Abstract
The immune system provides protection against infections and toxins and serves as a guard for all tissues against pathogens and malfunctioning cells. Modulation of the immune system to use immune cells as therapeutic agents has been an important target for the treatment of many diseases that could not be cured otherwise such as cancer and autoimmune diseases. In addition, modulation of the immune system is crucial for tissue regeneration, since tissue regeneration and wound healing processes consist of a complicated and ordered array of events, a considerable part of which include the involvement of immune cells. Nanomaterials in the form of nanoparticles and nanofibers provide a wide array of tools for modulation of the immune system. Different types of nanomaterials have been developed to be used for effective targeting and treatment of cancer, as vaccines, and for the treatment of autoimmune disorders. In this chapter, different nanomaterials with immunomodulatory effects will be reviewed with an emphasis on cancer, autoimmune diseases, and vaccine development. In addition, future perspectives for developing materials with more refined immunomodulatory characteristics will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
The immune system protects against infections and toxins, and plays crucial roles in the regeneration of the tissues after injury, including fighting against pathogens that might attack the regenerating tissue; removal of debris of the wounded and scar tissues; and degradation of the materials that are used for regenerative purposes. It is composed of many different types of cells and lymphoid organs that are tightly regulated and plays an invaluable role in the defense against invading microbes (Moon, Huang, & Irvine, 2012). Spleen, nasal-associated lymphoid tissue, Peyer’s patches in the gut, and lymph nodes distributed throughout the body are all parts of the immune system and immune cells are generated at the thymus and bone marrow.
There are two major types of immune response which are innate and adaptive immunity. Innate immunity is nonspecific and acts as the initial defense against invading microorganisms and foreign particles. Macrophages, dendritic cells, neutrophils, and mast cells perform phagocytosis to destroy the foreign cells and release cytokines. Adaptive immunity plays a role as the second line of defense. In that response, antigen presenting cells (APCs) bring specific antigens to highly specialized T cells and B cells. Antigen is recognized and an immune response that is specific to the invading microorganism is elicited and the target is cleared (Hussain, Vanoirbeek, & Hoet, 2012; Norman, 2005).
The immune system is important in the fight against diseases; however, incorrect regulation of the immune response, immunosuppression and immunostimulation may also lead to pathological conditions (Norman, 2005). Immunosuppression is the state in which the immune system functions are decreased and the response is weakened, which may result in invasion by the pathogens or rapid growth of tumor cells. Immunostimulation enhances the immune response, overactivity of which may lead to a strong adverse response and may result in autoimmune disorders. Since immune system is a very complex mechanism and is very tightly regulated, any effect, inhibition or activation of a pathway may cause unexpected side effects to other pathways or different cells or tissues. Thus, the inflammatory agents are used in a controlled manner due to possible side effects (Chou et al., 2013).
Vaccines are great examples of protection against diseases in which the immune system is stimulated to protect individuals from infectious microbial organisms (Pulendran & Ahmed, 2011). Although there are very successful vaccines against various diseases, there is still a need for vaccines against many other severely infectious pathogens, such as HIV, malaria, tuberculosis, and hepatitis C. Current vaccine development approaches are centered on rational design to have more potency and less immunogenicity, and the number and types of vaccine candidates are increasing rapidly (Mamo & Poland, 2012; Oberg, Kennedy, Li, Ovsyannikova, & Poland, 2011; Rappuoli, Mandl, Black, & De Gregorio, 2011).
Immune system may also provide protection from tumors by using cancer vaccines to stimulate the immune system to inhibit tumors (DeMaria & Bilusic, 2019; Hodi et al., 2010; Kalos et al., 2011; Lollini, Cavallo, Nanni, & Forni, 2006; Williams et al., 2011). Cancer immunotherapy is a popular field that aims to develop novel cancer therapy approaches by understanding and utilizing immune pathways (DeMaria & Bilusic, 2019). On the other side of the spectrum, over-stimulation of the immune system may cause autoimmune disorders that require suppression of the immune system (Feldmann & Steinman, 2005).
Immunomodulatory drugs have been highly coveted for balancing the immune system for autoimmune diseases or specifically enhancing certain immune cells for protection against or treatment of cancer or other infectious diseases and for aiding tissue regeneration. However, due to the complexity of the immune system, using a single-agent immunomodulatory drug may result in severe side effects. Nanomaterials are suggested to be ideal for selective delivery of immune regulating molecules.
4.2 Nanomaterials and Immune System
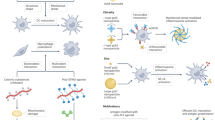
Nanotechnology gives the scientists ability to design nanomaterials at different sizes, shapes, chemical and physical properties and composition for use in the treatment of different diseases (Couvreur & Vauthier, 2006; Hubbell, Thomas, & Swartz, 2009; Mamo & Poland, 2012; Moghimi, Hunter, & Murray, 2005; L. Zhao et al., 2014). Nanomaterials have been the subject of research for being used as drug delivery vehicles or adjuvants for vaccines (Hubbell et al., 2009; Kasturi et al., 2011; Moon et al., 2011; Reddy et al., 2007). There is also interest in use of them in diagnostic systems (Cho et al., 2011; Noh, Jang, Ahn, Lim, & Chung, 2011). Moreover, they may be used for the treatment of cancer (Hellstrom et al., 2001; Kalos et al., 2011; Steenblock & Fahmy, 2008) and as delivery vehicles for immunotherapy drugs (Ali & Mooney, 2010; Peer et al., 2007; Svenson, 2012). The shape, size and surface properties of the nanoparticles can be engineered to aid in cell specific responses (Treuel, Jiang, & Nienhaus, 2013). An important advantage of the nanoparticles is their ease of functionalization through adding or removing properties.
Nanomaterials with immunomodulatory properties are highly coveted for regenerative medicine applications to enable the immune cells to work in a controlled manner. Depending on the material that they are made of, nanoparticles might be perceived as foreign materials when they enter the body, eliciting an immune response in the form of either suppression or stimulation (Dobrovolskaia & McNeil, 2007). Immunomodulatory properties of nanoparticles may be used in development of vaccines or anti-allergy drugs (Parween, Gupta, & Chauhan, 2011; Ryan et al., 2007).
There is ongoing research on targeted delivery of nanoparticles to specific tissues and cells for treatment or diagnosis. Nanoparticles should be relatively stable in the body and should not elicit immune response to prevent degradation, but they should also reach the target site and stay there. Nanoparticles may accumulate in the desired tissue through blood vessels or bind to specific biological structures at the target site (Pelaz et al., 2017). Also, nanoparticles may be tailored to prevent nonspecific binding. Recent advances in nanoengineering methods allowed production of nanoparticles that may be used to target diseased tissues, in diagnostic applications or to regulate the immune system to produce vaccines, inhibit tumors or prevent autoimmunity.
In the following sections, we will describe how nanomaterials can be used for different immunomodulatory purposes and for aiding immunotherapeutics. We will also summarize recent developments in the design and implementation of immunomodulatory particles.
4.3 Nanomaterials for Immunotherapy of Cancer
According to World Health Organization data, cancer is the second leading cause of death in the world, and approximately 1 in 6 deaths is caused by cancer. The economic burden of cancer among both developed and under-developed countries is also devastating, and even though an incredible amount of effort has been spent on cancer research and therapy, the currently available methods are still limited, which causes the high lethality rate of cancer.
Main methods for treating cancer are surgery, radiation and chemotherapy. Immunotherapy has recently been developed and it is suggested to be a promising approach for the treatment of various diseases including cancer, because it regulates the immune system to target and destroy cancer cells and tumors (Song, Musetti, & Huang, 2017). Immunotherapy is the regulation of the immune system (activation or suppression) for treatment of diseases. Immunosuppressive approaches may be used to reduce allergic reactions and reduce excessive inflammation in organ transplants. In contrast, in cancer patients, immune system is aimed to be more active and new methods are developed to activate the system against malignant cells. There are different approaches and materials used in cancer immunotherapy, which is expected to result in more durable antitumor responses and reduce metastasis and recurrence compared to previous treatment methods. Currently, immunotherapy is one of the most highly coveted treatment methods; however, it is also a very expensive treatment method and is not suitable for all cancer types.
4.3.1 Nanomaterials for Cancer Immunotherapy
Tumor microenvironment prevents the activity of the immune system, and tumor cells inhibit the activity of tumor-specific T-cells (Munn & Bronte, 2016; Vasievich & Huang, 2011). New research focuses on regulating the tumor microenvironment by inhibiting immunosuppressor molecules or activating soluble mediators, which in turn may elicit an immune response. Nanomaterials have the ability to activate or suppress immune system depending on their type, size, side groups, and functionalization and use of nanomaterials may reduce these side effects by achieving specific delivery to target tumors. Nanomaterials may be used to selectively distribute immune checkpoint regulators to the tumors to inhibit their growth. For example, PLGA nanoparticles that were conjugated with anti-OX40 mAb resulted in stronger cytokine production and increased overall antitumor cytotoxic cell response when injected into the tumor compared to the administration of free antibody (M. Chen, Ouyang, Zhou, Li, & Ye, 2014). Targeted inhibition of cytokines using various approaches is also important for preventing the immunosuppressive environment of the tumor. Park et al. developed nanoscale liposomal polymeric gels (nanolipogels, nLGs) for co-delivery of IL-2 and small molecule TGF-β receptor-I inhibitor SB505124 for this purpose (Park et al., 2012).
Targeted delivery to tumors may also allow the use of highly potent drugs with reduced side effects. Intratumoral injection of anticancer antibodies from functionalized nanoporous silica inhibited tumor growth more efficiently and at a longer duration than systemic antibody injections (Lei et al., 2010). In another study, anti-CD137 mAb and engineered IL-2Fc fusion protein that were anchored to PEGylated liposomes prevented lethal toxicity and increased systemic antitumor immunity compared to free anti-CD137 antibodies. In this study, it was also determined that the size of the nanomaterials is important. The nanoparticles that are small enough to reach into the tumor but large enough to join the systemic circulation were found to be highly efficient (Kwong, Gai, Elkhader, Wittrup, & Irvine, 2013). Heo et al. found that after injection with immunomodulating oligodeoxynucleotides or siRNA encapsulated PLGA’s in addition to chemotherapy, dendritic cells become active and migrate to the tumor-draining lymph nodes. This combination therapy using immunomodulatory nanomaterials inhibited tumor growth and increased the survival rate (Heo, Kim, Yun, & Lim, 2015).
Cell therapy is an alternative for treatment of many diseases; however, it has major limitations such as loss of function of the transplanted cells. A novel approach uses nanoparticles, created from liposomes and liposome-like synthetic nanoparticles 100–300 nm in diameter with a drug-loaded core and phospholipid surface layer, which carry adjuvants. These nanoparticles continuously stimulate donor cells and were shown to increase the efficiency of tumor elimination (Stephan, Moon, Um, Bersthteyn, & Irvine, 2010).
4.3.2 Cancer Vaccines
There are two types of cancer vaccines, therapeutic and prophylactic. Prophylactic vaccines are used to prevent cancers such as the hepatocellular carcinoma secondary to hepatitis B virus and squamous cell carcinoma secondary to human papillomavirus (HPV) (DeMaria & Bilusic, 2019). Therapeutic vaccines, on the other hand, are aimed to treat cancer.
Tumor cells express a variety of antigens, some of which are specific to them, and are not produced by healthy cells. In earlier studies, the cancer vaccines were developed with whole-cells together with adjuvants to target tumor cells. However, because of a need to develop more potent vaccines with fewer side effects, current research focuses on antigens that are specific to tumors (Herlyn & Birebent, 1999). Some of these targets are products of mutated oncogenes (p53, ras, PSA, GP-100, MART-1, B-raf). For cancer to occur, the cells have to have many mutations and escape through many checkpoints, thus every tumor has a different composition. Therefore, a personalized approach may be more effective for cancer treatment, and new developments in genomics allow scientists to determine the specific mutations in the patients. Together with the developments in nanotechnology, this information may be used to customize a specific therapeutic approach for each patient. Initial clinical trials of personalized cancer vaccines have shown the feasibility, safety, and immunotherapeutic activity of targeting individual tumors (Sahin & Türeci, 2018).
Cancer vaccines may not only target different antigens and immune adjuvants, but also use different vaccine platforms, such as peptides/proteins, whole tumor cells, recombinant vectors, dendritic cells (DCs), gangliosides, and genes (DeMaria & Bilusic, 2019). Cancer cells may also be coated with nanoparticles conjugated with tumor antigens to elicit an immune response (Fang et al., 2014). There are already approved cancer vaccines for treatment against early-stage bladder cancer, mCRPC, and metastatic melanoma, such as TheraCys® and TICE®, PROVENGE, and IMLYGIC®, and several new vaccines are undergoing clinical trials.
Cancer vaccines are promising; however, their impact in metastatic carcinomas is not very high. Cancer vaccines need to work in synergy with other therapeutic approaches to inhibit local (tumor-site) immune response and act as an immunosuppressive agent while stimulating antitumor response. Nanoparticles are very important in that respect, since they may be tailored for different functions.
There is an increased focus on vaccine development by using nanoparticles and nanotechnology methods. Tailored nanoparticles may have fewer side effects and can be more effective. Nanoparticle use in vaccines may protect antigens, allow for more specific targeted delivery and result in sustained slow release. The nanoparticle vaccine field is developing very rapidly and promising; however, there are some problems that need to be addressed, an important one of which is the lack of understanding of the behavior of nanoparticles in the body, when they are used as a shuttle system or an adjuvant in vaccines (L. Zhao et al., 2014).
Nanomaterials may be used for encapsulating antigen and adjuvants, protecting them from degradation and to increase the efficiency of T-cell response (Irvine, Hanson, Rakhra, & Tokatlian, 2015; Irvine, Swartz, & Szeto, 2013; Zhu, Zhang, Ni, Niu, & Chen, 2017). Some carbon-based nanomaterials, such as carbon nanotubes and graphene, might affect immune cells by specifically activating them and initiate an antitumor immune response (Orecchioni et al., 2014; Pescatori et al., 2013; Xu et al., 2013). In one example, PC7A nanoparticles were shown to deliver tumor antigens to cytosol and activating the stimulator of interferon genes (STING) pathway (Luo et al., 2017).
4.4 Nanomaterials for Development of Vaccines
Vaccines are microbial antigens or attenuated/killed microbes administered together with an adjuvant to induce antigen-specific immune responses that result in long-lasting immune memory against specific pathogens. In autoimmune disorders, the vaccine should work in an opposite manner: to inhibit immune responses against self-cells without affecting the capability of the immune system to act against foreign organisms or materials or cancer cells (Clemente-Casares, Tsai, Yang, & Santamaria, 2011).
The size of the particle effects the time required for drainage of that particle into the lymph node (Manolova et al., 2008). When antigens were covalently bound to nano-beads, the immune response differed with respect to the size of the nano-beads (Fifis et al., 2004). Nanoparticle size is also important in delivery. Amorphous silica nanoparticles that are greater than 100 nm have more difficulty in entering into cytosol compared to smaller nanoparticles (between 70 and 10 nm) (Hirai et al., 2012).
Dendritic cells ingest nanoparticles at varying efficiency. The rate of uptake differs by size, charge and hydrophobicity. Nanoparticles bigger than 500 nm are ingested at low rates (Foged, Brodin, Frokjaer, & Sundblad, 2005). Nanoparticles with positive charge are ingested more efficiently compared to neutral or negatively charged particles (Wischke, Borchert, Zimmermann, Siebenbrodt, & Lorenzen, 2006).
An advantage of nanoparticles is that they may be functionalized through addition of receptors on their surface to increase efficiency of delivery. Use of Fc receptors to deliver antigens to human dendritic cells have been suggested to be successful, by using intact antibodies or engineered fragments (Cruz et al., 2011; Mi et al., 2008).
Another advantage of nanoparticle vaccines is that they enter the APC via phagocytosis, unlike soluble antigens which enter by micropinocytosis. Thus nanoparticle vaccines present antigens more efficiently, which may also result in stronger immune responses (H. Shen et al., 2006).
Delivery of vaccines is another important subject. Several nanocarrier systems have been investigated for vaccine delivery, such as liposomes. Liposomes are important because of their adaptability and flexibility for use in different applications (Schwendener, 2014). Liposomes may be used to encapsulate different types of nanoparticles. Hydrophilic particles may be carried inside the liposome, while hydrophobic particles may be incorporated into the lipid bilayer. Liposomes may also be adjusted regarding their components, size and charge.
As a delivery system, nanoparticles may activate immune system by directly delivering antigen to the immune system cells or perform targeted delivery (Girija & Balasubramanian, 2018; Mody et al., 2013). To function as immunomodulators, nanoparticles may be tailored to activate immune pathways which might then enhance or inhibit antigen processing and immunogenicity. Gold and silica nanoparticles have been analyzed for their potential for use as cargo delivery system (Brito & O’Hagan, 2014; Shah, O’hagan, Amiji, & Brito, 2014). They may be tailored to strongly bind to antigens, co-deliver adjuvants and multi-epitope antigens into lymphoid organs and into antigen-presenting cells (Zhu et al., 2017). The antigens can be attached to nanoparticles by encapsulation, conjugation, or adsorption (L. Zhao et al., 2014). Adsorption is not very strong, and relies on electrical charge or hydrophobicity, which may lead to rapid dissociation of the antigen and the nanoparticle in vivo. Encapsulation and chemical conjugation results in stronger binding of the nanoparticle to the antigen (Pati, Shevtsov, & Sonawane, 2018). The antigen is released when the carrier particle is ingested and degraded by the cell. In chemical conjugation, antigen is coupled irreversibly to the nanoparticle (Andersson, Buldun, Pattinson, Draper, & Howarth, 2019). New research may focus on soft-matter nanoparticles that are based on emulsions which work as adjuvants when they are given into the body independently of the antigen, that is, no attachment (Morel et al., 2011; O’Hagan, Ott, & Van Nest, 1997). Further studies are required to fully understand the effects of these molecules on each other and to the immune system.
4.4.1 Nanoparticle Interactions with Antigen Presenting Cells
For the vaccines to work efficiently, antigens must be delivered to antigen presenting cells, dendritic cells and macrophages, so that these cells are activated and an immune response is elicited (Jones, 2008; Reddy, Swartz, & Hubbell, 2006). Interactions of these cells with nanoparticles and mechanisms of delivery into the cells are important for rational design of vaccines (Kumari & Yadav, 2011). Also, scientists have to be aware of the possible changes to the nanoparticle behavior with the changing size, shape, side-group, and overall functionalization (Khong et al., 2018; Xiang et al., 2006). For example, PLGA particles that are 300 nm in diameter were transported into the cells at a higher rate compared to 1, 7 and 17 μm particles (Joshi, Geary, & Salem, 2013). Internalization of positively charged polystyrene nanoparticles to the dendritic cells was also higher, possibly due to the interactions with the anionic cell membranes (Foged et al., 2005; Y. Shen, Hao, Ou, Hu, & Chen, 2018). However, it was not shown that this increased internalization is correlated with more potent immune response.
In addition, nanoparticles have been increasingly used to deliver not only antigen of interest but also co-adjuvants, such as poly(I:C), CpG and MPL (De Temmerman et al., 2011; Hafner, Corthésy, & Merkle, 2013). However, more work is needed in nano-vaccine research to overcome challenges including synthesizing nanoparticles that are cheap, uniform, and consistent, that are functionalized with desired properties, ability to target the particle to desired location, with fewer side effects. Thus, rational design of these nanoparticles with respect to the disorder is imperative. New technologies such as micro- and nanofluidics are also important for more efficient analyses of the effects of nanoparticles on different cell types (Hong, Lu, Liu, & Chen, 2019).
4.4.2 Polymeric Nanomaterials for Vaccine Development
Synthetic polymers have different compositions and properties and some of the most widely used ones to prepare nanoparticles are poly(d,l-lactide-co-glycolide) (PLG) (Kim et al., 1999; Köping-Höggård, Sánchez, & Alonso, 2005; C. Thomas, Rawat, Hope-Weeks, & Ahsan, 2011), poly(d,l-lactic-coglycolic acid) (PLGA) (Demento et al., 2012; Lü et al., 2009; Manish, Rahi, Kaur, Bhatnagar, & Singh, 2013; Silva et al., 2013), poly(g-glutamic acid) (g-PGA) (Akagi, Baba, & Akashi, 2012; Akagi, Kaneko, Kida, & Akashi, 2005), poly(ethylene glycol) (PEG) (Köping-Höggård et al., 2005), and polystyrene (Kalkanidis et al., 2006; Minigo et al., 2007). PLG and PLGA nanoparticles have high biocompatibility and biodegradability which are needed for use in drug delivery (D’souza et al., 2014; Danhier et al., 2012).
Importance of polymeric nanoparticles comes from their ability to deliver antigens to target cells or provide sustained release of their cargo antigens. These polymeric nanoparticles entrap antigens for delivery to certain cells or sustain antigen release by virtue of their slow biodegradation rate (Danhier et al., 2012). PLGA nanoparticles were shown to shuttle antigens against Plasmodium vivax with mono-phosphoryl lipid A as adjuvant (Moon, Suh, et al., 2012), hepatitis B virus (HBV) (C. Thomas, Rawat, et al., 2011), Bacillus anthracis (Manish et al., 2013), and model antigens such as ovalbumin and tetanus toxoid (Demento et al., 2012; Diwan, Tafaghodi, & Samuel, 2002).
Cationic alginate-polyethylenimine (PEI) nanogels have been proposed to be used as a vaccine delivery system (Li et al., 2013). Compared with the empty nanogels, nanoparticle loaded nanogels enhanced vaccine-induced antibody production more efficiently, showing the potential of this approach to be used to enhance vaccine-elicited humoral and cellular immune responses.
4.4.3 Metal Nanoparticles
Metal nanoparticles are widely used for different applications. They have well developed and controllable synthesis methods with precise sizes and shapes, and they can also be readily functionalized (Kalkanidis et al., 2006; L. Zhao et al., 2014).
Gold nanoparticles have been used in several studies for vaccine development (L. Zhao et al., 2014). The size of gold nanoparticles usually ranges from 2 to 150 nm, and they may be given spherical, rod or cubic shapes (Gregory, Titball, & Williamson, 2013; Niikura et al., 2013). In one study, the highly conserved extracellular region of the matrix 2 protein of influenza A virus was conjugated to gold nanoparticles to treat influenza. Intranasal administration of this nanoparticle system induced matrix 2 protein specific IgG serum antibodies (Tao, Ziemer, & Gill, 2014). Stone et al. fabricated gold nanorods which were attached to the respiratory syncytial virus by covalent binding of a viral protein. This gold nanorod construct contained the major protective antigen of the virus, the fusion protein (F), and was able to successfully induce immune response (Stone et al., 2013). In another study, gold nanoparticles were attached to a synthetic peptide resembling foot-and-mouth disease virus protein, with sizes ranging from 2 to 50 nm. These nanoparticles activated the antibody response at different strengths (Y. S. Chen, Hung, Lin, & Huang, 2010). Xu et al. conjugated the surface of the gold nanorods with cetyltrimethylammonium bromide (CTAB), poly(diallydimethylammonium chloride) (PDDAC), and PEI. PDDAC- or PEI-attached gold nanoparticles with DNA stimulated stronger immune response compared DNA only approach (Xu et al., 2012).
Surfaces of gold or iron oxide may also be functionalized by coating them with sugar molecules (glyconanoparticles). Carbohydrates and other molecules may be attached to metal nanoparticles, and the core may be filled with magnetic or fluorescence molecules (Marradi, Chiodo, García, & Penadés, 2013).
Some inorganic materials such as silica-based nanoparticles are biocompatible and are being investigated as nanovaccine constituents. Mesoporous silica nanoparticles may be tailored for targeted release of antigens by changing properties such as the shape, size and surface functionalization (Manzano et al., 2008). These mesoporous silica nanoparticles can carry more cargo compared to solid silica nanoparticles, and could be regulated to release the cargo in a controlled manner by changing mesoporous structures. Mesoporous silica nanoparticles are promising candidates for use in vaccines due to their controlled-release abilities.
4.4.4 Carbon-Based Nanomaterials
Carbon nanoparticles have been studied for their use in vaccine delivery (Gregory et al., 2013; T. Wang et al., 2011). Particles which were 450 nm in size and with 50 nm mesopores on the particle surface were produced. These pockets would be used to transport protein antigen, protecting the antigen and allowing oral administration (T. Wang et al., 2011). They are tolerated well inside the body and were given different shapes including mesoporous spheres (Bianco, Kostarelos, & Prato, 2005; Gupta et al., 2015). Carbon nanotubes are pure carbon molecules and are generally 0.8–2 nm in diameter with a length of 100–1000 nm (Parra, Abad-Somovilla, Mercader, Taton, & Abad-Fuentes, 2013; Villa et al., 2011).
4.4.5 Biological Materials for Vaccine Development
Proteins, peptides, carbohydrates, nucleic acids, and liposomal systems have all been proposed to be used for developing effective vaccines for various purposes. They can be used in the form of either nanoparticles and nanofibers or hydrogels. Recently, nanoparticles are proposed to be synthesized by self-assembling of proteins that assemble to form higher level quaternary structures. By using protein structure information, self-assembling nanoparticles that resulted in stronger immune response than influenza vaccines were designed (Kanekiyo et al., 2013). In this study, the viral hemagglutinin was genetically fused to ferritin, which is a protein composed of 24 identical polypeptides that can self-assemble into spherical 10 nm particles. This nanoparticle vaccine elicited hemagglutination inhibition antibody titers that were more than tenfold higher compared to the traditional vaccine. The advantages of protein nanoparticles in vaccine development include having highly organized structures and symmetry, biodegradability, and tailorability at three different interfaces and size (Neek, Kim, & Wang, 2019).
Vault nanoparticles are self-assembled to form an ellipsoid that contains an empty region inside (Buehler et al., 2014). Recent work which focused on the use of vault nanoparticles to carry antigens showed that these nanoparticles elicited potent immune response, that may also be useful in cancer vaccines (Kar et al., 2012). A new class of monodispersed, self-assembling vault nanoparticles comprises a protein shell exterior with a lipophilic core interior and these recombinant vaults contained a small amphipathic α-helix obtained from hepatitis C virus. This design resulted in a small area in which lipophilic compounds would be encapsulated and delivered to target cells (Buehler et al., 2014).
Liposomes are spherical structures composed of phospholipid bilayers with a core that may be used for antigen delivery. Liposomes are biodegradable, and range from 20 nm to 1 μm in size. Liposomes may be used as delivery systems for vaccines by encapsulation, adsorption, or surface coupling of antigens (Giddam, Zaman, Skwarczynski, & Toth, 2012). Liposomes do not have immunostimulatory or immunosuppression activity, so they are combined with adjuvants for vaccine development. An in-depth analysis of liposomal interaction with the immune system would allow scientists to design more potent vaccines. Liposomes may be used to deliver DNA vaccines or virosomes that contain viral envelope glycoproteins (Glück, Moser, & Metcalfe, 2004; Khatri et al., 2008). Liposomes also allow the use of intranasal approach for delivery of vaccines (S. Sharma, Mukkur, Benson, & Chen, 2009).
Recombinant vaccines using proteins have very little toxicity but their potency is not high. In one study, an interbilayer-cross-linked multilamellar vesicle system was produced by cross-linking headgroups of adjacent lipid bilayers. These vesicles internalize antigens in the core and immunostimulatory adjuvants on the vesicle walls. These vesicles were shown to have potency, eliciting very strong endogenous T-cell and antibody responses (Moon et al., 2011).
4.5 Nanomaterials for the Treatment of Autoimmune Diseases
Autoimmune disease is the name of around 80 disorders that share a common etiology: an immune attack on the body’s own cells. There are self-reactive B-cells and T-cells that recognize and attack the self-antigens. During T-cell development, self-reactive cells are normally deleted, and problems in removal or inhibition of these self-reactive cells may lead to autoimmunity (Notarangelo, Gambineri, & Badolato, 2006).
The prevalence of autoimmune diseases is increasing in industrialized countries, which may be due to environmental changes, such as air quality. There are no successful cures for these diseases. The disease progresses slowly and organ and tissue damage occur before diagnosis. To battle against autoimmune diseases, inhibitors against immunostimulatory molecules may be used, such as monoclonal antibodies or small receptor blockers. These inhibitors downregulate or result in the degradation of immunostimulatory agents.
Scientists have tried to develop techniques to transport anti-inflammatory drugs to target immune cells in affected tissues and inhibit or limit their pathological effects. For the treatment of autoimmune diseases, it would be extremely important to inhibit the T-cells that attack the body’s own tissues, but not effect T-cells in other tissues. A wide and very strong immunosuppression would result in infections. In the immune system, T-cells play a very critical role in the defense against diseases or degradation of tumor cells. However, they may be responsible from self-tissue damage in autoimmune disorders. Thus, it is imperative that T-cell activity is regulated to develop successful strategies against autoimmune diseases. This may be done by converting T-cells into other types, redirecting their program, such as conversion of effector cells to regulatory T-cells (Moon, Huang, & Irvine, 2012; O’Shea & Paul, 2010; Rose, 2016). Nanoparticles would be very important in these types of altering function activities. By specifically tailoring the abilities of the drugs, nanoparticles may regulate these cells (Park et al., 2011). Metal nanoparticles, liposomal systems, biomaterial-based nanomaterials and carbon-based nanoparticles have been widely studied for this purpose.
4.5.1 Carbon-Based Nanoparticles
Carbon nanotubes are formed by carbon atoms that arrange forming a two-dimensional hollow cylinder. Carbon nanotubes were shown to induce systemic immunosuppression in mice (L. A. Mitchell et al., 2007; Leah A. Mitchell, Lauer, Burchiel, & McDonald, 2009; Tkach et al., 2011; X. Wang, Podila, Shannahan, Rao, & Brown, 2013).
Fullerene is also formed by carbon atoms and forms a closed structure that may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. It has anti-inflammatory and antioxidant effects (Magoulas et al., 2012). Fullerenes inhibit the allergic response against Ag-driven type I hypersensitivity by decreasing the level of reactive oxygen species (ROS) (Ryan et al., 2007). Fullerene derivatives may defend against oxidative stress in ischemia-reperfused lungs (Y.-W. Chen, 2004). Fullerenes were also shown to inhibit the development of arthritis in a rat model (Yudoh, Karasawa, Masuko, & Kato, 2009). In another study, hydroxylated fullerenes inhibited neutrophil function in fathead minnows (Jovanović, Anastasova, Rowe, & Palić, 2011).
4.5.2 Metal Nanoparticles
Gold nanoparticles have diverse properties and their size can be tailored to modulate their immune response and biodistribution. In gold nanoparticles, gold core is inert and nontoxic, and the nanoparticles can be manufactured with a very wide size distribution ranging from 1 to 150 nm and can be easily synthesized by various methods (C. P. Sharma, 2010). Twenty-one nanometer spherical gold nanoparticles caused no apparent organ or cell toxicity in mice, but resulted in inhibition of inflammatory effects (H. Chen et al., 2013). Five and 15 nm gold nanoparticles reduce pro-inflammatory responses induced by interleukin-1 (Sumbayev et al., 2013).
Iron oxide nanoparticles have also been used for immunomodulatory purposes. When the mice were exposed to ovalbumin and to varying doses of iron oxide submicron- or nanoparticles, allergic response was significantly inhibited. Interestingly, low doses of submicron particles had no significant effect on the allergic response while the same dose of nanoparticles had an adjuvant effect on the response to ovalbumin. This study clearly showed that the particle dose and size affect the allergic response (Ban, Langonné, Huguet, Guichard, & Goutet, 2013). Administration of iron oxide nanoparticles (58.7 nm) also suppressed T-helper 1 cell-mediated immunity in ovalbumin sensitized mice (Shen, Liang, Wang, Liao, & Jan, 2012). Compared to single instillation, repeated instillations resulted in a reduction of inflammatory cell numbers in both bronchoalveolar lavages and pulmonary parenchyma (Ban, Langonné, Huguet, & Goutet, 2012).
Cerium oxide nanoparticles have the potential to reduce reactive oxygen species production and may be used to battle chronic inflammation (Hirst et al., 2009; Schanen et al., 2013). Also, cerium oxide nanoparticles (5–8 nm) protected the cardiac progenitor cells from H2O2-induced cytotoxicity (Pagliari et al., 2012).
Quantum dots are artificial semiconductor particles that are a few nanometers in size and their distinctive conductive properties are usually determined by their size. Cadmium telluride quantum dot nanoparticles suppressed the immune responses of macrophages to Pseudomonas aeruginosa by reducing NO, TNF, KC/CXCL-1, and IL-8 levels (Nguyen, Seligy, & Tayabali, 2013). Immunosuppression was also observed in Juvenile rainbow trout. When Juvenile rainbow trout were exposed to 5, 10, and 20 nM cadmium tellurium quantum dots, each form of dots resulted in a different pattern of gene expression and lowered fish immune response (Gagné et al., 2010). Sub-toxic levels of cadmium telluride quantum dot nanoparticles were also shown to suppress immune responses against bacteria in macrophages and epithelial cells (Nguyen et al., 2013).
4.5.3 Polymeric Nanoparticles
The size, shape and material of polymeric nanoparticles have also been shown to be important for suppression of immune responses. Twenty nanometer polystyrene particles decreased the efficiency of dendritic cells to degrade soluble antigens, without affecting their ability to induce antigen-specific CD4+ T-cell proliferation. Thousand nanometer polystyrene particles did not have such effects, while 20 nm particles accumulated in the lysosomes. Size-dependent accumulation of particles in lysosomes modulates dendritic cell function through impaired antigen degradation (Seydoux et al., 2014).
When dendritic cells (DCs) were treated with model biomedical poly(vinyl alcohol)-coated super-paramagnetic iron oxide nanoparticles (PVA-SPIONs), they were observed to exhibit decreased antigen processing capacity and CD4+ T-cell stimulation capacity (Blank et al., 2011).
Inert 50 nm polystyrene nanoparticles were also shown to inhibit allergic lung inflammation by modification of pulmonary dendritic cell function (Hardy et al., 2012). Particles composed of PLGA or PEG did not result in production of pro-inflammatory cytokines or inflammasome activation in macrophages. When instilled into the lungs, particle composition and size may increase the number and type of innate immune cells in the lungs without triggering inflammatory responses (Roberts et al., 2013). In another study, polystyrene or biodegradable poly(lactide-co-glycolide) microparticles encapsulating encephalitogenic peptides (500 nm diameter) were observed to induce long-term T-cell tolerance and ameliorated experimental autoimmune encephalomyelitis (Getts et al., 2012).
4.5.4 Biomaterial-Based Nanoparticles
Peptide, carbohydrate, nucleic acid, and liposomal formulations have also been used for immunomodulation for the treatment of autoimmune diseases. Nanoparticles carrying disease-related peptide-major histocompatibility complexes were shown to reduce polyclonal autoimmunity by activating the selective expansion of memory-like autoregulatory CD8+ T-cells (Clemente-Casares et al., 2011). These antigens interacted with CD8+ T-cells only in diseased but not healthy individuals, thus these nanoparticles coated with any relevant pMHC may be used as vaccines to inhibit polyclonal autoimmune responses in a disease and organ-specific manner (Tsai et al., 2010).
Liposomal encapsulation of glucocorticoids both enhances their efficacy in the treatment of encephalomyelitis and alters their target cell specificity and their mode of action compared to free glucocorticoids (Schweingruber et al., 2011). A study with folate-targeted nanoparticles showed promising results in the treatment of inflammatory arthritis (T. P. Thomas, Goonewardena, et al., 2011). Liposomal glucocorticoids inhibited proinflammatory macrophage functions and upregulate anti-inflammatory genes, but had little effect on T-cell apoptosis and function.
Injection of DNA plasmids encoding immunomodulatory proteins (OX40-TRAIL), and a cationic lipid was shown to ameliorate experimental autoimmune encephalomyelitis (Yellayi et al., 2011). Controlled release of immunomodulating peptide antigens from PLGA suppressed production of inflammatory cytokines and helped to reduce dosing without increased frequency (H. Zhao, Kiptoo, Williams, Siahaan, & Topp, 2010). A study in siRNA silencing in inflammatory monocytes to suppress expression of the chemokine receptor CCR2 showed that this approach prevented accumulation of inflammatory monocytes and their differentiation into highly activated antigen-presenting macrophages at the sites of inflammation. This therapeutic approach reduced inflammation in atherosclerotic plaques, decreased infarct size after coronary artery occlusion, prolonged survival of pancreatic islet allografts after transplantation, and suppressed tumor growth (Leuschner et al., 2011). Polylactide-cyclosporine A nanoparticles were produced at sub-100 nm sizes and narrow particle size distributions, and released cyclosporine A continuously for targeted immunosuppression. This study showed that polylactide-cyclosporine A nanoparticles were internalized into the dendritic cells with a continuous release to the culture medium, suppressing proliferation of T-cells without any systemic release of the drug (Azzi et al., 2010).
In another study, when leukemia inhibitory factor-loaded nanoparticles were directed to T-cells, vascularized heart grafts survived longer, Foxp3+ cells were induced and Th17 cells were expanded. These results show that engineered nanoparticles regulate immune pathways to elicit wanted response, thus enabling a new therapeutic approach for autoimmune disorders (Park et al., 2011).
4.6 Conclusions and Future Perspectives
Strategies to develop therapies based on inherent properties of different types of nanoparticles, which may be tailored for different diseases, should also employ methods to understand the mechanisms of the immunomodulatory action of these nanoparticles. There are many different types of nanoparticles with various chemical structures and sizes, and they can be functionalized with different side units. In addition to the size, particle composition, surface chemistry, ability to bind to plasma proteins, and drug excretion time and route is important for the immunomodulatory characteristics of the nanomaterials. These responses may be beneficial or harmful depending on the disease type. Research on immunomodulating nanomaterials to fight against cancer focuses on avoiding side effects and enhancing the tumor degrading ability of these nanoparticles. On the other hand, nanoparticles should have immune suppressing ability to treat autoimmune disorders.
It is extremely important that the correlation between the nanoparticle properties and immune response is elucidated for development of treatment and diagnosis methods in medicine. However, further detailed studies are required for tissue regeneration and diagnosis, prevention and treatment of diseases through immunomodulatory nanoparticles.
References
Akagi, T., Baba, M., & Akashi, M. (2012). Biodegradable nanoparticles as vaccine adjuvants and delivery systems: Regulation of immune responses by nanoparticle-based vaccine. Advances in Polymer Science, 247(1), 31–64. https://doi.org/10.1007/12_2011_150
Akagi, T., Kaneko, T., Kida, T., & Akashi, M. (2005). Preparation and characterization of biodegradable nanoparticles based on poly(γ-glutamic acid) with L-phenylalanine as a protein carrier. Journal of Controlled Release, 108(2–3), 226–236. https://doi.org/10.1016/j.jconrel.2005.08.003
Ali, O. A., & Mooney, D. J. (2010). Immunologically active biomaterials for cancer therapy. Current Topics in Microbiology and Immunology, 344(1), 279–297. https://doi.org/10.1007/82-2010-69
Andersson, A. M. C., Buldun, C. M., Pattinson, D. J., Draper, S. J., & Howarth, M. (2019). SnoopLigase peptide-peptide conjugation enables modular vaccine assembly. Scientific Reports, 9(1), 4625. https://doi.org/10.1038/s41598-019-40985-w
Azzi, J., Tang, L., Moore, R., Tong, R., El Haddad, N., Akiyoshi, T., … Abdi, R. (2010). Polylactide-cyclosporin A nanoparticles for targeted immunosuppression. FASEB Journal, 24(10), 3927–3938. https://doi.org/10.1096/fj.10-154690
Ban, M., Langonné, I., Huguet, N., & Goutet, M. (2012). Effect of submicron and nano-iron oxide particles on pulmonary immunity in mice. Toxicology Letters, 210(3), 267–275. https://doi.org/10.1016/j.toxlet.2012.02.004
Ban, M., Langonné, I., Huguet, N., Guichard, Y., & Goutet, M. (2013). Iron oxide particles modulate the ovalbumin-induced Th2 immune response in mice. Toxicology Letters, 216(1), 31–39. https://doi.org/10.1016/j.toxlet.2012.11.003
Bianco, A., Kostarelos, K., & Prato, M. (2005). Applications of carbon nanotubes in drug delivery. Current Opinion in Chemical Biology, 9(6), 674–679. https://doi.org/10.1016/j.cbpa.2005.10.005
Blank, F., Gerber, P., Rothen-Rutishauser, B., Sakulkhu, U., Salaklang, J., De Peyer, K., … Von Garnier, C. (2011). Biomedical nanoparticles modulate specific CD4 + T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology, 5(4), 606–621. https://doi.org/10.3109/17435390.2010.541293
Brito, L. A., & O’Hagan, D. T. (2014). Designing and building the next generation of improved vaccine adjuvants. Journal of Controlled Release, 190, 563–579. https://doi.org/10.1016/j.jconrel.2014.06.027
Buehler, D. C., Marsden, M. D., Shen, S., Toso, D. B., Wu, X., Loo, J. A., … Rome, L. H. (2014). Bioengineered vaults: Self-assembling protein shell-lipophilic core nanoparticles for drug delivery. ACS Nano, 8(8), 7723–7732. https://doi.org/10.1021/nn5002694
Chen, H., Dorrigan, A., Saad, S., Hare, D. J., Cortie, M. B., & Valenzuela, S. M. (2013). In vivo study of spherical gold nanoparticles: Inflammatory effects and distribution in mice. PLoS One, 8(2), e58208. https://doi.org/10.1371/journal.pone.0058208
Chen, M., Ouyang, H., Zhou, S., Li, J., & Ye, Y. (2014). PLGA-nanoparticle mediated delivery of anti-OX40 monoclonal antibody enhances anti-tumor cytotoxic T cell responses. Cellular Immunology, 287(2), 91–99. https://doi.org/10.1016/j.cellimm.2014.01.003
Chen, Y. S., Hung, Y. C., Lin, W. H., & Huang, G. S. (2010). Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology, 21(19), 195101. https://doi.org/10.1088/0957-4484/21/19/195101
Chen, Y.-W. (2004). Fullerene derivatives protect against oxidative stress in RAW 264.7 cells and ischemia-reperfused lungs. AJP: Regulatory, Integrative and Comparative Physiology, 287(1), R21–R26. https://doi.org/10.1152/ajpregu.00310.2003
Cho, N. H., Cheong, T. C., Min, J. H., Wu, J. H., Lee, S. J., Kim, D., … Seong, S. Y. (2011). A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nature Nanotechnology, 6(10), 675–682. https://doi.org/10.1038/nnano.2011.149
Chou, S. H., Shetty, A. V., Geng, Y., Xu, L., Munirathinam, G., Pipathsouk, A., … Zheng, G. (2013). Palmitate-derivatized human IL-2: A potential anticancer immunotherapeutic of low systemic toxicity. Cancer Immunology, Immunotherapy, 62(3), 597–603. https://doi.org/10.1007/s00262-012-1364-8
Clemente-Casares, X., Tsai, S., Yang, Y., & Santamaria, P. (2011). Peptide-MHC-based nanovaccines for the treatment of autoimmunity: A “one size fits all” approach? Journal of Molecular Medicine, 89(8), 733–742. https://doi.org/10.1007/s00109-011-0757-z
Couvreur, P., & Vauthier, C. (2006). Nanotechnology: Intelligent design to treat complex disease. Pharmaceutical Research, 23(7), 1417–1450. https://doi.org/10.1007/s11095-006-0284-8
Cruz, L. J., Rueda, F., Cordobilla, B., Simón, L., Hosta, L., Albericio, F., & Domingo, J. C. (2011). Targeting nanosystems to human DCs via Fc receptor as an effective strategy to deliver antigen for immunotherapy. Molecular Pharmaceutics, 8(1), 104–116. https://doi.org/10.1021/mp100178k
D’souza, M. J., Tawde, S. A., Akalkotkar, A., Chablani, L., D’souza, M., & Chiriva-Internati, M. (2014). Nanotechnology in vaccine delivery. In Molecular vaccines: From prophylaxis to therapy (Vol. 2, pp. 727–741). Wien: Springer. https://doi.org/10.1007/978-3-319-00978-0_19
Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., & Préat, V. (2012). PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release, 161(2), 505–522. https://doi.org/10.1016/j.jconrel.2012.01.043
De Temmerman, M. L., Rejman, J., Demeester, J., Irvine, D. J., Gander, B., & De Smedt, S. C. (2011). Particulate vaccines: On the quest for optimal delivery and immune response. Drug Discovery Today, 16(13–14), 569–582. https://doi.org/10.1016/j.drudis.2011.04.006
DeMaria, P. J., & Bilusic, M. (2019). Cancer vaccines. Hematology/Oncology Clinics of North America, 33(2), 199–214. https://doi.org/10.1016/j.hoc.2018.12.001
Demento, S. L., Cui, W., Criscione, J. M., Stern, E., Tulipan, J., Kaech, S. M., & Fahmy, T. M. (2012). Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials, 33(19), 4957–4964. https://doi.org/10.1016/j.biomaterials.2012.03.041
Diwan, M., Tafaghodi, M., & Samuel, J. (2002). Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. Journal of Controlled Release, 85(1–3), 247–262. https://doi.org/10.1016/S0168-3659(02)00275-4
Dobrovolskaia, M. A., & McNeil, S. E. (2007). Immunological properties of engineered nanomaterials. Nature Nanotechnology, 2(8), 469–478. https://doi.org/10.1038/nnano.2007.223
Fang, R. H., Hu, C.-M. J., Luk, B. T., Gao, W., Copp, J. A., Tai, Y., … Zhang, L. (2014). Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Letters, 14(4), 2181–2188. https://doi.org/10.1021/nl500618u
Feldmann, M., & Steinman, L. (2005). Design of effective immunotherapy for human autoimmunity. Nature, 435(7042), 612–619. https://doi.org/10.1038/nature03727
Fifis, T., Gamvrellis, A., Crimeen-Irwin, B., Pietersz, G. A., Li, J., Mottram, P. L., … Plebanski, M. (2004). Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. The Journal of Immunology, 173(5), 3148–3154. https://doi.org/10.4049/jimmunol.173.5.3148
Foged, C., Brodin, B., Frokjaer, S., & Sundblad, A. (2005). Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. International Journal of Pharmaceutics, 298(2), 315–322. https://doi.org/10.1016/j.ijpharm.2005.03.035
Gagné, F., Fortier, M., Yu, L., Osachoff, H. L., Skirrow, R. C., Van Aggelen, G., … Fournier, M. (2010). Immunocompetence and alterations in hepatic gene expression in rainbow trout exposed to CdS/CdTe quantum dots. Journal of Environmental Monitoring, 12(8), 1556–1565. https://doi.org/10.1039/c0em00031k
Getts, D. R., Martin, A. J., Mccarthy, D. P., Terry, R. L., Hunter, Z. N., Yap, W. T., … Miller, S. D. (2012). Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nature Biotechnology, 30(12), 1217–1224. https://doi.org/10.1038/nbt.2434
Giddam, A. K., Zaman, M., Skwarczynski, M., & Toth, I. (2012). Liposome-based delivery system for vaccine candidates: Constructing an effective formulation. Nanomedicine, 7(12), 1877–1893. https://doi.org/10.2217/nnm.12.157
Girija, A. R., & Balasubramanian, S. (2018). Theragnostic potentials of core/shell mesoporous silica nanostructures. Nanotheranostics, 3(1), 1–40. https://doi.org/10.7150/ntno.27877
Glück, R., Moser, C., & Metcalfe, I. C. (2004). Influenza virosomes as an efficient system for adjuvanted vaccine delivery. Expert Opinion on Biological Therapy, 4(7), 1139–1145. https://doi.org/10.1517/14712598.4.7.1139
Gregory, A. E., Titball, R., & Williamson, D. (2013). Vaccine delivery using nanoparticles. Frontiers in Cellular and Infection Microbiology, 3, 13. https://doi.org/10.3389/fcimb.2013.00013
Gupta, A., Liberati, T. A., Verhulst, S. J., Main, B. J., Roberts, M. H., Potty, A. G. R., … El-Amin, S. F., III. (2015). Biocompatibility of single-walled carbon nanotube composites for bone regeneration. Bone & Joint Research, 4(5), 70–77. https://doi.org/10.1302/2046-3758.45.2000382
Hafner, A. M., Corthésy, B., & Merkle, H. P. (2013). Particulate formulations for the delivery of poly(I: C) as vaccine adjuvant. Advanced Drug Delivery Reviews, 65(10), 1386–1399. https://doi.org/10.1016/j.addr.2013.05.013
Hardy, C. L., LeMasurier, J. S., Belz, G. T., Scalzo-Inguanti, K., Yao, J., Xiang, S. D., … Plebanski, M. (2012). Inert 50-nm polystyrene nanoparticles that modify pulmonary dendritic cell function and inhibit allergic airway inflammation. The Journal of Immunology, 188(3), 1431–1441. https://doi.org/10.4049/jimmunol.1100156
Hellstrom, I., Ledbetter, J. A., Scholler, N., Yang, Y., Ye, Z., Goodman, G., … Hellstrom, K. E. (2001). CD3-mediated activation of tumor-reactive lymphocytes from patients with advanced cancer. Proceedings of the National Academy of Sciences of the United States of America, 98(12), 6783–6788. https://doi.org/10.1073/pnas.021557498
Heo, M. B., Kim, S. Y., Yun, W. S., & Lim, Y. T. (2015). Sequential delivery of an anticancer drug and combined immunomodulatory nanoparticles for efficient chemoimmunotherapy. International Journal of Nanomedicine, 10, 5981–5993. https://doi.org/10.2147/IJN.S90104
Herlyn, D., & Birebent, B. (1999). Advances in cancer vaccine development. Annals of Medicine, 31(1), 66–78.
Hirai, T., Yoshioka, Y., Takahashi, H., Ichihashi, K., Yoshida, T., Tochigi, S., … Tsutsumi, Y. (2012). Amorphous silica nanoparticles enhance cross-presentation in murine dendritic cells. Biochemical and Biophysical Research Communications, 427(3), 553–556. https://doi.org/10.1016/j.bbrc.2012.09.095
Hirst, S. M., Karakoti, A. S., Tyler, R. D., Sriranganathan, N., Seal, S., & Reilly, C. M. (2009). Anti-inflammatory properties of cerium oxide nanoparticles. Small, 5(24), 2848–2856. https://doi.org/10.1002/smll.200901048
Hodi, F. S., O’Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., … Urba, W. J. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine, 363(8), 711–723. https://doi.org/10.1056/nejmoa1003466
Hong, T., Lu, A., Liu, W., & Chen, C. (2019). Microdroplet synthesis of silver nanoparticles with controlled sizes. Micromachines, 10(4), 274. https://doi.org/10.3390/mi10040274
Hubbell, J. A., Thomas, S. N., & Swartz, M. A. (2009). Materials engineering for immunomodulation. Nature, 462(7272), 449–460. https://doi.org/10.1038/nature08604
Hussain, S., Vanoirbeek, J. A. J., & Hoet, P. H. M. (2012). Interactions of nanomaterials with the immune system. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 4(2), 169–183. https://doi.org/10.1002/wnan.166
Irvine, D. J., Hanson, M. C., Rakhra, K., & Tokatlian, T. (2015). Synthetic nanoparticles for vaccines and immunotherapy. Chemical Reviews, 115(19), 11109–11146. https://doi.org/10.1021/acs.chemrev.5b00109
Irvine, D. J., Swartz, M. A., & Szeto, G. L. (2013). Engineering synthetic vaccines using cues from natural immunity. Nature Materials, 12(11), 978–990. https://doi.org/10.1038/nmat3775
Jones, K. S. (2008). Biomaterials as vaccine adjuvants. Biotechnology Progress, 24(4), 807–814. https://doi.org/10.1002/btpr.10
Joshi, V. B., Geary, S. M., & Salem, A. K. (2013). Biodegradable particles as vaccine delivery systems: Size matters. The AAPS Journal, 15(1), 85–94. https://doi.org/10.1208/s12248-012-9418-6
Jovanović, B., Anastasova, L., Rowe, E. W., & Palić, D. (2011). Hydroxylated fullerenes inhibit neutrophil function in fathead minnow (Pimephales promelas Rafinesque, 1820). Aquatic Toxicology, 101(2), 474–482. https://doi.org/10.1016/j.aquatox.2010.11.002
Kalkanidis, M., Pietersz, G. A., Xiang, S. D., Mottram, P. L., Crimeen-Irwin, B., Ardipradja, K., & Plebanski, M. (2006). Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods, 40(1), 20–29. https://doi.org/10.1016/j.ymeth.2006.05.018
Kalos, M., Levine, B. L., Porter, D. L., Katz, S., Grupp, S. A., Bagg, A., & June, C. H. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science Translational Medicine, 3(95), 95ra73. https://doi.org/10.1126/scitranslmed.3002842
Kanekiyo, M., Wei, C. J., Yassine, H. M., McTamney, P. M., Boyington, J. C., Whittle, J. R. R., … Nabel, G. J. (2013). Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature, 499(7456), 102–106. https://doi.org/10.1038/nature12202
Kar, U. K., Jiang, J., Champion, C. I., Salehi, S., Srivastava, M., Sharma, S., … Kelly, K. A. (2012). Vault nanocapsules as adjuvants favor cell-mediated over antibody-mediated immune responses following immunization of mice. PLoS One, 7(7), e38553. https://doi.org/10.1371/journal.pone.0038553
Kasturi, S. P., Skountzou, I., Albrecht, R. A., Koutsonanos, D., Hua, T., Nakaya, H. I., … Pulendran, B. (2011). Programming the magnitude and persistence of antibody responses with innate immunity. Nature, 470(7335), 543–550. https://doi.org/10.1038/nature09737
Khatri, K., Goyal, A. K., Gupta, P. N., Mishra, N., Mehta, A., & Vyas, S. P. (2008). Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine, 26(18), 2225–2233. https://doi.org/10.1016/j.vaccine.2008.02.058
Khong, H., Volmari, A., Sharma, M., Dai, Z., Imo, C. S., Hailemichael, Y., … Overwijk, W. W. (2018). Peptide vaccine formulation controls the duration of antigen presentation and magnitude of tumor-specific CD8 + T cell response. The Journal of Immunology, 200(10), 3464–3474. https://doi.org/10.4049/jimmunol.1700467
Kim, S. Y., Doh, H. J., Jang, M. H., Ha, Y. J., Chung, S. I. I., & Park, H. J. (1999). Oral immunization with Helicobacter pylori-loaded poly(D,L-Lactide-Co-Glycolide) nanoparticles. Helicobacter, 4(1), 33–39. https://doi.org/10.1046/j.1523-5378.1999.09046.x
Köping-Höggård, M., Sánchez, A., & Alonso, M. J. (2005). Nanoparticles as carriers for nasal vaccine delivery. Expert Review of Vaccines, 4(2), 185–196. https://doi.org/10.1586/14760584.4.2.185
Kumari, A., & Yadav, S. K. (2011). Cellular interactions of therapeutically delivered nanoparticles. Expert Opinion on Drug Delivery, 8(2), 141–151. https://doi.org/10.1517/17425247.2011.547934
Kwong, B., Gai, S. A., Elkhader, J., Wittrup, K. D., & Irvine, D. J. (2013). Localized immunotherapy via liposome-anchored anti- CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Research, 73(5), 1547–1558. https://doi.org/10.1158/0008-5472.CAN-12-3343
Lei, C., Liu, P., Chen, B., Mao, Y., Engelmann, H., Shin, Y., … Hellstrom, K. E. (2010). Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. Journal of the American Chemical Society, 132(20), 6906–6907. https://doi.org/10.1021/ja102414t
Leuschner, F., Dutta, P., Gorbatov, R., Novobrantseva, T. I., Donahoe, J. S., Courties, G., … Nahrendorf, M. (2011). Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature Biotechnology, 29(11), 1005–1010. https://doi.org/10.1038/nbt.1989
Li, P., Luo, Z., Liu, P., Gao, N., Zhang, Y., Pan, H., … Ma, Y. (2013). Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. Journal of Controlled Release, 168, 271. https://doi.org/10.1016/j.jconrel.2013.03.025
Lollini, P. L., Cavallo, F., Nanni, P., & Forni, G. (2006). Vaccines for tumour prevention. Nature Reviews Cancer, 6(3), 204–216. https://doi.org/10.1038/nrc1815
Lü, J. M., Wang, X., Marin-Muller, C., Wang, H., Lin, P. H., Yao, Q., & Chen, C. (2009). Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Review of Molecular Diagnostics, 9(4), 325–341. https://doi.org/10.1586/erm.09.15
Luo, M., Wang, H., Wang, Z., Cai, H., Lu, Z., Li, Y., … Gao, J. (2017). A STING-activating nanovaccine for cancer immunotherapy. Nature Nanotechnology, 12(7), 648–654. https://doi.org/10.1038/nnano.2017.52
Magoulas, G. E., Garnelis, T., Athanassopoulos, C. M., Papaioannou, D., Mattheolabakis, G., Avgoustakis, K., & Hadjipavlou-Litina, D. (2012). Synthesis and antioxidative/anti-inflammatory activity of novel fullerene-polyamine conjugates. Tetrahedron, 68(35), 7041–7049. https://doi.org/10.1016/j.tet.2012.06.066
Mamo, T., & Poland, G. A. (2012). Nanovaccinology: The next generation of vaccines meets 21st century materials science and engineering. Vaccine, 30(47), 6609–6611. https://doi.org/10.1016/j.vaccine.2012.08.023
Manish, M., Rahi, A., Kaur, M., Bhatnagar, R., & Singh, S. (2013). A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against bacillus anthracis spore challenge. PLoS One, 8(4), e61885. https://doi.org/10.1371/journal.pone.0061885
Manolova, V., Flace, A., Bauer, M., Schwarz, K., Saudan, P., & Bachmann, M. F. (2008). Nanoparticles target distinct dendritic cell populations according to their size. European Journal of Immunology, 38(5), 1404–1413. https://doi.org/10.1002/eji.200737984
Manzano, M., Aina, V., Areán, C. O., Balas, F., Cauda, V., Colilla, M., … Vallet-Regí, M. (2008). Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chemical Engineering Journal, 137(1), 30–37. https://doi.org/10.1016/j.cej.2007.07.078
Marradi, M., Chiodo, F., García, I., & Penadés, S. (2013). Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chemical Society Reviews, 42(11), 4728–4745. https://doi.org/10.1039/c2cs35420a
Mi, W., Wanjie, S., Lo, S.-T., Gan, Z., Pickl-Herk, B., Ober, R. J., & Ward, E. S. (2008). Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments. The Journal of Immunology, 181(11), 7550–7561. https://doi.org/10.4049/jimmunol.181.11.7550
Minigo, G., Scholzen, A., Tang, C. K., Hanley, J. C., Kalkanidis, M., Pietersz, G. A., … Plebanski, M. (2007). Poly-L-lysine-coated nanoparticles: A potent delivery system to enhance DNA vaccine efficacy. Vaccine, 25(7), 1316–1327. https://doi.org/10.1016/j.vaccine.2006.09.086
Mitchell, L. A., Lauer, F. T., Burchiel, S. W., & McDonald, J. D. (2009). Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nature Nanotechnology, 4(7), 451–456. https://doi.org/10.1038/nnano.2009.151
Mitchell, L. A., Gao, J., Wal, R. V., Gigliotti, A., Burchiel, S. W., & McDonald, J. D. (2007). Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicological Sciences, 100(1), 203–214. https://doi.org/10.1093/toxsci/kfm196
Mody, K. T., Popat, A., Mahony, D., Cavallaro, A. S., Yu, C., & Mitter, N. (2013). Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale, 5(12), 5167–5179. https://doi.org/10.1039/c3nr00357d
Moghimi, S. M., Hunter, A. C., & Murray, J. C. (2005). Nanomedicine: Current status and future prospects. The FASEB Journal, 19(3), 311–330. https://doi.org/10.1096/fj.04-2747rev
Moon, J. J., Huang, B., & Irvine, D. J. (2012). Engineering nano- and microparticles to tune immunity. Advanced Materials, 24(28), 3724–3746. https://doi.org/10.1002/adma.201200446
Moon, J. J., Suh, H., Bershteyn, A., Stephan, M. T., Liu, H., Huang, B., … Irvine, D. J. (2011). Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nature Materials, 10(3), 243–251. https://doi.org/10.1038/nmat2960
Moon, J. J., Suh, H., Polhemus, M. E., Ockenhouse, C. F., Yadava, A., & Irvine, D. J. (2012). Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLoS One, 7(2), e31472. https://doi.org/10.1371/journal.pone.0031472
Morel, S., Didierlaurent, A., Bourguignon, P., Delhaye, S., Baras, B., Jacob, V., … Van Mechelen, M. (2011). Adjuvant system AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine, 29(13), 2461–2473. https://doi.org/10.1016/j.vaccine.2011.01.011
Munn, D. H., & Bronte, V. (2016). Immune suppressive mechanisms in the tumor microenvironment. Current Opinion in Immunology, 39, 1–6. https://doi.org/10.1016/j.coi.2015.10.009
Neek, M., Kim, T. I., & Wang, S. W. (2019). Protein-based nanoparticles in cancer vaccine development. Nanomedicine: Nanotechnology, Biology, and Medicine, 15(1), 164–174. https://doi.org/10.1016/j.nano.2018.09.004
Nguyen, K. C., Seligy, V. L., & Tayabali, A. F. (2013). Cadmium telluride quantum dot nanoparticle cytotoxicity and effects on model immune responses to Pseudomonas aeruginosa. Nanotoxicology, 7(2), 202–211. https://doi.org/10.3109/17435390.2011.648667
Niikura, K., Matsunaga, T., Suzuki, T., Kobayashi, S., Yamaguchi, H., Orba, Y., … Sawa, H. (2013). Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano, 7(5), 3926–3938. https://doi.org/10.1021/nn3057005
Noh, Y.-W., Jang, Y.-S., Ahn, K.-J., Lim, Y. T., & Chung, B. H. (2011). Simultaneous in vivo tracking of dendritic cells and priming of an antigen-specific immune response. Biomaterials, 32(26), 6254–6263. https://doi.org/10.1016/j.biomaterials.2011.05.013
Norman, P. (2005). Immunobiology: The immune system in health and disease. Journal of Allergy and Clinical Immunology, 96(2), 274–274. https://doi.org/10.1016/s0091-6749(95)70025-0
Notarangelo, L. D., Gambineri, E., & Badolato, R. (2006). Immunodeficiencies with autoimmune consequences. Advances in Immunology, 89, 321–370. https://doi.org/10.1016/S0065-2776(05)89008-X
O’Hagan, D. T., Ott, G. S., & Van Nest, G. (1997). Recent advances in vaccine adjuvants: The development of MF59 emulsion and polymeric microparticles. Molecular Medicine Today, 3(2), 69–75. https://doi.org/10.1016/S1357-4310(96)10058-7
O’Shea, J., & Paul, W. E. (2010). Mechanisms underlying lineage commitment and plasticity of helper CD4 + T cells. Science, 327(5969), 1098–1102. https://doi.org/10.1126/science.1178334
Oberg, A. L., Kennedy, R. B., Li, P., Ovsyannikova, I. G., & Poland, G. A. (2011). Systems biology approaches to new vaccine development. Current Opinion in Immunology, 23(3), 436–443. https://doi.org/10.1016/j.coi.2011.04.005
Orecchioni, M., Bedognetti, D., Sgarrella, F., Marincola, F. M., Bianco, A., & Delogu, L. G. (2014). Impact of carbon nanotubes and graphene on immune cells. Journal of Translational Medicine, 12(1), 138. https://doi.org/10.1186/1479-5876-12-138
Pagliari, F., Mandoli, C., Forte, G., Magnani, E., Pagliari, S., Nardone, G., … Traversa, E. (2012). Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano, 6(5), 3767–3775. https://doi.org/10.1021/nn2048069
Park, J., Gao, W., Whiston, R., Strom, T. B., Metcalfe, S., & Fahmy, T. M. (2011). Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery. Molecular Pharmaceutics, 8(1), 143–152. https://doi.org/10.1021/mp100203a
Park, J., Wrzesinski, S. H., Stern, E., Look, M., Criscione, J., Ragheb, R., … Fahmy, T. M. (2012). Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature Materials, 11(10), 895–905. https://doi.org/10.1038/nmat3355
Parra, J., Abad-Somovilla, A., Mercader, J. V., Taton, T. A., & Abad-Fuentes, A. (2013). Carbon nanotube-protein carriers enhance size-dependent self-adjuvant antibody response to haptens. Journal of Controlled Release, 170(2), 242–251. https://doi.org/10.1016/j.jconrel.2013.05.019
Parween, S., Gupta, P. K., & Chauhan, V. S. (2011). Induction of humoral immune response against PfMSP-119 and PvMSP-1 19 using gold nanoparticles along with alum. Vaccine, 29(13), 2451–2460. https://doi.org/10.1016/j.vaccine.2011.01.014
Pati, R., Shevtsov, M., & Sonawane, A. (2018). Nanoparticle vaccines against infectious diseases. Frontiers in Immunology, 9(OCT), 2224. https://doi.org/10.3389/fimmu.2018.02224
Peer, D., Karp, J. M., Hong, S., Farokhzad, O. C., Margalit, R., & Langer, R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology, 2(12), 751–760. https://doi.org/10.1038/nnano.2007.387
Pelaz, B., Alexiou, C., Alvarez-Puebla, R. A., Alves, F., Andrews, A. M., Ashraf, S., … Parak, W. J. (2017). Diverse applications of nanomedicine. ACS Nano, 11(3), 2313–2381. https://doi.org/10.1021/acsnano.6b06040
Pescatori, M., Bedognetti, D., Venturelli, E., Ménard-Moyon, C., Bernardini, C., Muresu, E., … Delogu, L. G. (2013). Functionalized carbon nanotubes as immunomodulator systems. Biomaterials, 34(18), 4395–4403. https://doi.org/10.1016/j.biomaterials.2013.02.052
Pulendran, B., & Ahmed, R. (2011). Immunological mechanisms of vaccination. Nature Immunology, 12(6), 509–517.
Rappuoli, R., Mandl, C. W., Black, S., & De Gregorio, E. (2011). Vaccines for the twenty-first century society. Nature Reviews Immunology, 11(12), 865–872. https://doi.org/10.1038/nri3085
Reddy, S. T., Swartz, M. A., & Hubbell, J. A. (2006). Targeting dendritic cells with biomaterials: Developing the next generation of vaccines. Trends in Immunology, 27(12), 573–579. https://doi.org/10.1016/j.it.2006.10.005
Reddy, S. T., Van Der Vlies, A. J., Simeoni, E., Angeli, V., Randolph, G. J., O’Neil, C. P., … Hubbell, J. A. (2007). Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnology, 25(10), 1159–1164. https://doi.org/10.1038/nbt1332
Roberts, R. A., Shen, T., Allen, I. C., Hasan, W., DeSimone, J. M., & Ting, J. P. Y. (2013). Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano- and microscale particles. PLoS One, 8(4), e62115. https://doi.org/10.1371/journal.pone.0062115
Rose, N. R. (2016). Prediction and prevention of autoimmune disease in the 21st Century: A review and preview. American Journal of Epidemiology, 183(5), 403–406. https://doi.org/10.1093/aje/kwv292
Ryan, J. J., Bateman, H. R., Stover, A., Gomez, G., Norton, S. K., Zhao, W., … Kepley, C. L. (2007). Fullerene nanomaterials inhibit the allergic response. Journal of Immunology (Baltimore, Md. : 1950), 179(1), 665–672.
Sahin, U., & Türeci, Ö. (2018). Personalized vaccines for cancer immunotherapy. Science, 359(6382), 1355–1360. https://doi.org/10.1126/science.aar7112
Schanen, B. C., Das, S., Reilly, C. M., Warren, W. L., Self, W. T., Seal, S., & Drake, D. R. (2013). Immunomodulation and T helper TH 1/TH 2 response polarization by CeO 2 and TiO 2 nanoparticles. PLoS One, 8(5), e62816. https://doi.org/10.1371/journal.pone.0062816
Schweingruber, N., Haine, A., Tiede, K., Karabinskaya, A., van den Brandt, J., Wüst, S., … Lühder, F. (2011). Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis. The Journal of Immunology, 187(8), 4310–4318. https://doi.org/10.4049/jimmunol.1101604
Schwendener, R. A. (2014). Liposomes as vaccine delivery systems: A review of the recent advances. Therapeutic Advances in Vaccines, 2(6), 159–182. https://doi.org/10.1177/2051013614541440
Seydoux, E., Rothen-Rutishauser, B., Nita, I. M., Balog, S., Gazdhar, A., Stumbles, P. A., … von Garnier, C. (2014). Size-dependent accumulation of particles in lysosomes modulates dendritic cell function through impaired antigen degradation. International Journal of Nanomedicine, 9(1), 3885–3902. https://doi.org/10.2147/IJN.S64353
Shah, R. R., O’hagan, D. T., Amiji, M. M., & Brito, L. A. (2014). The impact of size on particulate vaccine adjuvants. Nanomedicine, 9(17), 2671–2681. https://doi.org/10.2217/nnm.14.193
Sharma, C. P. (2010). Biointegration of medical implant materials: Science and design (pp. 1–412). Cambridge: Woodhead Publishing. https://doi.org/10.1533/9781845699802
Sharma, S., Mukkur, T. K. S., Benson, H. A. E., & Chen, Y. (2009). Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. Journal of Pharmaceutical Sciences, 98(3), 812–843. https://doi.org/10.1002/jps.21493
Shen, C. C., Liang, H. J., Wang, C. C., Liao, M. H., & Jan, T.-R. (2012). Iron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. International Journal of Nanomedicine, 7, 2729. https://doi.org/10.2147/ijn.s31054
Shen, H., Ackerman, A. L., Cody, V., Giodini, A., Hinson, E. R., Cresswell, P., … Hanlon, D. J. (2006). Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology, 117(1), 78–88. https://doi.org/10.1111/j.1365-2567.2005.02268.x
Shen, Y., Hao, T., Ou, S., Hu, C., & Chen, L. (2018). Applications and perspectives of nanomaterials in novel vaccine development. MedChemComm, 9(2), 226–238. https://doi.org/10.1039/c7md00158d
Silva, A. L., Rosalia, R. A., Sazak, A., Carstens, M. G., Ossendorp, F., Oostendorp, J., & Jiskoot, W. (2013). Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: Low-burst release is crucial for efficient CD8+ T cell activation. European Journal of Pharmaceutics and Biopharmaceutics, 83(3), 338–345. https://doi.org/10.1016/j.ejpb.2012.11.006
Song, W., Musetti, S. N., & Huang, L. (2017). Nanomaterials for cancer immunotherapy. Biomaterials, 148, 16–30. https://doi.org/10.1016/j.biomaterials.2017.09.017
Steenblock, E. R., & Fahmy, T. M. (2008). A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Molecular Therapy, 16(4), 765–772. https://doi.org/10.1038/mt.2008.11
Stephan, M. T., Moon, J. J., Um, S. H., Bersthteyn, A., & Irvine, D. J. (2010). Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature Medicine, 16(9), 1035–1041. https://doi.org/10.1038/nm.2198
Stone, J. W., Thornburg, N. J., Blum, D. L., Kuhn, S. J., Wright, D. W., & Crowe, J. E. (2013). Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology, 24(29), 295102. https://doi.org/10.1088/0957-4484/24/29/295102
Sumbayev, V. V., Yasinska, I. M., Garcia, C. P., Gilliland, D., Lall, G. S., Gibbs, B. F., … Calzolai, L. (2013). Gold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responses. Small, 9(3), 472–477. https://doi.org/10.1002/smll.201201528
Svenson, S. (2012). Clinical translation of nanomedicines. Current Opinion in Solid State and Materials Science, 16(6), 287–294. https://doi.org/10.1016/j.cossms.2012.10.001
Tao, W., Ziemer, K. S., & Gill, H. S. (2014). Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine, 9(2), 237–251. https://doi.org/10.2217/nnm.13.58
Thomas, C., Rawat, A., Hope-Weeks, L., & Ahsan, F. (2011). Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Molecular Pharmaceutics, 8(2), 405–415. https://doi.org/10.1021/mp100255c
Thomas, T. P., Goonewardena, S. N., Majoros, I. J., Kotlyar, A., Cao, Z., Leroueil, P. R., & Baker, J. R. (2011). Folate-targeted nanoparticles show efficacy in the treatment of inflammatory arthritis. Arthritis and Rheumatism, 63(9), 2671–2680. https://doi.org/10.1002/art.30459
Tkach, A. V., Shurin, G. V., Shurin, M. R., Kisin, E. R., Murray, A. R., Young, S. H., … Shvedova, A. A. (2011). Direct effects of carbon nanotubes on dendritic cells induce immune suppression upon pulmonary exposure. ACS Nano, 5(7), 5755–5762. https://doi.org/10.1021/nn2014479
Treuel, L., Jiang, X., & Nienhaus, G. U. (2013). New views on cellular uptake and trafficking of manufactured nanoparticles. Journal of the Royal Society Interface, 10(82), 20120939. https://doi.org/10.1098/rsif.2012.0939
Tsai, S., Shameli, A., Yamanouchi, J., Clemente-Casares, X., Wang, J., Serra, P., … Santamaria, P. (2010). Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity, 32(4), 568–580. https://doi.org/10.1016/j.immuni.2010.03.015
Vasievich, E. A., & Huang, L. (2011). The suppressive tumor microenvironment: A challenge in cancer immunotherapy. Molecular Pharmaceutics, 8(3), 635–641. https://doi.org/10.1021/mp1004228
Villa, C. H., Dao, T., Ahearn, I., Fehrenbacher, N., Casey, E., Rey, D. A., … Scheinberg, D. A. (2011). Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano, 5(7), 5300–5311. https://doi.org/10.1021/nn200182x
Wang, T., Zou, M., Jiang, H., Ji, Z., Gao, P., & Cheng, G. (2011). Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. European Journal of Pharmaceutical Sciences, 44(5), 653–659. https://doi.org/10.1016/j.ejps.2011.10.012
Wang, X., Podila, R., Shannahan, J. H., Rao, A. M., & Brown, J. M. (2013). Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. International Journal of Nanomedicine, 8, 1733–1748. https://doi.org/10.2147/IJN.S44211
Williams, S. B., Lay, A. H., Lau, C. S., Josephson, D. Y., Wilson, T. G., Choueiri, T. K., & Pal, S. K. (2011). New therapies for castrate-resistant prostate cancer. Expert Opinion on Pharmacotherapy, 12(13), 2069–2074. https://doi.org/10.1517/14656566.2011.590133
Wischke, C., Borchert, H. H., Zimmermann, J., Siebenbrodt, I., & Lorenzen, D. R. (2006). Stable cationic microparticles for enhanced model antigen delivery to dendritic cells. Journal of Controlled Release, 114(3), 359–368. https://doi.org/10.1016/j.jconrel.2006.06.020
Xiang, S. D., Scholzen, A., Minigo, G., David, C., Apostolopoulos, V., Mottram, P. L., & Plebanski, M. (2006). Pathogen recognition and development of particulate vaccines: Does size matter? Methods, 40(1), 1–9. https://doi.org/10.1016/j.ymeth.2006.05.016
Xu, L., Liu, Y., Chen, Z., Li, W., Liu, Y., Wang, L., … Chen, C. (2012). Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Letters, 12(4), 2003–2012. https://doi.org/10.1021/nl300027p
Xu, L., Liu, Y., Chen, Z., Li, W., Liu, Y., Wang, L., … Chen, C. (2013). Morphologically virus-like fullerenol nanoparticles act as the dual-functional nanoadjuvant for HIV-1 vaccine. Advanced Materials, 25(41), 5928–5936. https://doi.org/10.1002/adma.201300583
Yellayi, S., Hilliard, B., Ghazanfar, M., Tsingalia, A., Nantz, M. H., Bollinger, L., … Hecker, J. G. (2011). A single intrathecal injection of DNA and an asymmetric cationic lipid as lipoplexes ameliorates experimental autoimmune encephalomyelitis. Molecular Pharmaceutics, 8(5), 1980–1984. https://doi.org/10.1021/mp2002413
Yudoh, K., Karasawa, R., Masuko, K., & Kato, T. (2009). Water-soluble fullerene (C60) inhibits the development of arthritis in the rat model of arthritis. International Journal of Nanomedicine, 4, 217–225.
Zhao, H., Kiptoo, P., Williams, T. D., Siahaan, T. J., & Topp, E. M. (2010). Immune response to controlled release of immunomodulating peptides in a murine experimental autoimmune encephalomyelitis (EAE) model. Journal of Controlled Release, 141(2), 145–152. https://doi.org/10.1016/j.jconrel.2009.09.002
Zhao, L., Seth, A., Wibowo, N., Zhao, C. X., Mitter, N., Yu, C., & Middelberg, A. P. J. (2014). Nanoparticle vaccines. Vaccine, 32(3), 327–337. https://doi.org/10.1016/j.vaccine.2013.11.069
Zhu, G., Zhang, F., Ni, Q., Niu, G., & Chen, X. (2017). Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano, 11(3), 2387–2392. https://doi.org/10.1021/acsnano.7b00978
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tekinay, T. (2019). Immunomodulatory Nanomaterials. In: Tekinay, A. (eds) Nanomaterials for Regenerative Medicine. Stem Cell Biology and Regenerative Medicine. Humana, Cham. https://doi.org/10.1007/978-3-030-31202-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-31202-2_4
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-31201-5
Online ISBN: 978-3-030-31202-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)