Abstract
In the development and implementation of the enhanced recovery after surgery (ERAS) program, there has been the need to understand the mechanism and the factors that affect the recovery process. Most of the elements considered by the ERAS® Society to have an impact on recovery have a physiological basis, and the interaction between them characterizes the modulation of the stress response. For example, besides surgical incision, some of them such as pain, hemorrhage, immobilization, and quasi starvation have a synergistic effect. The activation of the sympathetic system and the inflammatory response associated with all these surgical elements characterize the surgical stress response, thus leading to a state of low insulin sensitivity, which represents the most important pathogenic factor modulating the perioperative outcome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
In the development and implementation of the enhanced recovery after surgery (ERAS) program, there has been the need to understand the mechanism and the factors that affect the recovery process. Most of the elements considered by the ERAS® Society to have an impact on recovery have a physiological basis, and the interaction between them characterizes the modulation of the stress response. For example, besides surgical incision, some of them such as pain, hemorrhage, immobilization, and quasi starvation have a synergistic effect. The activation of the sympathetic system and the inflammatory response associated with all these surgical elements characterize the surgical stress response (Fig. 2.1), thus leading to a state of low insulin sensitivity, which represents the most important pathogenic factor modulating the perioperative outcome.
A rise in circulating glucocorticoids, catecholamines, and glucagon (i.e., counter-regulatory hormones) is elicited by activation of the hypothalamic-pituitary-adrenal axis and sympathetic nervous system. The response is mediated by afferent nerves and humoral factors including cytokines generated from the site of injury. Mobilization of energy reserves promotes hyperglycemia and catabolism. Hyperglycemia develops as a consequence of insulin resistance coupled with an inappropriately high hepatic glucose production. Proteolysis and lipolysis accelerate to provide precursors for gluconeogenesis. The resultant amino acid efflux also supports the synthesis of proteins involved in the acute-phase response. (Reprinted with permission from Gillis and Carli [1])
The low insulin sensitivity of the cell is characterized by an abnormal biological response to a normal concentration of insulin, the latter being responsible to control the metabolism of glucose, fat, and proteins. Therefore, a change in insulin sensitivity as a consequence of surgery impacts the whole metabolism. It results in an alteration in glucose metabolism with increased hepatic glucose production and decreased peripheral uptake leading to hyperglycemia. In addition, there is a breakdown of proteins at whole-body and muscle levels. These are the main metabolic characteristics of the surgical stress response.
The increased endogenous glucose production is correlated to the increased protein breakdown, and more precisely the breakdown into amino acids was shown to be directly responsible for the increase in hepatic endogenous glucose production. As there is a strong association between these two metabolic alterations and the postoperative rate of complications, it is plausible to assume that low insulin sensitivity can represent the main pathogenic mechanism.
This chapter covers the pathophysiology of glucose, insulin, and protein metabolism and the clinical relevance within recovery. Additionally, the chapter explores the attenuated response to surgical stress by the various elements of ERAS.
Glucose Metabolism
Pathophysiology
Fasting plasma glucose levels are normally kept between 3.3 and 6.4 mmol/L. Maintenance of normoglycemia is the result of a well-regulated balance of hepatic glucose production and tissue glucose uptake. Surgical stress triggers the release of counter-regulatory hormones (catecholamines, glucagon, cortisol, growth hormone) and pro-inflammatory cytokines (tumor necrosis factor-alpha [TNF-α]; interleukins: IL-1, IL-6), which lead to a state of insulin resistance. As a result, we observe a stimulated glucose production rate accompanied by decreased body glucose utilization causing an increase in the circulating blood glucose concentration (Fig. 2.2a–c).
The hyperglycemic response to surgery has long been recognized to depend on the type, severity, and extent of tissue trauma. Minor surgery is not associated with a clinically relevant increase in glycemia [1]. In fasting patients undergoing elective intraperitoneal procedures, however, blood glucose levels typically increase to 7–10 mmol/L. During cardiac surgery, mainly due to the profound inflammatory alterations associated with cardiopulmonary bypass, the disturbance of glucose homeostasis is severe, with glucose values frequently exceeding 15 mmol/L in nondiabetic and 20 mmol/L in diabetic patients.
Although the effect of surgical technique on glucose metabolism has not been widely studied, laparoscopic procedures may have less impact than the open approach. Possibly mediated through the reduction of tissue damage and the inhibition of inflammatory responses, patients following laparoscopic colon resection showed better glucose utilization when compared with laparotomy [2].
The choice of anesthetic drugs also is important. While intravenous anesthetics, such as propofol, appear to have no effect, inhalational agents are capable of impeding pancreatic insulin secretion. In contrast, opioids, particularly when administered in large doses, and neuraxial techniques mitigate the hyperglycemic response to surgery.
Perioperative use of corticosteroids, even in small doses, for the prevention of postoperative nausea and vomiting, as well as catecholamines, intravenous drugs, diluted in 5% dextrose,Footnote 1 blood products, and parenteral feeding exacerbate hyperglycemia, even in the absence of diabetes mellitus [3].
There is evidence to suggest that a large number of patients show abnormal glucose homeostasis before surgery. In a prospective study in 500 patients presenting for elective procedures, 26% of previously undiagnosed patients demonstrated blood glucose levels in the impaired-fasting glucose or the diabetic range [4]. Only 10% of diabetic patients in this observational study presented with a normal blood sugar prior to the operation.
Assessment
Accurate, precise, and timely measurement of blood glucose is an essential element of modern perioperative care. The circulating blood glucose concentration can be assessed using laboratory serum and plasma glucose analysis, whole blood and capillary glucose measurement by blood gas analyzers, or glucometers. Glucose analysis in the laboratory, the gold standard [6], may not provide results fast enough to promptly and effectively treat hypo- or hyperglycemic episodes in the operating theater. Hence, perioperatively glycemia is being routinely assessed by so-called point-of-care (POC) devices such as glucometers and blood gas analyzers. Blood glucose results obtained by older POC devices in the acute critical care setting need to be interpreted with caution, mainly because they do not correct for hematocrit [6,7,8] or other confounders such as body temperature, pH, pO2, tissue perfusion, hypoglycemia, and various medications [6]. Although the advent of newer technologies provided more reliable data in the critically ill [9], no studies addressed limitations and accuracy of glucometers during surgeries provoking the most profound alterations of glucose homeostasis. Hence, not unexpectedly, there are no clear recommendations by the US Food and Drug Administration (FDA) regarding specific glucometer safety requirements for patients warranting intravenous insulin therapy perioperatively.
In 2017 the use of the Nova StatStrip® Glucose Hospital Meter System in patients undergoing different types of surgery showed 100% accuracy of capillary and arterial glucose values based on the International Organization for Standardization (ISO) 15197:2013 criteria, i.e., all values were within zones A and B on the Parkes error grid for type 1 diabetes mellitus [10]. However, neither capillary nor arterial blood glucose results met the Clinical and Laboratory Standards Institute (CLSI) POCT12-A3 guidelines as required for intensive insulin protocols aimed at stricter glycemic control.
Results of a more recent study demonstrate that arterial blood glucose measurement by StatStrip® in cardiac surgery was accurate before the initiation of cardiopulmonary bypass (CPB) but lacked accuracy during and after CPB—most likely due to the interference of heparinization and anemia.
Clinical Relevance
Traditionally, the hyperglycemic response to surgery has been regarded as adaptive and beneficial because it ensures continuous provision of glucose for tissues that are glucose dependent, i.e., brain, erythrocytes, and immune cells.
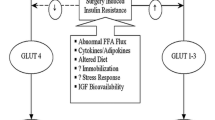
Surgical stress, however, triggers the release of mediators that, on one hand, inhibit the expression of the insulin-dependent membrane glucose transporter glut 4, which is mainly located in the myocardium and the skeletal muscle, and, on the other hand, stimulate the expression of the insulin-independent membrane glucose transporters glut 1, 2, and 3, which are located in blood cells, the endothelium, and the brain (Fig. 2.3).
In the healthy postprandial state, glucose concentration rises, and the subsequent increase in circulating insulin activates intracellular signaling cascades that ultimately result in the translocation of glucose transporter type 4 (GLUT-4) to the plasma membrane. Following elective surgery, hormonal and inflammatory mediators generated by the surgical stress response produce a state of insulin resistance. A reduction in peripheral insulin-mediated glucose uptake is observed and believed to be the cause of (1) a defect in insulin signaling pathways, particularly phosphoinositide-3-kinase-protein kinase (P13K) or (2) a defect in the translocation of GLUT-4 to plasma membrane. Akt serine/threonine protein kinase, IRS-1 insulin receptor substrate 1, P phosphorylation, PDK1/2 3-phosphoinositide-dependent protein kinase 1. (Reprinted with permission from Gillis and Carli [1])
As insulin-dependent cells appear to be protected by insulin resistance, most of the circulating glucose enters cells that do not require insulin for uptake resulting in a cellular glucose overload. Once inside the cell, glucose either nonenzymatically glycosylates proteins such as immunoglobulins and renders them dysfunctional or goes into glycolysis. That pathway generates excess superoxide radicals, which by binding to nitric oxide (NO) promote the formation of peroxynitrate that ultimately leads to mitochondrial dysfunction and apoptosis.
Hence, a growing body of evidence indicates that even moderate increases in blood glucose are associated with adverse outcomes after surgery [11]. Patients with cardiovascular, infectious, and neurological problems appear to be particularly sensitive.
In general surgical wards, patients with fasting blood glucose concentrations above 7 mmol/L or random blood glucose levels >11.1 mmol/L had an 18-fold greater in-hospital mortality, a longer stay, and a greater risk of infection than patients who were normoglycemic [12]. Acute hyperglycemia has been linked to an increased incidence of surgical site infections after cardiac procedures [13] and total joint arthroplasty [11], allograft rejection after renal transplantation [14], and functional deterioration following cerebrovascular accidents [15].
Hyperglycemia presumably contributes to increased mortality in patients after myocardial infarction [16], stroke [17], open heart [18], and general surgery [19]. Acute hyperglycemia—via manipulating nitric oxide synthase activity and the angiotensin II pathway—limits vascular reactivity and suppresses the immune system by inactivating immunoglobulins and inhibiting neutrophil chemotaxis/phagocytosis.
Acute changes in glucose levels may facilitate the development of post-traumatic chronic pain. In a chronic post-ischemia pain animal model, hyperglycemia, at the time of injury, increased, while strict glycemic control reduced mechanical and cold allodynia [20].
More recent evidence, mainly based on observational studies, indicates that perioperative hyperglycemia may increase the incidence of postoperative delirium and cognitive dysfunction in adults [21]. In children operated on for congenital heart problems, postoperative hyperglycemia had no effect on neurodevelopmental outcomes after 4 years [22].
Marked fluctuations in blood glucose may be harmful independent of the absolute glucose level [23]. Increased magnitudes of perioperative glycemic changes in patients undergoing elective coronary bypass surgery were associated with a greater risk of atrial fibrillation and length of intensive care unit (ICU) stay [24].
However, there is not a consistent definition of glycemic variability, and several metrics (e.g., the coefficient of variation of blood glucose levels or the glycemic lability index) have been used in critical illness. It also remains unclear whether variations within the normal glycemic range or periods of significant hypo- and hyperglycemia are problematic.
There is evidence to suggest that the quality of preoperative glycemic control is clinically important. Elevated levels of plasma glycosylated hemoglobin A (hemoglobin A1c), an indicator of glucose control in the preceding 3 months, were found to be predictive of complications after abdominal and cardiac surgery [25, 26]. In non-cardiac, nonvascular patients, preoperative blood glucose levels above 11.1 mmol L−1 were associated with a 2.1-fold higher risk in 30-day all-cause mortality and a 4-fold higher risk of 30-day cardiovascular mortality [27]. In a large cohort of 61,536 consecutive elective non-cardiac surgery patients, poor preoperative glycemic control was related to adverse in-hospital outcomes and 1-year mortality [28]. Diabetic patients undergoing open heart surgery with a HbA1c > 6.5% had a greater incidence of major complications, received more blood products, and spent more time in the ICU and the hospital than metabolically normal patients [29].
Insulin Metabolism
Pathophysiology
Insulin is the chief anabolic hormone in the human body. Although most recognized for its role in regulating glucose homeostasis, insulin plays a pivotal role in promoting protein synthesis and inhibiting protein breakdown. It is less known that insulin exerts non-metabolic effects including vasodilatory, anti-inflammatory, anti-oxidative, anti-fibrinolytic, and positive inotropic effects with potential clinical impact [30, 31].
Insulin resistance can be defined as any condition whereby a normal concentration of insulin produces a subnormal biological response. This umbrella term may comprise states of insulin insensitivity, insulin unresponsiveness, or a combination of both. Although the terms insulin sensitivity and insulin responsiveness are often used interchangeably, their difference stems from the classic sigmoidal dose-response curve of insulin action [32]. Insulin sensitivity is characterized by the insulin concentration required to achieve a half-maximal biological response, whereas insulin responsiveness is defined by the maximal effect attained. Impaired insulin sensitivity is, therefore, represented by a rightward shift in the insulin-dose response curve, and decreased responsiveness corresponds to a height reduction of the curve.
Proper use of these terms is important because they reflect different defects in insulin action: Insulin insensitivity appears to be more implicated in alterations at the pre-receptor and receptor level, whereas decreased responsiveness is related to post-receptor defects [32].
With regard to glucose metabolism, surgical patients should be called insulin insensitive because normoglycemia (= biological response) can be achieved by using large enough quantities of insulin. Whether similar relationships exist concerning the pharmacological effects of insulin on immunological and cardiovascular parameters or its anti-catabolic role in protein metabolism remains to be studied.
Much of the impairment of insulin function after surgery can be explained by the stress-induced release of counter-regulatory hormones. These hormones exert catabolic effects, either directly or indirectly, by inhibiting insulin secretion and/or counteracting its peripheral action. The observed association between the time course of perioperative interleukin 6 plasma concentrations and insulin resistance suggests that inflammatory mediators are also involved [33].
The main site for surgery-induced insulin resistance is skeletal muscle, because this is the quantitatively most important organ for insulin-mediated glucose uptake. The magnitude of whole-body insulin resistance is most pronounced on the day after surgery (up to 70% reduction) and lasts for about 3 weeks after uncomplicated elective abdominal operations. It has been primarily linked to the invasiveness of surgery [34]. Other factors may also contribute, such as the duration of trauma [35], bed rest and immobilization [36], type of anesthesia and analgesia [37], nutrition and preoperative fasting [37, 38], blood loss, physical status, and post-surgery rehabilitation [39].
Assessment
The gold standard for the assessment of insulin resistance in humans is the hyperinsulinemic-normoglycemic clamp technique, whereby insulin is infused at a constant rate to obtain a steady-state insulin concentration above the fasting level [40]. Based on frequent measurements of plasma glucose levels, glucose is intravenously infused at variable rates to maintain normoglycemia. Given that endogenous glucose production by the liver and kidneys is completely suppressed, the glucose infusion rate (under steady-state conditions) is reflective of glucose disposal and is, therefore, an indicator of peripheral insulin resistance: The greater the glucose infusion rate, the more sensitive the body is to insulin and vice versa.
Other indices traditionally used to quantitate insulin sensitivity in patients, such as the homeostasis model assessment (HOMA) index, the quantitative insulin-sensitivity check index (QUICKI) (both based on plasma insulin and glucose levels), or oral/intravenous (IV) glucose tolerance tests, have shown to be only poor indicators of insulin function.
Recent observations suggest that body mass index (BMI) and the quality of preoperative glycemic control (hemoglobin A1c) may be simple predictors of insulin sensitivity during major surgery [29, 41].
Clinical Relevance
Studies performed over a 6-year period in Sweden in the early 1990s demonstrate a significant correlation between the degree of the patient’s insulin sensitivity on the first postoperative day and length of hospital stay [33]. More recently a significant association was reported between the magnitude of insulin resistance during cardiac surgery and outcome [29]. Independent of the patient’s diabetic state, for every decrease in intraoperative insulin sensitivity by 20%, the risk of a serious complication including all-cause mortality, myocardial failure requiring mechanical support, stroke, need for dialysis, and serious infection (severe sepsis, pneumonia requiring mechanical ventilation, deep sternal wound infection) more than doubled after open heart surgery [29].
These findings lend support to the previously held contention that, perioperatively, alterations in glucose homeostasis are better predictors of adverse events than the presence of diagnosed or suspected diabetes mellitus itself. The outcome relevance of insulin resistance is also reflected by the problems associated with its metabolic sequelae, i.e., hyperglycemia and protein wasting, the “diabetes of the injury.”
Protein Metabolism
Pathophysiology
Normal protein metabolism is characterized by an equilibrium between anabolic and catabolic pathways. Surgical stress leads to biochemical and physiologic perturbations of neuroendocrine homeostasis, including stimulation of the sympathetic nervous system, parasympathetic suppression, and activation of the hypothalamic-pituitary axis (Fig. 2.4) [42].
The surgically stressed state is characterized by an elevation in protein turnover (i.e., protein synthesis and degradation), release of amino acids into circulation, urinary nitrogen losses, and impaired uptake of amino acids in skeletal tissue. Lean tissue is catabolized, releasing amino acids into circulation (including glutamine, alanine, and the branched chain amino acids [BCAAs]), while hepatic amino acid uptake is enhanced. This allows for reprioritization of protein synthesis to acute-phase reactants and the production of glucose via gluconeogenesis. Glutamine (Glu) and alanine (Ala) account for the majority of the amino acid efflux from peripheral tissues and are readily extracted from circulation by the liver. The excess nitrogen is converted in the liver to urea by combining ammonia (NH3) with CO2 (carbon dioxide). Urea is then released into circulation, traveling to the kidneys, where it can be filtered into urine. The BCAAs undergo irreversible degradation in skeletal tissue, in part for synthesis of glutamine and alanine, which reduces availability of these indispensable amino acids for reutilization in protein synthesis. Collectively, these metabolic changes promote whole-body protein catabolism. (Reprinted with permission from Gillis and Carli [1])
This results in a mobilization of substrates in order to improve the chance of survival. Metabolic pathways are shifted from anabolism toward catabolism [43]. Skeletal muscle protein stores are mobilized to provide amino acids for two main purposes: first, the amino acids can be converted to glucose by the liver as an energy source during a hypermetabolic state, and second, they serve as substrate for protein synthesis by the wound and the liver.
Typical features of protein catabolism are stimulated rates of whole-body protein breakdown and amino acid oxidation. The synthesis of rapidly turning over acute-phase plasma proteins is also upregulated; however, it is not to the same extent as protein breakdown, resulting in a net loss of functional and structural body protein [44,45,46,47]. Metabolically healthy patients lose between 40 g and 80 g of nitrogen after elective abdominal surgery, equivalent to 1.2–2.4 kg wet skeletal muscle [48]. Patients with burns or sepsis experience daily losses of up to 800 g of muscle mass. Protein loss in insulin-resistant patients, after colorectal cancer surgery, has been shown to be 50% greater than in patients with a normal insulin response [49]. More recent studies indicate a linear relationship between insulin sensitivity and protein balance in parenterally fed patients undergoing open heart surgery [50].
Muscle wasting occurs early and rapidly during the first week of critical illness and is more severe among patients with multiorgan failure [45]. Significant muscle weakness and physical disability can persist for more than 5 years after injury and critical illness [51, 52].
There is no evidence to suggest that the magnitude of catabolic changes in elderly patients differs from those in younger adults. Age, however, may be associated with reduced muscle mass and a decreased capacity to utilize nutrients. Older patients may, therefore, be more vulnerable to protein catabolism [53].
There are different rates of uptake or release of amino acids in specific regional vascular beds. During the acute phase of injury, amino acids are released from skeletal muscle as a result of accelerated proteolysis. These amino acids are extracted from the bloodstream of the splanchnic bed for hepatic synthesis of structural, plasma, and acute-phase proteins.
Two amino acids, alanine and glutamine, account for approximately 50–75% of the amino acid nitrogen released from skeletal muscle, although they make up only 6% of protein in muscle stores [54]. Alanine is an important glucose precursor and indirectly provides this fuel source, which is essential for several key tissues. Glutamine is a gluconeogenesis substrate but also serves as primary substrate for immune cells and enterocytes, participates in acid-base homeostasis, and serves as a precursor for glutathione, which is an important intracellular antioxidant. It has been hypothesized that the tissue requirements for glutamine may outstrip the ability for tissue (particularly skeletal muscle) to produce this amino acid. Hence a relative deficiency state exists, characterized by a fall in glutamine concentrations in both the plasma and tissue compartments [55].
The plasma concentration of albumin, a so-called negative acute-phase protein, typically decreases in response to surgical stress. Studies measuring the synthesis rate of albumin, however, provide more insight into the underlying mechanisms. While the synthetic rate of albumin decreases during surgery, it is upregulated during the early postoperative period and only returns to normal values after several weeks [56]. The physiologic significance of albumin synthesis and its regulation in patients undergoing surgery need to be further investigated. While under normal conditions, increased amino acid availability represents an important regulator of protein synthesis, it seems that in postoperative patients, other factors (inflammation, endocrine stress, and liver function) also play important roles [57, 58].
Bed Rest and Fatigue
Confining patients to bed for a prolonged period of time initiates a series of metabolic responses that can be deleterious if not corrected. Both muscle weakness and atrophy begin after only 1 day of bed rest, with the extent being greater in older people [59].
Malnourished Patients
Malnourished cancer patients experience a higher morbidity and mortality in response to surgical treatment, have a higher hospital readmission rate, and have a prolonged convalescence when compared with those who are normally nourished [60, 61]. Clinical outcome studies suggest that sarcopenic patients benefit more than their normal counterparts from a short course of intravenous nutrition, particularly if initiated before surgery [62,63,64]. Total parenteral nutrition in catabolic, depleted patients with gastrointestinal cancer, after trauma and during sepsis, resulted in a greater reduction of net protein catabolism than in nondepleted patients [65, 66].
In order to evaluate the efficacy of nutritional support, the patient’s baseline catabolic state must be quantified because sarcopenia is related to postoperative morbidity and mortality [61, 67]. A significant association exists between the degree of preoperative catabolism and the anabolic effect of nutrition, with catabolic patients benefiting the most [68]. These more recent observations support the previous demonstration of superior outcomes in perioperatively fed malnourished patients [64].
Assessment of Catabolism
Many clinical and biochemical indices have been used to characterize the nutritional status of surgical patients, but all techniques have limitations [69,70,71]. Anthropometric and body composition measurements need to be treated with caution in subjects who are dehydrated and/or have edema or ascites [69]. Serum proteins are pathophysiological markers influenced by factors other than malnutrition or catabolism, such as inflammation with redistribution and dilution [69, 72].
Protein economy in surgical patients has traditionally been characterized by measuring nitrogen balance, i.e., the difference between nitrogen entering and exiting the body. Nitrogen is mainly lost in the form of urea, which represents about 85% of the urinary nitrogen loss. This proportion, however, has been shown to vary widely. Because of the fixed relation between protein and nitrogen (1 g protein contains 6.25 g of nitrogen), urinary nitrogen excretion has commonly been assessed as a surrogate marker of whole-body protein loss. However, urinary nitrogen excretion measurements are unable to address the question of whether muscle wasting is a result of increased proteolysis, impaired protein synthesis, or, simply, the lack of proper anabolic response to nutrition. Furthermore, retention of nitrogen within the body and underestimation of nitrogen excretion in urine and other routes (feces, skin, wound secretion) invariably lead to false positive values [73, 74].
Tracer methods using amino acids labeled with stable isotopes (2H, 15N, 13C) are considered the technique of choice for the global assessment of catabolism in humans and its relation to protein and energy intake [75]. They provide a dynamic picture about the kinetics of glucose and amino acids on the whole-body (protein breakdown, oxidation and synthesis, glucose production and utilization) and organ tissue level [76,77,78].
Clinical Relevance
Because protein represents structural and functional components, the loss of lean tissue delays wound healing, compromises immune function, and diminishes muscle strength after surgery [79, 80]. The ensuing muscle weakness prolongs mechanical ventilation, inhibits coughing, and impedes mobilization, thereby causing morbidity and complicating convalescence [81, 82]. The length of time for return of normal physiologic function after discharge from the hospital is related to the extent of lean body loss during hospitalization [82].
Significant mortality occurs after critically ill patients are discharged from the ICU and hospital [51]. Many of these deaths are ascribed to the loss of muscle mass, inadequate physical activity, muscle weakness, and the inability to mobilize.
Metabolic Attenuation of the Stress Response
The pathophysiology of the surgical stress response is multifactorial, and therefore the therapeutic interventions should aim at identifying those metabolic components within the perioperative trajectory. Conceptually, the treatment of postoperative, low insulin sensitivity will normalize insulin action and the main components of metabolism. The implementation of several metabolic modalities and their use in an integrated fashion modulate the perioperative establishment of the state on insulin resistance, also called low insulin sensitivity.
Perioperative Nutrition
With the fed state insulin levels elevated, storage of substrates is made available, and insulin sensitivity is elevated in anticipation of the incoming stress. There is sufficient evidence that preoperative carbohydrate drink increases insulin sensitivity before surgery and attenuates the establishment of insulin resistance in the postoperative state [83]. Complex carbohydrates appear to have a greater insulin secretion response, which would have a pronounced effect on blocking gluconeogenesis.
The physiological advantage of feeding at time of catabolic stress is the stimulation of insulin production, which inhibits protein breakdown and facilitates the incorporation of supplied amino acids into protein synthesis [84].
Anabolism, a positive whole-body protein balance, is required for optimal patient recovery after surgery. Patients undergoing major elective surgery present with a negative whole-body protein balance, generated from an increase in proteolysis, as early as the first postoperative day [85, 86]. Therefore, the primary goal of perioperative nutritional care is thus the provision of protein to attenuate catabolism, as well as maintenance of normoglycemia, adequate hydration, and avoidance of fasting [87]. The extent to which anabolism is accomplished depends not only on the medical care provided, including ERAS, but also on the timing, route of delivery, and composition of the nutritional support regimens provided.
Insulin Therapy
Insulin sensitivity, rather than insulin responsiveness, is reduced throughout the period of surgical stress, probably as a result of the raised inflammatory response that affects insulin target cells. Insulin therapy is suggested when normoglycemia and protein balance need to be maintained. The perioperative administration of insulin to maintain blood glucose between 6 and 8 mmol/L is recommended in order to overcome postoperative insulin resistance and improve outcome [88].
Minimally Invasive Surgery
Activation of inflammatory pathways that could negatively impact on the recovery process can be reduced by limiting either the size or the orientation of the incision. Endoscopic techniques limit the size of the incision and the trauma to the abdominal wall by splitting the muscle fibers instead of cutting them. Changing the incision from vertical to horizontal could also decrease pain as a result of having less dermatomes involved in transporting nociceptive signals to the central nervous system. In addition, inflammation can be reduced by minimizing internal organ manipulation and direct peritoneal injury and blood loss [89].
Neural Deafferentation
Administration of epidural and spinal local anesthetics initiated before surgery and maintained during the first 48 hours after surgery (epidural only) has been shown to decrease perioperative insulin resistance, to attenuate the decrease in muscle protein synthesis and the rise in blood glucose, and facilitate the anabolic effect of amino acids in type 2 diabetics [90, 91]. The addition of nutrition while on neural blockade promotes protein synthesis and improves postoperative protein balance.
Maintenance of Intraoperative Normothermia
Maintaining patients normothermic during surgery has been shown to attenuate the perioperative release of catecholamines and decrease loss of body nitrogen [92]. Although no data on the metabolic effect of normothermia on insulin sensitivity are available, it is plausible to associate mechanistically the sparing protein loss process with improved insulin sensitivity.
Physical Activity and Mobilization
Long-term bed rest and sedentary activity produce marked changes in glucose and protein metabolism [93, 94]. Two weeks of limb immobilization has been shown to decrease the quadriceps lean mass by almost 5% and the strength by 25% and lowers peripheral insulin sensitivity [95].
Elderly and frail patients are particularly vulnerable, since loss of muscle mass impacts on their functional strength and functional capacity [96]. There is sufficient evidence that exercise training improves glucose metabolism and particularly insulin sensitivity. This is particularly evident in diabetic patients. The anabolic effect of exercise training can be enhanced by adequate intake of amino acids. Mobilization after surgery should therefore be considered an important factor in achieving anabolism, and this can be facilitated with adequate analgesia.
Conclusion
While we are aware of the implications of low insulin sensitivity associated with surgery on body metabolism, the connection between physiological and clinical outcomes is not always demonstrated.
The relative role of different pathogenic mechanisms in the development of postoperative insulin resistance leading to higher morbidity needs to be clarified. Hopefully, this can lead to better understanding and future therapeutic strategies. This implies that more work needs to be done to fill the gaps between what we know and what we do in clinical practice. Patients will be the ones who will gain from these advances in research and clinical care.
Notes
- 1.
Please note: the infusion of a 100 ml bag of dextrose 5% (=5 g of glucose) almost doubles the amount of circulating glucose in a 70 kg nondiabetic patient (assuming a glycemia level of 5 mmol/L = 0.9 g/L and a blood volume of 77 ml/kg) [5].
References
Polderman JA, Van Velzen L, Wasmoeth LG, et al. Hyperglycemia and ambulatory surgery. Minerva Anestesiol. 2015;81(9):951–9.
Carli F, Galeone M, Gzodzic B, et al. Effect of laparoscopic colon resection on postoperative glucose utilization and protein sparing: an integrated analysis of glucose and protein metabolism during the fasted and fed States using stable isotopes. Arch Surg (Chicago, IL: 1960). 2005;140(6):593–7.
Eberhart LH, Graf J, Morin AM, et al. Randomised controlled trial of the effect of oral premedication with dexamethasone on hyperglycaemic response to abdominal hysterectomy. Eur J Anaesthesiol. 2011;28(3):195–201.
Hatzakorzian R, Bui H, Carvalho G, Shan WL, Sidhu S, Schricker T. Fasting blood glucose levels in patients presenting for elective surgery. Nutrition (Burbank, Los Angeles County, CA). 2011;27(3):298–301.
Schricker T, Lattermann R, Wykes L, Carli F. Effect of i.v. dextrose administration on glucose metabolism during surgery. JPEN J Parenter Enteral Nutr. 2004;28(3):149–53.
Rice MJ, Pitkin AD, Coursin DB. Review article: glucose measurement in the operating room: more complicated than it seems. Anesth Analg. 2010;110(4):1056–65.
Ghys T, Goedhuys W, Spincemaille K, Gorus F, Gerlo E. Plasma-equivalent glucose at the point-of-care: evaluation of Roche Accu-Chek Inform and Abbott Precision PCx glucose meters. Clin Chim Acta. 2007;386(1–2):63–8.
Karon BS, Griesmann L, Scott R, et al. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther. 2008;10(2):111–20.
Mitsios JV, Ashby LA, Haverstick DM, Bruns DE, Scott MG. Analytic evaluation of a new glucose meter system in 15 different critical care settings. J Diabetes Sci Technol. 2013;7(5):1282–7.
Karon BS, Donato LJ, Larsen CM, et al. Accuracy of capillary and arterial whole blood glucose measurements using a glucose meter in patients under general anesthesia in the operating room. Anesthesiology. 2017;127(3):466–74.
Shohat N, Muhsen K, Gilat R, Rondon AJ, Chen AF, Parvizi J. Inadequate glycemic control is associated with increased surgical site infection in total joint arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2018;33(7):2312–2321.e2313.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82.
Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–60; discussion 360–352.
Thomas MC, Mathew TH, Russ GR, Rao MM, Moran J. Early peri-operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study. Transplantation. 2001;72(7):1321–4.
Baird TA, Parsons MW, Phan T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34(9):2208–14.
Stranders I, Diamant M, van Gelder RE, et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164(9):982–8.
Szczudlik A, Slowik A, Turaj W, et al. Transient hyperglycemia in ischemic stroke patients. J Neurol Sci. 2001;189(1-2):105–11.
Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–21.
Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8–14.
Ross-Huot MC, Laferriere A, Gi CM, Khorashadi M, Schricker T, Coderre TJ. Effects of glycemic regulation on chronic postischemia pain. Anesthesiology. 2011;115(3):614–25.
Hermanides J, Qeva E, Preckel B, Bilotta F. Perioperative hyperglycemia and neurocognitive outcome after surgery: a systematic review. Minerva Anestesiol. 2018;84(10):1178–88.
Krueger JJ, Brotschi B, Balmer C, Bernet V, Latal B. Postoperative hyperglycemia and 4-year neurodevelopmental outcome in children operated for congenital heart disease. J Pediatr. 2015;167(6):1253–1258.e1251.
Deane AM, Horowitz M. Dysglycaemia in the critically ill – significance and management. Diabetes Obes Metab. 2013;15(9):792–801.
Sim MA, Liu W, Chew STH, Ti LK. Wider perioperative glycemic fluctuations increase risk of postoperative atrial fibrillation and ICU length of stay. PLoS One. 2018;13(6):e0198533.
Halkos ME, Puskas JD, Lattouf OM, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008;136(3):631–40.
Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg. 2009;96(11):1358–64.
Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156(1):137–42.
Abdelmalak BB, Knittel J, Abdelmalak JB, et al. Preoperative blood glucose concentrations and postoperative outcomes after elective non-cardiac surgery: an observational study. Br J Anaesth. 2014;112(1):79–88.
Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95(9):4338–44.
Yki-Jarvinen H, Utriainen T. Insulin-induced vasodilatation: physiology or pharmacology? Diabetologia. 1998;41(4):369–79.
Das UN. Is insulin an anti-inflammatory molecule? Nutrition (Burbank, Los Angeles County, CA). 2001;17(5):409–13.
Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978;27(12 Suppl 2):1893–902.
Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2(1):69–78.
Thorell A, Loftenius A, Andersson B, Ljungqvist O. Postoperative insulin resistance and circulating concentrations of stress hormones and cytokines. Clin Nutr (Edinburgh, Scotland). 1996;15(2):75–9.
Tsubo T, Kudo T, Matsuki A, Oyama T. Decreased glucose utilization during prolonged anaesthesia and surgery. Can J Anaesth/Journal canadien d’anesthesie. 1990;37(6):645–9.
Nygren J, Thorell A, Efendic S, Nair KS, Ljungqvist O. Site of insulin resistance after surgery: the contribution of hypocaloric nutrition and bed rest. Clin Sci (London, England: 1979). 1997;93(2):137–46.
Uchida I, Asoh T, Shirasaka C, Tsuji H. Effect of epidural analgesia on postoperative insulin resistance as evaluated by insulin clamp technique. Br J Surg. 1988;75(6):557–62.
Wang ZG, Wang Q, Wang WJ, Qin HL. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br J Surg. 2010;97(3):317–27.
Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology. 2008;108(3):506–23.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23.
Nakadate Y, Sato H, Sato T, Codere-Maruyama T, Matsukawa T, Schricker T. Body mass index predicts insulin sensitivity during cardiac surgery: a prospective observational study. Can J Anaesth/Journal canadien d’anesthesie. 2018;65(5):551–9.
Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109–17.
Giannoudis PV, Dinopoulos H, Chalidis B, Hall GM. Surgical stress response. Injury. 2006;37 Suppl 5:S3–9.
Cuthbertson DP, Angeles Valero Zanuy MA, Leon Sanz ML. Post-shock metabolic response. 1942. Nutr Hosp. 2001;16(5):176–82; discussion 175–176.
Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600.
Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87(7):3378–84.
Schricker T, Meterissian S, Lattermann R, et al. Anticatabolic effects of avoiding preoperative fasting by intravenous hypocaloric nutrition: a randomized clinical trial. Ann Surg. 2008;248(6):1051–9.
Kinney JM, Elwyn DH. Protein metabolism and injury. Annu Rev Nutr. 1983;3:433–66.
Schricker T, Gougeon R, Eberhart L, et al. Type 2 diabetes mellitus and the catabolic response to surgery. Anesthesiology. 2005;102(2):320–6.
Donatelli F, Corbella D, Di Nicola M, et al. Preoperative insulin resistance and the impact of feeding on postoperative protein balance: a stable isotope study. J Clin Endocrinol Metab. 2011;96(11):E1789–97.
Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–93.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94.
Morais JA, Chevalier S, Gougeon R. Protein turnover and requirements in the healthy and frail elderly. J Nutr Health Aging. 2006;10(4):272–83.
Muhlbacher F, Kapadia CR, Colpoys MF, Smith RJ, Wilmore DW. Effects of glucocorticoids on glutamine metabolism in skeletal muscle. Am J Physiol. 1984;247(1 Pt 1):E75–83.
Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48(8):297–309.
Hulshoff A, Schricker T, Elgendy H, Hatzakorzian R, Lattermann R. Albumin synthesis in surgical patients. Nutrition (Burbank, Los Angeles County, CA). 2013;29(5):703–7.
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54.
Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293(2):E453–9.
Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(10 Suppl):S422–8.
Meguid MM, Debonis D, Meguid V, Hill LR, Terz JJ. Complications of abdominal operations for malignant disease. Am J Surg. 1988;156(5):341–5.
Jagoe RT, Goodship TH, Gibson GJ. The influence of nutritional status on complications after operations for lung cancer. Ann Thorac Surg. 2001;71(3):936–43.
Von Meyenfeldt MF, Meijerink WJ, Rouflart MM, Builmaassen MT, Soeters PB. Perioperative nutritional support: a randomised clinical trial. Clin Nutr (Edinburgh, Scotland). 1992;11(4):180–6.
Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24(1):7–14.
Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991;325(8):525–32.
Shaw JH, Wolfe RR. Glucose and urea kinetics in patients with early and advanced gastrointestinal cancer: the response to glucose infusion, parenteral feeding, and surgical resection. Surgery. 1987;101(2):181–91.
Shaw JH. Influence of stress, depletion, and/or malignant disease on the responsiveness of surgical patients to total parenteral nutrition. Am J Clin Nutr. 1988;48(1):144–7.
Hasselager R, Gogenur I. Core muscle size assessed by perioperative abdominal CT scan is related to mortality, postoperative complications, and hospitalization after major abdominal surgery: a systematic review. Langenbecks Arch Surg. 2014;399(3):287–95.
Schricker T, Wykes L, Meterissian S, et al. The anabolic effect of perioperative nutrition depends on the patient’s catabolic state before surgery. Ann Surg. 2013;257(1):155–9.
Downs JH, Haffejee A. Nutritional assessment in the critically ill. Curr Opin Clin Nutr Metab Care. 1998;1(3):275–9.
Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. Am J Clin Nutr. 2012;96(3):591–600.
Allison SP. Malnutrition, disease, and outcome. Nutrition (Burbank, Los Angeles County, CA). 2000;16(7–8):590–3.
Allison SP, Lobo DN, Stanga Z. The treatment of hypoalbuminaemia. Clin Nutr (Edinburgh, Scotland). 2001;20(3):275–9.
Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980;238(5):E473–9.
Prelack K, Dwyer J, Yu YM, Sheridan RL, Tompkins RG. Urinary urea nitrogen is imprecise as a predictor of protein balance in burned children. J Am Diet Assoc. 1997;97(5):489–95.
Berg A, Rooyackers O, Bellander BM, Wernerman J. Whole body protein kinetics during hypocaloric and normocaloric feeding in critically ill patients. Crit Care (London, England). 2013;17(4):R158.
Lattermann R, Carli F, Wykes L, Schricker T. Perioperative glucose infusion and the catabolic response to surgery: the effect of epidural block. Anesth Analg. 2003;96(2):555–62, table of contents.
Lattermann R, Carli F, Wykes L, Schricker T. Epidural blockade modifies perioperative glucose production without affecting protein catabolism. Anesthesiology. 2002;97(2):374–81.
Schricker T, Wykes L, Carli F. Epidural blockade improves substrate utilization after surgery. Am J Physiol Endocrinol Metab. 2000;279(3):E646–53.
Chandra RK. Nutrition, immunity, and infection: present knowledge and future directions. Lancet (London, England). 1983;1(8326 Pt 1):688–91.
Windsor JA, Hill GL. Weight loss with physiologic impairment. A basic indicator of surgical risk. Ann Surg. 1988;207(3):290–6.
Watters JM, Clancey SM, Moulton SB, Briere KM, Zhu JM. Impaired recovery of strength in older patients after major abdominal surgery. Ann Surg. 1993;218(3):380–90; discussion 390–383.
Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg. 1982;69(7):417–9.
Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol. 2009;23(4):401–9.
Hill GL, Douglas RG, Schroeder D. Metabolic basis for the management of patients undergoing major surgery. World J Surg. 1993;17(2):146–53.
Lopez-Hellin J, Baena-Fustegueras JA, Vidal M, Riera SS, Garcia-Arumi E. Perioperative nutrition prevents the early protein losses in patients submitted to gastrointestinal surgery. Clin Nutr (Edinburgh, Scotland). 2004;23(5):1001–8.
Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. 2006;(4):Cd004080.
Martindale RG, McClave SA, Taylor B, Lawson CM. Perioperative nutrition: what is the current landscape? JPEN J Parenter Enteral Nutr. 2013;37(5 Suppl):5s–20s.
Blixt C, Ahlstedt C, Ljungqvist O, Isaksson B, Kalman S, Rooyackers O. The effect of perioperative glucose control on postoperative insulin resistance. Clin Nutr (Edinburgh, Scotland). 2012;31(5):676–81.
Kim TK, Yoon JR. Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy. Korean J Anesthesiol. 2010;59(4):265–9.
Carli F, Halliday D. Continuous epidural blockade arrests the postoperative decrease in muscle protein fractional synthetic rate in surgical patients. Anesthesiology. 1997;86(5):1033–40.
Lugli AK, Donatelli F, Schricker T, Wykes L, Carli F. Epidural analgesia enhances the postoperative anabolic effect of amino acids in diabetes mellitus type 2 patients undergoing colon surgery. Anesthesiology. 2008;108(6):1093–9.
Carli F, Webster J, Nandi P, MacDonald IA, Pearson J, Mehta R. Thermogenesis after surgery: effect of perioperative heat conservation and epidural anesthesia. Am J Physiol. 1992;263(3 Pt 1):E441–7.
Glover EI, Phillips SM, Oates BR, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586(24):6049–61.
Wall BT, Snijders T, Senden JM, et al. Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J Clin Endocrinol Metab. 2013;98(12):4872–81.
Krogh-Madsen R, Thyfault JP, Broholm C, et al. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (Bethesda, MD: 1985). 2010;108(5):1034–40.
Kortebein P, Symons TB, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(10):1076–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Schricker, T., Lattermann, R., Carli, F. (2020). Physiology and Pathophysiology of ERAS. In: Ljungqvist, O., Francis, N., Urman, R. (eds) Enhanced Recovery After Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-33443-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-33443-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33442-0
Online ISBN: 978-3-030-33443-7
eBook Packages: MedicineMedicine (R0)