Abstract
Background: Although many methods are available for breast reconstruction, there are still times where finding the best solution remains a challenge. In selected cases, a laparoscopically harvested omental free flap (LHOFF) can be used for breast reconstruction with excellent results. Aims: The aim of this chapter is to discuss the anatomy, indication, limitations, and surgical technique of the LHOFF for primary or delayed breast reconstruction. Patient selection: Breast reconstruction using an LHOFF is indicated in selected cases: A lean patient with low breast volume and an intact skin envelope with unilateral reconstruction and patient choice to have a solely autologous reconstruction. Sufficient omentum volume must be confirmed through a diagnostic laparoscopy. Technique: The omentum is harvested laparoscopically by a general surgeon and extracted through a 1.5 inch (4 cm) Pfannenstiel incision. Simultaneously, the plastic surgeon’s team members prepare the mammary pocket and acceptor vessels. The gastroepiploic (GE) artery is anastomosed to the internal mammary artery in an end-to-end manner. The accompanying veins are anastomosed using a flow coupler device. The omentum is then carefully fixed in place in the pocket. Conclusion: Autologous breast reconstruction using a LHOFF can be an excellent option in select cases. It is a safe flap with minimal donor site morbidity and scarring with the use of laparoscopic harvesting. The aesthetic results are highly desirable with minimal scarring, good volume, and very soft tissue resembling the natural feeling of a nonreconstructed breast.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Omentum
- Free flap
- Autologous breast reconstruction
- Omental free flap
- Laparoscopically harvested omental free flap
Introduction
Abdominal flaps are still the most commonly used flap in autologous breast reconstruction today. Autologous fat transfer (AFT) is another reconstruction that is popular currently. These techniques are not always possible due to the woman’s characteristics, body shape, and previous abdominal operation(s). Patients may decline surgery that has risks of stark scarring or donor site morbidity.
Currently, there are many uses of the omental flap in abdominal thoracic surgery, such as massive sternotomy infection/wounds and reconstructive surgery after trauma or oncologic resection. The omentum appeared to be an attractive alternative to fill an empty skin envelope in breast reconstruction, but the risk of donor site morbidity from the pedicled flap (hernia, volvulus, etc.) was a major limitation. With the advent of laparoscopically harvested omentum, most of the drawbacks are avoided and we have used the omentum in select cases with excellent results. These surgeries are not yet widespread to our knowledge.
In 1880, Senn described the pedicled omental flap for the first time while using it to protect a suture line of an intestinal anastomosis [1]. In breast reconstruction, Kirikuta was the first to use the omentum as a pedicled flap in 1963 [2]. While the development of microvascular surgery progressed, free tissue transfer became popular. In 1993, Saltz et al. described the use of laparoscopy to harvest omental flaps [3], but breast reconstructions were not described. In 2006, Zaha et al. described the first significant case series of 40 cases, where the omentum was used for immediate breast reconstruction but from these only four were laparoscopically harvested [4]. Breast reconstruction using laparoscopically harvested omental free flap (LHOFF) can be an excellent option, provided there is a sufficient skin envelope and omentum.
Anatomy
“The omentum” in breast reconstruction/LHOFF refers to the greater omentum. The greater omentum is also called the omentum majus, gastrocolic omentum, or epiploon. It is a free-hanging mesenteric tissue apron in the abdominal cavity. It is attached to the greater gastric curvature and descends to the symphysis. It is a double sheet of peritoneum, folded on itself so that it has four layers. The omentum is composed of a connective tissue framework and consists of arteries, veins, lymphatics, and fat pads. The arterial vascularization of the omentum consists of a double blood supply from the left and right gastroepiploic arteries. The left artery obtains blood from the lineal artery and the right by the gastroduodenal artery. Both are branches of the celiac trunk. The right gastroepiploic artery is, most of the time, the stronger and slightly larger artery of the two. Either can be used to make a microvascular anastomosis. Both gastroepiploic arteries branch off gastric and epiploic arteries. The venous blood drainage of the omentum runs parallel to the arteries and empties into the portal system. The dimensions of the omentum vary from 5.5 to 14 inches (14–36 cm) in length and from 8 to 18 inches (20–46 cm) in width. The relationship between the weight of the patient and the weight of the omentum is unpredictable, and it cannot be accurately estimated with noninvasive techniques (e.g., echo, CT, MRI) [5, 6].
Patient Selection
Breast reconstruction using an LHOFF is indicated in selected cases [7].
In our clinic, LHOFF autologous breast reconstruction is indicated in the following group of patients:
-
A patient needing a unilateral reconstruction
-
Patient’s choice to have a solely autologous reconstruction, with a preference of minimal additional scarring
-
A lean patient with low breast volume, where it was not technically possible or desirable to use an abdominal-based flap, other perforator flaps (like buttocks or upper leg region), or AFT
-
A patient with an intact and sufficient skin envelope
-
A patient having sufficient omentum volume (as seen in diagnostic laparoscopy)
Preoperative Planning and Patient Preparation
The relationship between the patient’s characteristic (height and weight) and the volume of the omentum is unpredictable, and the volume of the omentum cannot be accurately estimated with noninvasive techniques (e.g., echo or MRI) [5, 6]. A diagnostic laparoscopy is therefore advised prior to the reconstruction. During the diagnostic laparoscopy, the volume of the omentum is estimated, and conflicting abdominal pathology (e.g., omental malignant nodules, abdominal adhesions) can be evaluated.
A sufficient skin envelope is required for successful LHOFF. If this was uncertain at the time of mastectomy, we usually performed a two-stage procedure. The initial stage included a subpectoral tissue expander (TE), which also preserves the pocket while awaiting definitive pathology reports. In the second stage, we performed the LHOFF breast reconstruction. In all other cases, an immediate reconstruction was done.
It is extremely important to mark on the pocket, prior to surgery, to show where filling is necessary to get the best postoperative outcome. We advise our patients to stop smoking for at least 6 weeks prior to surgery.
Surgical Technique
We use the technique as described in an earlier paper in Microsurgery [7].
Thirty minutes prior to the operation, we administer Kefzol (Cefazolin) antibiotics (2 g IV).
The plastic surgery and the general surgery teams are operating simultaneously.
The omentum is harvested laparoscopically by the general surgeon. The Veress needle is inserted at Palmer’s point, and the abdominal cavity is inflated with CO2 until intra-abdominal pressure is 10 mmHg. We use three trocars in the French position, 12 mm for a 30-degree fiber optic camera and two 5-mm trocars. The surgeon is positioned between the patient’s legs for a comfortable position to dissect the omentum from the bowel and stomach. The branches of the gastric vessels to the stomach are sealed using Harmonic Scalpel (Ethicon Endosurgery, Cincinnati, Ohio). The origin of both the artery and vein of the right gastroepiploic (GE) vessels is identified. To limit ischemia time, shortly after the preparation of the recipient site by the plastic surgeon, the vessels are clipped (the artery and vein are clearly identified, marked) separately and transected to limit ischemia time. Using an endobag, the free omentum is then extracted through a 4 cm Pfannenstiel incision at the superior edge of the mons pubis (See Fig. 21.1). After suturing the fascia, all wounds are closed intracutaneously.
Using an endobag, the greater omentum is extracted through a 4 cm Pfannenstiel incision at the superior edge of mons pubis. (With courtesy and permission of van Alphen et al. [7]. Wiley)
Simultaneously, the plastic surgeon’s team prepares the mammary area above the muscle pectoralis major to prevent muscle animation of the pectoralis major. It is important to remove all the fibrotic tissue of the chest wall to create the softest result and to maintain full range of motion in the shoulder. After the pocket is created, the internal mammary vessels at the level of the fourth rib are dissected as the recipient site. We place the free omentum in the pocket to measure the size of the omentum. If there is too much volume, the omentum can be shaped. The gastroepiploic artery is anastomosed to the internal mammary artery in an end-to-end manner. Due to the amount of fat in the omentum, the vessels tend to draw back in the fat and are therefore less easy to identify and handle. This is the reason why the general surgeon is asked to mark the artery (one clip) and vein (two clips) clearly and the vessels are mobilized longer than in other flaps. You can use the left or right gastroepiploic (GE) vessels for anastomosis, but often the right vessel is stronger and has a slightly larger caliber (See Fig. 21.2). A possible mismatch (in many cases, the GE artery is slightly wider) can be solved by using an oblique section from the smaller artery. The accompanying veins are anastomosed using a coupler device (even a mismatch of 2–3 sizes after cutting the vein, measured by the coupler measuring device, is acceptable here.) The omentum is then carefully fixed in place in the pocket, using six cranial monofilament 3.0 sutures. (The omentum tends to sag downward since it consists of a lot of fatty tissue which can slip.) One suction drain is placed in the anterior axillary line. The subcutaneous layer and skin are closed using a Monocryl 4.0 and Vicryl Rapide 4.0.
LHOFF after the anastomosis is completed. (Gastroepiploic artery to the internal mammary artery in an end-to-end fashion and the vein in an end-to-end fashion using flow coupler device). (With courtesy and permission of van Alphen et al. [7]. Wiley)
Note: Since the general surgeon sealed the gastroepiploic vessels using clips and cut them with diathermy, we shorten the vessels by 0.25 inch or 0.5 cm to minimize any complications with the anastomoses from thermal damage.
Note: The gastroepiploic vessels have a thicker adventitia than, for instance, the deep inferior epigastric artery perforator flap (DIEP) vessels, so carefully dissecting and cleaning the vessels prior to anastomosing prevent any interposition.
Technical Variations
Our preference is to create the pocket above the muscle pectoralis major, provided the skin envelope is thick and sufficient, to limit the change of animation while contracting the muscle. If additional volume is needed during the reconstruction, an implant can be placed under the omentum or in a dual-plane method subpectoral. In a second procedure, lipofilling can be used to increase the volume or to augment the thickness of the skin flap if any irregularities in thickness occurred.
Depending on the surgeon’s preference, the thoracodorsal vessels can also be used as recipient vessels. We prefer the internal mammary vessels for the anastomosis since they facilitate easy anastomosing of LHOFF and we have extensive experience with the internal mammary vessels from DIEP reconstructions. We also believe that by using the internal mammary vessels, it is easier to limit the pocket laterally and that less sagging or ptosis of the omentum toward the lateral border will occur. Another benefit is that you can still use a LD reconstruction as a rescue procedure because the thoracodorsal vessels have been preserved.
You can perform LHOFF as primary or delayed reconstruction. When planning a delayed reconstruction, we advise placing a tissue expander during the primary stage, to create or preserve the skin envelope. If the volume of the omentum is insufficient, the omentum can be used to cover an implant, but this negates the autologous aspect of the reconstruction (Table 21.1).
Postoperative Care
-
Evaluation of flap: The free omental flap is postoperatively monitored using a handheld Doppler ultrasound device to monitor in- and outflow. (Every hour for the first 24 hours, after that every 4 hours) When performing the operation for the first time, we used to use a flow monitoring coupler device for monitoring the venous outflow. Although the little cable of the device is very supple, kinking of the venous vessels proved a high risk due to the soft vessels of the omentum. A normal handheld Doppler ultrasound device can be easily used to monitor in- and outflow.
-
Drain: Vacuum drain for 4 days or until production <30 cc in 24 hours.
Note: Drain production can be significantly more than DIEP reconstruction. 50–100 cc clear or serosanguineous production is normal in the first 4–5 days. We advise leaving the drain in for 4 days and that the patient had been mobilized sufficiently.
-
Antibiotics: Kefzol (Cefazolin), 1 g IV every 8 hours for 72 hours.
-
Thrombosis prophylaxis: Leg pumps are used routinely. Prophylactic Fragmin (dalteparin) is administered during hospitalization. At discharge, the patient is given acetylsalicylic acid (Ascal) 80 mg every day for 1 month in total. Early mobilization during hospitalization is advised.
Complications
Since the arterial vascularization of the omentum consists of a double blood supply from the left and right gastroepiploic artery, it is well vascularized and reliable. The microsurgeon can choose the best vessels to use for the anastomosis, and the artery and vein can be easily lengthened to make the anastomosis easier. We advise performing a diagnostic laparoscopy prior to the reconstruction to estimate the volume and inspect the omentum and the abdomen. Literature showed that LHOFF is a safe flap with low complication rate [8].
Complications related to the amputation of the breast are skinflap necroses, since LHOFF is “buried” in a skin envelope. We now use a handheld Doppler ultrasound device for monitoring in- and outflow in the vessels to prevent flap necrosis.
Abdominal intervention-related complications are abdominal hernia ileus and visceral injury, although the complication rate of these abdominal complications is minimal when using the laparoscopic harvesting technique. Further general complications of surgery include: hematoma, wound infection, seroma, deep venous thrombosis, and pulmonary embolism.
Clinical Cases
A 61-year-old woman underwent mastectomy and secondary breast reconstruction with LHOFF. Breast size lift 75A. BMI 23.9.
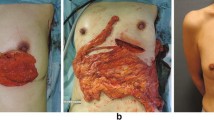
The LHOFF was indicated because of the following reasons: The choice to have a solely autologous reconstruction, with a preference of minimal additional scarring. A patient needing a unilateral reconstruction. Technically not possible to use an abdominal flap or latisumuss dorsi. A patient with an intact and sufficient skin envelope. And sufficient momentum volume was seen with the diagnostic laparoscopy. See Fig. 21.3 for preoperative result (pre-LHOFF reconstruction but after tissue expansion) TE in situ. See Fig. 21.4 for postoperative results after LHOFF at 6 months.
Postoperative results at 6 months are shown. (With courtesy and permission of van Alphen et al. [7]. Wiley)
Conclusions
Autologous breast reconstruction using a laparoscopically harvested omental free flap (LHOFF) can be an excellent option in select cases. It is a safe flap with minimal donor site morbidity and scarring with the use of laparoscopic harvesting. The aesthetic results are excellent with minimal scarring, good volume, and very soft tissue resembling the natural feeling of a nonreconstructed breast, which is highly appreciated by the patients.
References
Senn N. An experimental contribution to intestinal surgery, with special reference to the treatment of intestinal obstruction (Continued). Ann Surg. 1888;7(3):171–86.
Kiricuta I. The use of the great omentum in the surgery of breast cancer. Presse Med. 1963;71:15–7.
Saltz R, Stowers R, Smith M, Gadacz TR. Laparoscopically harvested omental free flap to cover a large soft tissue defect. Ann Surg. 1993;217(5):542–6; discussion 546–7
Zaha H, Inamine S, Naito T, Nomura H. Laparoscopically harvested omental flap for immediate breast reconstruction. Am J Surg. 2006;192:556–8.
Liebermann-Meffert D, White H. The greater omentum: anatomy, physiology, pathology, surgery, with an historical survey. In: Liebermann-Meffert D, White H, Vaubel E, editors. 1st ed. New York: Springer-Verlag Berlin Heidelberg GmbH; 1983. p. 1–372.
Liebermann-Meffert D. The greater omentum: anatomy, embryology, and surgical applications. Surg Clin North Am. 2000;80(1):275–93.
van Alphen TC, Fechner MR, Smit JM, Slooter GD, Broekhuysen CL. The laparoscopically harvested omentum as a free flap for autologous breast reconstruction. Microsurgery. 2017;37(6):539–45.
Ni C, Zhu Z, Xin Y, Xie Q, Yuan H, Zhong M, et al. Oncoplastic breast reconstruction with omental flap: a retrospective study and systematic review. J Cancer. 2018;9(10):1782–90.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
van Alphen, T.C., Slooter, G.D., Fechner, M.R., Broekhuysen, C.L. (2020). Breast Reconstruction with the Laparoscopically Harvested Omental Free Flap. In: Mayer, H. (eds) Breast Reconstruction. Springer, Cham. https://doi.org/10.1007/978-3-030-34603-4_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-34603-4_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34602-7
Online ISBN: 978-3-030-34603-4
eBook Packages: MedicineMedicine (R0)