Abstract
A new type of fluid, called nanofluid, has found numerous applications in engineering sector due to its outstanding properties. These are known as suspensions of nano-sized particles in fluids called basefluids. The suspension of these nanoparticles in the basefluid shows significant influence on its physical properties. In view of this, in the present book chapter, the properties of the nanofluid like its thermal conductivity, electrical conductivity and so on have been discussed and the notable studies carried out in the past have been summarized. Several factors that are responsible for the alteration of the properties of nanofluids at varying degrees are identified and discussed in this chapter. Further, these properties contribute to the distinctive applications of nanofluids in various engineering fields, which are reviewed and discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Owing to the ever-increasing need for heat management at micro level, such as computer chips, and macro-level, such as car engines, cooling and heat transfer has gained a lot of importance in domestic as well as industrial systems and technologies. It is a general truth about solids that they possess higher thermal conductivity than liquids. The great scientist James Clerk Maxwell (1881) developed the fact that solids dispersed in liquids enhance the thermal conductivity of those liquids, and that is when studies for enhancement of thermal properties of conventional heat transfer liquids gained an impetus. Millimetre or micrometre-sized particles were used to conduct these studies. But, the major issue coming in the way of using these particles was that they settle very rapidly in liquids and may also cause abrasion, clogging and additional pressure drop (Das et al. 2006). These issues limit the use of conventional solid–liquid suspensions as practical heat transfer fluids. The rise of nanotechnology has created the possibility of producing nanoparticles which are characterized by the particle sizes below 100 nm. Nanofluids, a new group of heat transfer fluids, acquired by dispersing and suspending nanoparticles with typical dimensions of the order of 1–100 nm were found by Choi (1995). The main objective behind using nanofluids is to attain higher thermal properties and uniform and stable dispersions of nanoparticles. In order to achieve this objective, it becomes essential to understand how nanoparticles intensify energy transport in liquids. Since Choi (1995) formulated this new concept of nanofluids, many scientists expeditiously developed nanofluids and found scientific facts not only on enhanced thermal properties of nanofluids, but signifying new mechanisms that play major role in enhancing the thermal properties of nanofluids and expanding new mathematical models for the nanofluids. The type of nanoparticle, its size, shape and distribution are dominant properties that cannot be effortlessly measured but affect the thermal transport properties of the nanofluids. Other important factors include type of basefluids used, the method for the preparation of nanofluid, usage of surfactants and dispersing additives, pH, temperature, viscosity and other physical properties. Two nanofluid samples with different type of nanoparticles and amount of surfactants and/or pH adjusters while keeping all other parameters constant may result in different thermo-physical properties.
Many researchers have studied nanofluids containing Al2O3 nanoparticles (Heyhat et al. 2013; Sokhansefat et al. 2014; Usri et al. 2015), Cu nanoparticles (Eastman et al. 2001; Yu et al. 2010) or carbon nanotubes (CNTs) (Jiang et al. 2015; Leong et al. 2016) and found remarkable increase in thermal conductivities. Hence, nanofluids have gained attention in various heat transfer applications. There are various possible applications of nanofluids, which include transportation (engine cooling/vehicle thermal management) (Azimi and Ommi 2013; Sidik et al. 2017), electronics cooling (Khaleduzzaman et al. 2015; Roberts and Walker 2010), nuclear systems cooling (Mahmud et al. 2016), heat exchangers (Bozorgan and Shafahi 2017; Li et al. 2018), fuel cell (Islam et al. 2015; Zakaria et al. 2016), solar water heating (Kasaeian et al. 2015), chillers (Liu et al. 2011), lubricants (Mao et al. 2014), thermal storage (Harikrishnana et al. 2013); (Chieruzzi et al. 2013) and many others.
As the thermal transport properties have been largely studied, researchers then turned towards finding and analysing the electrical conducting properties of the nanofluids. Maxwell’s model (Maxwell 1881) is found to be the pioneer in determining the electrical conductivity of the nanofluids theoretically based on the physical properties of the nano-sized particles and basefluid. These properties of the nanofluids are affected by many factors, one of which is the particle size distribution of the nano-sized particles in the nanofluids. Particle size distribution gives the amount of particles according to their sizes present in the nanofluid. It is basically the degree to which the sizes of the particles vary throughout the nanofluid. In a nanofluid, the dispersed nanoparticles are never of equal size and so its size distribution characterizes the nanofluid better than any exact value of size of the nanoparticles. This property is affected by many factors and affects other properties which are also discussed in this chapter. Several studies on nanofluids depicting the values of thermal conductivity, electrical conductivity as well as particle size are given in Table 1.
2 Synthesis Techniques of Nanofluids
Preparation of nanofluid is the technique of evenly and uniformly dispersing the nanoparticles in the basefluid. This may seem to be simple and not so important, but this process largely affects several properties of the nanofluid. Also, the lab-scale preparation of nanofluids may possibly determine the complexity of preparing and using nanofluids in large-scale applications. There are two methods of nanofluid preparation: the two-step and one-step methods.
2.1 Two-Step Method
Two-step method is the most common and has been extensively used for preparing nanofluids. As the name indicates, there are two steps that are carried out in this method during preparation of nanofluids. In the first step, dry powder is produced as nanoparticles, nanofibres or nanotubes. Then in the second processing step, nanomaterial is directly distributed in the basefluid with the use of dispersing devices like magnetic stirrer (Duangthongsuk and Wongwises 2009; Kavitha et al. 2012; Liu et al. 2005), homogenizer (Liu et al. 2005; Wen and Ding 2005) or by using ultrasound devices like probe or bath (Duangthongsuk and Wongwises 2009; Hwang et al. 2007; Manimaran et al. 2014). Mostly, ultrasound device or a higher shear homogenizer is commonly used to stir nanopowders with the basefluids. As this method permits a gap between the synthesis and dispersion steps, many researchers have used readily-available nano-sized particles for the preparation of nanofluids in various basefluids (Xuan and Li 2000). It is a common observation that the two-step process is highly used for preparing metal oxide nanoparticles-based nanofluids than the metallic nanoparticles-based nanofluids (Eastman et al. 2001). In the two-step method, drying the nanoparticles, their transportation and storage are further process steps that cannot be neglected. The main advantage of this method is that it is easily scalable. However, the major disadvantage is the formation of agglomerates because of the attraction between the particles due to their high surface energy (Mohammed et al. 2011). Two-step method is favourable to almost every kind of fluids (Wang and Mujumdar 2007). The illustration of two-step method is given in Fig. 1.
2.2 One-Step Method
In one-step method, the process takes place in single step in accordance to its name. This process is composed of both, the production of the nanoparticles and the synthesis of nanofluids. Numerous single-step processes have been developed for the production of nanofluid (Akoh et al. 1978; Eastman et al. 1997). Direct vapourization–condensation process that provided remarkable control over the size of nanoparticles produced a stable nanofluid without the addition of additive (Choi et al. 2001). Preparation of nanofluid by a novel process was introduced by Lo et al. (2005) using submerged arc nanoparticle synthesis system (SANSS) technique where a pure metal rod is heated by a submerged arc. SANSS is a constructive technique for avoiding nanoparticles agglomeration and also benefits in producing even dispersion of nanoparticles in deionized water.
In the one-step method, the costs for drying and dispersion of the nanoparticles in the basefluid can be avoided. The main disadvantages of this method are that it is not easily scalable because of its high cost of manufacture and that it is only applicable for the basefluids having low vapour pressure (Prakash et al. 2016; Wang and Mujumdar 2007). Zhu and Yin (2004) worked on a single-step chemical process for the synthesis of Cu nanofluids by reducing CuSO4·5H2O using NaH2PO2·H2O in ethylene glycol using microwave irradiation. This method is more efficiently proved to prepare mineral oil-based silver nanofluids. A vacuum-based submerged arc nanoparticle synthesis was studied by Lo et al. (2005) for the preparation of CuO, Cu2O and Cu-based nanofluids using different dielectric liquids. The nanofluids were prepared by using the vapourized metal which is condensed and then dispersed in deionized water. The illustration for one-step method is given in Fig. 2.
3 Thermal Conductivity of Nanofluids
Thermal conductivity is the ability of the nanofluids to transfer heat through them. There have been several studies to identify and study the thermal conductivity of fluids by incorporating various kinds of nanoparticles in them and to find out the enhancement in the thermal properties and the mechanism behind it.
3.1 Reason Behind Improved Thermal Performance of Nanofluids
Nanofluids as discussed earlier are suspensions of nanoparticles in a particular basefluid. We already know that the solids, due to the collision of their vibrating molecules, propagating phonons and diffused-free electrons transfer heat through them efficiently. This does not seem to happen in liquids and gases, because of their loosely packed molecules. On the other hand, the molecules of a solid are tightly packed, which makes it a good conductor of thermal energy. These solids, if incorporated into liquids affect the thermal properties as explained by Maxwell (1881). The solids act as “heat boats” that carry the thermal energy through the liquid and also as “stirrers” that generate convection currents, thus providing chances of collisions of molecules and augmentation of the thermal conductivity (Yang and Han 2006). Nanofluid can be called as a pseudo-homogenous suspension of solids into specific liquids, because of the very small size of the solid particles that are distributed throughout the base liquid called as the basefluid. They impart homogeneity to the mixture due to their size, which is between 1 and 100 nm.
There are many reasons which elevate the thermal properties of the basefluid when nanoparticles are contained in it. One of the reasons is the higher surface area of the nano-sized particles that is available to gain and distribute heat throughout their body. Major reasons include the Brownian motion of the nanoparticles in the fluid and the interfacial layer around the nanoparticles (Jang and Choi 2004). The Brownian motion is the movement that naturally occurs due to the movement of molecules of fluid around the particle. It gives rise to micro-mixing of the nanofluid, thus producing localized convection throughout the fluid. The thermal conductivity of the nanofluid is looked upon as a combined effect of the static and dynamic mechanisms which involve the thermal properties and interfacial layer phenomena and the Brownian motion phenomena, respectively (Sohel Murshed and Nieto de Castro 2011).
Another phenomenon that contributes to the enhanced thermal properties is the interfacial layer of liquid around the particle at the solid–liquid interface. The layer of liquid formed is an ordered molecule layer whose thickness plays a significant role in transportation of heat from solid surface to the bulk liquid (Yu and Choi 2003). It has been known that this interfacial layer has higher thermal conductivity than the bulk basefluid (Kotia et al. 2017). It has been found out by Yu and Choi (2003) that the thermal conductivity of the interfacial layer is ten times more than the bulk fluid. A correlation as given in Eq. (1) has been proposed by Leong et al. (2006) for determining the thermal conductivity of the interfacial layer where \( {\text{k}}_{\text{l}} \) is the interfacial layer thermal conductivity, C is a constant specific for a type of nanoparticle, t is the interfacial layer thickness, \( {\text{r}}_{\text{p}} \) is the radius of the nano-sized particle and \( {\text{k}}_{\text{f}} \) is the thermal conductivity of the basefluid.
The authors observed that the thermal conductivity of the layer is 2–3 times greater than that of the basefluid.
3.2 Measurement of Thermal Conductivity of the Nanofluids
Owing to the fact that there can be a lot of improvement in the thermal transport properties of the fluids, there has been a lot of increase in the investigation of thermal conductivity of the nanofluids. For this purpose, many techniques have been used by researchers which include the steady-state coaxial cylinder method (Glory et al. 2008), transient hot wire (THW) method (Garg et al. 2008; Lee et al. 2008; Rusconi et al. 2007), IR thermometry method (Gharagozloo and Goodson 2008) and so on. The newest technique for determining the thermal conductivity of nanofluids that has been used by numerous researchers lately is the instrument known as KD2 Pro thermal property analyser, developed by Decagon Devices Inc., USA. A simple arrangement of the KD2 Pro thermal conductivity analyser for thermal conductivity measurement of nanofluids is given in Fig. 3. This instrument follows the working principle of transient hot wire method. It comprises a KS-1 needle which is 60 mm long and has a diameter of 1.3 mm. This needle is immersed in the nanofluid which is maintained at a certain temperature. After 2 min, the instrument directly displays the value of thermal conductivity as measured by it. Owing to such simple and fast operation of the instrument, it gained a lot of attention and utilization by researchers working in the field of nanofluid (Esfe et al. 2015a; Leong et al. 2018; Zadkhast et al. 2017). The transient hot wire method involves the use of dynamic technique that measures the rise in temperature in a specific distance from a linear source of heat, that is, a hot wire immersed in the test material. Thus, if the heat source has constant heat output along the test material, the thermal conductivity is known directly from the effect of change in temperature over a period of time. An instrument that uses this method consists of a heating wire along with a temperature sensor together making up the probe that electrically insulates the probe from the test material.
Arrangement of KD2 Pro thermal conductivity analyser for thermal conductivity measurement of nanofluids (Zakaria et al. 2015)
3.3 Factors Affecting Thermal Conductivity of Nanofluids
Thermal conductivity of the nanofluid, being a characteristic of thermo-physical property of its components, is bound to vary with a lot of conditions. It includes nature of the nanoparticle and the basefluid, composition of the nanoparticle, concentration of the nanofluid, that is, its volume fraction in the nanofluid, temperature and pH. Following are the various factors explained along with some findings in the literature.
3.3.1 Nature of Nanoparticle and Basefluid
The nanofluid comprises two basic components, that is, the nanoparticle and basefluid, the properties of which shall definitely affect the properties of the prepared nanofluid. In fact, the nature of both these components has a big impact on the behaviour of the nanofluid. First of all, we know that the thermal conductivities of different nanomaterials vary over a wide range, right from polymeric materials having very low thermal conductivities to some carbon allotropes having very high thermal conductivities. Few of the thermal conductivity data found for different nanoparticles dispersed in water have been plotted in Fig. 4 (Ahammed et al. 2016; Minea and Manca 2017; Sundar et al. 2013).
Nanoparticles of different materials can be of different sizes and shapes. Chopkar et al. (2008) found that the relative thermal conductivity of the nanofluid increases nonlinearly with decrease in diameter of the nanoparticles dispersed in it. Similar outcome was obtained by Esfe et al. (2015a) for metal-based nanofluid and by Teng et al. (2010) for metal oxide-based nanofluid. For silica-ethanol nanofluid, it was found by Darvanjooghi and Esfahany (2016) that there are –OH groups on the surface of silica nanoparticles and that the hydrophilicity of the surface of nanoparticles and restricted movement of molecules of basefluid at the interface increases the intramolecular force field, thus enhancing the heat conductance through the interface. Increase in the nanoparticle size increases the amount of –OH groups on its surface and ultimately increasing the thermal conductivity of the nanofluid. At nanofluid concentration of 0.15 vol.%, the value of relative thermal conductivity was found to be 1.02 and 1.1 for nanoparticle size of 20 and 63 nm, respectively, at temperature of 25 °C.
Along with size, it is well established that the shape of nanoparticles affects its thermal properties (Alawi et al. 2018). Ghosh et al. (2012) investigated the influence of particle shape on the heat transfer characteristics of its nanofluid. It has been reported that the heat transfer of a nanoparticle of high aspect ratio is greater than nanoparticle of low aspect ratio. They studied a cylindrical-shaped Cu nanoparticle with aspect ratio (length to diameter) of 4 using molecular dynamics simulation and found out that a spherical-shaped Cu nanoparticle of same volume transfers heat lesser than that transferred by the cylindrical-shaped Cu nanoparticle. This is reported to be happening due to the high heat transfer caused by the increased contact area with increasing aspect ratio. Jeong et al. (2013) investigated the influence of spherical-shaped and rectangular-shaped ZnO nanoparticles based on nanofluid and found 16 and 19.8% thermal conductivity improvement, respectively, for 5 vol.% nanofluid concentration.
Zhu et al. (2018) found that the CuO nanowires have better thermal performance than the CuO nanospheres, which is due to the efficient thermal transport happening in 1-D nanostructure of the nanowires than the 0-D nanostructure of the nanospheres. They have found a 6.98% and a surprisingly high enhancement in thermal conductivity of 60.78% for CuO nanospheres and CuO nanowires, respectively. This is reported to be occurring because of the high aspect ratio and transport of heat in one controllable direction.
The basefluid used for the synthesis of nanofluid makes up most of the nanofluid quantity and governs the flow properties and thermal transport properties of the nanofluid. Even though nanoparticles alter the flow and thermal properties of the nanofluid, these properties are bound by the limits of the basefluid. For example, the thermal conductivity of a nanofluid made using a basefluid having inherently high thermal conductivity will be higher than the nanofluid made using a basefluid having inherently low thermal conductivity with the same nanoparticles. Also, the viscosity of the basefluid will determine the rheology of the nanofluid. Even though nanoparticles tend to alter the rheological behaviour of the nanofluid, the inherent flow property of the basefluid will rule the major part of the nanofluids’ rheology. The effect of basefluid on the thermal properties of the nanofluid is well studied by Syam Sundar et al. (2017). They have prepared graphene oxide/Co3O4 nanocomposite-based nanofluid and used water, ethylene glycol and mixtures of both in the ratios of EG/water as 20:80, 40:60 and 60:40 as basefluids. Although there is enhancement in thermal conductivity of all the nanofluids, the thermal conductivity of water being higher than that of ethylene glycol, the thermal conductivity of the nanofluid also shows the same trend. Also, in the mixtures of ethylene glycol and water, the thermal conductivity is found to be 0.619, 0.496 and 0.402 for EG/water mixture basefluid having ratios as 20:80, 40:60 and 60:40, respectively. The thermal conductivity of the nanofluid is bound by the thermal conductivity of the mixture of the two fluids. This shows how the basefluid composition alters the thermal conductivity of the nanofluid to a larger extent.
Agglomeration of nanoparticles has been a common observation as well as a serious problem in dealing with nanofluids and must be avoided so as to obtain a stable nanofluid. Nanoparticles do agglomerate and form aggregates that try to settle down, thus degrading the thermal properties of a nanofluid. The method of dispersion does affect the agglomerating property of the nanofluid but there is a limit after which the nanoparticles do not stay uniformly dispersed in the nanofluid. Use of surfactants or some dispersing agents is the most common method which is used to decrease agglomeration of nanoparticles in the fluids (Xuan et al. 2013). They are amphiphilic compounds having a tail and polar head group which are hydrophobic and hydrophilic, respectively (Schramm et al. 2003). This hydrophobic tail gets attached to the nanoparticles which are hydrophobic in nature. And the hydrophilic group interacts with the surrounding fluid. Thus, the wettability of the nanoparticle is improved by the surfactant. This reduces the surface tension and assists fluid continuity. On the other hand, it has also been found out by Xuan et al. (2013) that the use of surfactants in the nanofluids affects the thermal properties of the nanofluid and deteriorates heat transfer. They studied the effect of sodium dodecyl benzoic sulphate (SDBS) as surfactant on nanofluid and found out that the heat transfer coefficient offered by 0.34 vol.% Cu nanofluid decreases from 21,000 to 20,000 W/m2K as the amount of SDBS increases from 0.05 to 0.1 wt% in the nanofluid. Although this is the case for using surfactants, proper selection of surfactant must be done in order to control its effect over the crucial properties of a nanofluid. Xia et al. (2014) have studied the effect of two different surfactants on Al2O3/water nanofluids. They found out that polyvinylpyrrolidone (PVP), being a non-ionic surfactant has positive effects on the thermal conductivity of the nanofluid than sodium dodecyl sulphate (SDS) which is an anionic surfactant.
3.3.2 Composition of Nanoparticle
It has been already known that the nanoparticle nature influences the thermal conductivity of the nanofluid. Nanoparticles contained in a nanofluid may also be a composite of two or more nano-sized materials, which is known as nanocomposite. The components of a nanocomposite may have different properties and thus may impart their individual properties to the whole nanoparticle. So, there are chances that the amount of these components will determine the properties variation of a nanocomposite. Trinh et al. (2016) investigated the thermal conductivity of Cu/graphene nanocomposite-based nanofluid by changing the nanocomposite ratio using ethylene glycol as basefluid. They synthesized nanofluids containing Cu/graphene nanocomposite having graphene/Cu ratio of 7:1, 5:1, 3:1 and 1:1 by weight and found out that the thermal conductivity of Cu/graphene-based nanofluid is higher than that compared to graphene-based nanofluid. The thermal conductivity of Cu/graphene-based nanofluids containing nanoparticles of graphene/Cu ratio as 7:1 and 5:1 is 0.48 and 0.5 W/mK, respectively, at 60 °C. This is because of the combined effect of graphene sheets and Cu particles decorated over them, both having higher thermal conductivity. The decoration of graphene sheet with Cu particles decreases stacking of graphene sheets, thus elevating thermal properties of the nanofluid. Further, the nanofluid containing Cu/graphene nanoparticles having graphene/Cu ratio of 3:1 and 1:1 shows a decreasing trend of thermal conductivity values, that is, 0.42 and 0.415 W/mK, respectively. This is reported to be happening due to the formation of clusters of Cu particles as their amount is greater than that required to get attached to the functional groups over the graphene sheet. This is how the composition of nanoparticles plays an important role in altering the thermal conductivity of the nanofluid.
3.3.3 Volume Fraction of Nanoparticles in the Nanofluid
The increase in the thermal conductivity of a basefluid due to addition of nanoparticles is well known. But increasing the amount of nanoparticles in the basefluid also affects the thermal conductivity, as shown by many researchers (Alawi et al. 2018; Gupta et al. 2011; Khedkar et al. 2012; Kumar et al. 2018; Tijani and Sudirman 2018). The values of thermal conductivity for different ranges of volume fractions of nanoparticles in different nanofluids recorded in the past can be seen in Table 1. The nanoparticles acting as heat boats carry the heat through the nanofluid. Increasing the number of these heat boats ultimately leads to the increase in transport of the heat energy, thus augmenting the thermal conductivity of the nanofluid. This is due to the intensification of Brownian motion as the number of particles is high. Thermal conductivity of the nanofluid is reported to be having linear relationship with the concentration of the nanoparticles in it (Ali et al. 2010). Figure 5 depicts a trend of thermal conductivity of Al2O3/ethylene glycol nanofluids as a function of volume fraction at various temperatures as studied by Esfe et al. (2015a).
Effect of volume fraction of Al2O3 nanoparticles in Al2O3–ethylene glycol nanofluids on relative thermal conductivity of the nanofluids (Esfe et al. 2015a)
3.3.4 Temperature
Temperature is found to be greatly influencing the thermal conductivity of a nanofluid which is studied by several researchers. Mintsa et al. (2009) investigated the thermal conductivity of Al2O3 and CuO-based nanofluids as a function of temperature and reported an increase in thermal conductivity with rise in temperature for different size of nanoparticles and concentrations of nanofluids. Increase in temperature increases the nanoparticles’ surface energy leading to reduced agglomeration. Graphene, having attained most of the attention of the researchers, and its nanofluids have also found to show an increase in thermal conductivity with temperature, which is reported by Ahammed et al. (2016) at various concentrations of the nanofluid as shown in Fig. 6. It was clear that the factors like vibration of phonons and free electrons and molecular collision and molecular diffusion jointly affect the thermal conductivity of the graphene-based nanofluids.
Effect of temperature on thermal conductivity of graphene–water nanofluids (Ahammed et al. 2016)
One more reason behind the enhancement in the thermal conductivity with temperature is the increase in Brownian motion of the nanoparticles. Two factors responsible for this are the reduction of agglomeration of nanoparticles due to high surface energy at high temperatures and the reduction in viscosity of the basefluid which again facilitates swift Brownian motion of the nanoparticles (Yu-Hua et al. 2008).
It was also found that the solvent molecules get adhered and form layers of ordered arrangement of molecules to the hydrophilic colloidal particles, that is, nanoparticles added to it (Lee 2008). This layer possesses higher thermal conductivity and aids in increasing the heat transfer induced due to the nanoparticles (Keblinski et al. 2002). It is also well established by Suganthi et al. (2013) for ZnO-propylene glycol nanofluids that the thickness of this layer is high at lower temperatures where Brownian motion fails to be occurring due to higher viscosities of the basefluid. Thus, it was found that at a temperature of 10 °C, the liquid layer enhances the thermal conductivity of the nanofluid and attains a maximum, which further decreases as the temperature increases to 30 °C. This unusual but true mechanism of decrease in thermal conductivity with increase in temperature is also found out for ZnO-ethylene glycol nanofluids (Suganthi et al. 2014), as shown in Fig. 7.
Effect of temperature on thermal conductivity of ZnO–ethylene glycol nanofluids (Suganthi et al. 2014)
3.3.5 pH
Nanofluid is a suspension of nano-sized material in some basefluid. Its uniform dispersion in a typical basefluid shall definitely depend upon the charges on its surface. The surface charges will affect the degree of agglomeration, which ultimately influences the thermal properties of the nanofluid. pH of a nanofluid affects these surface charges and this is well defined by Wang and Zhu (2009) in their investigation of thermal conductivity of Al2O3 and Cu-based nanofluids, which is shown in Fig. 8. An increase in pH alters the charges on the surface of nanoparticles which raises the electrostatic forces of repulsion between two nanoparticles. This leads to reduction in agglomerative tendency of the nanoparticles in the nanofluid. Thus, the pH is found to be in direct relation with the electrical properties of the nanofluid, which will also be discussed later in this chapter.
Effect of pH of Al2O3 and Cu-based nanofluids on their thermal conductivity (Wang and Zhu 2009)
3.4 Models for Thermal Conductivity Prediction
Thermal conductivity has been of great interest in the convective heat transfer study of nanofluids on which theoretical and experimental studies have been done. Mechanism of heat conduction has been proposed, which is found to be dependent on the Brownian motion of nanoparticles, interfacial liquid layer of nanofluid (effect of nanolayer), nanoparticle clustering and nature of heat transport of the nanoparticles in the nanofluid. Many researchers have strived to derive models that can exactly predict the thermal behaviour of the nanofluid. It was Maxwell (1881), who was the first to develop the effective thermal conductivity model as given in Eq. (2). The equation can predict the effective thermal conductivity of the solid–liquid suspensions (\( k_{\text{eff}} \)), where \( k_{p} \) is the thermal conductivity of the dispersed particles, \( k_{l} \) is the thermal conductivity of the basefluid (continuous phase of liquid) and \( \emptyset \) is the volume concentration of the nanoparticles in the suspension.
Maxwell’s model (Maxwell 1881) assumes the thermal conductivity improvement due to the presence of nanolayer at the surface of the solid in a solid–liquid suspension. It has been expected that the thermal conductivity of the nanolayer on the surface of the nanoparticle is higher than that of the basefluid. Further, this model was modified by Maxwell (1881) so as to form a modified Maxwell model as given in Eq. (3), where \( \beta \) is the ratio of thickness of nanolayer (h) to the radius of the nanoparticle (r) and is given as \( \beta = h/r \). This equation is found to be valid for dispersion of spherical-shaped particles in the basefluid.
Further, Hamilton and Crosser (1962) introduced a model for a solid–liquid suspension as given in Eq. (4), where \( k_{p} \) is the thermal conductivity of particles, \( k_{f} \) is the thermal conductivity of basefluid, \( \emptyset \) is the volume fraction of particles, \( n \) is the empirical shape factor defined as \( n = \frac{3}{\psi } \) and \( \psi \) is the sphericity which is explained as the ratio of the surface area of a sphere having the same volume as that particle to the surface area of that particle. It is applicable for spherical and cylindrical particles.
Thermal conductivity model for randomly distributed spherical particles in a basefluid using the thermal conductivity of the particles and basefluid has been reported by Bruggeman (1935a), as given in Eq. (5).
In this equation, the effective thermal conductivity \( k_{eff} \) is determined as given in Eq. (6).
where the factor of \( \Delta \) is calculated by using Eq. (7)
Xue (2005) reported a model as given in Eq. (8) to calculate the thermal conductivity of carbon nanotube (CNT)-based nanofluid, which is explained as follows:
Xuan et al. (2003) developed a model as given in Eq. (9) that considered the effect of Brownian motion and nanoparticles clustering, where \( \mathop R\nolimits_{d} \) is the apparent radius of the nanoparticle clusters and \( \mathop K\nolimits_{B} \) is Boltzmann constant.
Later, Timofeeva et al. (2007) expressed a model, which is based on the effective medium theory as given in Eq. (10).
Prasher et al. (2005) stated that there is occurrence of convection-induced Brownian motion called as nanoconvection and developed a model as given in Eq. (11), where \( k_{m} = k_{f} \left( {1 + 0.25Re_{B} Pr} \right) \) is the matrix conductivity, \( Re_{B} = \frac{1}{\upsilon }\sqrt {\frac{{18K_{B} T}}{{\pi \rho_{p} d_{p} }}} \) is the Brownian Re number, m = 2.5%\( \pm \) 15% is a regression constant, \( \alpha_{B} = \frac{{2R_{b} k_{m} }}{{d_{p} }} \) is the particle Biot number and \( R_{b} \) is the interfacial thermal resistance existing between nanoparticle and liquid.
Later, Koo and Kleinstreuer suggested a model (Koo and Kleinstreuer 2004, 2005) as given in Eq. (12), which is a combined thermal conductivity model considering the Brownian motion and the volume fraction of the liquid with nanoparticles, where \( \theta \) is the fraction of the liquid volume which travels with a particle.
However, the difficulty of this model is that \( \theta \) and \( f \) are hard to obtain, and so they are to be expressed differently for different kinds of nanofluids. For example, for CuO nanofluid, the expression in Eq. (12) becomes the expression given in Eq. (13).
Yu and Choi (2003) proposed a renewed Maxwell model as given in Eq. (14) considering the solid–liquid interfacial layer on nanoparticles in the nanofluid.
Feng et al. (2007) reported a model as given in Eq. (15) to enhance the Yu and Choi (2003) model by presenting an equivalent thermal conductivity of the nanoparticles. They also investigated the influence of presence of the interfacial layer between nanoparticles and liquid.
Here, \( \beta = 1 + \frac{t}{{R, \,{\text{and}}\, k_{\text{pe}} }} \) is equal to the equivalent thermal conductivity of the nanoparticles and \( \gamma_{1} \) is the thermal conductivity ratio of interfacial layer to particles.
Further, Pak and Choi (1998) presented a new model as given in Eq. (16) considering that the thermal conductivity enhancement of the nanofluids is caused due to the dispersion of the suspended nanoparticles.
Jang and Choi (2007) established a model as given in Eq. (17) based on the influence of Brownian motion of nanoparticles. This thermal conductivity model is based on various four factors, like the collision of basefluid molecules, collision of nanoparticles driven by Brownian motion, thermal diffusion in nano-sized particle and fluids, and the thermal interaction of particle with fluid molecules, where \( Re_{d} = \frac{{C_{\text{RM}} d_{p} }}{\upsilon } \) in which \( C_{\text{RM}} = \frac{{K_{B} T}}{{3\pi \mu_{f} d_{p} l_{f} }} \), \( d_{f} \) is the equivalent diameter of particle and \( l_{f} \) mean free path.
3.5 Applications Based on Thermal Properties of the Nanofluids
As it has been well known that the nanofluids possess extraordinary thermal properties, compared with conventional fluids, there has also been a tremendous increase in the studies for different applications of the nanofluids in numerous heat transfer systems. Several researchers have investigated the heat transfer intensification of nanofluids by using different geometries of lab-scale heat exchanger setups and have proposed a possible and feasible application of nanofluids as an alternative to conventional heat transfer fluids. Heyhat et al. (2013) studied the convective heat transfer performance of Al2O3 nanofluids with water as basefluid flowing in a horizontal tube at constant wall temperature and laminar flow conditions. An increase in the heat transfer coefficient was reported for the nanofluid compared to that of basefluid and that it was further noticeable at higher Reynolds numbers. At fully developed flow region, the improvement in heat transfer coefficient was reported to be 32% for 2 vol.% of Al2O3 nanofluid. Convective heat transfer performance of TiO2/water nanofluid in a helical coiled tube heat exchanger has been studied by Kahani et al. (2014). A thermal performance factor of 3.72 was achieved for 2 vol.% TiO2 nanofluid flowing at Reynolds number of 1750. A heat transfer coefficient enhancement of 105% has been found by Bhanvase et al. (2014) for TiO2-based nanofluid using ethylene glycol/water mixture as basefluid flowing in a straight tube heat exchanger suggesting a great alternative for applications in heat transfer equipments. Huang et al. (2015) investigated the convective heat transfer and pressure drop of Al2O3-based and multi-walled carbon nanotubes-based (MWCNT) nanofluid. A higher heat transfer was achieved by using the nanofluids; however, increasing concentrations of the nanofluids increased the pressure drop. But, this was only found to be happening at higher concentrations of nanofluid due to increased viscosity, whereas viscosities of nanofluids with low concentrations did not seem to affect the pressure drop due to negligible increase in viscosity as compared to the basefluid. Convective heat transfer coefficient of Fe3O4/graphene nanocomposite-based nanofluid was found to enhance by 14.5% compared to the basefluid in a straight tube heat exchanger by Askari et al. (2017). Bhanvase et al. (2018) investigated the boost in heat transfer of polyaniline-based (PANI) nanofluids using water as a basefluid. They found a 69.62% enhancement in heat transfer coefficient for 0.5 vol.% PANI nanofluid.
Applications of nanofluid as car radiator coolant have also been found by many researchers. Naraki et al. (2013) experimentally examined the heat transfer coefficient of CuO/water nanofluids in a car radiator coolant under laminar flow conditions. It has been reported that there is 8% increase in the heat transfer coefficient of the CuO nanofluids compared to water. However, it has been further pointed out that even though there is an increase in the thermal performance of the car radiator with application of the nanofluid, the factors like sedimentation and stability should also be considered before their application. Studies on thermo-physical properties comprising thermal conductivity, density, viscosity and specific heat of Al2O3-based nanofluids as car radiator coolants have been conducted by Elias et al. (2014). Similarly, Al2O3-based nanofluids using ethylene glycol as a basefluid have been studied for car radiator application by Goudarzi and Jamali (2017). For this purpose, the authors used a radiator with wire coil inserts and reported that the thermal performance of the radiator can be enhanced up to 14% by the use of Al2O3/ethylene glycol nanofluids. Ali et al. (2015) investigated the application of ZnO nanofluids as car radiator coolants and found an enhancement in heat transfer of 46% for a nanofluid of concentration 0.2 vol.%.
Nanofluids for cooling applications in electronic systems have also been studied widely. Selvakumar and Suresh (2012) studied the application of CuO/water nanofluid in a thin-channelled copper heat sink. A highest value of convective heat transfer coefficient of 29.63% was reported for the water block by using 0.2 vol.% CuO nanofluid compared to deionized water. Similar study has been presented by Sohel et al. (2014) using Al2O3/water nanofluids in an electronic heat sink that helped them achieve 18% improvement in the heat transfer coefficient compared to distilled water. They compared their study with the one previously made by Selvakumar and Suresh (2012) and found that the Al2O3/water nanofluid reduces the heat sink base temperature more than that reduced using the CuO/water nanofluid, even though the heat input is thrice the heat input for study using CuO/water nanofluid. Khaleduzzaman et al. (2015) studied the compatibility of Al2O3/water nanofluid to be used in a heat sink by investigating its stability. They studied Al2O3/water nanofluids in the volume concentration range of 0.1–0.25 vol.% and found that they are satisfactorily stable and that there is no sedimentation and clogging occurring using these nanofluids. Khatak et al. (2015) studied the effect of ZnO nanofluid in spray cooling of electronic devices. They observed that a 0.05 vol.% ZnO nanofluid was successful in decreasing the specimen surface temperature by 15% at a heat input and nanofluid flowrate of 180 W and 20 ml/min, respectively.
Nanofluids have also found applications in effective extraction of the solar energy. Efficiency of a solar collector has been studied by applying Al2O3/water nanofluid as an absorbing medium by Yousefi et al. (2012). For the study, they used a flat plate solar collector and passed the nanofluid to study the effect of nanofluid flowrate, concentration of nanoparticles in the nanofluid and use of surfactant. 28.3% increase in the thermal efficiency of the solar collector has been reported when 0.2 wt% Al2O3/water nanofluid was passed through the collector. Gupta et al. (2015) also studied the Al2O3/water nanofluid in a direct absorber solar collector (DASC) of tube-in-plate type. The reported increase in the collector efficiency was 8.1% when 0.005 vol.% Al2O3/water nanofluid was passed through the DASC at a flowrate of 1.5 l/min. Sokhansefat et al. (2014) investigated the simulation of performance of Al2O3 nanofluid using synthetic oil as a basefluid in a parabolic trough collector tube and suggested a possible and beneficial application of it in the solar thermal energy collection in a parabolic trough geometry. Experimental study of CuO/water nanofluid application in a direct absorption concentrating parabolic solar collector (DAPSC) was reported to exhibit an increase in the thermal efficiency of 52% for CuO/water nanofluid with volume concentration of 0.008 vol.% (Menbari et al. 2016).
Nanofluids have also found to have gained pace in applications in refrigeration systems. Kumaresan et al. (2012) experimentally investigated convective heat transfer using MWCNT-based nanofluid as a secondary refrigerant using water/ethylene glycol mixture as basefluid in a tubular heat exchanger. An enhancement of nearly 160% was found to be occurring by the use of 0.45 vol.% of the MWCNT-based nanofluids, which was reportedly happening due to the higher thermal conductivity, larger aspect ratio of the nanoparticles, particles rearrangement and delayed development of boundary layer. Similar study using single-walled carbon nanotubes-based (SWCNT) nanofluid has been conducted by Vasconcelos et al. (2017). Influence of shape of ZnO-based nanorefrigerant on the heat transfer using the refrigerant R-134a as a basefluid is studied by Maheshwary et al. (2018). Spherical-shaped ZnO nanoparticles in the R-134a refrigerant found to exhibit a thermal conductivity enhancement of the refrigerant by 25.26%. Thus, the thermal properties of the nanofluids have been exploited in a numerous ways to make heat transfer processes more efficient with an augmentation in the thermal transport caused by the presence of nanoparticles in the nanofluid.
4 Electrical Conductivity of Nanofluids
Nanofluids are well known in the field of heat transfer as numerous researchers have already studied its heat transfer characteristics and have reported several applications of the nanofluids in thermal transport system as seen earlier in this chapter. But there are several other properties of nanofluids that cannot be ignored. One of them is the electrical conductivity of the nanofluids. As known already, the electrical conductivity is the ability to transport or conduct electric current. Awareness about the fact that the nanofluid may have superior electrical conductivity values than other materials makes one to analyse it and apply a certain nanofluid in a typical system which require fluids that conduct electrical energy. As the nanofluids possess higher thermal conductivity, it is possible for them to also possess higher electrical conductance.
Maxwell (1881) has very well defined that the electrical conductivity is affected by the physical properties of the nanoparticles and the basefluid. But many researchers have found that it is not only affected by the properties of the components of the nanofluid but also their interaction with each other (Ganguly et al. 2009; Minea and Luciu 2012; Shen et al. 2012). Chakraborty and Padhy (2008) found that the agglomeration of the nanoparticles leads to efficient electrical conductivity due to the nanoparticles making physical contact with each other. But in contrast to that, they have stated that the agglomeration of the nanoparticles also leads to larger particle size having larger mass that may reduce the electrophoretic mobility due to increased viscosity of the nanofluid.
The DLVO theory developed for explaining the stability of colloids in suspension states that there is a formation of a charged layer on a colloidal particle in the nanofluid. This layer is composed of ions that are charged opposite to the particle surface and so form a charged diffuse layer over the particles. These ionic charges are found to be forming due to the adsorption and desorption of ions present in the solution on the particle surface (Cruz et al. 2005). These charged particles can then transfer the charge and behave as charge carriers. In a nanofluid, the nanoparticles act as carriers of electrical charge. The electrical double layer (EDL) has also found to be one of the mechanisms responsible for the electrical transport properties of the nanofluids by many researchers (Chakraborty and Padhy 2008; Ganguly et al. 2009; Minea and Luciu 2012).
4.1 Measurement of Electrical Conductivity of the Nanofluids
Electrical conductivity of nanofluids has mostly been measured by researchers all around the world by using electrical conductivity metre consisting of a probe, whose electrical circuit with the circular electrode that makes up the working principle of the electrical conductivity metre is shown in Fig. 9a. The one shown in Fig. 9b is a typical electrical conductivity measuring device known as a four-cell conductivity electrode metre (CyberScan CON 11) made by Eutech Instruments Pte Ltd in Singapore having in-built automatic temperature compensation (ATC). This metre gives instant value of electrical conductivity and temperature.
Setup for electrical conductivity measurement. a Electrical circuit with electrode and b Electrical conductivity metre setup (Sarojini et al. 2013)
4.2 Factors Affecting the Electrical Conductivity of the Nanofluids
Electrical conductivity being the property dependent on the components of the nanofluid can be affected by many factors. As discussed earlier, the electrical conductivity of the nanofluids is dependent on the charge over the particles. They can also be dependent on the amount and size of these charge carriers, that is, the nanoparticles. These charges on the nanoparticles are dependent on the pH (Sigmund et al. 2000). Also, other nanofluid environment factors like temperature may prove to be affecting the electrical conductivity similar to its governing effect on the thermal conductivity.
4.2.1 Concentration of Nanoparticles
It has been already known that the electrical conductivity is due to the electrical double layer formed over the nanoparticles, making them the electric charge carriers. Many researchers studied the effect of amount of these carriers, that is, nanoparticles dispersed in various kinds of basefluids on their respective nanofluid electrical conductivity (Baby and Ramaprabhu 2010; Glory et al. 2008; White et al. 2011). Increasing the nanoparticle concentration increases the interaction between the nanoparticles resulting in increase in electrical conductivity (Shoghl et al. 2016). Liu et al. (2004) investigated the electrical conductivity of the multi-walled carbon nanotube (MCNT) dispersed in chloroform and toluene and reported that the electrical conductivity of the nanofluids intensifies with increase in the concentration of the nanofluids. Lisunova et al. (2006) also studied the electrical conductivity of MCNTs nanofluid using water as a basefluid and Trixton X-305 as a dispersant. It has been reported that augmentation in the electrical conductivity is more pronounced as the volume fraction of the nanofluid exceeds 0.01, which happens reportedly due to the aggregation and percolation behaviour of the nanotubes. The concentration where this phenomenon occurs is known as the percolation threshold. The nanotubes having a high aspect ratio form networks and behave as electro-conductive clusters at higher concentrations, which ultimately lead to a high electrical conductivity. However, Glover et al. (2008) reported that there is no percolation threshold for SWNT-based aqueous nanofluids and that there is a linear relationship between the electrical conductivity and the concentration depicting ionic conduction behaviour. The electrical conductivity of the water-based single-walled carbon nanotube (SWNT) nanofluids reported to be increased from 0.12 × 10−3 to 1.6 × 10−3 S/m when nanofluid concentration increases from 0 to 0.5 wt%, which was around 13 times more. It was shown for alumina nanofluids that the electrical conductivity also linearly increases with increase in the concentration of the nanofluid by Ganguly et al. (2009) and it was recorded to be 258 µS/cm for nanofluid volume fraction of 0.03 at room temperature. An enhancement of electrical conductivity exhibited by graphene-based nanofluids using mixture of water and ethylene glycol as basefluids was studied by Baby and Ramaprabhu (2010). They found that a 0.03% concentrated graphene nanofluid shows an electrical conductivity enhancement of almost 1400% at 25 °C. A 973 times higher electrical conductivity was recorded by Shen et al. (2012) by adding ZnO nanoparticles to insulating oil at a concentration of 0.75 vol.%. An outstanding enhancement of 25678% in electrical conductivity was recorded by Hadadian et al. (2014) for water-based graphene oxide nanofluid at a very low graphene oxide mass fraction of 0.0006. Electrical conductivity of nitrogen-doped graphene-based nanofluids was studied by Mehrali et al. (2015) and they found a maximum electrical conductivity enhancement of 1814.96% for a 0.06 wt% nanofluid. Adio et al. (2015a) investigated the effect of volume fraction on the electrical conductivity of MgO-based nanofluids using ethylene glycol as basefluids. For the MgO nanofluid concentrations of 0.1, 0.5, 1, 2 and 3 vol.%, the electrical conductivity was found to be 3.01, 6.68, 8.73, 11.74 and 14.05 µS/cm, respectively. Al2O3 nanofluids prepared using bio-glycol/water mixtures as basefluid were studied by Abdolbaqi et al. (2016) and they found a decrease in electrical conductivity with an increase in the concentration of the nanofluid. A nanofluid prepared using bio-glycol/water mixture in the ratio of 40:60 by volume showed a decrease in electrical conductivity from 620 to 472 µS/cm when the concentration of Al2O3 nanoparticles increased from 0 to 2 vol.%. It has been found that the effect of nanoparticle concentration is more pronounced than that of temperature on the electrical conductivity of the nanofluids (Heyhat and Irannezhad 2018).
4.2.2 Size of Nanoparticles
Sarojini et al. (2013) studied that for alumina nanoparticles, the reduction in particle size leads to an increment in the electrical conductivity of the nanofluids in which they are dispersed. This is found to be happening due to the higher electrophoretic mobility of the smaller-sized particles compared to the larger-sized particles. A 1 vol.% alumina nanofluid prepared using water as a basefluid show electrical conductivity of nearly 95, 240 and 300 µS/cm for dispersed nanoparticles of size 150, 80 and between 20 and 30 nm, respectively.
Different types of nanofluids were prepared by Konakanchi et al. (2011) to study the effect of different parameters affecting the electrical conductivity of the nanofluids. A decrease in the electrical conductivity has been reported with an increase in the particle size of Al2O3 nanoparticles. 1% Al2O3 nanofluid showed electrical conductivity of 165, 80 and 15 µS/cm when 10, 20 and 45 nm-sized Al2O3 nanoparticles were dispersed in propylene glycol and water mixture at 60:40 mass ratio, respectively, at around 40 °C. Further, 1% ZnO nanofluid showed electrical conductivity of 36 and 24 µS/cm, when 36 and 70 nm-sized Al2O3 nanoparticles were dispersed in the same basefluid, respectively, at around 40 °C. ZnO nanofluids containing different sizes of ZnO nanoparticles prepared using propylene glycol as basefluid have been studied by White et al. (2011). Also, a decrease in the electrical conductivity from 9.6 to 1.2 µS/cm with an increase in the ZnO nanoparticle size from 20 to 60 nm in 7 vol.% concentrated nanofluid has been reported. The effect of increasing the size of the nanoparticles is opposite to the effect of increasing volume fraction of the nanoparticles in the nanofluids, leading to a decrease in the electrical conductivity of the nanofluids.
Azimi and Taheri (2015) investigated the effect of particle size of CuO nanoparticles dispersed in water on the electrical conductivity of the water-based nanofluids. An optimum particle size of the CuO nanoparticles, which is 95 nm, has been determined that exhibits maximum electrical conductivity of 0.108 µS/cm for 0.18 g/l concentration of CuO nanofluid at 25 °C. A decrease of diameter below 95 nm or increase beyond 95 nm of the CuO nanoparticles in the nanofluid leads to a decrease in the electrical conductivity of their nanofluid.
4.2.3 Temperature
The electrical conductivity of nanofluids relies on the efficiency of electron transfer through the nanofluids due to the nanoparticles. With an increase in temperature, the electrons can transfer through the energy barriers very easily as found out by Liu et al. (2004) or multi-walled carbon nanotube nanofluids. Several researchers have reported an increase in electrical conductivity with an increase in temperature (Hadadian et al. 2014; Konakanchi et al. 2011; Shen et al. 2012). However, it has also been known that the influence of temperature on electrical conductivity is lesser than that of the concentration (Mehrali et al. 2015, Goharshadi and Azizi-Toupkanloo 2013). The increase in electrical conductivity of nanofluids becomes more pronounced at higher temperatures (Adio et al. 2015a; Heyhat and Irannezhad 2018). Also, the mechanism for an enhancement in the electrical conductivity of nanofluids is different from the mechanism of enhancement of their thermal conductivity (Sarojini et al. 2013). Ganguly et al. (2009) found an increase of electrical conductivity of 0.03 volume fraction of alumina/water nanofluids from 258 to 351 µS/cm for an increase in temperature from 24 °C to 45 °C. Baby and Ramaprabhu (2010) also studied the effect of temperature on the graphene-based nanofluids using water as well as ethylene glycol as basefluids and reported an increase in electrical conductivity if the temperature of the nanofluid was increased. Konakanchi et al. (2011) also showed almost a linear relationship for electrical conductivity and temperature of Al2O3 and SiO2 nanofluids prepared using mixture of propylene glycol and water as a basefluid. On the other hand, electrical conductivity of water-based Al2O3 nanofluids was found to remain constant with respect to temperature by Minea and Luciu (2012). Dong et al. (2013) showed that for aluminium nitride-transformer oil-based nanofluids, the electrical conductivity shows a decreasing trend from 25 °C to 40 °C, but it became stable after 40 °C. Higher temperature of nanofluids is found to promote aggregation and thus formation of transport paths for conduction of electric charge leads to enhancement in electrical conductivity of the nanofluids (Bagheli et al. 2015). Naddaf and Heris (2018) studied the electrical conductivity of MWCNT-based nanofluids using diesel oil as a basefluid and oleic acid as a surfactant and recorded the electrical conductivities as 0.18, 135.2, 299.9 and 444.9 µS/cm for MWCNT-based nanofluids of concentrations 0.05, 0.1, 0.2 and 0.5 wt%, respectively, at a temperature of 20 °C.
4.3 Role of Zeta Potential
When nanoparticles are dispersed in the basefluid, there is a certain layer of the basefluid surrounding it. The thin layer of the liquid formed on the particle in a nanofluid is called as the Stern layer. There is also a layer known as diffuse layer that comprises the loosely associated ions at the outer surface of the Stern layer. Both of these layers are responsible for the formation of the electrical double layer. The loosely associated ions in the diffuse layer shear with the ions in the bulk fluid when the particle undergoes a motion, most commonly the Brownian motion. Zeta potential is the electric potential at this shear surface. Schematic of zeta potential is shown in Fig. 10. The zeta potential is known by measuring the velocity of the particle moving towards the electrode in the presence of an electric field externally maintained across the nanofluid sample. A value of zeta potential of ± 30 mV is considered as a value exhibited by a stable nanofluid and that exhibiting a value above or below this value is known as stable nanofluid or unstable nanofluid, respectively. Zeta potential is measured by determining the electrophoretic mobility of the particles.
Schematic of zeta potential (Chakraborty 2019)
4.4 Relation of Stability and Electrical Conductivity of Nanofluids
Stability is the degree of uniform dispersion of the nanoparticles in the basefluid and also one of the factors considered in electrical conductivity of the nanofluids (Shoghl et al. 2016). Nanofluids contain nanoparticles that are susceptible to surface charges. These surface charges that have major role in electrical conductivity also have a major role in the stability of the nanofluid (White et al. 2011). The surface charge on a nanoparticle is due to the protonation and de-protonation of functional groups on its surface (Lee et al. 2006), which causes the formation of electrical double layer (EDL) at the surface of the particles. For example, the stability of graphene oxide nanofluids is attributed to the charge developed on its surface due to the de-protonation of acidic groups on its surface (Hadadian et al. 2014). Use of dispersant or surfactants affect the ionic charges on the nanoparticles. They alter the pH of a nanofluid, and so, ultimately, the stability of the nanofluid is affected (Sarojini et al. 2013). The purpose of changing the pH is to deviate the charge of the nanoparticles from their isoelectric point (IEP), so as to decrease the agglomeration (Zawrah et al. 2016). IEP is the point when there is zero charge on the nanoparticles and which causes maximum aggregation of the nanoparticles due to maximum van der Waals forces of attraction. An increase in the pH will increase the ionic strength causing a decrease in the van der Waals forces and ultimately reduction in the aggregation (Younes et al. 2012). As we have seen that aggregation and de-aggregation is important as far as electrical conductivity of the nanofluid is concerned, the change in pH and stability is related to the electrical properties of the nanofluid. The study of electrical conductivity in relation to stability of the nanofluid has been done by some researchers (Cruz et al. 2005; Ganguly et al. 2009). In fact, the electrical conductivity helps determining the stability of the nanofluid (Shoghl et al. 2016).
4.5 Models for Electrical Conductivity Prediction
Models present an additional insight to the variations of certain parameter with respect to changes in other parameter and provide a prediction of the experimental results for the same. Similar to thermal conductivity, there are model equations which are derived for predicting the electrical conductivity as well. A classical model developed by Maxwell (1881) for conductivity in heterogeneous media as given in Eq. (18) is being used since long ago for the prediction of electrical conductivity of nanofluids. This equation gives the relation between the effective conductivity of the nanofluid (\( \lambda_{\text{eff}} \)) and conductivity of the basefluid (\( \lambda_{\text{bf}} \)) as a function of conductivity ratio of the two phases (\( \alpha \)) and volume fraction of the nanoparticles in the nanofluid (\( \phi \)). This correlation given by Maxwell is valid for spherical particles which are randomly distributed in the dispersions. Also, it assumes that there is no formation of aggregates and the distances between two particles is greater than their diameters.
Here, ‘\( \alpha \)’ as given in Eq. (19) is the ratio of conductivity of the nanoparticles (\( \lambda_{p} \)) to the conductivity of the basefluid (\( \lambda_{bf} \)).
Certain approximations made by Cruz et al. (2005) to simplify the Maxwell’s equation, are presented in Table 2. Maxwell’s model only considers the properties of the individual components of the solid–liquid mixture and not their interaction.
Several researchers have tested this model for electrical conductivity of diverse nanofluids so as to verify whether it can predict their experimental results, but have reached a conclusion that it fails to predict the behaviour of nanofluid and do not comply with the practical findings (Ganguly et al. 2009; Lisunova et al. 2006). Lisunova et al. (2006) stated that the classical Maxwell model fails to predict the electrical conductivity of MWCNT-based nanofluids due to the elongated shape and high aspect ratio of the nanotubes that is not valid for usage of the Maxwell model. A higher electrical conductivity of the suspension for the concentration higher than 0.01 volume fraction was found, which was reportedly happening due to the aggregation and networking of the MWCNTs which form electroconductive clusters that behave as a pathway for electrical conductance. But this is not true for every kind of nanoparticles as proposed by Chakraborty and Padhy (2008) as the reduction in the density of particles due to their agglomeration shall reduce the electrical conductivity or if the particles are naturally non-conductive. Ganguly et al. (2009) also suggested that the Maxwell’s model cannot predict the electrical conductivity. They have reported that the Maxwell model underpredicts the electrical conductivity of water-based Al2O3 nanofluids when compared to the experimental values. This was due to the dependence of electrical conductivity of the Al2O3 nanofluids on some additional factors rather than only the physical properties of the fluid and particles. Therefore, they presented a new correlation, as given in Eq. (20), to predict the electrical conductivity of the nanofluids.
Further, Konakanchi et al. (2011) studied the electrical conductivity of three types of nanofluids, namely Al2O3, SiO2 and ZnO nanofluids, using propylene glycol/water mixture as the basefluid. They developed a correlation given in Eq. (21) for prediction of electrical conductivity of the Al2O3 nanofluids (\( \lambda_{nf} \)) with respect to temperature (T) which has correlation coefficient of 0.9923.
Also, for electrical conductivity of the SiO2 nanofluids (\( \lambda_{\text{nf}} \)), they have confirmed the agreement of the experimental data with respect to temperature (T) with correlation given in Eq. (22) which has correlation coefficient of 0.99.
Similarly, for electrical conductivity of ZnO nanofluids (\( \lambda_{nf} \)), a correlation was developed. But, unlike the equation for Al2O3 nanofluid and SiO2 nanofluid electrical conductivity, the equation for ZnO nanofluid electrical conductivity was a second-order polynomial equation as given in Eq. (23) which has correlation coefficient of 0.99.
Similarly, for the modelling of electrical conductivity of Al2O3 nanofluid (\( \lambda_{\text{nf}} \)) in relation with the percentage volumetric concentration of the nanofluid (ϕ) the authors present a polynomial equation, as given in Eq. (24), having correlation coefficient of 0.9994.
Further, they also developed correlations for electrical conductivity (\( \lambda_{\text{nf}} \)) in relation with the average particle size (d) for Al2O3, SiO2 and ZnO nanofluids as given in Eqs. (25), (26) and (27), respectively.
Ohshima (2003) investigated the electrokinetic phenomena of a dilute colloidal suspension consisting spherical particles in a salt-free medium that contains counter-ions. Further derivation for electrophoretic mobility of the suspended particles has been presented and expression for determining the electrical conductivity of the suspensions has been obtained. Further two types of cases have been stated for the model depending upon the relation between the actual charge, that is, amount of nanoparticles and its critical value. The first case states that if the charge is lower than the critical charge value, then there is a linear increase in the electrical conductivity and electrophoretic mobility occurring due to counter-ions with the increase in the charge. The second case states that if the charge is higher than the critical charge value, then the electrical conductivity and electrophoretic mobility become constant and are independent of the charge, that is, amount of nanoparticles due to counter-ion condensation effects. White et al. (2011) studied the electrical conductivity of ZnO-based nanofluids prepared using propylene glycol as a basefluid. They have used the model developed by Ohshima and clearly found that their experimental values are consistent with those given by the model. At lower volume fractions, the first case of the model is found to be satisfactorily predicting the electrical conductivity values arising due to the counter-ions and gives a linear fit. This model departs from a certain critical concentration proving the counter-ion condensation occurring at concentrations higher than the critical value. So they have also stated that this condition of the nanofluid is due to the elongated geometry which is different from that assumed by the model and that the optimization of the counter-ion condensation effects can increase their applicability. Minea and Luciu (2012) studied the Maxwell model along with the Bruggeman model (1935a, b) as given in Eq. (28) for Al2O3 nanofluids prepared in water, but both the models could not predict the experimental data.
So, they presented a new model, by performing a regression analysis, having a correlation coefficient of 0.9975 which is given in Eq. (29) relating the thermal conductivity of the Al2O3/water nanofluids (\( \lambda \)) with temperature (T) and volume fraction (ϕ).
Again, as found out by Shen et al. (2012) for ZnO nanofluids prepared using insulated oil as a basefluid, the Maxwell model underpredicts the electrical conductivity of the nanofluid. They concluded that the electrical conductivity of the nanofluid depend on two additional factors along with Maxwell electrical conductivity (\( \lambda_{M} \)). Those two factors are the electrical conductivity due to electrophoresis (\( \lambda_{E} \)) and due to the Brownian motion (\( \lambda_{B} \)). The equation thus derived by them to predict the electrical conductivity of the ZnO-insulated oil nanofluid is as given in Eq. (30) where \( \lambda_{\text{bf}} \) is the electrical conductivity of the basefluid, \( \phi \) is the volume fraction of the nanoparticles in the nanofluid, \( \varepsilon_{r} \) is the relatively dielectric constant of the nanofluid, \( \varepsilon_{0} \) is the dielectric constant of the vacuum, \( U_{0} \) is the zeta potential of the nanoparticles relative to the basefluid, r is the radius of the spherical nanoparticle, R is the thermodynamic constant, t is the temperature, L is the Avagadro’s constant, \( \lambda \) is the viscosity index of the fluid, \( T_{0} \) is the temperature of the nanofluid at which the viscosity is measured, ρ is the nanofluid density and ν is the nanofluid kinematic viscosity. This equation is valid for particles with higher electrical conductivity than the basefluid as the first term, that is the term for Maxwell conductivity, is approximated for such solid–liquid systems.
Similar study was done by Dong et al. (2013) for transformer oil-based aluminium nitride (AlN) nanofluids in which they reported that the experimental electrical conductivity is in good agreement with the one predicted using the model given in Eq. (31), which is similar to the previous one by Shen et al. (2012) ignoring the effect of Brownian motion.
The Shen’s model (2012) has been used by Bagheli et al. (2015) to predict electrical conductivity of Fe3O4 nanofluid and found that it can satisfactorily predict the electrical conductivity at lower volume fraction. However, at higher concentrations the Shen’s model fails to predict the electrical conductivity of Fe3O4 nanofluid which is reportedly due to the agglomeration effects which are not considered in the model.
Sarojini et al. (2013) found that the Maxwell model fits well for conducting particles like Cu but not for non-conducting particles like Al2O3 and CuO dispersed in polar solvents. In fact, the Maxwell model underestimates the electrical conductivity of Al2O3 and CuO nanofluids. Same has been found by Zakaria et al. (2015) for water/ethylene glycol mixture-based Al2O3 nanofluids with ethylene glycol concentration in the basefluid above 40%. At higher ethylene glycol concentration, that is, above 80%, there is negligible error between the experimental value and the predicted value by the Maxwell model. Hadadian et al. (2014) studied the electrical conductivity of graphene oxide-based nanofluids and found out an equation for predicting electrical conductivity for the sheet-like material dispersed in water. They developed an equation for a range of temperature amongst which at 25 °C, the empirical relationship is as given in Eq. (32) having a correlation coefficient of 0.9998, where \( f_{m} \) is the mass fraction of the graphene oxide sheets in the nanofluid.
Water-based nitrogen-doped graphene nanofluids using Trixton X-100 as a surfactant for dispersion were prepared by Mehrali et al. (2015) and similar equation was found out for determining the electrical conductivity of the nanofluid in relation with the weight percentage (wt%) at 25 °C having a correlation coefficient of 0.999 as given in Eq. (33).
The electrical conductivity of MgO/ethylene glycol nanofluids is also incorrectly predicted by the Maxwell as well as the Ohshima’s model as per the study of Adio et al. (2015). Also, for Al2O3 nanofluids prepared using bio-glycol/water mixtures as basefluid, the Maxwell’s model shows similar characteristics to those obtained by experiment but underpredicts the experimental data as found out by Abdolbaqi et al. (2016). Shoghl et al. (2016) studied a wide range of water-based nanofluids, namely Al2O3 nanofluid, carbon nanotube (CNT) nanofluid, CuO nanofluid, MgO nanofluid, TiO2 nanofluid and ZnO nanofluid and found that the electrical conductivity of all these nanofluids cannot be satisfactorily predicted by the Maxwell model. So, they proposed new models for electrical conductivity (λ) of each nanofluid as given in Eqs. (34), (35), (36), (37), (38) and (39), each with a correlation coefficient of 0.999, 0.962, 0.998, 0.998, 0.999 and 0.976, respectively, as a function of volume percent of the nanofluid (ϕ).
Nurdin and Satriananda (2017) reported that the electrical conductivity of maghemite (γ-Fe2O3)/water nanofluids also cannot be predicted by the Maxwell and Bruggeman model. Modern modelling techniques like the use of artificial neural network (ANN) have equipped researchers with newer methods to predict the electrical conductivities of nanofluids as studied by Aghayari et al. (2018). They found that the ANN can well predict the electrical conductivity of CuO/glycerol nanofluids. Cruz et al. (2005) have stated that the electric double layer (EDL) plays a major role in determining whether the nature of the suspended particles is insulating or conducting and also that this nature can be altered which is bound to affect the stability of the nanofluid.
4.6 Applications Based on Electrical Conductivity of Nanofluid
Applications of nanofluids particularly exploiting their electrical conductivity have not been yet discovered. It has been only studied by Zakaria et al. (2015) that the electrical conductivity of nanofluid applied for thermal application shall affect its thermal conductivity. It is reported that the Al2O3/water/ethylene glycol nanofluid in the role of coolant in the proton exchange membrane fuel cell (PEMFC) receives ions due to the contamination of the bipolar plate of the cell and also because of the oxidation of ethylene glycol which is a component of the nanofluid’s basefluid due to its degradation during the process (Dill 2005; Zhang and Kandlikar 2012). Also, it has been known that the electrical conductivity of the coolant can cause the occurrence of shunt current and electrolysis of the coolant on the electrical appliance (Elhamid et al. 2004; Gershun et al. 2009). Such occurrence of shunt current leads to the decrease in the efficiency of the appliance and can prove harmful to the user. They have analysed the thermo-electrical conductivity (TEC) ratio of the nanofluid which is advantageous at a higher value for its nanofluid application in fuel cell. Thus, this shows that the significance of electrical conductivity is in determining the feasibility of the application of a nanofluid for thermal applications in an electrically active environment.
5 Particle Size Distribution of Nanofluids
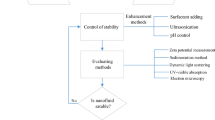
Particle size distribution (PSD) as specified earlier is the amount of particles present in the nanofluid classified according to their sizes. It gives the estimate of the variance of the sizes of the particles dispersed in the nanofluid. Nanofluids containing particles of a same size are called as monodisperse nanofluids and that containing particles of different sizes are called as polydisperse nanofluids. A monodisperse nanofluid exhibits a narrow particle size distribution, whereas a polydisperse nanofluid exhibits a wide particle size distribution, as shown in Fig. 11. As we already know that the various thermal, optical, electrical, mechanical and physical properties of the nanoparticles rely on their size (Dhamoon et al. 2018), so the study of the size distribution of these nanoparticles in the nanofluid is of great importance. It is to be first made clear that the size of the particle and the particle size distribution are two different terminologies even though the latter is dependent on the earlier. There are many factors that affect the particle size of the nanoparticles in the nanofluid and so they indirectly affect the particle size distribution.
5.1 Characterization Techniques Used for Particle Size Distribution
There are several methods that can be used for determining the particles size of the nanoparticles dispersed in the nanofluid and characterize its particle size distribution (Lin et al. 2014). Dynamic light scattering is the method in which the intensities at which the laser beam is scattered by the nanoparticles are measured, which depends on their Brownian motion in the nanofluid. So, the hydrodynamic diameter (\( D_{h} \)) as a measure of particle size can thus be known by using the Stokes–Einstein relation given in Eq. (40) where \( k_{b} \) is the Boltzmann’s constant, T is the thermodynamic temperature, n is the viscosity and \( D_{t} \) is the translational diffusion coefficient.
In DLS, the hydrodynamic diameter is considered, which is equivalent to the diameter of a spherical particle that would have same translational diffusion coefficient. The Raman scattering technique is another technique that uses the differences in frequencies of the photons scattered after they are incident on a material and interact with the dipoles of its molecules. It gives the indirect measure of the size distribution of the nanoparticles.
There are electron microscopy techniques, like SEM and TEM, which examine the nanofluid stability and thus give an estimate of the particles size distribution. These methods use high-resolution microscopic techniques to capture images using electron beam. Both the methods include the evaporation of the basefluid and then capturing the image of the particles remaining on the grid of the microscopes. A direct determination of the size of all the particles seen in the image can give a size distribution of the nanofluid. Another method for determination of the size distribution of the nanoparticles, known as atomic force microscopy (AFM), uses a cantilever machined at micro-size having a sharp tip to detect its deflection caused by the repulsion forces and thus generate an image of the material. Another method is the UV–visible spectrophotometry that makes use of the amount of light absorbed by the nanoparticles to classify them into different sizes. Nanomaterials of different sizes absorb light at different wavelengths. The absorbance of light by a nanofluid is a function of size of the nanoparticles present in it and so their size can be indirectly known from the UV–visible spectra of the nanofluid.
5.2 Factors Affecting Size Distribution of Particles or Aggregation in Nanofluids
The distribution of particles throughout the nanofluid can be definitely a function of the nanomaterial synthesis method as well as nanofluid synthesis method. But there are several other factors that completely alter the distribution of the sizes of the particles when dispersed in the nanofluid. Some of them are discussed here.
5.2.1 Time of Sonication During Synthesis of Nanofluid
As we have already seen in previous sections of this chapter, there are basically two types of nanofluid synthesis methods. Amongst them, the two-step method involves drying of nanoparticles and then re-dispersing them before application using either mechanical means or ultrasonication, the latter being used at most of the times. But this method also gives scope for the nanoparticles to form clusters and aggregates and form a polydisperse nanofluid due to inefficient dispersion due to insufficient ultrasonication (Mahbubul et al. 2015). Ma and Banerjee (2017) have specified that there are four steps involved in the formation of particles, namely thermal decomposition, nucleation, diffusion growth and particle coagulation. Under the assumption that there is no coagulation of the nanoparticles, they have observed that the nanofluid shows almost monodisperse characteristics of size distribution and that the particle size increases as the reaction is proceeding. Also, the coagulation of the particles at the end of reaction happening due to Brownian motion-induced collisions produces a polydisperse nanofluid.
Suganthi and Rajan (2012) studied the effect of ultrasonication time on the particle size distribution of ZnO-based nanofluids prepared using water as a basefluid. They have clearly mentioned that the hydrodynamic size of the nanoparticles reduces as the ultrasonication time for dispersion increases. An optimum particle size distribution that gives a minimum hydrodynamic size of the nanoparticles has been found out as 3 h for 0.5 vol.% ZnO-based nanofluid. A further increase in ultrasonication time leads to agglomeration of the nanoparticles resulting in a higher particle size. Silambarasan et al. (2012) prepared TiO2 particles using the stirred bead milling approach and then dispersed it in water to produce nanofluid using ultrasonication method. They found a wide size distribution of the particles produced by the stirred bead milling method ranging from 40 to 900 nm. They found that sonication of 6 and 7 h almost de-agglomerates the larger-sized particles and narrows down the particle size distribution of the TiO2 nanoparticles between the sizes of 35 and 300 nm. Sonication time of 7 h increases the percentage of smaller-sized particles even more.
5.2.2 Concentration of Nanoparticles in Nanofluid
The concentration of the nanoparticles affects the particle size distribution of the nanoparticles in the nanofluid in a way that a large quantity of nanoparticles may form larger agglomerates and broaden the particle size distribution. Colla et al. (2014) prepared ZnO nanofluids and studied the effect of concentration on the intensity of size distribution of the particles in the nanofluid. They found that the intensity of particles in the narrow range increases as the ZnO nanofluid concentration increases from 1 to 5%. But the intensity again decreases as the nanofluid concentration further increases to 10% which can be due to the agglomeration of the some particles and formation of larger size particles, thus broadening the size distribution curve. Juneja and Gangacharyulu (2017) studied the effect of concentration of particle size distribution of Al2O3-based nanofluid prepared using distilled water, ethylene glycol and mixture of water/ethylene glycol (in the ratio 75:25). They prepared Al2O3 nanofluids in the volume fraction range of 0.1–1%. For water-based Al2O3 nanofluids, the mean diameter of the nanoparticles first increased till a volume concentration of 0.25 vol.% and then showed a decreasing trend till 1% nanofluid concentration. The one prepared using ethylene glycol as a basefluid showed a straight decrease in the mean diameter of Al2O3 nanoparticles, while the nanofluid prepared in water/ethylene glycol mixture as a basefluid exhibited no change in the mean diameter after increasing the nanofluid concentration above 0.5 vol.%.
5.2.3 Addition of Surfactants
Surfactants in the nanofluids play an important role in the dispersion of the nanoparticles and in preventing formation of agglomerates. Wang et al. (2009) studied the effect of addition of sodium dodecylbenzene sulfonate (SDBS) surfactant on the particle size distribution of Al2O3 and Cu nanoparticle in water-based nanofluids. They found that the presence of SDBS shifts the whole size distribution curve of both the nanofluids prepared at 0.05 wt% concentration to lower particle size range. In a similar study, Das et al. (2016) found that the TiO2–water nanofluids can be well stabilized using acetic acid and cetyl trimethylammonium bromide (CTAB) as dispersants. They purchased 21 nm-sized TiO2 nanoparticles, but their dispersion in water produced nanofluid containing average particle diameter of 147.6 and 207.7 nm with surfactants acetic acid and CTAB, respectively, at respective nanofluid concentrations of 1.5 and 1 vol.%. The higher size of the particles detected in the nanofluid occurred due to thermodynamically stable TiO2 nanoparticle cluster formed in the nanofluid.
Saterlie et al. (2011) conducted a comparative study of the effect of two types of surfactants, namely cetyl trimethylammonium bromide (CTAB) and oleic acid, on the particle size distribution of Cu nanoparticles in water-based nanofluids at 0.55 and 1 vol.% concentrations of the nanoparticles. They observed that oleic acid and CTAB are able to produce Cu nanofluids with particle size distribution at lower ranges having an average particle size of nearly 120 and 80 nm, respectively, at a nanofluid concentration of 0.55 vol.%. A similar size distribution is also obtained by using CTAB in 1 vol.% Cu nanofluid. But when oleic acid is used for dispersing same concentration of Cu nanoparticles in water, a substantial increase in the size of the particles is observed and the size distribution shifted to higher ranges exhibiting an average particle size of 800 nm. This was reported to be happening due to the heavy agglomeration of the nanoparticles due to inefficiency of oleic acid to keep them de-agglomerated and its failure in producing stable nanofluid.
5.2.4 Temperature
The temperatures, as known earlier, tend to affect the surface charges of the particles, which may affect their tendency to agglomerate. Also, the Brownian motion of the particles is greatly affected by temperature, which may have an indirect effect on the particle size. It was found by Suganthi and Rajan (2012) for ZnO-based nanofluids that the average particle hydrodynamic size increases with increase in temperature of the nanofluid. This was found to be happening due to the increase in Brownian motion of the particles. They prepared water-based ZnO nanofluids using sodium hexametaphosphate (SHMP) as a stabilizing agent. The adsorption of PO3− ions generated due to dissociation of SHMP when dissolved in water on the surface of the ZnO nanoparticles reduces their agglomeration. This chemisorption of the PO3− ions over the surface of the particles is a function of temperature and so an increase in the temperature resulted in the shift of the equilibrium of this exothermic adsorption towards desorption which is favourable at higher temperatures. Ultimately, this process leads to production of vacant sites on the surface of the ZnO nanoparticles, facilitating particle–particle interaction and thus agglomeration of the nanoparticles. Being dependent on the equilibrium of the adsorption of the PO3− ions, this process is reversible and so restoration of the PO3− ions took place as the temperature of the nanofluid was decreased and the difference between the particle size during the heating and cooling cycle was 2 nm at 25 °C.
5.3 Influence of Particle Size Distribution on Properties of Nanofluids
Particle size distribution of nanofluids determines whether the nanofluid is monodisperse or polydisperse. Size of the particles dispersed in the nanofluid impacts a lot of thermo-physical properties of the nanofluid. Below is given how the particle size distribution affects the thermal and optical properties of nanofluids.
5.3.1 Thermal Properties
Polydispersity of the nanofluids has a significant impact on the nanofluid thermal conductivity (Karthikeyan et al. 2008). It has been found out by Feng et al. (2008) that the thermal conductivity of the nanofluids is better when they exhibit uniform size distribution and that it is less when the size distribution is non-uniform.
Zhou and Wu (2014) developed a model of thermal conductivity as a function of PSD considering the clustering of the nanoparticles in tightly packed aggregates, as given in Eq. (41), where \( k_{\text{nf}} \) is the thermal conductivity of nanofluid containing primary particles as well as clusters, \( k_{\text{bf}} \) is the thermal conductivity of basefluid, \( k_{\text{pm}} \) is the thermal conductivity containing primary particles, \( k_{p} \) is the thermal conductivity of the particles, \( \xi \) is the shape factor defined as \( \xi = 3/\psi \) (\( \psi \) is the sphericity of the nanoparticle clusters), \( \phi_{c} \) is the volume fraction of clusters in the nanofluid and is the product of volume fraction of nanoparticle clusters in the nanofluid, \( \phi_{cs} \), and volume fraction of nanoparticles in the spherical clusters, \( \phi_{\text{ins}} \).
5.3.2 Optical Properties
Most of the times while characterizing the nanofluid we take into consideration its optical properties, a monodisperse system is assumed, even though practically, the particles are always dispersed in a polydisperse manner to some degree (Qin and Lee 2018). This is due to the fact that the larger-sized particles enhance the scattering of long wavelengths, unlike the small-sized particles that absorb only short wavelengths (Du and Tang 2015). Thus, the absorption of wavelengths by the nanofluid depends on the various sizes of nanoparticles dispersed in it, that is, on the size distribution of the nanoparticles. Agglomeration in such case plays a very important role. Agglomerates of nanoparticles shift the wavelength of absorption of the nanofluid. Agglomeration leads to conversion of a nearly monodisperse nanofluid into polydisperse nanofluid that distorts its absorption spectra. Optical properties of nanofluids play a major role in the solar thermal applications of nanofluids and so the particle size distribution of the nanofluids gains importance (Crisostomo et al. 2017; Hjerrild et al. 2016).
6 Summary
The field of nanotechnology has given rise to nanofluids that are proved to be having superior properties than conventional fluids. The two preparation methods of nanofluids are discussed in this chapter along with few properties. The main properties of nanofluids are their thermal, electrical and optical properties which are very important as far as the various applications of nanofluids are concerned. Accordingly, there are some factors that affect these properties. There is certain mechanism which is responsible for the extraordinary behaviour of nanofluids.
Enhanced thermal properties of the nanofluids are an outcome of the high surface area provided by the nanoparticles in the nanofluid, their Brownian motion in the nanofluid and the interfacial layer of the basefluid that surrounds the nanoparticles and possesses higher thermal conductivity than the rest of the fluid in bulk. These are helpful in various heat transfer enhancement processes as studied by various researchers. Also, the formation of EDL on the surface of the nanoparticles is responsible for electrical conductivity of the nanofluids. There are model relationships that are useful in predicting the thermal conductivity as well as electrical conductivity of the nanofluids, as found out by various researchers. The role of zeta potential in the electrical properties of the nanofluids is also a factor to be considered. Researchers have used several methods to measure the thermal and electrical conductivities of the nanofluids, amongst which few have become very famous. There are several applications based on the thermal properties of the nanofluids but very few are based on the electrical properties of the nanofluids. Particle size distribution is yet another property that has importance as far as thermal and optical properties of the nanofluids are concerned. There are several factors that affect the particle size distribution. Agglomeration is one basic property that must be considered while determining the polydispersity of the nanofluids.
References
Abdolbaqi MKh, Azmi WH, Mamat R, Sharma KV, Najafi G (2016) Experimental investigation of thermal conductivity and electrical conductivity of bioglycol-water mixture based Al2O3 nanofluid. Appl Therm Eng 102:932–941
Adio SA, Sharifpur M, Meyer JP (2015a) Factors affecting the pH and electrical conductivity of MgO-ethylene glycol nanofluids. Bull Mater Sci 38:1345–1357
Adio SA, Sharifpur M, Meyer JP (2015b) Investigation into effective viscosity, electrical conductivity, and pH of γ-Al2O3-glycerol nanofluids in Einstein concentration regime. Heat Trans Eng 38:1241–1251
Aghayari R, Maddah H, Ahmadi MH, Yan W, Ghasemi N (2018) Measurement and artificial neural network modeling of electrical conductivity of CuO/glycerol nanofluids at various thermal and concentration conditions. Energies 11:1190
Ahammed N, Asirvatham LG, Titus J, Bose JR, Wongwises S (2016) Measurement of thermal conductivity of graphene–water nanofluid at below and above ambient temperatures. Int Commun Heat Mass 70:66–74
Akoh H, Tsukasaki Y, Yatsuya S, Tasaki A (1978) Magnetic properties of ferromagnetic ultrafine particles prepared by vacuum evaporation on running oil substrate. J Crystal Growth 45:495–500
Alawi OA, Sidik NAC, Xian HW, Kean TH, Kazi SN (2018) Thermal conductivity and viscosity models of metallic oxides nanofluids. Int J Heat Mass Transf 116:1314–1325
Ali FM, Yunus MM, Moksin MM, Talib ZA (2010) The effect of volume fraction concentration on the thermal conductivity and thermal diffusivity of nanofluids: numerical and experimental. Rev Sci Instrum 81:074901
Ali HM, Ali H, Liaquat H, Maqsood HTB, Nadir MA (2015) Experimental investigation of convective heat transfer augmentation for car radiator using ZnO-water nanofluids. Energy 84:317–324
Askari S, Koolivand H, Pourkhalil M, Lotfi R, Rashidi A (2017) Investigation of Fe3O4/Graphene nanohybrid heat transfer properties: experimental approach. Int Commun Heat Mass 87:30–39
Azimi M, Ommi F (2013) Using nanofluid for heat transfer enhancement in engine cooling process. J Nano Energy Power Res 2:1–3
Azimi HR, Taheri R (2015) Electrical conductivity of CuO nanofluids. Int J Nano Dimens 6:77–81
Baby TT, Ramaprabhu S (2010) Investigation of thermal and electrical conductivity of graphene based nanofluids. J Appl Phys 108:124308
Bagheli S, Fadafan HK, Orimi RL, Ghaemi M (2015) Synthesis and experimental investigation of the electrical conductivity of water based magnetite nanofluids. Powder Technol 274:426–430
Bhanvase BA, Sarode MR, Putterwar LA, Abdullah KA, Deosarkar MP, Sonawane SH (2014) Intensification of convective heat transfer in water/ethylene glycol based nanofluids containing TiO2 nanoparticles. Chem Eng Process 82:123–131
Bhanvase BA, Sayankar SD, Kapre A, Fule PJ, Sonawane SH (2018) Experimental investigation on intensified convective heat transfer coefficient of water based PANI nanofluid in vertical helical coiled heat exchanger. Appl Therm Eng 128:134–140
Bozorgan N, Shafahi M (2017) Analysis of gasketed-plate heat exchanger performance using nanofluid. J Heat Mass Trans Res 4:65–72
Bruggeman DAG (1935a) Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. Ann Phys (Leipzig) 24:636
Bruggeman DAG (1935b) Dielectric constant and conductivity of mixtures of isotropic materials. Ann Phys (Leipzig) 24:636–679
Chakraborty S (2019) An investigation on the long-term stability of TiO2 nanofluid. Mater Today Proc 11:714–718
Chakraborty S, Padhy S (2008) Anomalous electrical conductivity of nanoscale colloidal suspensions. ACS Nano 2:2029–2036
Chieruzzi M, Cerritelli GF, Miliozzi A, Kenny JM (2013) Effect of nanoprticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res Lett 8:448
Choi SUS (1995) Enhancing thermal conductivity of fluids with nanoparticles. ASME Publications FED-vol. 231/MD 66:99–105
Choi SUS, Yu W, Hull JR, Zhang ZG, Lockwood FE (2001) Nanofluids for vehicle thermal management. In: Proceedings of the 2001 vehicle thermal management systems conference, society of automotive engineers
Chopkar M, Sudarshan S, Das PK, Manna I (2008) Effect of particle size on thermal conductivity of nanofluid. Metall. Mater. Trans Metall Mater Trans A 39A:1535–1542
Coelho MF, Rivas MA, Vilao G, Nogueira EM, Iglesias TP (2019) Permittivity and electrical conductivity of copper oxide nanofluid (12 nm) in water at different temperatures. J Chem Thermodyn 132:164–173
Colla L, Marinelli L, Fedele L, Bobbo S, Manca O (2014) Characterization and simulation of the heat transfer behavior of water-based ZnO nanofluids. J Nanosci Nanotechnol 14:1–11
Crisostomo F, Hjerrild N, Mesgari S, Li Q, Taylor RA (2017) A hybrid PV/T collector using spectrally selective absorbing nanofluids. Appl Energy 193:1–14
Cruz RCD, Reinshagen J, Oberacker R, Segadães AM, Hoffmann MJ (2005) Electrical conductivity and stability of concentrated aqueous alumina suspensions. J Colloid Interface Sci 286:579–588
Darvanjooghi MHK, Esfahany MN (2016) Experimental investigation of the effect of nanoparticle size on thermal conductivity of in-situ prepared silica–ethanol nanofluid. Int Commun Heat Mass 7:148–154
Das SK, Choi SUS, Patel HE (2006) Heat transfer in nanofluids—a review. Heat Transf Eng 27:3–19
Das PK, Mallik AK, Ganguly R, Santra AK (2016) Synthesis and characterization of TiO2–water nanofluids with different surfactants. Int Commun Heat Mass Transf 75:341–348
Dhamoon RK, Popli H, Aggarwal G, Gupta M (2018) Particle size characterization techniques, factors and quality-by-design approach. Int J Drug Deliv 10:1–11
Dill NJ (2005) Fuel cell stack coolant conductivity monitoring circuit, ed: US Patent 6, 838, 201
Dong M, Shen LP, Wang H, Wang HB, Miao J (2013) Investigation on the electrical conductivity of transformer oil-based AlN nanofluid. J Nanomater 2013; 7 pages
Du M, Tang GH (2015) Optical property of nanofluids with particle agglomeration. Sol Energy 122:864–872
Duangthongsuk W, Wongwises S (2009) Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp Therm Fluid Sci 33:706–714
Eastman JA, Choi US, Li S, Thompson LJ, Lee S (1997) Enhanced thermal conductivity through the development of nanofluids. In: Materials research society symposium proceedings. Materials research society, vol 4576. Pittsburgh, PA, USA, Boston, MA, USA, pp 3–11
Eastman JA, Choi SUS, Li S, Yu W, Thompson LJ (2001) Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl Phys Lett 78:718–720
Elhamid MHA, Mikhail YM, Blunk RH, Lisi DJ (2004) Inexpensive dielectric coolant for fuel cell stacks, ed: Google Patents
Elias MM, Mahbubul IM, Saidur MR, Shahrul IM, Khaleduzzaman SS, Sadeghipour S (2014) Experimental investigation on the thermo-physical properties of Al2O3 nanoparticles suspended in car radiator coolant. Int Commun Heat Mass 54:48–53
Esfe MH, Saedodin S, Bahiraei M, Toghraie D, Mahian O, Wongwises S (2014) Thermal conductivity modeling of MgO/EG nanofluids using experimental data and artificial neural network. J Therm Anal Calorim 118:287–294
Esfe MH, Karimipour A, Yan W, Akbari M, Safaei MR, Dahari M (2015a) Experimental study on thermal conductivity of ethylene glycol based nanofluids containing Al2O3 nanoparticles. Int J Heat Mass Transf 88:728–734
Esfe MH, Saedodin S, Wongwises S, Toghraie D (2015b) An experimental study on the effect of diameter on thermal conductivity and dynamic viscosity of Fe/water nanofluids. J Therm Anal Calorim 119:1817–1824
Esfe MH, Yan WM, Akbari M, Karimipour A, Hassani M (2015c) Experimental study on thermal conductivity of DWCNT-ZnO/water-EG nanofluids. Int Commun Heat Mass Transf 68:248–251
Feng Y, Xu P, Zou M, Yu B (2007) The effective thermal conductivity of nanofluids based on the nanolayer and the aggregation of nanoparticles. J Phys D Appl Phys 40:3164
Feng Y, Yu B, Feng K, Xu P, Zou M (2008) Thermal conductivity of nanofluids and size distribution of nanoparticles by Monte Carlo simulations. J Nanopart Res 10:1319–1328
Ganguly S, Sikdar S, Basu S (2009) Experimental investigation of the effective electrical conductivity of aluminium oxide nanofluids. Powder Technol 196:326–330
Garg J, Poudel B, Chiesa M, Gordon JB, Ma JJ, Wang JJ, Wang JB, Ren ZF, Kang YT, Ohtani H, Nanda J, McKinley GH, Chen G (2008) Enhanced thermal conductivity and viscosity of copper nanoparticles in ethylene glycol nanofluid. J Appl Phys 103:074301
Gershun AV, Jeffcoate CS, Marinho FJ, Woyciesjes PM (2009) Heat transfer compositions with high electrical resistance for fuel cell assemblies, ed: Google Patents
Gharagozloo PE, Goodson KE (2008) Diffusion, aggregation and the thermal conductivity of nanofluids. Appl Phys Lett 93:103110
Ghosh MM, Ghosh S, Pabi SK (2012) Effects of particle shape and fluid temperature on heat-transfer characteristics of nanofluids. J Mater Eng Perform 22:1525–1529
Glory J, Bonetti M, Helezen M, Hermite ML, Reynaud C (2008) Thermal and electrical conductivities of water-based nanofluids prepared with long multiwalled carbon nanotubes. J Appl Phys 103:094309
Glover B, Whites KW, Hong H, Mukherjee A, Billups WE (2008) Effective electrical conductivity of functional single-wall carbon nanotubes in aqueous fluids. Synth Met 158:506–508
Goharshadi EK, Azizi-Toupkanloo H (2013) Silver colloid nanoparticles: Ultrasound-assisted synthesis, electrical and rheological properties. Powder Technol 237:97–101
Goharshadi EK, Toupkanloo HA, Karimi M (2015) Electrical conductivity of water-based palladium nanofluids. Microfluid Nanofluidics 18:667–672
Goudarzi K, Jamali H (2017) Heat transfer enhancement of Al2O3-EG nanofluid in a car radiator with wire coil inserts. Appl Therm Eng 118:510–517
Guo Y, Zhang T, Zhang D, Wang Q (2018) Experimental investigation of thermal and electrical conductivity of silicon oxide nanofluids in ethylene glycol/water mixture. Int J Heat Mass Trans 117:280–286
Gupta SS, Siva VM, Krishnan S, Sreeprasad TS, Singh PK (2011) Thermal conductivity enhancement of nanofluids containing graphene nanosheets. J Appl Phys 110:084302
Gupta HK, Agrawal GD, Mathur J (2015) Investigations for effect of Al2O3-H2O nanofluid flow rate on the efficiency of direct absorption solar collector. Case Stud Thermal Eng 5:70–78
Hadadian M, Goharshadi EK, Youssefi A (2014) Electrical conductivity, thermal conductivity, and rheological properties of graphene oxide-based nanofluids. J Nanopart Res 16:2788
Hamilton RL, Crosser OK (1962) Thermal conductivity of heterogeneous two-component system. Ind Eng Chem Fundam 1:187–191
Harikrishnan S, Magesh S, Kalaiselvam S (2013) Preparation and thermal energy storage behaviour of stearic acid-TiO2 nanfluid as a phase change material for solar heating system. Thermochima Acta 565:137–145
Heyhat MM, Irannezhad A (2018) Experimental investigation on the competition between enhancement of electrical and thermal conductivities in water-based nanofluids. J Mol Liq 268:169–175
Heyhat MM, Kowsary F, Rashidi AM, Momenpour MH, Amrollahi A (2013) Experimental investigation of laminar convective heat transfer and pressure drop of water–based Al2O3 nanofluids in fully developed flow regime. Exp Therm Fluid Sci 44:483–489
Hjerrild NE, Mesgari S, Crisostomo F, Scott JA, Amal R, Taylor RA (2016) Hybrid PV/T enhancement using selectively absorbing Ag–SiO2/carbon nanofluids. Sol Energy Mater Sol Cells 147:281–287
Huang D, Wu Z, Sunden B (2015) Pressure drop and convective heat transfer of Al2O3/water and MWCNT/water nanofluids in a chevron plate heat exchanger. Int J Heat Mass Transf 89:620–626
Hwang Y, Lee JK, Lee CH, Jung YM, Cheong SI, Lee CG, Ku BC, Jang SP (2007) Stability and thermal conductivity characteristics of nanofluids. Thermochim Acta 455:70–74
Ijam A, Saidur R, Ganesan P, Golsheikh AM (2015) Stability, thermo-physical properties, and electrical conductivity of graphene oxide-deionized water/ ethylene glycol based nanofluid. Int J Heat Mass Trans 87:92–103
Islam MR, Shabani B, Rosengarten G, Andrews J (2015) The potential of using nanofluids in PEM fuel cell cooling systems: a review. Renew Sust Energy Rev 48:523–539
Jang SP, Choi SUS (2004) Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl Phys Lett 84:4316–4318
Jang P, Choi SUS (2007) Effect of various parameters on nanofluid thermal conductivity. J Heat Trans 129:617–623
Jeong J, Li C, Kwon Y, Lee J, Kim SH, Yun R (2013) Particle shape effect on the viscosity and thermal conductivity of ZnO nanofluids. Int J Refrig 36:2233–2241
Jiang H, Zhang Q, Shi L (2015) Effective thermal conductivity of carbon nanotube – based nanofluid. J Taiwan Inst Chem Eng 55:76–81
Juneja M, Gangacharyulu D (2017) Effect of volume fraction on particle size distribution in aumina based nanofluids. J Thermal Energy Syst 2:27–32
Kahani M, Heris SZ, Mousavi SM (2014) Experimental investigation of TiO2/water nanofluid laminar forced convective heat transfer through helical coiled tube. Heat Mass Transf 50:1563–1573
Karthikeyan NR, Philip J, Raj B (2008) Effect of clustering on the thermal conductivity of nanofluids. Mater Chem Phys 109:50–55
Kasaeian A, Eshghi AT, Sameti M (2015) A review on the applications of nanofluids in solar energy systems. Renew Sust Energy Rev 43:584–598
Kavitha T, Rajendran A, Durairajan A (2012) Synthesis, characterization of TiO2 nano powder and water based nanofluids using two step method. Eur J Appl Eng Sci Res 1:235–240
Keblinski P, Phillpot SR, Choi SUS, Eastman JA (2002) Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int J Heat Mass Transf 45:855–863
Khaleduzzaman SS, Sohel MR, Saidur R, Selvaraj J (2015) Stability of Al2O3–water nanofluid for electronic cooling system. Procedia Eng 105:406–411
Khaleduzzaman SS, Sohel MR, Saidur R, Selvaraj J (2015) Stability of Al2O3-water nanofluid for electronics cooling system. Procedia Eng 105:406–411
Khatak P, Jakhar R, Kumar M (2015). Enhancement in cooling of electronic components by nanofluids. J Inst Eng India Ser C 96:245–251
Khdher AIM, Sidik NAC, Hamzah WAW, Mamat R (2016) An experimental determination of thermal conductivity and electrical conductivity of bio glycol based Al2O3 nanofluids and development of new correlation. Int Commun Heat Mass Transf 73:75–83
Khedkar RS, Sonawane SS, Wasewar KL (2012) Influence of CuO nanoparticles in enhancing the thermal conductivity of water and monoethylene glycol based nanofluids. Int Commun Heat Mass 39:665–669
Kole M, Dey TK (2013) Investigation of thermal conductivity, viscosity and electrical conductivity of graphene based nanofluids. J Appl Phys 113:084307
Konakanchi H, Vajjha R, Misra D, Das D (2011) Electrical conductivity measurements of nanofluids and development of new correlations. J Nanosci Nanotechnol 11:1–8
Koo J, Kleinstreuer C (2004) A new thermal conductivity model for nanofluids. J Nanopart Res 6:577–588
Koo J, Kleinstreuer C (2005) Impact analysis of nanoparticle motion mechanism on the thermal conductivity of nanofluids. Int Commun Heat Mass Trans 32:1111–1118
Kotia A, Borkakoti S, Deval P, Ghosh SK (2017) Review of interfacial layer’s effect on thermal conductivity in nanofluid. Heat Mass Transf 53:2199–2209
Kumar N, Sonawane SS, Sonawane SH (2018) Experimental study of thermal conductivity, heat transfer and friction factor of Al2O3 based nanofluid. Int Commun Heat Mass 90:1–10
Kumaresan V, Velraj R, Das SK (2012) Convective heat transfer characteristics of secondary refrigerant based CNT nanofluids in a tubular heat exchanger. Int J Refrig 35:2287–2296
Lee YS (2008) Self-assembly and nanotechnology: a force balance approach. Wiley, New York
Lee D, Jae-Won K, Kim BG (2006) A new parameter to control heat transport in nanofluids: surface charge state of the particle in suspension. J Phys Chem B 110:4323–4328
Lee JH, Hwang KS, Jang SP, Lee BH, Kim JH, Choi SUS, Choi CJ (2008) Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf 51:2651–2656
Leong KC, Yang C, Murshed SMS (2006) A model for the thermal conductivity of nanofluids—the effect of interfacial layer. J Nanopart Res 8:245–254
Leong KY, Ibrahim IC, Amer NH, Risby MS (2016) Thermal conductivity of carbon based nanofluids as heat transfer fluids. Appl Mech Mater 819:29–33
Leong KY, Razali I, Ahmad KZK, Amer NH, Akmal HN (2018) Thermal conductivity characteristic of titanium dioxide water based nanofluids subjected to various types of surfactant. J Eng Sci Technol 13:1677–1689
Li Z, Sheikholeslami M, Jafaryar M, Shafee A, Chamkha AJ (2018) Investigation of nanofluid entropy generation in a heat exchanger with helical twisted tapes. J Mol Liq 266:797–805
Lin P, Lin S, Wang PC, Sridhar R (2014) Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv 32:711–726
Lisunova MO, Lebovka NI, Melezhyk OV, Boiko YP (2006) Stability of the aqueous suspensions of nanotubes in the presence of nonionic surfactant. J Colloid Interface Sci 299:740–746
Liu L, Yang Y, Zhang Y (2004) A study on the electrical conductivity of multi-walled carbon nanotube aqueous solution. Physica E 24:343–348
Liu MS, Lin MCC, Huang IT, Wang CC (2005) Enhancement of thermal conductivity with carbon nano-tube for nanofluids. Int Commun Heat Mass 32:1202–1210
Liu MS, Lin MCC, Wang C (2011) Enhancement of thermal condutivities with Cu, CuO, and carbon nanotube nanofluids and application of MWNT/water nanofluid on a water chiller system. Nanoscale Res Lett 6:297
Lo CH, Tsung TT, Chen LC, Su CH, Lin HM (2005) Fabrication of copper oxide nanofluid using submerged arc nanoparticle synthesis system (SANSS). J Nanopart Res 7:313–320
Ma B, Banerjee D (2017) Predicting particle size distribution in nanofluid synthesis. In: Proceedings of the ASME 2017 Heat Transfer Summer Conference HT2017, USA
Mahbubul IM, Shahrul IM, Khaleduzzaman SS, Saidur R, Amalina MA, Turgut A (2015) Experimental investigation on effect of ultrasonication duration on colloidal dispersion and thermophysical properties of alumina–water nanofluid. Int J Heat Mass Transf 88:73–81
Maheshwary PB, Handa CC, Nemade KR (2018) Effect of shape on thermophysical and heat transfer properties of ZnO/R-134a nanorefrigerant. Mater Today ProcMater Today: Proc 5:1635–1639
Mahmud KM, Yudistiram SA, Ramadhan AI (2016) Analytical study of forced convection in fluid cooling use nanofluid Al2O3- water on nuclear reactor core based fuel cylinder with hexagonal sub channel. Int J Energy Eng 6:8–15
Manimaran R, Palaniradja K, Alagumurthi N, Sendhilnathan S, Hussain J (2014) Preparation and characterization of copper oxide nanofluid for heat transfer applications. Appl Nanosci 4:163–167
Mao C, Huang Y, Zhou X, Gan H, Zhang J, Zhou Z (2014) The tribological properties of nanofluid used in minimum quantity lubrication grinding. Int J Adv Manuf Technol 71:1221–1228
Maxwell JC (1881) A treatise on electricity and magnetism, 2nd edn. Clarendon Press, Oxford, p 1881
Mehrali M, Sadeghinezhad E, Rashidi MM, Akhiani AR, Latibari ST, Mehrali M, Metselaar HSC (2015) Experimental and numerical investigation of the effective electrical conductivity of nitrogen-doped graphene nanofluids. J Nanopart Res 17:267
Menbari A, Alemrajabi AA, Razaei A (2016) Heat transfer analysis and the effect of CuO/Water nanofluid on direct absorption concentrating solar collector. Appl Therm Eng 104:176–183
Minea AA, Luciu RS (2012) Investigations on electrical conductivity of stabilized water based Al2O3 nanofluids. Microfluid Nanofluidics 13:977–985
Minea AA, Manca O (2017) Field-synergy and figure of merit analysis of two oxide water based nanofluid flow in heated tubes. Heat Transfer Eng 38:909–918
Mintsa HA, Roy G, Nguyen CT, Doucet D (2009) New temperature dependent thermal conductivity data for water-based nanofluids. Int J Thermal Sci 48:363–371
Mohammed HA, Al-aswadi AA, Shuaib NH, Saidur R (2011) Convective heat transfer and fluid flow study over a step using nanofluids: a review. Renew Sust Energy Rev 15:2921–2939
Naddaf A, Heris SZ (2018) Experimental study on thermal conductivity and electrical conductivity of diesel oil-based nanofluids of graphene nanoplatelets and carbon nanotubes. Int Commun Heat Mass Transf 95:116–122
Naraki M, Peyghambarzadeh SM, Hashemabadi SH, Vermahmoudi Y (2013) Parametric study of overall heat transfer coefficient of CuO/water nanofluids in a car radiator. Int J Therm Sci 66:82–90
Natarajan E, Sathish R (2009) Role of nanofluid in solar water heater. Int J Adv Manuf Technol 8:1–5
Nurdin I, Satriananda (2017) Investigation on electrical conductivity enhancement of water based maghemite (γ-Fe2O3) nanofluids. Int J Mater Sci Appl 6:32–36
Ohshima H (2003) Electrokinetic phenomena in a dilute suspension of spherical colloidal particles in a salt-free medium. Colloids Surf Physicochem Eng Asp 222:207–211
Pak BC, Choi YI (1998) Hydraulic Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp Heat Trans 11:151–170
Prakash SB, Kotin KN, Kumar PM (2016) Preparation and characterization of nanofluid (CuO/Water, TiO2/Water). Int J Sci Eng 1:14–20
Prasher R, Bhattacharya P, Phelan PE (2005) Brownian-motion-based convective–conductive model for the effective thermal conductivity of nanofluids. J Heat Trans 128:588–595
Qin C, Lee BJ (2018) Effect of particle size distribution on optical property of nanofluids and DASC performance. Korean Soc Mech Eng 4:392–393
Roberts NA, Walker DG (2010) Convective performance of nanofluids in commercial electronics cooling systems. Appl Therm Eng 30:2499–2504
Rusconi R, Williams WC, Buongiorno J, Piazza R, Hu LW (2007) Numerical analysis of convective instabilities in a transient short-hot-wire setup for measurement of liquid thermal conductivity. Int J Thermophys 28:1131–1146
Sarojini KGK, Manoj SV, Singh PK, Pradeep T, Das SK (2013) Electrical conductivity of ceramic and metallic nanofluids. Colloids Surf Physicochem Eng Asp 417:39–46
Saterlie M, Sahin H, Kavlicoglu B, Liu Y, Graeve O (2011) Particle size effects in the thermal conductivity enhancement of copper-based nanofluids. Nanoscale Res Lett 6:217
Schramm LL, Stasiuk EN, Marangoni DG (2003) Surfactants and their applications. Ann Rep Program Chem Sect 99:3–48
Selvakumar P, Suresh S (2012) Convective performance of CuO/water nanofluid in an electronic heat sink. Exp Therm Fluid Sci 40:57–63
Shen LP, Wang H, Dong M, Ma ZC, Wang HB (2012) Solvothermal synthesis and electrical conductivity model for the zinc oxide-insulated oil nanofluid. Phys Lett A 376:1053–1057
Shoghl SN, Jamali J, Moraveji MK (2016) Electrical conductivity, viscosity, and density of different nanofluids: an experimental study. Exp Therm Fluid Sci 74:339–346
Sidik NAC, Yazid MNAWM, Mamat R (2017) Recent advancement of nanofluids in engine cooling system. Renew Sust Energy Rev 75:137–144
Sigmund WM, Bell NS, Bergstrom (2000) Novel powder‐processing methods for advanced ceramics. J Am Ceram Soc 83:1557–1574
Sikadar S, Basu S, Ganguly S (2011) Investigation of electrical conductivity of titanium dioxide nanofluids. Int J Nanoparticles 4: 336–349
Silambarasan M, Manikandan S, Rajan KS (2012) Viscosity and thermal conductivity of dispersions of sub-micron TiO2 particles in water prepared by stirred bead milling and ultrasonication. Int J Heat Mass Transf 55:7991–8002
Sohel Murshed SM, Nieto de Castro CA (2011) Contribution of Brownian motion in thermal conductivity of nanofluids. In: Proceedings of the world congress on engineering, vol III. London, UK
Sohel MR, Khaleduzzaman SS, Saidur R, Hepbasli A, Sabri MFM, Mahbubul IM (2014) An experimental investigation of heat transfer enhancement of a minichannel heat sink using Al2O3–H2O nanofluid. Int J Heat Mass Transf 74:164–172
Sokhansefat T, Kasaeian AB, Kowsary F (2014) Heat transfer enhancement in parabolic trough collector tube using Al2O3/synthetic oil nanofluid. Renew Sust Energy Rev 33:636–644
Suganthi KS, Rajan KS (2012) Temperature induced changes in ZnO-water nanofluid: zeta potential, size distribution and viscosity profiles. Int J Heat Mass Trasnf 55:7969–7980
Suganthi KS, Parthasarathy M, Rajan KS (2013) Liquid-layering induced, temperature-dependent thermal conductivity enhancement in ZnO–propylene glycol nanofluids. Chem Phys Lett 561–562:120–124
Suganthi KS, Leela Vinodhan V, Rajan KS (2014) Heat transfer performance and transport properties of ZnO-ethylene glycol and ZnO–ethylene glycol–water nanofluid coolants. Appl Energy 135:548–559
Sundar LS, Singh MK, Sousa ACM (2013) Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int Commun Heat Mass 44:7–14
Syam Sumdar L, Singh MK, Ferro MC, Sousa ACM (2017) Experimental investigation of the thermal transport properties of graphene oxide/Co3O4 hybrid nanofluids. Int Commun Heat Mass 84:1–10
Teng T, Hung Y, Teng T, Mo H, Hsu H (2010) The effect of alumina/water nanofluid particle size on thermal conductivity. Appl Therm Eng 30:2213–2218
Tijani AS, Sudirman ASB (2018) Thermos-physical properties and heat transfer characteristics of water/anti-freezing and Al2O3/CuO based nanofluid as a coolant for car radiator. Int J Heat Mass Transf 118:48–57
Timofeeva EY, Gavriloy AN, McCloskey JM, Tolmachey YV, Sprunt S, Lopatina LM, Selinger JV (2007) Thermal conductivity and particle agglomeration in alumina nanofluids: experiment and theory. Phys Rev E 76:061203
Trinh PV, Anh NN, Quang LD, Thang BH, Hong PN, Hong NT, Khoi PH, Minh PN (2016) Thermal conductivity of ethylene glycol based copper nanoparticle decorated graphene nanofluids. Commun Phys 26:351–360
Usri NA, Azmi WH, Mamat R, Hamid KA, Najafi G (2015) Thermal conductivity enhancement of Al2O3 nanofluid in ethylene glycol and water mixture. Energy Procedia 79:397–402
Vasconcelos AA, Gomez AOC, Filho EPB, Parise JAR (2017) Experimental evaluation of SWCNT-water nanofluid as a secondary fluid in a refrigeration system. Appl Therm Eng 111:1487–1492
Wang XQ, Mujumdar AS (2007) Heat transfer characteristics of nanofluids: a review. Int J Therm Sci 46:1–19
Wang XJ, Zhu DS (2009) Investigation of pH and SDBS on enhancement of thermal conductivity in nanofluids. Chem Phys Lett 470:107–111
Wang X, Zhu D, Yang S (2009) Investigation of pH and SDBS on enhancement of thermal conductivity in nanofluids. Chem Phys Lett 470:107–111
Wen D, Ding Y (2005) Experimental investigation into the pool boiling heat transfer of aqueous based γ-alumina nanofluids. J Nanopart Res 7:265–274
White SB, Shih AJ, Pipe KP (2011) Investigation of the electrical conductivity of propylene glycol-based ZnO nanofluids. Nanoscale Res Lett 6:346
Wong KFV, Kurma T (2008) Transport properties of alumina nanofluids. Nanotechnology 19:345702
Xia G, Jiang H, Liu R, Zhai Y (2014) Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int J Therm Sci 84:118–124
Xie H, Yu W, Chen W (2010) MgO nanofluids: higher thermal conductivity and lower viscosity among ethylene glycol-based nanofluids containing oxide nanoparticles. J Exp Nano Sci 5:463–472
Xuan Y, Li Q (2000) Heat transfer enhancement of nanofluids. Int J Heat Fluid Flow 21:58–64
Xuan Y, Li Q, Hu W (2003) Aggregation structure and thermal conductivity of nanofluids. AIChE J 49:1038–1043
Xuan Y, Li Q, Tie P (2013) The effect of surfactants on heat transfer feature of nanofluids. Exp Therm Fluid Sci 46:259–262
Xue QZ (2005) Model for thermal conductivity of mixture of carbon-nanotube based composites. Physisca B Condens Matter 368:302–307
Yang B, Han ZH (2006) Temperature-dependent thermal conductivity of nanorod-based nanofluids. Appl Phys Lett 89:083111
Younes H, Christensen G, Luan X, Hong H, Smith P (2012) Effects of alignment, pH, surfactant, and solvent on heat transfer nanofluids containing Fe2O3 and CuO nanoparticles. J Appl Phys 111:064308
Yousefi T, Veysi F, Shojaeizadeh E, Zinadini S (2012) An experimental investigation on the effect of Al2O3-H2O nanofluid on the efficiency of flat-plate solar collectors. Renew Energ 39:293–298
Yu W, Choi SUS (2003) The role of interfacial layers in the enhanced thermal conductivity of nanofluids: a renovated Maxwell model. J Nanopart Res 5:167–171
Yu W, Xie H, Chen L, Li Y (2010) Investigation on the thermal transport properties of ethylene glycol-based nanofluids containing copper nanoparticles. Powder Technol 197:218–221
Yu-Hua L, Wei Q, Jian-Chao F (2008) Temperature dependence of thermal conductivity of nanofluids. Chin Phys Lett 25:3319–3322
Zadkhast M, Toghraie D, Karimipour A (2017) Developing a new correlation to estimate the thermal conductivity of MWCNT-CuO/water hybrid nanofluid via an experimental investigation. J Therm Anal Calorim 129:859–867
Zakaria I, Azmi WH, Mohamed WANW, Mamat R, Najafi G (2015) Experimental investigation of thermal conductivity and electrical conductivity of Al2O3 nanofluid in water-ethylene glycol mixture for proton exchange membrane fuel cell application. Int Commun Heat Mass 61:61–68
Zakaria I, Azmi WH, Mamat AMI, Mamat R, Saidur R, Talib SFA, Mohamed WANW (2016) Thermal analysis of Al2O3-water ethylene glycol mixture nanofluid for single PEM fuel cell cooling plate: an experimental study. Int J Hydrogen Energy 41:5096–5112
Zawrah MF, Khattab RM, Girgis LG, Daidamony HEl, Aziz REA (2016) Stability and electrical conductivity of water-base Al2O3 nanofluids for different applications. HBRC J 12:227–234
Zhang G, Kandlikar SG (2012) A critical review of cooling techniques in proton exchange membrane fuel cell stacks. Int J Hydrog Energy 37:2412–2429
Zhou D, Wu H (2014) A thermal conductivity model of nanofluids based on particle size distribution analysis. Appl Phys Lett 105:083117
Zhu HT, Yin YS (2004) A novel one-step chemical method preparation of copper nanofluids. J Colloid Inter Sci 227:100–130
Zhu D, Wang L, Yu W, Xie H (2018) Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci Rep Sci Rep 8:5282
Zyla G, Fal J (2016) Experimental studies on viscosity, thermal and electrical conductivity of aluminium nitride-ethylene glycol (AlN-EG) nanofluids. Themochim Acta 637:11–16
Zyla G, Fal J (2017) Viscosity, thermal and electrical conductivity of silicon dioxide-ethylene glycol transparent nanofluids: an experimental studies. Thermochima Acta 650:106–113
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barai, D.P., Chichghare, K.K., Chawhan, S.S., Bhanvase, B.A. (2020). Synthesis and Characterization of Nanofluids: Thermal Conductivity, Electrical Conductivity and Particle Size Distribution. In: Ledwani, L., Sangwai, J. (eds) Nanotechnology for Energy and Environmental Engineering. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-33774-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-33774-2_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33773-5
Online ISBN: 978-3-030-33774-2
eBook Packages: EnergyEnergy (R0)