Abstract
Food allergies are increasing in prevalence, have a highly variable clinical presentation, and can result in life-threatening reactions. In order for clinicians to accurately diagnose and manage food allergies, they must have a clear understanding of the symptoms and underlying pathophysiology. Food allergies by definition are immune-mediated responses that occur reproducibly on food ingestion, which differentiates them from non-immunologic adverse food reactions like lactose intolerance or food poisoning. Food allergies can be IgE mediated, non-IgE/cell mediated, or mixed IgE and non-IgE mediated with varying clinical presentations depending on the underlying immune mechanisms. IgE-mediated food allergies produce symptoms that affect the cutaneous (hives, pruritus, angioedema), gastrointestinal (abdominal pain, vomiting, diarrhea), respiratory (rhinorrhea, dyspnea, wheezing), and cardiovascular (hypotension, syncope) systems. IgE-mediated food allergies can progress to anaphylaxis, which is a severe and potentially fatal systemic reaction that requires timely recognition and treatment. Non-IgE- or cell-mediated reactions are typically delayed or chronic, and include food protein-induced allergic proctocolitis (FPIAP), food protein-induced enterocolitis syndrome (FPIES), celiac disease, and food-induced pulmonary hemosiderosis (Heiner syndrome). Mixed IgE- and non-IgE-mediated reactions occur in eosinophilic esophagitis or eosinophilic gastroenteritis, which present with symptoms of dysphagia, vomiting, reflux, and abdominal pain related to eosinophilic infiltrates in the GI tract. This chapter describes the clinical manifestations of food allergies with an emphasis on anaphylaxis and the pathophysiology behind these reactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
About 40% of children in the United States with a food allergy will experience a severe reaction [1]. Food allergy is one of the most common causes of anaphylaxis and accounts for 30–50% of all anaphylaxis cases and up to 81% of anaphylaxis cases in children [2].
Given the increasing prevalence and life-threatening consequences of food allergy, accurate and timely diagnosis of food allergies is critical. Clinicians require the tools to distinguish food allergy versus intolerance and describe the pathophysiology that contributes to the development of food allergies. This review will describe and categorize food allergies by clinical presentations and their underlying immune mechanisms. History and exam findings that contribute to the diagnosis will be reviewed.
Food Allergy Versus Intolerance
The 2010 Expert Panel Report sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) defined food allergy as “an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food” and food intolerance as “foods or food components that elicit reproducible adverse reactions but do not have established or likely immunologic mechanisms” [3]. Food intolerances can result from metabolic, pharmacologic, toxins, and chemical reactions. Examples of each will now be discussed and are summarized in Table 2.1 [4].
Metabolic causes of food intolerance include lactose intolerance, galactosemia, and alcohol intolerance. Patients who are lactose intolerant are unable to digest lactose leading to excess fluid in the gut, resulting in abdominal pain, bloating, and diarrhea. This is important to differentiate from an allergy to cow’s milk, which is an immunologic response to cow’s milk protein that can result in anaphylaxis [3]. Galactosemia is a metabolic food intolerance due to a deficiency in the enzyme required to process galactose. This results in vomiting, diarrhea, failure to thrive, jaundice, and lethargy in infants but is commonly detected before the onset of clinical symptoms on newborn metabolic screening. Pharmacologic food intolerances result from chemically active compounds including caffeine and alcohol. Toxic effects of foods can result from scombroid poisoning due to elevated histaminic chemicals in decomposing dark-flesh fish like tuna, mackerel, mahi-mahi, and sardine. Symptoms of scombroid poisoning closely mimic a food allergy and can involve urticaria, angioedema, flushing, vomiting, diarrhea, dizziness, and hypotension after eating spoiled fish [5]. While the symptoms are histamine mediated and very closely mimic an allergy, scombroid poisoning is not a food allergy because it does not have an immunologic mechanism. Chemical reactions can occur due to vagally mediated gustatory rhinitis [6] or auriculotemporal syndrome, also called Frey Syndrome, which results in redness and sweating of the cheek following salivation [7]. Non-immunologic reactions can also occur due to sulfites, nitrites, and monosodium glutamate (MSG). Sulfites have been implicated in asthma reactions, and these patients generally have a history of asthma exacerbations triggered by sulfite-rich foods like dried fruit or wine [8]. Finally, some patients have food intolerances that are psychologically driven including food phobias or aversions.
Key questions in the patient’s history, as reviewed later in this chapter, can help clinicians distinguish food allergy from non-immune adverse food reactions. When the diagnosis remains uncertain, referral to an allergist/immunologist should be considered.
Immune Responses
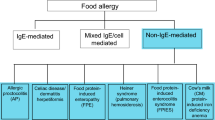
Immune responses to foods can be grouped into three types: (1) immunoglobulin E (IgE)-mediated reactions, (2) non-IgE- or cell-mediated reactions, and (3) mixed reactions.
IgE-Mediated Food Reactions
Patients with IgE-mediated FA present with a variety of symptoms most commonly involving the skin, gastrointestinal tract, respiratory tract, and cardiovascular systems. It is important to note that the timing, sequence, and severity of symptoms vary widely between reactions even in the same individual [9]. The highly unpredictable nature and potential for high morbidity and mortality make recognizing and treating reactions of utmost importance.
IgE-specific antibodies for food allergens develop during initial sensitization to a food. Once sensitization occurs, food antigen-specific IgE is present in the circulation and on the surface of tissue mast cells and circulating basophils bound to the high-affinity FcεRI receptor. After re-exposure to the food, cross-linking of the food protein-specific IgE bound to FcεRI results in degranulation of mast cells and basophils releasing preformed histamine and proteases along with synthesis of leukotrienes, prostaglandins, and cytokines [10].
Because IgE cross-linking releases preformed allergic mediators, signs and symptoms of IgE-mediated food allergies develop rapidly and should be considered in patients who develop signs and symptoms within minutes up to 2 hours after ingesting the suspected food allergen. The delayed release of mediators that are synthesized following IgE cross-linking may result in a delayed phase of symptoms hours after the initial reaction though the mechanisms behind delayed and biphasic reactions are not well understood.
The key features of reactions by organ system will now be described and are also noted in Table 2.2.
Skin Reactions
Symptoms involving the cutaneous and subcutaneous tissue are very common in IgE-mediated food reactions and include urticaria, diffuse pruritis, flushing, and angioedema. Urticarias are raised erythematous wheals that are pruritic, typically well circumscribed or coalescing, and evanescent. Angioedema is non-pitting edema that involves non-gravitationally dependent areas. It commonly affects the face (especially the lips and eyelids), extremities, and upper airway. IgE-mediated FA should be suspected in a patient who develops urticaria or angioedema within minutes to 2 hours after ingestion of a suspected food allergen. Both ingestion of food and direct contact can cause urticaria. For example, a child who is peanut allergic may develop contact urticaria after touching peanut butter to their skin without actually ingesting it. In this case, hives are typically limited to the area in contact with the allergenic food. Urticaria secondary to food reactions typically fade shortly after exposure, within several minutes to several hours, though this is highly variable depending on the trigger, severity of the reaction, ongoing exposure, and treatments. Urticarias that persist for greater than 6 weeks are chronic and FA is unlikely the cause [11].
Urticaria is the result of cross-linking of antigen-specific IgE on cutaneous mast cells in the superficial dermis. Angioedema, on the other hand, is due to cross-linking of IgE on mast cells in the deeper dermis and subcutaneous tissues. While urticaria can be the result of a wide variety of allergies including medications and insect bites, and non-allergic causes like infections, it is estimated that at least 20% of acute urticaria is due to food allergy [12, 13]. While the majority of anaphylactic reactions include skin symptoms, it is worth noting that up to 20% of cases of anaphylaxis do not, and the lack of skin symptoms may result in delayed diagnosis of an allergic reaction.
Respiratory Tract Reactions
Reactions can be divided into those affecting the upper and lower respiratory tract. Upper respiratory tract symptoms include sneezing, rhinorrhea, congestion, nasal and/or eye itching, conjunctival erythema, and tearing. Rhinoconjunctivitis is more commonly seen during systemic reactions and is rarely the only presenting symptom [14].
Lower respiratory signs and symptoms are present in up to 70% of anaphylactic reactions and include dyspnea, chest tightness or pain, cough, wheezing, dysphonia, and stridor [15]. Respiratory manifestations such as edema of the glottis and wheezing are the primary cause of death in patients with food-induced anaphylaxis and need to be treated aggressively, especially in asthmatic patients [2]. Bronchospasm can be due to inhalation of food allergens, specifically vapors from cooking fish and shellfish [16, 17].
Gastrointestinal Symptoms
GI symptoms occur in 45% of cases of anaphylaxis [15]. Many patients experience tingling or itching in their mouth. Young children may scratch at their mouth, tongue, throat, or ears. Nausea and vomiting may occur within minutes of ingestion, whereas abdominal pain, cramping, and diarrhea may occur either immediately or with a delay of up to several hours after ingestion [14]. These symptoms can result in dehydration and electrolyte disturbances in infants and young children, and volume loss from vomiting/diarrhea can cause hypovolemic shock.
Cardiovascular Symptoms
Cardiovascular symptoms occur in 45% of cases of anaphylaxis and most commonly include tachycardia, hypotension with resulting dizziness and/or syncope, and urinary incontinence [15]. Up to 35% of the intravascular volume can shift to the extravascular space within 10 minutes of onset of a reaction due to increased vascular permeability from histamine and other vasodilatory mediators, which can result in hypotensive shock and cardiac arrest.
Cardiovascular symptoms may include syncope, a feeling of faintness, palpitations, and/or chest pain. Hypotension or shock may be the result of vascular collapse, cardiac arrhythmia, or asphyxia. Anaphylaxis may be complicated by myocardial ischemia [15].
Anaphylaxis
Anaphylaxis is an IgE-mediated acute life-threatening systemic allergic reaction that affects up to 2% of the population [18]. FAs are the most common cause of anaphylaxis in infants and children [3, 19]. Peanuts are the most common food to cause anaphylaxis in children and shellfish is the most common in adults [20, 21]. Risk factors for fatal reaction from food-induced anaphylaxis are adolescent or young adult age, coexistent asthma, and reactions due to peanut or tree nut [14]. Anaphylaxis is caused by cross-linking of antigen-specific IgE on mast cells and basophils. This cross-linking causes the mast cells and basophils to release allergic mediators including histamine, tryptase, chymase, platelet-activating factor, prostaglandin D2, cysteinyl leukotrienes, IL-6, and TNF-α [22], resulting in multi-organ effects that can present with up to 40 potential signs and symptoms and result in death secondary to respiratory or cardiovascular compromise.
The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network have developed diagnostic criteria for anaphylaxis (Table 2.3) [23]:
-
1.
Acute onset (minutes to hours) of involvement of skin, mucosal tissue, or both (hives, flushing, pruritis, angioedema) and either respiratory or cardiovascular compromise
-
2.
Two or more of the following that occur rapidly after exposure to a likely allergen for that patient: involvement of the skin or mucosal tissue (generalized hives, itch, flushing, swelling), respiratory compromise (dyspnea, wheeze-bronchospasm, stridor, hypoxia), cardiovascular compromise (hypotension, collapse), persistent gastrointestinal symptoms (crampy abdominal pain, vomiting)
-
3.
Hypotension after exposure to known allergen for that patient
Fifteen percent of patients exhibit neurologic symptoms of impending doom, headache, or confusion. Young children and infants can exhibit sudden behavioral changes like cessation of play, irritability, and clinginess [15].
Biphasic reactions are estimated to occur in 1–20% of anaphylactic reactions and involve a recurrence of symptoms after the apparent resolution of the initial reaction. Biphasic reactions typically occur about 8 hours after the initial reaction but have been reported up to 72 hours later [3]. The pathophysiology of biphasic reactions is not well understood, but it is more likely to occur in cases of moderate to severe anaphylaxis or when treatment with epinephrine is delayed [24]. It has been hypothesized that due to the large variation in time intervals, biphasic reactions may be due to a multitude of factors with earlier onset indicating medication wear-off or inadequate initial treatment and later onset due to biphasic release of mediators like histamine and platelet-activating factor or activation of secondary inflammatory pathways [25].
Alpha-Gal Allergy
The only known IgE-mediated food allergy that characteristically has a delayed reaction is allergy to galactose-alpha-1,3-galactose (alpha-gal). This is an allergic reaction to a carbohydrate epitope on mammalian meats, for example, beef, pork, and lamb. This reaction typically occurs 4–6 hours after ingestion [26]. Symptoms are similar to other IgE-mediated food allergies with hives, pruritus, and gastrointestinal symptoms being most common. Patients can experience severe anaphylaxis with cardiovascular and respiratory compromise. In most patients, eliminating beef, pork, lamb, and all other sources of non-primate mammalian meat is sufficient to avoid further allergic reactions. However, some patients continue to have reactions and require additional elimination of dairy and gelatin to fully avoid reactions since alpha-gal is also found in mammalian milk and bovine gelatin [27, 28]. The reaction is related to sensitization to the carbohydrate antigen galactose-alpha-1,3-galactose, which occurs after the bite of the lone star tick (Amblyomma americanum) in the United States and Ixodes species in Europe and Australia. The lone star tick is common in the East, Southeast, and Midwest United States [29]. The pathophysiology underlying IgE sensitization to alpha-gal after tick bite and resulting mammalian meat allergy is not well understood. The delay in reaction time is likely related to the time it takes for antigen digestion and/or processing, and it is likely that the allergic form of the oligosaccharide does not enter the circulation until several hours after eating. Alpha-gal is the only known carbohydrate antigen to induce an IgE-mediated reaction as all the remainder are due to proteins [26, 27, 30]. Alpha-gal allergy should be considered in children who live in areas where the lone star tick is common and who have a history of delayed reactions to red meat or recurrent urticaria, angioedema, or idiopathic anaphylaxis [31].
Food-Dependent Exercise-Induced Anaphylaxis
Food-dependent exercise-induced anaphylaxis results in typical symptoms of anaphylaxis only after patients physically exert themselves within a few hours after eating. The symptoms are the same as anaphylaxis due to other causes. Symptoms typically begin during vigorous exercise, but the level of exertion that precipitates symptoms is unpredictable [32]. Patients do not have reactions if they consume the foods to which they are sensitized without exercising afterwards. Most patients only experience symptoms to specific foods to which they are sensitized, but some patients experience anaphylaxis if they exercise after consuming any food or drink. The foods most commonly implicated are wheat, shellfish, and nuts; however, a wide variety of triggering foods have been identified including celery, oranges, apples, rice, tomatoes, and cow’s milk with geographic variability in sensitization patterns [9, 33]. Patients typically have positive IgE on skin prick or serum testing. Pathogenesis of this type of anaphylaxis is believed to similarly be due to mast cell degranulation and release of mediators. Patients have demonstrated skin biopsies with mast cell degranulation and transient elevations in tryptase. The specific mechanism by which exercise precipitates anaphylaxis in these patients has yet to be elucidated, but a number of hypotheses exist suggesting changes in serum osmolality, pH, gut permeability, and blood flow redistribution [33,34,35].
Pitfalls in Making the Diagnosis of Anaphylaxis
Identifying IgE-mediated food allergy and anaphylaxis is challenging due to the high variability between symptoms and timing of reactions. The same individual can have widely variable reactions to the same food, and there is no way to predict the type or severity of the reaction. Anaphylaxis is often underreported and underdiagnosed in part due to this variability. Especially if it is a patient’s first reaction, it can be difficult to identify a trigger. Young children are often not able to describe their symptoms, and patients who have altered consciousness or impaired judgment will also not be able to describe the reaction. Hypotension can be challenging to recognize in very young children or infants. Skin symptoms (hives, itching, angioedema) are very helpful in identifying reactions; however, these are absent in 10–20% of all anaphylactic reactions [15]. Diagnosis of anaphylaxis can be aided by the expert guidelines (Table 2.3), and is highly likely when any one of the criteria is fulfilled. The presence of one of the three criteria predicts diagnosis of anaphylaxis 95% of the time [36]. Food allergy anaphylaxis plans are also available to caregivers to aid in the diagnosis and treatment of anaphylactic reactions [37]. Additionally, there are scoring tools available to grade the severity of anaphylaxis ranging from mild including only skin and subcutaneous involvement, moderate including respiratory, cardiovascular, or gastrointestinal involvement, and severe with hypoxia, hypotension, or neurologic compromise [38].
Pollen-Food Allergy Syndrome (PFS) or Oral Allergy Syndrome (OAS)
Patients sensitized to pollen aeroallergens can experience symptoms after eating raw fruits, vegetables, nuts, or certain spices. Symptoms are typically limited to the oropharynx, though systemic reactions have been rarely reported. The cause of PFS is cross-reactivity that develops due to shared epitopes between the structure of pollen and fresh fruit and vegetables. The cross-reactive antigen in food is degraded by digestive enzymes and heat; therefore, patients can typically tolerate the heated versions of these foods, and symptoms are most often limited to local reactions in the oral cavity without progression to systemic symptoms. Symptoms characteristically occur within minutes and are usually mild and transient. The most common symptom is oropharyngeal pruritus, typically described as itching or tingling of the mouth and palate. Some patients describe throat tightness, and there has been reported oral and perioral angioedema, mucosal vesicles, conjunctivitis, congestion, and coryza. Rare other symptoms are facial rash, and nasal and otic pruritus. Patients with atopic dermatitis sensitized to birch pollen can experience worsening of eczema after consumption of a cross-reactive food. Less than 5% of patients progress to a more generalized reaction with nausea, vomiting, abdominal pain, upper respiratory obstruction and rarely progress to anaphylaxis [39]. PFS is further discussed in Chap. 5.
Pathophysiology of Food Allergy Sensitization
Mechanisms underlying allergic sensitization are currently the subject of widespread investigation and are likely a complex interaction of genetic and environmental factors [40]. Sensitization can occur through the GI tract, the skin, and the respiratory tract and is thought to be related to impaired or inflamed mucosal barrier [41]. Tolerance to food antigens requires food antigen-specific regulatory T cells (T-regs) and is mediated by antigen presentation by CD103+ dendritic cells (DCs) in the GI tract and CD11b + dermal DCs and Langerhans cells in the skin. These cells migrate to lymph nodes where they induce antigen-specific Treg cells [42]. CD103 + DCs in the gut produce TGF-β and retinoic acid which drive Treg differentiation [43], and T-regs in turn produce suppressor cytokines like TGF-β, IL-10, and IL-35 [41]. In patients with food allergies, instead of induction of Treg cells, they develop antigen-specific TH2 cells that produce IL-4, 5, and 13 and induce IgE class switching [40].
The specific factors that lead to this breakdown in tolerance and allergic sensitization are not yet understood, but it is likely that multiple factors are involved. The microbiota has been shown to have a strong association with allergic disease; however, the exact mechanisms through which the microbiome influences the immune system have yet to be fully elucidated [44]. Route of allergen exposure also likely plays a role, with increased exposure through non-oral routes resulting in allergic sensitization. For example, children with atopic dermatitis have impaired skin barrier function, which is hypothesized to lead to allergen exposure through the skin before the GI tract. This may contribute to increased rates of food allergies in these patients [43].
Despite the clear role of IgE in mediating anaphylaxis, IgE levels do not always correlate with clinical symptoms. Patients with high levels of allergen-specific IgE do not always develop clinical symptoms with food exposure, and similarly patients can have low or undetectable serum levels of allergen-specific IgE and still develop anaphylaxis to a specific food, indicating the causes of anaphylaxis are more complex than is currently understood.
Non-IgE-/Cell-Mediated Food Allergies
Non-IgE- or cell-mediated food allergies are mediated by T cells and commonly result in delayed or chronic reactions. These reactions include food protein-induced allergic proctocolitis (FPIAP), food protein-induced enterocolitis syndrome (FPIES), celiac disease, and food-induced pulmonary hemosiderosis (Heiner syndrome). Key clinical features of each are summarized in Table 2.4.
Food Protein-Induced Allergic Proctocolitis (FPIAP)
FPIAP presents with blood, and sometimes mucous, in the stools of otherwise healthy appearing, normally growing, and developing infants. Symptoms typically develop in the first 2–8 weeks of life [45]. Infants can have increased gas, colic, and increased frequency of bowel movements but are otherwise well appearing with normal growth [46]. Cow’s milk is the most common trigger followed by soy and egg [45]. FPIAP can occur in breast- or formula-fed infants [45, 47]. Symptom improvement results after maternal avoidance of the triggering food or starting a hypoallergenic formula typically within 72 hours, but it can take up to 2 weeks for symptoms to fully resolve [48]. FPIAP typically completely resolves within 1–3 years, with the majority resolving within the first year [45]. The pathophysiology is largely unknown but is thought to be due to dietary proteins in breastmilk or formula causing inflammation in the lower GI tract [49, 50].
Food Protein-Induced Enterocolitis Syndrome (FPIES)
FPIES affects infants and young children and presents with gastrointestinal symptoms of repetitive severe projective emesis 1–4 hours after ingestion of trigger foods. Diarrhea can occur 5–10 hours after ingestion [51, 52]. Infants triggered by cow’s milk and soy typically present in the first 3 months of life, while children triggered by solids like rice, oat, or poultry typically present between 4 and 7 months [52, 53]. FPIES in older children has been reported to seafood and egg but is highly uncommon [52]. Symptoms are often severe and can result in significant dehydration and hypovolemic shock with pallor, lethargy, and hypothermia with hypotension reported in 15% of cases [46]. Associated lab findings of acidemia, methemoglobinemia, anemia, and leukocytosis with left shift are common and contribute to the condition being commonly misdiagnosed as sepsis. FPIES is considered a medical emergency given the rapid progression and clinical consequences of shock. Symptoms generally resolve within 24 hours with supportive care with anti-emetics and intravenous fluid resuscitation. A more chronic form of FPIES can be seen in cow’s milk or soy formula-fed infants under 6 months old and presents with chronic vomiting, diarrhea, and failure to thrive. Symptoms resolve within several days of removing the triggering formula. If re-exposed, patients can present with acute FPIES [51].
The pathophysiology of FPIES is currently unknown, but hypothesized mechanisms involve antigen-specific T-cell-mediated inflammation causing increased intestinal permeability. FPIES is not an IgE-mediated food allergy; however, it is associated with comorbid atopic disease including eczema and allergic rhinitis. In addition, some patients with FPIES have positive IgE to the trigger food, especially casein in patients with cow’s-milk-induced symptoms. The relationship between IgE and non-IgE mechanisms in patients with FPIES is still under investigation, and recent research has also suggested the role of innate immunity in the pathogenesis as well [54]. Ondansetron, a serotonin 5-HT3 receptor antagonist, is highly effective in improving FPIES symptoms, suggesting possible involvement of neuroimmune mechanisms [46, 51, 52].
Celiac Disease
Celiac disease (CD) is caused by chronic mucosal inflammation in the small bowel. Symptoms are commonly chronic diarrhea, bloating, abdominal pain, and malabsorption with resulting failure to thrive in young children. Older children and adolescents can present with similar symptoms along with short stature and delayed puberty. Other findings are variable and can include osteoporosis, dental enamel hypoplasia, oral aphthae, arthritis, neurologic problems (headaches, cerebellar ataxia, idiopathic epilepsy, peripheral neuropathy), unexplained elevation of transaminases, and as the disease progresses vitamin deficiencies like iron, vitamin D, and vitamin K [55]. Dermatitis herpetiformis, an intensely pruritic vesicular rash, is a classic dermatologic finding. Extra-intestinal manifestations tend to increase with age [56]. Symptoms and histologic abnormalities typically resolve after gluten is removed from the diet within weeks to months.
CD is caused by autoreactivity resulting in destruction of the villi in the small intestine [57]. The pathogenesis is determined by a combination of genetic factors, exposure to gluten, and environmental influences. There is a strong genetic predisposition, and virtually all patients with CD have Human Leukocyte Antigen (HLA)-DQ2 and HLA-DQ8. Gluten is digested into gliadin fragments that are taken up by B cells, macrophages, and dendritic cells expressing HLA class II DQ2 and/or DQ8 molecules on their surface. These cells then present the antigen to gliadin-specific CD4+ TH1 cells. Inflammatory cytokines including IFN-gamma and IL-15 contribute to the differentiation of intraepithelial lymphocytes into cytotoxic CD8+ T cells resulting in the classic histologic findings of villous atrophy and crypt hyperplasia. While almost all patients with CD are HLA-DQ2 or DQ8 positive, these alleles are also prevalent in about 30% of the general population who do not develop CD, indicating there are environmental factors that are yet to be understood [58]. The infant microbiota and the timing and amount of initial gluten exposure are hypothesized mechanisms still under investigation [56].
Heiner Syndrome
Heiner syndrome or food-induced pulmonary hemosiderosis is a rare condition that affects infants exposed to cow’s milk who develop chronic respiratory symptoms that can progress to pulmonary infiltrates and hemosiderosis with iron-laden macrophages in the bronchial fluid. Patients also frequently have eosinophilia, iron deficiency anemia, and failure to thrive. It is associated with milk-specific IgG antibodies. Avoidance of milk protein results in resolution of symptoms and pulmonary infiltrates [59,60,61]. The pathophysiology is poorly understood. A high index of suspicion is required as the presentation is variable and symptoms and imaging findings can be mistaken for recurrent or persistent infections.
Mixed IgE-Mediated and Non-IgE-Mediated Reactions
Eosinophilic Esophagitis and Eosinophilic Gastroenteritis
Eosinophilic esophagitis (EoE) and eosinophilic gastroenteritis (EG) are examples of mixed IgE and non-IgE (T-cell mediated) reactions. Patients with EoE have a diverse clinical presentation depending on their age. Young children commonly present with feeding difficulties, failure to thrive, vomiting, reflux, and abdominal pain, while older children present with dysphagia and food impaction. Patients have been found to cut food into small pieces or drink large amount of liquids during meals [62, 63]. EoE is a histologic diagnosis that requires biopsy to confirm the presence of greater than 15 eosinophils per high-powered field in the esophagus [8]. Patients with EoE commonly have other atopic conditions and up to 90% have either allergic rhinitis, asthma, or an IgE-mediated food allergy [64]. Many cases of EoE exhibit seasonal variability with symptoms and eosinophilic infiltration worsening during high aeroallergen counts to which the patient is sensitized. For example, some patients with tree pollen allergies have been shown to exhibit worsening of disease in the spring [63]. EG is diagnosed when eosinophils are found distal to the esophagus in the stomach or lower GI tract and symptoms are variable, in part dependent on the location of eosinophil inflammation.
The pathophysiology of EoE and EG remains largely unknown but is likely multifactorial with genetic and environmental factors contributing. Although EoE is not solely an IgE-driven food allergy, it is primarily TH2-driven with increased levels of IL-5, IL-9, and IL-13, increased eosinophilia, mucosal mast cells, and TH2 lymphocytes in the esophageal tissue [63, 65]. There is likely a contribution of impaired epithelial barrier. Males are disproportionately affected with a 3:1 male to female ratio, and evidence points to a strong genetic component with a sibling risk ratio of 80, which is 40 times higher than for asthma [64].
Key Questions in the History of Patients with Possible Food Allergy
Overall, a detailed history is the key in approaching a suspected food allergy. When obtaining a reaction history, clinicians should obtain details about all potential food triggers, timing of the reaction, response to treatments, and categorize the type of food allergy to determine whether it is IgE-mediated or another mechanism [8]. Important aspects of the history are detailed in Table 2.5 [4].
Conclusion
FAs are a common threat in the pediatric population that is increasing in prevalence. FAs can have life-threatening consequences when they progress to anaphylaxis, and as such the accurate diagnosis is critical for preventing morbidity and mortality. An understanding of the underlying pathophysiology is key to differentiating the different types of FAs and anticipating their clinical consequences and anticipated management.
Abbreviations
- CD:
-
Celiac disease
- DCs:
-
Dendritic cells
- EG:
-
Eosinophilic gastroenteritis
- EOE:
-
Eosinophilic esophagitis
- FA:
-
Food allergy
- FPIAP:
-
Food protein-induced allergic proctocolitis
- FPIES:
-
Food protein-induced enterocolitis syndrome
- HLA:
-
Human leukocyte antigen
- MSG:
-
Monosodium glutamate
- OAS:
-
Oral allergy syndrome
- PFS:
-
Pollen-food allergy syndrome
References
Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142(6):e20181235.
Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012;32(1):165–95.
Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6):S1–58.
Sharma HP, Bansil S, Uygungil B. Signs and symptoms of food allergy and food-induced anaphylaxis. Pediatr Clin North Am. 2015;62(6):1377–92.
Hungerford JM. Scombroid poisoning: a review. Toxicon. 2010;56(2):231–43.
Settipane RA. Other causes of rhinitis: mixed rhinitis, rhinitis medicamentosa, hormonal rhinitis, rhinitis of the elderly, and gustatory rhinitis. Immunol Allergy Clin North Am. 2011;31(3):457–67.
Motz KM, Kim YJ. Auriculotemporal syndrome (Frey syndrome). Otolaryngol Clin North Am. 2016;49(2):501–9.
Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016–25.e43.
Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111(3):1601–8.
Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140(2):335–48.
Kobza Black A, Greaves MW, Champion RH, Pye RJ. The urticarias 1990. Br J Dermatol. 1991;124(1):100–8.
Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema: a review of 554 patients. Br J Dermatol. 1969;81(8):588–97.
Sehgal VN, Rege VL. An interrogative study of 158 urticaria patients. Ann Allergy. 1973;31(6):279–83.
Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327(6):380–4.
Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125(2):S161–81.
Crespo JF, Pascual C, Dominguez C, Ojeda I, Muñoz FM, Esteban MM. Allergic reactions associated with airborne fish particles in IgE-mediated fish hypersensitive patients. Allergy. 1995;50(3):257–61.
Roberts G, Lack G. Relevance of inhalational exposure to food allergens. Curr Opin Allergy Clin Immunol. 2003;3(3):211–5.
Lieberman P. Epidemiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8(4):316–20.
Yu JE, Lin RY. The epidemiology of anaphylaxis. Clin Rev Allergy Immunol. 2018;54(3):366–74.
Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114(1):159–65.
Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120(3):491–503.
Ono E, Taniguchi M, Mita H, Fukutomi Y, Higashi N, Miyazaki E, et al. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin Exp Allergy. 2009;39(1):72–80.
Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J Allergy Clin Immunol. 2006;117(2):391–7.
Waleed A, Ellis AK. Do corticosteroids prevent biphasic anaphylaxis? JACI. 2017;5(5):1194–205.
Tole JW, Lieberman P. Biphasic anaphylaxis: review of incidence, clinical predictors, and observation recommendations. Immunol Allergy Clin North Am. 2007;27(2):309–26.
Commins SP, Platts-Mills TAE. Delayed anaphylaxis to red meat in patients with IgE specific for galactose alpha-1,3-galactose (alpha-gal). Curr Allergy Asthma Rep. 2013;13(1):72–7.
Tripathi A, Commins SP, Heymann PW, Platts-Mills TAE. Delayed anaphylaxis to red meat masquerading as idiopathic anaphylaxis. J Allergy Clin Immunol Pract. 2014;2(3):259–65.
Mullins RJ, James H, Platts-Mills TAE, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy Clin Immunol. 2012;129(5):1334–42.
Centers for Disease Control and Prevention. Approximate distribution of the Lone Star Tick. https://www.cdc.gov/ticks/maps/lone_star_tick.html (2011). Accessed 6 Dec 2018.
Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–33.
Kennedy JL, Stallings AP, Platts-Mills TAE, Oliveira WM, Workman L, James HR, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013;131(5):e1545–52.
Castells MC, Horan RF, Sheffer AL. Exercise-induced anaphylaxis (EIA). Clin Rev Allergy Imunol. 1999;17(4):413–24.
Wong GK, Krishna MT. Food-dependent exercise-induced anaphylaxis: is wheat unique? Curr Allergy Asthma Rep. 2013;13(6):639–44.
Feldweg AM. Exercise-induced anaphylaxis. Immunol Allergy Clin North Am. 2015;35(2):261–75.
Barg W, Medrala W, Wolanczyk-Medrala A. Exercise-induced anaphylaxis: an update on diagnosis and treatment. Curr Allergy Asthma Rep. 2011;11(1):45–51.
Kemp SF. Navigating the updated anaphylaxis parameters. Allergy Asthma Clin Immunol. 2007;3(2):40–9.
Food Allergy Research & Education (FARE). Food Allergy & Anaphylaxis Emergency Care Plan. https://www.foodallergy.org/sites/default/files/2018-06/emergency-care-plan.pdf (2018). Accessed 1 Dec 2018.
Brown SGA. Clinical features and severity grading of anaphylaxis. JACI. 2004;114(2):371–6.
Price A, Ramachandran S, Smith GP, Stevenson ML, Pomeranz MK, Cohen DE. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26(2):78–88.
Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58.
Sampson HA, O’Mahony L, Burks AW, Plaut M, Lack G, Akdis CA. Mechanisms of food allergy. J Allergy Clin Immunol. 2018;141(1):11–9.
Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity. 2017;47(1):32–50.
Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137(4):984–97.
Gensollen T, Blumberg RS. Correlation between early-life regulation of the immune system by microbiota and allergy development. J Allergy Clin Immunol. 2017;139(4):1084–91.
Leonard SA. Non-IgE-mediated adverse food reactions. Curr Allergy Asthma Rep. 2017;17(12):84.
Nowak-Wegrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol. 2015;135(5):1114–24.
Feuille E, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome, allergic proctocolitis, and enteropathy. Curr Allergy Asthma Rep. 2015;15(8):50.
Lake AM. Food-induced eosinophilic proctocolitis. J Pediatr Gastroenterol Nutr. 2000;30:S58–60.
Lake AM, Whitington PF, Hamilton SR. Dietary protein-induced colitis in breast-fed infants. J Pediatr. 1982;101(6):906–10.
Kaya A, Toyran M, Civelek E, Misirlioglu E, Kirsaclioglu C, Kocabas CN. Characteristics and prognosis of allergic proctocolitis in infants. J Pediatr Gastroenterol Nutr. 2015;61(1):69–73.
Cherian S, Varshney P. Food protein-induced enterocolitis syndrome (FPIES): review of recent guidelines. Curr Allergy Asthma Rep. 2018;18(4):28.
Nowak-Wegrzyn A, Chehade M, Groetch ME, Spergel JM, Wood RA, Allen K, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary – workgroup report of the adverse reactions to foods committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017;139(4):1111–1126.e4.
Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics. 2003;111(4):829–35.
Goswami R, Blazquez AB, Kosoy R, Rahman A, Nowak-Wegrzyn A, Berin MC. Systemic innate immune activation in food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2017;139(6):1885–96.e9.
Barker JM, Liu E. Celiac disease: pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr Infect Dis. 2008;55:349–65.
Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr. 2014;168(3):272–8.
Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3(7):797–801.
Green PHR, Lebwohl B, Greywoode R. Celiac disease. J Allergy Clin Immunol. 2015;135(5):1099–106.
Heiner DC, Sears JW, Kniker WT. Multiple precipitins to cow’s milk in chronic respiratory disease. A syndrome including poor growth, gastrointestinal symptoms, evidence of allergy, iron deficiency anemia, and pulmonary hemosiderosis. Am J Dis Child. 1962;103:634–54.
Moissidis I, Chaidaroon D, Vichyanond P, Bahna SL. Milk-induced pulmonary disease in infants (Heiner syndrome). Pediatr Allergy Immunol. 2005;16(6):545–52.
Lee SK, Kniker WT, Cook CD, Heiner DC. Cow’s milk-induced pulmonary disease in children. Adv Pediatr Infect Dis. 1978;25:39–57.
Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351(9):940–1.
Furuta GT, Katzka DA. Eosinophilic esophagitis. N Engl J Med. 2015;373(17):1640–8.
Cianferoni A, Spergel JM. Eosinophilic esophagitis and gastroenteritis. Curr Allergy Asthma Rep. 2015;15(9):58.
Caldwell JM, Paul M, Rothenberg ME. Novel immunologic mechanisms in eosinophilic esophagitis. Curr Opin Immunol. 2017;48:114–21.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Widge, A.T., Sharma, H.P. (2020). Pathophysiology and Symptoms of Food Allergy and Anaphylaxis. In: Gupta, R. (eds) Pediatric Food Allergy . Springer, Cham. https://doi.org/10.1007/978-3-030-33292-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-33292-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33291-4
Online ISBN: 978-3-030-33292-1
eBook Packages: MedicineMedicine (R0)