Abstract
Chemical admixtures constitute indispensable ingredients for the production of modern advanced concrete. In developed countries, at least 80% of the concrete produced contains one or several admixtures. They include plasticizers, superplasticizers, retarders, accelerators, stabilizers, defoamers, foamers and shrinkage reducers, to name the most important classes. With their help it is possible to optimize the properties of fresh and hardened concrete in such way as to adapt better to local climate and processing conditions and to enhance the mechanical properties and durability. Furthermore, highly sophisticated products such as ultra-high strength concrete (UHPC) or self-levelling and self-compacting concrete (SCC) became possible only with the invention of specific high performance admixtures.
This article gives an overview of major classes of chemical admixtures (e.g. PCE superplasticizers, C-S-H-PCE nanocomposites, stabilizers for SCC, shrinkage-reducing agents) and their current status of development. The main technologies will be described and their role in the formulation of modern advanced concrete will be highlighted. Finally, an outlook on potential developments in the future (e.g. improved curing agents, admixtures which enhance the ductility of concrete) will be provided.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Admixtures
- PCE superplasticizers
- Shrinkage-reducing agents
- C-S-H-PCE nanocomposites
- Viscosity modifying agents
1 Polycarboxylate (PCE) Superplasticizers
1.1 Current PCE Technology
Polycarboxylate superplasticizers are added to the fresh concrete for the dispersion of the cement particles. They produce a highly flowable concrete which can be placed at the construction site much easier. Additionally, lower w/c ratios can be applied thus facilitating the manufacturing of building materials with high mechanical strength and long durability. PCE-based admixtures have taken an unprecedented rise since their invention in 1981 (Hirata 1981). It is estimated that in 2014, the global volume of PCE produced exceeded 3 mio. tons, based on 30% liquid concentration. Meanwhile, the term “PCE” includes a huge variety of chemically often substantially different polymers, with significant variances in performance characteristics. In the following, the main classes of PCE products on the market are described and their general chemical composition is exhibited in Fig. 1.
MPEG-Type PCEs:
They constitute the first type of PCE which was invented in Japan. MPEG-PCEs can be synthesized either via aqueous free radical copolymerization of methacrylic acid with an ω-methoxy poly(ethylene glycol) methacrylate ester macromonomer (this route is predominantly used by the industry) (Plank et al. 2008) or by esterification (“grafting”) of short chain poly(meth)acrylic acid with ω-methoxy poly(ethylene glycol) (Guicquero et al. 1999). Note that both synthesis routes can lead to substantially different products, even when exactly the same molar ratios of monomers are used. Via esterification, a PCE polymer exhibiting a regular (statistical) repartition of side chains along the main chain is achieved while gradient polymers exhibiting a decreasing side chain density along the backbone are formed from the copolymerization process as a result of the higher reactivity of the ester macromonomer versus methacrylic acid (Pourchet et al. 2012). Performance tests have revealed that in many cases gradient polymers perform better, because their blocks of polymethacrylic acid allow higher adsorption on cement. One major disadvantage of MPEG-PCEs is their limited stability (especially when acrylate instead of methacrylate ester macromonomers are used) which derives from hydrolysis of the ester linkage between the main and the side chain. Furthermore, the diol or diester content present in the raw materials must be kept below 1% to avoid undesirable crosslinking (Paas 2015).
APEG-Type PCEs:
This kind is prepared via free radical copolymerization from α-allyl-ω-methoxy or ω-hydroxy poly(ethylene glycol) ether and maleic anhydride or acrylic acid as key monomers, either in bulk or in aqueous solution (Akimoto 1992). APEG-PCEs always possess a strictly alternating monomer sequence (ABAB), because the allyl ether macromonomer does not homopolymerize as a consequence of mesomeric stabilization of the allyl radical. This stabilization causes allyl ethers to react rather slowly and can lead to low conversion rates for the macromonomer. Polymerization in bulk works well for side chain lengths of up to 34 EO units while polymerization in water typically yields copolymers possessing very short trunk chains (“star polymers”) made of ~10 repeating units only which however were found to exhibit superior dispersing performance. The disadvantages of aqueous copolymerization are longer reaction times, lower conversion rates and lower concentration of the finished PCE solution.
Initially, APEG-PCEs suffered from a reputation of causing delayed plastification (i.e. the slump of concrete first increased over ~30 min to reach a maximum, and then dropped). Meanwhile, this problem has been solved, for example by incorporation of specific comonomers as spacer molecules such as styrene or allyl maleate which can modulate the conformational flexibility of the trunk chain (Plank and Lange 2012). This method provides PCE molecules with pronounced stiffness which can adsorb faster and thus avoid the effect of delayed plastification.
VPEG-Type PCEs:
Such PCEs are obtained by aqueous free radical copolymerization of e.g. 4-hydroxy butyl poly(ethylene glycol) vinyl ether and maleic anhydride or acrylic acid (Albrecht 1996). Their polymerization must be conducted at temperatures <30 °C to avoid vinyl ether monomer degradation. As a result, a specific low temperature initiator such as Vazo 50 (2,2’-Azobis (2-methyl propionamidine) dihydrochloride) is required. The advantage of the vinyl over the allyl ether technology is the much higher reactivity of vinyl ethers.
HPEG-Type PCEs:
Here, α-methallyl-ω-methoxy or ω-hydroxy poly(ethylene glycol) are used as macromonomers in copolymerization with e.g. acrylic acid (Hamada et al. 2001). This kind of PCE which is easy to manufacture in large industrial scale emerged a few years ago, especially in China. There, even a process has been developed where copolymerization is performed at room temperature and is applied in many factories (Wang et al. 2013). Most HPEG-PCEs can outperform the MPEG- or APEG-PCEs with respect to their dispersing ability.
IPEG-Type PCEs:
This type of PCE (sometimes also referred to as TPEG-PCE) is synthesized from isoprenyl oxy poly(ethylene glycol) ether as macromonomer by copolymerization with e.g. acrylic acid (Yamamoto 2004). In recent years, this PCE has become quite popular, especially in Japan and China, because of its excellent performance which often exceeds that of any other type of PCE, and its simple preparation utilizing free radical copolymerization. A disadvantage of IPEG-PCEs is their potential to decompose into isoprene, water and glycol (Nagare 2006). To prevent this undesired process, the IPEG macromonomer and the IPEG-PCE should not be handled in bulk, but always kept in aqueous solution.
XPEG-Type PCEs:
It has been established before that the ability of an individual PCE molecule to cover as much surface area on cement as possible directly correlates to its dosage (Ohta 1997). Hence, polymers which stretch out further on the surface are believed to present more effective PCEs. Following this concept, slightly crosslinked PCE molecules utilizing diesters (e.g. synthesized from PEG and methacrylic acid or maleic anhydride) were shown to provide enhanced dispersion (Tahara 1995).
PAAM-Type PCEs:
These zwitterionic PCEs possess mixed side chains composed of polyamidoamine (PAAM) and PEO segments. This structural motif distinguishes them fundamentally from all other PCEs which exclusively contain PEO/PPO side chains. The PAAM-type PCE is said to fluidify cement at w/c ratios as low as 0.12 (Amaya 2000). Its disadvantage is the high cost of the PAAM side chain.
1.2 New PCE Products
Industrial and academic researchers continue to develop and introduce new and improved polymers, despite of the great diversity of already existing PCE products. Those include:
Organo-Silane (OSi) Modified PCEs:
They can be prepared by incorporating either 3-trimethoxysilyl propyl methacrylate (MAPTMS) or N-maleic γ-amidopropyl triethoxy silane (MAPS) as a new comonomer into a conventional PCE, e.g. the MPEG-type (Fig. 2) (Fan et al. 2012, Witt 2012). The consideration behind this concept was to achieve a chemical bond between C-S-H and the superplasticizer, made possible through condensation of silanol (-Si-OH) groups present in both compounds. If formed, such a bond would anchor the PCE molecule irreversibly on the surface of hydrating cement and prevent its desorption e.g. by sulfate ions or anionic retarders resulting from competitive adsorption.
Phosphated (PHOS) PCEs:
Superplasticizers generally achieve their dispersing power through adsorption on the surface of cement, especially on ettringite (Yoshioka et al. 2002). Such adsorption is facilitated through anionic anchoring groups which typically include carboxylate or dicarboxylate groups. Some years ago it has been shown that phosphonate presents a more powerful anchoring group than carboxylate (Mosquet et al. 1997). Very recently, novel superplasticizers have been presented which incorporate phosphate as an anchoring group (Kraus 2011, Dalas et al. 2015). Phosphatation can be accomplished by esterification of e.g. hydroxyethyl methacrylate with phosphoric acid, leading to the PCE copolymer shown in Fig. 2. The phosphated PCEs are said to adsorb on cement almost instantaneously which presents a major advantage in specific concrete and dry-mix mortar applications. Furthermore, they appear to be more sulfate-tolerant, compared to conventional PCE superplasticizers, and often require lower dosages (Stecher and Plank 2019).
1.3 Tailoring PCEs to Specific Applications
Recently, substantial progress has been made in the optimization of current PCE products for difficult applications. Those include concretes of particularly low w/c ratios (<0.30) and the compatibility of PCEs with clay contaminants occurring in aggregates.
Stickiness of Concrete at Low w/c Ratio:
The problem of stickiness and slow flow of concrete prepared at low w/c ratio is well-known and was solved as follows: It was found that the hydrophilic-lipophilic balance (HLB) value of a PCE molecule determines whether the concrete admixed with this polymer exhibits slow or fast flow (Lange et al. 2014). According to this study, PCE molecules should be as hydrophilic as possible and their HLB value should be >18.5. Such PCEs (preferably of IPEG- and APEG-type) produce cement pastes with particularly low plastic viscosity and exhibit fast flow without any stickiness. Such rheologically optimized concrete is easier to pump, spread and compact and presents a huge step forward in improving the workability of high-strength concretes of low w/c ratios.
Enhanced Clay Tolerance:
Over the last years, applicators have observed that PCE superplasticizers – unlike polycondensates – exhibit a pronounced sensitivity to clay and silt contaminants (Jeknavorian et al. 2003, Atarashi et al. 2004). As a result, their performances are greatly reduced or the PCEs become entirely ineffective. Montmorillonite, a 2:1 smectite clay, has been found to be more harmful than other clay minerals such as kaolinites or muscovites (Lei and Plank 2014). Generally, the capacity of clays to sorb water, hydrate and swell leads to more viscous cement pastes. This effect results in a loss of workability or a higher water demand, independent of whether a superplasticizer is present or not.
Previous research has established that in cement pore solution, the surfaces of bentonite clay particles become positively charged as a result of Ca2+ adsorption onto the negative alumosilicate layers. Onto these surfaces, polyanionic superplasticizers such as polycondensates or polycarboxylates adsorb, thus resulting in a partial depletion of superplasticizer from the pore solution. This way, clay competes with cement for superplasticizer molecules. Moreover, PCE polymers can intercalate chemically into the interlayer space between the individual alumosilicate layers of specific clay minerals, especially montmorillonite (bentonite), resulting in an organo-mineral phase whereby their poly(ethylene glycol) side chains occupy the interlayer space, as is shown in Fig. 3. This reaction with clay is specific for PCEs and is a consequence of their PEO side chains, as was evidenced by XRD measurements (Ng 2012a). Consequently, PCEs can be used up by clay by both surface adsorption and chemical sorption whereas polycondensates such as BNS are consumed only by surface interaction (Jardine 2002, Ng and Plank 2012b). This explains why PCEs are significantly more affected by clay than polycondensates.
The industry has developed several strategies to mitigate the negative effects of clay on PCEs. The first concept includes the use of sacrificial agents.
Analysis of sorbed amounts of individual PCE constituents (backbone, represented by poly(methacrylic acid) and side chain, represented by poly(ethylene glycol)) revealed that the side chain sorbs in large amounts on clay (~400 mg MPEG/g clay) while the polymer trunk is consumed much less (~30 mg PMA/g clay) (Ng 2012a). This not only signifies that the PEO side chain present in PCE provides the main interaction with clay; it also offers a remedy for the problem whereby pure PEG or MPEG are utilized as sacrificial agents to occupy the interlayer spaces while the PCE molecule which exhibits a lower tendency to intercalate as a result of its anionic charge is preserved and can thus interact with the cement to achieve dispersion (Ng and Plank 2012b). As another remedy, addition of cationic polymers which inhibit the swelling of clay entirely has been proposed (Jacquet 2006). This method offers the advantages of zero water consumption because the clay will not hydrate at all. Additionally, the interlayer spacing will not be accessible for the PCEs.
Obviously, the best solution to the incompatibility problem of PCE and clay would be a novel PCE structure which does not contain PEO side chains. Recently, such polymers have been synthesized using either hydroxy alkyl esters of methacrylic acid or vinyl ethers as side chain bearing macromonomers (Lei and Plank 2012). Utilizing XRD analysis, it was found that indeed these novel polycarboxylates do not undergo side chain intercalation with clay and adsorb in small quantities only (~25 mg polymer/g clay). Consequently, they exhibit robust performance even in the presence of clay contaminants. This behavior perfectly confirms the concept of non-PEO side chains as a remedy for the intercalation problem of conventional PCEs into clay structures.
2 Early Strength Enhancing Admixtures
A recent invention includes the application of C-S-H-PCE nanocomposites as seed crystals for the hydration of the silicate phases C3S and C2S (Nicoleau et al. 2011, 2013). The nanocomposites can be prepared by combining aqueous solutions of e.g. sodium silicate and calcium nitrate with a PCE solution. The resulting precipitate contains nanofoils of C-S-H with surface adsorbed and possibly intercalated PCE (Fig. 4).
The nanofoils greatly accelerate the silicate hydration by reducing the free activation energy ΔG of the crystallization to zero. In cement hydration this barrier needs to be overcome to initiate C-S-H nucleation. The result is a much enhanced early strength development, especially after 6–12 h of hydration, without sacrificing the final strength as is the case for most common accelerators such as e.g. calcium nitrate, sodium silicate, sodium aluminate or aluminum dihydroxy formate (Fig. 4). Recently, it has been found that C-S-H-PCE nanocomposites also enhance the early strength of blended cements containing e.g. fly ash or calcined clays by accelerating the pozzolanic reaction (Kanchanason and Plank 2018).
3 Stabilizers
For highly dispersed concretes such as e.g. self-compacting concrete (SCC), polymeric stabilizers (also referred to as viscosity modifying agents, VMAs) are frequently applied to prevent disintegration and bleeding. Common stabilizers include welan gum, curdlan, hydroxypropyl cellulose, polyethylene glycol (Hibino 2000), and ATBS-based copolymers. Among the latter, two types have become quite popular in SCC mixes. The first one constitutes a terpolymer prepared via aqueous free radical copolymerization from 2-acrylamido-2-methylpropane sulfonic acid (ATBS), N-vinyl acetamide (NVA), acrylonitrile (ACN) and acrylamide (AA) while the second one comprises ATBS, N,N-dimethyl acrylamide (NNDMA) and, in some versions, tristyrylphenol poly(ethylene glycol) methacrylate ester as a third monomer. The ATBS-NNDMA copolymers can be prepared either via aqueous free radical copolymerization or through gel polymerization utilizing the Norrish-Trommsdorf effect (Futami 2003, Schinabeck 2005). The chemical structures of the ATBS-based stabilizers are displayed in Fig. 5. Both ATBS copolymers constitute linear molecules exhibiting high stiffness, owed to hydrogen bridging between ATBS and the neighboring NVA or NNDMA monomer.
Applicators of VMAs are well aware that these products not only can provide the desired effect, but also significantly impact on the rheology of concrete in a way that the fluidizing effect from PCE can be lost. Hence, a counterproductive (antagonistic) effect can occur which renders application of those stabilizing polymers tricky. To improve this situation, the interaction of PCE superplasticizers with ATBS/NNDMA and welan gum VMAs has been studied thoroughly.
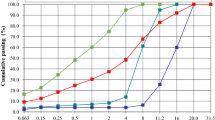
Surprisingly, for the ATBS/NNDMA stabilizer it was found that at low dosages (0–0.1 wt%) it acts as a viscosifier in concrete while at higher additions it provides a strong dispersing effect (Fig. 6).
Furthermore, when combined with PCE it is the stabilizing polymer which determines the flow regime, and not the PCE (Plank 2015). The reason behind this effect is that the ATBS copolymer preferably adsorbs on cement and thus prevents the PCE from adsorbing and becoming effective. The results suggest that when PCEs are combined with this ATBS copolymer, then a stabilizer dosage of >0.1% bwoc should be applied to avoid its thickening effect.
For welan gum VMA, a different scenario was found. According to these results, the stabilizing effect of welan gum biopolymer solely relies on its strong viscosifying effect on the cement pore solution which originates from its high adsorption on cement. Thus, with increased concentrations welan gum starts to destroy the fluidity generated by PCEs (Üzer and Plank 2016). Consequently, opposite to the ATBS/NNDMA stabilizer which requires a minimum dosage to avoid thickening, additions of welan gum to the PCE concrete should be kept as low as possible to avoid its negative effect on concrete rheology.
The investigations presented here suggest that admixture combinations are by no means trivial, and that understanding their mechanism of interaction with cement can help to optimize their performance.
4 Shrinkage-Reducing Admixtures
During its hydration and hardening, mortar and concrete undergo autogenous (= chemical) and dry (= physical) shrinkage (Lura et al. 2003, Tazawa et al. 1995). The latter is the consequence of water evaporation at the surface which causes a contractive force in the capillary pores and thus results in compaction. Previous studies have revealed that occurrence of physical shrinkage is dependent on the presence of pores exhibiting specific diameters, namely from ~10–50 nm (Wittmann 1982). Effective shrinkage-reducing agents (SRAs) are those which reduce the surface tension of the pore solution and which can modulate the pore size distribution in the cementitious matrix in such way that the shrinkage causing pore diameters are avoided. Diols, glycols, glycol ethers and amino terminated poly(ethylene-propylene) glycols have been identified as suitable SRAs (Fig. 7).
It is, however, well established that diols of quite similar structure and surface activity than those displayed in Fig. 7 do not provide any shrinkage-reducing effect at all, whereby the reason is still unknown. Furthermore, effective SRAs require extremely high dosages of 2–4% bwoc which are far beyond those for common functional admixtures used in concrete. Also, the reduction in shrinkage achieved from these admixtures is limited. Hence, it becomes obvious that a considerable gap with respect to the potency of SRAs and a thorough understanding of their working mechanism exists.
In recent years, two contributions on this subject were published. The first work presented that the pore-size modulating effect of SRAs is linked to their ability to form micelles of specific, large enough diameters which are the templates for pores which do not induce shrinkage (Kayello 2014). These micelles form at a stage in cement hydration when a significant amount of water has already been consumed and the SRAs are present in the pore solution at concentrations of 6–10%. Compounds which form micelles too early or too late in cement hydration cannot provide any shrinkage-reducing effect.
The second contribution utilized molecular modeling to identify potentially effective SRAs and then tested them in mortar to confirm the concept (Shlonimskaya et al. 2014). Based on a computer-aided molecular design (CAMD) approach that used the signature molecular descriptor, 2-propoxyethanol and 3-ethoxypropylamine were found to provide exceptional reduction in the surface tension of water. Their high shrinkage-reducing potential was confirmed in actual mortar tests.
Inspite of all this it obvious that our current technology of SRAs is quite limited and – compared to that existing in the field of e.g. superplasticizers or retarders – is far behind. More intense research is required to fill this gap in the future and to bring its technology to a level which allows a more effective control of physical shrinkage compared to the state of the art.
5 New Admixture Technologies – What Can We Expect in the Future?
5.1 Improved Curing Agents
Until now, a significant gap in current curing technology exists. The current situation on construction sites where large concrete slabs or decks are poured is that significant efforts have to be undertaken to reduce dry shrinkage and cracking on the surfaces. The most common practices include the spraying of water onto the concrete surface or coverage with a plastic foil to reduce water evaporation. Both methods are often not very effective, and on top they require a substantial amount of labor. Hence, the industry is challenged with developing admixtures which e.g. can be mixed into the fresh concrete and then prevent its surface desiccation, thus eliminating the need for post-curing of concrete. In light of this, superabsorbent polymers (SAPs) seem to be a promising candidate for the internal curing of concrete (Mignon et al. 2017). These cross-linked polymers which are typically synthesized from acrylic acid and/or acrylamide start to swell upon the contact with the pore solution. Consequently, a hydrogel is formed which gradually releases the absorbed water during the self-dessication of the concrete, thus mitigating the autogeneous shrinkage during hardening (Snoeck et al. 2017). Another type of curing agents are water evaporation retardants (e.g. poly lauryl methacrylate emulsions) which are applied on the surface of the plastic concrete to prevent the formation of plastic shrinkage cracks (Liu et al. 2010).
5.2 Admixtures Improving the Ductility of Concrete
Concrete presents a unique building material because of its easy preparation from abundantly available raw materials, its low cost and its enormous strength. Those excellent features have propelled the global volume of concrete poured to more than 30 billion tons per year. Inspite of these extraordinary properties, concrete suffers from one major deficiency which greatly limits its application: low ductility (= tensile or bending strength) and low fracture toughness (Fig. 8).
Compared to human bone for example, the fracture toughness of concrete is about 100 times lower. For a conventional concrete (w/c ratio ~0.5), the tensile strength reaches only ~10% of its compressive strength, thus rendering concrete a very brittle material. The problem becomes even worse when the w/c ratio is low. For example, in ultra-high strength concrete (UHPC, w/c = 0.25) the tensile strength develops to only 5% of the compressive strength. Consequently, such concrete is prone to crack formation through vibrational impact (on bridges e.g. from traffic, on buildings from wind forces, etc.).
In the future, the industry will be challenged with developing concepts which can reduce in-situ the brittleness of concrete. Potential solutions involve the addition of textile fibers or the generation of organo-mineral phases which are more flexible than conventional cement hydrates (e.g. meso crystals similar to those described for CaCO3-PCE precipitates (Keller and Plank 2013), or Ca2Al-polymer-LDH composites (Plank and Ng 2012)). In this respect, an interesting concept would be the in-situ formation of C-S-H-polymer nanocomposites similar to those described in Sect. 2 for C-S-H-PCE which potentially can improve the bending strength of concrete. Considering the magnitude of the task it might be useful to study concepts from nature such as they occur in mollusk shells which consist of calcite tablets with interstitial chitin (Mann 1993). Such biomimetic approaches will hopefully inspire researchers to propose solutions for this problem.
6 Conclusion
Chemical admixtures have truly revolutionized modern concrete technology. They present a major driver for innovation in concrete and will continue to do so for many years to come. In the future, it would be extremely attractive to have admixtures which allow the safe application of self-compacting concrete delivered as ready-mixed concrete to the job site. Even more, to be able to control concrete consistency (fluidity) during delivery through the energy uptake of the rotating container of the concrete truck and energy-dependent PCE dosage would be most intriguing. Undoubtedly, the current admixture products will be refined further to become even more effective, and they will be tailored more specifically to distinct applications.
References
Akimoto, S., Honda, S., Yasukohchi, T.: Additives for Cement, EP 0,291,073 (1992)
Albrecht, G., Weichmann, J., Penkner, J., Kern, A.: Copolymers based on Oxyalkylene Glycol Alkenyl Ethers and Derivatives of Unsaturated Dicarboxylic Acids, EP 0,736,553 (1996)
Amaya, T., Ikeda, A., Imamura, J., Kobayashi, A., Saito, K., Danzinger, W., Tomoyose, T.: Cement Dispersant and Concrete Composition containing the Dispersant, WO 0,039,045 (2000)
Atarashi, D., Sakai, E., Obinata, R., Daimon, M.: Interactions between superplasticizers and clay minerals, Cement Sci. Concr. Technol. 58, 387–392 (2004)
Dalas, F., Nonat, A., Pourchet, S., Mosquet, M., Rinaldi, D., Sabio, S.: Tailoring the anionic function and the side chains of comb-like superplasticizers to improve their adsorption. Cem. Concr. Res. 67, 21–30 (2015)
Fan, W., Stoffelbach, F., Rieger, J., Regnaud, L., Vichot, A., Bresson, B., Lequeux, N.: A new class of organosilane-modified polycarboxylate superplasticizers with low sulfate sensitivity. Cem. Concr. Res. 42, 166–172 (2012)
Futami, T., Yamaguchi, T., Tagoshi, H.: Use of a Polymer as a High-Flow Concrete Additive and Concrete Material Containing the Additive, EP 0,757,998 (2003)
Guicquero, J.P., Maitrasse, P., Mosquet, M.A., Sers, A.: A Water Soluble or Water Dispersible Dispersing Agent, FR 2,776,285 (1999)
Hamada, D., Yamato, F., Mizunuma, T., Ichikawa, H.: DE 10,048,139 A1 (2001)
Hibino, M.: Effect of viscosity enhancing agent on self-compactibility of fresh concrete. In: Sixth International Conference on Superplasticizers and other Chemical Admixtures in Concrete (CANMET/ACI), Nice, SP-195, pp. 305-320 (2000)
Hirata, T.: Cement dispersants, JP 842,022 (S59-018338) (1981)
Jacquet, A., Villard, E., Watt, O.: Method for inserting impurities, WO 2006,032,785 (2006)
Jardine, L., Koyata, H., Folliard, K., Ou, C.C., Jachimowicz, F., Chun, B., Jeknavorian, A.A., Hill, C.L.: Admixture and method for optimizing addition of EO/PO superplasticizer to concrete containing smectite clay-containing aggregates, U.S. 6,352,952 (2002)
Jeknavorian, A.A., Jardine, L., Ou, C.C., Koyata, H., Folliard, K.J. (2003) Interaction of superplasticizers with clay-bearing aggregates, In: Malhotra, V.M. (ed.) 7th CANMET/ ACI International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Berlin/Germany, ACI, SP-217, pp. 1293–1316
Kanchanason, V., Plank, J.: Effectiveness of a calcium silicate hydrate – polycarboxylate ether (C-S-H-PCE) nanocomposite on early strength development of fly ash cement. Constr. Build. Mater. 169, 20–27 (2018)
Kayello, H.M., Naresh, K.R., Tadisina, R., Shlonimskaya, N., Biernacki, J.J., Visco, D.P.: An application of computer-aided molecular design (CAMD) using the signature molecular descriptor – Part 1. identification of surface tension reducing agents and the search for shrinkage reducing admixtures. J. Am. Ceram. Soc. 97(2), 365–377 (2014)
Keller, H., Plank, J.: Mineralisation of CaCO3 in the presence of polycarboxylate comb polymers. Cem. Concr. Res. 54, 1–11 (2013)
Kraus, A., Dierschke, F., Becker, F., Schuhbeck, T., Grassl, H., Groess, K.: Method for producing phosphate polycondensation products and the use thereof, US patent 2011/0281975 A1 (2011)
Lange, A., Hirata, T., Plank, J.: Influence of the HLB value of polycarboxylate superplasticizers on the flow behavior of mortar and concrete. Cem. Concr. Res. 60, 45–50 (2014)
Lei, L., Plank, J.: A concept for a polycarboxylate superplasticizer possessing enhanced clay tolerance. Cem. Concr. Res. 42, 1299–1306 (2012)
Lei, L., Plank, J.: A study on the impact of different clay minerals on the dispersing force of conventional and modified vinyl ether based polycarboxylate superplasticizers. Cem. Concr. Res. 60, 1–10 (2014)
Liu, J.P., Li, L., Miao, C.W., Tian, Q., Ran, Q.P., Wang, Y.J.: Characterization of the monolayers prepared from emulsions and its effect on retardation of water evaporation on the plastic concrete surface. Colloids Surf. A: Pysicochem. Eng. ASP. 366(1–3), 208–212 (2010)
Lura, P., Jensen, O.M., van Breugel, K.: Autogenous shrinkage in high-performance cement paste: an evaluation. Cem. Concr. Res. 33, 223–232 (2003)
Mann, S.: Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature 365, 499–505 (1993)
Mignon, A., Snoeck, D., Dubruel, P., Van Vlierberghe, S., De Belie, N.: Crack mitigation in concrete: superabsorbent polymers as key to success? Materials 10(3), 237 (2017)
Mosquet, M., Chevalier, Y., Brunel, S., Guicquero, J.-P.: Polyethylene di-phosphonates as efficient dispersing polymers for aqueous suspensions. J. Appl. Pol. Sci. 65, 2545–2555 (1997)
Nagare, K.: Storage and/or Transportation Method of Polyalkylene Glycol Monomers, US 7,030,282 B2 (2006)
Ng, S., Plank, J.: Study on the interaction of Na-montmorillonite clays with polycarboxylate based superplasticizers. In: Malhotra, V.M. (ed.) 10th CANMET/ACI Conference on Superplasticizers and Other Chemical Admixtures in Concrete (Proceeding Papers), ACI, Prague, pp. 407–421 (2012a)
Ng, S., Plank, J.: Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem. Concr. Res. 42, 847–854 (2012b)
Nicoleau, L., Albrecht, G., Lorenz, K., Jetzlsperger, E., Fridrich, D., Wohlhaupter, T., Dorfner, R., Leitner, H., Vierle, M., Schmitt, D., Braeu, M., Hesse, C., Montero, Pancera, S., Zuern, S., Kutschera, M.: Plasticizer-Containing Hardening Accelerator Composition, US 2011,0269,875 A1 (2011)
Nicoleau, L., Gädt, T., Chitu, L., Maier, G., Paris, O.: Oriented aggregation of calcium silicate hydrate platelets by the use of comb-like copolymers. Soft Matter 9, 4864–4874 (2013)
Ohta, A., Sugiyama, T., Tanaka, Y.: Fluidizing mechanism and application of polycarboxylate-based superplasticizers, In: Malhotra, V.M. (ed.) 5th CANMET/ACI Conference on Superplasticizers and Other Chemical Admixtures in Concrete (Proceedings volume), Rome, ACI, SP-173, pp. 359–378 (1997)
Paas, J., Müller, M.W., Plank, J.: Influence of diester content in macromonomers on performance of MPEG-Based PCEs. In: Malhotra, V.M., Gupta, P.R., Holland, T.C. (eds.) 11th CANMET/ACI Conference on Superplasticizers and Other Chemical Admixtures in Concrete (Proceedings), ACI SP-302, Ottawa (Canada), pp. 199–210 (2015)
Plank, J., Pöllmann, K., Zouaoui, N., Andres, P.R., Schaefer, C.: Synthesis and performance of methacrylic ester based polycarboxylate superplasticizers possessing hydroxy terminated poly(ethylene glycol) side chains. Cem. Concr. Res. 38, 1210–1216 (2008)
Plank, J., Lange, A.: Concrete Admixtures, EP 12,002,354.4 (2012)
Plank, J., Ng, S., Foraita, S.: Intercalation of Microbial Biopolymers Welan gum and EPS I into Double Layered Hydroxides, Zeitschrift für Naturforschung B 67b, 479-487 (2012)
Plank, J., Meyer, L.: New insights into physicochemical interactions occurring between polycarboxylate superplasticizers and a stabilizer in self-compacting concrete. J. Sustain. Cem.-Based Mat. 4, 164–175 (2015)
Pourchet, S., Liautaud, S., Rinaldi, D., Pochard, I.: Effect of the repartition of the PEG side chains on the adsorption and dispersion behaviors of PCP in presence of sulfate. Cem. Concr. Res. 42, 431–439 (2012)
Schinabeck, M., Friedrich, S., Holland, U., Pfeuffer, T., Eberwein, M., Schuhbeck, T.: Water- soluble copolymers containing sulfo groups, method for the production and use thereof, EP 1,763,546 (2005)
Shlonimskaya, N., Biernacki, J.J., Kayello, H.M., Visco, D.P.: An application of computer- aided molecular design (camd) using the signature molecular descriptor – part 2: evaluating newly identified surface tension-reducing substances for potential use as shrinkage-reducing admixtures. J. Am. Ceram. Soc. 97(2), 378–385 (2014)
Snoeck, D., Pel, L., De Belie, N.: The water kinetics of superabsorbent polymers during cement hydration and internal curing visualized and studied by NMR. Sci. Rep. 7, 9514 (2017)
Stecher, J., Plank, J.: Novel concrete superplasticizers based on phosphate esters. Cem. Concr. Res. 119, 36–43 (2019)
Tahara, H., Ito, H., Mori, Y., Mizushima, M.: Cement Additive, Method for Producing the same, and Cement Composition, US 5,476,885 (1995)
Tazawa, E., Miyazawa, S., Kasai, T.: Chemical shrinkage and autogenous shrinkage of hydrating cement paste. Cem. Concr. Res. 25, 288–292 (1995)
Üzer, E., Plank, J.: Impact of welan gum stabilizer on the dispersing performance of polycarboxylate superplasticizers. Cem. Concr. Res. 82, 100–106 (2016)
Wang, Z.M., Xu, Y., Wu, H., Liu, X., Zheng, F.Y., Li, H.Q., Cui, S.P., Lan, M.Z., Wang, Y.L.: A Room Temperature Synthesis Method for Polycarboxylate Superplasticizer, CN 101974135 B (2013)
Witt, J., Plank, J.: A novel type of PCE possessing Silyl functionalities. In: Malhotra, V.M. (ed.) 10th CANMET/ACI Conference on Superplasticizers and Other Chemical Admixtures in Concrete (Proceedings), ACI, Prague, SP-288.04, pp. 57–70 (2012)
Wittmann, F.H.: Creep and Shrinkage in Concrete Structures, pp. 129–161. John Wiley & Sons Ltd, Hoboken (1982)
Yamamoto, M., Uno, T., Onda, Y., Tanaka, H., Yamashita, A., Hirata, T., Hirano, N.: Copolymer for Cement Admixtures and its Production Process and Use, US 6,727,315 (2004)
Yoshioka, K., Tazawa, E., Kawai, K., Enohata, T.: Adsorption characteristics of superplasticizers on cement component minerals. Cem. Concr. Res. 32, 1507–1513 (2002)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 RILEM
About this paper
Cite this paper
Plank, J., Ilg, M. (2020). The Role of Chemical Admixtures in the Formulation of Modern Advanced Concrete. In: Boshoff, W., Combrinck, R., Mechtcherine, V., Wyrzykowski, M. (eds) 3rd International Conference on the Application of Superabsorbent Polymers (SAP) and Other New Admixtures Towards Smart Concrete. SAP 2019. RILEM Bookseries, vol 24. Springer, Cham. https://doi.org/10.1007/978-3-030-33342-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-33342-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33341-6
Online ISBN: 978-3-030-33342-3
eBook Packages: EngineeringEngineering (R0)