Abstract
Outcome after traumatic brain injury (TBI) is worsened by hemorrhagic shock (HS); however, the existing volume expansion approach with resuscitation fluids (RF) is controversial as it does not adequately alleviate impaired microvascular cerebral blood flow (mCBF). We previously reported that resuscitation fluid with drag reducing polymers (DRP-RF) improves CBF by rheological modulation of hemodynamics. Here, we evaluate the efficacy of DRP-RF, compared to lactated Ringers resuscitation fluid (LR-RF), in reducing cerebral microthrombosis and reperfusion mitochondrial oxidative stress after TBI complicated by HS. Fluid percussion TBI (1.5 ATA, 50 ms) was induced in rats and followed by controlled HS to a mean arterial pressure (MAP) of 40 mmHg. DRP-RF or LR-RF was infused to restore MAP to 60 mmHg for 1 h (pre-hospital period), followed by blood re-infusion to a MAP = 70 mmHg (hospital period). In vivo 2-photon laser scanning microscopy over the parietal cortex was used to monitor microvascular blood flow, nicotinamide adenine dinucleotide (NADH) for tissue oxygen supply and mitochondrial oxidative stress (superoxide by i.v. hydroethidine [HEt], 1 mg/kg) for 4 h after TBI/HS, followed by Dil vascular painting during perfusion-fixation. TBI/HS decreased mCBF resulting in capillary microthrombosis and tissue hypoxia. Microvascular CBF and tissue oxygenation were significantly improved in the DRP-RF compared to the LR-RF treated group (p < 0.05). Reperfusion-induced oxidative stress, reflected by HEt fluorescence, was 32 ± 6% higher in LR-RF vs. DRP-RF (p < 0.05). Post-mortem whole-brain visualization of DiI painted vessels revealed multiple microthromboses in both hemispheres that were 29 ± 3% less in DRP-RF vs. LR-RF group (p < 0.05). Resuscitation after TBI/HS using DRP-RF effectively restores mCBF, reduces hypoxia, microthrombosis formation, and mitochondrial oxidative stress compared to conventional volume expansion with LR-RF.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Traumatic brain injury (TBI)
- Hemorrhagic shock (HS)
- Microthrombi
- Superoxide
- Resuscitation fluid (RF)
- Drag reducing polymer (DRP)
1 Introduction
Outcome after traumatic brain injury (TBI) is significantly worsened by hemorrhagic shock (HS) due to increased severity of reduced cerebral blood flow (CBF) leading to capillary microthrombosis, hypoxia, reactive oxygen species formation due to mitochondrial dysfunction, neuronal death and a two-fold increase in contusion volume [1, 2]. The existing volume expansion approach with resuscitation fluids (RF) is controversial as it does not adequately alleviate impaired microvascular cerebral blood flow (mCBF) and is thus not neuroprotective. We previously reported that resuscitation fluid with drag-reducing polymers (DRP-RF) improved CBF and reduced cerebral hypoxia and neuronal necrosis by rheological modulation of hemodynamics. Here, we evaluate the efficacy of DRP-RF compared to lactated Ringers resuscitation fluid (LR-RF), in reducing cerebral microthrombosis and reperfusion mitochondrial oxidative stress after TBI complicated by HS.
2 Methods
Most of the procedures used in these studies have already been described [3]. Protocol #200640 was approved by the Institutional Animal Care and Use Committee of the University of New Mexico and the studies were conducted according to the NIH Guide for the Care and Use of Laboratory Animals.
Surgical Preparation
Laboratory-acclimated male Sprague-Dawley rats (250–300 g) were mechanically ventilated on isoflurane (2%), nitrous oxide (69%) oxygen anesthesia (29%). Femoral vein and artery catheters were inserted. For imaging and TBI, a 5-mm craniotomy over the left parietal cortex was filled with 2% agarose in saline and sealed by a cover glass. The fluid percussion was used as a model of TBI and was induced by 1.5 atm 50 ms pulse from a custom-built Pneumatic Impactor connected to the brain through a pressure transducer filled with artificial cerebrospinal fluid. HS was performed in a way similar to that described by Robertson et al. [2].
Overall Design of the Study
TBI was induced after baseline in-vivo 2-photon laser scanning microscopy (2PLSM) and followed by a 1-h hemorrhagic phase, where blood was slowly withdrawn through the femoral vein to reduce mean arterial pressure (MAP) to 40 mmHg. In the following 1-h pre-hospital care phase, resuscitation fluids (LR-RF or DRP-RF) were slowly infused i.v. to raise MAP to ~55 mmHg and CBF to ~65% of baseline. In a subsequent 3-h definitive hospital care phase, shed blood was re-infused to a MAP of 70 mmHg and CBF of ~75% of baseline. In vivo 2PLSM was done throughout the study over the peri-contusion area of the parietal cortex of the rat brain. Monitored variables included: cerebral microvascular blood flow velocity, number of perfused capillaries, tissue oxygen supply (NADH autofluorescence) and superoxide production by i.v. Hydroethidine (HEt, Sigma-Aldrich, USA). The laser Doppler flux was measured via a lateral temporal window using a 0.9-mm diameter probe (DRT4, Moor Inst., Axminster, UK) in the same region of the brain studied by 2PLSM. Brain and rectal temperatures were monitored and maintained at 38 ± 0.5 °C. Arterial blood gases, electrolytes, hematocrit and pH were measured hourly (epoc Blood Analysis System, Alere Inc., Waltham, MA, USA). At the end of experiments animals were subjected to perfusion with Vessel Painting.
Superoxide Production Evaluation
0.5 mg of HEt in a concentration of 1 mg/ml in 0.1 M phosphate buffer solution (PBS) containing 20% DMSO, was injected intravenously during surgical preparation [4]. Hydroethidine (HEt) is a fluorescent dye that is oxidized to ethidium (ET) by superoxide. ET fluoresces at a different wavelength (Em = 595 nm) than HEt (Em = 415 nm) and thus may be used to visualize superoxide production.
Animal perfusion and Vessel Painting
Vessel painting was done during cardiac perfusion according to Hughes with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen, USA) which binds directly and preferentially to endothelial cells [5]. The Vessel Painting included the following sequential order for delivery of perfusion solutions: a) PBS (150 ml), b) 50 ml of DiI (13 μg/ml), and c) paraformaldehyde (4%, 200 ml). After fixation and perfusion, the brain was extracted from the cranium and all meninges were removed with care. Imaging was done with 2PLSM in a custom-made fixed tissue imaging chamber.
DRP Preparation
Polyethylene oxide (PEO, MW ~4000 kDa) was dissolved in saline to 0.1% (1000 ppm), dialyzed against saline using a 50 kD cutoff membrane, diluted in saline to 50 ppm, slow rocked for ~2 h and then sterilized using a 0.22 μm filter [6]. DRP-RF was prepared before infusion by adding DRP to Lactated Ringer to reach the final DRP concentration of 0.0005% (5 ppm).
Two-Photon Laser Scanning Microscopy
Fluorescent serum (i.v. fluorescein isothiocyanate (FITS) dextran, 150 kDa in physiological saline, 5% wt/vol) was visualized using an Olympus BX 51WI upright microscope and water-immersion LUMPlan FL/IR 20X/0.50 W objective. Excitation was provided by a PrairieView Ultima multiphoton microscopy laser scan unit powered by a Millennia Prime 10 W diode laser source pumping a Tsunami Ti: Sapphire laser (Spectra-Physics, Mountain View, CA, USA) tuned to 750 nm center wavelength. Band-pass-filtered epifluorescence (510–530 nm for FITS, 445–475 nm for NADH and 565–600 for ET) was collected by photomultiplier tubes of the Prairie View Ultima system. Images (512 x 512 pixels, 0.15 um/pixel in the x- and y-axes) or line scans were acquired using Prairie View software. Red blood cell flow velocity was measured in microvessels ranging from 3–50 μm diameter up to 500 μm below the surface of the parietal cortex, as described previously [5]. Tissue hypoxia was assessed by measurement of NADH autofluorescence. In offline analyses using NIH ImageJ software, a three-dimensional anatomy of the vasculature in areas of interest were reconstructed from two-dimensional (planar) scans of the fluorescence intensity obtained at successive focal depths in the cortex (XYZ stack).
Statistical analyses were done using GraphPad Prism software 6.0 (La Jolla, CA, USA) by Student’s t-test or Kolmogorov-Smirnov test where appropriate. Differences between groups were determined using two-way analysis of variance (ANOVA) for multiple comparisons and post-hoc testing using the Mann-Whitney U-test.
3 Results
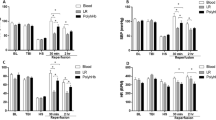
As in our previous studies, TBI followed by HS decreased mCBF resulting in capillary microthrombosis and tissue hypoxia . Microvascular CBF, the number of perfused capillaries and tissue oxygenation in peri contusion areas were significantly better in the DRP-RF compared to the LR-RF treated group.
Post-mortem whole-brain visualization of DiI painted vessels revealed multiple microthromboses and reduction of microvascular density in both hemispheres in rats after TBI with HS (Fig. 1A, B): i.e., in the injured hemisphere in DRP-RF, microvascular density was higher than in LR-RF (% vessel/total area∗100 was 4.9 ± 0.4 vs. 3.1 ± 0.3, respectively, p < 0.05) as opposed to 6.8 ± 0.4 in Sham rats (Fig. 1C). In contralateral to the injury hemisphere, microvascular density was also reduced (% vessel/total area∗100 was 6.1 ± 0.5 vs 5.2 ± 0.5, in DRP-RF, vs. LR-RF, respectively, p < 0.09) (Fig. 1C).
Resuscitation with DRP-RF reduces microthrombosis in both hemispheres after TBI with HS as shown by post-mortem DiI vascular painting. (a) Cortical microvascular network in Sham mouse brain; and (b) after TBI with HS; (c) Graph showing reduced cortical microvasculature in LR-RF group and better-preserved microvasculature in DRP-RF group in both, traumatized and contralateral hemispheres. Mean ± SEM, N = 10 rats per group, ∗P < 0.05 from the LR-RF group
Superoxide production was determined by oxidation of HEt to ET. Under normal physiological conditions at baseline, ET fluorescence appeared as small particles in the cytosol, suggesting mitochondrial generation of superoxide (Fig. 2A). In pathological conditions, the ET fluorescent signal filled the cytosolic space and allowed clear visualization and differentiation of individual soma and dendritic processes. Neurons in which the accumulated fluorescence demonstrated the entire body of the soma and obscured the nucleus were considered positive for diffuse cytosolic ET fluorescence.
Resuscitation with DRP-RF reduces superoxide production in cortical neurons after TBI with HS: (a) Representative image of a rat cortex at baseline without ET positive neurons; (b) Neurons with diffuse cytosolic ET fluorescence in a rat cortex from LR-RF group by the end of experiment; (c) and from DRP-RF group; The dynamics of the increase in ET positive cortical neurons. Mean ± SEM, n = 10 rats per group, ∗P < 0.05 from the LR-RF group
TBI with subsequent HS caused generation of superoxide; in both groups the number of Et positive neurons increased to 58.1 ± 5.2 per 0.075 mm3 (Fig. 2D). In the pre-hospital phase, in the group in which the conventional LR-RF was slowly infused in the amount of 5.6 ± 2.2 ml, the number of Et positive neurons further increased to 137.9 ± 5.6 per 0.075 mm3 (Fig. 2D). In the group in which DRP-RF was slowly infused in a smaller amount of 2.0 ± 0.3 ml, the number of Et positive neurons increased to only 62.3 ± 4.8 per 0.075 mm3 (Fig. 2D). By the end of the hospital phase with re-infusion of blood, in the LR-RF group, the number of Et positive neurons further increased to 176.5 ± 6.2 per 0.075 mm3 (Fig. 2B, D), reflecting active reactive oxygen species (superoxide) formation probably due to excessive oxidative phosphorylation in metabolically stressed mitochondria. In the DRP-RF group, the number of Et positive neurons increased to only 84.2 ± 5.3 per 0.075 mm3 (Fig. 2C, D), reflecting lower oxidative stress.
4 Discussion
The most common free radical in TBI, responsible for tissue damage, is superoxide that is produced when oxygen molecules gain an electron from other molecules [7, 8]. The major source of superoxide in brain injury is the mitochondria [8, 9]. As demonstrated in this work, attenuation of oxidative stress by DRP-RF after TBI with HS is related to the mitigation of microthrombosis formation and hypoxia reduction. Hypoxia, induced by TBI and exacerbated by HS, causes mitochondrial impairment leading to excessive and altered oxidative phosphorylation in mitochondria and, as a result, superoxide hyperproduction [10]. Resuscitation re-perfusion exacerbates this process [11]. Attenuation of reduction of cerebral microvascular density by mitigation of microthrombosis formation by DRP-RF does not allow hypoxia to reach the critical level which, possibly, reduces mitochondrial injury and neuronal excitotoxicity and, as a result, reactive oxygen species overproduction. The mechanisms of hemorheological modulation by DRP include increasing the arteriolar blood volume flow via the increase of flow velocity by reduction of flow separations and vortices at vessel bifurcations and decreasing pressure loss across the arterial network due to the viscoelastic properties of DRP [11]. This leads to a rise in pre-capillary blood pressure thus enhancing capillary perfusion, countering capillary stasis, increasing the density of functioning capillaries and the number of red blood cells passing through capillaries to improve tissue oxygenation [11].
5 Conclusion
Rheological modulation of blood flow using advanced resuscitation fluid with DRP in nanomolar amounts effectively restores microvascular CBF, reduces hypoxia, microthrombosis formation and mitochondrial oxidative stress compared to conventional volume expansion with lactated Ringer.
References
Manley G, Knudson MM, Morabito D et al (2001) Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg 136(10):1118–1123
Navarro JC, Pillai S, Cherian L et al (2012) Histopathological and behavioral effects of immediate and delayed hemorrhagic shock after mild traumatic brain injury in rats. J Neurotrauma 29(2):322–334
Bragin DE, Kameneva MV, Bragina OA et al (2017) Rheological effects of drag-reducing polymers improve cerebral blood flow and oxygenation after traumatic brain injury in rats. J Cereb Blood Flow Metab 37(3):762–775
Hughes S, Dashkin O, Defazio RA (2014) Vessel painting technique for visualizing the cerebral vascular architecture of the mouse. Methods Mol Biol 1135:127–113
Peterson SL, Morrow D, Liu S et al (2002) Hydroethidine detection of superoxide production during the lithium-pilocarpine model of status epilepticus. Epilepsy Res 49(3):226–238
Kameneva MV, Wu ZJ, Uraysh A et al (2004) Blood soluble drag-reducing polymers prevent lethality from hemorrhagic shock in acute animal experiments. Biorheology 41(1):53–64
Abdul-Muneer PM, Chandra N, Haorah J (2015) Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51(3):966–979
Hiebert JB, Shen Q, Thimmesch AR et al (2015) Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci Aug 350(2):132–138
Yonutas H, Vekaria HJ, Sullivan PG (2016) Mitochondrial specific therapeutic targets following brain injury. Brain Res 1640:77–93
Fiskum G (2000) Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma 17(10):843–855
Kameneva MV (2012) Microrheological effects of drag-reducing polymers in vitro and in vivo. Int J Eng Sci 59:168–183
Acknowledgments
Supported by DOD DM160142.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bragin, D.E., Bragina, O.A., Kameneva, M.V., Nemoto, E.M. (2020). Resuscitation with Drag Reducing Polymers after Traumatic Brain Injury with Hemorrhagic Shock Reduces Microthrombosis and Oxidative Stress. In: Ryu, PD., LaManna, J., Harrison, D., Lee, SS. (eds) Oxygen Transport to Tissue XLI. Advances in Experimental Medicine and Biology, vol 1232. Springer, Cham. https://doi.org/10.1007/978-3-030-34461-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-34461-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34459-7
Online ISBN: 978-3-030-34461-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)