Abstract
Molecular and functional imaging aims to assess oncologic therapy response by integrating molecular and functional tumor biology in order to assess therapeutic efficacy and improve patient outcome. Most oncologic processes reflect heterogeneous disease both functionally and morphologically. Further, clonal proliferations of cells may evolve with time becoming resistant to specific therapies. It is important to identify those cancer patients who derive benefit from therapy, such that expensive, toxic, or futile treatment is avoided in those who will not respond. The ultimate goal is to offer the right treatment to the right patient over time. Molecular and functional imaging either using positron emission tomography (PET) or gamma cameras often through hybrid scanners that also include computed tomography (CT) and/or magnetic resonance imaging (MRI) are sensitive techniques with a major role in the precision medicine algorithm of oncology patients. These modalities provide insight prior to, during, and following therapy. Further, they often serve as a biomarker of tumoral heterogeneity helping to direct the selection of appropriate treatment, and detect early response to therapy. Also, molecular and functional imaging is a powerful prognostic biomarker in oncology that can suggest patient outcome based on treatment response.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Cancer is a spectrum of disease that is morphologically and functionally heterogeneous. Further, the genetic profile of the disease can evolve with time leading to the development of resistance, and this evolution is not uniform throughout the body. Although localized disease may be cured following resection, metastatic disease is a leading cause of cancer-related death. Over the past few years, several new therapies have become available for oncology patients. Today, there is a suite of therapies available including surgery, radiation, chemotherapy, immunotherapy, and radionuclide therapy, among others. Further, technological advances have led to the creation of hybrid scanners such as positron emission tomography (PET)/ computed tomography (CT), single photon computed tomography(SPECT)/CT, and PET/magnetic resonance imaging (MRI). These scanners noninvasively assess morphological and functional tumor heterogeneity throughout the body, evaluate disease extent and biologic behavior before and after therapy, and identify sites of disease that are developing resistance. Multi-modality imaging is helpful, not only for staging but also to suggest the most appropriate ongoing therapy at a metabolic-molecular level. Understanding the genetic underpinnings and imaging signature of cancer is key if we wish to develop treatment algorithms that use the most effective therapy tailored to individual patients while limiting futile, toxic treatment.
2 A Bird’s Eye View of Radiopharmaceuticals
There are many radionuclides, such as 99mTc, 111In, 123I, 131I, 18F, 11C, 68Ga, 64Cu, and 89Zr, among others, that can be used to label pharmaceuticals and create radiopharmaceuticals. Once all legal requirements and regulatory issues have been met (Schwarz et al. 2019), these radiopharmaceuticals can be administered to patients and the patients can be imaged to determine functional and molecular information. Radiopharmaceuticals labeled with positron-emitting radionuclides are imaged with PET, while those labeled with single photon-emitting radionuclides are typically imaged using gamma cameras with SPECT capability. Malignant cells often demonstrate increased glucose metabolism compared with normal tissue (Warburg et al. 1927; Warburg 1956), and 18F-labeled 2-fluoro-2-deoxy-d-glucose (18F-FDG), a radioactive glucose analogue that decays by positron emission, is the most ubiquitous PET radiopharmaceutical used in oncology today. Since glucose metabolism changes faster than tumor size, 18F-FDG PET often shows therapy response much earlier than anatomic imaging with CT or MRI. Of course, it is important to recall that the intensity of 18F-FDG uptake is affected by several factors including cellular histology, density, aggressiveness, and technical parameters, among others. Thus, imaging should be performed with standardized techniques, and evaluated in the correct clinical context. There are many radiopharmaceuticals used in oncology, often designed to target a cellular process, metabolism, receptor, or cell trafficking. For example: 3′-deoxy-3′-[18F] fluorothymidine (FLT) is used to study proliferation by imaging the DNA salvage pathway, [18F]fluoromisonidazole(1-(2-nitroimidazolyl)-2-hydroxy-3-fluoropropane (FMISO) and [18F]fluoroazomycin arabinofuranoside (FAZA) are used to assess tumor hypoxia, and O-[18F]fluoromethyl-L-tyrosine (FMT) is used to study amino acid transport. The idea is that through the use of different PET radiopharmaceuticals, imaging signatures will detail disease phenotype, genotype, and heterogeneity (Gerbaudo and Garcia 2016). What is becoming evident is that more than one biomarker may be needed to determine the effectiveness of therapy and for the assessment of treatment response.
3 Functional and Molecular Imaging for Therapy Assessment in Oncology
Functional and molecular imaging has been used in therapy assessment for many years. Two examples are: (1) 99mTc-labeled methylene diphosphonate (99mTc-MDP) bone scans to assess response across a spectrum of oncologic disease and therapies (Fig. 1) (Scher et al. 2016) and (2) Iodine (123I or 131I) labeled metaiodobenzylguanidine (MIBG) in neuroblastoma (Fig. 2) (Ady et al. 1995). Depending on the radionuclide chosen and the amount of activity administered, radiolabeled MIBG can serve as an imaging agent and/or a therapeutic agent. For imaging, 123I is preferred because of the shorter half-life, ideal gamma photon energy (159 keV), lack of beta emission, and lower radiation dose to the patient; however, access may be limited and expense is higher. For therapy, 131I is required. In general, planar imaging is standard of care. The addition of SPECT increases the contrast of the planar scintigraphic images, thus providing improved functional information. The CT portion of the SPECT/CT, if performed, provides improved anatomical information by pinpointing the location of the abnormal activity seen on the SPECT images. Therefore, the addition of SPECT/CT usually provides a more accurate diagnosis than planar imaging alone. However, due to the increased time of acquisition and image interpretation as well as the radiation exposure from the CT component of the study, SPECT/CT is often done as needed on an ad hoc basis.
Whole body planar 99mTc-MDP bone scan images in a man with symptomatic castration resistant prostate cancer bone metastases obtained prior to (a) and following (b) therapy with 223RaCl2 show decrease in intensity of osseous disease in the right proximal humerus, lumbar spine, and left iliac bone following therapy
When interpreting functional and molecular imaging, it is important to recall the underlying mechanism that leads to the imaging obtained. On 99mTc-MDP bone scans, radiopharmaceutical uptake correlates with increased osteoblastic activity and findings suggestive of osseous disease reflect bone reaction to malignant cells, not the presence of the malignant cells themselves. Osteoblastic activity from healing following therapy is difficult to distinguish from progressive metastatic disease, confounding image interpretation. The flare phenomenon is defined as apparent “disease progression” occurring until approximately 3 months of therapy due to increased lesion intensity or number in the context of improved clinical findings and stability or improvement of bone scan findings on repeat bone scan after 6 months of therapy (Cook et al. 2011; Pollen et al. 1984; Coleman et al. 1988). Also, it is challenging to accurately quantify the burden of osseous metastatic disease on 99mTc-MDP bone scans. Larson et al. proposed the Bone Scan Index (BSI) as a method to measure total skeletal disease by summing the product of the weight and fractional involvement of each of 158 individual bones, where each bone is expressed as a percentage of the entire skeleton (Dennis et al. 2012). However, this is time consuming and rarely used in clinical practice. Quantitative analysis is easier with PET, and 18F-labeled sodium fluoride (18F-NaF) is a high-affinity bone-seeking agent with higher affinity for osteoblastic activity and superior imaging characteristics than 99mTc-MDP (Grant et al. 2008). Even-Sapir et al. compared MDP bone scans and 18F-NaF PET/CT in patients with localized high-risk or metastatic prostate cancer and found the sensitivity and specificity of 99mTc-MDP planar bone scans was 70% and 57%, respectively, whereas for 18F-NaF PET/CT it was 100% and 100%, respectively (Even-Sapir et al. 2006). Similar to 99mTc-MDP bone scans, 18F-NaF PET/CT detects bone turnover, not malignant cells themselves, and thus generate an indirect marker of osseous malignancy. 18F-FDG is used to image glucose metabolism and has been compared with 18F-NaF in the evaluation of therapy response, for example, in men with prostate cancer. 18F-FDG is taken up at sites of disease, while 18F-NaF is taken up at sites of osteoblastic reaction to the disease (Fig. 3). However, 18F-FDG uptake is variable and may be low at sites of specific cancer histology. Recently, there has been growing interest in radiopharmaceuticals targeting the prostate-specific membrane antigen (PSMA), a cell surface transmembrane glycoprotein that is overexpressed on prostate cancer cells (Bouchelouche et al. 2010; Evans et al. 2011; Barrett et al. 2013). This has potential for detection of disease, therapy planning as well as for the assessment of therapy response (Rowe et al. 2016; Koerber et al. 2018; Emmett et al. 2018). Early results suggest response assessment may be confounded by flare (Zacho and Petersen 2018; Zukotynski et al. 2018) and mixed interval change following therapy. Also, not all sites of disease show uptake of PSMA targeting radiopharmaceuticals, and the most helpful radiopharmaceutical to assess therapy response may be case specific (Figs. 4 and 5).
Mechanism of radiopharmaceutical uptake. Axial PET, CT, and fused PET/CT images from an 18F-NaF PET/CT shows radiotracer uptake at the periphery of a site of prostate cancer due to osteoblastic turnover (a), while axial PET, CT, and fused PET/CT images from an 18F-FDG PET/CT show subtle radiotracer uptake within the tumor, likely involving the bone marrow (b)
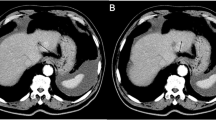
More than one radiopharmaceutical may be helpful to assess therapy response in oncology. Change in radiopharmaceutical uptake is more pronounced on the 18F-FDG PET/CT than on 18F-DCFPyL PET/CT at a site of lytic metastatic prostate cancer. Axial CT at baseline, 3 months and 10 months of therapy (a), axial fused 18F-DCFPyL PET/CT (b) and 18F-FDG PET/CT (c) at baseline and 3 months of therapy
More than one radiopharmaceutical may be helpful to assess therapy response in oncology. Change in radiopharmaceutical uptake is more pronounced on 18F-DCFPyL PET/CT than on 18F-FDG PET/CT at a site of lytic metastatic prostate cancer. Axial CT images at baseline and 3 months of therapy (a), axial fused 18F-DCFPyL PET/CT (b) and 18F-FDG PET/CT (c) images at baseline and 3 months of therapy
There are numerous cell-surface receptors involved in cell-signaling pathways and radiopharmaceuticals targeting cell receptors have become powerful imaging and therapy tools. The somatostatin receptor (SSTR)-binding radiopharmaceutical [68Ga-DOTA0,Tyr3]octreotate (68Ga-DOTATATE) and peptide receptor radionuclide therapy (PRRT) with SSTR-binding peptide [177Lu-DOTA0,Tyr3]octreotate (177Lu-DOTATATE) have been used to image and treat neuroendocrine disease, respectively (Figs. 6 and 7). Since radiopharmaceutical uptake is affected by tumor heterogeneity, volumes of interest obtained from imaging done prior to therapy can be used to compute the fraction of administered radiopharmaceutical sequestered in normal parenchyma as well as at sites of disease (Beauregard et al. 2012). This can then be used to adjust the amount of administered therapeutic radiopharmaceutical to minimize toxicity while maximizing patient benefit.
Whole body planar images of the subject from Fig. 6 show multifocal osseous and soft tissue disease immediately following cycle 1 (a) and cycle 2 (b) of 177Lu-DOTATATE therapy with interval decrease in intensity and extent of radiopharmaceutical uptake at sites of disease following therapy
4 Prognostic Value of Functional and Molecular Imaging Oncologic Imaging Response
Molecular and functional imaging response assessment has been studied across the spectrum of oncologic disease. Since metabolic and pathophysiological changes often precede alterations in morphology, PET is helpful to assess response to cytotoxic and cytostatic therapy and often predicts response before morphologic imaging (i.e., CT and MRI). In general, the earlier the response, the better the progression-free survival (PFS) and overall survival (OS) of the oncology patient. Thus, there is a concept of prognostic value of the reduction in FDG uptake related to treatment. For example, Weber et al. showed that in stage IIIB and IV non-small cell lung cancer (NSCLC), a reduction in tumor FDG uptake of more than 20% after one cycle of platinum-based chemotherapy was predictive of long-term survival (Weber et al. 2003). Vansteenkiste et al. found that in stage IIIA-N2 NSCLC, a reduction in tumor uptake by more than 50% on FDG-PET after 3 cycles of neoadjuvant chemotherapy was predictive of longer survival (Vansteenkiste et al. 2004). Hoekstra et al. reported that in stage IIIA-N2 NSCLC, a 35% reduction in tumor FDG uptake after one cycle of induction therapy showed prolonged overall survival (Hoekstra et al. 2005). MacManus and colleagues showed that tumor metabolic response predicts outcome following radiation therapy (Mac Manus et al. 2005). Complete metabolic responders had a 1-year survival rate of 93% compared to 47% for nonresponders, and 2-year survival rate of 62% versus 30%, respectively. Although imaging patients 3–4 months after radiotherapy minimizes false-positive FDG uptake in radiation-induced inflammation, a shorter time frame may be acceptable in certain cases (Hicks et al. 2004).
5 The Development of Molecular and Functional Therapy Response Assessment Criteria
Determining the effectiveness of cancer therapy requires a standardized, reproducible, and objective method for evaluating therapy response. Over the years several efforts were made to meet this clinical need resulting in the creation of multiple guidelines. The history of therapy response assessment in oncology is complex. As imaging techniques developed, so too did criteria for therapy response assessment. Morphologic imaging therapy response assessment criteria such as Response Evaluation Criteria In Solid Tumors (RECIST 1.1 (Eisenhauer et al. 2009)) are effective to monitor cytolytic therapy effect, in which clinical efficacy typically translates into tumor mass reduction. However, targeted cytostatic therapies (e.g., tyrosine kinase inhibitors such as erlotinib and gefitinib) primarily slow or stop tumor cell proliferation and may not result in a significant change in tumor mass, limiting size-based criteria for therapy response assessment. Initial 18F-FDG-PET studies showed that successful response to erlotinib and gefitinib could be predicted within days of therapy (Sunaga et al. 2008; Takahashi et al. 2012). Also, metabolic treatment response was linked with survival and quality of life (Sunaga et al. 2008; Takahashi et al. 2012; van Gool et al. 2014a, b; Benz et al. 2011; Hachemi et al. 2014).
In 1999, the European Organization for Research and Treatment of Cancer (EORTC) published criteria for tumor response classification which were among the first to include the assessment of tumor metabolism using functional imaging with FDG PET (Young et al. 1999). These criteria used the standardized uptake value (SUV) as a metric for quantifying radiopharmaceutical uptake at sites of disease, a metric that reflects radiopharmaceutical uptake corrected for total body mass (patient weight) and injected radiopharmaceutical activity. According to EORTC criteria: (1) A complete metabolic response (CMR) was when there was no site of disease distinguishable from adjacent background activity; (2) progressive metabolic disease (PMD) was an increase in maximum SUV (SUVmax) of 25% or more from baseline or the appearance of new disease sites; (3) a partial metabolic response (PMR) was a reduction in SUVmax between 15 and 25% after one or more cycles of chemotherapy; and (4) stable metabolic disease (SMD) was disease response that could not be classified into another category. The number of lesions to measure and minimum measurable lesion activity was not defined. Anatomic information was not included.
In 2009, Wahl et al. proposed Positron Emission Tomography Response Criteria In Solid Tumors (PERCIST) for FDG PET (Wahl et al. 2009). Main differences between EORTC and PERCIST were (Table 1, Aide et al. 2018): (1) use of SULpeak (radiopharmaceutical activity measured in a 1 cm3 sphere at the site of highest tumor activity corrected for lean body mass) rather than SUVmax, (2) specification of five sites of disease (up to two per organ) or target lesions to be measured, and (3) definition of a measurable lesion as having at least 1.5 times the mean SUL of liver.
With the advent of standardized criteria for molecular and functional imaging therapy response assessment, debate flourished concerning the value of using a qualitative (visual) versus a quantitative (objective) approach. A study by Lin et al. comparing qualitative and quantitative FDG PET analysis in patients with diffuse large B cell lymphoma DLBCL (Lin et al. 2007) found the qualitative analysis predicted event-free survival with an accuracy of 65.2%, whereas the quantitative SUV-based analysis had an accuracy of 76.1%. However, quantitative analyses have limitations: (1) There are several methods for calculating and reporting radiopharmaceutical uptake at disease sites e.g., correcting for total body mass versus lean body mass, reporting maximal activity (SUVmax) versus average activity in a defined region (SUVpeak, SUVmean), use of metabolically active tumor bulk defined by indices of metabolic tumor volume (MTV) and total lesion glycolysis (TLG) as well as tumor metabolic heterogeneity estimated through texture analysis, among others. (2) Differences in scanner hardware, image reconstruction, and patient characteristics, among other factors, affect radiopharmaceutical uptake at disease sites and can impact metrics of response assessment (Ziai et al. 2016).
In an effort to achieve repeatability and reproducibility of response assessment metrics, guidelines were produced detailing how oncologic PET/CT scans should be performed (Boellaard et al. 2015; Fendler et al. 2017). Recommendations include the use of a standardized protocol for scan acquisition and maintenance of consistency between scanners, image acquisition and reconstruction parameters, dose of radiopharmaceutical administered and uptake time between baseline and follow-up imaging, among others. Also phantom derived parameters may help align quantification metrics between scanners and image reconstructions (Lasnon et al. 2013, 2017; Quak et al. 2016). Finally, inclusion of activity in a reference region of interest (ROI) such as liver or aortic blood pool is suggested in an oncologic PET/CT report to serve as an alert for potential technical issues if/when this is outside the expected range.
Currently, therapy response assessment criteria often include a combination of anatomic, molecular, and functional imaging. There are criteria for response assessment that are used in clinical trials and are not specific to cancer histology. In most cases there are no clinical guidelines or standards directing the use of these measurements in patient care and these criteria (such as PERCIST) are rarely used in routine clinical practice. A few criteria for molecular and functional disease response classification are specific to cancer histology (e.g., Deauville/Lugano). These criteria are incorporated into clinical guidelines (e.g., NCCN [National Comprehensive Cancer Network]) and included in clinical PET/CT reporting. Although the clinical and research communities remain fragmented in their use of molecular and functional imaging therapy response assessment criteria, there is momentum to converge on a common approach for the purposes of PET/CT reporting and the most illustrative example of this is lymphoma.
6 Molecular and Functional Imaging Response Assessment in Lymphoma
Lymphoma is a heterogeneous spectrum of lymphoproliferative disease classified as Hodgkin’s lymphoma (HL) or non-Hodgkin’s lymphoma (NHL) that encompasses a spectrum of disease of variable metabolic activity. It is estimated that approximately 40% of non-Hodgkin’s lymphoma patients and 20% of Hodgkin’s lymphoma patients have a residual mediastinal or abdominal mass following therapy, and that most are non-malignant on pathology (Orlandi et al. 1990; Aisner and Wiernik 1982; Mikhaeel et al. 2000). It is difficult to distinguish inflammatory, necrotic, or fibrotic tissue from residual lymphoma based on anatomic evaluation alone (Canellos 1988; Reske 2003; Lewis et al. 1982; Surbone et al. 1988). Molecular and functional imaging with PET can distinguish metabolically active from non-metabolically active disease and helps overcome the limitation of anatomically based response assessment for lymphoma. Molecular and functional response criteria have been used in the evaluation of patients with lymphoma for many years.
Following a workshop held in Deauville, France, in 2009 (Meignan et al. 2009), the Deauville 5-point scoring system was created based on FDG PET, with treatment response assessed qualitatively on a 5-point scale according to the intensity of uptake at sites of disease relative to reference activity in mediastinal blood pool and liver. Scores of 3 or below (comparable to liver activity or less) are considered negative for metabolically active residual disease (Fig. 8). Scores of 4–5 (above liver activity) are considered positive for residual metabolically active disease. Several studies have shown interobserver agreement of this system. For example, Barrington et al., Furth et al., and Gallamini et al. comparing interobserver agreement in HL reported κ values of 0.79–0.85, 0.748, and 0.69–0.84, respectively (Barrington et al. 2010; Furth et al. 2011; Gallamini et al. 2009, 2014). The system was easy to apply and was the first molecular and functional response criteria to become part of routine clinical oncologic PET/CT reporting for patients with HL (Meignan et al. 2010, 2012; Le Roux et al. 2011). In 2014, following the 12th International Conference on Malignant Lymphomas (ICML) in Lugano, Switzerland, the Lugano classification system was created (Barrington et al. 2014; Cheson et al. 2014). The Lugano classification includes both PET and CT response assessment as well as a combination of qualitative and quantitative metrics. The PET criteria are based on the Deauville 5-point scoring system, while the inclusion of CT criteria overcame the limitation of response in lymphomas with low or variable FDG avidity. Reproducibility of the Lugano classification system is being determined.
Among the advantages of a standardized response assessment in lymphoma is the predictive value and ability to modify treatment early in the disease course to improve outcome. In limited HL, the prognosis is excellent and so characterization of functional and molecular imaging therapy response on interim FDG PET/CT (typically after 2 or 4 chemotherapy cycles) has failed to distinguish between patients in terms of outcome. However, as the disease becomes more extensive, an interim positive PET suggests poorer outcome (Moghbel et al. 2017). Further, inclusion of both PET and CT response assessment typically show improved patient stratification and clinical outcome. For example, a study of interim PET and CT in HL reported 2-year PFS of 95%, 78%, 71%, and 36% with PET−/CT−, PET−/CT+, PET+/CT−, and PET+/CT+ patients, respectively (Kostakoglu et al. 2012). Further, the results of interim PET can show complications of therapy (Fig. 9) and enable early treatment modification resulting in improved outcome. For example, in a study of patients with HL and positive interim PET after 2 cycles of ABVD, escalating therapy (2 cycles of BEACOPP + involved node radiotherapy) resulted in improved PFS (90.6% versus 77.4%) (André et al. 2017).
Baseline 18F-FDG PET/CT MIP image in a man with Hodgkin’s lymphoma shows metabolically active lymph nodes above and below the diaphragm as well as osseous and right renal disease (a). Interim 18F-FDG PET/CT MIP image shows response to therapy; however, there was development of pneumonitis likely related to drug toxicity (b). 18F-FDG PET/CT MIP image at the completion of therapy shows response to therapy with resolution of the pneumonitis
Of course, in specific scenarios such as patients on immunotherapy, certain modifications to the criteria must be considered. In 2016, modification to the Lugano criteria (LYRIC criteria) was suggested to account for immunotherapy response assessment. The main change compared with the Lugano criteria was the addition of an indeterminate response category (Cheson et al. 2016).
7 Molecular and Functional Therapy Response Assessment and Immune Therapy
In recent years, there has been investigation into immunotherapy (Popovic et al. 2018). Today, the most ubiquitous agents include: (1) T lymphocyte-associated protein 4 (CTLA-4) inhibitors (e.g., ipilimumab) and (2) programmed cell death protein 1 (PD1) or PD1/programmed cell death protein ligand 1 (PD1/PD-L1) axis inhibitors (e.g., pembrolizumab and nivolumab). The idea is that CTLA-4 is a protein recruited to the surface of regulatory T cells where it interacts with B7 receptors on antigen-presenting cells resulting in T cell downregulation. Thus, inhibition of CTLA-4 results in enhanced T cell activation and immune response expansion. PD1 is a transmembrane glycoprotein expressed on immune cells and PD-L1 is a ligand for PD1 that may be expressed on tumor cells. When PD1 is bound by PD-L1, it inhibits kinases involved in T cell activation. Thus, inhibition of this process can also enhance immunity. Current research in the area is focused, at least in part, on blocking additional immune regulatory checkpoints, inducing immune responses with vaccines or increasing tumor traffic of lymphocytes. The literature suggests ipilimumab monotherapy results in overall benefit for about 20% of patients with melanoma (Hodi et al. 2010) and that this can be improved to over 50% using a combination of ipilimumab and nivolumab (Fig. 10), albeit with higher risk of toxicity (Larkin et al. 2015). Interestingly, radiation provides immune co-stimulatory signals, hence the rationale for combining external beam or radionuclide therapy with immunotherapy. It has been postulated that PET may noninvasively provide information of the tumor microenvironment predictive of response; however, this remains to be rigorously proven.
18F-FDG PET/CT images in a woman with metabolically active melanoma. Axial CT, PET, fused and MIP images obtained prior to (a) and following (b) ipilimumab and nivolumab therapy show complete response. The focal radiopharmaceutical uptake in the right central neck (orange arrow) was in a thyroid nodule and likely reflects primary thyroid pathology (results of biopsy pending)
By enhancing the immune response, immune-related adverse events may be induced (e.g., dermatitis (pruritus/rash/vitiligo), endocrine disorders (hypophysitis, thyroiditis, etc.), pneumonitis, gastrointestinal symptoms (diarrhea, colitis, etc.), hepatitis, pancreatitis, and myalgia among other things). Following immunotherapy, reactive splenic enlargement and reactive lymph node enlargement in the tumor drainage basin are also common. Since inflammation is typically FDG-avid, PET can detect immune-related adverse events, sometimes weeks before these become clinically apparent (Fig. 11) (Kwak et al. 2015). Although this is helpful since rapid initiation of systemic therapy (e.g., systemic corticosteroids) can improve patient outcome, it can make the disease response difficult to assess.
Clinical and imaging response to immunotherapy is variable. Often an early response is seen. Inflammatory reactions can occur at tumor sites within days of therapy (Reusch et al. 2006). In some cases a response can be delayed for weeks or months (Le et al. 2013). Further, tumor flare cannot be distinguished from progression based on morphologic, imaging or even on FDG PET/CT. It is estimated that approximately 15% of patients with melanoma on ipilimumab show increasing disease burden on imaging despite clinical benefit (e.g., pseudoprogression or flare), although this is lower (less than 3%) with other agents (Wolchok et al. 2009). In a small number of cases, immunotherapy can provoke rapid disease progression or hyperprogression (Champiat et al. 2017; Saâda-Bouzid et al. 2017). As such it is key to correlate imaging findings with the patient’s clinical condition: (1) those patients with improving or stable clinical condition and progression on imaging may be experiencing pseudoprogression and, in this case, treatment may be continued with response confirmed by follow-up imaging; (2) those patients who are deteriorating are most likely progressing and discontinuing therapy may be warranted since waiting for imaging confirmation could lead to deterioration rendering a new therapy nonviable.
Recently, two new molecular and functional imaging response assessment criteria have been proposed in the setting of immunotherapy: (1) PET/CT Criteria for Early Prediction of Response to Immune Checkpoint Inhibitor Therapy (PECRIT) (Cho et al. 2017) and (2) PET Response Evaluation Criteria for Immunotherapy (PERCIMT) (Anwar et al. 2018; Sachpekidis et al. 2018). In both cases, the evaluation of clinical benefit is incorporated as well as the use of morphologic and functional metrics (Table 1). Currently, it is suggested that a baseline PET be performed prior to immunotherapy with follow-up 8–9 weeks or more after immunotherapy initiation (typically after 2 or 3 cycles of therapy) and at therapy completion. It is thought that the value of FDG PET is most pronounced in patients with limited morphological response on anatomic imaging, or who develop signs/ symptoms of immune-related adverse events. Further, clinical benefit and the presence of a metabolic response despite morphologic progression can be helpful for clinical decision-making.
Functional and molecular response assessment imaging in oncologic patients receiving immunotherapy remains imperfect, and research into more specific imaging biomarkers is ongoing, including clinical trials using 89Zr-labeled immune checkpoint inhibitors as well as investigation into the use of radiolabeled antibody fragments.
8 Conclusion
We have come a long way from the crude manual disease assessment of yesterday to the standardized staging and response assessment criteria of today. Further, as our technology improves, so too does the possibility of more advanced imaging assessment including complex structural and functional data acquisition with parametric mapping and kinetic modeling allowing evaluation of tumor heterogeneity throughout the body. Also, the recent proliferation of hybrid scanners that include anatomic, functional, and molecular imaging capabilities has enhanced our ability to assess disease response, adjust therapy regimens, and develop an accurate measure of patient prognosis. It has been recognized that standardization of image acquisition and analysis parameters as well as harmonization of criteria used for response assessment across the clinical and research landscape is important. As our understanding of the biological effects of therapeutic interventions improves, so too does our understanding of the best time-points for therapy response assessment. Although further studies are necessary we are starting to converge on a universal system, particularly in certain tumors such as lymphoma.
References
Ady N, Zucker JM, Asselain B et al (1995) A new 123I-MIBG whole body scan scoring method-application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer 31A(2):256–261
Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E (2018) FDG PET/CT for assessing tumour response to immunotherapy—report on the EANM symposium n immune modulation nd recent review of the literature. Eur J Nucl Med Mol Imaging 46:238–250
Aisner J, Wiernik PH (1982) Restaging laparotomy in the management of the non-Hodgkin lymphomas. Med Pediatr Oncol 10:429–438
André MPE, Girinsky T, Federico M et al (2017) Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 35:1786–1794
Anwar H, Sachpekidis C, Winkler J et al (2018) Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 45:376–383
Barrett JA, Coleman RE, Goldsmith SJ et al (2013) First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med 54:380–387
Barrington SF, Qian W, Somer EJ et al (2010) Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 37:1824–1833
Barrington SF, Mikhaeel NG, Kostakoglu L et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol 32:3048–3058
Beauregard JM, Hodman MS, Kong GK et al (2012) The tumor sink effect on the biodistribution of 68Ga-DOTA-octreotate: implications for peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 39:50–56
Benz MR, Herrmann K, Walter F et al (2011) 18F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J Nucl Med 52(11):1684–1689
Boellaard R, Delgado-Bolton R, Oyen WJG et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42:328–354
Bouchelouche K, Choyke PL, Capala J (2010) Prostate specific membrane antigen—a target for imaging and therapy with radionuclides. Discov Med 9:55–61
Canellos G (1988) Residual mass in lymphoma may not be residual disease. J Clin Oncol 6:931–933
Champiat S, Dercle L, Ammari S et al (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920–1928
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Cheson BD, Ansell S, Schwartz L et al (2016) Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 128:2489–2496
Cho SY, Lipson EJ, Im HJ et al (2017) Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med 58:1421–1428
Coleman RE, Mashiter G, Whitaker KB, Moss DW, Rubens RD, Fogelman I (1988) Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med 29:1354–1359
Cook GJR, Venkitaraman R, Sohaib AS (2011) The diagnostic utility of the flare phenomenon on bone scintigraphy in staging prostate cancer. Eur J Nucl Med Mol Imaging 38:7–13
Dennis ER, Jia X, Mezheritskiy IS et al (2012) Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer. J Clin Oncol 30:519–524
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Emmett L, Crumbaker M, Ho B et al (2018) Metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. [Epub ahead of print]
Evans MJ, Smith-Jones PM, Wongvipat J et al (2011) Noninvasive measurement of androgen receptor signaling with positron emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A 108:9578–9582
Even-Sapir E, Metser U, Mishani E et al (2006) The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med 47:287–297
Fendler W, Eiber M, Beheshti M et al (2017) 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 44:1014–1024
Furth C, Amthauer H, Hautzel H et al (2011) Evaluation of interim PET response criteria in paediatric Hodgkin’s lymphoma—results for dedicated assessment criteria in a blinded dual-centre read. Ann Oncol 22:1198–1203
Gallamini A, Fiore F, Sorasio R, Meignan M (2009) Interim positron emission tomography scan in Hodgkin lymphoma: definitions, interpretation rules, and clinical validation. Leuk Lymphoma 50:1761–1764
Gallamini A, Barrington SF, Biggi A et al (2014) The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica 99:1107–1113
Gerbaudo VH, Garcia CA (2016) PET/CT of lung cancer. Springer, Cham
van Gool MH, Aukema TS, Schaake EE et al (2014a) NEL Study Group. Timing of metabolic response monitoring during erlotinib treatment in non-small cell lung cancer. J Nucl Med 55(7):1081–1086
van Gool MH, Aukema TS, Schaake EE et al (2014b) 18F-fluorodeoxyglucose positron emission tomography versus computed tomography in predicting histopathological response to epidermal growth factor receptor-tyrosine kinase inhibitor treatment in resectable non-small cell lung cancer. Ann Surg Oncol 21(9):2831–2837
Grant FD, Fahey FH, Packard AB et al (2008) Skeletal PET with 18F-fluoride: applying a new technology to an old tracer. J Nucl Med 49:68–78
Hachemi M, Couturier O, Vervueren L et al (2014) [18F]FDG positron emission tomography within two weeks of starting erlotinib therapy can predict response in non-small cell lung cancer patients. PLoS One 9(2):e87629
Hicks RJ, Mac Manus MP, Matthews JP et al (2004) Early FDG-PET imaging after radical radiotherapy for non-small-cell lung cancer: inflammatory changes in normal tissues correlate with tumor response and do not confound therapeutic response evaluation. Int J Radiat Oncol Biol Phys 60(2):412–418
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Hoekstra CJ, Stroobants SG, Smit EF et al (2005) Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-d-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol 23(33):8362–8370
Koerber SA, Will L, Kratochwil C et al (2018) 68Ga-PSMA-11 PET/CT in Primary and recurrent prostate carcinoma: implications for radiotherapeutic management in 121 patients. J Nucl Med [Epub ahead of print]
Kostakoglu L, Schöder H, Johnson JL et al (2012) Interim FDG PET imaging in stage I–II non-bulky Hodgkin lymphoma: would using combined positron emission tomography and computed tomography criteria better predict response than each test alone? Leuk Lymphoma 53:2143–2150
Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA (2015) Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics 35:424–437
Larkin J, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:1270–1271
Lasnon C, Desmonts C, Quak E et al (2013) Harmonizing SUVs in multicentre trials when using different generation PET systems: prospective validation in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 40:985–996
Lasnon C, Quak E, Le Roux PY et al (2017) EORTC response criteria are more influenced by reconstruction inconsistencies than PERCIST but both benefit from the EARL harmonization program. EJNMMI Phys 4:17
Le Roux P-Y, Gastinne T, Le Gouill S et al (2011) Prognostic value of interim FDG PET/CT in Hodgkin’s lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging 38:1064–1071
Le DT, Lutz E, Uram JN et al (2013) Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 36:382–389
Lewis E, Bernardino ME, Salvador PG, Cabanillas FF, Barnes PA, Thomas JL (1982) Post-therapy CT-detected mass in lymphoma patients: is it viable tissue? J Comput Assist Tomogr 6:792–795
Lin C, Itti E, Haioun C et al (2007) Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med 48:1626–1632
Mac Manus MP, Hicks RJ, Matthews JP, Wirth A, Rischin D, Ball DL (2005) Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer 49(1):95–108
Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C (2009) Report on the first international workshop on interim-PET scan in lymphoma. Leuk Lymphoma 50:1257–1260
Meignan M, Gallamini A, Haioun C, Polliack A (2010) Report on the Second International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma 51:2171–2180
Meignan M, Gallamini A, Itti E, Barrington S, Haioun C, Polliack A (2012) Report on the third international workshop on interim positron emission tomography in lymphoma held in Menton, France, 26-27 September 2011 and Menton 2011 consensus. Leuk Lymphoma 53:1876–1881
Mikhaeel N, Timothy A, Hain S, O’Doherty M (2000) 18-FDG-PET for the assessment of residual masses on CT following treatment of lymphomas. Ann Oncol 11:S147–S150
Moghbel MC, Mittra E, Gallamini A et al (2017) Response assessment criteria and their applications in lymphoma: Part 2. J Nucl Med 58(1):13–22
Orlandi E, Lazzarino M, Brusamolino E et al (1990) Residual mediastinal widening following therapy in Hodgkin’s disease. Hematol Oncol 8:125–131
Pollen JJ, Witztum KF, Ashburn WL (1984) The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR Am J Roentgenol 142:773–776
Popovic A, Jaffee EM, Zaidi N (2018) Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest 128:3209–3218
Quak E, Le Roux PY, Lasnon C et al (2016) Does PET SUV harmonization affect PERCIST response classification? J Nucl Med 57:1699–1706
Reske S (2003) PET and restaging of malignant lymphoma including residual masses and relapse. Eur J Nucl Med Mol Imaging 30(Suppl 1):S89–S96
Reusch U, Sundaram M, Davol PA et al (2006) Anti-CD3 × anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res 12:183–190
Rowe SP, Macura KJ, Blackford AL et al (2016) PSMA-based [18F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients wit metastatic prostate cancer. Mol Imaging Biol 18(3):411–419
Saâda-Bouzid E, Defaucheux C, Karabajakian A et al (2017) Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 28:1605–1611
Sachpekidis C, Anwar H, Winkler J et al (2018) The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging 45:1289–1296
Scher HI, Morris MJ, Stadler WM et al (2016) Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol 34:1402–1418
Schwarz SW, Decristoforo C, Goodbody AE et al (2019) Harmonization of United States, European Union and Canadian first-in-human regulatory requirements for radiopharmaceuticals—is this possible? J Nucl Med [Epub ahead of print]
Sunaga N, Oriuchi N, Kaira K et al (2008) Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer 59(2):203–210
Surbone A, Longo DL, DeVita V et al (1988) Residual abdominal masses in aggressive non-Hodgkin’s lymphoma after combination chemotherapy: significance and management. J Clin Oncol 6:1832–1837
Takahashi R, Hirata H, Tachibana I et al (2012) Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res 18(1):220–228
Vansteenkiste J, Fischer BM, Dooms C, Mortensen J (2004) Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol 5:531–540
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Warburg O (1956) On respiratory impairment in cancer cells. Science 124(3215):269–270
Warburg O, Wind F, Negelein E (1927) The metabolism of tumours in the body. J Gen Physiol 8(6):519–530
Weber WA, Petersen V, Schmidt B et al (2003) Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol 21:2651–2657
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–1782
Zacho HD, Petersen LJ (2018) Bone flare to androgen deprivation therapy in metastatic, hormone-sensitive prostate cancer on 68Ga-prostate-specific membrane antigen PET/CT. Clin Nucl Med 43(11):e404–e406
Ziai P, Hayeri MR, Salei A et al (2016) Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. Radiographics 36:481–496
Zukotynski KA, Valliant J, Benard F et al (2018) Flare on serial prostate-specific membrane antigen-targeted 18F-DCFPyL PET/CT examinations in castration-resistant prostate cancer: first observations. Clin Nucl Med 43(3):213–216
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zukotynski, K.A., Kuo, P.H., Kim, C.K., Subramaniam, R.M. (2020). Molecular and Functional Imaging in Oncology Therapy Response. In: Nishino, M. (eds) Therapy Response Imaging in Oncology. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-030-31171-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-31171-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31170-4
Online ISBN: 978-3-030-31171-1
eBook Packages: MedicineMedicine (R0)