker than the direct addition of oxygen, which is due to the decreasing efficiency of ore reduction process under the high-ore/coal-ratio condition. For example, the carbon monoxide proportion in Figs. 4a and 5a show the minor variation (72.67–64.26%) with the significantly increased ore/coal ratio from 0.4 to 1.6, while the similar variation can be observed with different oxygen/coal ratio from 0.6 to 0.7 in Fig. 4b.
The utilization ratios of the reductive components with complete condition combinations were illustrated in Fig. 6. Both carbon monoxide and hydrogen utilization ratio show an upward trend with the increasing ore/coal ratio on the whole. However, the utilization ratio of CO in the high-oxygen-ratio interval (1.0–1.6) decreased. The reasonable explanation was that the reaction rate of hydrogen reduction was faster than that of carbon monoxide reduction . In addition, the reduction efficiency also reduced with increasing hematite feed, and the utilization ratio decreased eventually with higher ore/coal ratio. The realization of the flash reduction process depended on the reaction temperature , which influenced the reaction rate significantly.
However, the utilization ratio with different cases shows an unusual rule that the hydrogen utilization ratio almost kept zero in Fig. 6c. The kinetic parameter of the water gas shift reaction (WGSR) was borrowed from the coal gasification research, while the temperature should have a range of application. The equilibrium temperature of cases with high oxygen/coal ratio in our model may be beyond the reasonable scope of temperature , and the exaggerated reaction ratio of WGSR made the carbon monoxide consume the generated steam rapidly. The unreasonable utilization ratio variation was attributed to this distortion. The reverse water gas shift reaction (rWGSR) was included to offset the too fast reaction rate at the high-temperature condition to make up for this distortion in the next version model.
Figure 7 shows the exhaust temperature of all combinations of conditions that represent the temperature level in the reaction shaft to a certain extent. The significant positive proportional relationship of the oxygen/coal ratio on the temperature was observed, while the ore/coal ratio shows the negative influence. The excessive oxygen combusted the reductive gas and released a large amount of the chemical energy, which was essential for the ore reduction . The cold ore particles absorbed much heat to reach the flash reduction temperature range (1473–1873 K). It should be noticed that the reduction of hematite by hydrogen (Fe2O3 + H2) was an endothermic reaction. However, the carbon monoxide reduction (Fe2O3 + CO) was an exothermic process, that was also a reasonable explanation of the flatter temperature variation in the high-oxygen/coal-ratio cases.
Predicted Reduction Degree of Hematite
For the two-step smelting reduction processes, the corrosive ferrous oxide caused the severe erosion of the refractory brick. Therefore, a large amount of pulverized coal or even coke had to be sprayed into the settler to avoid corrosion . However, the CG-FI process was expected to reduce the hematite to a certain degree in the reaction shaft to avoid erosion of ferrous oxides.
Figure 8 shows the predicted reduction degree in all of the material ratio combinations, and the corresponding effective-gas ratio was also presented. In the low-oxygen/coal-ratio cases, the reduction degree decreased continuously. The worst reduction degree was obtained at the trial (oxygen/coal ratio = 0.6, ore/coal ratio = 1.6) as low as 52.47%. With the increasing oxygen/coal ratio, the reduction degree increased significantly and kept 100% in the low-ore/coal-ratio cases. The reduced reduction degree should mainly attribute to the decreasing temperature , as shown in Fig. 7, which leads to the lower reduction efficiency of flash ironmaking .
The consistent and credible trends were observed between the cases with ore/coal ratio = 0.6 and 0.7 according to the quality variation curves. However, the cases with oxygen/coal ratio were already beyond the reasonable reduction temperature range (1473–1873 K). According to the mentioned reason, the variation curved may exaggerate the reduction efficiency due to the too fast WGSR.
Conclusion
The numerical model was established based on the gasification–reduction coupling process , and the predictive modeling was conducted to investigate the cases with different material ratio combinations. The variation rule of the product quality was explored, and optimum conditions were determined. The conclusions were listed as follows:
-
(1)
With the increasing oxygen/coal ratio, the syngas quality deteriorated according to the decreasing effective-gas ratio and hydrogen –carbon ratio. However, the temperature was increased significantly, and corresponding reduction degree kept rising until it reached 100%.
-
(2)
With the increasing ore/coal ratio, the effective-gas rate decreased continuously, and the utilization ratio of the reductive gas raised in the certified range of ore/coal ratio (0.2–1.6). Since the excessive ore feed absorbs a large amount of heat, the lower efficiency of the ore reduction process was observed in high-ore/coal-ratio cases.
-
(3)
Although the excellent quality of reductive gas and reduced iron can be obtained simultaneously according to the variation curves, the production efficiency of the reduced iron was the essential restrictive link. The range that oxygen/coal ratio = 0.6–0.7 and ore/coal ratio = 0.2–1.2 can be reasonable according to the actual production requirements.
References
Zhang CX, Qi YH, Yan DL, Hu CQ, Zhang XX (2016) Energy-saving and environmental protection of ironmaking system in China. Iron and Steel 41(11):1–5
Meijer K, Zeilstra C, Teerhuis C et al (2013) Developments in alternative ironmaking. Trans Indian Inst Met 66(5–6):475–481
Hasanbeigi A, Arens M, Price L (2014) Alternative emerging ironmaking technologies for energy-efficiency and carbon dioxide emissions reduction: a technical review. Renew Sustain Energy Rev 33:645–658
Sohn HY, Mohassab Y (2016) Development of a novel flash ironmaking technology with greatly reduced energy consumption and CO2 emissions. J Sustain Metall 2(3):216–227
Wang H, Sohn HY (2012) Hydrogen reduction kinetics of magnetite concentrate particles relevant to a novel flash ironmaking process. Metall Mater Trans B 44(1):133–145
Chen F, Mohassab Y, Jiang T, Sohn HY (2015) Hydrogen reduction kinetics of hematite concentrate particles relevant to a novel flash ironmaking process. Metall Mater Trans B 46(3):1133–1145
Zhou J, Zhou J, Chen Z, Mao Y (2014) Influence analysis of air flow momentum on concentrate dispersion and combustion in copper flash smelting furnace by CFD simulation. JOM-US 66(9):1629–1637
Ahokainen T, Jokilaakso A (2013) Numerical simulation of the outokumpu flash smelting furnace reaction shaft. Can Metall Quart 37(3–4):275–283
Sohn HY, Perez-Fontes SE (2016) Computational fluid dynamics modeling of hydrogen-oxygen flame. Int J Hydrog Energy 41(4):3284–3290
Chen F, Mohassab Y, Zhang S, Sohn HY (2015) Kinetics of the reduction of hematite concentrate particles by carbon monoxide relevant to a novel flash ironmaking process. Metall Mater Trans B 46(4):1716–1728
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Yang, Y., Guo, L., Bao, Q., Guo, Z. (2020). Predictive Modeling and Optimization of the Variant Combinations of Material Ratios in the Gasification–Reduction Coupling Process. In: Peng, Z., et al. 11th International Symposium on High-Temperature Metallurgical Processing. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-36540-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-36540-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36539-4
Online ISBN: 978-3-030-36540-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)