Abstract
Covering ancient geomorphological landscapes, and surrounded by some of the most diverse forests on Earth, the Neotropical savannas were once perceived by naturalists as ancient environments. However, current evidence suggests that tropical forests have existed in the Neotropics since the Paleocene, whereas most plant lineages present in South American savannas are recently derived from clades from the surrounding forested biomes. This chapter provides a multidisciplinary overview on the origin, assembly and expansion of Neotropical savannas, with focus on South America. For this, we consider available evidence from the fossil record, paleoenvironmental proxies (phytoliths), and phylogenetic information for both plants and animals. Paleoenvironmental reconstructions indicate suitable climates for central South American savannas since the middle Miocene, which is also when molecular phylogenies indicate the origin of some vertebrate groups typical of savannas. Fossil data indicate the ecological expansion of both C3 and C4 grasses in southern South America by the late Miocene. Fossil information also indicates the onset of savannas in northern South America during the Pliocene, a period in which most woody plants of the largest extension of Neotropical savannas (the Cerrado) are thought to have diversified, as inferred by dated phylogenies. Although the combined lines of evidence indicate that Neotropical savannas in South America are indeed younger than their surrounding forests, the precise timing and factors that influenced the origin, assembly and expansion of Neotropical savannas remain contentious. Future research should aim at (1) increasing and integrating knowledge about the diversification of important taxa characteristic to Neotropical savannas, (2) establishing continuous sequences of fossils, and (3) building accurate paleoenvironmental reconstructions for the entire Neogene.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The tropical savannas of South America were once considered to be among the oldest ecosystems on the continent—a notion mostly based on their geographical distribution, especially in the Cerrado domain (Fig. 12.1), which largely occurs on ancient geomorphological surfaces (Cole 1986; Ratter et al. 1997; Oliveira-Filho and Ratter 2002). The high species richness of the Neotropical savannas has also been interpreted as evidence of their antiquity, reflecting long periods of time for biological diversification over the Cenozoic (Cole 1986; Oliveira-Filho and Ratter 2002). However, this view has been challenged based on data from molecular phylogenies inferred for vascular plants, which indicate a more recent origin of the lineages that currently inhabit the Neotropical savannas. According to molecular results, the diversification of Neotropical savanna lineages started only in the late Miocene and early Pliocene, ca. 10–4 million of years ago, mya (Pennington et al. 2006a; Simon et al. 2009). In contrast, molecular evidence provides support for a much more ancient origin of Neotropical rainforest taxa, as exemplified by the radiation of major lineages of Malpighiales in the mid-Cretaceous, ca. 112–94 mya, and the origin of palms—characteristic of most current tropical rainforests—by ca. 100 mya (Couvreur et al. 2011). Although such lineages originated mainly in the Cretaceous, the establishment of rainforest ecosystems occurred only by the early Paleogene, as indicated by the fossil record (Davis et al. 2005; Wing et al. 2009). Consequently, South America may be considered a land of ancient forests co-occurring with relatively young savannas. However, the precise timing and factors that influenced the origin, assembly, and expansion of tropical savannas remain debated. For that, as exemplified by studies of the tropical forests, data should be sought from multiple sources (Antonelli et al. 2018b).
The term savanna has a wide and diverse use in the literature. Here we consider tropical savannas the ecosystems characterized by vegetation with continuous grassy ground cover, regardless of tree cover density, and with an ecological prominence of C4 grasses as in the Cerrado sensu lato in central Brazil (the terms C3 and C4 refer to pathways that distinct groups of plants use to capture CO2 for photosynthesis; the latter is often employed by plants found in warmer, drier, and more seasonal climates; Bourlière 1983; Sarmiento 1984; Lehmann et al. 2011). Globally, biomes in which tropical savannas predominate are also referred to as “savannas”, with additional vegetation types occurring within the savanna biome (e.g., gallery forests; Olson et al. 2001; Moncrieff et al. 2016). Here we use the term “domain” to refer to each of the geographically separated core areas of the savanna biome (Ab’Sáber 2003). The savanna domains of the Neotropics are mainly coincident with the “terrestrial ecoregions” of Olson et al. (2001) and the “biogeographical provinces” of Morrone (2017), but the two former classifications treat parts of the Amazonian and the Atlantic forest domains as distinct ecoregions and provinces, which is less convenient for our discussion in this chapter.
In the Neotropics, there are three main domains of the savanna biome: the Llanos and the Guianan domains in northern South America, and the Cerrado domain in central South America—here we do not adtapt the classification of the Chaco and the Caatinga as tropical savannas, due to their more extreme climatic seasonality and their distinct vegetation structure (Olson et al. 2001; Ab’Sáber 2003; Fig. 12.1). There are also several smaller areas of savanna scattered across the Neotropics, such as within lowland Amazonia, in the Atlantic Forest, in Central America, and in the Caribbean islands (Pennington et al. 2006b). For simplicity, in this chapter we consider forests as any year-round vegetated ecosystem without a grassy ground cover, and with varying degrees of closed canopy (Lehmann et al. 2011). We distinguish between two main categories of forests: seasonally-dry tropical forest or SDTF (sensu Pennington et al. 2000) and tropical rain forests. We use these simplified definitions of forests and savannas in order to gather data across a wider geographical and temporal extent. Although in the dry season SDTFs have a similar physiognomy to savannas, a major factor distinguishing them is that savannas burn regularly and naturally, while SDTFs do not. This difference in fire regime is also reflected by their vegetation: fire-intolerant cacti, for instance, are particularly conspicuous in SDTFs (Banda et al. 2016). As these ecosystems and biomes are within the Neotropical region, they share approximately the same pool of lineages, making such simplifications less problematic than when comparing biomes across different continents (Corlett and Primack 2006; Moncrieff et al. 2016).
In terms of its geographical extent, its species richness, endemism, and floristic composition, the Cerrado domain can be considered the most cohesive of the Neotropical savannas (Silva and Bates 2002). It is mainly distributed across ancient well-drained plateaus (500–1500 m elevation), which are covered primarily by tropical savannas (Eiten 1972). It also extends across more recently dissected, adjacent peripheral depressions and valleys, where tropical savannas are less widespread. In some areas, they become replaced by SDTFs (Ratter et al. 1978). In contrast, the Llanos are not as geographically extensive and are located atop relatively recently formed geomorphological surfaces, which were subject to sporadic marine transgressions during the Neogene (Diaz de Gamero 1996; Jaramillo et al. 2017b). Additionally, the establishment of the climatic conditions associated with savannas in northern South America seems to be more recent. This recent formation is probably reflected in the relatively low species richness and endemism observed in the savanna of these northern domains (particularly for animals, but less so for plants) relative to the Cerrado savannas (Huber et al. 2006). Nevertheless, even young Neotropical systems, such as the high-elevation Páramos, may be extraordinarily diverse (Madriñán et al. 2013).
Most phylogenetic information regarding the ages of origination, assembly, and expansion of Neotropical savanna clades are derived from plant lineages of the Cerrado domain. However, phylogenetic information based on molecular data can at most only provide hypotheses for the ages of origination and diversification of extant lineages. Those dates may closely reflect the biomes they currently inhabit (e.g., Bytebier et al. 2011) but may also be of much more recent origin, and not necessarily reflect the ecological expansion of those biomes. The fossil record represents direct evidence, but, in the area currently occupied by the Cerrado domain, the number of available records is scarce; most of the information about the expansion of savannas or their fundamental components (e.g., C4 grasses) relates to the northern and southern parts of South America. Given the pros and cons of each data source, to understand the evolution of tropical savannas in South America, it is necessary to integrate available data from all suitable sources and from across the whole continent.
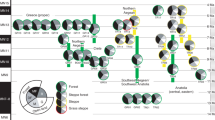
Our goal in this chapter is to review and integrate different lines of evidence that can inform on the evolutionary history of Neotropical savannas in South America. Specifically, we combine information from dated molecular phylogenies, the fossil record, and paleoenvironmental reconstructions (Fig. 12.2) to better understand the origins, assembly and expansion of South American tropical savannas.
Approximate timeline (not to scale) summarising the origin and expansion of South American tropical savannas. Arrows indicate earliest evidences. Rain forest ecosystems appeared in the early Paleocene (indicated by macrofossils and pollen). The first non-analog open biomes (shrublands) appeared in the middle Eocene (indicated by macrofossils, phytoliths, and pollen data). A decline in the concentration of CO2 started already during the end of the Oligocene, preceding the Miocene global cooling. First divergences in clades of typical savanna vertebrates occurred during the middle Miocene (indicated by molecular phylogenies), the same time in which climatic modeling indicates the presence of appropriate conditions for the occurrence of savannas. C4 grasses and the woody flora diversified from the late Miocene to the Pleistocene (indicated by molecular phylogenies). Savanna expansion occurred after the late Miocene (indicated by macrofossils, phytoliths, and pollen data). Finally, the Great American Biotic Interchange may have been increased by the expansion of savannas in South America (indicated by molecular phylogenies and fossil data)
2 Evolutionary Patterns of Extant Clades
Here we present an account of phylogenetic and biogeographical patterns of extant organisms, with a focus on the patterns of organisms in the savannas of the Cerrado domain, where such data are more complete. We do not aim at providing a comprehensive review, but rather highlight some of the earliest pieces of evidence of organisms typical of the Neotropical savannas available in the literature. Beyond the importance of phylogenetic and biogeographical information derived from plant clades, the presence of animals adapted to specific environmental conditions such as precipitation seasonality, fire regimes, and open vegetation structure can also give clues on the evolution of tropical savannas.
Plants
Most phylogenetic evidence for the recent origin of tropical savannas is based on woody legumes (Fabaceae), which dominate most Neotropical biomes. In the Cerrado, the colonization of savannas by legume clades has been influenced by adaptations to fire and drought that emerged independently in several clades (Simon et al. 2009; Batalha et al. 2011; Simon and Pennington 2012). This suggests that habitat shifts may have been important in determining the high number of plant species and morphological diversity in savannas (Souza-Neto et al. 2016). For instance, species of the genera Andira, Bauhinia, Lupinus, Stryphnodendron and Mimosa have adapted to drought and fire, diversifying in savannas only recently from rainforest and SDTF lineages, ca. 10–4 mya (Simon et al. 2009, 2016; Souza-Neto et al. 2016).
An increasing amount of fossil and molecular data, particularly from woody legumes, supports the view that more ancient ecosystems surrounded the Neotropical savannas at the time of their assembly. For instance, woody legume clades that occur both in the Cerrado and in the Caatinga domains are generally older than in situ Cerrado radiations (e.g., Cassia clade, ca. 18–9 mya, Souza-Neto et al. 2016). The same is true for some clades of Neotropical SDTF legumes, such as Coursetia, Poissonia, and Ruprechtia, which are older than the savanna clades within these genera, ranging from ca. 20–8 mya (Pennington et al. 2004; de Queiroz and Lavin 2011). Estimations based on dated phylogenies are also congruent with the fossil record, which shows that SDTFs were already present around 13–12 mya (Burnham and Johnson 2004).
Other plant clades corroborate the results found within the Fabaceae, such as the Bignonieae lineages Fridericia and Xylophragma, in the Cerrado (Lohmann et al. 2012). Within Pradosia (Sapotaceae), savanna lineages evolved even more recently (less than ca. 1 mya), and the adaptation to savanna dry conditions and fire involved important morphological changes such as the enlargement of woody structures underneath the soil (geoxylic habits, Terra-Araujo et al. 2015). Moreover, colonization of the Cerrado and other seasonal habitats has involved adaptations to drought and fire-tolerance in palm species such as Sabal palmetto and Serenoa repens (McPherson and Williams 1998; Abrahamson 2007). Further, while the palm lineages Allagoptera and Attalea (Arecaceae) diversified ca. 10 mya, the savanna lineages of this clade diversified only after ca. 1 mya (Freitas et al. 2016; Bacon et al. 2017). In addition, some species of Allagoptera show progressive shifts towards increasingly dry habitats through time, with origins in tropical rain forests, followed by colonizations of SDTFs, the savannas of the Cerrado domain, and, finally, the sandy, coastal Restinga ecosystems (Bacon et al. 2017).
In summary, phylogenetic patterns of woody plants support the hypothesis of recent assembly of the savannas of the Cerrado domain, as proposed by Pennington et al. (2004) and Simon et al. (2009). However, it is important to mention that the major diversity in savanna ecosystems is composed of non-woody elements (more than 75% of all plant species; Filgueiras 2002). Therefore, we cannot reject the possibility that other plants in Neotropical savannas belong to lineages that are more ancient, or at least older than the woody elements of the Cerrado flora. For example, the typical open fields on rock outcrops in the higher elevation areas in the Cerrado domain (“campos rupestres”) might include lineages potentially older and more diverse than the ones mentioned in this chapter (de Souza et al. 2013). However, we do not cover in detail the evolution of the campos rupestres here, as molecular data is still scarce, and in which evolution might have involved adaptations more specific to the poor soils and relatively colder climates where this physiognomy occurs (Neves et al. 2018).
Arthropods
Most biogeographical studies of arthropods in South America examined diversification in the context of the set of eastern South American dry vegetation domains (Cerrado, Chaco and Caatinga), or the “South American Dry Diagonal” (Prado 1993; Werneck 2011). Most such studies rely on ancestral state reconstructions on molecular phylogenies, rather than the arthropod fossil record—which is scarce in the region. As such, they only provide indirect clues into the origins of the savannas in the region.
For example, a phylogenetic analysis indicates that turtle ants (Myrmicinae: Cephalotes) had a burst in speciation beginning ca. 12 mya, followed by a significant increase in dispersal out of the Cerrado and Chaco (Price et al. 2014). In addition, around 9 mya, the Cerrado and Chaco clades of Zoniopoda grasshoppers (Orthoptera: Romaleidae) diverged from each other (Pocco et al. 2018). Moreover, lineages adapted to wet environments of the eastern Andes and Atlantic Forest were apparently widespread until the appearance/expansion of Chaco and Cerrado vegetation. For instance, extant Forsterinaria satyr butterflies (Nymphalidae: Satyrinae) from the Andes and southeastern Brazil diverged at ca. 7–11 mya, and ancestral state reconstruction suggests that dispersal across dry domains has become less likely towards the present than before 11 mya (Matos-Maraví et al. 2013). Similarly, the divergence of southeastern and northwestern South American Cayenne ticks (Acari: Ixodidae: Amblyomma cajennense) at ca. 10 mya may have been driven by the onset of drier climatic regimes in the Cerrado domain (Beati et al. 2013).
On the other hand, some of the arthropod clades of the Cerrado are derived from ancestral clades of the surrounding forested domains, instead of the South American Dry Diagonal, as reported for butterflies and Tropidopedia bees (Brown and Gifford 2002; Aguiar and Melo 2007). Also, Cerrado spiders of the genus Oligoxystre (Theraphosidae) and Araneus venatrix (Araneidae) have their closest relatives in the Atlantic Forest, the former diverging from its sister clade in the late Miocene (Guadanucci 2011; Peres et al. 2017). In general, studies that focused on extant arthropods indicate a late Miocene origin of taxa associated to savannas, with open domains acting as a strong geographical barrier for taxa that occur in the surrounding rain forest domains (Morrone 2006, 2014; Ferrari et al. 2015). However, the absence of data on extinctions, together with several gaps in our understanding of the origins of important ecological and functional groups (e.g., termites), prevent more conclusive answers about the origins of the South American savanna arthropods.
Tetrapods
Both mammals and birds show low levels of species endemism in the Cerrado (<10%; Macedo 2002; Marinho-Filho et al. 2002). In both groups, the oldest divergences of Cerrado lineages have been dated back to the late Miocene, with diversification inferred to the Pliocene and Pleistocene—thus temporally congruent with those reported for woody plants.
For birds, it has been hypothesized that the open ecosystems (both savannas and grasslands) of the Cerrado domain have been historically occupied by ancient endemic lineages, whereas younger endemic lineages have primarily occupied the forested areas (Silva 1997). The few published studies involving birds in the region indicate Pliocene (Passeriformes: Neopelma) or even Pleistocene (Passeriformes: Paroaria capitate and P. gularis) divergences between lineages from the Cerrado domain and lineages typical of Amazonia or the Atlantic Forest (Lopes and Gonzaga 2013; Capurucho et al. 2018). Divergences between lineages of the open ecosystems of the Cerrado domain and those of other open domains in South America (mostly grasslands) occurred from the Miocene to the Pleistocene (Chaves et al. 2015), suggesting a more complex assembly of birds in the Cerrado domain than proposed by Silva (1997).
For mammals, one of the most ancient divergences for a Cerrado endemic has been estimated for the monotypic rodent genus Calassomys (Muroidea), ca. 7 mya (Pardiñas et al. 2014). The divergence time between the rodent genus Podoxymys (Muroidea) of the tepuis and its closest relatives in the Cerrado has been used to suggest a connection between the central and northern savannas ca. 3 mya (Leite et al. 2015), although long-distance dispersal and extinctions should not be disregarded to explain the same pattern (Fine and Lohmann 2018). It has also been proposed that, still during the Pliocene, the Cerrado served as the center of diversification for the rodent genus Calomys (Muroidea; Almeida et al. 2007). For marsupials, a complex scenario has been inferred with groups colonizing the Cerrado domain from the Caatinga (e.g., Didelphidae: Thylamys karimii), between the Miocene and Pliocene whereas lineages derived from Amazonia colonized the Cerrado during the Pleistocene (e.g., Didelphidae: Monodelphis domestica; Pavan et al. 2016). In Neotropical primates, which are strongly associated with forests, there are cases of inferred diversification in savanna biomes during the Pleistocene, ca. 1–2 mya (Alfaro et al. 2015).
Amphibians and reptiles (collectively, the herpetofauna) are represented by more than 200 endemic species in the Cerrado domain, indicating their potential to unveil the history of South American savannas (Valdujo et al. 2012; Werneck 2011; Nogueira et al. 2011; Azevedo et al. 2016; Guedes et al. 2018). It can be argued that due to their relatively low mobility, herpetofaunal lineages preserve signals of the first evolutionary events in the savannas of the Cerrado domain. The oldest divergence times of herpetofaunal lineages studied so far in the Cerrado date back to the early and middle Miocene. Available studies suggest, for example, that the anole lizard Norops meridionalis diverged from its Amazonian sister clade ca. 21 mya, 15 mya lower bound of confidence interval (Guarnizo et al. 2016). Data indicate that the savanna clade of the lizard genus Kentropyx also diverged from its Amazonian sister lineage during the middle to late Miocene (Werneck et al. 2009), similar to some of the leptodactylid frogs of the genus Adenomera that colonized the Cerrado domain from Amazonia (Fouquet et al. 2014). The split of early diverging lineages in the region indicates a relatively early arrival of this group, before the origination and diversification of the woody plant species typical of today’s savannas.
The topography of the region seems to have influenced the coincident patterns of geographical range restriction of herpetofaunal species in the highlands of central Brazil (Azevedo et al. 2016). This is also suggested by phylogenetic studies of the lizard lineages Ameiva, Kentropyx, Phyllopezus, Polychrus acutirostris, and Gymnodactylus amaralli, as well as the frog genus Rhinella and snakes of the Bothrops neuwedi group (Werneck et al. 2009; Maciel et al. 2010; Thomé et al. 2010; Giugliano et al. 2013; Machado et al. 2013; Domingos et al. 2014; Fonseca et al. 2018). Although indicating relatively old initial divergences, geological and climatic events of the Pliocene and Pleistocene also appear to have affected the geographical distribution of lineages of the aforementioned clades, although we cannot disregard the influence of idiosyncratic colonization histories of individual clades (see discussion in Smith et al. 2014), nor the role of biotic interactions and other factors influencing the diversification of these taxa.
3 Inferences from the Fossil Record
While most information regarding extant groups of organisms of Neotropical savannas is derived from clades occurring in the Cerrado domain, the fossil record in this area is virtually absent for the proposed periods of origin and expansion of tropical savannas. For these reasons, we expand our focus to other regions of South America to find clues to the origin and expansion of environmental conditions and organisms associated with savannas.
Northern South America
In this region, the plant fossil record indicates that most areas currently covered by savannas were occupied by rainforest during the Paleogene (Jaramillo and Cárdenas 2013). The Neogene witnessed a large expansion of several modern ecosystems, including savannas, which replaced areas formerly occupied by lowland forests. Several areas that nowadays are dominated by SDTFs and savannas were occupied by a different ecosystem during the early Neogene. For example, the upper Magdalena valley of Colombia was covered by humid forests ca. 13–11 mya (Kay et al. 1997), and northwestern Venezuela and northeastern Colombia were occupied by a humid forest up to at least the early Pliocene, ca. 3.5 mya (Hambalek 1993; Hambalek et al. 1994; Jaramillo et al. 2015; Carrillo et al. 2018). The extensive savannas of the Llanos of Colombia and Venezuela were occupied by a humid forest up to ca. 6 mya (Jaramillo et al. 2006, 2017b), which, during glacial times (last ca. 2.7 mya), developed extensive sand dunes free of vegetation (Morales 1979; Khobzi 1981; Carr et al. 2015; Tripaldi and Zarate 2016). However, the magnitude of local sand dune fields, and their effect on modern savanna distribution, are still poorly understood. Such northern South American sites indicate a recent expansion of dry climates and associated vegetation across a portion of the Neotropics.
Southern South America
The functional morphology of endemic extinct groups of native ungulates (meridiungulates) was traditionally used to reconstruct the ecological shift of forest to grasslands during the Cenozoic. Specifically, the evolution of meridiungulates with high-crowned teeth, a trait that characterizes herbivorous ungulates in grassland ecosystems today, was long interpreted as an indication of the expansion of grasslands or savannas in the middle Eocene of Patagonia (e.g., Stebbins 1981; Jacobs et al. 1999). Analyses of middle and late Eocene fossil plant silica assemblages (phytoliths, see below), from fossilized soil horizons at Gran Barranca, Patagonia, Argentina, has supported this notion—pointing to the emergence of subtropical savannas already by ca. 40 mya (Mazzoni 1979; Zucol et al. 1999, 2007, 2010).
More recent and detailed studies have led to a very different interpretation of the paleosol biosilica record at Gran Barranca and nearby outcrops, namely that grasses were a relatively minor element in the landscape prior to the late Miocene (Strömberg et al. 2013, 2014; Selkin et al. 2015). This inference is consistent with the low relative abundance of grass phytoliths recorded in the outer shell of dung beetle ball trace fossils from Gran Barranca and other Patagonian sites (Strömberg and Stidham 2001; Sánchez et al. 2010; Strömberg 2011), as well as pollen data (Barreda and Palazzesi 2007; Palazzesi and Barreda 2012). However, these results contrast sharply with the previous interpretation of high abundance of grasses in the early-middle Eocene (e.g., Zucol et al. 2010). Dunn and co-authors (2015) further used epidermal phytolith shape to show that, despite being nearly grass-free, the vegetation in southern South America became increasingly open during the middle and late Eocene, culminating in non-analogue shrublands with abundant palms. This opening of landscapes may have been a consequence of the establishment of arid climates in southern South America by the middle Eocene, as inferred from stable isotopic, sedimentological, and paleosol climate proxy data (Bellosi 2010; Bellosi and Krause 2013; Kohn et al. 2015). Together, these lines of evidence are consistent with a hypothesis that the high-crowned cheek teeth of many meridiungulates may have evolved in response to eating plant tissues partially covered by dust or volcanic ash in the dry shrublands of Patagonia, rather than as an adaptation to feeding on grass (Dunn et al. 2015).
During the early Miocene, habitats became increasingly wet in Patagonia, a shift that seems to have preceded an expansion of more closed habitats by the middle Miocene, as shown by stable isotope data and biosilica records (Dunn et al. 2015; Kohn et al. 2015). A similar increase in forested areas in Patagonia can also be deducted from the fossil record of New World monkeys, which are commonly associated with closed canopies (Silvestro et al. 2018). Interestingly, palms decreased and grasses became more abundant, albeit still not dominant, during approximately the same time (Strömberg et al. 2013, 2014), lending support to the hypothesis that these open-habitat grasses were more mesic. The exact timing for an expansion of grass-dominated habitats in Patagonia is not known from phytoliths, but pollen data from southern South America indicate that typical grassland species became abundant only in the last ca. 10 myr (Barreda and Palazzesi 2007; Palazzesi and Barreda 2012). The spread of C4 grasses appears to have occurred relatively soon thereafter (ca. 5 mya), based on stable carbon isotope ratios from mammal tooth enamel, paleosol carbonates, and leaf waxes (Latorre et al. 1997; Kleinert and Strecker 2001; Bywater-Reyes et al. 2010; Hynek et al. 2012; Rohrmann et al. 2016). A study using phytoliths, combined with stable isotopes, further showed that high-latitude C3 environments existed alongside the lowland C4-dominated vegetation (Cotton et al. 2014).
In summary, fossil evidence from southern South America suggest that the appearance of open vegetation predated that inferred for the northern parts of the continent by over 30 myr. Open, shrubland habitats that were not analogous to savannas appeared first in the south, as early as the middle Eocene, likely influencing the evolution of large, herbivorous mammals. Grass-dominated ecosystems, however, started to dominate the landscape only over the last 10 myr.
Savannas and the Great American Biotic Interchange
After the opening of environments in both northern and southern South America in the late Miocene and early Pliocene, a large number of mammalian herbivores from North America arrived in South America in the Pliocene and Pleistocene following an expansion of savannas in Central America (Bacon et al. 2016). Prior to the Great American Biotic Interchange (GABI), the South American mid-Miocene large herbivore fauna was relatively disparate, at least at the ordinal level, consisting of giant sloths (Pilosa), armadillos/glyptodonts (Cingulata), a few large rodents, and three closely related clades of endemic south American ungulates [part of the Meridiungulata mentioned earlier (sensu McKenna and Bell 1997), i.e., Astrapotheria, Notoungulata, Litopterna; MacFadden 2006]. After the GABI, the record indicates the arrival of a diverse array of North American herbivores, such as ungulates (Artiodactyla and Perissodactyla) and relatives of elephants (Proboscidea) in South America. This resulted in an extremely diverse megaherbivore fauna in the late Pleistocene, which may have been the most diverse of any area of the world at that time in both species and phylogenetic diversity (Faurby and Svenning 2015). Because there is ample evidence from contemporary ecosystems that reductions in megafauna can sometimes lead to drastic increases in tree cover (Daskin et al. 2016), we postulate a coupling between the arrival of the North American mammals and an increase in the South American savannas, a hypothesis that could be potentially tested with the fossil record.
4 Climate Evolution and Savannas
The global distribution of savannas is largely constrained by annual precipitation and precipitation seasonality (Lehmann et al. 2011). Yet, extensive areas in the world in which tropical savannas currently occur are predicted to support forests instead (Bond 2005). Such areas are generally in more mesic environments and disturbances such as fires or herbivory help to keep the vegetation open (Bond and Midgley 2012). In less mesic environments, such as extensive areas of well-drained plateaus in central Brazil, precipitation seasonality can still be the main determinant of the predominance of savanna ecosystems, although soil composition is also an important predictor of the distribution of riparian forests, SDTFs and savannas (Ruggiero et al. 2002; Bueno et al. 2018).
Several factors interact to produce the precipitation seasonality in the area currently occupied by the Cerrado domain, including the geography of the whole continent and the latitudinal location, orientation and height of the Andean mountains—which according to global circulation models determine moisture transportation across the continent (Sepulchre et al. 2010). Seasonal and annual changes in the atmospheric circulation are in turn related to the thermal differences between the continental landmass and the surrounding oceans, characterized by a monsoonal system—the South American Convergence Zone (Gan et al. 2004; Liebmann and Mechoso 2011). This climatic system appeared first sometime during the Miocene, probably related to global scale changes in ocean circulation, tectonic movements, and variation in pCO2 (Herbert et al. 2016). Due to its complexity, it is difficult to model more precisely when and where in South America a monsoonal system was first established, and how the climatic changes affected the origin and spread of savannas in central South America.
In contrast, in northern South America, a simple mechanism helps explains the origin of the climate seasonality determining the occurrence of savannas. In this region, precipitation is largely controlled by the amplitude and degree of migration of the Intertropical Convergence Zone (ITCZ). During the austral summer, the ITCZ migrates to the south and positions itself over southern Colombia, Ecuador, and the Amazon basin (Poveda et al. 2006), leaving large portions of northern South America under dry conditions. Precipitation increases over northern South America when the ITCZ migrates north during the boreal summer (Poveda et al. 2006). This shift of the ITCZ produces a long dry season over the region occupied by savannas and xerophytic forests in northern South America. We hence propose that the ITCZ has shifted at some point within the last 6 million years, yielding the modern climate configuration. Two events have been proposed to affect the ITCZ during the late Neogene. The first one relates to the onset of the thermohaline circulation ca. 10–12 mya, as a consequence of the closure of the Central American Seaway, which pushed the ITCZ southward to its modern position (Sepulchre et al. 2014; Bacon et al. 2015; Montes et al. 2015; Jaramillo et al. 2017a; Jaramillo 2018). The second relates to the onset of permanent extensive ice in the Northern Hemisphere ca. 2.7 mya, which would have pushed the ITZC south to its current position (Flohn 1981; Shackleton et al. 1984; Chiang and Bitz 2005), generating conditions for the expansion of the savannas in northern South America. However, no fossil record is yet available to document the period covering the late Pliocene to the early Pleistocene and hence test these alternatives.
Modeling Approaches
Paleomodeling can provide insight on the origin and expansion of environmental conditions correlated to the occurrence of savannas. Beyond atmosphere-ocean circulation models, paleosols and paleotopography can also be modeled (Bragg et al. 2012; Dowsett et al. 2016), allowing the prediction of palaeovegetation (Henrot et al. 2017). The potential distribution of vegetation since the Last Glacial Maximum (LGM) inferred through such models suggests a surprising contraction of savannas and expansion of tropical forests (Costa et al. 2017), although former studies indicated precisely the contrary (Braconnot et al. 2007). Reconstruction of the forest/savanna cover for the Pliocene (mid-Piacenzian, ca. 3 mya) indicates the potential presence of savannas in the eastern part of current Amazonia and central Brazil. During this time, forests are predicted to have occupied the southern part of the current Cerrado distribution, connecting the Atlantic Forest and Amazonia (as also predicted for the LGM; Dowsett et al. 2016). A reconstruction for the late Miocene biomes indicates the potential presence of savannas in wide areas of the eastern Amazon basin and the central and southern areas of the current Cerrado domain (Pound et al. 2011). Simulations for the predominance of trees, shrubs, or grasses (instead of modelling whole domains) for the late Miocene, predict that grasslands occurred in the eastern and northern Amazon basin but were not coincident with the current distribution of the Cerrado (Bradshaw et al. 2015). A model for the middle Miocene (17–15 mya) still indicates the potential occurrence of savannas, but with a predominance of forests for most areas of South America (Henrot et al. 2017). However, paleontological sites and data are rare in South America; in fact, middle Miocene fossil localities are almost all located in the temperate zone of the Northern Hemisphere (Henrot et al. 2017), preventing model validation. There is also great uncertainty in inferring modern vegetation analogues. For instance, Eocene-Oligocene (ca. 33.9–33.5 mya) biome classifications from fossilized plant communities do not point to the presence of any grass-dominated biomes in South America, but instead different types of forests and shrublands (Palazzesi and Barreda 2012; Strömberg et al. 2013; Pound and Salzmann 2017). In summary, despite the caveats and the difficulties involved in modeling biomes in deep geological time, the existing analyses suggest that environmental conditions favorable to savannas have existed since the middle Miocene, whereas environmental conditions suitable for forests and non-analog open ecosystems dominated the continent in earlier times.
5 Origin, Assembly, and Expansion of Neotropical Savannas
Origins
The relatively recent emergence inferred from current evidence for Neotropical savannas provides a great opportunity to understand the origins, taxonomic assembly, and expansion of modern ecosystems and biomes.
Within the general framework of biome shifts and niche evolution (Donoghue and Edwards 2014), there are two main scenarios for the origin of savanna ecosystems. One envisions that multiple forest species gradually responded to a changing climate and independently developed adaptations to fire and seasonal drought. The competing alternative is that, once precipitation seasonality and a fire regime developed and created an open environment, species in surrounding environments and which were pre-adapted to those conditions succeeded in colonizing and diversifying the new environment. This latter model has been suggested for fire-prone habitats such as the South African fynbos, the chaparral in California and the kwongan in southwest Australia (see discussion in Bytebier et al. 2011).
In the Neotropics, information derived from molecular phylogenies and the biogeography of extant taxa appears to support the second scenario: early diversification of some vertebrate clades (e.g., herpetofauna) indicate the presence of open ecosystems in the early and middle Miocene, preceding the diversification of woody plants in the late Miocene and early Pliocene. In addition, the diversification of some lineages typical of the high elevation open grasslands in central South America (campo rupestre) pre-dates the diversification of the Cerrado woody-flora (Silveira et al. 2016), a pattern that may indicate that grassland ecosystems arose first in a region that remains under-sampled for fossil data.
Assembly
Over evolutionary time, it has been suggested that lineages rarely cross the boundaries of major biomes across continents (Crisp et al. 2009). This does not seem to be the case of the savannas in Cerrado, since many congeners are found in Amazonia, the Atlantic Forest, and SDTFs. This indicates that habitat shifts have been an important process in the generation of savanna diversity (Souza-Neto et al. 2016). A recent study examining connectivity amongst all Neotropical biomes found that the Cerrado and Chaco have been colonized primarily by Amazonian species (Antonelli et al. 2018a). The telling cases revised earlier in this chapter suggest that the assembly of plants, arthropods and tetrapods typical of savannas in the Cerrado domain occurred mainly during the late Miocene to the Pleistocene, with lineages colonizing mainly from surrounding biomes, although, generally, very little is known on the degree of niche conservatism of such lineages.
Expansion of Savannas
At a global scale, savanna expansion appears to differ in time among continents. Although the fossil record underlying this conclusion is still scarce (Edwards et al. 2010), most empirical data indicate that savannas had not yet expanded ca. 15 mya (Jacobs et al. 1999; Edwards et al. 2010; Strömberg 2011). What factors could have induced the expansion of Neotropical savannas in South America? Beyond changes in precipitation and seasonality (Lehmann et al. 2011), levels of CO2 may also have played a critical role, as grasses cannot compete with trees under high levels of CO2 or reduced water stress (e.g., Higgins and Scheiter 2012). Levels of CO2 are thought to have been high (>500 parts per million, ppm) during most of the Paleogene (Royer et al. 2011), reaching relatively low levels by the mid-late Oligocene (ca. 34 mya). Although proxy data suggest that atmospheric CO2 temporarily rose during the middle Miocene, to levels above 400 ppm (Kürschner et al. 2008), the issue is far from settled. It is believed that CO2 levels decreased drastically to reach <200 ppm during glacial times in the beginning of the Pleistocene (Royer 2006, 2010; de Boer et al. 2010; Royer et al. 2011). During the glacial/interglacial cycles of the Pleistocene (starting at 2.6 mya), CO2 oscillated in tandem with global temperature, ranging from ca. 280 ppm during interglacial periods to 180 ppm during glacial periods (Monnin et al. 2001; Siegenthaler et al. 2005; Lüthi et al. 2008; Tripati et al. 2009). Neogene global climate change leading to less precipitation and higher temperatures, coupled with lower atmosphere CO2 concentrations favoring C4 grasses has therefore been suggested to have driven vegetation changes in Neotropical savannas (Beerling and Osborne 2006; Osborne and Beerling 2006).
Although a coupling between CO2 levels and the expansion of savannas is suggested, this is not coincident with the origins and diversification of C4 grasses. Different groups of C4 grasses have originated since at least ca. 30 mya, with most origins occurring since the early Miocene (ca. 20 mya; Spriggs et al. 2014). The diversification of C4 grasses that dominate in South America (e.g., Andropogonae, Paspalum) occurred since the middle Miocene (ca. 15 mya; Spriggs et al. 2014). This predates, by a few million years, the commonly cited ages for when C4 grasses become ecologically dominant on other continents (<10 mya). Given that CO2 had reached relatively low levels already by the mid-late Oligocene, additional factors are also linked to the more recent rise and dominance of C4 grasses (summarized in e.g., Edwards et al. 2010). Therefore, the causes of the late expansion of C4-dominated ecosystems are still under debate. The fossil record revised in this chapter agrees with a late Miocene (Patagonia) to early Pliocene (north South America) increase in dominance and geographical extension of open ecosystems in South America.
6 Advancing Knowledge on the Origin and Evolution of Savannas
Phylogenies and the Age of Savannas
Phylogenies of extant organisms can provide important information on the age of biomes, but the evidence should be corroborated with other data. Survival in savanna ecosystems likely requires drought adaptations for lineages originally from forested ecosystems and physiological and morphological adaptations are proposed to be linked to the transitions among biomes (Donoghue and Edwards 2014). However, a phylogeny cannot easily tell us if such adaptations originated after the biome transitions (Zanne et al. 2014). Therefore the lack of old lineages of plants or animals that are currently characteristic of the savannas in the Cerrado domain cannot be seen as definitive proof against an older origin of this ecosystem (Wang 1994; Wang et al. 1999). If dispersal between biomes is predominantly from forested to open biomes, as recent data for the Neotropics suggest (Antonelli et al. 2018a), we would not expect to observe more than a few old endemic lineages in the savannas of the Cerrado domain, especially if newly arising lineages regularly outcompete the existing ones. Distinct lineages of organisms can have an old and continuous history, but without continuous and reliable fossil data, all estimates of the ages of organisms in a certain area may be biased towards younger ages (Nagalingum et al. 2011; Matzke and Wright 2016). Clearly, phylogenetic estimation and the current distribution of taxa needs to be complemented by integrating information from the fossil record and about Earth’s climatic conditions through time (Fritz et al. 2013), as reviewed in this chapter.
Modeling Savanna/Forest Transitions
Tropical forests and savannas seem to represent alternative states in certain areas, and their transition may be associated with thresholds or tipping points in environmental variables, especially precipitation (Archibald et al. 2011; Hirota et al. 2011; Lehmann et al. 2014). Savannas can potentially shift to SDTFs in drier areas with high soil fertility, or shift to semideciduous or evergreen forest in areas with higher water availability (Souza-Neto et al. 2016; Bueno et al. 2018). Therefore, modeling the past distribution of biomes without considering the different kinds of environmental thresholds from their different composing ecosystems can be imprecise. Modelling biomes or ecosystems can be also misleading due to the various assumptions made, such as the concordance between the distribution of linages and the distribution of a particular biome (Särkinen et al. 2011; Collevatti et al. 2013). Finally, although climatic conditions probably varied considerably in the past, recent evidence shows that stable biome states are possible for some combinations of climatic drivers, challenging the climatic determinism needed for biome reconstructions through time (Moncrieff et al. 2016). Despite these uncertainties, paleoclimatic reconstructions would benefit from the integration of specific knowledge on savanna thresholds and tipping points, as well as the validation and refinement of models based on fossil and phytolith data.
Filling Gaps in the Fossil Record
Fossil sites in northern South America could offer a poorly explored window to study the origination and evolution of tropical savannas, such as the fossil faunas of the Falcón basin in north-western Venezuela and in the Cocinetas basin in northern Colombia (Sánchez-Villagra et al. 2010; Jaramillo et al. 2015; Carrillo et al. 2018). The Pliocene-Pleistocene terrestrial mammal fauna of this region includes a high diversity of herbivores with a wide range of body sizes, which suggests there was enough vegetation cover to sustain a complex community of herbivores (Amson et al. 2016; Pérez et al. 2017; Carrillo et al. 2018). Quantifying the relationships between ecomorphological traits of the local fossil community (e.g., tooth structure, limb proportions) and the expected environmental properties (e.g., temperature, precipitation) may help characterize the replacement of forests by savannas in the region.
Fossil plant silica assemblages—phytoliths—have not been employed yet to study specifically the evolutionary history of the region currently occupied by the Cerrado biome. Such data could be a valuable addition to the scarce pollen and macrofossil information currently available. Studies described above using phytoliths from other parts of South America point to the potential of this record to uncover the non-analogue vegetation types and conditions that shaped the assembly of all grassland ecosystems on the continent. As amply demonstrated in recent work on modern grassy ecosystems (Hirota et al. 2011; Staver et al. 2011; Lehmann et al. 2014), such historical contingencies matter for predicting future vegetation responses to ongoing environmental change and therefore have direct conservation implications (Griffith et al. 2017).
7 Conclusions
Current knowledge indicates that the striking diversity found today in South American Neotropical savannas is most probably not the result of a long geological history or stability of this ecosystem, at least not relative to Neotropical rainforests. Environmental conditions for the occurrence of savannas have existed since the middle Miocene, and molecular phylogenies of some vertebrate groups indicate the presence of open ecosystems in central South America around then. This timing coincides broadly with the initial diversification of C4 grasses inferred from phylogenies. However, the fossil data indicates a time lag for the expansion and ecological dominance of both C3 and C4 grasses a few million years later, in the late Miocene. The period of expansion and ecological dominance of C4 grasses is coincident with the timing in which the woody flora of the savannas in the Cerrado started to diversify, as suggested by molecular phylogenies. This time lag between the origin and expansion of savannas in central South America could potentially suggest that lineages of trees that are adapted to the open fire-prone savannas diversified and became ecologically dominant after open habitats, presumably grasslands (e.g., “campos rupestres”) and associated fauna, had emerged.
Further research on tropical savannas should focus on poorly known but potentially ecologically dominant or/and ancient taxa in the tropical savannas, which might include many herbs, small shrubs, termites, ants, and fossorial lizards (Filgueiras 2002; Constantino 2005; Costa et al. 2010; Colli et al. 2016). Unfortunately, areas in which the tropical savannas occur are also particularly favorable to agriculture and farming, undergoing an alarming rate of deforestation. Protecting those species-rich and fragile ecosystems will be crucial for our ability to answer many of the remaining questions about the origins and evolution of Neotropical savannas and their biotas.
References
Ab’Sáber A (2003) Os domínios de natureza no Brasil: potencialidades paisagísticas. Ateliê Edi, São Paulo
Abrahamson WG (2007) Leaf traits and leaf life spans of two xeric-adapted palmettos. Am J Bot 94:1297–1308
Aguiar AJC, Melo GAR (2007) Taxonomic revision, phylogenetic analysis, and biogeography of the bee genus Tropidopedia (Hymenoptera, Apidae, Tapinotaspidini). Zool J Linnean Soc 151:511–554
Alfaro JWL, Cortés-Ortiz L, Di Fiore A, Boubli JP (2015) Comparative biogeography of Neotropical primates. Mol Phylogenet Evol 82:518–529
Almeida FC, Bonvicino CR, Cordeiro-Estrela P (2007) Phylogeny and temporal diversification of Calomys (Rodentia, Sigmodontinae): implications for the biogeography of an endemic genus of the open/dry biomes of South America. Mol Phylogenet Evol 42:449–466
Amson E, Carrillo JD, Jaramillo C (2016) Neogene sloth assemblages (Mammalia, Pilosa) of the Cocinetas basin (La Guajira, Colombia): implications for the Great American biotic interchange. Palaeontology 59:563–582
Antonelli A, Zizka A, Carvalho FA, Scharn R, Bacon CD, Silvestro D, Condamine FL (2018a) Amazonia is the primary source of Neotropical biodiversity. Proc Natl Acad Sci U S A 115(23):6034–6039
Antonelli A, Ariza M, Albert J, Andermann T, Azevedo J, Bacon CD, Faurby S, Guedes T, Hoorn C, Lohmann LG, Matos-Maraví P, Ritter CD, Sanmartin I, Silvestro D, Tejedor M, ter Steege H, Tuomisto H, Werneck FP, Zizka A, Edwards SV (2018b) Conceptual and empirical advances in Neotropical biodiversity research. PeerJ 6:e5644
Archibald S, Levin S, Archibald SA, Hoffmann WA, Bond WJ, Hanan N, Ratnam J, Hawthorne W, Orgle T, Roy D, Wilgen B, Van Scholes R, Duff G, Bowman D, Cook G, Batalha MA, Pivello VR, Meirelles ST, Caylor KK, Kolle O, Lloyd J, Stocks B, Levine J, Jeugd HVD, Stock WD, Rensburg SJV, Waldram MS, Bond W, Stock W, Fairbanks D (2011) Global resilience of tropical forest. Science 334:232–235
Azevedo JAR, Valdujo PH, Nogueira C (2016) Biogeography of anurans and squamates in the Cerrado hotspot: coincident endemism patterns in the richest and most impacted savanna on the globe. J Biogeogr 43:2454–2464
Bacon CD, Silvestro D, Jaramillo C, Smith BT, Chakrabarty P, Antonelli A (2015) Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc Natl Acad Sci U S A 112:6110–6115
Bacon CD, Molnar P, Antonelli A, Crawford AJ, Montes C, Vallejo-Pareja MC (2016) Quaternary glaciation and the Great American biotic interchange. Geology 44:375–378
Bacon CD, Moraes RM, Jaramillo C, Antonelli A (2017) Endemic palm species shed light on habitat shifts and the assembly of the Cerrado and Restinga floras. Mol Phylogenet Evol 110:127–133
Banda K, Delgado-Salinas A, Dexter KG, Linares-Palomino R, Oliveira-Filho A, Prado D, Pullan M, Quintana C, Riina R, Rodriguez M GM, Weintritt J, Acevedo-Rodriguez P, Adarve J, Alvarez E, Aranguren BA, Arteaga JC, Aymard G, Castano A, Ceballos-Mago N et al (2016) Plant diversity patterns in neotropical dry forests and their conservation implications. Science 353:1383–1387
Barreda V, Palazzesi L (2007) Patagonian vegetation turnovers during the Paleogene-early Neogene: origin of arid-adapted floras. Bot Rev 73:31–50
Batalha MA, Silva IA, Cianciaruso MV, De Carvalho GH (2011) Trait diversity on the phylogeny of cerrado woody species. Oikos 120:1741–1751
Beati L, Nava S, Burkman EJ, Barros-Battesti DM, Labruna MB, Guglielmone AA, Cáceres AG, Guzmán-Cornejo CM, León R, Durden LA, Faccini JLH (2013) Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae), the Cayenne tick: phylogeography and evidence for allopatric speciation. BMC Evol Biol 13:267
Beerling DJ, Osborne CP (2006) The origin of the savanna biome. Glob Chang Biol 12:2023–2031
Bellosi E (2010) Loessic and fluvial sedimentation in Sarmiento formation pyroclastics, middle Cenozoic of central Patagonia. In: Madden RH, Carlini AA, Vucetich MG, Kay RF (eds) The paleontology of Gran Barranca. Cambridge University Press, Cambridge, pp 278–292
Bellosi ES, Krause JM (2013) Onset of the Middle Eocene global cooling and expansion of open-vegetation habitats in central Patagonia. Andean Geol 1:29–48
Boer B, van de Wal RSW, Bintanja R, Lourens LJ, Tuenter E (2010) Cenozoic global ice-volume and temperature simulations with 1-D ice-sheet models forced by benthic δ18O records. Ann Glaciol 51:23–33
Bond WJ (2005) Large parts of the world are brown or black: a different view on the “Green World” hypothesis. J Veg Sci 16:261–266
Bond WJ, Midgley GF (2012) Carbon dioxide and the uneasy interactions of trees and savannah grasses. Philos Trans R Soc Lond B Biol Sci 367:601–612
Bourlière F (1983) Ecosystems of the world. In: Goodall DW (ed) Tropical savannas, vol 13. Elsevier, Amsterdam, pp 1–730
Braconnot P, Otto-Bliesner B, Harrison S, Joussaume S, Peterchmitt JY, Abe-Ouchi A, Crucifix M, Driesschaert E, Fichefet T, Hewitt CD (2007) Results of PMIP2 coupled simulations of the mid-Holocene and last glacial maximum—part 1: experiments and large-scale features. Clim Past 3:261–277
Bradshaw CD, Lunt DJ, Flecker R, Davies-Barnard T (2015) Disentangling the roles of late Miocene palaeogeography and vegetation—implications for climate sensitivity. Palaeogeogr Palaeoclimatol Palaeoecol 417:17–34
Bragg FJ, Lunt DJ, Haywood AM (2012) Mid-Pliocene climate modelled using the UK Hadley Centre Model: PlioMIP experiments 1 and 2. Geosci Model Dev 5:1109–1125
Brown KS, Gifford DC (2002) Lepidoptera in the Cerrado landscape and the conservation of vegetation, soil, and topographical mosaics. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil: ecology and natural history of a neotropical savana. Columbia University Press, New York, pp 201–222
Bueno ML, Dexter KG, Pennington RT, Pontara V, Neves DM, Ratter JA, de Oliveira-Filho AT (2018) The environmental triangle of the Cerrado domain: ecological factors driving shifts in tree species composition between forests and savannas. J Ecol 106:2109–2120
Burnham RJ, Johnson KR (2004) South American palaeobotany and the origins of neotropical rainforests. Philos Trans R Soc Lond B Biol Sci 359:1595–1610
Bytebier B, Antonelli A, Bellstedt DU, Linder HP (2011) Estimating the age of fire in the Cape flora of South Africa from an orchid phylogeny. Proc R Soc B Biol Sci 278:188–195
Bywater-Reyes S, Carrapa B, Clementz M, Schoenbohm L (2010) Effect of late Cenozoic aridification on sedimentation in the Eastern Cordillera of northwest Argentina (Angastaco basin). Geology 38:235–238
Capurucho JMG, Ashley MV, Ribas CC, Bates JM (2018) Connecting Amazonian, Cerrado, and Atlantic Forest histories: Paraphyly, old divergences, and modern population dynamics in tyrant-manakins (Neopelma/Tyranneutes, Aves: Pipridae). Mol Phylogenet Evol 127:696–705
Carr AS, Armitage SJ, Berrio JC, Bilbao BA, Boom A (2015) An optical luminescence chronology for late Pleistocene aeolian activity in the Colombian and Venezuelan Llanos. Quat Res 85:299–312
Carrillo JD, Amson E, Jaramillo C, Sánchez R, Quiroz L, Cuartas C, Rincón AF, Sánchez-Villagra MR (2018) The Neogene record of northern South American native ungulates. Smithson Contrib Paleobiol 101:1–67
Chaves AV, Freitas GHS, Vasconcelos MF, Santos FR (2015) Biogeographic patterns, origin and speciation of the endemic birds from eastern Brazilian mountaintops: a review. Syst Biodivers 13:1–16
Chiang JCH, Bitz CM (2005) Influence of high latitude ice cover on the marine intertropical convergence zone. Clim Dyn 25:477–496
Cole MM (1986) The savannas: biogeography and geobotany. Academic, London, pp 1–438
Collevatti RG, Terribile LC, de Oliveira G, Lima-Ribeiro MS, Nabout JC, Rangel TF, Diniz-Filho JAF (2013) Drawbacks to palaeodistribution modelling: the case of South American seasonally dry forests. J Biogeogr 40:345–358
Colli GR, Fenker J, Tedeschi LG, Barreto-Lima AF, Mott T, Ribeiro SLB (2016) In the depths of obscurity: knowledge gaps and extinction risk of Brazilian worm lizards (Squamata, Amphisbaenidae). Biol Conserv 204:51–62
Constantino R (2005) Padrões de diversidade e endemismo de térmitas no bioma Cerrado. In: Scariot A, Silva JCS, Felfili JM (eds) Cerrado Ecologia biodiversidade e Conservação. Ministério do Meio Ambient, Brasília, pp 319–333
Corlett RT, Primack RB (2006) Tropical rainforests and the need for cross-continental comparisons. Trends Ecol Evol 21:104–110
Costa GC, Nogueira CC, Machado RB, Colli GR (2010) Sampling bias and the use of ecological niche modeling in conservation planning: a field evaluation in a biodiversity hotspot. Biodivers Conserv 19:883–899
Costa GC, Hampe A, Ledru MP, Martinez PA, Mazzochini GG, Shepard DB, Werneck FP, Moritz C, Carnaval AC (2017) Biome stability in South America over the last 30 kyr: inferences from long-term vegetation dynamics and habitat modelling. Glob Ecol Biogeogr 27:285–297
Cotton JM, Hyland EG, Sheldon ND (2014) Multi-proxy evidence for tectonic control on the expansion of C4 grasses in northwest Argentina. Earth Planet Sci Lett 395:41–50
Couvreur TLP, Forest F, Baker WJ (2011) Origin and global diversification patterns of tropical rain forests: inferences from a complete genus-level phylogeny of palms. BMC Biol 1:1–144
Crisp MD, Arroyo MT, Cook LG, Gandolfo MA, Jordan GJ, McGlone MS, Weston PH, Westoby M, Wilf P, Linder HP (2009) Phylogenetic biome conservatism on a global scale. Nature 458:754–756
Daskin JH, Stalmans M, Pringle RM (2016) Ecological legacies of civil war: 35-year increase in savanna tree cover following wholesale large-mammal declines. J Ecol 104:79–89
Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ (2005) Explosive radiation of malpighiales supports a mid-cretaceous origin of modern tropical rain forests. Am Nat 165:E36–E65
de Souza ER, Lewis GP, Forest F, Schnadelbach AS, Berg CVD, de Queiroz LP (2013) Phylogeny of Calliandra (Leguminosae: Mimosoideae) based on nuclear and plastid molecular markers. Taxon 62:1200–1219
Domingos FMCB, Bosque RJ, Cassimiro J, Colli GR, Rodrigues MT, Santos MG, Beheregaray LB (2014) Out of the deep: cryptic speciation in a Neotropical gecko (Squamata, Phyllodactylidae) revealed by species delimitation methods. Mol Phylogenet Evol 80:113–124
Donoghue MJ, Edwards EJ (2014) Biome shifts and niche evolution in plants. Annu Rev Ecol Evol Syst 45:547–572
Dowsett H, Dolan A, Rowley D, Pound M, Salzmann U, Robinson M, Chandler M, Foley K, Haywood A (2016) The PRISM4 (mid-Piacenzian) palaeoenvironmental reconstruction. Clim Past Discuss 4:1–39
Dunn RE, Strömberg CAE, Madden RH, Kohn MJ, Carlini AA (2015) Linked canopy, climate and faunal evolution in the Cenozoic of Patagonia. Science 347:258–261
Edwards EJ, Osborne CP, Strömberg CAE, Smith SA, Bond WJ, Christin PA, Cousins AB, Duvall MR, Fox DL, Freckleton RP, Ghannoum O, Hartwell J, Huang Y, Janis CM, Keeley JE, Kellogg EA, Knapp AK, Leakey ADB, Nelson DM, Saarela JM, Sage RF, Sala OE, Salamin N, Still CJ, Tipple B (2010) The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328:587–591
Eiten G (1972) The cerrado vegetation of Brazil. Bot Rev 38:201–341
Faurby S, Svenning JC (2015) Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers Distrib 21:1155–1166
Ferrari A, Barão KR, Bianchi FM, Campos LA, Grazia J (2015) Classification and biogeography of neotropical true bugs. In: Panizzi Antônio R, Jocélia G (eds) True bugs (Heteroptera) of the Neotropics. Springer, Dordrecht, pp 57–87
Filgueiras T (2002) Herbaceous plant communities. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil: ecology and natural history of a neotropical savana. Columbia University Press, New York, pp 121–139
Fine PVA, Lohmann LG (2018) Importance of dispersal in the assembly of the Neotropical biota. Proc Natl Acad Sci U S A 115:201807012
Flohn H (1981) A hemispheric circulation asymmetry during late Tertiary. Geol Rundsch 70:725–736
Fonseca EM, Gehara M, Werneck FP, Lanna FM, Colli GR, Sites JW, Rodrigues MT, Garda AA (2018) Diversification with gene flow and niche divergence in a lizard species along the South American “diagonal of open formations”. J Biogeogr 45:1688–1700
Fouquet A, Cassini SC, Haddad CFB, Pech N, Rodrigues MT (2014) Species delimitation, patterns of diversification and historical biogeography of the Neotropical frog genus Adenomera (Anura, Leptodactylidae). J Biogeogr 41:855–870
Freitas C, Meerow AW, Pintaud JC, Henderson A, Noblick L, Costa FRC, Barbosa CE, Barrington D (2016) Phylogenetic analysis of Attalea (Arecaceae): insights into the historical biogeography of a recently diversified Neotropical plant group. Bot J Linn Soc 182:287–302
Fritz SA, Schnitzler J, Eronen JT, Hof C, Böhning-Gaese K, Graham CH (2013) Diversity in time and space: wanted dead and alive. Trends Ecol Evol 28:509–516
Gamero MLD (1996) The changing course of the Orinoco river during the Neogene: a review. Palaeogeogr Palaeoclimatol Palaeoecol 123:385–402
Gan MA, Kousky VE, Ropelewski CF (2004) The South America monsoon circulation and its relationship to rainfall over west-central Brazil. J Clim 17:47–66
Giugliano LG, Nogueira C, Valdujo PH, Collevatti RG, Colli GR (2013) Cryptic diversity in South American Teiinae (Squamata, Teiidae) lizards. Zool Scr 42:473–487
Griffith DM, Lehmann CER, Strömberg CAE, Parr CL, Pennington RT, Sankaran M, Ratnam J, Still CJ, Powell RL, Hanan NP (2017) Comment on “The extent of forest in dryland biomes”. Science 358:27–30
Guadanucci JPL (2011) Cladistic analysis and biogeography of the genus Oligoxystre Vellard 1924 (Araneae: Mygalomorphae: Theraphosidae). J Arachnol 39:320–326
Guarnizo CE, Werneck FP, Giugliano LG, Santos MG, Fenker J, Sousa L, D’Angiolella AB, dos Santos AR, Strüssmann C, Rodrigues MT, Dorado-Rodrigues TF, Gamble T, Colli GR (2016) Cryptic lineages and diversification of an endemic anole lizard (Squamata, Dactyloidae) of the Cerrado hotspot. Mol Phylogenet Evol 94:279–289
Guedes TB, Sawaya RJ, Zizka A, Laffan S, Faurby S, Pyron RA, Bernils RS, Jansen M, Passos P, Prudente ALC et al (2018) Patterns, biases and prospects in the distribution and diversity of Neotropical snakes. Glob Ecol Biogeogr 27:14–21
Hambalek N (1993) Palinoestratigrafia del Mioceno-Pliocene de la región de Urumaco, Falcon Noroccidental. Universidad Central de Venezuela, Caracas
Hambalek N, Rull V, Digiacomo E, Gamero MLD (1994) Evolución paleoecológica y paleoambiental de la secuencia del Neógeno en el surco de Urumaco. Estudio palinológico y litológico. Bol Soc Venez Geol 191:7–19
Henrot AJ, Utescher T, Erdei B, Dury M, Hamon N, Ramstein G, Krapp M, Herold N, Goldner A, Favre E, Munhoven G, François L (2017) Middle Miocene climate and vegetation models and their validation with proxy data. Palaeogeogr Palaeoclimatol Palaeoecol 467:95–119
Herbert TD, Lawrence KT, Tzanova A, Peterson LC, Caballero-Gill R, Kelly CS (2016) Late Miocene global cooling and the rise of modern ecosystems. Nat Geosci 9:843–847
Higgins SI, Scheiter S (2012) Atmospheric CO2 forces abrupt vegetation shifts locally, but not globally. Nature 488:209–212
Hirota M, Holmgren M, Van Nes EH, Scheffer M (2011) Global resilience of tropical forest and savanna to critical transitions. Science 334:232–235
Huber O, de Stefano RD, Aymard G, Riina R (2006) Flora and vegetation of the Venezuelan Llanos: a review. In: Ratter JA, Pennington RT (eds) Neotropical savannas and seasonally dry forests: plant diversity, biogeography, and conservation. CRC, Boca Raton, FL, pp 95–120
Hynek SA, Passey BH, Prado JL, Brown FH, Cerling TE, Quade J (2012) Small mammal carbon isotope ecology across the Miocene–Pliocene boundary, northwestern Argentina. Earth Planet Sci Lett 321:177–188
Jacobs BF, Kingston JD, Jacobs LL (1999) The origin of grass-dominated ecosystems. Ann Mo Bot Gard 86:590–643
Jaramillo C (2018) Evolution of the Isthmus of Panama: biological, paleoceanographic, and paleoclimatological implications. In: Hoorn C, Perrigo A, Antonelli A (eds) Mountains, climate and biodiversity. Wiley, Oxford, pp 323–338
Jaramillo C, Cárdenas A (2013) Global warming and neotropical rainforests: a historical perspective. Annu Rev Earth Planet Sci 41:741–766
Jaramillo C, Rueda M, Mora G (2006) Cenozoic plant diversity in the Neotropics. Science 311:1893–1896
Jaramillo C, Moreno F, Hendy AJW, Sánchez-Villagra MR, Marty D (2015) Preface: La Guajira, Colombia: a new window into the Cenozoic neotropical biodiversity and the Great American biotic interchange. Swiss J Palaeontol 134:1–4
Jaramillo C, Montes C, Cardona A, Silvestro D, Antonelli A, Bacon C (2017a) Comment (1) on “Formation of the Isthmus of Panama” by O’Dea et al. Sci Adv 3:e1602321
Jaramillo C, Romero I, D’Apolito C, Bayona G, Duarte E, Louwye S, Escobar J, Luque J, Carrillo-Briceno J, Zapata V, Mora A, Schouten S, Zavada M, Harrington G, Ortiz J, Wesselingh F (2017b) Miocene flooding events of Western Amazonia. Sci Adv 3:e1601693
Kay RF, Madden RH, Cifelli RL, Flynn JJ (1997) Vertebrate paleontology in the Neotropics. The Miocene Fauna of La Venta, Colombia. Smithsonian Institution Press, Washington, DC
Khobzi J (1981) Los campos de dunas del norte de Colombia y de los Llanos del Orinoco (Colombia y Venezuela). Rev CIAF 6:257–292
Kleinert K, Strecker MR (2001) Climate change in response to orographic barrier uplift: paleosol and stable isotope evidence from the late Neogene Santa Maria basin, northwestern Argentina. Geol Soc Am Bull 113:728–742
Kohn MJ, Strömberg CAE, Madden RH, Dunn RE, Evans S, Palacios A, Carlini AA (2015) Quasi-static Eocene-Oligocene climate in Patagonia promotes slow faunal evolution and mid-Cenozoic global cooling. Palaeogeogr Palaeoclimatol Palaeoecol 435:24–37
Kürschner WM, Kvaček Z, Dilcher DL (2008) The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proc Natl Acad Sci 105:449–453
Latorre C, Quade J, McIntosh WC (1997) The expansion of C4 grasses and global change in the late Miocene: stable isotope evidence from the Americas. Earth Planet Sci Lett 146:83–96
Lehmann CE, Archibald SA, Hoffmann WA, Bond WJ (2011) Deciphering the distribution of the savanna biome. New Phytol 191:197–209
Lehmann CER, Anderson TM, Sankaran M, Higgins SI, Archibald S, Hoffmann WA, Hanan NP, Williams RJ, Fensham RJ, Felfili J, Hutley LB, Ratnam J, San Jose J, Montes R, Franklin D, Russell-Smith J, Ryan CM, Durigan G, Hiernaux P, Haidar R, Bowman D, Bond WJ (2014) Savanna vegetation-fire-climate relationships differ among continents. Science 343:548–552
Leite YLR, Kok PJR, Weksler M (2015) Evolutionary affinities of the “Lost World” mouse suggest a late Pliocene connection between the Guiana and Brazilian shields. J Biogeogr 42:706–715
Liebmann B, Mechoso CR (2011) The South American monsoon system. In: Chang CP, Ding Y, Lau NC, Johnson RH, Wang B, Yasunari T (eds) The global monsoon system: research and forecast. World Scientific Publishing, New Jersey, pp 137–157
Lohmann LG, Bell CD, Calió MF, Winkworth RC (2012) Pattern and timing of biogeographical history in the Neotropical tribe Bignonieae (Bignoniaceae). Bot J Linn Soc 171:154–170
Lopes LE, Gonzaga LP (2013) Taxonomy, natural history, and conservation of Paroaria baeri (Aves: Thraupidae). Trop Zool 26:87–103
Lüthi D, Le Floch M, Bereiter B, Blunier T, Barnola JM, Siegenthaler U, Raynaud D, Jouzel J, Fischer H, Kawamura K, Stocker TF (2008) High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 453:379–382
Macedo RHF (2002) The avifauna: ecology, biogeography, and behavior. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil: ecology and natural history of a neotropical savana. Columbia University Press, New York, pp 242–265
MacFadden BJ (2006) Extinct mammalian biodiversity of the ancient New World tropics. Trends Ecol Evol 21:157–165
Machado T, Silva VX, Silva MJJ (2013) Phylogenetic relationships within Bothrops neuwiedi group (Serpentes, Squamata): geographically highly-structured lineages, evidence of introgressive hybridization and Neogene/Quaternary diversification. Mol Phylogenet Evol 71:1–14
Maciel NM, Collevatti RG, Colli GR, Schwartz EF (2010) Late Miocene diversification and phylogenetic relationships of the huge toads in the Rhinella marina (Linnaeus, 1758) species group (Anura: Bufonidae). Mol Phylogenet Evol 57:787–797
Madriñán S, Cortés AJ, Richardson JE (2013) Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Front Genet 4:1–7
Marinho-Filho J, Rodrigues FHG, Juarez KM (2002) The Cerrado mammals: diversity, ecology, and natural history. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil: ecology and natural history of a neotropical savana. Columbia University Press, New York, pp 266–284
Matos-Maraví P, Peña C, Willmott KR, Freitas AVL, Wahlberg N (2013) Systematics and evolutionary history of butterflies in the “Taygetis clade” (Nymphalidae: Satyrinae: Euptychiina): towards a better understanding of Neotropical biogeography. Mol Phylogenet Evol 66:54–68
Matzke NJ, Wright A (2016) Inferring node dates from tip dates in fossil Canidae: the importance of tree priors. Biol Lett 12:1–4
Mazzoni MM (1979) Contribución al conocimiento petrográfico de la Formación Sarmiento, barranca sur del lago Colhue Huapi, provincia de Chubut. Rev Asoc Argent Mineral Petrol Sedimentol 10:33–54
McKenna MC, Bell SK (1997) Classification of mammals above the species level. Columbia University Press, New York
McPherson K, Williams K (1998) Fire resistance of cabbage palms (Sabal palmetto) in the southeastern USA. For Ecol Manag 109:197–207
Moncrieff GR, Bond WJ, Higgins SI (2016) Revising the biome concept for understanding and predicting global change impacts. J Biogeogr 43:863–873
Monnin E, Indermühle A, Dällenbach A, Flückiger J, Stauffer B, Stocker TF, Raynaud D, Barnola J-M (2001) Atmospheric CO2 concentrations over the last glacial termination. Science 291:112–114
Montes C, Cardona A, Jaramillo C, Pardo A, Silva JC, Valencia V, Ayala C, Pérez-Angel LC, Rodriguez-Parra LA, Ramirez V, Niño H (2015) Middle Miocene closure of the Central American Seaway. Science 348:226–229
Morales PR (1979) Estudio de los médanos de los Llanos Centrales de Venezuela: evidencias de un clima desértico. Acta Biol Venez 10:19–49
Morrone JJ (2006) Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol 51:467–494
Morrone JJ (2014) Biogeographical regionalisation of the Neotropical region. Zootaxa 3782:1–110
Morrone JJ (2017) Neotropical biogeography: regionalization and evolution. CRC/Taylor & Francis Group, Boca Raton, FL
Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S (2011) Recent synchronous radiation of a living fossil. Science 334:796–799
Neves DM et al (2018) Lack of floristic identity in campos rupestres—a hyperdiverse mosaic of rocky montane savannas in South America. Flora 238:24–31
Nogueira C, Ribeiro S, Costa GC, Colli GR (2011) Vicariance and endemism in a Neotropical savanna hotspot: distribution patterns of Cerrado squamate reptiles. J Biogeogr 38:1907–1922
Oliveira-Filho AT, Ratter JA (2002) Vegetation physiognomies and wood flora of the Cerrado biome. In: The cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 91–120
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC et al (2001) Terrestrial ecoregions of the world: a new map of life on earth. A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 51:933–938
Osborne CP, Beerling DJ (2006) Nature’s green revolution: the remarkable evolutionary rise of C4 plants. Philos Trans R Soc Lond B Biol Sci 361:173–194
Palazzesi L, Barreda V (2012) Fossil pollen records reveal a late rise of open-habitat ecosystems in Patagonia. Nat Commun 3:1294
Pardiñas UFJ, Lessa G, Teta P, Salazar-Bravo J, Câmara EMVC (2014) A new genus of sigmodontine rodent from eastern Brazil and the origin of the tribe Phyllotini. J Mammal 95:201–215
Pavan SE, Jansa SA, Voss RS (2016) Spatiotemporal diversification of a low-vagility Neotropical vertebrate clade (short-tailed opossums, Didelphidae: Monodelphis). J Biogeogr 43:1299–1309
Pennington RT, Prado DE, Pendry CA (2000) Neotropical seasonally dry forests and Quaternary vegetation changes. J Biogeogr 27:261–273
Pennington RT, Lavin M, Prado DE, Pendry CA, Pell SK, Butterworth CA (2004) Historical climate change and speciation: neotropical seasonally dry forest plants show patterns of both tertiary and quaternary diversification. Philos Trans R Soc Lond B Biol Sci 1443:515–537
Pennington RT, Richardson JE, Lavin M (2006a) Insights into the historical construction of species-rich biomes from dated plant phylogenies, neutral ecological theory and phylogenetic community structure. New Phytol 172:605–616
Pennington TR, Lewis GP, Ratter JA (2006b) Neotropical savannas and seasonally dry forests: plant diversity, biogeography, and conservation. CRC, London
Peres EA, Silva MJ, Solferini VN (2017) Phylogeography of the spider Araneus venatrix (Araneidae) suggests past connections between Amazon and Atlantic rainforests. Biol J Linn Soc 121:771–785
Pérez ME, Vallejo-Pareja MC, Carrillo JD, Jaramillo C (2017) A new Pliocene Capybara (Rodentia, Caviidae) from northern South America (Guajira, Colombia), and its implications for the Great American biotic interchange. J Mamm Evol 24:111–125
Pocco ME, Guzmán N, Plischuk S, Confalonieri V, Lange CE, Cigliano MM (2018) Diversification patterns of the grasshopper genus Zoniopoda Stål (Romaleidae, Acridoidea, Orthoptera) in open vegetation biomes of South America. Syst Entomol 43:290–307
Pound MJ, Salzmann U (2017) Heterogeneity in global vegetation and terrestrial climate change during the late Eocene to early Oligocene transition. Sci Rep 7:1–12
Pound MJ, Haywood AM, Salzmann U, Riding JB, Lunt DJ, Hunter SJ (2011) A Tortonian (Late Miocene, 11.61–7.25Ma) global vegetation reconstruction. Palaeogeogr Palaeoclimatol Palaeoecol 300:29–45
Poveda G, Waylen PR, Pulwarty RS (2006) Annual and inter-annual variability of the present climate in northern South America and southern Mesoamerica. Palaeogeogr Palaeoclimatol Palaeoecol 234:3–27
Prado DE (1993) What is the Gran Chaco vegetation in South America? Candollea 145:29
Price SL, Powell S, Kronauer DJC, Tran LAP, Pierce NE, Wayne RK (2014) Renewed diversification is associated with new ecological opportunity in the Neotropical turtle ants. J Evol Biol 27:242–258
Queiroz LP, Lavin M (2011) Coursetia (Leguminosae) from eastern Brazil: nuclear ribosomal and chloroplast DNA sequence analysis reveal the monophyly of three Caatinga-inhabiting species. Syst Bot 36:69–79
Ratter JA, Askew GP, Montgomery RF, Gifford DR (1978) Observations on forests of some mesotrophic soils in Central Brazil (Observações sobre florestas de alguns solos mesotroficos no Brasil Central). Rev Bras Bot 1:47–58
Ratter JA, Ribeiro JF, Bridgewater S (1997) The Brazilian cerrado vegetation and threats to its biodiversity. Ann Bot 80:223–230
Rohrmann A, Sachse D, Mulch A, Pingel H, Tofelde S, Alonso RN, Strecker MR (2016) Miocene orographic uplift forces rapid hydrological change in the southern central Andes. Sci Rep 6:35678
Royer DL (2006) CO2-forced climate thresholds during the Phanerozoic. Geochim Cosmochim Acta 70:5665–5675
Royer DL (2010) Fossil soils constrain ancient climate sensitivity. Proc Natl Acad Sci U S A 107:517–518
Royer DL, Pagani M, Beerling DJ (2011) Geologic constraints on earth system sensitivity to CO2 during the Cretaceous and early Paleogene. Earth Syst Dyn Discuss 2:1–30
Ruggiero PGC, Batalha MA, Pivello VR, Meirelles ST (2002) Soil-vegetation relationships in cerrado (Brazilian savanna) and semideciduous forest, Southeastern Brazil. Plant Ecol 160:1–16
Sánchez MV, Laza JH, Bellosi ES, Genise JF (2010) Ichnostratigraphy of middle Cenozoic Coprinisphaera from central Patagonia: insights into the evolution of dung beetles, herbivores and grass-dominated habitats. Palaeogeogr Palaeoclimatol Palaeoecol 297:633–648
Sánchez-Villagra MR, Aguilera OA, Carlini AA (2010) Urumaco and Venezuelan Paleontology. In: Orangel A et al (eds) The fossil record of the northern Neotropics The fossil record of the northern Neotropics. Indiana University Press, Bloomington, IN, pp 192–213
Särkinen T, Iganci JRV, Linares-Palomino R, Simon MF, Prado DE (2011) Forgotten forests—issues and prospects in biome mapping using seasonally dry tropical forests as a case study. BMC Ecol 11:1–15
Sarmiento G (1984) The ecology of neotropical savannas. Harvard University Press, Cambridge
Selkin PA, Strömberg CAE, Dunn RE, Kohn MJ, Carlini AA, Davies-Vollum KS, Madden RH (2015) Climate, dust, and fire across the Eocene-Oligocene transition, Patagonia. Geology G36664.1:1–4
Sepulchre P, Sloan LC, Fluteau F (2010) Modelling the response of Amazonian climate to the uplift of the Andean mountain range. In: Hoorn MC, Wesselingh FP (eds) Amazonia, landscape and species evolution: a look into the past. Wiley-Blackwell, Oxford, pp 211–222
Sepulchre P, Arsouze T, Donnadieu Y, Dutay JC, Jaramillo C, Le Bras J, Martin E, Montes C, Waite AJ (2014) Consequences of shoaling of the Central American Seaway determined from modeling Nd isotope. Paleoceanography 29:176–189
Shackleton NJ, Backman J, Zimmerman H, Kent DV, Hall MA, Roberts DG, Schnitker D, Baldauf JG, Desprairies A, Homrighausen R, Huddlestun P, Keene JB, Kaltenback AJ, Krumsiek KAO, Morton AC, Murray JW, Westberg-Smith J (1984) Oxygen isotope calibration of the onset of ice-rafting and history of glaciation in the North Atlantic region. Nature 307:620–623
Siegenthaler U, Stocker TF, Monnin E, Lüthi D, Schwander J, Stauffer B, Raynaud D, Barnola JM, Fischer H, Masson-Delmotte V, Jouzel J (2005) Stable carbon cycle-climate relationship during the late Pleistocene. Science 310:1313–1317
Silva JMC (1997) Endemic bird species and conservation in the Cerrado Region, South America. Biodivers Conserv 6:435–450
Da Silva JC, Bates J (2002) Biogeographic patterns and conservation in the South American Cerrado: a tropical savanna hotspot. Bioscience 52:225–233
Silveira FAO, Negreiros D, Barbosa NPU, Buisson E, Carmo FF, Carstensen DW, Conceição AA, Cornelissen TG, Echternacht L, Fernandes GW, Garcia QS, Guerra TJ, Jacobi CM, Lemos-Filho JP, Le Stradic S, Morellato LPC, Neves FS, Oliveira RS, Schaefer CE, Viana PL, Lambers H (2016) Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant Soil 403:129–152
Silvestro D, Tejedor MF, Serrano-Serrano ML, Loiseau O, Rossier V, Rolland J, Zizka A, Höhna S, Antonelli A, Salamin N (2018) Early arrival and climatically-linked geographic expansion of New World monkeys from tiny African ancestors. Syst Biol 68:78–92
Simon MF, Pennington T (2012) Evidence for adaptation to fire regimes in the tropical savannas of the Brazilian Cerrado. Int J Plant Sci 173:711–723
Simon MF, Grether R, Queiroz LP, Skema C, Pennington RT, Hughes CE (2009) Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc Natl Acad Sci U S A 106:20359–20364
Simon MF, Pastore JFB, Souza AF, Borges LM, Scalon VR, Ribeiro PG, Santos-Silva J, Souza VC, Queiroz LP (2016) Molecular phylogeny of Stryphnodendron (Mimosoideae, Leguminosae) and generic delimitations in the Piptadenia group. Int J Plant Sci 177:41–59
Smith BT, McCormack JE, Cuervo AM, Hickerson MJ, Aleixo A, Cadena CD, Pérez-Emán J, Burney CW, Xie X, Harvey MG, Faircloth BC, Glenn TC, Derryberry EP, Prejean J, Fields S, Brumfield RT (2014) The drivers of tropical speciation. Nature 515:406–409
Souza-Neto AC, Cianciaruso MV, Collevatti RG (2016) Habitat shifts shaping the diversity of a biodiversity hotspot through time: insights from the phylogenetic structure of Caesalpinioideae in the Brazilian Cerrado. J Biogeogr 43:340–350
Spriggs EL, Christinb PA, Edwards EJ (2014) C4 photosynthesis promoted species diversification during the Miocene grassland expansion. PLoS One 9:e97722
Staver AC, Archibald S, Levin SA (2011) The global extent and determinants of savanna and forest as alternative biome states. Science 334:230–232
Stebbins GL (1981) Coevolution of grasses and herbivores. Ann Mo Bot Gard 68:75–86
Strömberg CAE (2011) Evolution of grasses and grassland ecosystems. Annu Rev Earth Planet Sci 39:517–544
Strömberg CAE, Stidham TA (2001) Dung beetle brood balls and notoungulate diet. J Vertebr Paleontol 21:105A
Strömberg CAE, Dunn RE, Madden RH, Kohn MJ, Carlini AA (2013) Decoupling the spread of grasslands from the evolution of grazer-type herbivores in South America. Nat Commun 4:1478
Strömberg CAE, Dunn RE, Madden RH, Kohn MJ, Carlini AA (2014) Evolution of grazer morphologies in the absence of grasslands in southern South America. In: North American paleontological convention. The Paleontological Society Special Publications, Gainesville, FL, p 113
Terra-Araujo MH, de Faria AD, Vicentini A, Nylinder S, Swenson U (2015) Species tree phylogeny and biogeography of the Neotropical genus Pradosia (Sapotaceae, Chrysophylloideae). Mol Phylogenet Evol 87:1–13
Thomé MTC, Zamudio KR, Giovanelli JGR, Haddad CFB, Baldissera FA Jr, Alexandrino J (2010) Phylogeography of endemic toads and post-Pliocene persistence of the Brazilian Atlantic Forest. Mol Phylogenet Evol 55:1018–1031
Tripaldi A, Zarate M (2016) A review of Late Quaternary inland dune systems of South America east of the Andes. Quat Int 410:96–110
Tripati AK, Roberts CD, Eagle RA (2009) Coupling of CO2 and ice sheet stability over major climate transitions of the last 20 million years. Science 326:1394
Valdujo PH, Silvano DL, Colli G, Martins M (2012) Anuran species composition and distribution patterns in Brazilian Cerrado, a Neotropical hotspot. S Am J Herpetol 7:63–78
Wang X (1994) Phylogenetic systematics of the Hesperocyoninae (Carnivora, Canidae). Bull Am Mus Nat Hist 221:1–27
Wang X, Tedford RH, Taylor BE (1999) Phylogenetic systematics of the Borophaginae (Carnivora, Canidae). Bull Am Mus Nat Hist 243:1–391
Werneck FP (2011) The diversification of eastern South American open vegetation biomes: historical biogeography and perspectives. Quat Sci Rev 30:1630–1648
Werneck FP, Giugliano LG, Collevatti RG, Colli GR (2009) Phylogeny, biogeography and evolution of clutch size in South American lizards of the genus Kentropyx (Squamata: Teiidae). Mol Ecol 18:262–278
Wing SL, Herrera F, Jaramillo CA, Gomez-Navarro C, Wilf P, Labandeira CC (2009) Late Paleocene fossils from the Cerrejon formation, Colombia, are the earliest record of Neotropical rainforest. Proc Natl Acad Sci U S A 106:18627–18632
Zanne AE, Tank DC, Cornwell WK, Eastman JM, Smith SA, Fitzjohn RG, McGlinn DJ, O’Meara BC, Moles AT, Reich PB, Royer DL, Soltis DE, Stevens PF, Westoby M, Wright IJ, Aarssen L, Bertin RI, Calaminus A, Govaerts R, Hemmings F, Leishman MR, Oleksyn J, Soltis PS, Swenson NG, Warman L, Beaulieu JM (2014) Three keys to the radiation of angiosperms into freezing environments. Nature 506:89–92
Zucol AF, Mazzoni MM, Madden RH (1999) Análisis fitolíticos en la secuencia sedimentaria de Gran Barranca, Chubut. In: Frenguelli J (ed) Primer Encuentro Argentino de Investigaciones Fitolíticas. Asociación de Ciencias Naturales del Litoral, Diamante, pp 11–12
Zucol AF, Brea M, Bellosi E, Carlini AA, Vucetich MG (2007) Preliminary phytolith analysis of Sarmiento formation in the Gran Barranca (central Patagonia, Argentina). In: Madella M, Zurro D (eds) Plants, people and places. Recent studies in phytolith analysis. Oxbow Books, Oxford, pp 189–195
Zucol AF, Brea M, Bellosi E (2010) Phytolith studies in Gran Barranca (central Patagonia, Argentina): the middle-late Eocene. In: Madden RH, Carlini AA, Vucetich MG, Kay RF (eds) The paleontology of Gran Barranca. Cambridge University Press, Cambridge, pp 317–340
Acknowledgments
We thank Valentí Rull and Ana Carnaval for the invitation to contribute a chapter on this topic, an anonymous reviewer for the valuable comments on our manuscript, our research groups for valuable discussion, and S. Pineda-Muñoz for comments and discussion on ecometrics. Funding for this work was provided by the Swedish Research Council (B0569601), the Swedish Foundation for Strategic Research, the Biodiversity and Ecosystems in a Changing Climate (BECC) programme, and a Wallenberg Academy Fellowship to A.A.; continuous productivity grants from CNPq to R.G.C.; the Smithsonian Tropical Research Institute, the Anders Foundation, 1923 Fund and Gregory D. and Jennifer Walston Johnson to C.J.; FAPESP scholarship 2014/18837-7 and the State Univ. of Maranhão for the Senior Research fellowship to T.B.G.; the Swiss National Science Foundation fund P2ZHP3_174749 to J.D.C.; NSF EAR-1253713 and EAR-1349749 to C.A.E.S.; the Marie Skłodowska-Curie fellowship (project MARIPOSAS-704035) and the PPLZ programme of the Czech Academy of Sciences (grant L200961951) to P.M.M; Swedish Research Council (2017-04980) to C.D.B; Swedish Research Council (2017-03862) to S.F.; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—99999.001292/2015-03) to J.A.R.A.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Azevedo, J.A.R. et al. (2020). On the Young Savannas in the Land of Ancient Forests. In: Rull, V., Carnaval, A. (eds) Neotropical Diversification: Patterns and Processes. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-31167-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-31167-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-31166-7
Online ISBN: 978-3-030-31167-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)