Abstract
Mountain ranges are important centers of biodiversity around the world. This high diversity is the result of the presence of different soil types and underlying bedrock, a variety of micro-climatic regimes, high topographic heterogeneity, a heterogeneous and complex vegetation cline, and a dynamic geo-climatic history. Neotropical research on mountains has focused on the Andes, while other mountain ranges are lacking in biodiversity and biogeographic studies. However, the non-Andean mountains comprise important elements of the South American relief, are home to a substantial proportion of Neotropical species, and exhibit a complex and reticulate history of diversification of their biota. Here, we provide a brief review of the biological and biogeographical importance of the major non-Andean South American mountain ranges, discussing their role for diversification and maintenance of Neotropical biodiversity. We focus on six regions: the Serra do Mar Range, the Mantiqueira Mountains, the Espinhaço Mountains, the Northeastern Highlands, the Central Brazilian Highlands, and the Pantepui region. We summarize the main geophysical and biotic characteristics of each mountain range, as well as key results from phylogenetic studies, the fossil record, and studies tackling biogeographical patterns of diversity, richness, and endemism. Moreover, mountain biodiversity studies can incorporate not only environmental data, but also information on more recent man-made landscape shifts. Here, we provide an example of how human population density interacts with climate and species traits to explain richness patterns in one group of montane organisms particularly vulnerable to environmental changes: anuran amphibians. Our results and the evidence published to date indicate that the Neogene and Quaternary were pivotal periods of Neotropical diversification for many terrestrial taxa, promoting endemism in non-Andean mountains. In general, all non-Andean mountain ranges have high levels of species richness and endemism as compared to their surrounding lowlands. Biotic interchange among them, the Andes, and their surrounding biotas has been intensive over tens of millions of years, greatly contributing to the outstanding levels of Neotropical biodiversity observed today. Despite their vast and understudied biodiversity, mountain ecosystems are fragile, facing severe challenges in the face of climate change, habitat loss, and extinctions. Efforts to better understand and protect South American mountain ecosystems are urgently needed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Atlantic Forest
- Caatinga
- Campos de altitude
- Campos rupestres
- Cerrado
- Diversification
- Montane habitats
- Tepuis
1 Introduction
There is a strong connection between mountains and biodiversity. Topographic variation, heterogeneity of soil types, and altitudinal gradients are important factors that, together with climatic variability, generate habitat diversity in mountains and increase species richness and endemism (Tuomisto et al. 2003; Körner 2004; Fischer et al. 2011; Fjeldså et al. 2012; Luebert and Muller 2015; Badgley et al. 2017; Antonelli et al. 2018a). Furthermore, the composition and spatial distribution of biodiversity in mountain ranges may also reflect environmental tolerances, habitat fragmentation, and distinct life histories of species (Marquet et al. 2004; Leibold et al. 2004; Ricklefs 2004; Schipper et al. 2008). With their often-isolated positions on continents, like islands in a surrounding ocean, mountain ranges are key to understanding evolutionary processes since they generate, receive, and maintain biodiversity (Hughes and Atchison 2015; Antonelli et al. 2009, 2018a)—thereby influencing spatial patterns of biological diversity (Homeier et al. 2010; Bonaccorso and Guayasamin 2013; Hoorn et al. 2013, 2018a; Guedes et al. 2014; Moura et al. 2016; Bacon et al. 2018a). Mountain ranges around the world are known to hold about one-third of all terrestrial species and are recognized as important centers of biological diversity (Körner et al. 2017). These areas are crucial for the maintenance of biodiversity and have been of interest since the days of early naturalists, including Alexander von Humboldt and Charles Darwin (Wulf 2016).
Owing to their remoteness and, sometimes, extremely difficult access, the biodiversity of mountains remains poorly explored. In the Neotropics, much attention has been paid to the patterns and processes of diversity of the rich Andean biota, the longest mountain range on Earth (e.g., Rundel et al. 1994; Kessler 2001; Cadena 2007; Castroviejo-Fisher et al. 2014; Luebert and Weigend 2014; Bacon et al. 2016, 2018a, b; Sanín et al. 2016; Hoorn et al. 2018b). However, besides the Andes, the Neotropics harbor several other important mountain ranges.
In the Neotropics, outside South America, there is pronounced relief (<3100 m) on several Caribbean islands such as Cuba, Hispaniola, and Jamaica. Mexican and Central American mountains and plateaus are also extensive and highly complex, including volcanos that reach above 4200 m of altitude in Guatemala, and several volcanos in Mexico that exceed 5000 m.
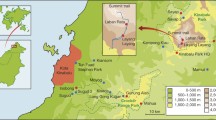
In South America, non-Andean mountain ranges are generally lower than 3000 m and include the Serra do Mar Range, the Mantiqueira Mountains, the Espinhaço Mountains, the Diamantina Plateau, the Central Brazilian Highlands (e.g., the Guimarães Plateau, the Serra Geral Plateau, the Caiaponia Mountain, the Veadeiros Plateau, the Central Brazilian Plateau, the Canastra Range, the Mesas Plateau region, the Parecis Plateau, and the Bodoquena Range), the Northeastern Highlands and the Pantepui Region (Fig. 13.1). These non-Andean mountains act as refugia and centers of endemism and diversification for Neotropical organisms (Rull 2005; Carnaval et al. 2009; Désamoré et al. 2010; Bonacorso and Guayasamin 2013; Chaves et al. 2014; Azevedo et al. 2016; Huber et al. 2018).
The non-Andean Mountains of South America. Map of the Neotropical region showing the major non-Andean Mountains of continental South America reviewed here, according to the indication and naming of the Global Mountains Biodiversity Assessment (GMBA 2018). The map was produced on QGis 2.14.10 using a relief mask provided by Natural Earth Data (https://www.naturalearthdata.com)
The Atlantic Forest is a hotspot of biodiversity (Myers et al. 2000) that comprises three non-Andean mountain ranges: the Serra do Mar Range, the Mantiqueira Mountains, and Espinhaço Mountains. The biodiversity of this complex rainforest system is one of the best documented in South America (e.g., Bello et al. 2017; Bovendorp et al. 2017; Culot et al. 2019; Santos et al. 2018; Vancine et al. 2018). However, most of its original vegetation has been degraded, and a large portion of it has been completely lost as a result of human disturbances (Ribeiro et al. 2009), leaving only 9%–16% of its original extent. The effects of degradation are seen at a broad range of spatial scales, including changes in microclimatic conditions (Didham and Lawton 1999), species abundances and community composition (Ewers and Didham 2005), geographic distributions of species (Ewers and Didham 2005), and effects on global climate change (Travis 2003). There is also evidence that deforestation has changed the distributional patterns of some species in the Atlantic Forest (Sancha et al. 2014).
The scarcity of species inventories for most biological groups inhabiting South American mountains, combined with an even more severe lack of phylogenetic and fossil information, are major obstacles to understanding the origin and maintenance of the huge biological diversity on mountains (Zizka and Antonelli 2018). In this chapter, we (1) provide an overview of non-Andean mountains in South America from an environmental and a biological perspective, and (2) summarize what is known about the mechanisms potentially underlying local diversification. To address these goals, we combine evidence from phylogenies, the fossil record, and biogeographical patterns. As an empirical example of how biodiversity data can be studied and interpreted in the light of anthropogenic pressures, we (3) select one specific group (amphibians), and one mountain range (the Serra do Mar, in the Brazilian Atlantic Forest), to analyze the influence of bioclimatic variables and human population density on species richness.
2 Non-Andean South American Mountains: What Are They?
Central and Eastern South America have several mountain ranges that often support relatively high levels of biodiversity. In this section, we focus on the most prominent non-Andean mountains (Fig. 13.1) based on a recent shapefile provided by The Global Mountain Biodiversity Assessment (GMBA 2018). Our aim is to discuss the origin and diversification of their biota, as well their patterns of species richness, endemism, and distribution. Below, we summarize the main geophysical and biotic characteristics of each mountain range.
Serra do Mar Range, or Serra do Mar. This is a continuous mountain range extending about 1500 km along the east coast of Brazil between the states of Rio de Janeiro in the north to northern Rio Grande do Sul in the south (Fig. 13.1). The Serra do Mar Range forms a narrow strip of cliffs and eroded escarpments on granite-gneiss bedrock (Gontijo-Pascutti et al. 2012). In the region that faces to the coast, these cliffs drop 1000–1300 m (all altitudes are provided as meters above sea level), while the interior (continental) face has small peaks where elevation reaches between 500 and 1100 m. The range’s highest peaks are in the Serra dos Órgãos (e.g., 2366 m in Maior Peak, 2257 m in Caledônia Peak, and 2255 m in Pedra do Sino) in the state of Rio de Janeiro, and in the Serra da Bocaina (1550 m) in the state of São Paulo (Almeida 1964; Ab’Saber 1971; Gontijo-Pascutti et al. 2012). The Serra do Mar Range is mostly covered by the Atlantic Forest (with the exception of barren granite outcrops), with a complex set of physiognomies dominated by ombrophilous montane forest (Fig. 13.2a, b) (Veloso et al. 1991; Morellato and Haddad 2000; Medeiros et al. 2012), and patches of highland grasslands above 1000 m (Garey and Provete 2016). The region has outstanding levels of species richness and endemism and is therefore recognized as a global hotspot of biodiversity (Fig. 13.2i–l; Myers et al. 2000). Currently, less than 10% of the original area of the Atlantic Forest remains, of which most is concentrated in the Serra do Mar region (Galindo-Leal and Câmara 2003; Ribeiro et al. 2009).
Landscapes and biodiversity of non-Andean South American Mountains. Examples of the diversity of habitats in mountains along with some characteristic taxa: (a) Serra do Mar Range, dominated by ombrophilous Atlantic Forest in the state of São Paulo, Brazil; (b) View from the top of Serra da Bocaina, one of the highest peaks of the Serra do Mar in the state of São Paulo, Brazil, reaching 1500 m above sea level; (c) View of the mountainous relief of the Itatiaia massif, in the Mantiqueira Mountains at the border between the states of Minas Gerais and Rio de Janeiro, Brazil; (d) The highland grassland campos de altitude of the Serra da Bocaina; (e) Forested areas in the Northeastern Highlands in Areia, Paraíba, Brazil; (f) View of the Pantepui Region, Guiana Highland, in French Guiana; (g) General view of the Diamantina Plateau showing a complex physiognomy including savanna (Cerrado), semi-arid (Caatinga), and forested vegetation; (h) Blue lily Vellozia sp., common in the Serra da Canastra after the fire season; (i) Dendropsophus elegans, an endemic frog species of the Atlantic Forest, shown here in the Serra do Mar Range; (j) The Black-cheeked Gnateater Conopophaga melanops, an endemic species of the Atlantic Forest photographed in the limits of the Serra do Mar Range, São Paulo, Brazil; (k) Echinanthera amoena, an endemic snake of the Atlantic Forest, whose distribution coincides with the limits of the Serra do Mar Range; (l) Mazama guazoubira, a species of mammal found on Serra do Mar Range, state of São Paulo, Brazil. Photo credits: a, e, h, i, k: TBG; b–d: DBP; f: AA; g: Daniela Coelho; j: Giulia B. D’Angelo; l: Marcela Nascimento
Mantiqueira Mountains, or Serra da Mantiqueira. These mountains derive from the same tectonic events that formed the Serra do Mar Range, making their exact delimitation difficult (Modenesi-Gauttieri et al. 2002; Gontijo-Pascutti et al. 2012). The Mantiqueira region stretches for about 900 km along the borders of three Brazilian states (Minas Gerais, Rio de Janeiro, and São Paulo; Figs. 13.1 and 13.2c). It is divided into two distinct geomorphological units, Campos do Jordão (in the states of São Paulo and Minas Gerais) and Itatiaia (reaching the states of Minas Gerais and Rio de Janeiro) massifs, formed by crystalline rocks at altitudes between 1700 and 2000 m (Ab’Saber and Bernardes 1958; Almeida 1964; Ab’Saber 1970; Gontijo-Pascutti et al. 2012). The highest portions of the Mantiqueira Mountains are the Agulhas Negras Peak (2792 m), Três Estados Peak (2665 m), Mina Peak (2798 m), and the isolated Bandeira Peak (inside Serra do Caparó, 2891 m). The region is also part of the Atlantic Forest bioregion and besides the typical rainforests of the region, it also includes highland grasslands or campos de altitude. The campos de altitude are a series of cool-humid, grass-dominated formations found exclusively on the uplifted blocks of igneous or high-grade metamorphic rocks above the treeline (up to 1000 m; Fig. 13.2d). The vegetation consists mainly of grasses and herbaceous daises and allies (Asteraceae) and Melastomataceae, with many endemic species (Safford 1999a, b; Almeida et al. 2004; Garey and Provete 2016; Silva et al. 2018).
Espinhaço Mountains, or Cadeia do Espinhaço. This is the second largest South American mountain range after the Andes, extending for about 1000 km and up to 75 km wide (Fig. 13.1). The Espinhaço range is formed by sets of smaller ranges, with many local names, and the altitude generally varies between 800 and 1000 m. Some higher elevations can be found along its extension, the highest ones being the Sol (2072 m) and Itambé Peaks (2002 m), both in the state of Minas Gerais. The Espinhaço Mountains separate two large river basins: the São Francisco to the west and the Doce to the east; and they also form a border between the Atlantic Forest and the open, savanna landscapes of the Cerrado and Caatinga bioregions. The Espinhaço Mountains are an important geographical and ecological barrier that shaped the distribution and diversification of many Neotropical organisms occurring across the region (Ab’Saber 1977; Alkmin 2012). The special soil conditions (acidic, nutrient-impoverished and low in phosphorus), climate, and relief harbor a unique physiognomy, the rocky grasslands (campos rupestres) being the most peculiar one. The campos rupestres are not a single homogeneous vegetation type, but instead are a mosaic of related communities composed mainly of grasses (Poaceae) and Asteraceae, also containing many narrowly distributed and threatened taxa, such as orchids growing directly on rocky outcrops (Antonelli et al. 2010). This landscape is interspersed with savanna vegetation patches, including denser shrubs and sparse treelets, which cover the higher slopes and mountaintops (between 700 and 2000 m of elevation), especially in the Espinhaço Mountains (Giulietti and Pirani 1988; Almeida et al. 2004; Chaves et al. 2014). The campos rupestres, also present over other non-Andean highlands, cover less than 1% of the Brazilian territory, yet they harbor a disproportionate 17% of the country’s estimated plant diversity, and almost half of the diversity of the Cerrado (Fernandes et al. 2018).
Diamantina Plateau, or Chapada Diamantina. This is the northernmost portion of the Espinhaço Mountains. The Diamantina Plateau comprises an area of about 35,000 km2 in the state of Bahia, northeastern Brazil (Fig. 13.1). The highest altitudes can be found in Almas, Itobira, and Barbados Peaks, reaching from 1958 to 2033 m, respectively (Alkmin 2012). The Diamantina Plateau is part of the Caatinga bioregion (Ab’Saber 1977; Morrone 2014), but it contains a diverse set of landscapes and physiognomies, including typical elements of the Caatinga, open grasslands typical of the Cerrado, campos rupestres, and semi-deciduous Atlantic Forest (Fig. 13.2g). The altitudinal variation along the relief and the complex mosaic of vegetation provide a large variety of habitats, supporting a high diversity of species and an endemic biota (Funch et al. 2005; Harley et al. 2005; Queiroz et al. 2005; Rocha et al. 2005; Conceição et al. 2005; Conceição and Pirani 2007; Echternacht et al. 2011; Fernandes and Hamdan 2014).
Central Brazilian Highlands, or Planalto Brasileiro. This is set of distinct plateaus or hills covered mostly by the Cerrado vegetation, including pre-Cambrian formations such as the Central Brazilian Plateau, Veadeiros Plateau and the Canastra Range as well as multiple other plateaus or hills of diverse geological origins, generally uplifted Phanerozoic sedimentary basins (e.g., Guimarães Plateau, Serra Geral Plateau, Mesas Plateau region, Parecis Plateau, Bodoquena Range) (Fig. 13.1; Bartorelli 2012; Petri and Sanches 2012; Carvalho Junior et al. 2015). The altitude across the Central Brazilian Highlands ranges from 400 to 1650 m. The Veadeiros Plateau is one of the highest areas (600–1600 m). The plateau’s topographic heterogeneity determines many features of the Cerrado bioregion, such as soil composition, local climate, vegetation mosaics, and distribution of many groups of organisms simultaneously (Brown and Gifford 2002; Azevedo et al. 2016). The Central Brazilian Highlands is characterized by the most diverse physiognomies among the regions surveyed here, including several types of savannas (from open grasslands—campinas, cerrado limpo—to more densely forested areas or campo sujo), campos rupestres or rocky grasslands, and seasonal forests, all regulated by seasonality of precipitation and fire (Henriques 2005). The Cerrado harbors a relatively high species richness and endemism level as compared to other savannas around the world, which could be related to the presence of such diverse plateau systems, and is a global biodiversity hotspot (Myers et al. 2000; DRYFLOR 2016; Bacon et al. 2017; Guedes et al. 2018).
Northeastern Highlands, or Brejos Nordestinos. The region comprises about 43 mountains and plateaus between 600 and 1200 m altitude, situated in northeastern Brazil in the states of Bahia, Ceará, Pernambuco, and Paraíba (Fig. 13.1; Ab’Sáber 2003; Tabarelli and Santos 2004). The vegetation on each of these mountains (Caatinga moist forest enclaves, according to WWF’s ecoregion delimitation) is recognized as an archipelago of “exception landscapes” with higher humidity than the semi-arid surrounding Caatinga (Andrade-Lima 1966; Ab’Sáber 2003; Tabarelli and Santos 2004). In general, the vegetation in these Northeastern Highlands is complex and includes caatinga vegetation, elements of the Cerrado such as campos rupestres, carrasco (a kind of xerophilous vegetation in sandy soils present on some plateaus contiguous to the Caatinga bioregion; Andrade-Lima 1978; Araújo et al. 1999), and dense and humid relictual forested areas (Fig. 13.2e; Ab’Saber 1967, 1974).
The Pantepui Region, or Pantepui. This is the third major mountain system in South America by area (Figs. 13.1 and 13.2f). It is a biogeographic province composed of extensive table mountains—“tepuis”—derived from the sandstone of the Roraima Group, which in turn is part of the Guiana Shield that covers a large portion of northern South America (Fig. 13.1) (Rull 2004, 2005; Rull and Nogué 2007). The summits of the tepuis range from ~1500 to 3000 m, are sometimes flat and in those cases termed “table mountains” (Gibbs and Barron 1993; McDiarmid and Donnelly 2005; Rull 2005; Rull and Nogué 2007). The Pantepui Region has a varied and specialized diversity of flowering plants comprising about 2500 species (Huber 1988). Since the surface of the tepui summits represents only about 0.5% of the total area of the Pantepui Region, its density of endemic species is one of the highest on Earth, making the Pantepui one of the most important centers of species endemism in the Neotropics (Huber 1988, 1995; Berry and Riina 2005; Rull 2004, 2005; Rull and Nogué 2007; Désamoré et al. 2010).
3 Non-Andean South American Mountains: What Lives There?
Due to its large area and high topographic and habitat heterogeneity, the Serra do Mar Range is a center of endemism for many animal and plant taxa (Cardoso-da-Silva et al. 2004; Loyola et al. 2009; Villalobos et al. 2013). A recent spatial prioritization study (Loyola et al. 2009) identified the Serra do Mar Range as an important area for conservation of terrestrial vertebrates, including both endemic and threatened species. Villalobos et al. (2013) found similar results and pointed to the uniqueness of the central portion of the Atlantic Forest bioregion as having high richness of small-ranged anuran species. The concentration of small-ranged species in this region suggests that the complex topography of the region, coupled with the dispersal limitation of anurans, may have contributed to the speciation process. A biogeographic regionalization scheme based on taxonomic species composition (Vasconcelos et al. 2014) also found a similar result, further highlighting the distinctive anuran species composition between the southeastern and northern portion of the Atlantic Forest. Those authors also found that temperature, topographic variation, and precipitation seasonality best predicted the formation of the regionalization pattern. Overall, these results agree with the division of the Atlantic Forest bioregion into two distinct regions: one north and one south of the Doce River, as distinguished by many lineages of vertebrates and a major shift in climatic space through time (Carnaval et al. 2014).
A particular habitat in the montaintops of the Serra do Mar Range and Mantiqueira Mountains known to comprise several endemic taxa is the campos de altitude (Garey and Provete 2016; Silva et al. 2018). Open vegetation physiognomies of the Andes and campos de altitude of the Serra do Mar Range and Mantiqueira Mountains share about one third of plant genera (Safford 1999b, 2007). Among animals, an example of disjunct distribution is the sister relationship between the bufonid frog genera Amazophrynella and Dendrophryniscus, in which the former occurs in the Amazonia and Andes, whereas the latter occurs in the Atlantic rainforest, including the Serra do Mar Range; their split dates to the Eocene (Fouquet et al. 2012).
Several phylogeographic studies found evidence that birds, squamate reptiles (Cabanne et al. 2007; Batalha-Filho and Miyaki 2011), anuran amphibians (Thomé et al. 2014), and small mammals (Dantas et al. 2011) have only recently expanded their ranges towards the southern portion of the Atlantic Forest, as a result of the warmer climate compared to the Last Glacial Maximum. Many of these species appear to have diverged recently from their ancestors (Gaston 2003). The speciation mode of most anuran species is allopatric (Lynch 1989; Hua and Wiens 2010; Skeels and Cardillo 2019), which points to the role of mountains as vicariant barriers to lowland species, promoting endemism. Taken together, these results help explain the high number of small-ranged species in the mountainous areas of southeastern Brazil in general, and the Serra do Mar Range in particular.
The Espinhaço Mountains are geologically more stable than the Andes, with a Precambrian origin followed by comparatively fewer tectonic events in the Cenozoic (Saadi 1995). Similar to the Andes, the Espinhaço Mountains are an orographic barrier (Derby 1906), important in maintaining a minimum level of precipitation and humidity in the windward side of the range through geological time (Magalhães et al. 2015). In general, such geologic and climatic stability are related to high levels of endemism of both recent and relatively unique and ancient lineages (Loarie et al. 2009). Endemism levels are also high in different portions of the mountain range, which from a biogeographic viewpoint may be considered as a series of sky islands with various levels of differentiation among different lineages (Bonatelli et al. 2014; Ramos et al. 2018) or distinct species of the same genera occurring on different mountain tops (Echternacht et al. 2011). The Espinhaço Mountains harbor more than 4000 species of vascular plants, with endemism levels around 30% (Giulietti et al. 1997). Most of the endemic species of plants and animals across the region are associated with high altitude open fields on rock outcrops. These include one endemic monotypic genus of rodent (Calassomys apicalis), four bird species, and 10 Mimosa plant species (Silva 1997; Simon and Proença 2000; Almeida et al. 2007). The herpetofauna is narrowly distributed, with at least 18 species of frogs and five endemic reptiles only found in the southern portion of the Espinhaço (Azevedo et al. 2016). Although the campos rupestres harbor the majority of the endemics of the Espinhaço Mountains, some species are endemic to other environments, such as gallery forests on mountaintops, as is the case of some Bokermannohyla frogs and the lizard Enyalius erythroceneus (Napoli and Juncá 2006; Rodrigues et al. 2006; Leite et al. 2008).
The Central Brazilian Highlands have a higher diversity of plants than lower elevation areas within the Cerrado (Munhoz and Felfili 2006). The plant genus Mimosa (Fabaceae) alone contains more than 20 species restricted to the Veadeiros Plateau (Simon and Proença 2000). The same pattern of high richness and endemism is known for amphibians of the Veadeiros Plateau, with the occurrence of five endemic frogs from a total of 54 species, one of the highest levels of amphibian richness in South America (Santoro and Brandão 2014). Some other species are more widespread within the Central Brazilian Highlands, occurring also in other highlands to the south of the Veadeiros Plateau (500–1300 m). The Central Brazilian Plateau harbors several small-ranged species of reptiles, amphibians, plants, and rodents, but no endemic bird species (Silva 1997; Simon and Proença 2000; Marinho-Filho et al. 2002; Azevedo et al. 2016). However, comparisons of the whole Central Brazilian Plateau with other mountain ranges are difficult, given the many differences in area and climate, and the fact that the plateau has a more gentle and eroded relief and deeper and well drained soils than other mountain systems discussed here (Ab’Sáber 2003). The Central Brazilian Plateau does not serve as an orogenic barrier (Derby 1906; Bookhagen and Strecker 2008) as the Espinhaço Mountains do, the latter being more subject to the extreme climatic seasonality of central South America. To the west of the Cerrado domain, there are several small isolated plateaus and table mountains (e.g., Bodoquena, Guimarães, Huanchaca, and Parecis Ranges), generally below 900 m. Although lower in elevation and with relatively more gentle relief, these mountains and plateaus also harbor endemic species of reptiles, amphibians, and plants (Simon and Proença 2000; Azevedo et al. 2016), but most of them do not have any endemic mammal or bird species (with exception of the rodent Jucelinomis huanchaca from the Huanchaca Plateau; Emmons 1999).
The summits of the Pantepui Region harbor vegetation more similar to the Páramos and the campos de altitude of the Atlantic Forest than to the surrounding lowland Amazonian Forest. However, sometimes these table mountains can also be surrounded by tropical savanna (Huber 1995). The entire region harbors 2100 species of vascular plants, of which around 1300 are endemic (Huber et al. 2018). The difference in endemism levels for the Pantepui Region compared to other Brazilian ranges is remarkable, with 43 species of birds compared to only four for the (southern) Espinhaço (Borges et al. 2018), and nine in the Diamantina Plateau (Guedes et al. 2014). Regional endemism for the herpetofauna is even more extreme, reaching 87.6% of the amphibians and 74.2% of the reptiles (McDiarmid and Donnelly 2005). In contrast to plants and birds, there are fewer/lower affinities between the Pantepui Region and the Andean herpetofauna (Kok 2013). This is an interesting pattern that contrasts with most biogeographical hypotheses proposed based on the distribution of organisms with good dispersal abilities (many vascular plants and birds; Antonelli et al. 2009; Borges et al. 2018; Huber et al. 2018).
Campos rupestres from different mountains share more vascular plant species with the surrounding Cerrado savannas than to any other mountains or bioregions (Neves et al. 2018a, b), which contrasts with the situation for the tepuis and their distant biotic affinities. High mountains in the Atlantic Forest domain also harbor campos de altitude (Safford 1999a, b; Ribeiro et al. 2007). Besides being primarily related to central Brazilian campos rupestres, the vascular flora of the campos de altitude share a strong floristic similarity to the Páramos in the Andean mountains, and to southern temperate grasslands (DeForest Safford 2007). This can be exemplified by the species radiation of the fern genus Jamesonia (Pteridaceae) in the Páramos, which is the sister taxon of Eriosorus myriophyllus, a fern typical of the highland grasslands in the Atlantic Forest (Sánchez-Baracaldo 2004). The birds Asthenes luizae and Cinclodes espinhacensis from the campos rupestres of the Espinhaço Mountains also show biogeographical connections among southern Brazilian mountains and the Andes, probably connected by montane regions across the Patagonian-Chacoan region c. 8 million years ago (Ma) (Derryberry et al. 2011).
Biotic connections, or interchange, across montane habitats have taken place across different routes, including the Southern route (southern Brazilian mountains—Patagonia—southern Andes). The hummingbird Colibri delphinae has a disjunct distribution across the northern Espinhaço Mountains, Pantepui Region, northern Andes, Central America, and the island of Trinidad (Schuchmann 1999). Similarly, Chaves et al. (2014) provide an extensive list of widespread plant species across the Pantepui Region, and south-eastern and southern Brazil, including ferns, grasses, bromeliads, and plants of the families Cyperaceae, Eriocaulaceae, Velloziaceae, and Xyridaceae (Costa et al. 2008; Rapini et al. 2008; Salino and Almeida 2008; Versieux et al. 2008; Viana and Filgueiras 2008; Mello-Silva 2010). Finally, the rodent species Podoxymys roraimae also suggests an old biogeographical connection between the Cerrado of the Central Brazilian Plateau and the Pantepui Region during the Pliocene (Leite et al. 2015).
Alternatively, at least some disjunct distributions may not reflect bioregion connectivity, but rather represent sporadic dispersal events. Indeed, intensive dispersal among Neotropical mountain ranges and with surrounding lowlands may explain the reticulate evolutionary history of many Neotropical bioregions, as recently shown by a large cross-comparative analysis of dated phylogenies of plants and animals (Antonelli et al. 2018b; Fine and Lohmann 2018).
4 Evolutionary Origins and Species Diversification in Non-Andean Mountains: Insights from Phylogenetic Studies
One of the main goals of evolutionary biology is to understand the mechanisms that drive spatial variation in biodiversity (Quintero et al. 2015). One way to investigate such mechanisms is by using molecular phylogenies, which provide insights into the origin and diversification of extant lineages. When integrated with fossil information, phylogenies are particularly powerful tools for historical inference by enabling time calibration through node or tip constraints (Ronquist et al. 2012) or through diversification rates and biogeographic analyses (Silvestro et al. 2016). When lineages are endemic to an area—or are highly characteristic of a particular bioregion—their evolutionary history can be used to interpret the history of the region itself (Eiserhardt et al. 2017). Further, the inference of ancestral areas enables the investigation of how entire biotas and local communities are assembled through time (Bacon 2013; Antonelli et al. 2018b). The similarity of divergence times for diverse taxa can inform on the formation of a bioregion or geological feature (e.g., Bacon et al. 2015), although a conceptual and methodological reliance on concordance may be unnecessary (Papadopoulou and Knowles 2016; Zamudio et al. 2016).
Molecular data indicate that the diversification of many Neotropical terrestrial organisms took place during the Neogene, with lineages accruing their present diversity and distribution in the Quaternary (since 2.6 Ma; Rull 2011). For example, Machado et al. (2014) found a Neogene origin for snakes of Bothrops neuwiedi species group, with population differentiation likely driven by Quaternary climate change. Specifically, the species from the campos de altitude of the Mantiqueira Mountains diverged around 1.2–0.58 Ma, similar to the even younger ages of the Espinhaço Mountains (less than 1 Ma; Machado et al. 2014). Neogene origins were also found in the radiation of Minaria plants (Apocynaceae), comprising 21 species that are primarily endemic to the campos rupestres (Ribeiro et al. 2014). Minaria arose in the Diamantina Plateau during the Late Miocene and Early Pliocene in campos rupestres, and during the Pliocene the genus diversified across its entire range, particularly in the northern Diamantina Plateau of Bahia. These plant divergence times are older than those of the endemic birds in Cinclodes (Late Pleistocene; Freitas et al. 2012) and younger than those inferred for another characteristic campos rupestres plant group, Hoffmannseggella (syn. Cattleya) orchids (Orchidaceae; 14–11 Ma; Antonelli et al. 2010).
Historical biogeography uses phylogenies to infer the ancestral areas of lineages through time, which can in turn be used to suggest geological and/or climatic connectivity among regions. Yet, evidence about the origins and diversification along the non-Andean mountains seems far from settled, and more data are needed to understand temporal patterns. Sometimes, the reticulate and complex history of clades may not always reflect the geological history of the area. For instance, although the final upheaval of the Central Brazilian Highlands took place c. 4–2 Ma, Beerling and Osborne (2006) and Werneck et al. (2012) inferred that widespread lizard populations (Phyllopezus pollicaris) of the Caatinga and Cerrado diverged during the Miocene. In contrast, Carnaval and Bates (2007) found the divergence of two frog species from the Northeastern Highlands of Ceará to be much more recent (ca. 0.1 Ma). These mountain enclaves of the Northeastern Highlands are some of the least sampled among all non-Andean mountains, with few phylogenetic studies published to date. In contrast, the mountains of Central Brazil are fairly well known, and increased interest in the patterns and causes of high Cerrado diversity has shown divergence of endemic clades primarily occurring since 4 Ma (e.g., Almeida et al. 2007; Giugliano et al. 2007; Simon et al. 2009).
Climatic changes through time also have an important role in shaping the evolutionary trajectory of species, which may affect both geographic distribution patterns and species composition, as well as genetic variability across landscapes. The evolutionary history of the titi monkey genus Callicebus suggests a separation between the Central Brazilian Highlands and Northeastern Highlands lineages from those of the Serra do Mar Range in the Pleistocene (crown ages of ca. 2 and 6 Ma, respectively; Carneiro et al. 2018). Similarly, the manakin bird Neopelma pallescens originated in the Serra do Mar Range and dispersed to areas including Central Brazilian Highlands in the Late Miocene (Capurucho et al. 2018). In recent geological times, besides glacial cycling during the Quaternary the central Serra do Mar Range remained climatically more stable in comparison to southern parts of the range (Carnaval et al. 2009), leading to complex patterns of genetic diversity within frog species across their distribution. These types of climatic changes have also led to different responses among communities, creating different evolutionary trajectories for lizard species (Prates et al. 2016).
Usually, ancient geomorphological landscapes also have ancient environments occupied by ancient lineages. However, the Pantepui mountain system is one of the oldest in South America, but its biota contains both ancient and more recent lineages. Among the earliest diverging lineages are tepuian bromeliads (Bromeliaceae; Givnish et al. 2011), dating to c. 9.1 Ma. More recent endemic lineages include a species of opossum (Podoxymys roraimae; Pavan et al. 2016) and a toucanet (Aulacorhynchus whitelianus; Bonaccorso and Guayasamin 2013), which diverged in the Pleistocene. A review of the diversification of the Pantepui biota is provided by Huber et al. (2018).
5 Connecting Studies of Biodiversity and Human Impacts on Non-Andean Montane Biotas: A Case Study from the Serra Do Mar Range
Among the non-Andean montane regions, the Serra do Mar Range is one of the most heavily altered by humans, with a long history of deforestation dating back to colonial times between 1500 and 1815 (Dean 1997). This is one of the most densely populated areas in Brazil, comprising São Paulo and Rio de Janeiro, the two largest Brazilian cities (ca. 19 million inhabitants). Because evidence suggests that deforestation has changed the distributional patterns of small mammals in the Atlantic Forest (Sancha et al. 2014), possibly driven by extinction, we expect that more densely occupied regions within this range will generally have lower species richness.
Anurans are especially diverse in the Serra do Mar Range (Villalobos et al. 2013). However, it is unknown whether the anthropogenic impacts detected in small mammals (Sancha et al. 2014) are reflected in these taxa, especially because the effect of deforestation in natural communities varies depending on life history and species traits. In general, species exhibiting narrow environmental requirements should be more heavily affected than ecological generalists.
To further understand whether and how human population density interacts with climate (reflecting climatic niche space; Wiens et al. 2006) and the evolutionary history of the local biota, and hence influences species richness patterns along the Serra do Mar Range, we deconstructed the total species richness into groups based on three life-history traits correlated with resource use: habitat use (terrestrial pond dwellers, burrowing, stream dwellers, and arboreal species), reproductive mode (species with free-swimming larvae vs. direct developers), and body size (50.99 mm ≤ SVL ≥ 51 mm, based on breaks of the snout-vent length data; Fig. 13.3), following Marquet et al. (2004). With this, we sought to test how important life-history traits are in determining species richness patterns at a broad spatial scale, given the human footprint. Direct-developing anuran species (i.e., without larval phase) require high levels of humidity (da Silva et al. 2012). Thus, we expect them to show a nested distributional pattern along a rainfall seasonality gradient. We expect the highest species richness of direct-developing species to be in areas with high temperature and precipitation and low human influence. Since streams are more common in regions with high altitude, we expect the richness of stream-dwellers to be higher in high altitude areas. Yet it is unknown if these areas have been preferentially altered by humans.
Observed richness patterns of the 233 anuran species occurring in the Serra do Mar Range. (a) all species; (b) arboreal; (c) fossorial; (d) terrestrial; (e) terrestrial stream dwellers; (f) terrestrial with free-swimming larvae; (g) direct developers; (h) large-bodied species; (i) small-bodied species. The figure shows that the richness of most species peaks in the northern portion of the Serra do Mar Range, in the state of Rio de Janeiro, decreasing towards the south. The region with the highest richness is also the one with the highest peaks (see main text)
For our analysis, we built a grid with 57 cells of 0.5° covering the Serra do Mar Range in the SAM software (Rangel et al. 2010), using the limits of this ecoregion as provided by Olson et al. (2001). To calculate species richness for each cell, we overlaid polygons depicting the extent of occurrence (‘range maps’) of all anuran species available in IUCN (2009). Species were considered present if at least 50% of the range polygon covered the cell. We then used six climatic variables (following Wiens et al. 2006; Qian and Ricklefs 2007) obtained from Worldclim v. 1.4 (Hijmans et al. 2005). These variables (see Table 13.1) are related to physiological limits of amphibian species and influence their broad-scale distribution (e.g., Wiens et al. 2006). We built a global stationary Generalized Least Squares (GLS) model to predict the total richness and the richness of each group of species separately (response variables) as a function of macroclimatic variables and human population density (predictor variables; Fig. 13.4), while also accounting for the spatial autocorrelation in the data. Finally, we used a semi-variogram to explicitly model the residuals and to build their variance-covariance matrix (Dormann et al. 2013) into the model. Moran’s I of residuals was used to diagnose if the GLS model successfully accommodated spatial autocorrelation.
Predicted richness patterns of the 233 anuran species occurring in the Serra do Mar Range, using a generalized least square (GLS) approach. (a) all species; (b) arboreal; (c) fossorial; (d) terrestrial; (e) terrestrial stream dwellers; (f) terrestrial with free-swimming larvae; (g) direct developers; (h) large-bodied species; (i) small-bodied species. A visual comparison between this and the previous figure shows that patterns predicted by the model closely resemble the observed ones, with small extensions towards the borders of the Serra do Mar Range in the predicted richness. The results suggest that including human population size in the statistical niche model, as well as analyzing both total species richness and smaller groups of species, can improve the prediction of species richness in a global biodiversity hotspot
We found that the variable that most influenced species richness was temperature seasonality (Table 13.1). This supported our expectations, since the Serra do Mar Range has generally low rainfall seasonality and high species richness. However, the relative importance of each individual variable analyzed differed for each group of species, depending on their life-history traits. Surprisingly, altitude and human population size were negatively related to the richness of small-bodied and pond-dweller species, yet positively related to the richness of other species groups (Fig. 13.4; Table 13.1). Ponds are much more common in lowlands, where flat terrain necessary for their formation is found, while streams are more common in the escarpments and high-altitude areas, due to the sloped terrain. Data available for ectotherms demonstrate that larger species occur in cooler places as predicted by Bergmann’s rule (Ashton 2002; Ashton and Feldman 2003; Morrison and Hero 2003; Vinarski 2014; Zamora-Camacho et al. 2014; Amado et al. 2018; but see Adams and Church 2008; Romano and Ficetola 2010 for contrasting results). Together, these factors may explain the negative relationship between altitude and richness of small-bodied species we found.
Overall, our empirical results show that life-history traits are important for assessing and explaining species–climate relationships in mountains. Such traits are part of the functional diversity of ecosystems and should be included alongside total richness in similar analyses whenever possible. Also, some predictor variables showed shifts in coefficient, such as temperature seasonality, positively affecting the richness of fossorial species, but negatively affecting that of stream-dwellers and large species, besides total richness.
Ecological theory predicts that highly seasonal environments present harsh conditions for most species, acting like a strong environmental filter (Ricklefs 2004; Wiens et al. 2006; Qian and Ricklefs 2007). We therefore expected temperature seasonality to negatively affect species richness. However, surprisingly, fossorial species were positively correlated with it, probably because belowground habitats would buffer them against variations in ambient temperature.
Contrary to our initial hypothesis, we found human population density to be positively correlated with richness of direct-developing species, and also free-swimming larvae, fossorial species, stream dwellers, and arboreal species (Table 13.1), with standardized slope varying from 0.047 to 0.21. This unexpected result may be an artefact of sampling bias, since urban centers also concentrate more researchers and research institutions (Oliveira et al. 2016).
6 Future Directions
The generally low level of biological sampling carried out in most mountain ranges surveyed to date precludes robust evolutionary inferences. Only fragmentary evolutionary stories have been told, showing how particular species are related and when they derived from common ancestors in the surrounding landscapes or from distant regions. How general are the patterns described? We still do not know. Our review motivates several additional questions, which could be addressed by a more thorough genetic, and if possible paleontological, sampling in each mountain range. For instance, are the rainforest species in mountain enclaves in the Caatinga remnants of a once much larger Atlantic rainforest, or the result of dispersals from the Andean slopes, Amazonia, or eastern Brazil? How did surface uplift and associated landscape changes influence the rates of speciation and extinction in Neotropical mountains, and how were those related to biotic and abiotic changes? How often have biotic corridors been created in deep history that linked the montane biotas across South America, and what biological impact did such connections have on the distribution and diversification of Neotropical diversity (e.g., Costa et al. 2017)? What was the importance of in situ adaptation of lowland ancestors versus long-distance establishment of pre-adapted species for the high levels of montane endemicity (Antonelli 2015; Merckx et al. 2015)? What role did Neotropical mountains play in protecting species from episodes of past climate changes, due to their lower climatic velocity in comparison with lowlands (Sandel et al. 2011)? Will mountains provide refugia for biodiversity from escalating human pressures in the future? Addressing these important questions will require concerted efforts from researchers across disciplines.
Across the world, mountains play key roles as cradles and reservoirs of biodiversity. Although the contribution of the Andes to Neotropical diversity cannot be understated (Antonelli et al. 2018c; Rangel et al. 2018), it is now time to increase efforts to better understand the evolution and distribution patterns of the rich and highly endemic biota found on other Neotropical mountain ranges. On an even more urgent level, we need to investigate the effects of deforestation and other changes in land use on distributional patterns among communities, in order to facilitate the prioritization of areas for conservation. Through a case study of the amphibians of the Serra do Mar Range, we provide one example of how human impacts can be assessed alongside current climatic conditions and species traits, which reflect the evolutionary history of local lineages, in correlative studies of observed biodiversity patterns. Although we were unable to detect a strong human imprint in the system of focus, we hope that our review will inspire work on the various aspects of research outlined here, and most importantly lead to a recognition of the complexity, challenges, and amazing prospects ahead of us.
References
Ab’Saber AN (1967) Domínios morfoclimáticos e províncias fitogeográficas do Brasil. Orient Dep Geografia USP São Paul 3:45–48
Ab’Saber AN (1970) Províncias geológicas e domínios morfoclimáticos no Brasil. Geomorfologia 20:1–26
Ab’Saber AN (1971) A organização natural das paisagens inter e subtropicais brasileiras In: Modenesi-Gauttieri A, Bartorelli V, Mantesso Neto CDR, Carneiro MA, Lisboa MA (Orgs) 2010 A obra de Aziz Nacib Ab’Saber. Beca, São Paulo, pp 404–414
Ab’Saber AN (1974) O domínio morfoclimático semi-árido das Caatinga brasileiras. Geomorfologia 43:1–39
Ab’Saber AN (1977) Os domínios morfoclimáticos da América do Sul. Primeira Aproximação. Geomorfologia 52:1–21
Ab’Sáber AN (2003) Os domínios de natureza no Brasil. Potencialidades paisagísticas. Ateliê, Cotia
Ab’Saber AN, Bernardes N (1958) Vale do Paraíba, Serra da Mantiqueira e arredores de São Paulo: guia da excursão no 4, realizada por ocasião do XVIII Congresso Internacional de Geografia. Conselho Nacional de Geografia, Rio de Janeiro
Adams DC, Church JO (2008) Amphibians do not follow Bergmann’s rule. Evolution 62:413–420
Alkmin FF (2012) Serra do Espinhaço e Chapada Diamantina. In: Hasui Y, Carneiro CDR, Almeida FFM, Bartorelli A (Orgs) Geologia do Brasil. Beca, São Paulo, pp 236–244
Almeida FFM (1964) Fundamentos geológicos do relevo paulista. In: Inst Geol Geogr Geologia do Estado de São Paulo. IGG, São Paulo, pp 167–262
Almeida AM, Prado PI, Lewinsohn TM (2004) Geographical distribution of Eupatorieae (Asteraceae) in the south-eastern and South Brazilian mountain ranges. Plant Ecol 174:163–181
Almeida FC, Bonvicino CR, Cordeiro-Estrela P (2007) Phylogeny and temporal diversification of Calomys (Rodentia, Sigmodontinae): implications for the biogeography of an endemic genus of the open/dry biomes of South America. Mol Phylogenet Evol 42:449–466
Amado TF, Bidau CJ, Olalla-Tárraga MÁ (2018) Geographic variation of body size in New World anurans: energy and water in a balance. Ecography 41:1–11
Andrade-Lima D (1966) Esboço fitoecológico de alguns “brejos” de Pernambuco. Bol Técnico Inst Pesq Agron Pernamb 8:3–9
Andrade-Lima D (1978) Vegetação. In: Lins RC (ed) Bacia do Parnaíba: aspectos fisiográficos. Instituto Joaquim Nabuco de Pesquisas Sociais, Recife, pp 131–135
Antonelli A (2015) Multiple origins of mountain life. Nature 524:300–301
Antonelli A, Nylander JAA, Persson C, Sanmartín I (2009) Tracing the impact of the Andean uplift on Neotropical plant evolution. Proc Natl Acad Sci U S A 106:9749–9754
Antonelli A, Verola CF, Parisod C, Gustafsson ALS (2010) Climate cooling promoted the expansion and radiation of a threatened group of South American orchids (Epidendroideae: Laeliinae). Biol J Linn Soc 100:597–607
Antonelli A, Kissling WD, Flantua SGA, Bermúdez MA, Mulch AM, Muellner-Riehl AN, Kreft H, Linder HP, Badgley C, Fjeldså J, Fritz SA, Rahbek C, Herman F, Hooghiemstra H, Hoorn C (2018a) Geological and climatic influences on mountain biodiversity. Nat Geosci 11:718–725
Antonelli A, Zizka A, Carvalho FA, Scharn R, Bacon CD, Silvestro D, Condamine FL (2018b) Amazonia is the primary source of Neotropical biodiversity. Proc Natl Acad Sci U S A 115:6034–6039
Antonelli A, Ariza M, Albert J, Andermann T, Azevedo JAR, Bacon CD, Faurby S, Guedes TB, Hoorn C, Lohmann LG, Matos-Maraví P, Ritter CD, Sanmartín I, Silvestro D, Tejedor M, ter Steege H, Tuomisto H, Werneck FP, Zizka A, Edwards S (2018c) Conceptual and empirical advances in Neotropical biodiversity research. PeerJ 6:e5644. 1–53
Araújo FS, Martins FR, Shephers GJ (1999) Variações estruturais e florísticas do carrasco no Planalto da Ibiapaba, estado do Ceará. Rev Bras Biol 59:663–678
Ashton KG (2002) Do amphibians follow Bergmann’s rule? Can J Zool 80:708–716
Ashton KG, Feldman CR (2003) Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57:1151–1163
Azevedo JAR, Valdujo PH, Nogueira C (2016) Biogeography of anurans and squamates in the Cerrado hotspot: coincident endemism patterns in the richest and most impacted savanna on the globe. J Biogeogr 43:2454–2464
Bacon CD (2013) Biome evolution and biogeographical change through time. Front Biogeogr 5:227–231
Bacon CD, Silvestro D, Jaramillo CA, Tilston Smith B, Chakrabarty P, Antonelli A (2015) Biological evidence shows earlier emergence of the Isthmus of Panama. Proc Natl Acad Sci U S A 112:6110–6115
Bacon CD, Velásquez-Puentes F, Flórez-Rodríguez A, Balslev H, Galeano G, Bernal R, Antonelli A (2016) Phylogenetics of Iriarteeae (Arecaceae), cross-Andean disjunctions and convergence of clustered infructescence morphology in Wettinia. Bot J 182:272–286
Bacon CD, Moraes M, Jaramillo C, Antonelli A (2017) Endemic palm species shed light on habitat shifts and the assembly of the Cerrado and Restinga Floras. Mol Phylogenet Evol 110:127–133
Bacon CD, Velásquez-Puentes F, Hoorn C, Antonelli A (2018a) Iriarteeae palms tracked the uplift of Andean Cordilleras. J Biogeogr 45:1653–1663
Bacon CD, Velásquez-Puentes FJ, Hinojosa LF, Schwartz T, Oxelman B, Pfeil B, Arroyo MTK, Wanntorp L, Antonelli A (2018b) Evolutionary persistence in Gunnera and the contribution of southern plant groups to the tropical Andes biodiversity hotspot. PeerJ 6:e4388. 1–21
Badgley C, Smiley TM, Terry R, Davis EB, DeSantis LRG, Fox DL, Hopkins SSB, Jezkova T, Matocq MD, Matzke N, McGuire JL, Mulch A, Roddle BR, Roth VL, Samuels JX, Strômberg CAE, Yanites BJ (2017) Biodiversity and topographic complexity: modern and geohistorical perspectives. Trends Ecol Evol 32:211–226
Bartorelli A (2012) Chapada dos Veadeiros. In: Hasui Y, Carneiro CDR, Almeida FFM, Bartorelli A (Orgs) Geologia do Brasil. Beca, São Paulo, pp 326–330
Batalha-Filho H, Miyaki CY (2011) Filogeografia da Mata Atlântica. Revista da Biologia, USP Volume Especial Biogeografia, pp 31–34
Beerling DJ, Osborne CP (2006) The origin of the savanna biome. Glob Chang Biol 12:2023–2031
Bello C, Galetti M, Montan D, Pizo MA et al (2017) Atlantic frugivory: a plant-frugivory interaction data set for the Atlantic Forest. Ecology 98:1729–1729
Berry PE, Riina R (2005) Insights into the diversity of the Pantepui Flora and the biogeographic complexity of the Guayana Shield. Biol Skr 55:145–167
Bonaccorso E, Guayasamin JM (2013) On the origin of Pantepui montane biotas. A perspective based on the phylogeny of Aulacorhynchus toucanets. PLoS One 8:e67321
Bonatelli IAS, Perez MF, Peterson AT, Taylor NP, Zappi DC, Machado MC, Koch I, Pires AHC, Moraes EM (2014) Interglacial microrefugia and diversification of a cactus species complex: phylogeography and palaeodistributional reconstructions for Pilosocereus aurisetus and allies. Mol Ecol 23:3044–3063
Bookhagen B, Strecker MR (2008) Orographic barriers, high-resolution TRMM rainfall, and relief variations along the eastern Andes. Geophys Res Lett 35:1–6. https://doi.org/10.1029/2007GL032011
Borges SH, Santos MPD, Moreira M, Baccaro F, Capurucho JMG, Ribas C (2018) Dissecting bird diversity in the Pantepui area of endemism, northern South America. J Ornithol 159:1–14
Bovendorp RS, Villar N, Abreu-Junior EF, Bello C, Regolin AL, Percequillo A, Galetti M (2017) Atlantic small-mammal: a dataset of communities of rodents and marsupials of the Atlantic forests of South America. Ecology 99:2226–2226
Brown KS Jr, Gifford DR (2002) Lepidoptera in the Cerrado landscape and conservation of vegetation, soil and topographical mosaics. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, NY, pp 201–222
Cabanne GS, Santos FR, Miyaki CY (2007) Phylogeography of Xiphorhynchus fuscus (Passeriformes, Dendrocolaptidae): vicariance and recent demographic expansion in southern Atlantic forest. Biol J Linn Soc 91:73–84
Cadena CD (2007) Testing the role of interspecific competition in the evolutionary origin of elevational zonation: an example with Buarremon brush-finches (Aves, Emberizidae) in the Neotropical Mountains. Evolution 61:1120–1136
Capurucho JMG, Ashley MV, Ribas CC, Bates JM (2018) Connecting Amazonian, Cerrado, and Atlantic forest histories: paraphyly, old divergences, and modern population dynamics in tyrant-manakins (Neopelma/Tyranneutes, Aves: Pipridae). Mol Phylogenet Evol 127:696–705
Cardoso-da-Silva JM, Cardoso-de-Sousa M, Castelletti CHM (2004) Areas of endemism for passerine birds in the Atlantic forest, South America. Glob Ecol Biogeogr 13:85–92
Carnaval AC, Bates JM (2007) Amphibian DNA shows marked genetic structure and tracks Pleistocene climate change in northeastern Brazil. Evolution 61:2942–2957
Carnaval AC, Moritz C, Hickerson M, Haddad C, Rodrigues M (2009) Stability predicts diversity in the Brazilian Atlantic forest hotspot. Science 323:785–789
Carnaval AC, Waltari E, Rodrigues MT, Rosauer D, VanDerWal J, Damasceno R, Prates I, Strangas M, Spanos Z, Rivera D, Pie MR, Firkowski CR, Bornschein MR, Ribeiro LF, Moritz C (2014) Prediction of phylogeographic endemism in an environmentally complex biome. Proc R Soc B Biol Sci 281:1–8
Carneiro J, Sampaio I, de Sousa e Silva-Júnior J, Farias I, Hrbek T, Pissinatti A, Silva R, Martins-Junior A, Boubli J, Ferrari SF, Schneider H (2018) Phylogeny, molecular dating and zoogeographic history of the titi monkeys (Callicebus, Pitheciidae) of eastern Brazil. Mol Phylogenet Evol 124:10–15
Carvalho Junior OA, Guimarães RF, Martins ES, Gomes RAT (2015) Chapada dos Veadeiros: The highest landscapes in the Brazilian Central Plateau. In: Vieira B, Salgado A, Santos L (eds) Landscapes and landforms of Brazil. world geomorphological landscapes. Springer, Dordrecht, pp 221–230
Castroviejo-Fisher S, Guayasamin JM, Gonzales-Voyer A, Vilà C (2014) Neotropical diversification seen through glassfrogs. J Biogeogr 41:66–80
Chaves AV, Freitas GHS, Vasconcelos MF, Santos FR (2014) Biogeographic patterns, origin and speciation of the endemic birds from eastern Brazilian mountaintops: a review. Syst Biodivers 13:1–16
Conceição AA, Pirani JR (2007) Diversidade em quatro áreas de campos rupestres na Chapada Diamantina, Bahia, Brazil: Espécies distintas, mas riquezas similares. Rodriguesia 58:193–206
Conceição AA, Rapini A, Pirani JR, Giulietti AM, Harley RM, Silva TRS, Santos AKA, Correia C, Andrade IM, Costa JAS, Souza LRS, Andrade MJG, Funch RR, Freitas AMM, Oliveira AA (2005) Campos Rupestres. In: Juncá FA, Funch L, Rocha W (Orgs) Biodiversidade e Conservação da Chapada Diamantina. Ministério do Meio Ambiente, Brasília, pp 153–168
Costa FN, Trovó M, Sano PT (2008) Eriocaulaceae na Cadeia do Espinhaço: riqueza, endemismo e ameaças. Megadiversidade 4:91–99
Costa GC, Hampe A, Ledru M, Martinez PA, Mazzochini GG, Shepard DB, Werneck FP, Moritz C, Carnaval AC (2017) Biome stability in South America over the last 30 kyr: inferences from long-term vegetation dynamics and habitat modelling. Glob Ecol Biogeogr 27:285–297
Culot L, Pereira LA, Agostini I, Almeida MAB et al (2019) Atlantic-primates: a dataset of communities and occurrences of primates in the Atlantic forest of South America. Ecology 100:2525–2525
da Silva FR, Almeida-Neto M, do Prado VHM, Haddad CFB, Rossa-Feres DC (2012) Humidity levels drive reproductive modes and phylogenetic diversity of amphibians in the Brazilian Atlantic Forest. J Biogeogr 39:1720–1732
Dantas GPMA, Cabanne GSN, Santos FCR (2011) How past vicariant events can explain the Atlantic Forest biodiversity? In: Grillo O, Venora G (eds) Ecosystems biodiversity. InTech, Rijeka, pp 429–442
de Mello-Silva R (2010) Circumscribing Vellozia hirsuta and V. tubiflora (Velloziaceae). Hoehnea 37:617–646
Dean W (1997) With broadax and firebrand: the destruction of the Brazilian Atlantic Forest. University of California Press, Berkeley, CA
Derby OA (1906) The Serra do Espinhaço, Brazil. J Geol 14:374–401
Derryberry EP, Claramunt S, Derryberry G, Chesser RT, Cracraft J, Aleixo A, Pérez-Emán J, Remsen JV, Brumfield RT (2011) Lineage diversification and morphological evolution in a large-scale continental radiation: the Neotropical ovenbirds and woodcreepers (Aves: Furnariidae). Evolution 65:2973–2986
Désamoré A, Vanderpoorten A, Laenen B, Gradstein SR, Kok PJR (2010) Biogeography of the lost world (Pantepui region, northeastern South America): insights from bryophytes. Phytotaxa 9:254–265
Didham RK, Lawton JH (1999) Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments. Biotropica 31:17–30
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, García Marquéz JR, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Kurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46
DRYFLOR (2016) Plant diversity patterns and their conservation implications in Neotropical dry Forests. Science 353:1383–1387
Echternacht L, Trovó M, Oliveira CT, Pirani JR (2011) Areas of endemism in the Espinhaço Range in Minas Gerais, Brazil. Flora 206:782–791
Eiserhardt WL, Couvreur TLP, Baker WJ (2017) Plant phylogeny as a window on the evolution of hyperdiversity in the tropical rainforest biome. New Phytol 214:1408–1422
Emmons LH (1999) Two new species of Juscelinomys (Rodentia: Muridae) from Bolivia. Am Mus Novit 3280:1–15
Ewers RM, Didham RK (2005) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:11–142
Fernandes D, Hamdan B (2014) A new species of Chironius Fitzinger, 1826 from the state of Bahia, Northeastern Brazil (Serpentes: Colubridae). Zootaxa 3881:563–575
Fernandes GW, Barbosa NPU, Alberton B, Barbieri A, Dirzo R, Goulart F, Guerra TJ, Morellato LPC, Solar RRC (2018) The deadly route to collapse and the uncertain fate of Brazilian rupestrian grasslands. Biodivers Conserv 27:1–17
Fine PVA, Lohmann LG (2018) Importance of dispersal in the assembly of the Neotropical biota. Proc Natl Acad Sci U S A 115:5829–5831
Fischer A, Blaschke M, Bässler C (2011) Altitudinal gradients in biodiversity research: the state of the art and future perspectives under climate change aspects. Waldökologie, Landschaftsforschung und Naturschutz 11:35–47
Fjeldså J, Bowie RCK, Rahbek C (2012) The role of mountain ranges in the diversification of birds. Annu Rev Ecol Evol Syst 43:249–265
Fouquet A, Recoder R, Teixeira M Jr, Cassimiro J, Amaro RC, Camacho A, Damasceno R, Carnaval AC, Moritz C, Rodrigues MT (2012) Molecular phylogeny and morphometric analyses reveal deep divergence between Amazonia and Atlantic Forest species of Dendrophryniscus. Mol Phylogenet Evol 62:823–838
Freitas GHS, Chaves AV, Costa LM, Santos FR, Rodrigues M (2012) A new species of Cinclodes from the Espinhaço Range, southeastern Brazil: insights into the biogeographical history of the South American highlands. Int J Avian Sci 154:738–755
Funch LS, Funch RR, Harley R, Giulietti AM, Queiroz LP, França F, de Melo E, Gonçalves CN, Santos T (2005) Florestas Estacionais Semideciduais. In: Juncá FA, Funch L, Rocha W (Orgs) Biodiversidade e Conservação da Chapada Diamantina. Ministério do Meio Ambiente, Brasília, pp 180–193
Galindo-Leal C, Câmara IG (2003) Atlantic Forest hotspots status: an Overview. In: Galindo-Leal C, Câmara IG (eds) The Atlantic Forest of South America: biodiversity status, threats, and outlook. Island Press, Washington, DC, pp 3–11
Garey MV, Provete DB (2016) Conservation status, threat sources, and endemism of anurans in highland grasslands in Southern and Southeastern Brazil. Oecol Aust 20:94–108
Gaston KJ (2003) The structure and dynamics of geographic ranges. Oxford University Press, New York
Gibbs AK, Barron CN (1993) The geology of the Guiana Shield. Oxford University Press, New York
Giugliano LG, Collevatti RG, Colli GR (2007) Molecular dating and phylogenetic relationships among Teiidae (Squamata) inferred by molecular and morphological data. Mol Phylogenet Evol 45:168–179
Giulietti AM, Pirani JR (1988) Patterns of geographic distribution of some plant species from the Espinhaço Range, Minas Gerais and Bahia, Brazil. In: Vanzolini PE, Heyer WR (eds) Proceedings of a workshop on Neotropical distribution patterns. Academia Brasileira de Ciências, Rio de Janeiro, pp 39–69
Giulietti AM, Pirani JR, Harley RM (1997) Espinhaço range region, eastern Brazil. In: Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J, Hamilton AC (eds) Centres of plant diversity: a guide strategy for their conservation, vol 3. IUCN Publication Unity, Cambridge, pp 397–404
Givnish TJ, Barfuss MH, Van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabailey RS, Crayn DM, Smith JA, Winter K, Brown GK, Evans TM, Holst BK, Luther H, Till W, Zizka G, Berry PE, Systsma KJ (2011) Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. Am J Bot 98:872–895
GMBA (2018) Global Mountain Biodiversity Assessment. http://www.gmba.unibe.ch/about_us/background/. Accessed 23 July 2018
Gontijo-Pascutti AHF, Hasui Y, Santos M, Júnior AVS, Souza IA (2012) As Serras do Mar e Mantiqueira. In: Hasui Y, Carneiro CDR, Almeida FFM, Bartorelli A (Orgs) Geologia do Brasil. Beca, São Paulo, pp 549–571
Guedes TB, Sawaya RJ, Nogueira CC (2014) Biogeography, vicariance and conservation of snakes of the neglected and endangered Caatinga region, northeastern Brazil. J Biogeogr 41:19–931
Guedes TB, Sawaya R, Zizka A, Laffan S, Faurby S, Pyron RA, Bérnils RS, Jansen M, Passos P, Prudente ALC, Cisneros-Heredia DF, Braz HB, Nogueira CC, Antonelli A (2018) Patterns, biases and prospects in the distribution and diversity of Neotropical snakes. Glob Ecol Biogeogr 27:14–21
Harley RM, Giulietti AM, Grilo AS, Silva TRS, Funch L, Funch RR, Queiroz LP, França F, Melo E, Gonçalves CN, Nascimento FHF (2005) Cerrado. In: Juncá FA, Funch L, Rocha W (Orgs) Biodiversidade e Conservação da Chapada Diamantina. Ministério do Meio Ambiente, Brasília, pp 121–152
Henriques RPB (2005) Influência da história, solo e fogo na distribuição e dinâmica das fitofisionomias no bioma Cerrado. In: Souza Silva JC, Felfili JM (eds) Cerrado: ecologia, biodiversidade e conservação. Ministério do Meio Ambiente, Brasília, pp 73–92
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Homeier J, Breckle SW, Günter S, Rollenbeck RT, Leuschner C (2010) Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich Ecuadorian montane rain forest. Biotropica 42:140–148
Hoorn C, Mosbrugger V, Mulch A, Antonelli A (2013) Mountain building and biodiversity. Nat Geosci 6:154
Hoorn C, Perrigo A, Antonelli A (2018a) Mountains, climate and biodiversity: an introduction. In: Hoorn C, Perrigo A, Antonelli A (eds) Mountains, climate and biodiversity. Wiley-Blackwell, Hoboken, NJ, pp 1–15
Hoorn C, Perrigo A, Antonelli A (2018b) Mountains, climate and biodiversity. Wiley-Blackwell, Hoboken, NJ
Hua X, Wiens JJ (2010) Latitudinal variation in speciation mechanisms in frogs. Evolution 64:429–443
Huber O (1988) Vegetation y Flora de Panteui, Region Guayana. Acta Bot Bras 1:41–52
Huber O (1995) Geographical and physical features. In: Berry PE, Holst BK, Yatskievych K (eds) Flora of the venezuelan Guayana. Voli. 1. Introduction. Missouri Botanical Garden, St Louis, pp 1–61
Huber O, Prance GT, Kroonenberg SB, Antonelli A (2018) The Tepuis of the Guiana Highlands. In: Hoorn C, Perrigo A, Antonelli A (eds) Mountains, climate and biodiversity. Wiley-Blackwell, Hoboken, NJ, pp 339–353
Hughes CE, Atchison GW (2015) The ubiquity of alpine plant radiations: from the Andes to the Hengduan Mountains. New Phytol 207:275–282
IUCN (International Union for Conservation of Nature), Conservation International, and NatureServe (2009) Global amphibian assessment. IUCN, Gland
Kessler M (2001) Patterns of diversity and range size of selected plant groups along an elevation transect in the Bolivia Andes. Biodivers Conserv 10:1897–1921
Kok PJR (2013) Islands in the sky: species diversity, evolutionary history, and patterns of endemism of the Pantepui Herpetofauna. Institute of Biology (IBL)/National Herbarium Nederland (NHN), Faculty of Science, Leiden University
Körner C (2004) Mountain biodiversity, its causes and function. Ambio 13:11–17
Körner C, Jetz W, Paulsen J, Payne D, Rudmann-Maurer K, Spehn EM (2017) A global inventory of mountains for bio-geographical applications. Alp Bot 127:1–15
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacomunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Leite FSF, Juncá FA, Eterovick PC (2008) Status do conhecimento, endemismo e conservação de anfíbios anuros da Cadeia do Espinhaço, Brasil. Megadiversidade 4:158–176
Leite YLR, Kok PJR, Weksler M (2015) Evolutionary affinities of the “Lost World” mouse suggest a late Pliocene connection between the Guiana and Brazilian shields. J Biogeogr 42:706–715
Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD (2009) The velocity of climate change. Nature 462:1052
Loyola RD, Kubota U, Fonseca GAB, Lewinsohn TM (2009) Key Neotropical ecoregions for conservation of terrestrial vertebrates. Biodivers Conserv 18:2017–2031
Luebert F, Muller LAH (2015) Effects of mountain formation and uplift on biological diversity. Front Genet 6:1–2
Luebert F, Weigend M (2014) Phylogenetic insights into Andean plant diversification. Front Ecol Evol 2:1–17
Lynch JD (1989) The gauge of speciation: on the frequencies of modes of speciation. In: Otte D, Endler J (eds) Speciation and its consequences. Sinauer Associates, Sunderland, MA, pp 527–553
Machado T, Silva VX, de Silva MJ (2014) Phylogenetic relationships within Bothrops neuwiedi group (Serpentes, Squamata): geographically high-structured lineages, evidence of introgressive hybridization and Neogene/Quaternary diversification. Mol Phylogenet Evol 71:1–14
Magalhães AP, Barros LF, Felippe MF (2015) Southern Serra do Espinhaço: the impressive plateau of quartzite ridges. In: Vieira B, Salgado A, Santos L (eds) Landscapes and landforms of Brazil. World geomorphological landscapes. Springer, Dordrecht, pp 359–370
Marinho-Filho J, Rodrigues FHG, Juarez KM (2002) The Cerrado mammals: diversity, ecology, and natural history. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savana. Columbia University Press, New York, pp 266–284
Marquet PA, Fernández M, Navarrete SA, Valdovinos C (2004) Species richness emerging: toward a deconstruction of species richness patterns. In: Lomolino M, Lawrence R (eds) Frontiers in biogeography: new directions in the geography of nature. Sinauer Associates, Sunderland, MA, pp 191–209
McDiarmid RW, Donnelly MA (2005) The herpetofauna of the Guayana Highlands: amphibians and reptiles of the lost world. In: Donnelly MA, Crother BI, Guyer C, Wake MH, White ME (eds) Ecology and evolution in the tropics: a herpetological perspective. University of Chicago Press, Chicago, IL, pp 461–560
Medeiros MCMP, Mattos IFA, Kanashiro MM, Tamashiro JY, Aidar MPM (2012) Vegetation mapping in an area of Ombrophilous Dense Forest at Parque Estadual da Serra do Mar, São Paulo State, Brazil, and floristic composition of the tree component of some physiognomies. Hoehnea 39:219–233
Merckx VSFT, Hendriks KP, Beentjes KK et al (2015) Evolution of endemism on a young tropical mountain. Nature 524:347–350
Modenesi-Gauttieri MC, Hiruma ST, Riccomini C (2002) Morphotectonics of a high plateau on the northwestern flank of the Continental Rift of southeastern Brazil. Geomorphology 43:257–271
Morellato LPC, Haddad CFB (2000) Introduction: the Brazilian Atlantic Forest. Biotropica 32:786–792
Morrison C, Hero JM (2003) Geographic variation in life-history characteristics of amphibians: a review. J Anim Ecol 72:270–279
Morrone J (2014) Biogeographical regionalisation of the Neotropical region. Zootaxa 3782:1–110
Moura MR, Villalobos F, Costa GC, Garcia PCA (2016) Disentangling the role of climatic, topography and vegetation in species richness gradients. PLoS One 3:1–16
Munhoz CBR, Felfili JM (2006) Floristics of the herbaceous and subshrub layer of a moist grassland in the Cerrado biosphere reserve (Alto Paraíso de Goiás), Brazil. Edinb J Bot 63:343–354
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Napoli MF, Juncá FA (2006) A new species of the Bokermannohyla circumdata group (Amphibia: Anura: Hylidae) from Chapada Diamantina, State of Bahia, Brazil. Zootaxa 1244:57–68
Neves DM, Dexter KG, Pennington RT, Bueno ML, de Miranda PLS, Oliveira-Filho AT (2018a) Lack of floristic identity in campos rupestres: a hyperdiverse mosaic of rocky montane savannas in South America. Flora 238:24–31
Neves MO, Pereira EA, Sugai JLMM, Rocha SB, Da Feio RN, Santana DJ (2018b) Distribution pattern of anurans from three mountain complexes in southeastern Brazil and their conservation implications. An Acad Bras Cienc 90:1611–1623
Oliveira U, Paglia AP, Brescovit AD, Carvalho CJB, Silva DP, Rezende DT, Leite FS, Batista JA, Barbosa JPP, Stehmann JR, Ascher JS, Vasconcelos MF, De Marco P Jr, Löwenberg-Neto P, Dias PG, Ferro VG, Santos AJ (2016) The strong influence of collection bias on biodiversity kwnoledge shortfalls of Brazilian terrestrial biodiversity. Divers Distrib 22:1232–1244
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura HE, Lamoreux JF, Wettngel WW, Hedao P, Kassem KR (2001) Terrestrial ecoregions of the world: a new map of life on earth. BioSciences 51:933–938
Papadopoulou A, Knowles LL (2016) Toward a paradigm shift in comparative phylogeography driven by trait-based hypothesis. Proc Natl Acad Sci U S A 29:8018–8024
Pavan SE, Jansa S, Voss RS (2016) Spatiotemporal diversification of a low-vagility Neotropical vertebrate clade (short-tailed opossums, Didelphidae: Monodelphis). J Biogeogr 43:1299–1309
Petri S, Sanches EAM (2012) Chapada dos Guimarães. In: Hasui Y, Carneiro CDR, Almeida FFM, Bartorelli A (Orgs) Geologia do Brasil. Beca, São Paulo, pp 413–418
Prates I, Xue AT, Brown JL, Alvarado-Serrano DF, MTU R, Hickerson MJ, Carnaval AC (2016) Inferring responses to climate dynamics from historical demography in neotropical forest lizards. Proc Natl Acad Sci U S A 113:7978–7985
Qian H, Ricklefs RE (2007) A latitudinal gradient in large-scale beta diversity for vascular plants in North America. Ecol Lett 10:737–744
Queiroz LP, França F, Giulietti AM Melo E, Gonçalves CN, Funch LS, Harley RM, Funch RR, Silva TS (2005) Caatinga. In: Juncá FA, Funch L, Rocha W (Orgs) Biodiversidade e Conservação da Chapada Diamantina. Ministério do Meio Ambiente, Brasília, pp 95–120
Quintero I, Keil P, Jetz W, Crawford FW (2015) Historical biogeography using species grographical ranges. Syst Biol 64:1059–1073
Ramos EKS, de Magalhães RF, Sari EHR, Rosa AHB, Garcia PCA, Santos FR (2018) Population genetics and distribution data reveal conservation concerns to the sky island endemic Pithecopus megacephalus (Anura, Phyllomedusidae). Conserv Genet 19:99–110
Rangel TF, Diniz-Filho JAF, Bini LM (2010) SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33:46–50
Rangel TF, Edwards NR, Holden PB, Diniz-Filho JAF, Gosling WD, Coelho MTP, Cassemiro FAS, Rahbek C, Colwell RK (2018) Modeling the ecology and evolution of biodiversity: biogeographical cradles, museums, and graves. Science 361:1–15
Rapini A, Ribeiro PL, Lambert S, Pirani JR (2008) A flora dos campos rupestres da Cadeia do Espinhaço. Megadiversidade 4:16–24
Ribeiro KT, Medina BMO, Scarano FR (2007) Species composition and biogeographic relations of the rock outcrop flora on the high plateau of Itatiaia, SE-Brazil. Rev Bras Bot 30:623–639. https://doi.org/10.1590/S0100-84042007000400008
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ribeiro PL, Rapini A, Damascena LS, van den Berg C (2014) Plant diversification in the Espinhaço Range: insights from the biogeography of Minaria (Apocynaceae). Taxon 63:1253–1264
Ricklefs RE (2004) A comprehensive framework for global patterns in biodiversity. Ecol Lett 7:1–15
Rocha WJSF, Chaves JM, Rocha CC, Lobão JB (2005) Unidades de paisagem da Chapada Diamantina-BA. In: Juncá FA, Funch L, Rocha W (Orgs) Biodiversidade e Conservação da Chapada Diamantina. Ministério do Meio Ambiente, Brasília, pp 47–64
Rodrigues MT, de Freitas MA, Silva TFS, Bertolotto CEV (2006) A new species of lizard genus Enyalius (Squamata, Leiosauridae) from the highlands of Chapada Diamantina, state of Bahia, Brazil, with a key to species. Phyllomedusa 5:11–24
Romano A, Ficetola GF (2010) Ecogeographic variation of body sixe in the spectacled salamanders (Salamandrina): influence of genetic structure and local factors. J Biogeogr 27:2358–2370
Ronquist F, Klopfstein S, Vilhelmsen L, Schulmeister S, Murray DL, Rasnityn AP (2012) A total-evidence approach to dating with fossils applied to the early radiation of the hymenoptera. Syst Biol 61:973–999
Rull V (2004) Biogeography of the ‘lost world’: a palaeoecological perspective. Earth Sci Rev 67:125–137
Rull V (2005) Biotic diversification in the Guayana Highlands: a proposal. J Biogeogr 32:921–927
Rull V (2011) Neotropical biodiversity: timing and potential drivers. Trends Ecol Evol 26:508–513
Rull V, Nogué S (2007) Potential migration routes and barriers for vascular plants of the Neotropical Guyana Highlands during the Quaternary. J Biogeogr 34:1327–1341
Rundel PW, Smith AP, Meinzer FC (1994) Tropical alpine environments: plant form and function. Cambridge University Press, Cambridge
Saadi A (1995) A Geomorfologia da Serra Do Espinhaço em Minas Gerais e de suas Margens. Geonomos 3:41–63
Safford HD (1999a) Brazilian Páramos I. An introduction to the physical environment and vegetation of the campos de altitude. J Biogeogr 26:693–712
Safford HD (1999b) Brazilian Páramos II. Macro- and mesoclimate of the Campos de Altitude and affinities with high mountain climates of the tropical Andes and Costa Rica. J Biogeogr 26:713–737
Safford HD (2007) Brazilian Páramos IV. Phytogeography of the Campos de Altitude. J Biogeogr 34:1701–1722
Salino A, Almeida TE (2008) Diversidade e conservação das pteridófitas na Cadeia do Espinhaço, Brasil. Megadiversidade 4(1–2):78–98
Sancha NU, Higgins CL, Presley SJ, Strauss RE (2014) Metacommunity structure in a highly fragmented forest: has deforestation in the Atlantic Forest altered historic biogeographic patterns? Divers Distrib 20:1058–1070
Sánchez-Baracaldo P (2004) Phylogenetics and biogeography of the Neotropical fern genera Jamesonia and Eriosorus (Pteridaceae). Am J Bot 91:274–284
Sandel B, Arge L, Dalsgaard B, Davies RG, Gaston KJ, Sutherland WJ, Svenning JC (2011) The influence of Late Quaternary climate-change velocity on species endemism. Science 334:660–664
Sanín MJ, Kissling WD, Bacon CD, Borchsenius F, Galeano G, Svenning JC, Olivera J, Ramírez R, Trénel P, Pintaud JC (2016) The Neogene rise of the tropical Andes facilitated diversification of wax palms (Ceroxylon: Arecaceae) through geographical colonization and climatic niche separation. Bot J 182:303–317
Santoro GRCC, Brandão RA (2014) Reproductive modes, habitat use, and richness of anurans from Chapada dos Veadeiros, central Brazil. North West J Zool 10:365–373
Santos JP, Freitas AVL, Brown KS Jr, Carreira JYO et al (2018) Atlantic butterflies: a data set of fruit-feeding butterfly communities from the Atlantic Forests. Ecology 99:2875–2875
Schipper J, Chanson JS, Chiozza F et al (2008) The status of the world’s land and marine mammals: diversity, threat, and knowledge. Science 322:225–230
Schuchmann KL (1999) Family Trochilidae (Hummingbirds). In: Del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world. Lynx Edicions, Barcelona, pp 468–680
Silva JMC (1997) Endemic bird species and conservation in the Cerrado Region, South America. Biodivers Conserv 6:435–450
Silva ET, Peixoto MAA, Leite FSF, Feio RN, Garcia PCA (2018) Anuran distribution in a highly diverse region of the Atlantic Forest: the Mantiqueira Mountain Range in Southeastern Brazil. Herpetologica 74:294–305
Silvestro D, Zizka A, Bacon CD, Cascales-Miñna B, Salamin N, Antonelli A (2016) Fossil biogeography: a new model to infer dispersal, extinction and sampling from palaeontological data. Philos Trans R Soc B 371:20150225
Simon MF, Proença C (2000) Phytogeographic patterns of Mimosa (Mimosoideae, Leguminosae) in the Cerrado biome of Brazil: an indicator genus of high-altitude centers of endemism? Biol Conserv 96:279–296
Simon MF, Grether R, Quieroz LP, Skema C, Pennington RT, Hughes CE (2009) Recent assembly of the Cerrado, a Neotropical plant diversity hotspot. Proc Natl Acad Sci U S A 106:20359–20364
Skeels A, Cardillo M (2019) Reconstructing the geography of speciation from contemporary biodiversity data. Am Nat 193:000–000
Tabarelli M, Santos AMM (2004) Uma breve descrição sobre a história natural dos brejos nordestinos. In: Pôrto KC, Cabral JJP, Tabarelli M (Orgs) Brejos de altitude em Pernambuco e Paraíba. História natural, ecologia e conservação. Ministério do Meio Ambiente, Brasília, pp 17–24
Thomé MTC, Zamudio KR, Haddad CFBF, Alexandrino JO (2014) Barriers, rather than refugia, underlie the origin of diversity in toads endemic to the Brazilian Atlantic Forest. Mol Ecol 23:6152–6164
Travis JMJ (2003) Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc R Soc Lond Ser B Biol Sci 270:467–473
Tuomisto H, Ruokolainen K, Yli-Halla M (2003) Dispersal, environment, and floristic variation of Wester Amazonian Forests. Science 299:241–244
Vancine MH, Duarte KS, Souza YS, Giovanelli JGR et al (2018) Atlantic Amphibians: a data set of amphibian communities from the Atlantic Forests of South America. Ecology 99:1692–1692
Vasconcelos TS, Prado VHM, da Silva FR, Haddad CFB (2014) Biogeographic distribution patterns and their correlates in the diverse frog fauna of the Atlantic Forest hotspot. PLoS One 9:e104130
Veloso HP, Rangel-Filho ALR, Lima JC (1991) Classificação da vegetação brasileira adaptada a um sistema universal. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro
Versieux LM, Wendt T, Louzada RB, Wanderleu MGL (2008) Bromeliaceae da Cadeia do Espinhaço. Megadiversidade 4:99–110
Viana PL, Filgueiras TS (2008) Inverntário e distribuicão geográfica das gramíneas (Poaceae) na Cadeia do Espinhaço, Brasil. Megadiversidade 4:71–88
Villalobos F, Dobrovolski R, Provete DB, Gouvea SF (2013) Is rich and rare the common share? Describing biodiversity and informing conservation practices for South American amphibians. PLoS One 8:e56073
Vinarski MV (2014) On the applicability of Bergmann’s rule to ectotherms: the state of the art. Biol Bull Rev 4:232–242
Werneck FP, Gamble T, Colli GR, Rodrigues MT, Sites JW Jr (2012) Deep diversification and long-term persistence in the South American “dry diagonal”: integrating continent-wide phylogeography and distribution modeling of Gekos. Evolution 66:3014–3034
Wiens JJ, Graham CH, Moen DS, Smith SA, Reeder TW (2006) Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am Nat 168:579–596
Wulf A (2016) The invention of nature: Alexander von Humboldt’s New World. Vintage Publisher, London
Zamora-Camacho FJ, Reguera S, Moreno-Rueda G (2014) Bergmann’s rule rules body size in an ectotherm: heat conservation in a lizard along a 2200-metre elevational gradient. J Evol Biol 27:2820–2828
Zamudio KR, Bell RC, Mason NA (2016) Phenotypes in phylogeography: species’ traits, environmental variation, and vertebrate diversification. Proc Natl Acad Sci U S A 113:8041–8048
Zizka A, Antonelli A (2018) Mountains of diversity. Nature 555:173–174
Acknowledgments
We are grateful to Valentí Rull and Ana Carnaval for inviting us to contribute this chapter. One anonymous reviewer and Ana Carnaval provided excellent feedback that improved the manuscript. We thank various colleagues in our research groups and scientific networks who have discussed the ideas presented here. We also thank Giulia B. D’Angelo and Marcela Nascimento for providing the bird and mammal photos from the Serra do Mar Range in Fig. 13.2. Funding for this work was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2014/18837-7) and State University of Maranhão, “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES 99999.001292/2015-03), the Swedish Research Council (B0569601), the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013, ERC Grant Agreement n. 331024), the Swedish Foundation for Strategic Research, the Biodiversity and Ecosystems in a Changing Climate (BECC) programme, and a Wallenberg Academy Fellowship to A.A.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guedes, T.B., Azevedo, J.A.R., Bacon, C.D., Provete, D.B., Antonelli, A. (2020). Diversity, Endemism, and Evolutionary History of Montane Biotas Outside the Andean Region. In: Rull, V., Carnaval, A. (eds) Neotropical Diversification: Patterns and Processes. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-31167-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-31167-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31166-7
Online ISBN: 978-3-030-31167-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)