Abstract
This chapter discusses the utilization of ultrafiltration membrane (UF) in water treatment system. The UF membrane overview including the type, preparation and characterization is concisely reviewed. The two major types of membrane namely polymeric and ceramic membranes are subdivided into two distinguished subchapters focusing on their fabrication and physicochemical properties. Additionally, the main converge of this chapter is the application of these UF membranes on multidisciplinary industries such as textile, dairy, beverages, microelectronics, petrochemical, cosmetic and pharmaceutical, and few others. The advantages and limitations of UF carefully addressed in their respective subchapters. At the end of this chapter, an attempt is also made to show the future direction of the UF membrane towards the advance membrane technology system such as membrane distillation, membrane contactor and many others.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction and History
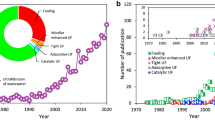
Membrane technologies have been extensively used in wastewater treatment owing to their precious properties such as cost effective, ease of fabrication and simple operation system. Accordingly, membrane technologies are divided into four types based on their pore sizes which are microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO) [6, 34, 61]. In term of rejection performance, NF and RO showed excellent performances due to their smaller pore size. Unfortunately, both of these membranes show drawbacks of having low flux, needing another pretreatment and high pressure consumption. It should be mentioned here that UF membrane offers advantages of high flux, simple operation and low pressure consumption. The average pore diameter for UF membranes is 10–1000 Å. In early 1900s, Bechhold pioneering the fabrication of UF membrane by prepared it from nitro cellulose [8]. Later in 1918, Zsigmondy and Bachmann explained in detail in their publication on how to modify the MF membrane into UF membrane [70]. In 1922, they got their invention patented in US patent by exposing a thin coating of a nitrocellulose solution [71]. The most highlighted breakthrough of UF membrane was in 1963, in which the first cellulose acetate membrane prepared by Loeb and Sourirajan [45]. Three years later, Abcor installed the first commercial UF membrane to recover electrocoat paint from automobile paint shop rinse water [6]. Interestingly, UF membrane received widely attention and started its commercialization with various configuration such as spiral wound and hollow fibre. Figure 1 shows the flow of the development of UF membranes.

From Baker [6]
Milestones in the development of UF membranes.
Nowadays, there has been an increased interest in development of UF membrane, not only towards polymeric membrane type but also towards ceramic membrane. In 1983, Tsapyuk et al. introduced the first UF ceramic membrane by forming a layer of cellulose acetate on porous tubular ceramic membrane for treating wastewater containing colloidal solutions of gelatin and silver [62]. A recent study on UF membrane has been investigated by investigating ceramic membrane incorporating with copper oxide nanoparticles through dip coating for chromium (VI) removal [12]. A detailed XPS analysis (Fig. 2) revealed the binding properties of the CuO and clay-alumina ceramic membrane surface and the rejection mechanism of chromium (VI).

From Choudhury et al. [12]
XPS analysis of UF ceramic membrane derived from clay-alumina surface and CuO.
Herein, it is also interesting to note that the application of UF ceramic membranes have been widely applied as photocatalytic membrane [3, 59]. For example, a research team from Dr. Othman’s lab has been successfully prepared a low-cost ceramic membrane derived from kaolin. The ceramic membrane was then modified with TiO2 via hydrothermal method for decolorization of reactive black 5 [51]. Figure 3 shows the SEM images of the prepared membrane and proved a well-dispersed TiO2 nanorods (Fig. 3) have been obtained. Accordingly, the prepared membrane induced high water permeation of 165 L/h m2bar and photocatalytic activity of 80.3% under UV irradiation.

From Mohtor et al. [51]
Morphology images of well-dispersed of TiO2 nanorods on kaolin-ceramic membrane at a 2000x and b 5000x of (1) kaolin support and kaolin/TNR membrane that prepared at (2) 2 h, (3) 6 h, and (4) 10 h of reaction time.
2 UF Membrane Type, Characteristics and Preparation
2.1 Polymeric Membrane
Polymeric membranes is known as the major source of membrane that commercially available in the market due to its low cost and abundance of production [66]. Unlike the MF range process, the UF membranes are mainly operating via sieving process and rather in a wider separation range to that of MF membranes. The pore size of the membranes generally between 0.01 and 0.1 μm, are capable of removing particles, colloids, viruses and pathogens as well as allows the permeability of microsolutes with molecular weight (MW) of less than 300 through the membrane [20]. Although these UF membranes are capable of removing those species, the selectivity of the membrane is basically based on the surface charge and size difference of the components to be separated, besides the properties of the membranes itself and its hydrodynamic conditions.
There are numerous types of polymers have been used for the fabrication of UF membranes and few have become an emerged selection as a leading choice for potable reuse applications. The two major considerations that determining the potable recycle of the membrane technologies are the properties of the material that composed the membrane and the mechanisms of the membrane formation. The materials selection of the membranes is mainly depending on the physicochemical properties of the polymer including distribution of the membrane pore size, porosity, wetting vulnerability, polymer flexibility, cost, mechanical strength, durability, stability and chemical resistance [64]. Apart of that, the other advantageous properties of the polymer that associated to the fabrication of the membrane are namely as low tortuosity besides the surface properties including the surface charge that mainly attributed to the rejection performance. Additionally, there are few characteristics that principally influence the membrane performance by enhancing the membrane regeneration and fouling recovery resulted from the weakly adhering materials, resistance towards aggressive cleaning agents as well as low surface roughness [67].
Of all the polymeric materials chosen for the membranes production, there are few reported and widely used attributable to their superior properties contributable to the high performance of the membranes. Table 1 summarizes a list of frequently used polymeric membranes with their respective properties [2, 52, 67]. As been aforementioned earlier, the selection of such polymers is primarily due to their physicochemical properties contributing to the high performance of the fabricated membranes.
There were variety of polymeric membrane have been utilized for the potable reuse membrane facilities. One of its kind is the polypropylene membrane which controls the largest potable reuse membrane in the world. The higher mechanical strength accompanied with the high resistivity towards many solvents has attributed to this successfulness. Over the time, the industries have now shifted to PVDF as a material of choice. This could be possibly due to the improved membrane lifespan, reduced capital cost relatable to the production and declined nominal pore size as compared to that of polypropylene [44]. On the other hand, while the new materials along with advantageous operation continue to develop, the contemplation of the polymer constituents of the current facilities can involve a substantial principal cost for the system retrofit because the low pressure of the UF membrane systems have normally not homogeneous to the common platform and thus would permit the latest product materials to be installed promptly to the system with prior modification. Therefore, these UF membrane systems are unique and proposed in a variation of shapes in both submerged and pressurized patterns [63].
Apart from the materials selection, the fabrication of the polymeric UF membranes plays a vital role that consequently determine the performance of the membranes. There are many techniques attempted by the manufacturers in fabricating the membranes. These fabrication techniques are mainly depending more on the membrane materials rather than the class of membrane (i.e. MF, UF, etc.). The most common techniques used for the membrane fabrication are phase inversion and electrospinning process. Notably, phase inversion techniques are varied including non-solvent induced phase separation (NIPS), vapor induced phase inversion (VIPS), thermal phase separation and controlled evaporation. On the other hand, more complicated approaches are utilized for the composite-type membranes especially those acquired the surface modifications [65]. These included the film casting, hydrothermal synthesis process, dip coating, surface adsorption, spray coating, sputtering and etching, aerosol deposition and layer-by-layer deposition [41]. Besides that, the chemical process is also attempted to modify the membranes chemically, depending on the applications. These techniques included radical grafting, chemical coupling and hydrophilization with plasma.
The membranes package in reactors and modules has become a vital step in developing the membrane filtration technology. The module forms frequently occupy the great limitations on the membrane constituents attributable to the existing fabrication techniques. Therefore, a broad range of membrane components and configurations have been established that well-matched to a variation of appliances. Figure 4 depicts the four conventional polymeric membrane modules namely flat sheet, tubular, spiral wound and hollow fibre configurations.
Of all the membrane configurations, flat sheet and the uncommonly used tubular membrane modules with diameter of 1–3 cm have a lower packing density and thus lead to the higher costs. The stacked membrane modules of flat sheet configure are constructed to bear an elevated pressure that can overload of 100 bar. However, their applications are only restricted to cutting-edge water treatment process attributable to the low surface area per unit volume densities. Extensively, these membranes are exposed to severe membrane fouling and thus possessed low treatment efficiencies [1]. Alternatively, these modules have largely replaced by the spiral wound and hollow fibre configurations for reuse applications and water treatment processes.
Unlike the flat sheet counterpart, hollow fibre components may comprise of quite a few or several thousands of hollow fibre membranes of various lumen dimension of 0.5–1.5 mm in diameter. This feature would subsequently enhance the surface area per unit volume thus, mostly preferred over other membrane configurations. Additionally, hollow fibre configuration fundamentally has a favourable mass transfer coefficient and higher packing densities and thus making it suitable for the UF processes [29]. Moreover, the filtration in hollow fibre can be achieved from inside-out or vice versa. This could be attributable to the removal of foulants by backwashing process. Unfortunately, most of the polymeric hollow fibre membranes are limited to the applications to pressures below that 4 bar, even after the surface modifications [44].
On the other hand, the spiral wound membrane module is known as the most common module used for the UF processes [46]. The spiral wound design is such a success due to the relative ease of manufacturing and high packing densities of the modules. This module contains of a small diameter tube that firmly loaded with flat sheet membrane and split up by the mesh spacer between the feed and permeate outlets. The compact configurations of the spiral wound modules offer higher surface area for a filtration unit and thus defeating the restrictions faced by the flat sheet and tubular membrane modules. These spiral wound membrane modules have been found and reported to be successfully removing traditional contaminants from the feed, and rather emerging contaminants from wastewater sources [21, 47].
2.2 Ceramic Membrane
Ceramic membrane has been known to be the counter for polymeric membrane [28, 34]. Normally, ceramic membrane is fabricated from inorganic materials such as silica, titania, alumina and zirconia. The membrane mostly has been used for filtration due to its permselective barrier which acts as a fine sieve especially to be used in water treatment process. Porosity is the main characteristic possessed by ceramic membrane which make it able to be used in waste water filtration which can be divided into porous and dense together with other criteria including pore size, membrane’s thickness and surface porosity. The summary of membranes type is shown in Table 2 used in filtration according to its pore size and porosity.
Divided into four layers kind of porosity, categorization of the membrane will be choose based on the pore size and the type of filtration as well as the contaminants or pollutants that will be used according to its application.
Basically, there are four layers possessed by ceramic membrane where the performance of the membrane was taken for consideration in term of separation and permeability properties. These layers are characterized as macroporous (the biggest particle size), followed by mesoporous, microporous and dense (the smallest particle size). The mechanism of the ceramic membrane can be described in Fig. 5 where the lowest layer act as a mechanical support for the membrane, the top layer is the place for the separation and is bridged by a middle layer which compromising by two or more mesoporous structure with varied of pore size.

From Hubadillah et al. [34]
Schematic diagram of ceramic membrane.
Depending on its application, silica, titania, alumina and zirconia or even the combination or derivative of these materials are the most commonly used raw ceramic materials for ceramic membrane. Recently, alumina has been the most commonly commercialized materials for ceramic membrane owing to the excellent structure both chemically as well as thermally of the material. However, due to its high cost and some drawbacks, the emphasising of low cost and alternative materials for ceramic materials has drawn more interest. Alternative materials from clay such as kaolin, bauxite, bentonite, ball clay, sagger clay and fire clay has been proposed due to its excellent performance similar to conventional ceramic membrane materials [32, 33, 35]. Other than that, zeolite also has been proposed as starting materials for ceramic membrane due to its adsorption and ion exchange capacity properties [34].
Filtration of membrane can be defined as a technique which utilized the use of strong, thin and semi-permeable membrane for separation process. Due to gradient pressure or driving force, the dissolved components and suspended solids in the water or waste water will be halted from pass through the membrane. MF comprises of 0.1–10 μm considered larger compared to UF which utilized to remove large colloids, cells, viruses and microbes. On the hand, the size of UF which comprises of 0.001–0.1 µm pores useful for removing smaller colloids, other macromolecules from various media such as protein. The separation by these membranes were accomplished by the size of the pore size where the smaller pore size membrane will reject large solutes while smaller solutes such as water will flow through the pore of the membrane [48].
Currently, most UF are in the shape of pressurized, multi-bore and spiral wound hollow fibre membrane depending on its application [24]. The fabrication process of ceramic membrane normally involved few stages which are (1) preparation of suspension; (2) spinning or pressing of suspension and lastly (3) final sintering.
Commonly, slip casting has been the well-known used method to fabricate ceramic membrane. Not only requires a very long time to cast, the wall thickness of the membrane is also hard to control which eventually lead to unstable thickness and very thick. Another casting method is tape casting where the method was adapted from casting of polymeric membrane involved pressure press technique. The method uses a casting knife which acts as moving carrier for the powder suspension later let to be dried in a certain desired area. This method suitable to be employed in gas application where the ceramic membrane was used for oxygen and hydrogen. In addition, there is another method which is extrusion method which very common especially in industry to fabricate ceramic or inorganic membrane such as tubular support. Normally being used in large scale production, this method also being applied in traditional construction materials such as porcelain, tiles, electronic materials, brick etc. The development of sol-gel method of ceramic UF membranes was first introduced by Leenaars et al. [42]. Sol-gel technique is advantageous in term of small pore-size as this technique allows controlling the pore-size hence make it the most important technique in ceramic membranes fabrication (Figs. 6 and 7).

From Hubadillah et al. [31]
FESEM image of ceramic membrane showing different pore size.

From Hubadillah et al. [36]
Ceramic hollow fibre membrane from kaolin at different ceramic content.
Membrane morphology of ceramic membrane plays one huge factor in its separation performance. Associated with its shape, distribution, density, pore size, and the membrane’s surface which determine the membrane’s category to be either dense or porous. Depending on its application such as to be used for gas or liquid separation, it is very crucial for manufacturers to be able to specify the properties of the ceramic membrane. For gas separation application, dense ceramic membrane is suitable as this membrane possess gas tightness, crystal structures and permeation characteristics. Unlike porous ceramic membranes which suitable to be used for liquid application, few parameters need to be considered such as the particle or pore size, packing, surface area etc. which is very important to be taken prior to manufacturing. Therefore, it is essential to analysis the characterization and properties of the membranes according to its application.
A membrane’s pore structure will determine how the solutes will pass through, while the membrane surface determines the uptake of these solutes and potential for fouling. Many membranes are asymmetric, so a complete set of information about each membrane layer is very important. From those studies, it is remarkable the significant influence of the solute–solute and solute–membrane interactions on the final performance of the filtration process. However, most of the studies on UF membranes involve the use of tight UF membranes [43].
The used of ceramic membrane for UF has been quite popular among researchers in membrane technology due to its excellent chemical and physical properties. Many applications have been used and described in detail in followed subtopics. Basically, ceramic membranes for UF requires the pore size of the membrane within range more than 50 nm to be taken place. However, it is hard to be applied in actual projects, due to its shortages of high cost, great investment, poor stain- resistant capacity, and high energy consumption. Researchers have utilized different methods to overcome these problems, concluding optimized structural design, adding turbulence promoter, and coupling with other treating processes. On the other hand, membrane fouling is still the most difficult problem to solve. Thus, it is necessary to enrich the theoretical aspects of ceramic membrane process by investigating the membrane fouling control and cleaning procedure.
3 Application of UF Membrane in Industrial Influent
3.1 Textile Industry
Another remarkable problem related to wastewater is textile industry due to the production of dyes. Salts and toxic contaminants in its wastewater. This is due to the various activities done by textile industry such as printing, bleaching and dyeing that used colors and other chemicals. Among all technologies, NF membranes were found to be effective in treating this wastewater because of its precious behaviour of having synergistic effects of steric hindrance exclusion and electrostatic repulsion [9]. Unfortunately, NF membranes is negatively charged membrane in which having strong electrostatic repulsion that prohibited the salt particles contained in the wastewater from being treated [19]. Due to this, the usage of NF membrane in the textile wastewater has been replaced with UF membrane. Table 3 summarizes the investigation of UF membrane towards wastewater treatment from textile industry.
3.2 Dairy Industry
The membrane technology has transfigured along with the diary industries. The membranes have been utilized for many purposes in this field including the calibration of the main constituents of the milk for fitting with the new product, extending the shelf life of the dairy products in the absence of heat treatment as well as enhancing the yield and quality of the dairy food productions. Additionally, the membranes have also used for concentrating, purifying and fractionating the valuable milk proteins in its natural states [39].
The milk is considered as an essential and complete food for the human diet especially for the vegetarians. It is also known as the ideal liquid for the membrane separation due to its compositions. The utilization of the membrane technologies for the diary production has been started since the 1960s and currently has become the second largest industries utilizing the membranes behind the wastewater treatment process [57]. It is reported that several hundreds of thousands square meters of membranes (400,000 m2) is currently used in these industries [56]. From that figure, about 2/3 of the membranes set up in these industries is utilized for the whey treatment and the remaining 1/3 for the milk. The application of the membrane technology in the dairy industries have been significantly boosted with the establishment of the superior substances namely polyamide, polysulfone and cellulose acetate accompanied by the technological processes of UF. Figure 8 shows the milk component indicated by size and suitable membrane processes.

From Daufin et al. [15]
The milk components due to size and appropriate membrane processes for the treatment.
There are numerous types of membranes with diverse properties presented in the market and frequently utilized in the dairy industries. These membrane filtrations have been utilized in many areas of the industries including the whey processing, milk protein processing, shelf life of milk, cheese industry, demineralization or desalting as well as fractionation of milk fat. The extended shelf life of milk products has been applied in a manner of reducing the microbial activities thus, prolong the lifetime under the chilled surroundings [54]. On the other hand, the membrane technology in cheese industry used for the concentration of milk by a factor of 1.2–2.0 times and increases the casein: protein ratio thus, reducing the requirement of the processing equipment as well as enhancing the yielding of cheese production [26].
The application of the membrane technology could be beneficial in these industries due to a wide range of advantages offer by this technology. The membrane filtration is capable of producing a desired quality of product via non-thermal environmentally friendly approaches that possibly minimizes the adverse effect rise from the temperature. The effect of denaturation of proteins, changes in phase as well as sensory attributed by the heat treatment could be avoided using the membrane technology. Additionally, the membranes can be utilized for the removal of the undesirable constituents namely microorganisms, sediments or drugs that consequently have a adverse effect of the product quality. Furthermore, there were reported that these membrane technology have used the fusion of membranes relatively to that a single membrane systems [7].
3.3 Beverage Industry
Over the past few years, the growth of beverage industry can be seen is likely increased especially in juice manufacturing. The search to develop the best method to keep the highest percentage of originality of its original source of food such as fruit juice so that the original flavour of the juice can be retained. Converting from lab scale to manufacturing or pilot scale has been one of the most challenging things encountered by many manufacturing plants. Not only problem may arise during the process, another problem could occur after the process which is fruit-waste-containing water. Membrane technology has been discovered could minimize these problems especially UF or MF membrane, however, here comes another problem which is membrane fouling which could hinder the expansion on the use of the membranes in pilot plants.
The utilization of UF is not solely for filtration, instead, fractionation and fixation also can be considered to be another usage of UF. Clara et al. [13] stated that UF can be exploited for fractionation of milk to generate cheese where lactose and solved salts containing in the permeate and proteins, fat and some insoluble salts containing in the retentate part. It has been discovered that skimmed milk produced by UF contain high concentration of calcium and protein which is very vital in dairy industry application. On the other hand, in fruit juice application where UF has been used for clarification of the desired juice depending on the molecular weight cut-off. Instead of collecting from the permeate part, the juice was collected from the retentate part. UF also has been used in order to keep the juice from any miscellaneous such as bacteria, undesired proteins and polysaccharides which polluting the clarity of the juice.
Membrane technology has been considered one important technology to be developed in beverage industry specially to separate varieties of wastes from beverage industries such as fruit juices, wines and some vegetables juices wastes. UF has been known to allow the separation process from retentate containing the concentrated fibrous or pulp and a permeate containing solution after clarification of free from microorganisms. Not only in the process in waste water treatments, UF has been used to clarify fruit juice to prevent pasteurized fraction. In addition, UF also brings huge impact in beverage industry where suspended solid separated from clear final products such as in liqueur, clear fruit juice and carbonated soft drinks has been considered to possess negative impact to the quality of the products [16]. Study by Galaverna et al. [22] stated that apart from separation process, UF was also used in the recovery of bioactive components from fruit juice. In the study, the bioactive components from depectinized kiwi fruit juice were mostly recovered from the process of clarification using UF.
Mohamad et al. [50], has summarised some studies by several researches by using ceramic membranes in UF process. The polymeric membranes that has been used in UF process include polysulfone, polyethersulfone, regenerated and acetate cellulose. However, during cleaning process by using strong chemicals, the membranes tend to break which leads to membrane fouling due to chemical degradation in long term period which shortened the lifespan of the membranes. Due to this drawback, ceramic membranes specifically to be made to be used in UF process has drawn much interests among researchers due to its great performance in term of chemically stable in long term period, thermally and mechanically. UF ceramic membranes which comprised of alumina oxide, zirconia oxide and titania oxide and some supported by these materials or carbon substructure were made according to its MWCO to identify the membrane compatibility to be used in apple juice separation/clarification process. From the observation, the reduction of permeate flux over time up was due to the formation of layer on the surface of the membranes because of the retained solids juice which inhibited a smooth transmembrane process. Also, at pressure below 300 kPa, the membranes also shown some improvement in term of reducing the fouling resistance. On the other hands, ceramic membranes also have been reported showing a good performance to separate protein and lactose where almost PR ~80% content of high protein and LR ~7% content of low lactose were retained considering high permeate flux at approximately 40 L/m2 h (Fig. 9).

From Cheryan and Alvarez [11]
Schematic of a UF fruit juice in plant.
According to Girard and Fukumoto [25], compounds present in fruit juices are mostly made up of organic and amino acids, sugars, pigments, vitamins, phenolic compounds, essential oils, nucleotides, minerals, esters, alcohols and aldehydes. Low molecular size of these molecules which is <1 kDa together with pore size of the membrane was observed to influence the enzyme depectinization. During this process, pectin was broken down making components of MW below 10 kDa increased. Due to huge range of MW of pectin, which is about 10–500 kDa, these large molecules could disperse which later formed a solid suspensions or colloids called polysaccharides. This colloid not only will produce unclear solution to the juice, it also endures large particles and hence allowing transmembrane with UF membrane to take place.
Another fouling on membrane was also examined by Saha et al. [55]. By using polymeric membrane of PS/PES, the fouling on the membrane caused by polysaccharides in sugarcane juice was examined and it has been found that, the large particles size of polysaccharide contributed to the major fouling of the membrane. Apparently, arabinogalactan, lipids and some phenolics components have become the major contributor for membrane fouling in which this researcher used a method to modify the membrane’s surface by grafting a thin layer of polymer made up from hydrogel to overcome this problem. This modified approach literally has been the best option proposed in order to solve the problem of membrane fouling due to large molecules such as polysaccharides (Fig. 10).

From Saha et al. [55]
SEM images of UF-PS-100H membrane: a pristine (surface), b pristine (cross-section), c polysaccharide fouled (surface) and d polysaccharide fouled (cross-section).
3.4 Petrochemical Industries
Unlike the other applications, the utilization of the membrane technology in petrochemical industries is mainly aimed for the separation and recovery process of several chemicals attained in the petrochemical fields. Since most of the chemicals relatable to the petrochemical industries are that of highly volatile compounds, the UF membranes for these processes are principally using the pervaporation approaches. Compared to the conventional approaches, membranes can possibly offer easy-operate, low maintenance and simple process option. Additionally, the membrane modules can be added and improved to achieve the anticipated separation process [53].
The separation of light solvent and the dewaxing process of solvent are among the common focus of the usage of membrane system in this area. The waste stream of petrochemical process may contain aromatic amines and/or phenolic compounds. Pervaporation filtration could possibly offer the separation of solutions or mixture of components that close boiling points. This process is normally difficult to be attained by other means including distillation. The first pervaporation plant was built and installed commercially by a German company Gesellschaft für Trenntechnik GmbH (GFT) in 1982 for the splitting of alcohol from water. The plant has utilized the polyvinyl alcohol as composite membrane since it is more permeable to water than the alcohol. The membrane was capable of reducing the water content of feed ethanol from ~10 to <1% as water permeate. Figure 11 depicts the flow scheme of the GFT plant for the ethanol recovery.

From Baker [5]
The flow scheme of GFT plant for ethanol recovery process.
On the other hand, membrane technology has also been used for the recovery of phenol besides the polymer adsorption, solvent extraction and activated carbon utilization [27]. The membrane aromatic recovery system (MARS) is fairly innovative procedure for the reclamation of aromatic acids and bases. In this procedure, the aromatics are selectively eradicated from the wastewater into the stripping solution. The process normally used a tubular silicon rubber membrane with 500 μm wall thickness. For the recovery of the aromatic acids such as phenol, the stripping solution is preserved at basic condition using NaOH whilst the acidic stripping solution of HCl is used for the reclamation of aromatic bases such as aniline.
Therefore, it can be clearly seen that the reaction and separation procedure possessed by the membrane play a crucial role in achieving the desired product of the petrochemical industries. Membrane that works in very low energy, do not require any heating-cooling system and do not produced any environmental pollution. The recovery of the small amount component from the stream using membrane can be achieved without the requirement of any substantial additional energy costs. Additionally, the system usually composed of low capital cost due to compact size, low power consumption and modular configuration will be subsequently reducing the production cost.
4 Application of UF Membrane in Industrial Effluent
4.1 Cosmetic and Pharmaceutical Industries
Recently, quality issue in wastewater especially from industry correspond to the risen of personal care products (PPCPs) and endocrine disrupting compounds, pharmaceuticals (EDC) has been concerning to fresh water supply. EDC mainly can be found in pharmaceutical products and can be considered as one of micropollutants in water or wastewater especially from industries. The removal of EDC/PPCPs to water stream from cosmetic and pharmaceutical industries one of the greatest micropollutants that need to be taken seriously.
According to Clara et al. [13], normally, the concentration of EDC in water was measured from nanogram per litre (ng/L) to micrograms per litre (μg/L) which be later specified the size of membrane should be used in treating the wastewater also according to its molecular weight cut-off (MWCO). Study by this researcher shown that, during wastewater treatment process, some substances such as carbamazepine which is one type of EDC, was not really removed by using membrane bioreactor during the process. This may due to the size of the molecules which is considered large and the membrane operated was not suitable to be used in the process. Hence, solution to this matter would the use of dense membrane with UF in reverse or forward osmosis process to enhance the power consumption of the membrane reactor.
Another study by Yoon et al. [68] has indicated that hydrophobic adsorption has been the biggest factor to assist high retention in a NF and UF dead-end stirred-reactor measurements. The results presented that less polar retention exhibit high volatile and more hydrophobic behaviour and vice versa. The membrane used for the removal of EDC/PPCPs has shown NF has better performance rather than UF in term of retention due to the size of the EDC which can be considered large. The pore size of the UF was investigated and it has shown that performance of the membrane was affected by the pore size. Other factors including source of waste water, electrostatic repulsion, feed and retentate solution, as well as size molecules which may affect the hydrophobicity adsorption of the membrane (Fig. 12).

From Yoon et al. [69]
Comparison on average percentage retention of EDC/PPCPs by the NF and UF membranes.
Another study was done by Yoon et al. [69] using the same membrane and reactor in order to investigate EDC/PPCPs removal from various effluents sources. Rejection of retention by size of membrane hugely affected the removal especially once the operation has reached a stable or steady state. With the enhance of hydrophobic adsorption, it was found that based on the result of average of 0 to <2.5 ng/cm2 adsorbed mass of EDC/PPCPs was calculated.
On the other hand, Taheran et al. [60] has pointed that estimation on these parameters including pore size, porosity, hydrophobicity, molecular size, weight and charge of the molecules in the feed, as well as morphology of the membranes should be included in order to remove pharmaceutically active compounds (PhAC) from water by using separation method. Salt rejection is one of the keys in membrane separation and to ensure an efficient removal of PhACs. Normally, MWCO of MF and UF membrane could be more than thousands Dalton where range for most of MWCO molecules only in between 200 and 800 Daltons. Hence, the adsorption capacity of the membrane should be evaluated for better performance to avoid any failure in separation process. Apart from membrane separation process which involved UF and NF, adsorption should be taken as another alternative due to the size of PhACs which hindering the used of membrane separation method (Fig. 13).

From Taheran et al. [60]
Proposed mechanism of PhACs removal from wastewater.
4.2 Food Processing Industries
Another remarkable problem related to wastewater is from food processing industries. This wastewater produced from the equipment cleaning process where all the contaminants flow out together into the drainage. Accordingly, wastewater from food industries contains very high BOD and protein, thus was automatically polluted the environment when pumped out from the industries.
A recent study by Miron et al. towards the treatment of protein-based solution have been investigated with ceramic membrane technology [49]. In the study, ceramic membrane was prepared and treated via hydrothermal process with partially irreversible cleaning operations. As a result, 100% lysosome rejection was successfully obtained. To be noted, HPLC test of the protein sample showed changes in its peaks during permeation as well as retention. For example, a peak with value of 0.4 min indicating a hydrodynamic radius of protein was shown during retention process, showing that the protein was denatured after the process. Figure 14 shows the chromatograms of retentate samples obtained during filtration test 2 of lysozyme using prepared UF membrane.

From Miron et al. [49]
Chromatograms of retentate samples taken at different pressures.
Later, another recent study by Ding et al. was towards protein treatment in detail together with membrane fouling problem [18]. A filtration system with 76 mm diameter stirres ultrafiltration cell and conducted by a pressure of 0.1 MPa. Accordingly, the performance was conducted by using 250 mg/L BSA solution as protein-based solution. As a result, the regression results for flux decline using the four fouling models and are shown in Fig. 15. Interestingly, the results proved that the corresponding correlation coefficient (R2) values regarding 20 mg/L BSA were 0.993, 0.995, 0.995, and 0.998 for complete blocking, intermediate blocking, cake filtration, and standard blocking, respectively. All these R2 values fit the flux decline well, indicating that there was a possibility of the four fouling mechanisms (pore blocking, adsorption fouling, cake layer and combined fouling) having occurred. Of the fouling models, complete blocking deviated farther from the flux decline than the other three model equations did.
4.3 Iron and Steel Industries
The implementation of major resources efficiency by minimizing the freshwater intake and emission has become vital due to the stringent environmental regulations and awareness amongst the environment activist across the globe. Due to that, the industries have become the most pressured entities regulates by this sanction worldwide [38]. One of its kind is the steel industries that use a large amount of water. Nonetheless, a little amount of water is spent as the largest part of the water is recycled and restored to the source.
In the iron and steel industries, water is utilized not only for cooling purposes, but rather uses for another procedures namely dust scrubbing and descaling. Thus, all forms of water (from fresh to seawater) are used in this steelmaking processes. The freshwater is primarily used for the straight and secondary cooling processes, whilst the seawater is consuming for one-time cooling upon the antifouling pretreatment [37]. In average, the water consumption for an assimilated steelmaking process is about 28.6 m3/tonne of produced steel whereas the water release is of 25.3 m3/tonne of produced steel. Therefore, the inclusive water usage per tonne of steel is rather low of about 1.6 m3. This water consumption and losses is mainly attributed by the evaporation.
The water resource management is a vital challenge to be monitored to increase the sustainability of the manufacture cycle as the freshwater accessibility and quality become the foremost interests. The increase in water recycling upon the cooling and cleaning processes can decline the water quality. Upon the cooling and desalination of this water, the escalate of salt content in the water rotation system attributable to the evaporation will not merely possess the ecological harmfulness, but rather undesirably disrupt the factory equipment due to corrosion.
Both conventional and advanced wastewater treatments such as chemical precipitation/clarification in the combination with flocculation have been attempted in the focus of producing high-grade water and thus, high proficiency of water reutilizing. Similarly, there were studies reported that membrane technology have been carried out in many industries for the treatment and reclaim of wastewater [23]. Additionally, the employment of UF membranes has efficiently removed the tiny colloidal suspended solid organic matter thus, causing in the premium water for iron and steel industries purposes. This subsequently reduced the cost of operation for the industries [30]. Figure 16 illustrates the integrated membrane systems for wastewater reclamation from iron and steel industries.

From Huang et al. [30]
The integrated membrane systems for wastewater reclamation in iron and steel industries.
5 Conclusion and Future Direction
In summary, UF membranes considered to be one the viable technology to treat wastewater due to possessed precious behaviour include low pressure, high flux, cost-effective investment and operational simplicity. UF membrane induced smaller pore size ranging from 10 to 1000 Å. Accordingly, it was found that UF membranes has an ability to treat various industrial wastewater such as textile, diary and food processing industries. Interestingly, the development of UF membrane is not only referred to polymeric membrane, but also ceramic membrane. Compared to polymeric membrane, ceramic membrane showed better performance especially in term of its ability that can endure harsh condition. Due to this, development of UF ceramic membrane has been developed by incorporating nanoparticles on membrane surface or membrane matrix through coating and dope mixing, respectively. It was also reported that UF membranes have become attention in photocatalytic application to treat dye or other recalcitrant compounds contained in wastewater. However, ceramic membrane has micro-size pores and the incorporation of nanoparticles onto ceramic membrane surface will be a great challenge. Another remarkable encounter is that the cost of production of ceramic membrane from alumina is relatively high due to expensive starting materials and requirement of high sintering temperature. Due to this, low cost and green ceramic material from clays and wastes have been used to fabricate low cost ceramic membranes. The most prominent thing here is that these green materials possessed the presence of natural photocatalytic material such as TiO2 in small amount. Therefore, further research and development of dual-layer or triple-layer ceramic membrane that prepared in single step is an interesting topic to be studied towards photocatalytic process of dyes in wastewater.
References
Adham, S., Chiu, K.-P., Lehman, G.: Optimization of Membrane Treatment for Direct and Clarified Water Filtration. American Water Works Association, USA (2006)
Alspach, B., Adham, S., Cooke, T., Delphos, P., Garcia-Aleman, J., Jacangelo, J.: Microfiltration and ultrafiltration membranes for drinking water. J. Am. Water Works Assoc. 100, 84–97 (2008)
Athanasekou, C.P., Moustakas, N.G., Morales-Torres, S., Pastrana-Martínez, L.M., Figueiredo, J.L., Faria, J.L., Silva, A.M.T., Dona-Rodriguez, J.M., Romanos, G.E., Falaras, P.: Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 178, 12–19 (2015)
Babu, J., Murthy, Z.V.P.: Treatment of textile dyes containing wastewaters with PES/PVA thin film composite nanofiltration membranes. Sep. Purif. Technol. 183, 66–72 (2017)
Baker, R.W.: Membrane Technology. Wiley, USA (2000)
Baker, R.W.: Membrane Technology and Applications. Wiley, USA (2012)
Balannec, B., Vourch, M., Rabiller-Baudry, M., Chaufer, B.: Comparative study of different nanofiltration and reverse osmosis membranes for dairy effluent treatment by dead-end filtration. Sep. Purif. Technol. 42, 195–200 (2005)
Bechhol, H.: Kolloidstudien mit der Filtrationsmethode. Z. Physik. Chem. 60, 257–318 (1907)
Bejaoui, I., Mnif, A., Hamrouni, B.: Performance of reverse osmosis and nanofiltration in the removal of fluoride from model water and metal packaging industrial effluent. Sep. Sci. Technol. 49, 1135–1145 (2014)
Bouazizi, A., Breida, M., Achiou, B., Ouammou, M., Calvo, J.I., Aaddane, A., Younssi, S.A.: Removal of dyes by a new nano–TiO2 ultrafiltration membrane deposited on low-cost support prepared from natural Moroccan bentonite. Appl. Clay Sci. 149, 127–135 (2017)
Cheryan, M., Alvarez, J.R.: Food and beverage industry applications. Membr. Sci. Technol. (USA, Elsevier) (1995)
Choudhury, P., Mondal, P., Majumdar, S., Saha, S., Sahoo, G.C.: Preparation of ceramic ultrafiltration membrane using green synthesized CuO nanoparticles for chromium (VI) removal and optimization by response surface methodology. J. Clean. Prod. 203, 511–520 (2018)
Clara, M., Strenn, B., Gans, O., Martinez, E., Kreuzinger, N., Kroiss, H.: Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 39, 4797–4807 (2005)
Crowder, M.L., Gooding, C.H.: Spiral wound, hollow fiber membrane modules: a new approach to higher mass transfer efficiency. J. Membr. Sci. 137, 17–29 (1997)
Daufin, G., Escudier, J.P., Carrere, H., Berot, S., Fillaudeau, L., Decloux, M.: Recent and emerging applications of membrane processes in the food and dairy industry. Food Bioprod. Process. 79, 89–102 (2001)
De Barros, S., Andrade, C., Mendes, E., Peres, L.: Study of fouling mechanism in pineapple juice clarification by ultrafiltration. J. Membr. Sci. 215, 213–224 (2003)
Dilaver, M., Hocaoğlu, S.M., Soydemir, G., Dursun, M., Keskinler, B., Koyuncu, İ., Ağtaş, M.: Hot wastewater recovery by using ceramic membrane ultrafiltration and its reusability in textile industry. J. Clean. Prod. 171, 220–233 (2018)
Ding, Y., Ma, B., Liu, H., Qu, J.: Effects of protein properties on ultrafiltration membrane fouling performance in water treatment. J. Environ. Sci. 77, 273–281 (2018)
Dow, N., Villalobos Garcia, J., Niadoo, L., Milne, N., Zhang, J., Gray, S., Duke, M.: Demonstration of membrane distillation on textile waste water: assessment of long term performance, membrane cleaning and waste heat integration. Environ. Sci. Water Res. Technol. 3, 433–449 (2017)
Fane, A.G., Wang, R., Jia, Y.: Membrane technology: past, present and future. In: Wang, L.K., Chen, J.P., Hung, Y.T., Shammas, N.K. (eds.) Membrane and Desalination Technologies. Humana Press, Totowa (2011)
Fujioka, T., Khan, S.J., McDonald, J.A., Roux, A., Poussade, Y., Drewes, J.E., Nghiem, L.D.: Modelling the rejection of N-nitrosamines by a spiral-wound reverse osmosis system: mathematical model development and validation. J. Membr. Sci. 454, 212–219 (2014)
Galaverna, G., Di Silvestro, G., Cassano, A., Sforza, S., Dossena, A., Drioli, E., Marchelli, R.: A new integrated membrane process for the production of concentrated blood orange juice: effect on bioactive compounds and antioxidant activity. Food Chem. 106, 1021–1030 (2008)
Galiana-Aleixandre, M., Iborra-Clar, A., Bes-Piá, B., Mendoza-Roca, J., Cuartas-Uribe, B., Iborra-Clar, M.: Nanofiltration for sulfate removal and water reuse of the pickling and tanning processes in a tannery. Desalination 179, 307–313 (2005)
Gille, D., Czolkoss, W.: Ultrafiltration with multi-bore membranes as seawater pre-treatment. Desalination 182, 301–307 (2005)
Girard, B., Fukumoto, L.R.: Membrane processing of fruit juices and beverages: a review. Crit. Rev. Food Sci. Nutr. 40(2), 91–157 (2000)
Guinee, T., O’kennedy, B., Kelly, P.: Effect of milk protein standardization using different methods on the composition and yields of Cheddar cheese. J. Dairy Sci. 89, 468–482 (2006)
Han, S., Ferreira, F.C., Livingston, A.: Membrane aromatic recovery system (MARS)—a new membrane process for the recovery of phenols from wastewaters. J. Membr. Sci. 188, 219–233 (2001)
Harun, Z., Hubadillah, S.K., Hasan, S., Yunos, M.Z.: Effect of thermodynamic properties on porosity of ceramic membrane prepared by phase inversion. Appl. Mech. Mater. 575, 31–35 (2014)
Hernández, S., Lei, S., Rong, W., Ormsbee, L., Bhattacharyya, D.: Functionalization of flat sheet and hollow fiber microfiltration membranes for water applications. ACS Sustain. Chem. Eng. 4, 907–918 (2016)
Huang, X.F., Ling, J., Xu, J.C., Feng, Y., Li, G.M.: Advanced treatment of wastewater from an iron and steel enterprise by a constructed wetland/ultrafiltration/reverse osmosis process. Desalination 269, 41–49 (2011)
Hubadillah, S.K., Harun, Z., Othman, M.H.D., Ismail, A.F., Gani, P.: Effect of kaolin particle size and loading on the characteristics of kaolin ceramic support prepared via phase inversion technique. J. Asian Ceram. Soc. 4, 164–177 (2016)
Hubadillah, S.K., Othman, M.H.D., Harun, Z., Ismail, A.F., Iwamoto, Y., Honda, S., Rahman, M.A., Jaafar, J., Gani, P., Mohd Sokri, M.N.: Effect of fabrication parameters on physical properties of metakaolin-based ceramic hollow fibre membrane (CHFM). Ceram. Int. 42, 15547–15558 (2016)
Hubadillah, S.K., Othman, M.H.D., Ismail, A.F., Rahman, M.A., Jaafar, J., Iwamoto, Y., Honda, S., Dzahir, M.I.H.M., Yusop, M.Z.M.: Fabrication of low cost, green silica based ceramic hollow fibre membrane prepared from waste rice husk for water filtration application. Ceram. Int. 44, 10498–10509 (2018)
Hubadillah, S.K., Othman, M.H.D., Matsuura, T., Ismail, A.F., Rahman, M.A., Harun, Z., Jaafar, J., Nomura, M.: Fabrications and applications of low cost ceramic membrane from kaolin: a comprehensive review. Ceram. Int. 44, 4538–4560 (2018)
Hubadillah, S.K., Othman, M.H.D., Matsuura, T., Rahman, M.A., Jaafar, J., Ismail, A.F., Amin, S.Z.M.: Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep. Sci. Technol. 205, 22–31 (2018)
Hubadillah, S.K., Othman, M.H.D., Rahman, M.A., Ismail, A.F., Jaafar, J.: Preparation and characterization of inexpensive kaolin hollow fibre membrane (KHFM) prepared using phase inversion/sintering technique for the efficient separation of real oily wastewater. Arab. J. Chem. (2018)
Jamalludin, M.R., Harun, Z., Hubadillah, S.K., Basri, H., Ismail, A.F., Othman, M.H.D., Shohur, M.F., Yunos, M.Z.: Antifouling polysulfone membranes blended with green SiO2 from rice husk ash (RHA) for humic acid separation. Chem. Eng. Res. Des. 114, 268–279 (2016)
Klemeš, J.J., Varbanov, P.S., Kravanja, Z.: Recent developments in process integration. Chem. Eng. Res. Des. 91, 2037–2053 (2013)
Kumar, P., Sharma, N., Ranjan, R., Kumar, S., Bhat, Z., Jeong, D.K.: Perspective of membrane technology in dairy industry: a review. Asian-Australas. J. Anim. Sci. 26, 1347 (2013)
Lafi, R., Gzara, L., Lajimi, R.H., Hafiane, A.: Treatment of textile wastewater by a hybrid ultrafiltration/electrodialysis process. Chem. Eng. Process. Process Intens. 132, 105–113 (2018)
Lalia, B.S., Kochkodan, V., Hashaikeh, R., Hilal, N.: A review on membrane fabrication: structure, properties and performance relationship. Desalination 326, 77–95 (2013)
Leenaars, A., Keizer, K., Burggraaf, A.: The preparation and characterization of alumina membranes with ultra-fine pores. J. Mater. Sci. 19, 1077–1088 (1984)
Li, K.: Ceramic Membranes for Separation and Reaction. Wiley, USA (2007)
Liu, F., Hashim, N.A., Liu, Y., Abed, M.R.M., Li, K.: Progress in the production and modification of PVDF membranes. J. Membr. Sci. 375, 1–27 (2011)
Loeb, S., Sourirajan, S.: Sea water demineralization by means of an osmotic membrane. In: Saline Water Conversion-II. American Chemical Society (1963)
Loo, S.L., Fane, A.G., Krantz, W.B., Lim, T.T.: Emergency water supply: a review of potential technologies and selection criteria. Water Res. 46, 3125–3151 (2012)
Madaeni, S.S., Koocheki, S.: Application of taguchi method in the optimization of wastewater treatment using spiral-wound reverse osmosis element. Chem. Eng. J. 119, 37–44 (2006)
Miller, D.J., Dreyer, D.R., Bielawski, C.W., Paul, D.R., Freeman, B.D.: Surface modification of water purification membranes. Angew. Chem. Int. Ed. 56, 4662–4711 (2016)
Miron, S.M., Dutournié, P., Thabet, K., Ponche, A.: Filtration of protein-based solutions with ceramic ultrafiltration membrane. Study of selectivity, adsorption, and protein denaturation. C. R. Chim. (2018)
Mohamad, A.W., Ching, Y.N., Gen, H.N.: Ultrafiltration in food processing industry: review on application, membrane fouling, and fouling control. Food Bioprocess Technol. 5, 1143–1156 (2012)
Mohtor, N.H., Othman, M.H.D., Bakar, S.A., Kurniawan, T.A., Dzinun, H., Norddin, M.N.A.M., Rajis, Z.: Synthesis of nanostructured titanium dioxide layer onto kaolin hollow fibre membrane via hydrothermal method for decolourisation of reactive black 5. Chemosphere 208, 595–605 (2018)
Pearce, G.K.: UF/MF Membrane Water Treatment: Principles and Design. Water Treatment Academy Bangkok (2011)
Ravanchi, M.T., Kaghazchi, T., Kargari, A.: Application of membrane separation processes in petrochemical industry: a review. Desalination 235, 199–244 (2009)
Rysstad, G., Kolstad, J.: Extended shelf life milk—advances in technology. Int. J. Dairy Technol. 59, 85–96 (2006)
Saha, N., Balakrishnan, M., Ulbricht, M.: Sugarcane juice ultrafiltration: FTIR and SEM analysis of polysaccharide fouling. J. Membr. Sci. 306, 287–297 (2007)
Saxena, A., Tripathi, B.P., Kumar, M., Shahi, V.K.: Membrane-based techniques for the separation and purification of proteins: an overview. Adv. Colloid Interface Sci. 145, 1–22 (2009)
Shi, X., Tal, G., Hankins, N.P., Gitis, V.: Fouling and cleaning of ultrafiltration membranes: a review. J. Water Process Eng. 1, 121–138 (2014)
Srivastava, H.P., Arthanareeswaran, G., Anantharaman, N., Starov, V.M.: Performance of modified poly(vinylidene fluoride) membrane for textile wastewater ultrafiltration. Desalination 282, 87–94 (2011)
Szymański, K., Morawski, A.W., Mozia, S.: Humic acids removal in a photocatalytic membrane reactor with a ceramic UF membrane. Chem. Eng. J. 305, 19–27 (2016)
Taheran, M., Brar, S.K., Verma, M., Surampalli, R.Y., Zhang, T.C., Valéro, J.R.: Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 547, 60–77 (2016)
Tamime, A.Y.: Membrane Processing: Dairy and Beverage Applications. Wiley, USA (2012)
Tsapyuk, E.A., Medvedev, M.I., Badekha, V.P., Kucheruk, D.D.: Treating wastewater from the production of light-sensitive photographic and cinematographic materials and the treatment of the resulting wastes. J. Water Chem. Technol. 5, 67–72 (1983)
Turken, T., Sengur-Tasdemir, R., Koseoglu-Imer, D.Y., Koyuncu, I.: Determination of filtration performances of nanocomposite hollow fiber membranes with silver nanoparticles. Environ. Eng. Sci. 32, 656–665 (2015)
Van Der Bruggen, B., Vandecasteele, C., Van Gestel, T., Doyen, W., Leysen, R.: A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 22, 46–56 (2004)
Vrouwenvelder, J.S., Graf Von Der Schulenburg, D.A., Kruithof, J.C., Johns, M.L., Van Loosdrecht, M.C.M.: Biofouling of spiral-wound nanofiltration and reverse osmosis membranes: a feed spacer problem. Water Res. 43, 583–594 (2009)
Warsinger, D.M., Chakraborty, S., Tow, E.W., Plumlee, M.H., Bellona, C., Loutatidou, S., Karimi, L., Mikelonis, A.M., Achilli, A., Ghassemi, A., Padhye, L.P., Snyder, S.A., Curcio, S., Vecitis, C.D., Arafat, H.A., Lienhard, J.H.: A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 81, 209–237 (2018)
Wilf, M., Tech, T.: Membrane Types and Factors Affecting Membrane Performance. National Water Research Institute, Fountain Valley, CA (2008)
Yoon, Y., Westerhoff, P., Snyder, S.A., Wert, E.C.: Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membrane Sci. 270, 88–100 (2006)
Yoon, Y., Westerhoff, P., Snyder, S.A., Wert, E.C., Yoon, J.: Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 202, 16–23 (2007)
Zsigmondy, R., Bachmann, W.: Über neue Filter. Z. Anorg. Allg. Chem. 103, 119–128 (1918)
Zsigmondy, R., Wilhelm, B.: Filter and method of producing same. United State Patent Application (1916)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Othman, M.H.D., Adam, M.R., Pauzan, M.A.B., Hubadillah, S.K., Rahman, M.A., Jaafar, J. (2020). Ultrafiltration Membrane for Water Treatment. In: Inamuddin, Boddula, R., Asiri, A. (eds) Self-standing Substrates. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-29522-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-29522-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29521-9
Online ISBN: 978-3-030-29522-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)