Abstract
In many practical outdoor insulation applications, silicone rubber materials have outperformed others due to its inherent water repellence or hydrophobicity. In some cases, however, it has been found that the leakage current of such insulators exceed that of glass, especially under prolonged water exposure, while still showing a lower pollution flashover probability. In this paper, the effect of water saturation on silicone rubber material is studied with particular interest in the surface discharge behaviour. We will show that the presence of moisture may reduce the severity of surface discharges under some conditions. The polar nature of water and its dipole relaxation time will change the surface charge distribution thereby hindering the growth of anodic (+) surface discharges. Experiments are performed below 10 kV on both dry and moisture saturated silicone-rubber test samples. The moisture-saturated samples were aged in a climatic chamber and in hot water respectively. The test arrangement consists of a sharp point against a plane electrode for the initial tests and a flat washer vs. plane for the remaining trials; the electrodes are separated (interposed) by a 2 mm thick material specimen. This arrangement yields an electric field with a strong tangential component, which is favourable for obtaining surface discharges. Electrical measurements are performed using standard partial discharge detection equipment to obtain phase resolved partial discharge patterns. A camera with an intensified charge coupled device, or ICCD, is used to obtain time resolved images. Tests show that surface moisture affects both the magnitude and position of the discharge pulses as well as the physical shape of the electrical discharges, whereas prolonged moisture exposure appears to have a minimal effect on filler free silicone rubber materials, which were wiped dry.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In many practical outdoor insulation applications, silicone rubber materials have outperformed others. Its superior hydrophobic properties and its ability to recover has made silicone rubber a popular choice for power line insulators in areas having a high pollution severity. Its hydrophobic properties are due to the presence of low molecular weight mobile silicone fragments. The mobility of the Polydimethylsiloxane groups and the orientation of its side methyl groups provide a low surface energy, which makes the surface non-polar, thereby repelling (polar) water [1]. This in turn controls the surface wettability. Furthermore, low molecular weight components within the bulk of the material migrates to the surface [2], thereby encapsulating pollutants and rendering the surface hydrophobic.
Hydration, Ultraviolet radiation, surface discharges and dry band arcing (causing heat) lead to irreversible damage (aging) and the ultimate failure of polymeric materials due to tracking and erosion. The reduced hydrophobicity will encourage water filming during a wetting event and if soluble pollutants or salts are present on the surface of the insulator, leakage currents will flow which in turn causes discharges.
1.1 Dielectric Degradation Due to Hydration
The degradation of polymers due to water ingress or the process of hydration has been found to be one of the factors contributing to eventual failure of polymers used for outdoor insulators. Tokoro and Hackham [3] studied the hydrophobic properties of Ethylene-propylene-diene-monomer (EPDM) material immersed in salt water. They have shown that the presence of salt has deleterious effects on the hydrophobicity of the material; furthermore, they have found that the presence of salt reduces the moisture absorption rate of the material. An investigation of water permeation into Silicone rubbers by Gao and Cai [4], showed that temperature does not influence the moisture saturation level of materials, but that the amount of filler material has a large influence thereupon; lower filler concentration had shown a reduced moisture absorption value. Similarly, it was shown that higher concentrations of NaCl reduced the absorption rate of liquids into the material. These findings have also been confirmed for silicone rubber materials with other filler materials like fly-ash [5, 6] as well as for carbon black filled polymers [7] and cable sheath materials for subsea cables [8].
The presence of moisture both within and on the surface of an insulator will cause increased leakage currents to flow. In some cases, it has been found that the leakage current on silicone insulators exceed that of porcelain insulators with the same profile under conditions of prolonged rain [9]. Periods of high humidity have shown a similar increase in leakage currents for silicone rubber materials with natural pollutants, since they absorb moisture [5]. In addition to these leakage currents (mainly conduction losses), interactions between a time varying field and water molecules contained within a dielectric material, or upon its surface will lead to further energy losses due to dielectric heating. This is due to the polar nature of the water molecules and its dipole relaxation time. These losses are in general much lower than the conduction losses from spark discharges and surface currents.
1.2 Main Hypothesis
Since the dielectric heating losses are small, they are generally ignored, however it is believed that the dipole relaxation phenomena may also affect the overall charge distribution and hence the surface discharge behaviour. It is postulated that the presence of moisture may reduce the severity or length of surface discharges due to the build-up of localised surface charge. To investigate this hypothesis, an experiment was performed on silicone rubber materials without filler to determine the effect of moisture on the surface discharge behaviour for a non-uniform field arrangement in air.
2 Test Arrangement
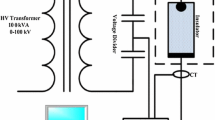
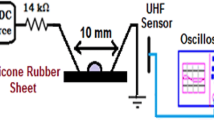
The test arrangement consists of a high voltage power source, a test specimen holder, a partial discharge detector and an optical recording system. The test specimen holder and general arrangement as shown in Fig. 1, was designed and constructed at TU Braunschweig – elenia [10]. It was purpose built to create an electrical stress on the material test sample, with a strong tangential electrical field component, which is favourable for obtaining surface discharges. The specimen holder, to the right of Fig. 1, has two electrodes: a vertical flat plate electrode, shown to the right and an angled rod, which is set against the surface of the test material sample, which is placed between the electrodes. A high voltage supply is connected across the electrodes with the rod at ground. For sample set A, a sharp point was used and for sample sets B – D, a round disc washer of 6 mm diameter placed between the rod and the sample was used. An intensified or ICCD camera, to the left of Fig. 1, is placed ahead of the rod to view the surface discharges at right angles. A commercial partial discharge detector was used together with a coupling capacitor Cm, and measuring impedance Zm, as shown in Fig. 2, to obtain phase resolved partial discharge or PRPD patterns for the test specimen Cx.
Note that a positive supply voltage creates a negative plasma at the surface due to the inverted arrangement.
3 Experimental Procedure
3.1 Sample Preparation
The test sample consisted of 12 round silicone rubber slabs with a thickness of 2 mm and a diameter of 90 mm. The specimens were moulded from a commercial two-pack RTV material without ATH filler materials. Sets of three specimens each were prepared. For the first trial set ‘A’, the specimens were immersed (aged) in distilled water for one month at \(90\,^\circ \)C leading to a complete loss of hydrophobicity. To investigate the effects of alternate aging or wetting methods, three more sample sets were prepared:
-
Set B was dried for three months at \(50\,^\circ \)C
-
Set C was set in a climate chamber for three months at \(23\,^\circ \)C & 97% relative humidity (RH)
-
Set D was immersed in distilled water at room temperature for three months, followed by 5 Days at \(90\,^\circ \)C
Sample set C had shown only a slight reduction to its hydrophobicity and sample set D a severe, but not complete loss of hydrophobicity.
3.2 Test Method
The specimens were stored in airtight containers and tested within 1 h of removing it from its aging environment. All specimens were thoroughly dried with paper before inserting it into the test arrangement. The voltage was gradually increased to 8 kV (RMS equivalent for sine wave) and the discharges was allowed to stabilise for two minutes. Following this period, a cumulative PRPD pattern was recorded using the PD detector, while at the same time, recording the surface discharge behaviour with the ICCD camera. For each test, twenty images were recorded at each polarity. The images were synchronised with the zero crossings using a special triggering circuit similar to [10] to record sequential images 20 ms apart, each with a 10 ms exposure time.
4 Test Results and Discussion
4.1 Sample Set A
Initial tests were performed on sample set A, which had been preconditioned as given in Sect. 3.1. Figures 3 and 4 shows the logarithmically scaled PRPD patterns for a dry reference sample and the preconditioned sample respectively.
It can be shown in Fig. 4, that the PD activity is reduced for the wet preconditioned sample Set A, especially at the peaks. The discharge pattern band at the negative polarity is narrower with a sudden decrease in activity at the voltage maximum. As stated before, the electrode tip is positive when the voltage is negative. This reduction in the positive or Anode plasma can furthermore be observed in the photographic results obtained with the ICCD camera. Figure 5 shows the image results for the dry reference sample and the wet preconditioned sample A.
The discharges are sharply divergent for the wet preconditioned sample and appear to be bound to a specific radius, whereas the discharges for the dry sample appear more random, radiating further out. The negative glow discharges (not shown) also appeared less intense for the wet preconditioned sample. This discharge behavior is probably caused by the formation of localized surface charges, which may be attributed to the polar nature of the moisture contained within the surface layer.
4.2 Sample Sets B, C, D
In an attempt to investigate the phenomena further, additional sample sets B, C & D was prepared. See Sect. 3.1 for further details. In each case, the surface was wiped with ethanol and dried with paper shortly before each experiment to remove surface moisture. Since water and ethanol are dissolvable in each other, due to the hydroxyl (–OH) groups, the traces of surface moisture will be evaporated together with the volatile ethanol. In this way, an assessment could be made to determine the effects of possible moisture ingress into the bulk of the material.
Surface and volume resistivity was measured using a Megger MIT515 insulation tester and a guarded concentric electrode geometry at a voltage of 5 kV for each sample set as given in Table 1.
All volume resistivity values were between one and ten terra-ohm, showing that the bulk insulating properties were not affected by moisture. The surface resistivity had shown some degree of reduction for the sample that was fully immersed for 3 months, however since the samples were aged with de-ionized water, a high surface resistivity was expected. After preparing the samples, PRPD patterns and discharge images were recorded for sample sets B, C and D as shown in Figs. 6, 7, 8, 9, 10 and 11.
As can be seen, there appears to be only a slight deviation in the PRPD patterns and no observable changes in the surface discharge patterns. It should be noted that a slightly different electrode geometry was used for sample set A (sharp point) as shown in Fig. 5 and that these images should not be compared to those of sample sets B through D, using a flat circular electrode (Figs. 9, 10 and 11).
5 Conclusions
For sample set A, with a complete loss of hydrophobicity and some traces of surface moisture, diminished partial discharge activity was measured at the voltage peaks. Furthermore, the magnitude of the discharge pulses in the positive and negative half-cycles were reduced for the wet preconditioned sample set. The negative polarity plasma (positive voltage, negative tip) was less intense for the wet sample. The positive polarity plasma streamers (negative voltage, positive tip) showed a shorter, divergent discharge pattern, bound to a definite boundary or radius. For sample sets B, C and D, having their surfaces dried before testing, and with sets C and D subjected to prolonged moisture, no definite effects could be shown as a result of prolonged moisture. The surface resistivity remained high and the discharge patterns were unaltered. Since these specimens were without fillers, it is believed that no significant water ingress resulted from these aging methods. For sample set A, however, which was only dried with paper (no visible surface moisture), the observed effects confirmed that surface moisture has a reducing effect on the surface discharges, both in magnitude and physical length. Materials with fillers would absorb more moisture as shown by others for Silicone rubber [4,5,6], EPDM [3] and other polymers [7, 8]. In addition, materials with a surface pollution layer would hold more moisture in the pollution layer when subjected to prolonged wetting [5, 9]. Therefore, it may be concluded that charge accumulation due to water absorption in the surface layer, has a reducing effect on surface discharge behaviour for silicone rubber materials.
References
Kuo, A.C.M.: Polymer Data Handbook. Oxford University Press Inc., Oxford (1999)
Mavrikakis, N., Siderakis, K., Koudoumas, E., Drakakis, E., Kymakis, E.: Laboratory investigation of the hydrophobicity transfer mechanism on composite insulators aged in coastal service. Eng. Technol. Appl. Sci. Res. 6(5), 1124–1129 (2016)
Tokoro, T., Hackham, R.: Effect of water salinity and temperature on the hydro-phobicity of ethylene propylene diene monomer insulator. In: IEEE Annual Report, Conference on Electrical Insulation and Dielectric Phenomena, San-Francisco, October 1996
Gao, Y., Cai, Y.: Investigation on permeation properties of liquids into HTV silicone rubber materials. IEEE Trans. Dielectr. Electr. Insul. 21(6), 2428–2437 (2014)
Chrzan, K.L., Greyling, C.: Hygroscopic properties of natural pollutants on silicone rubber surfaces. In: International Symposium on High Voltage Engineering, Cape Town, South Africa (2009)
Manjang, S., Kitta, I., Muhammadia, K.B., Mulya, R.N.I.: Effect of water diffusion on dielectric behaviour of polymer insulators. In: The 5th International Conference on Electrical Engineering and Informatics 2015, Bali, Indonesia, August 2015
Schultz, J., Papirer, E., Jacquemart, C.: Mechanisms of diffusion of water in carbon black filled polymers. Eur. Polym. J. 22, 499–503 (1985)
Nordas, S., Hvidsten, S.: Correction to: water diffusion in high voltage subsea cable outer sheath materials. In: IEEE International symposium on electrical insulation, ISEI 2008, July 2008. https://doi.org/10.1109/ELINSL.2008.4570401
Chrzan, K.L., Vosloo, W., Holtzhausen, J.P.: Leakage current on porcelain and silicone insulators under sea or light industrial pollution. IEEE Trans. Power Deliv. 26(3), 2051–2052 (2011)
Gortschakow, S., Kozakov, R., Pieterse, P.J., Hilbert, M., Kurrat, M.: Application of optical methods for diagnostics of partial discharges. In: VDE Hochspannungstechnik 2018, VDE-Conference, Berlin, VDE VERLAG GMBH, 12–14 November 2018, pp. 415-420. ISBN 978-3-8007-4807-5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Pieterse, P., Hilbert, M., Gortschakow, S., Uhrlandt, D. (2020). The Effect of Water Saturation on Silicone Rubber Surface Discharge Behaviour. In: Németh, B. (eds) Proceedings of the 21st International Symposium on High Voltage Engineering. ISH 2019. Lecture Notes in Electrical Engineering, vol 599. Springer, Cham. https://doi.org/10.1007/978-3-030-31680-8_59
Download citation
DOI: https://doi.org/10.1007/978-3-030-31680-8_59
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31679-2
Online ISBN: 978-3-030-31680-8
eBook Packages: EngineeringEngineering (R0)