Abstract
Biofilms formed by microbes are the aggregates of bacterial masses that are fixed in the matrix produced by itself comprising of extracellular polymeric substances (EPS). Microbial biofilms pose serious threat to the hospital-based infections as well as other types of infections. This is because biofilm provides highly enhanced protection and tolerance to the pathogens towards antimicrobial compounds. Moreover, the pathogen also survives the immune response of the host. This leads to extremely intractable, prolonged infections resulting in high tolls of morbidity and mortality. The fact that around 80% of human diseases are biofilm-based; the scientists have started to explore effective remedies to precisely aim at the disruption of biofilm, thus, diffusing the cells of microbes into their more susceptible planktonic type of life. With the advent of the significance of biofilm disruption to combat serious infections, various antibiofilm agents have been investigated for their efficacy. This includes some primary metabolites including complex carbohydrates, peptides and fats and various categories of secondary metabolites. Many enzymatic biofilm dispersal agents have also attracted the attention of those working in the given area. Other dispersal compounds include anti-matrix molecules, dispersal signals and sequestration molecules. These antibiofilm agents have shown high effectiveness in inhibiting clinically relevant pathogens. These biofilm dispersal agents will pave a way for a new approach towards future drug development for the treatment of clinically severe infections.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Microbial biofilms are the masses of cells which are fixed in a matrix produced by itself in extracellular polymeric substances (EPS). These biofilms pose serious damage to human health by causing various lethal infections which are strong and show resistance to antibiotics and immune system of host. This makes the treatments of pathogens challenging and expensive.
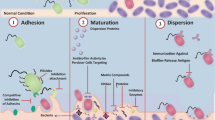
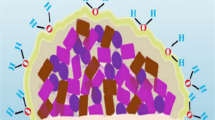
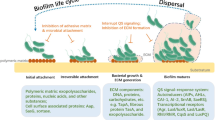
In the environment under natural conditions, microorganisms frequently grow on various biotic and abiotic planes in the form of multi-cellular populations which are recognized as biofilms. Microbes can form biofilms by their capability of attachment to household items like showers, taps and water tanks besides living in the form of biofilms in nature (Mullis and Falkinham 2013; Rozej et al. 2014; Xu et al. 2014). Pseudomonas aeruginosa is the most common organism which forms biofilm in the household system (Mullis and Falkinham 2013). Bacterial biofilm populations can comprise of multiple cell layers and also in the form of mushroom-like assemblies as in case of Staphylococcus epidermidis and various Pseudomonas species. The morphology of biofilms vary from dry, flat and wrinkled colonies on agar plates as in case of Bacillus subtilis and Vibrio cholerae (Bester et al. 2010; Lopez et al. 2010; Seper et al. 2014) to small yellow air balloons as in Myxococcus xanthus (Dubey 2003; Jiyoung et al. 2009). Anoxybacillus flavithermus is capable of forming biofilms in silica and is a danger in processing of food (Saw et al. 2008). But the common character of all microbial biofilms is a matrix surrounding the cells, a slimy layer on the surface of each bacterial cell that provides defence to the cells and provides food and water to bacteria (Bester et al. 2010; Lopez et al. 2010). The matrix comprises of complex polysaccharides, proteins, phospholipides and extracellular DNA (Christner et al. 2010, 2012; Linnes et al. 2013; Reichhardt et al. 2014; Becker et al. 2014). Occasionally, the matrix appears in different colours (pink, brown or blackish) so as to prevent DNA impairment by solar radiations (Xu et al. 2014). Nutrients and other materials can be pooled amongst the microbial cells by passive diffusion through the porous matrix. In this manner, they work as cell communication machineries, and this phenomenon is known as quorum sensing (Banat et al. 2014). The most significant consequence of the matrix is the defence against external stimuli and mechanical destruction. Moreover, the matrix also guards the cells from chemical compounds for instance antibiotics, antimicrobial agents and disinfectants. Additionally, the biofilm also safeguards bacterial cells against shearing forces, physical and chemical forces and inadequate availability of nutrient (Taylor et al. 2014; Banat et al. 2014). The cells present in the matrix share mutual benefit from one another and help in each other’s progression and survival (Xu et al. 2014; Bester et al. 2010).
15.2 Development of Biofilms as a Threat to Human Health
In the early 1940s, antibiotics have been introduced to human medicine and now emerged as a major threat to public health at an alarming rate. This problem is amplified by pathogenic bacteria existing most commonly in biofilm form, creating additional bacterial tolerance to antimicrobial agents and has been considered as primary cause of chronic infection, transforming bacteria into antibiotic-resistant form in biofilm formulation (Bowler 2018). Biofilm-associated bacteria are 1000 times more resistant to antibiotics than their planktonic counterparts and are often insensitive towards host immune system (Olson et al. 2002). The resistant mechanism attributed by biofilm bacteria due to resistant capsules, enzyme-mediated resistance, heterogeneity in metabolism and growth rate, metabolic state of the organisms in the biofilm, genetic adaptation and most important effective quorum sensing and other membrane modification (Singh et al. 2017).
The mono-species of bacteria which have the capacity to synthesize a biofilm in clinical surroundings are accountable for biofilm-related diseases, in both animals and humans (Dubey 2003). These are most frequently seen not only close to oral areas like dental caries and periodontitis, or in infections of respiratory tract in cystic fibrosis patients (Lambiase et al. 2009; Hall-Stoodley et al. 2004), but also found on the exteriors of implanted medical devices. Amongst clinical pathogens, the most dangerous are gram-positive, coagulase-negative staphylococci (e.g. S. epidermidis) and coagulase-positive S. aureus that make biofilms on abiotic surfaces (Rohde et al. 2006; Mack et al. 2006; Moretro et al. 2003; Rupp and Archer 1994; Mack et al. 1992). Microbes have enhanced pathogenicity in the form of biofilm and frequently cause infections on artificial biomedical parts such as surgical pins or hip joints (McCann et al. 2008). Infections can occur in cardiac pacemakers with a trailing endocarditis and intravenous catheters because of extracellular matrix of human and coating of the implant by serum being rich in nutrient and provide environment for the growth (Hall-Stoodley et al. 2004; McCann et al. 2008). This is the reason that the disease and death of hospital acquired, nosocomial infections are increasing each year (Rohde et al. 2006; Rupp and Archer 1994). Majority of the cells associated with material-associated infections are due to S. epidermidis cells, as they are skin inhabitant where wounds and implants are easily accessible (Gotz 2002). Another deleterious effect of biofilm synthesis on abiotic planes is the infection of edible items during production of food and its processing. Meat and milk can be contaminated through multi-resistant staphylococcal species on coming in contact with the biofilm-coated surface (Moretro et al. 2003; Mettler and Carpentier 1998).
The approaches for the inhibition and elimination of these biofilms are very restricted. This is because of the increasing antibiotic resistance of microbial species, but also because of the increased resistance of biofilm-based structure of microbes against antimicrobial agents and disinfectants (Mack et al. 2006; Gotz 2002; Ganeshnarayan et al. 2008). The extracellular polymeric compounds of the matrix are responsible for providing the protection to the microbes against external forces which comprise of polysaccharides, proteins, lipids, nucleic acids and humic acids. These matrix components also result in the characteristic mushroom-like biofilm assembly (Flemming and Wingender 2010). Considering the complicated structural organization and regulatory network resulting in the formation of biofilm, the understanding of the complexity of the matrix in detail and also the innovative strategies are essential to disperse established microbial biofilms and prevent their occurrence on abiotic surfaces to improve the management of medical patient and food safety. Up to now, novel targets for the screening of antimicrobial agents and vaccine development for the prevention of biofilms are promising (Gotz 2004). The present chapter reviews the latest developments in the area of biofilm disruption taking into consideration various agents which have shown potential towards this.
Biofilms can be developed on almost every moist surface which is most often unwanted as they cause serious complications in various sectors, including the food division. They are recognized as the preferential microbial lifestyle due to the numerous advantages offered by them for the embedded cells. Biofilm cells show strong resistance to stress conditions, mainly to antimicrobials, since their multifarious and compact structure hinders the permeation of antimicrobials and the contact with the cells present deep in the biofilm. The increased resistance to the presently employed control strategies accentuates the urgent requirement of new alternative and/or complementary eradication approaches. To this direction, the use of enzymes is an interesting alternative antibiofilm approach due to their capability to degrade crucial components of the biofilm matrix, cause cell lysis, promote biofilm disruption and interrupt the cell-to-cell signalling events monitoring biofilm formation and maintenance. This review provides an overview of the enzymes used for biofilm control, their targets and examples of effective applications (Meireles et al. 2016).
15.3 Natural Antibiofilm Agents
15.3.1 Fatty Acids as Antibiofilm Agent
15.3.1.1 Myriads Role of Fatty Acid Inhibitor (FAI) in Biofilm Formulation
The mode of growth of microorganisms could be manipulated either by preventing foaming of biofilms or by disrupting the existing ones. In this context, extensive literature are available that have detailed signal (extracellular) accountable for biofilm scattering coupled with an array of factors that have been shown to arouse biofilm disturbance. For example, addition of chemicals, rapid reduction in oxygen and increased concentration of organic carbon resulted in cell cluster disaggregation in P. aeruginosa (Chen and Stewart 2000). In this section, emphasis will be primarily upon the different fatty acids as inhibitor molecules to augment the vulnerability of biofilm cells by weakening the normal biological processes that maintain biofilm integrity, and also critically discussed process in controlling bacterial virulence, alteration in cellular phenotype and promote biofilm tolerance (Table 15.1).
15.3.1.2 FAI Interactions with Quorum Sensing (QS)
Owing to its importance in nutrition, fatty acids (FA) are representative of quorum sensing chemicals capable of modulating virulence-associated behaviour of bacterial population. Bacterial pathogen component actively up-regulated the expression of QS system to induce virulence factors that promote biofilm formation and infectious diseases. Recently, it was documented that chains of monounsaturated fatty acids such as palmitoleic acid and myristoleic acids significantly diminished biofilm synthesis of Acinetobacter baumannii with drastically reduced motility (Nicol et al. 2018). This might be due to fatty acids involvement in down-regulation of LuxIR-type quorum sensing (QS) communication system, thus consequently reduced the N-acyl-homoserine lactone production (AHL). Another medium-chain fatty acid derivative, cis-2 decenoic acid (C2DA), showed lethality towards multiple bacterial strains, including gram-positive, gram-negative and yeast strains by inducing dispersion of biofilms, although this biofilm inhibition was only demonstrated in P. aeruginosa (Stoodley et al. 2011). Furthermore, combination of antibiotic (linezolid) with C2DA resulted in 16% inhibition of biofilm either individually or in combination with daptomycin and vancomycin. The easy incorporation of linezolid and C2DA into the plasma membrane of bacterial cells and also due to cis-conformation increased membrane permeability are the fundamental mechanisms for enhancement of antibiofilm activity of these compounds (Jennings et al. 2012). Global gene expression analysis lyngbyoic acid (LA)-treated P. aeruginosa revealed that LA down-regulates gene controlled by quorum sensing (Kwan et al. 2011). Another study conducted by Zhen Cai et al. clearly revealed that fatty acid diffusible signal factor (DSF) binds and allosterically activates histidine kinase RpfC of phytopathogenic bacterium Xanthomonas campestris to regulate quorum sensing and virulence. The role of fatty acid or its derivative is directly or indirectly involved in inhibitor of biofilm formulation as well as virulence, but précised mode is doubtful in various studies hence extensive effort is required for better understanding.
15.3.1.3 Fatty Acid as a Signalling Molecule
In pathogenicity research, fatty acid molecules have already been identified as signalling as well as inducer molecules, ranging from yeast to gram-positive and gram-negative bacteria which regulate a wide range of cellular functions (Marques et al. 2014). In addition to this, signals generated by fatty acid are engrossed in intra-species, inter-species and cross-kingdom communication. These signals are known to regulate motility, virulence, polymer production, biofilm development, biofilm dispersion, bacterial growth and persistence. The different fatty acids as signalling molecules in various organisms have been summarized in Table 15.1. More than 50 molecules including autoinducer-1 (AI-1) also known as N-acylhomoserine lactones (N-AHL), autoinducer-2 (AI-2) a furanosyl borate, PQS, oligopeptides (5–10 amino acid cyclic thiolactone) known as autoinducer peptides (AIP) and short-chain fatty acids which are typically unsaturated at the number 2 carbon in a cis configuration have been identified till date (Parsek and Greenberg 2005; Ryan and Dow 2011; Kalia et al. 2015). Signals of fatty acids are known to increase aggregative behaviour and biofilm formation capability. For example, isolation from Xylella fastidiosa, i.e. 12-Me-C14, has been reported to eradicate swarming motility and to subside biofilm formation by capability of X. fastidiosa and in P. aeruginosa. Apart from bacteria, fungi like C. albicans are also documented for the induction of fatty acid signals which reported the production of farnesoic acid that inhibits the formation of germ tube by regulating the morphological transition from a yeast-form to a hyphal-form (Estrela and Abraham 2010).
Biofilm dispersion autoinducers have been reported the experience of involvement of the fatty acid. Like in P. aeruginosa commonly known fatty acid signal, cis-2-decenoic acid (C10:Δ2) is identified and has been established as a biofilm dispersion auto inducer (Davies and Marques 2009). Moreover, Propionibacterium acnes, Actimomyces naeslundii, Lactobacillus casei and Streptococcus mutans when cultured as single or mixed species are seen to be susceptive towards cis-DA which induces biofilm dispersion in them. However, an entire comprehensive signalling mechanism of the cis-DA system is yet to be elucidated.
15.3.1.4 Fatty Acid Regulation in Biofilm Dispersion
Studies in the past decades related to the effect of fatty acids and its derivatives on the biofilm resulted in concluding on the intracellular mechanisms involved in some species of bacteria. P. aeruginosa produces Cis-2-decenoic acid in batch cultures, and biofilm cultures induce a dispersion response in biofilms formed by a range of gram-negative and gram-positive bacteria and yeast, as well as in P. aeruginosa (Huang and Wong 2007; Chatterjee et al. 2008). It is also well cited that DSF has also played roles for the regulation of pathogenicity in X. campestris (Barber et al. 1997) together with sysnthesis of extracellular proteases and exopolysaccharide production, flagellum synthesis, aggregative behaviour, biofilm formation and resistance to toxins. Along with cis-2-decenoic acid and DSF, small chain-monounsaturated fatty acids and BDSF have activity across a wide array of bacteria as extracellular signals. If microorganisms depend on the degradation of extracellular polymers produced by neighbouring microorganisms of other species as well as their own species will relieve the cells from the biofilm matrix during a dispersion response. Cross-kingdom activity has been proposed previously for fatty acid messengers from evidence that DSF is recognized by C. albicans binding to the receptor of farnesoic acid, leading to an arrest in filamentation. The application of a dispersion inducer mainly fatty acid prior to, or in combination with, treatment by antimicrobial agents provides a novel mechanism for enhancing the activity of these treatments through the disruption of existing biofilms; in this context, broad-spectrum activity of cis-2-decenoic acid suggests that this and other short-chain cis-2-monounsaturated fatty acids likely have deep evolutionary roots. It is interesting that fatty acid communication has been found to be present in many plant and animal species, and the connection to cell dispersion in these systems may be an interesting area for future investigation.
15.3.2 Enzymes as Antibiofilm Agent
15.3.2.1 Amylases and Cellulases
Amylase and cellulase enzyme complex was produced from Penicillium janthinellum, a mutant EU2D-21 under submerged fermentation. Good specific enzyme activities were found after eight days of incubation at 30 ℃. This enzyme complex was evaluated for its capability to target and disrupt the biofilms of different bacteria. It was seen that it disrupted biofilms of Escherichia coli (85.5%), Salmonella enterica (79.72%), P. aeruginosa (88.76%) and Staphyloccus aureus (87.42%) within 1 h of incubation at 50 ℃. The exopolysaccharide matrix of the biofilm and bacteria from the cell surface were detached by the enzyme complex as shown by the scanning electron microscopy (SEM), quantitative analysis of biofilm removal assay and crystal violet assay (Nagraj and Gokhale 2018).
15.3.2.2 Proteases
The cell surface proteins in Staphylococcus aureus strains promote the development of biofilm. Proteinase-mediated biofilm dispersion was investigated in the present study in different isolates of S. aureus. It was shown by microtitre plate-based biofilm assay demonstrated that Proteinase K (2 μg/mL) markedly checked the development of biofilm in bap-positive S. aureus as well as other S. aureus strains but not in weak biofilm-producing strains, that is, bap-mutant M556 and SA392. However, there was no effect of Proteinase K treatment on the planktonic growth of S. aureus. It was indicated by the results of the study that Bap might also play role in eDNA retention in the matrix of biofilm that supports biofilm stability. A synergistic response in antibiotic efficiency was seen against all biofilm forming S. aureus strains when a combination of Proteinase K was applied in combination with antibiotics (Mukherji et al. 2015; Shukla and Rao 2017).
The treatment of biofilms with broadly specific proteases, such as Proteinase K and trypsin results into biofilm disassembly (Boles and Horswill 2008; Mootz et al. 2013). The serine proteases Proteinase K (from Tritirachium album) and trypsin have often been utilized as efficient biofilm disruption agents that hamper bacterial adherence and biofilm formation in S. aureus (Gilan and Sivan 2013) presumably through degradation of surface structures (Boles and Horswill 2008; Gilan and Sivan 2013; Loughran et al. 2014). Shukla and Rao (2013) also reported that biofilms formed by S. aureus with the help of Bap proteins were vulnerable to Proteinase K-mediated detachment and dispersion. Biofilm assays done in 96-well-plates showed that Proteinase K obstructed both biofilm adherence and progression in Bap expressing S. aureus cultures.
A number of proteases have been recognized as an antibiofilm disrupting agents, with varying degrees of success (Loughran et al. 2014; Craik et al. 2011). Serine proteases, in particular, have been effective at disrupting the matrix. This is not entirely surprising, as serine proteases have been produced number of biofilm-forming microbes which likely aid in active dispersal and biofilm structural arrangement (Loughran et al. 2014; Marti et al. 2010; Chen et al. 2013).
15.3.2.3 Hydrolase
Glycoside hydrolases were evaluated for potential therapeutic effect on P. aeruginosa, and it was showed that glycoside hydrolases specifically target and degrade the exopolysaccharide constituent of the biofilm matrix. Bacterial biofilms present a significant clinical challenge because they are recalcitrant to existing therapeutic regimes. The main part of biofilm production in the opportunistic human pathogen P. aeruginosa is the biosynthesis of the exopolysaccharides Pel and Psl, which are responsible for the formation and maintenance of the structural biofilm scaffold and defence against antimicrobials and host defenses. Knowing that the glycoside hydrolases PelAh and PslGh encoded in the Pel and Psl biosynthetic operons, respectively, are utilized for in vivo exopolysaccharide processing, it was anticipated that these would provide specificity to target P. aeruginosa biofilms. Evaluating these enzymes as potential therapeutics, it was demonstrated that these glycoside hydrolases selectively target and degrade the exopolysaccharide component of the biofilm matrix. PelAh and PslGh restrict biofilm synthesis over a 24-h period with a half-maximal effective concentration (EC50) of 69.3 ± 1.2 and 4.1 ± 1.1 nM, respectively, and are capable of disrupting pre-existing biofilms in 1 h with EC50 of 35.7 ± 1.1 and 12.9 ± 1.1 nM, respectively. This treatment was effective against clinical and environmental isolates of P. aeruginosa and reduced biofilm biomass by 58–94%. These noncytotoxic enzymes potentiated antibiotics because the addition of either enzyme to a sublethal concentration of colistin reduced viable bacterial counts by 2.5 orders of magnitude when used either prophylactically or on established 24-h biofilms. In addition, PelAh was able to enhance neutrophil killing by ~50%. This work illustrates the feasibility and benefits of using bacterial exopolysaccharide biosynthetic glycoside hydrolases to develop novel antibiofilm therapeutics (Baker et al. 2016).
Aspergillus fumigatus and P. aeruginosa produce galactosaminogalactan and Pel, respectively, which are cationic heteropolysaccharides. These exopolysaccharides both contain 1,4-linked N-acetyl-D-galactosamine and play a vital function in biofilm formation by these microorganisms. Proteins comprising glycoside hydrolase domains have been identified recently as a part of the anabolic pathway of each exopolysaccharide. Recombinant hydrolase domains from these proteins degrade their respective polysaccharides under in vitro conditions. These glycoside hydrolases were shown to exhibit antibiofilm activity against varied microorganisms and may be useful as novel therapeutic agents for the degradation of biofilms and reducing virulence (Snarr et al. 2017).
In another study, a Psl-specific glycoside hydrolase (PslG) was covalently bound to numerous, chemically different planes using amine functionalization (APTMS) and glutaraldehyde (GDA) linking. Since bacterial colonization and biofilm synthesis on surfaces are typically facilitated by the accumulation of exopolysaccharides and conditioning protein layers. P. aeruginosa is a nosocomial opportunistic pathogen that employs strain-specific exopolysaccharides such as Psl, Pel or alginate for both initial surface attachment and biofilm formation. To generate surfaces that resist P. aeruginosa colonization, in situ quartz crystal microbalance (QCM) experiments and fluorescence microscopy confirmed a complete lack of Psl adsorption on the PslG-bound surfaces. Covalently bound PslG was also seen to markedly reduce P. aeruginosa surface adherence and biofilm formation over-extended growth periods (8 days). The PslG surfaces showed a ∼99.9% (∼3 − log) reduction in surface-associated bacteria compared to control surfaces or those treated with inactive enzyme. This work showed a non-eluting ‘bioactive’ surface that specifically targets a mechanism of cell adhesion, and that surface-bound glycoside hydrolase can significantly reduce surface colonization of bacteria through local, continuous enzymatic degradation of exopolysaccharide (Psl). These results have significant implications for the surface design of medical devices to keep bacteria in a planktonic state, and therefore, susceptible to antibiotics and antimicrobials (Asker et al. 2018).
Poly-β(1,6)-N-acetyl-D-glucosamine (PNAG) is a main constituent of biofilm of many pathogenic bacteria. The synthesis, modification and export of PNAG in E. coli and Bordetella species need the protein products encoded by the pgaABCD operon. PgaB is a two-domain periplasmic protein that contains an N-terminal deacetylase domain and a C-terminal PNAG-binding domain that is crucial for export. In the current study, it was shown that the C-terminal domains of Bordetella bronchiseptica PgaB (PgaB) and E. coli PgaB (PgaB) work as glycoside hydrolases. These enzymes hydrolyze purified deacetylated PNAG (dPNAG) from S. aureus, degrade PNAG-dependent biofilms formed by Bordetella pertussis, Staphylococcus carnosus, S. epidermidis and E. coli, and potentiate bacterial killing by gentamicin. Furthermore, it was seen that PgaB was only able to hydrolyze PNAG produced in situ by the E. coli PgaCD synthase complex when an active deacetylase domain was present. Mass spectrometry analysis of the PgaB-hydrolyzed dPNAG substrate showed a GlcN-GlcNAc-GlcNAc motif at the new reducing end of detected fragments. This work magnifies the role of PgaB within the PNAG biosynthesis apparatus, defines a new glycoside hydrolase family GH153 and identifies PgaB as a possible therapeutic agent for treating PNAG-dependent biofilm infections. The work provides further insight into the mechanism of periplasmic PNAG modification, and suggests PgaB could be utilized as a therapeutic agent to eliminate biofilms (Little et al. 2018).
15.3.2.4 Lactonase
The tenacity of bacterial infection is often linked to quorum sensing-mediated biofilm synthesis. Thus, the interruption of this signalling circuit presents an attractive anti-virulence strategy. Quorum sensing is an important aspect of biofilm formation. Quorum-quenching lactonases have been shown to be effective in disrupting of quorum sensing circuits. The present study pronounces a method to degrade biofilm in a clinically significant A. baumannii S1 strain by the application of an engineered quorum-quenching lactonase. This treatment by engineered lactonase attained significant reduction in A. baumannii S1 biofilm (Tay et al. 2016).
15.3.2.5 Dispersin B
An artificial gene encoding dispersin B of Aggregatibacter actinomycetemcomitans was cloned and expressed in E. coli cells. Procedure for purification of recombinant dispersin B was established, and its in vitro activity was determined. The enzyme was used in experiments on disruption of the biofilms formed by various microorganisms. It exhibited high activity against S. epidermidis biofilms. The biofilms formed by Burkholderia cenocepacia and Achromobacter xylosoxidans were more resistant to the recombinant enzyme (Dobrynina et al. 2015).
15.3.2.6 Papain
Considering the proteolytic nature of papain and the biopolymer matrix structure of bacterial biofilms, the present study was aimed to evaluate the ability of papain to act as an inhibitor of biofilms in different concentrations. The effect of different concentrations of papain in biofilm production by several methicillin-resistant S. epidermidis (MRSE) and methicillin-resistant Staphylococcus haemolyticus (MRSHa) isolates was explored. When papain was mixed into the culture, the biofilm formation by MRSE was restricted. However, the enzymatic action of papain exhibited more efficiency for MRSHa isolates. The experiment suggested that papain is able to affect the ability of cells to form biofilm, thus affecting the bacterial attachment (de Oliveira et al. 2014).
15.3.2.7 DNAses
DNases have been applied for degradation of mucous in cystic fibrosis patients, demonstrating its viable potential for human use as a therapy (Shak 1995; Sawicki et al. 2015; Shak et al. 1990). In vivo studies utilizing DNase have shown that degradation of eDNA can disrupt biofilms present in a mammalian host (Hymes et al. 2013; Conover et al. 2011).
15.3.2.8 Polysaccharide Depolymerase
In the current work, bacterial exopolysaccharide was degraded by a heat-stable polysaccharide depolymerase which was prepared from the phage infecting Klebsiella. Treatment at 75 °C for 10 min led to the complete inactivate of phage. However, there was no loss of phage enzyme activity after the treatment. The colony counting showed the phage enzyme could rapidly decrease the number of biofilm-associated bacteria. The rate of inhibition reached the maximum (80%) after 4 h of treatment. Enzyme pre-treatment could also enhance the fumigation effect of chlorine dioxide. Approximately, 92% of the BF bacteria were eliminated after treatment with the phage enzyme followed by 30 min of treatment with chlorine dioxide. According to the results of colonies counting and scanning electron microscopy, the phage enzyme could effectively decrease the bacterial adherence as well as the adhesion of extracellular polymeric substances in the BF. This study has demonstrated that the phage-borne polysaccharide depolymerase enzyme is valuable for eradicating the bacterial BF (Chai et al. 2014).
15.3.3 Inhibitors of Quorum Sensing
15.3.3.1 Implication of Quorum Sensing in Biofilm Development
The interactions and communication amongst bacteria done collaboratively for biofilm formation are known as quorum sensing (QS). This mechanism was elucidated initially by Professor Greenberg where he proposed different ways to intervene with pathogenic microflora and to moderate microbiome for better health approaches (Fuqua et al. 1994). Many species of bacteria are inhibitors of biofilm. This communication system includes induction of a specific set of bacterial genes that hold potential of response to expanded volume of cell population (Platt and Fuqua 2010). Today, targeting the inter-bacterial interactions has become the foremost in healthcare researches, especially with multi-drug resistance amongst the pathogens (Haque et al. 2018; Golberg et al. 2013; Saurav et al. 2016).
Recently, the use of natural products to interfere with pathogenic bacterial quorum-sensing systems (Jamal et al. 2018; Hirakawa and Tomita 2013; Brackman and Coenye 2015; Rémy et al. 2018) has been proposed for development of new antimicrobial agents. Since this strategy does not require bacterial killing, it is proposed to reduce the development of resistant strains (Tang and Zhang 2014).
Autoinducers like acyl homoserine lactones (AHL) are synthesized and secreted by the phenomenon of quorum sensing (Newton and Fray 2004). Gram-positive bacteria secrete quorum-sensing peptides as a regulatory system consisting of two components, a membrane-bound histidine kinase receptor and an intracellular response regulator (Platt and Fuqua 2010). Besides these, gram-negative as well as gram-positive bacteria can use a common entity, borate furanosyl, an autoinducer-2 (IA-2) and (IA-3). The mechanism of quorum sensing is also reported for the control of growth of biofilm (Abee et al. 2011).
15.3.3.2 Bacterial QS Inhibitors
Quorum sensing affects the structure of biofilm, and lack of quorum sensing is related with development of thin biofilm as well as increased susceptibility to antibiotics (Shih and Huang 2002).
In aeromonads, the QS regulates biofilm formation, motility, multicellular synchronized social life and virulence (Talagrand-Reboul et al. 2017).
Numerous bacteria produce certain substances (Table 15.2) on their surface that prevents biofilm formation by others, such as extracellular polysaccharides, which are crucial in formation of biofilm in bacteria. They also prevent biofilm formation in adjacent cells (Rendueles et al. 2013).
Actinobacillus pleuropneumoniae serotype 5 which exhibits antibiofilm activity has extracellular polysaccharide which prevents intra- and inter-cellular communications amongst microbes, thereby exhibiting an example of natural anti-biolfilm phenomena (Karwacki et al. 2013).
Another study of Schertzer et al. exhibited similar activity of glycolipid and glycoprotein structure and QS signals to sense and stop external positive-charged compounds (Schertzer et al. 2009).
Similarly, the extracts of coral-associated bacteria have been shown to induce a decrement in S. aureus and Serratia marcescens biofilm development. Ethyl acetate extracts of Bacillus firmus—a coral-associated bacterium–show antibiofilm activity against multi-drug resistant (MDR) S. aureus (Gowrishankar et al. 2012). A new natural compound, 4-phenylbutanoic acid, extracted from Bacillus pumilus, demonstrated antagonistic activity against biofilms (Nithya et al. 2011).
Another example is seen with Streptococcus salivarius, that exhibit antibiofilm activity by use of two enzymes, viz. fructosyltransferase (FTF) and exo-β-D-fructosidase (FruA), that decrease EPS production (Ogawa et al. 2011). However, excess production of FruA may have an involvement in development of sucrose-dependent biofilm in the oral cavity.
15.3.4 Inhibition of QS by Phytochemicals
Phytochemicals from fruits and vegetables hold the potential to hinder quorum sensing in human pathogens (Vattem et al. 2007). This is appealing, since the regular intake of such anti-QS associated food may be of therapeutic value in preventing the invasion of intestinal pathogens.
15.3.4.1 Polyphenols or Phenolic Compounds
Range from simple molecules to polymerized tannins, and have one aromatic ring with six carbons. These secondary metabolites affect biofilm development in microbes (Huber et al. 2003; Sarabhai et al. 2013).
15.3.4.2 Phenolic Acids
Are organic compounds which have one carboxylic functional group and phenolic hydroxyl group. In plants, the acid phenols are mostly in the form of esters of aliphatic alcohol or of quininic acid, glycosides or rosmarinic acid. Hydroxylation of the phenolic group has exhibited direct effect upon their antimicrobial activity. The mechanism for this phenolic damage involves inhibition of enzymes by oxidized compounds (Mason and Wasserman 1987).
15.3.4.3 Flavonoids
Belong to the polyphenol family. They have a structure with 15 carbon atoms, constituting two aromatic units, two C6 rings joined by a C3 chain (Heim et al. 2002); flavonoids are the compounds which give colours to flowers, fruits as well as leaves.
Flavanones, found enormously in citrus spps, show interference with quorum sensing and also affect other physiological process (Truchado et al. 2012). Naringenin, quercetin and apigenin are inhibitors of HAI-1 or Al-2-mediated bioluminescence production in Vibrio harveyi. Naringenin and taxifolin decrease the synthesis of pyocyanin and elastase in P. aeruginosa where growth of bacteria was unaffected. Naringenin and taxifolin also hindered the quorum sensing by affecting the gene expression in P. aeruginosa PAO1. Naringenin holds the potential of hindering the synthesis of quorum-sensing mediators such as N-(3-oxododecanoyl), lactone-1-homoserine (3-oxo-C12-HSL), acylhomoserine lactone and N-butanoyl-1-homoserine lactone (C4-HSL) (Vandeputte et al. 2011).
15.3.4.4 Quercetin, Sinensetin, Apigenin
Also inhibit the development of biofilms in V. harveyi BB120 as well as E. coli O157:H7 (Truchado et al. 2012; Vikram et al. 2010). In P. aeruginosa PAO1, flavonoids such as the catechin decrease virulence factors (pyocyanin and elastase) influenced by quorum signalling, leading to inhibition of biofilm development.
The HLA molecules are deteriorated by legume products including alfalfa, clover, lotus, peas and yam beans (Delalande et al. 2005; Gotz et al. 2007).
15.3.4.5 Terpenes
Are natural compounds having hydrocarbons of either cyclic or open-chain skeleton. The basic molecule is isoprene, i.e. C5H8. The term terpenoid refers to terpene skeleton substances with one or more functional groups (alcohol, aldehyde, ketone, acid, lactone, etc.). The terpenoid classification is based on the number of repetitions of the isoprene base unit: hemiterpens (C30), tetratepens (C40) and polyterpens.
Essential oils are plant extracts that mainly contain mono- and sesqui-terpenes. Essential oil from medicinal plants such as Citrus reticulate (Luciardi et al. 2016), Eucalyptus radiate, Eucalyptus globulus (Luis et al. 2016) and Thymus vulgare (Myszka et al. 2016) have shown anti-QS effects.
In addition, compounds such as vanillin in Vanilla planifolia (Ponnusamy et al. 2009), eriodictyol in Eriodictyon californicum (Vandeputte et al. 2011), methyl eugenol in Cuminum cyminum (Packiavathy et al. 2012), erucin in Brassica oleracea (Ganin et al. 2013), ajoene in Allium sativum (Jakobsen et al. 2012a) and naringin, naringenin, kaempferol, quercetin, rutin, neoeriocitrin in Citrus sinensis (Vikram et al. 2011) were found to cease quorum sensing.
15.3.5 Medicinal Plants as QS Inhibitors
Different parts and organs of various plants have been reported to possess medicinal values. Examples include Glycyrrhiza glabra (Bhargava et al. 2015), Psoralea corylifolia (Husain et al. 2018), Piper bredemeyeri (Olivero et al. 2011), Bauhinia acuruana, Pityrocarpa moniliformis, Commiphora leptophloeos (Trentina et al. 2011), Cocos nucifera (Viju et al. 2013) and Terminalia catappa (Taganna et al. 2011).
Rubus rosaefolius (Oliveira et al. 2016), Centella asiatica (Vasavi et al. 2016), Areca catechu (Koh and Tham 2011) and Sclerocarya birrea (Sarkar et al. 2014) have also been reported to have an inhibitory effect against QS signalling.
15.3.6 Plants as a Source of Anti-QS Drugs: Mechanism of Action
Anti-quorum sensing role of essential oils and their major constituents: amongst the essential oils, lavender, clove and rosemary have shown anti-QS activity. Recently, Luciardi et al. revealed essential oil from Citrus reticulata to possess antibiofilm and anti-quorum sensing activity against P. aeruginosa. At 0.1 mg/mL, this compound showed 41% decrease in biofilm cell viability and a 33% decrement in AHL production (Luciardi et al. 2016).
Thymus vulgare and its derivatives carvacrol and thymol exhibited antibiofilm and anti-quorum sensing activity against Pseudomonas fluorescens KM121, thus revealing a tremendous decrease in AHL production and biofilms (Myszka et al. 2016).
Organic compounds extracted from the medicinal plants also act as inhibitory agents of quorum sensing (Thakur et al. 2016). Organic compounds from the medicinal plants (Table 15.3) have same chemical skeleton to those of quorum sensing signals or possess the potential to deteriote signalling receptor (LuxR/LasR) (Vattem et al. 2007; Al-Hussaini and Mahasneh 2009).
-
Indeed, GABA (gaminobutyric acid), synthesised by some plants, promotes signal degradation by OHC8HSL HLA lactonase (ATTM) in Agrobacterium tumefaciens, thus hindering the process of quorum sensing-dependent infection (Chevrot et al. 2006).
-
Medicago truncatula modulating AhyR, CVIR and LuxR activities in various microorganisms (Gao et al. 2003) and quorum sensing in P. aeruginosa and Sinorhizobium meliloti (Mathesius et al. 2003).
-
Curcuma longa produces curcumin which hinders the expression of the virulence genes of P. aeruginosa PA01.
-
Polyphenols like hydroxycinnamic acids, rutin and epicatechin found in the extracts of apples and its derivatives have shown a significant role in anti-quorum sensing activity; they also show synergistic activity against Chromobacterium violaceum (Fratianni et al. 2011, 2012).
The anti-quorum sensing activity has also been exhibited by various other medicinal plants like Laurus nobilis, Rosmarinus officinalis and Pityriasis alba. These plants had the potential to decrease the synthesis of violacein (Vattem et al. 2007; Al-Hussaini and Mahasneh 2009).
The hydroalcoholic extracts of Berberi saristata and Camellia sinensis exhibited anti-quorum sensing activity against E. coli (Thakur et al. 2016). In addition, the in silico studies affirm the anti-quorum activity of the phytomolecules present in these extracts (flavonoids, alkaloids and tannins) through the antagonistic activity of LuxS.
The extract fractionation permits screening of chemical molecules and moieties for a significant outcome. Vasavi et al. showed that the flavonoids-rich ethyl acetate fraction of C. asiatica decreased pyocyanin synthesis and biofilms development by inhibition of elastolitic and proteolytic activity in these bacteria (Vasavi et al. 2016).
The ethanol and ethyl acetate extracts from Hypericum connatum have been shown to possess an anti-quorum sensing effect against C. violaceum, which hinders in the synthesis of violacein (Fratianni et al. 2013).
Gallic acid containing polyphenolics such as epigallocatechin gallate, ellagic acid and tannic acid from medicinal plants, hold the potential of interfering and inhibiting the interaction amongst bacterial cells via AHL mediated signalling (Sarabhai et al. 2013). Similarly, grenades and berries are rich in ellagitannins such as punicalagin and ellagic acid (Larrosa et al. 2010). In the intestinal flora, the conversion of ellagitanins to ellagic acid is done by the micro-gut flora and then metabolized to form urolithin A and urolithin B. The resulting metabolites get easily assimilated in the human intestine, playing their vital roles. In recent studies, it has also been revealed that urolithin A and B hinder quorum-sensing activity and also lower the levels of AHL produced by entherocolitica entheropathogen.
15.3.7 Anti-QS Action of Isolated Molecules from Medicinal Plants
With the application of bio-guided fractional approach, numerous secondary metabolites extracted from medicinal value plants have been obtained which exhibit anti-quorum sensing activities. For example, furanones have been exhibited to inhibit AHL processes (Manefield et al. 2002). Numerous plant secondary metabolites imitate AHL of bacteria, hence influencing quorum sensing and biofilm development in bacteria (Teplitski et al. 2000).
Cinnamaldehyde (major constituent in essential oils) and their derivatives have been estimated to hinder quorum sensing and biofilm development (Brackman et al. 2008; Niu et al. 2006). Cinnamaldehyde has been shown to inhibit bioluminescence in V. harveyi BB170 and eugenol in P. aeruginosa and C. violaceum via inhibition of virulence factor production such as violacein, elastase, pyocyanin and biofilm (Zhou et al. 2013).
Iso-limonic acid and ichangin (molecules contained in bigaradier seed extracts) include limonoids that inhibit the growth of V. harveyi at a very low concentration and these mechanisms are related to the inhibition of HAI- and AI-2-mediated bioluminescence (Vikram et al. 2011).
Curcumin, obtained from C. longa, has anti-quorum sensing activity. This molecule can attenuate the QS dependent factors such as EPS production of several pathogenic strains such as E. coli, P. aeruginosa, Proteus mirabilis and S. marcescens. In addition, curcumin has been reported to reduce a few phenotypes concerned with QS inhibition including swimming, swarming and motility. Curcumin also inhibits biofilms and pyoacin formation in P. aeruginosa via modulation at gene expression related to QS signalling pathways (Rudrappa and Bais 2008).
Usnic acid, a lichen metabolite, has anatagonistic activity against biofilm of fungus and bacteria. QS inhibitors can increase the susceptibility of biofilms to antibiotics and do not pose threat to humans (Sun et al. 2013).
Garlic inhibits quorum sensing at the gene expression level. Ajoene is obtained after crushing the garlic, and it has sulphur. Ajoene also shows antagonistic activity against synthesis of rhamnolipid, which protects biofilms from white blood cells. The combination of ajoene and the antibiotic tobramycin has been shown to kill ~90% of biofilm bacteria. Garlic possesses anti-viral, anti-fungal and anti-protozoal properties and is useful for the cardiovascular and immune systems (Jakobsen et al. 2012b). The only drawback with these sulphur-containing compounds is that their activity is lost as soon as they get in contact with oxygen.
Hamamelitannin extracts from the willow bark, also hinders quorum sensing (Morgan 2015).
Bacterial extracts with anti-quorum sensing activity are also analyzed using a mass spectrometry-based metabolomics Global Natural Products Social Molecular Networking platform (GNPS; https://gnps.ucsd.edu/) for compound dereplication (Allard et al. 2016). In addition, an integrated biological, genomic and metabolomic approach using 16SrRNA sequences is being lately employed to discover anti-quorum sensing molecules from bacterial strains (Ong et al. 2019).
15.3.8 Conclusion
Biofilm-associated bacteria are less sensitive to antibiotics than free-living (planktonic) cells. Furthermore, with variations in the concentration of antibiotics throughout a biofilm, microbial cells are often exposed to levels below inhibitory concentrations and may develop resistance. This, as well as the irresponsible use of antibiotics, leads to the selection of pathogens that are difficult to eradicate. Biofilms comprising of multicellular, surface-adherent communities help the microorganisms to survive in various stress conditions which include antibiotics, lack of nutrient, heat shock and immune responses. In view of the increment in the numbers of old patients who require artificial medical devices such as knees and hips, there will be an extended need for novel agents and strategies to treat biofilm-related infections. Some strategies have been described recently which appears to play an important role in future antibiofilm therapies. Both established and novel experimental treatments targeted at various stages of wound healing that are specifically aimed at reducing and eliminating biofilm bacteria. Importantly, the highly tolerant nature of these bacterial communities suggests that most singular approaches could be circumvented and a multifaceted, combinatorial approach will be the most effective strategy for treating these complicated infections. The usage of enzyme complex as antibiofilm therapeutics to eradicate biofilms is feasible and beneficial. This can also be used as a promising strategy to improve treatment of multidrug-resistant bacterial infections. The biofilm-forming microorganisms pose great threat to frequent infections in immune-compromised patients and is problematic to eliminate from medical devices. There are several classes of antibiofilm agents which need proper investigation in terms of their efficiency in inhibiting the microbial growth, understanding of the complex matrix organization in biofilms to deduce the mechanism of their action. The potential biofilm inhibiting compounds include natural bioactive compounds, enzymes that disturb the biofilm structure and other nonenzymatic molecules (Fig. 15.1).
References
Abee T, Kovács AT, Kuipers OP, van der Veen S (2011) Biofilm formation and dispersal in gram-positive bacteria. Curr Opin Biotechnol 22(2):172–179
Al-Hussaini R, Mahasneh AM (2009) Microbial growth and quorum sensing antagonist activities of herbal plants extracts. Molecules 14:3425–3435
Allard PM, Peresse T, Bisson J, Gindro K, Marcourt L, Pham VC, Roussi F, Litaudon M, Lv Wolfender J (2016) Integration of molecular networking and in-silico MS/MS fragmentation for natural products dereplication. Anal Chem 88:3317–3323
Asker D, Awad TS, Baker P, Howell PL, Hatton BD (2018) Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials 167:168–176
Baker P, Hill PJ, Snarr BD, Alnabelseya N, Pestrak MJ, Lee MJ, Jennings LK, Tam J, Melnyk RA, Parsek MR, Sheppard DC, Wozniak DJ, Howel PL (2016) Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci Adv 2:e1501632
Banat IM, Diaz de Rienzo MA, Quinn GA (2014) Microbial biofilms: biosurfactants as antibiofilm agents. Appl Microbiol Biotechnol 98:9915–9929
Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJG, Slater H, Do JM, Williams P, Daniels MJ (1997) A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24:555–566
Beaulieu ED, Ionescu M, Chatterjee S, Yokota K, Trauner D, Lindow S (2013) Characterization of a diffusible signaling factor from Xylella fastidiosa. MBio 4:9–14
Becker K, Heilmann C, Peters G (2014) Coagulase-negative Staphylococci. Clin Microbiol Rev 27:870–926
Bester E, Kroukamp O, Hausner M, Edwards EA, Wolfaardt GM (2010) Biofilm form and function: carbon availability affects biofilm architecture, metagbolic activity and planktonic cell yield. J Appl Microbiol 110:387–398
Bhargava N, Singh SP, Sharma A, Sharma A, Capalash N (2015) Attenuation of quorum sensing-mediated virulence of Acinetobacter baumannii by Glycyrrhiza glabra flavonoids. Future Microbiol 10:1953–1968
Boles BR, Horswill AR (2008) Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052
Boon C, Deng Y, Wang LH, He Y, Xu JL, Fan Y, Pan SQ, Zhang LH (2008) A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J 2:27–36
Bowler PG (2018) Antibiotic resistance and biofilm tolerance: a combined threat in the treatment of chronic infections. Adv Wound Care 27:273–277
Brackman G, Coenye T (2015) Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des 21:5–11
Brackman G, Defoirdt T, Miyamoto C, Bossier P, van Calenbergh S, Nelis H et al (2008) Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol 8:149–162
Chai Z, Wang J, Tao S, Mou H (2014) Application of bacteriophage-borne enzyme combined with chlorine dioxide on controlling bacterial biofilm. LWT-Food Sci Technol 59:1159e1165
Chatterjee S, Newman KL, Lindow SE (2008) Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol Plant Microbe Interact 21:1309–1315
Chen C, Krishnan V, Macon K, Manne K, Narayana SV, Schneewind O (2013) Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J Biol Chem 288:29440–29452
Chen X, Stewart PS (2000) Biofilm removal caused by chemical treatments. Water Res 34:4229–4233
Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, Ron E et al (2006) GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 103:7460–7464
Christner M, Franke GC, Schommer NN, Wendt U, Wegert K, Pehle P, Kroll G, Schulze C, Buck F, Mack D, Aepfelbacher M, Rohde H (2010) The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol Microbiol 75:187–207
Christner M, Heinze C, Busch M, Franke GC, Hentschke M, Bayard Dühring S, Büttner H, Kotasinska M, Wischnewski V, Kroll G, Buck F, Molin S, Otto M, Rohde H (2012) sarA negatively regulates Staphylococcus epidermidis biofilm formation by modulating expression of a 1 MDa extracellular matrix binding protein and autolysis-dependent release of eDNA. Mol Microbiol 86:394–410
Colnaghi Simionato AV, da Silva DS, Lambais MR, Carrilho E (2007) Characterization of a putative Xylella fastidiosa diffusible signal factor by HRGC-EI-MS. J Mass Spectrom 42:490–496
Conover MS, Mishra M, Deora R (2011) Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice. PLoS ONE 6:e16861
Craik CS, Page MJ, Madison EL (2011) Proteases as therapeutics. Biochem J 435:1–16
Davies DG, Marques CN (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403
de Oliveira HLCD, Fleming MECK, Silva PV, de Paula GR, Futuro DO, Velarde GC, Esper LMR, Teixeira LA (2014) Influence of papain in biofilm formed by methicillin-resistant Staphylococcus epidermidis and methicillin-resistant Staphylococcus haemolyticus isolates. Braz J Pharm Sci 50
Delalande L, Faure D, Raffoux A, Uroz S, D’Angelo-Picard C, Elasri M et al (2005) N-hexanoyl-L-homoserine lactone, a mediator of bacterial quorum-sensing regulation, exhibits plant-dependent stability and may be inactivated by germinating Lotus corniculatus seedlings. FEMS Microbiol Ecol 52:13–20
Deng Y, Wu J, Eberl L, Zhang LH (2010) Structural and functional characterization of diffusible signal factor family quorum-sensing signals produced by members of the Burkholderia cepacia complex. Appl Environ Microbiol 76:4675–4683
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Dobrynina OY, Bolshakova TN, Umyarov AM, Boksha S, Lavrova NV, Grishin AV, Lyashchuk AM, Galushkina ZM, Avetisian LR, Chernukha MY, Shaginian IA, Lunin VG, Karyagina AS (2015) Disruption of bacterial biofilms using recombinant dispersin B. Microbiology 84:498–501
Dubey JP (2003) Review of Neospora caninum and neosporosis in animals. Korean J Parasitol 14(1):1–16
Estrela AB, Abraham WR (2010) Combining biofilm-controlling compounds and antibiotics as a promising new way to control biofilm infections. Pharmaceuticals 3:1374–1393
Flavier AB, Clough SJ, Schell MA, Denny TP (1997) Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol Microbiol 26:251–259
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Fratianni F, Coppola R, Nazzaro F (2011) Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar Annurca. J Med Food 14:957–963
Fratianni F, de Giulio A, Sada A, Nazzaro F (2012) Biochemical characteristics and biological properties of annurca apple cider. J Med Food 15:18–23
Fratianni F, Nazzaro F, Marandino A, Fusco MDR, Coppola R, de Feo V et al (2013) Biochemical composition, antimicrobial activities, and anti-quorum-sensing activities of ethanol and ethyl acetate extracts from Hypericum connatum Lam. (Guttiferae). J Med Food 16:454–459
Fuqua WC, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275
Ganeshnarayan K, Shah SM, Libera MR, Santostefano A, Kaplan JB (2008) Poly-N-acetylglucosamine matrix polysaccharide impedes fluid convection and transport of the cationic surfactant cetylpyridinium chloride through bacterial biofilms. Appl Environ Microbiol 75:1308–1314
Ganin H, Rayo J, Amara N, Levy N, Krief P, Meijler MM et al (2013) Sulforaphane and erucin, natural isothiocyanates from broccoli, inhibit bacterial quorum sensing. Med Chem Comm 4:175–184
Gao M, Teplitski M, Robinson JB, Bauer WD (2003) Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol Plant Microbe Interact 16:827–834
Gilan I, Sivan A (2013) Effect of proteases on biofilm formation of the plastic-degrading actinomycete Rhodococcus ruber C208. FEMS Microbiol Lett 342:18–23
Golberg K, Pavlov V, Marks RS, Kushmaro A (2013) Coral-associated bacteria, quorum sensing disrupters, and the regulation of biofouling. Biofouling 29:669–682
Gotz C, Fekete A, Gebefuegi I, Forczek ST, Fuksova K, Li X et al (2007) Uptake, degradation and chiral discrimination of N-acyl-D/L-homoserine lactones by barley (Hordeum vulgare) and yam bean (Pachyrhizus erosus) plants. Anal Bioanal Chem 389:1447–1457
Götz F (2002) Staphylococcus and biofilms. Mol Microbiol 43:1367–1378
Götz F (2004) Staphylococci in colonization and disease: prospective targets for drugs and vaccines. Curr Opin Microbiol 7:477–487
Gowrishankar S, Mosioma ND, Pandian SK (2012) Coral-associated bacteria as a promising antibiofilm agent against methicillin-resistant and-susceptible Staphylococcus aureus biofilms. Evid-Based Complement Alternat Med. Article ID 862374, 16 pages
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108
Haque S, Ahmad F, Dar SA, Jawed A, Mandal RK, Wahid M, Lohani M, Khan S, Singh V, Akhter N (2018) Development in strategies for quorum sensing virulence factor inhibition to combat bacterial drug resistance. Microb Pathog 121:293–302
He YW, Wu J, Cha JS, Zhang LH (2010) Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol 10:187
Heim KE, Tahliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584
Hirakawa H, Tomita H (2013) Interference of bacterial cell-to-cell communication: a new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front Microbiol 24:114
Huang TP, Wong ACL (2007) A cyclic AMP receptor protein-regulated cell-cell communication system mediates expression of a FecA homologue in Stenotrophomonas maltophilia. Appl Environ Microbiol 73:5034–5040
Huber B, Eberl L, Feucht W, Polster J (2003) Influence of polyphenols on bacterial biofilms formation and quorum-sensing. Z Naturforsch 58:879–884
Husain FM, Ahmad I, Khan FI, Al-Shabib NA, Baig MH, Hussain A, Rehman MT, Alajmi MF, Lobb KA (2018) Seed extract of Psoralea corylifolia and its constituent bakuchiol impairs AHL-based quorum sensing and biofilm formation in food- and human-related pathogens. Front Cell Infect Microbiol 8:351
Hymes SR, Randis TM, Sun TY, Ratner AJ (2013) DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis 207:1491–1497
Jakobsen TH, Bragason SK, Phipps RK, Christensen LD, van Gennip M, Alhede M et al (2012a) Food as a source for quorum sensing inhibitors: Iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl Environ Microb 78:2410–2421
Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M et al (2012b) Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chem 56:2314–2325
Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA (2018) Bacterial biofilm and associated infections. J Chin Med Assoc 81:7–11
Jennings J, Courtney H, Haggard W (2012) Cis-2-decenoic acid inhibits S. aureus growth and biofilm in vitro: a pilot study. Clin Orthop Relat Res 470:2663–2670
Jiyoung K, Hoi JN, Kim P, Sok DE, Nam SW, Lee CH (2009) LC-MS/MS profiling-based secondary metabolites screening of Myxococcus xanthus. J Microbiol Biotechnol 19:51–54
Kalia M, Yadav VK, Singh PK, Sharma D, Pandey H, Narvi SS, Agarwal V (2015) Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa. PLoS ONE 10:e0135495
Karwacki MT, Kadouri DE, Bendaoud M et al (2013) Antibiofilm activity of Actinobacillus pleuropneumoniae serotype 5 capsular polysaccharide. PLoS ONE 8(5):e63844
Koh K, Tham F (2011) Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J Microbiol Immunol Infec 44:144–152
Kwan JC, Meickle T, Ladwa D, Teplitski M, Paul V, Luesch H (2011) Lyngbyoic acid, a “tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol Biosyst 7:1205–1216
Lambiase A, Rossano F, Del Pezzo M, Raia V, Sepe A, de Gregorio F, Catania MR (2009) Sphingobacterium respiratory tract infection in patients with cystic fibrosis. BMC Res Notes 2:262
Lang S, Wullbrandt D (1999) Rhamnose lipids—biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32
Larrosa M, García-Conesa MT, Espín JC, Tom´as-Barber´an FA (2010) Ellagitannins, ellagic acid and vascular health. Mol Asp Med 31:513–539
Linnes JC, Ma H, Bryers JD (2013) Giant extracellular matrix binding protein expression in Staphylococcus epidermidis is regulated by biofilm formation and osmotic pressure. Curr Microbiol 66:627–633
Little DJ, Pfoh R, Le Mauff F, Bamford NC, Notte C, Baker P et al (2018) PgaB orthologues contain a glycoside hydrolase domain that cleaves deacetylated poly-β(1,6)-N-acetylglucosamine and can disrupt bacterial biofilms. PLoS Pathog 14:e1006998
López D, Vlamakis H, Kolter R (2010) Biofilms. Cold Spring Harb Perspect Biol 2:a000398
Loughran AJ, Atwood DN, Anthony AC, Harik NS, Spencer HJ, Beenken KE et al (2014a) Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. Microbiology 3:897–909
Luciardi MC, Blazquez MA, Cartagena E, Bardon A, Arena ME (2016) Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT Food Sci Technol 68:373–380
Luís A, Duarte A, Gominho J, Domingues F, Duarte AP (2016) Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiate essential oils. Ind Crops Prod 79:274–282
Mack D, Rohde H, Harris LG, Davies AP, Horstkotte MA et al (2006) Biofilm formation in medical device-related infection. Int J Artif Organs 29:343–359
Mack D, Siemssen N, Laufs R (1992) Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun 60(5):2048–2057
Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, Kjelleberg S et al (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148:1119–1127
Marques CN, Morozov A, Planzos P, Zelaya HM (2014) The fatty acid signaling molecule cis-2-decenoic acid increases metabolic activity and reverts persister cells to an antimicrobial-susceptible state. Appl Environ Microbiol 80:6976–6991
Marti M, Trotonda MP, Tormo-Mas MA, Vergara-Irigaray M, Cheung AL, Lasa I et al (2010) Extracellular proteases inhibit protein-dependent biofilm formation in Staphylococcus aureus. Microbes Infect 12:55–64
Mason TL, Wasserman BP (1987) Inactivation of red beet betaglucan synthase by native and oxidized phenolic compounds. Phytochemistry 26:2197–2202
Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG et al (2003) Extensive and specific responses of a eukaryote to bacterial quorum-sensing signals. Proc Natl Acad Sci USA 100:1444–1449
McCann MT, Gilmore BF, Gorman SP (2008) Staphylococcus epidermidis device related infections: pathogenesis and clinical management. J Pharm Pharmacol 60:1551–1571
Meireles A, Borges A, Giaouris E, Simões M (2016) The current knowledge on the application of anti-biofilm enzymes in the food industry. Food Res Int 86:140–146
Mettler E, Carpentier B (1998) Variations over time of microbial load and physicochemical properties of floor materials after cleaning in food industry premises. J Food Prot 61:57–65
Mootz JM, Malone CL, Shaw LN, Horswill AR (2013) Staphopains modulate Staphylococcus aureus biofilm integrity. Infect Immun 81:3227–3238
Moretro T, Hermansen L, Holck AL, Sidhu MS, Rudi K et al (2003) Biofilm formation and the presence of the intercellular adhesion locus ica among staphylococci from food and food processing environments. Appl Environ Microbiol 69:5648–5655
Morgan B (2015) Microbial gangs are organised killers. Cosmos. https://cosmosmagazine.com/life-sciences/microbial-gangs-are-organised-killers
Mukherji R, Patil A, Prabhune A (2015) Role of extracellular proteases in biofilm disruption of gram positive bacteria with special emphasis on staphylococcus aureus biofilms. Enz Eng 4:1
Mullis SN, Falkinham JO III (2013) Adherence and biofilm formation of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium abscessus to household plumbing materials. J Appl Microbiol 115:908–914
Myszka K, Schmidt MT, Majcher M, Juzwa W, Olkowicz M, Czaczyk K (2016) Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare essential oil and its major bioactive compounds. Intern Biodet Biodeg 114:252–259
Nagraj AK, Gokhale D (2018) Bacterial biofilm degradation using extracellular enzymes produced by Penicillium janthinellum EU2D-21 under submerged fermentation. J Adv Microbiol 8:687–698
Newton JA, Fray RG (2004) Integration of environmental and host-derived signals with quorum sensing during plant-microbe interactions. Cell Microbiol 6(3):213–224
Nicol M, Alexandre S, Luizet JB, Skogman M, Jouenne T, Salcedo S, Dé E (2018) Unsaturated fatty acids affect quorum sensing communication system and inhibit motility and biofilm formation of Acinetobacter baumannii. Int J Mol Sci 19:214
Nithya C, Devi MG, Karutha Pandian S (2011) A novel compound from the marine bacterium Bacillus pumilus S6-15 inhibits biofilm formation in gram-positive and gram-negative species. Biofouling 27:519–528
Niu S, Afre S, Gilbert ES (2006) Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett Appl Microbiol 43:489–494
Ogawa A, Furukawa S, Fujita S et al (2011) Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl Environ Microbiol 77:1572–1580
Oliveira BD, Rodrigues AC, Cardoso BMI, Ramos ALCC, Bertoldi MC, Taylor JG et al (2016) Antioxidant, antimicrobial and anti-quorum sensing activities of Rubus rosaefolius phenolic extract. Ind Crop Prod 84:59–66
Olivero J, Pajaro N, Stashenko E (2011) Anti-quorum sensing activity of essential oils isolated from different species of the genus Piper. Vitae 18:77–82
Olson ME, Ceri H, Morck DW, Buret AG, Read RR (2002) Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res 66:86
Ong JFM, Goh HC, Lim SC, Pang LM, Chin JSF, Tan KS et al (2019) The discovery of potential anti-quorum sensing natural products from microbes associated with marine samples from Singapore. Mar Drugs 17(72):1–15
Packiavathy I, Agilandeswari P, Musthafa K, Pandian S, Ravi A (2012) Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against gram negative bacterial pathogens. Food Res Int 45:85–92
Parsek MR, Greenberg EP (2005) Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13:27–33
Platt TG, Fuqua C (2010) Whats in a name? The semantics of quorum sensing. Trends Microbiol 18(9):383–387
Ponnusamy K, Paul D, Kweon JH (2009) Inhibition of quorum sensing mechanism and Aeromonas hydrophila biofilm formation by vanillin. Environ Eng Sci 26:1359–1363
Rahmani-Badi A, Sepehr S, Mohammadi P, Soudi MR, Babaie-Naiej H (2014) A combination of cis-2-decenoic acid and antibiotics eradicates pre-established catheter-associated biofilms. J Med Microbiol 63:1509–1515
Reichhardt C, Fong JCN, Yildiz F, Cegelski L (2014) Characterization of the Vibrio cholerae extracellular matrix: a top-down solid-state NMR approach. Biochim Biophys Acta 1848:378–383
Rémy B, Mion S, Plener L, Elias M, Chabrière E, Daudé D (2018) Interference in bacterial quorum sensing: a biopharmaceutical perspective. Front Pharmacol 9:203
Rendueles O, Kaplan JB, Ghigo JM (2013) Antibiofilm polysaccharides. Env Microbiol 15:334–346
Rohde H, Mack D, Christner M, Burdelski C, Franke GC et al (2006) Pathogenesis of staphylococcal device-related infections: from basic science to new diagnostic, therapeutic and prophylactic approaches. Rev Med Microbiol 17:45–54
Rozej A, Cydzik-Kwiatkowska A, Kowalska B, Kowalski D (2014) Structure and microbial diversity of biofilms on different pipe materials of a model drinking water distribution systems. World J Micriobiol Biotechnol 31:37–47
Rudrappa T, Bais HP (2008) Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J Agric Food Chem 56:1955–1962
Rupp ME, Archer GL (1994) Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis 19:231–243
Ryan RP, Dow JM (2011) Communication with a growing family: diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol 19:145–152
Sarabhai S, Sharma P, Capalash N (2013) Ellagic acid derivatives from Terminalia chebula downregulate the expression of quorum sensing genes to attenuate Pseudomonas aeruginosa PAO1 virulence. PLoS ONE 8:e53441
Sarkar R, Chaudhary S, Sharma A, Yadav K, Nema N, Sekhoachad M et al (2014) Anti-biofilm activity of marulae a study with the standardized bark extract. J Ethnopharmacol 154:170–175
Saurav K, Bar-Shalom R, Haber M, Burgsdorf I, Oliviero G, Costantino V, Morgenstern D, Steindler L (2016) In search of alternative antibiotic drugs: quorum-quenching activity in sponges and their bacterial isolates. Front Microbiol 7:416
Saw JH, Mountain BW, Feng L, Omelchenko MV, Hou S, Saito JA, Stott MB, Li D, Zhao G, Wu J, Galperin MY, Koonin EV, Makarova KS, Wolf YI, Rigden DJ, Dunfield PF, Wang L, Alam M (2008) Encapsulated in silica: genome, proteome and physiology of the thermophilic bacterium Anoxybacillus flavithermus WK I. Genome Biol 9:161
Sawicki GS, Chou W, Raimundo K, Trzaskoma B, Konstan MW (2015) Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. J Cyst Fibros 14:777–783
Schertzer JW, Boulette ML, Whiteley M (2009) More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol 17(5):189–195
Sepehr S, Rahmani-Badi A, Babaie-Naiej H, Soudi MR (2014) Unsaturated fatty acid, cis-2- decenoic acid, in combination with disinfectants or antibiotics removes pre-established biofilms formed by food-related bacteria. PLoS ONE 9:e101677
Seper A, Pressler K, Kariisa A, Haid AG, Roier S, Leitner DR, Reidl J, Tamayo R, Schild S (2014) Identification of genes induced in Vibrio cholerae in a dynamic biofilm system. Int J Med Microbiol 304:749–763
Shak S (1995) Aerosolized recombinant human DNase I for the treatment of cystic fibrosis. Chest 107:65S–70S
Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL (1990) Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci USA 87:9188–9192
Shih PC, Huang CT (2002) Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J Antimicrob Chemother 49(2):309–314
Shukla SK, Rao TS (2013) Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. J Antibiot (Tokyo) 66:55–60
Shukla SK, Rao TS (2017) Staphylococcus aureus biofilm removal by targeting biofilm-associated extracellular proteins. Indian J Med Res 146:S1–S8
Singh S, Singh SK, Chowdhury I, Singh R (2017) Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J 11:53
Snarr BD, Baker P, Bamford NC, Sato Y, Liu H, Lehoux M, Gravelat FN, Ostapska H, Baistrocchi SR, Cerone RP, Filler EE, Parsek MR, Filler SG, Howell PL, Sheppard DC (2017) Microbial glycoside hydrolases as antibiofilm agents with cross-kingdom activity. Proc Natl Acad Sci USA 114:7124–7129
Stoodley P, Conti SF, DeMeo PJ, Nistico L, Melton-Kreft R, Johnson S, Kathju S (2011) Characterization of a mixed MRSA/MRSE biofilm in an explanted total ankle arthroplasty. FEMS Immunol Med Microbiol 62:66–74
Sun F, Qu F, Ling Y et al (2013) Biofilm-associated infections, antibiotic resistance and novel therapeutic strategies. Future Microbiol 8:877–886
Taganna J, Quanico J, Perono R, Amor E, Rivera W (2011) Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilms maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. J Ethnopharmacol 134:865–871
Talagrand-Reboul E, Jumas-Bilak E, Lamy B (2017) The social life of aeromonas through biofilm and quorum sensing systems. Front Microbiol 8:37
Tang JL, Liu YN, Barber CE, Dow JM, Wootton JC, Daniels MJ (1991) Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol Gen Genet 226:409–417
Tang K, Zhang XH (2014) Quorum quenching agents: resources for antivirulence therapy. Mar Drugs 12:3245–3282
Tay SB, Chow JY, Go MK, Yew WS (2016) Anti-virulent disruption of pathogenic biofilms using engineered quorum-quenching lactonases. J Vis Exp 107:53243
Taylor P, Yeung ATY, Hancock REW (2014) Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies. J Biotechnol 191:121–130
Teplitski M, Robinson JB, Bauer WD (2000) Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant Microbe Interact 13:637–648
Thakur P, Chawala R, Tanwar A, Singh A, Chakotiya AS, Narula A, Goel R, Arora R, Sharma RK (2016) Attenuation of adhesion, quorum sensing and biofilm mediated virulence of carbapenem resistant Escherichia coli by selected natural plant products. Microb Pathog 92:76–85
Trentina D, Giordania R, Zimmerb K, da Silva A, da Silva M, Correiac M et al (2011) Potential of medicinal plants from the Brazilian semi-arid region (Caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J Ethnopharmacol 137:327–335
Truchado P, Gim´enez-Bastida JA, Larrosa M, Castro-Ib´añez I, Espín JC, Tom´as-Barber´an FA et al (2012) A inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J Agri Food Chem 60:8885–8894
Vandeputte OM, Kiendrebeogo M, Rasamiravaka T, Stévigny C, Duez P, Rajaonson S, Diallo B, Mol A, Baucher M, El Jazir M (2011) The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 157:2120–2132
Vasavi HS, Arun PD, Rekha PD (2016) Anti-quorum sensing activity of flavonoid rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J Microbiol Immunol Infect 49:8–15
Vattem DA, Mihalik K, Crixell SH, McClean RJC (2007) Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 78:302–310
Viju N, Satheesh S, Vincent S (2013) Antibiofilm activity of coconut (Cocos nucifera Linn.) husk fibre extract. Saudi J Biol Sci 20:85–91
Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS (2010) Suppression of bacterial cell-cell signalling, biofilms formation and type III secretion system by citrus flavonoids. J Appl Microbiol 109:515–527
Vikram A, Jesudhasan PR, Jayaprakasha GK, Pillai SD, Patil BS (2011) Citrus limonoids interfere with Vibrio harveyi cell-cell signaling and biofilm formation by modulating the response regulator LuxO. Microbiology 157:99–110
Vílchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Wagner-Döbler I, Sztajer H (2010) Streptococcus mutants inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). ChemBioChem 11:1552–1162
Wang LH, He Y, Gao Y, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L et al (2004) A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51:903–912
Xu FF, Morohoshi T, Wang WZ, Yamaguchi Y, Liang Y, Ikeda T (2014) Evaluation of intraspecies interactions in biofilm formation by Methylobacterium species isolated from pink-pigmented household biofilms. Microbes Environ 29:388–392
Zhou L, Zheng H, Tang Y, Yu W, Gong Q (2013) Eugenol inhibits quorum sensing at sub-inhibitory concentrations. Biotechnol Lett 35:631–637
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sharma, R., Bajpai, P., Sayyed, U., Ahmad, I.Z. (2019). Approaches Towards Microbial Biofilm Disruption by Natural Bioactive Agents. In: Kumar, S., Chandra, N., Singh, L., Hashmi, M., Varma, A. (eds) Biofilms in Human Diseases: Treatment and Control. Springer, Cham. https://doi.org/10.1007/978-3-030-30757-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-30757-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30756-1
Online ISBN: 978-3-030-30757-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)