Abstract
Conventional morphological magnetic resonance imaging (MRI) represents the standard imaging method for neuro-oncologic assessment; its primary roles consist of initial brain tumor evaluation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
5.1 Morphological MRI
Conventional morphological magnetic resonance imaging (MRI) represents the standard imaging method for neuro-oncologic assessment; its primary roles consist of initial brain tumor evaluation.
Conversely, after diagnosis, each treatment alters anatomy and structural framework evaluated at baseline imaging, so interpretation of images has to be supported from a set of knowledge that include physiology of the tissue response to any type of treatment: chemotherapy, surgical, radiation therapy, and new immunological treatments.
Standardized brain tumor MRI protocol should include the following sequences: TSE T1, TSE T2, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and 3-D gadolinium contrast-enhanced FFE T1, performed on a minimum 1.5 T MR system. T2∗-weighted imaging, such as susceptibility-weighted imaging (SWI), is usually performed as part of the routine brain MRI examination. If 3-D sequences cannot be performed due to time constraints or technical limitations, 2-D sequences can be substituted.
These sequences provide exquisite anatomic detail, and the use of a gadolinium-based contrast agent in this protocol allows for the detection of areas where the blood–brain barrier is compromised.
In general, gliomas are hypointense on T1W images and hyperintense on T2W images. Contrast enhancement, necrosis, hemorrhage, infiltration of surrounding brain and abundant peritumoral edema are commonly considered imaging characteristics of aggressive lesions and raise the possibility of a high-grade glial neoplasm.
Specific presurgical sequences such as high-resolution isovolumetric 3D T2-weighted and postcontrast 3D T1 spoiled gradient echo imaging can be obtained for intraoperative navigation or with a head frame for stereotactic radiosurgical planning.
MRI after treatment imaging includes the same sequences of a standard evaluation [1, 2].
5.2 Susceptibility-Weighted Imaging
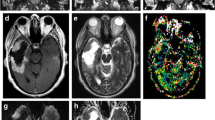
High-resolution 3D T2∗ gradient echo sequences such as SWI are highly sensitive to magnetic susceptibility effects from blood products or mineralization. This technique is useful to depict internal vascular architecture and hemorrhage in tumors, which can be used to suggest grade, as well as calcification to narrow the differential diagnosis. Both blood products and mineralization appear dark on magnitude images and can be differentiated on filtered phase images in which paramagnetic blood products appear dark and diamagnetic calcium appears bright. Minimum intensity projection images can also be reviewed to more clearly visualize normal venous structures, tumoral vascularity, and parenchymal foci of susceptibility.
SWI is also used in noninvasive grading of primary brain neoplasms by assessment and counting, of intratumoral susceptibility signals (ITSS) that refers to linear dot-like or small mass-like areas of low signal within or along the edges of the tumor on non-CE SWI. SWI is sensitive to hemorrhage, calcification, deoxiHb, and all show low signal on SWI.
The morphological pattern of ITSS has been shown to correlate with neoangiogenesis (linear), calcifications or necrosis (dot like and conglomerated). The number of ITSS can reflect the WHO histological grading, so ITSS enables estimating of tumor grade as well as imaging surveillance of tumor progression or more malignant transformation.
The additional ITSS observed on CE-SWI when compared with the unenhanced/conventional SWI image comes from the CIPS effect: contrast-induced phase shift, that are T2∗ effects more frequently observed at the border zone of high-grade glioma.
Postcontrast SW images can show bright enhancement along the tumor that suggested leakage of contrast material due to breakdown of the blood–brain barrier. All the features described about SWI imaging make this sequence essential in the assessment of gliomas especially in posttreatment imaging and follow-up [3, 4, 5].
5.3 Diffusion-Weighted Imaging
DWI is a technique with a multitude of applications within neuroimaging as well as in other areas of radiology. The technique employs MRI sequences sensitized to the movement of water molecules. Pulse sequences are generated so that water molecules that do not move between pulse applications are refocused, and thus are able to generate signal, whereas those that do move lose their ability to generate signal in the reconstructed image. Thus areas of restricted diffusion are bright on DWI, whereas areas of free water motion are dark.
DWI offers significant value in the evaluation of brain tumors, allowing for the assessment of tumor cellularity, peritumoral edema, regions of tumor hypoxia, integrity of white matter tracts, and postoperative injury. Corresponding apparent diffusion coefficient (ADC) values, reflecting the magnitude of diffusivity, are derived for each voxel and displayed as a calculated ADC map.
DWI best serves to characterize tumor cellularity on the premise that water diffusivity within the extracellular compartment is inversely correlated to the volume of the intracellular space.
Low ADC values, representing decreased water diffusivity, can be used to suggest highly cellular tumors. Additionally, low ADC values can be used as a surrogate for increasing tumor grade or as an independent biomarker signifying poor outcomes both in glioma and lymphoma. ADC values have also been used to better localize tumor infiltrated foci among regions of vasogenic edema to better direct tissue sampling and therapy [2].
5.4 MR Perfusion Imaging
It is now established that tumors produce factors which initiate the development of new blood vessels, a process known as neoangiogenesis; perfusion-weighted imaging (PWI) is the MR imaging technique able in assessing the neoangiogenic properties of proliferating gliomas.
Several MR perfusion techniques are currently employed: dynamic susceptibility contrast (DSC), dynamic contrast-enhanced (DCE), and arterial spin labeling (ASL). Of these, DSC perfusion is the most studied and widely applied, while ASL, which does not require intravenous contrast, has been the subject of increasing investigation and clinical implementation.
DSC is based on the detection of susceptibility induced signal loss on T2∗-weighted sequences after the administration of an intravenous gadolinium contrast agent. A signal intensity time curve is generated from which relative cerebral blood volume (rCBV) and other perfusion metrics are derived. rCBV is elevated in tumor, where it is seen as a marker of angiogenesis.
rCBV has been positively correlated to glioma grade, although some lower-grade gliomas such as oligodendrogliomas may have elevated rCBV; rCBV has been noted to be increased in infiltrative edema of gliomas compared to acellular vasogenic edema; rCBV may also predict areas of progression in glioma prior to changes on contrast-enhanced MRI.
The new underlying principle behind DCE is that disordered tumor vasculature permits intravascular contrast diffusion into the interstitial compartment which is then quantifiable over a dynamic MR acquisition. A modified version of the Tofts pharmacokinetic model is the most commonly used analytic approach in DCE, producing three main imaging biomarkers: estimates of the vascular fraction (vp), extravascular extracellular space fraction (ve), and the transfer contrast coefficient (Ktrans). The volume transfer constant or ktrans, a measure of capillary permeability, is the primary metric derived from DCE perfusion; ktrans can be used to grade tumors, particularly gliomas, as gliomas with increased capillary permeability are more likely to be higher grade than lower grade. Another metric quantified by DCE is ve, an estimate of fractional extracellular extravascular space, which has been shown to be related to tumor cellularity.
ASL is a noninvasive perfusion imaging technique which quantitatively measures cerebral blood flow. It uses an inversion pulse to label inflowing blood proximal to the area of imaging with subsequent subtraction of these labeled spins from control static images. ASL is of particular clinical interest due to its noncontrast technique, relative speed, ability to image the whole brain, and minimal postprocessing.
Several studies have shown a promising role for ASL differentiating high- from low-grade gliomas based on a degree of microvascular proliferation [2, 10].
5.5 MR Spectroscopy
Proton MRI is dependent on signal generated from free protons, which are most abundant in water. In addition to free protons, the MRI signal is also affected to a much lesser degree by protons bound to macromolecules, which are found in low relative concentrations in biologic tissues. Despite their scarcity, these bound protons have specific frequency variations, which are expressed in parts per millions (ppm) in relation to a known reference frequency. Acquiring data which allows separation of these small frequency peaks, each representing a specific macromolecular component, is known as MR spectroscopy (MRS).
Proton (1H) MRS can be performed with long (288 or 144 ms) and short (35 ms) echo times. MRS can be obtained using a single-voxel technique to a targeted region of interest or a multivoxel technique to cover a broader area and better evaluate regional biochemical differences. The most recognizable metabolite peaks on long echo 1H MRS include N-acetylaspartate (NAA) at 2.0 parts per million (ppm), creatine (Cr) at 3.0 ppm, choline (Cho) at 3.2 ppm, and myo-inositol (MI) at 3.5 ppm. NAA is a marker of neuronal viability, Cr reflects normal cellular metabolism, Cho is a marker of cell membrane turnover, and MI reflects astrocyte integrity. Lipid and lactate, which have a broad peak at 1.3 ppm, are not seen in normal tissue and considered markers of necrosis and hypoxia, respectively.
Brain tumor spectra reflect cellular turnover and loss of normal neuronal metabolites, typically as elevated Cho and decreased NAA.
Generally, absolute heights of metabolite peaks are not used, and rather the peaks are analyzed as ratios such as Cho/NAA and Cho/Cr.
MRS has been shown to differentiate gliomas by grade on the basis of a positive correlation between Cho/NAA and Cho/Cr ratios and grade. Additionally, lower-grade gliomas have been associated with elevated MI/Cr ratio.
Within regions of nonenhancing signal abnormality, elevated Cho/NAA and Cho/Cr ratios have been observed in infiltrative edema compared to vasogenic edema reflecting the increased cellularity underlying the signal abnormality. In this way, MRS can be used to differentiate glioma from noninfiltrative tumor such as metastasis or for biopsy targeting and treatment planning.
Widespread adoption of MRS is limited by technical issues such as variability in acquisition techniques, differences in metabolite ratio calculations, and volume averaging due to lesion location or voxel size [1, 2, 10].
5.6 Chemical Exchange Saturation Transfer (CEST): AMID Amide Proton Transfer Imaging
CEST creates imaging contrast based on sensitivity to chemical exchange of protons on functional metabolic groups rather than exogenously administered contrast. The CEST technique which has primarily been applied to brain tumor imaging is the exchange of amide protons of endogenous tissue proteins and peptides, known as amide proton transfer (APT). Early studies have shown a potential use of APT in the differentiation of tumor from edema and true progression versus pseudoprogression, although these techniques have yet to be widely applied clinically.
Quantitative APT parameters have been proposed as prognostic indicators of brain gliomas by reflecting the cellular proliferation levels that correlated with Ki-67 and as a sensitive biomarker of treatment responses [1, 10].
5.7 Diffusion Tensor Imaging
An advanced application of diffusion imaging is DTI, which interrogates the 3D shape of diffusion using both diffusivity (eigenvalues) and direction (eigenvectors). The principle metrics obtained from DTI include mean diffusivity (MD) and fractional anisotropy (FA). In presurgical planning, DTI-based tractography is used to guide surgical resection by analyzing the integrity of white matter fiber trajectory in order to determine whether there is tumor invasion or tumor displacement of the adjacent white matter tracts.
FA represents the degree of directionality of water diffusion and in the normal brain reflects the presence of intact myelinated white matter tracts. In brain tumors, disrupted cellular architecture results in altered FA that correlates to cellularity. Longer progression-free survival and overall survival were seen in glioblastoma patients in whom more DTI abnormality was resected. Additionally, FA has been reported to be increased in the infiltrative peritumoral edema surrounding high-grade gliomas as compared to the vasogenic edema surrounding metastases.
Often, tumor boundaries are not clearly delineated by conventional imaging, and DTI tractography may improve border characterization leading to greater resection and improved outcomes. The identification and preservation of white matter tracts is also important in preserving the neurological functional integrity of patients undergoing resection of lesions near eloquent cortex [2].
5.7.1 Diffusion Kurtosis Imaging
With free diffusion, the distribution of diffusion-driven molecular displacements obeys the Gaussian law. In biologic tissue, the complex microstructures in biological tissue result in hindered and restricted diffusion of water molecules, and which leads to a non-Gaussian distribution. The non-Gaussianity of water diffusion is thought to depend on cell membranes, organelles, and water compartments which represent the microstructure of the tissue. DKI has been used to measure non-Gaussian diffusion which has the potential to characterize both normal and pathologic tissue better than diffusion tensor imaging. Compared to the DTI which describes the unrestricted but hindered anisotropic diffusion of water protons, DKI reflects unrestricted diffusion determined by the cytoarchitectonic complexity and can measure the degree of tissue organization. The mean kurtosis (MK), which is one of the principle DKI parameters, is thought to be an index of microstructural complexity. Increased values of the kurtosis parameters in high-grade gliomas may reflect a higher degree of tissue complexity resulting from tumor invasion, increased tumor cellularity, necrosis, hemorrhage, and endothelial proliferation. Whereas low-grade gliomas usually have relatively homogeneous areas of tumor cells with sparse tumor-cell density, and thus resulting in lower kurtosis parameter values [6].
5.8 Functional MRI
fMRI indirectly measures neuronal activity using the ratio of deoxyhemoglobin to oxyhemoglobin as a contrast mechanism, known as blood oxygen level-dependent (BOLD) signal. fMRI can be used for sensory motor, language, and memory mapping, all of which have important implications for presurgical planning and intraoperative navigation.
In task-based fMRI, the patient alternates between a passive resting state and task performance, usually motor or language function, while relative changes in BOLD signal are measured and used to infer areas of cortical activation. Anatomic areas localized with task-based fMRI have been validated to approximate functional sites identified with cortical stimulation mapping. Apart from localizing eloquent cortex, task-based fMRI can be used to characterize tumors. Decreased BOLD signal is noted in cortex involved by tumor and differences are also seen between high- and low-grade tumor suggesting alterations in cerebral blood volume of the tumor affected area.
Recently, there has been increased interest in resting-state functional MRI (rs-fMRI), which does not require patient cooperation with task paradigms and can be performed under anesthesia. rs-fMRI detects spontaneous low-frequency fluctuations in the BOLD signal between regions that are spatially distinct to identify functional networks, so-called resting-state networks (RSNs).
The most fundamental RSN is the default mode network (DMN) and evidence regarding other RSNs including somatosensory, visual, auditory, language, attention, and cognitive control networks is evolving. Compared with task-based fMRI, rs-fMRI has the ability to identify many networks simultaneously, thereby providing more comprehensive information on the functional architecture of the brain while reducing imaging time [2, 10].
5.9. MRI Molecular Characterization
The 2016 WHO classification of central nervous system tumors represented a substantial change in comparison to the 2007 predecessor for defining and categorizing intracranial malignancies. The 2016 guidelines, for the first time, specified the importance of molecular findings, in addition to histology, for the identification of tumor types.
Most clinically relevant molecular markers are the following [1, 6,7,8,9].
5.9.1 Codeletion of 1p/19q
The 1p/19q co-deletion in oligodendroglioma is a prognostic factor for better chemoradiotherapy response and longer survival. An indistinct tumor border on T1W and T2W images and heterogeneous signal intensity on T2W images are some of the conventional MR features that have been correlated with 1p/19q co-deletion status. 1p/19q co-deleted tumors also appear to have higher rCBV than their non-deleted counterparts [1].
5.9.2 Isocitrate Dehydrogenase Mutation
Mutations of the IDH gene are associated with improved survival irrespective of tumor grade. They are found in secondary but only rarely in primary GBM.
Several studies have suggested that IDH1 mutant gliomas have a predilection for the frontal lobe and have larger volumes of nonenhancing tumor. Low-grade wildtype IDH1 glioma may be more infiltrative than IDH1 mutant tumors. rCBV is higher in IDH1 wildtype GBM than in IDH1 mutants.
IDH1 mutations cause the production of 2-hydroxyglutarate (2-HG), an oncometabolite that can be detected with MR spectroscopy.
The presence of 2-HG is highly specific for IDH1 mutant tumors. In a recent study, MRS was used to measure 2-HG in patients with IDH mutant gliomas before and after radiochemotherapy. 2-HG levels declined significantly on posttreatment scans raising the possibility that measurements of 2-HG levels could be a biomarker of drug response [1].
5.9.3 MGMT Promoter Methylation
Methylation of the MGMT repair protein promoter inhibits transcription of MGMT, increases sensitivity to TMZ, and positively impacts patient prognosis. Tumor features derived from standard and advanced MRI have been investigated as a way of noninvasively identifying MGMT promoter methylation status. For instance, ring enhancement is associated with nonmethylated MGMT promoter.
A study of 43 GBM patients using DCE-MRI found that MGMT methylated tumors had increased permeability (higher Ktrans) compared to unmethylated tumors.
A recent study combined both structural and physiologic MR imaging found that MGMT promoter methylation was associated with increased ADC and decreased rCBF (from ASL imaging) [1].
5.10 Imaging of Treatment Response
Assessing brain tumor treatment response by MRI presents considerable challenges such as differentiating progression from treatment-related changes.
Therapies can either mimic or mask disease progression, serial imaging is often the most helpful and reliable noninvasive method to assess disease activity.
Brain tumor follow-up after treatment imaging reflects both treatment effect and natural evolution of tumor [1, 2, 10].
5.10.1 Pseudoprogression
Pseudoprogression consist of inflammatory response marked by a transient increase in contrast enhancement and edema upon completion of chemoradiotherapy, which is observed in up to 30% of high-grade glioma patients and can also be seen in the setting of low-grade glioma. It occurs within the first 3 months following therapy and more frequently in tumors harboring O6-methylguanine DNA methyltransferase (MGMT) promoter methylation.
Since both pseudoprogression and true tumor progression share pathophysiology characterized by an underlying disruption of the BBB, it is difficult to differentiate using conventional imaging.
5.10.2 Pseudoresponse
Pseudoresponse represents a marked decrease in contrast enhancement on MRI related to diminished leakiness of the BBB following treatment with antiangiogenic agents, most commonly bevacizumab, in patients with recurrent glioblastoma. The marked decrease in contrast enhancement, and often in peritumoral edema, can be observed as early as 1 day after initiation of antiangiogenic therapy and does not necessarily reflect biological antitumor effect of therapy.
Antiangiogenic agents may select for a hypoxic and invasive tumor phenotype that is capable of co-opting existing vasculature and therefore growing as nonenhancing infiltrative tumor before manifesting as progressive enhancing disease.
Decreased enhancement should persist for greater than 4 weeks to be considered a true response.
5.10.3 Long-Term Complications of Therapy
Several other chronic changes attributable to brain tumor therapy are well cataloged. Symmetric white matter signal abnormality representing gradual demyelination, gliosis, and vascular injury following chemotherapy, radiotherapy, or both is associated with progressive neurocognitive decline and disordered white matter diffusion. In extreme cases, a diffuse necrotizing leukoencephalopathy can develop following intrathecal chemotherapy without or with radiotherapy.
Rarely, patients with a remote history of intracranial irradiation present with headaches and neurological deficits and are found to have abnormal cortical enhancement.
References
Pope WB, Brandal G. Conventional and advanced magnetic resonance imaging in patients with high-grade glioma. Q J Nucl Med Mol Imaging. 2018;62(3):239–53.
Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017;81(3):397–415.
Fahrendorf D, Schwindt W, Wölfer J, et al. Benefits of contrast-enhanced SWI in patients with glioblastoma multiforme. Eur Radiol. 2013;23(10):2868–79.
Hsu CC, Watkins TW, Kwan GN, et al. Susceptibility weighted imaging of glioma: update on current imaging statusand future directions. J Neuroimaging. 2016;26(4):383–90.
Hori M, Ishigame K, Kabasawa H, et al. Precontrast and postcontrast susceptibility-weighted imaging in the assessment of intracranial brain neoplasms at 1.5 T. Jpn J Radiol. 2010;28(4):299–304.
Kim M, Kim HS. Emerging techniques in brain tumor imaging: what radiologists need to know. Korean J Radiol. 2016;17(5):598–619.
Patel SH, Poisson LM, Brat DJ, et al. T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res. 2017;23(20):6078–85.
Batchala PP, TJE M, Donahue JH, et al. Neuroimaging-based classification algorithm for predicting 1p/19q-codeletion status in IDH-mutant lower grade gliomas. AJNR Am J Neuroradiol. 2019;40(3):426–32.
Delfanti RL, Piccioni DE, Handwerker J, et al. Imaging correlates for the 2016 update on WHO classification of grade II/III gliomas: implications for IDH, 1p/19q and ATRX status. J Neurooncol. 2017;135(3):601–9.
Hyare H, Thust S, Rees J. Advanced MRI techniques in the monitoring of treatment of gliomas. Curr Treat Options Neurol. 2017;19(3):11.
Acknowledgments
The current chapter is a revision made by Saverio Pollice, Michela Capuano, and Tommaso Scarabino of the original chapter written by Saverio Pollice, Teresa Popolizio, and Tommaso Scarabino in the previous edition of the book.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pollice, S., Capuano, M., Scarabino, T. (2020). Magnetic Resonance Technique. In: Scarabino, T., Pollice, S. (eds) Imaging Gliomas After Treatment. Springer, Cham. https://doi.org/10.1007/978-3-030-31210-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-31210-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31209-1
Online ISBN: 978-3-030-31210-7
eBook Packages: MedicineMedicine (R0)