Abstract
Treatment of diseases using conventional drugs is often limited by their low bioavailability, short circulation half-lives, poor solubility, and nonspecificity which results in high-dosage requirements. The high dosage of drug molecules results in higher toxicity, increasing the side effects of the conventional drugs used for treatment of diseases. Nanomedicine is the use of nanotechnology for healthcare with clinical applications ranging from disease diagnosis to formulation of carriers for drug and gene delivery applications. Use of nanotechnology-based delivery vehicles, such as nanoparticles, nanocapsules, micelles, or dendrimers, has emerged as a promising strategy to deliver conventional drugs, recombinant proteins, vaccines, and, more recently, genetic material by addressing the problems related to poor solubility, high toxicity, nonspecific delivery, in vivo degradation, and short circulation half-lives of the conventional drugs, which often limits optimal dosage at the target site. The rapidly growing nanomedicine industry not only caters to the treatment of various diseases including cancer, pain, asthma, multiple sclerosis, and kidney diseases but also helps in differentiating normal and diseased cells. Metallic, polymeric, semiconductor, and magnetic nanoparticles have been employed in engineering nanostructures that are increasingly being employed for disease diagnosis. While the unique optical, magnetic, and size-dependent properties of nanoparticles make them suitable candidates for disease diagnosis, their ability to undergo surface modification with polymers, antibodies, or aptamers helps in increasing their circulation time and reduces their potential toxicity. Conjugation of these nanoparticles with aptamers has been utilized for development of sensors with fluorescence, optical, and electrochemical detection signals which are sensitive, highly specific, reusable, and label-free. Nanostructures have improved medical diagnosis by providing inexpensive, reproducible, sensitive, and highly specific methods for disease diagnosis either in terms of sensors or as imaging agents. Nanomedicine not only includes the fields of therapeutics and diagnostics but also involves development of implantable materials and devices. Despite the innumerable advantages of nanostructures in the field of nanomedicine, only a handful of products have been able to reach the market due to several disadvantages that these magic bullets are associated with including toxicity of the said materials. However, maintenance of a balance between the advantages and disadvantages would definitely open up avenues for personalized medicine through therapeutics, diagnostics, and theranostics. The present chapter discusses the current state-of-the-art materials used in nanomedicine for disease diagnosis or treatment, problems associated with them, and future prospects of nanomedicine toward personalized medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Nanomedicine is the use of nanotechnology for healthcare with clinical applications ranging from disease diagnosis to formulation of carriers for drug and gene delivery applications. It is often regarded as the use of unique nanoscale properties which includes transition in physicochemical properties and physiological interactions of the nanostructured materials, i.e., structures with at least one dimension up to 300 nm, for human health. Nanostructures have a greater surface area to volume ratio which increases their carrying capacity and possibility of surface modifications. They exhibit unique size-dependent properties which are fairly distinct from those of bulk material, for example, 20 nm gold nanoparticles exhibit red color which changes to blue on increasing their size. The unique optical properties of nanoparticles make them suitable candidates for optical imaging. The use of nanostructures as drug carriers offers several advantages including higher stability, ability to incorporate both hydrophobic and hydrophilic drugs, and ability of controlled drug release. Moreover, nanostructures can be administered through various routes including inhalation, oral, intravenous, or intramuscular, reducing the intrusions and hence increasing patient compliance. The unique advantages of nanostructures including targeted delivery of drug, reduced side effects of free drug molecules, increased bioavailability, smaller and highly sensitive diagnostic tools along with reduced degree of invasiveness have resulted in a new field of medicine, “nanomedicine.”
The first nanomedicine which was a liposomal formulation of anticancer drug doxorubicin was introduced in the year 1995, and since then, about 50 other nanostructure-based drugs have entered clinical practice (Min et al. 2015). A plethora of nanostructures including nanoparticles, nanocapsules, liposomes, micelles, dendrimers, and quantum dots are being used as nanomedicines for the diagnosis and treatment of a wide variety of diseases (Fig. 9.1) including cancer, infectious diseases, multiple sclerosis, chronic pain, asthma, and emphysema (Hobbs et al. 1998). Nanomaterials are being used not only for drug and gene delivery applications but also for detection of pathogens (Edelstein et al. 2000), for tissue engineering (Ma et al. 2003; de la Isla et al. 2003), as contrast agents (Weissleder et al. 1990), for tumor destruction (Shinkai et al. 1999), and also as fluorescent biological labels (Bruchez et al. 1998; Chan and Nie 1998). According to a recent review on nanomedicine, by 2013, about 247 nanomedicine products were approved or were in various stages of clinical study (Etheridge et al. 2013). Nanomedicine has become a vast industry in a small span of just two decades due to its ability to address several issues related to poor solubility, high toxicity, nonspecific delivery, in vivo degradation, and short circulation half-lives of the conventional drugs, which often limits optimal dosage at the target site (Gelderblom et al. 2001). Nanostructures used for diagnosis due to their distinct optical, magnetic, and structural properties offer several advantages over the traditional diagnosis methods including but not limited to minimal invasiveness, simplicity, low-detection limits, rapid analysis at room temperature, and capability of in situ analysis (Jyoti and Tomar 2017).
Current application of nanostructures in the nanomedicine arena includes development of (a) implantable materials for tissue engineering including repair and replacement, (b) implantable devices including implantable sensors and surgical aids, (c) diagnostic tools for disease detection, and (d) biopharmaceuticals for drug and gene delivery (Agrawal 2016). A wide variety of nanomedicines are available commercially or are in clinical trials to treat diseases including breast cancer, non-small cell lung cancer, pancreatic cancer, ovarian cancer, and multiple myeloma as well as diagnostic tools to detect disease-causing pathogens or to locate the tumor location (Agrawal 2016).
Nanostructures have presented themselves as promising nano-therapeutic agents, i.e., a combination of diagnosis and therapy. While the optical and magnetic properties of nanostructures make them great imaging agents, simultaneous drug loading in these nanostructures could help cure the disease at the same time. Nanotheranostics open up new avenues for personalized medicine. While an imaging agent can assist localization in the diseased area, its amalgamation with a therapeutic drug would help in treatment of the disease. The imaging nanostructure could also be utilized to monitor the effectiveness of the therapy in being site specific while predicting the side effects (accumulation in healthy cells) at the same time. Despite the promising potential of nanostructures in diagnosis and treatment of diseases, the technical developments in nanomedicine raise concerns related to the safety of the said materials, making toxicity of nanomaterials a major concern for their future developments. Moreover, the effects of these nanostructures on the biochemical pathways are yet unknown.
The current chapter discusses the application of different nanostructures in the field of nanomedicine for diagnostic as well as therapeutic purposes, followed by a brief overview of the drawbacks that these magic bullets face and the future directions to expand the nanomedicine market.
9.2 Nanostructures for Disease Diagnosis
A biosensor is a chemical sensor which comprises of a biological recognition element that recognizes the analyte and transducer which transmits the signal. Different biological interactions, e.g., antigen-antibody or antigen-aptamer, are utilized to generate signals such as optical, electrochemical, thermal, or piezoelectric that help in the detection of different disease-causing agents such as bacteria or virus. Nanostructures have high surface area per unit volume which locates all the constituent atoms near the surface leading to different physicochemical properties at nanoscale compared to the bulk solid. They have high electrical conductivity, better mechanical shock-bearing ability, show piezoelectric effect and color depending on the size of the nanoparticles. Features of different nanostructures like high electrical conductivity, colorimetric properties, and strong mechanical strength allow them to be suitable for conjugation with aptamers and antibodies in biosensing applications. Nanotubes (e.g., carbon nanotubes), nano-wires, nanorods, metallic nanoparticles (e.g., gold), quantum dots, and thin films made up of nanocrystalline matter are some widely used nanostructures for nano biosensing applications.
The emerging synergy between nanotechnology and biosensors has been utilized over the past few years due to their potential to recognize threat agents in real time and also perform their detection with extremely high sensitivity and selectivity. Nanostructures-based sensors help in rapid, sensitive, easy, and cost-effective detection of different biological molecules including disease-causing pathogens, proteins expressed in different cancer cells, blood analytes such as glucose, cholesterol, uric acid, and albumin along with distinguishing normal versus cancer cells (Manoharan et al. 2018). Such sensors either use antigen-antibody interactions or functional nucleic acids as bio-recognition elements. Functional nucleic acids including aptamers, DNAzymes (catalytic DNA that can catalyze biochemical reaction in the presence of cofactors), and Aptazymes (a combination of aptamer and DNAzyme) (Li and Lu 2000; Jhaveri et al. 2000; Yi 2002; Navani and Li 2006), due to ability of in vitro production and higher selectivity are now rivaling the antibodies (Jayasena 1999). The following section discusses different nanostructures including metallic, polymeric, or magnetic nanoparticles, quantum dots, and aptamer-nanoparticle conjugates used for molecular diagnostics.
9.2.1 Metallic Nanoparticles for Diagnostics
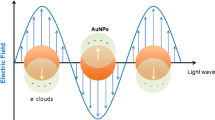
The most widely used nanostructures for disease diagnosis are metallic nanoparticles made up of gold, silver, or metal oxides such as titanium dioxide. Gold and silver nanoparticles exhibit surface plasmon resonance which results in sharp, intense absorption band in the visible range, making them suitable candidates for development of optical nanosensors. Moreover, the properties exhibited by these nanoparticles can be tailored by controlling their size and structure. Gold and silver nanoparticles detect different disease-causing analytes, such as antigens, nucleic acids, or aptamers, based on interparticle distance. Reduction in the interparticle distance results in aggregation of particles which can be detected by a visible color change mainly red to purple or blue in case of gold nanoparticles (Fig. 9.2) and yellow to brown for silver nanoparticles, making them suitable candidates for visible optical detection (Boisselier and Astruc 2009). These particles are often surface modified to increase circulation time (generally with polyethylene glycol) and achieve targeted diagnosis (with antibodies or aptamers).
Surface-modified gold nanoparticles have been employed for detection of Mycobacterium tuberculosis and Staphylococcus aureus based on the aggregation principle. In presence of the microorganisms bound to the particles, they are unable to come together and aggregate (no color change), whereas the absence of microorganism results in visible aggregation in the form of color change on addition of sodium chloride (NaCl) (Baptista et al. 2006; Huang 2007). Gold nanorods, surface modified with polyethylene glycol and covalently attached to monoclonal antibody ‘Herceptin’ for targeting breast cancer cells, have demonstrated their potential in vitro as well as in vivo in tumor-bearing nude mice (Eghtedari et al. 2009). The most common use of metallic nanoparticles is for detection of hydrogen peroxide (H2O2), the most extensively studied reactive oxygen species in medicine, overproduction of which is associated with many diseases including cardiovascular diseases, diabetes, neurodegeneration, cancer, and aging. Recent advances in use of metallic nanoparticles have focused on development of bimetallic sensors. Zhang et al. (2016) combined the advantages of nano gold and silver to prepare core-shell nanoparticles for detection of H2O2 in human fluids. The developed nanosensor exhibited greater stability provided by nano silver and higher surface plasmon effect displayed by nano gold. Gold nanoparticles exhibit a surface plasmon peak at 520 nm (wine red), whereas the developed bimetallic sensor exhibits a surface plasmon peak at 375 nm (orange). In the presence of H2O2 and glucose or cholesterol, a redox reaction results in reduction of silver. As a consequence, only gold nanoparticles are left in the solution, shifting the absorbance peak to 520 nm (wine red). The shift in peak can then be quantified to determine the concentration of H2O2 or cholesterol in the fluid being tested (Zhang et al. 2016). Other bimetallic sensors being explored for sensing of peroxide, glucose, and cholesterol include Au/Pt (Che et al. 2009; Yanyan et al. 2011), Pt/Pd, and TiO2/graphene-supported Pt/Pd nanocomposites (Cao et al. 2013; Safavi and Farjami 2011).
Though metallic nanoparticles constitute the major market in disease diagnosis nanostructures made from polymers, magnetic material and semiconductors (quantum dots) discussed below are also being increasingly employed for effective disease diagnosis.
9.2.2 Polymeric Nanoparticles for Diagnostics
Synthesis of nanostructures using polymers such as polypyrrole and polyaniline for different sensing applications has recently gained attention due to their greater biocompatibility and biodegradability. Conducting properties of polypyrrole, carboxylated polypyrrole, polyaniline, and poly (3, 4-ethylenedioxythiophene) were employed by Park et al. (Park et al. 2016) and Song et al. (Song et al. 2013) to develop bioelectronic-based nanosensor for detection of hydrogen peroxide (H2O2). Hydrogen peroxide is a reactive oxygen species which is essential for cell growth and is thus related to several diseases including cancer and Alzheimer’s. Polypyrrole-coated silver nanostrips integrated in an electrochemical sensor have also been developed for catalytic detection of H2O2. The enlarged surface area of silver nanostructures has been reported to facilitate rapid sensing (response time less than 5 s) and reducing minimum detection limit (Mahmoudian et al. 2014). Glucose sensing, important for monitoring diabetes, has been done using polymeric nanoparticles by employing a hybrid nanostructure entrapping glucose oxidase and gold nanoparticles in polyaniline layers. Gold nanoparticles were reported to assist polyaniline layer formation and the developed biosensor consisting of glucose oxidase as catalyst resulted in formation of hydrogen peroxide which was used as an indicator for presence of glucose (Mazeiko et al. 2013).
9.2.3 Magnetic Nanoparticles for Diagnostics
Magnetic nanoparticles are being used as a versatile diagnostic tool in nanomedicine. They are attached to antibodies or aptamers specific to the target molecule or microorganism, which on binding to specific targets result in magnetic signals that can be detected in the presence of magnetic field using a sensitive magnetometer. Magnetite, iron, nickel, and cobalt nanoparticles possess superparamagnetic properties at nanoscale, making them suitable candidates for being used as diagnostic tools. However, these nanoparticles are easily oxidized and tend to aggregate.
Prevention of oxidation and aggregation of magnetic nanoparticles has been achieved by constructing nanocomposites employing polymers on the outer layers. Iron nanoparticles of 15–20 nm have been embedded in beads of styrene and glycidyl methacrylate, resulting in 100–200 nm nanocomposites which are stable, exhibit a zeta potential of −58.4 mV, and show superparamagnetic properties that have been used for tracking cells and also for calcium sensing. Such nanoparticles at a size range of 2–3 nm have been used to diagnose undetectable lymph nodes (Atanasijevic et al. 2006). Polymer coatings also prevent nonspecific protein adsorption, an important requisite to biosensing (Jain 2007). Dextran-coated iron oxide nanoparticles have also been used to enhance visualization of intracranial tumors in magnetic resonance imaging. CellTracks are commercially available ferrofluids, i.e., a magnetic core surrounded by a polymer layer which has antibodies attached for capturing cells. The conjugated antibodies bind to the antigen on target cells. This commercially available ferrofluid has been used to detect circulating cancer cells and also for isolation of bacteria, making it easier to manage patients with serious infections (Manoharan et al. 2018). The toxicity of magnetic nanoparticles is determined by the constituents of the particle including the magnetic core (e.g., magnetite, iron, nickel, and cobalt), the outer polymeric shell, along with the shape and size of the particle.
9.2.4 Quantum Dots for Diagnostics
Quantum dots or semiconductor nanoparticles have unique optical properties including simple, broad excitation range with a very narrow or sharp emission with the emitted wavelength being dependent on size and composition of quantum dots. These molecules exhibit size-dependent properties because of the presence of “bandgap.” Bandgap is defined as the difference in the energy levels of the valence band, the primary residence of electrons, and the conduction band, the energy level to which the electrons reach after excitation. The movement of electron from conduction band to valence band when the excitation is stopped results in release of energy in the form of light. The high sensitivity, simple instrumentation, and higher photostability in comparison to organic or inorganic fluorescent dyes make them suitable for various diagnostic applications including tagging viruses and cancer cells. They can also be easily attached to cells, proteins, and nucleic acids making them powerful tagging agents. The availability of quantum dots in red and infrared colors enables whole blood analysis.
Carbohydrate-encapsulated quantum dots have been successfully employed in imaging of cancer. Immunofluorescent labeling of breast cancer marker Her2 has been achieved using polyacrylate-coated quantum dots that are covalently linked to antibodies (Jain 2007). Cell surface proteins of respiratory syncytial virus have been detected using quantum dots linked to antibodies (Bentzen et al. 2005). Dual color quantum dots, capable of excitation with a single light source, have also been developed for detection of respiratory syncytial virus within few hours. Respiratory syncytial virus while infecting lungs leaves its coat containing F and G proteins on the cell’s surface. Antibody-linked quantum dots when come in contact with viral particles or infected cells stick to their surface assisting their diagnosis during the course of infection itself. However, the most commonly used quantum dots made of CdSe (cadmium selenide)-ZnS (zinc sulfide) release potentially toxic cadmium and zinc ions into the cells. The toxicity of such quantum dots can be prevented by capping them with zinc oxide which prevents Cd2+ formation on exposure to air (Jain 2007).
The synergy between metallic, polymeric, semiconductor, and magnetic nanoparticles has contributed in development of several nanomedicines for disease diagnosis. While the unique optical, magnetic, and size-dependent properties of nanoparticles make them suitable candidates for disease diagnosis, their ability to undergo surface modification with polymers, antibodies, or aptamers helps in increasing their circulation time and reduces their potential toxicity. Conjugation of these nanoparticles with aptamers has helped in development of more robust diagnostic tools which have been discussed in the following section.
9.2.5 Aptamer Nanoparticle Conjugates for Diagnostics
The current decade witnessed many advancements in aptamer-nanoparticle conjugates for diagnostic applications (Sharma et al. 2017; Dhiman et al. 2017; Sharma et al. 2016; Sharma and Shukla 2014). Aptamers modified with nanomaterials have great potential to be used in clinics for diagnostics purposes (Kalra et al. 2018; Kaur et al. 2018; Chopra et al. 2014). The conjugate can bind with a broad range of diverse targets ranging from small molecules and proteins to intact viruses and whole cells. Aptamers have been selected for small molecules like cocaine, aspartame, growth factors, toxins, peptides, viral proteins, and bacterial cells. Based on the transduction principle, the diagnostic and imaging applications can be further categorized into electrochemical, colorimetric, fluorescence, or magnetism based methods outlined in the following subsections.
9.2.5.1 Fluorescence-Based Sensors
The unique optical properties of quantum dots or semiconductor nanoparticles make them suitable for use in conjugation with aptamers for diagnostic and imaging applications. The first reported use of quantum dots in conjugation with aptamer was done by Levy et al. (Levy et al. 2005). In this work, they detected thrombin using “Fluorescence Resonance Energy Transfer (FRET)” principles (Fig. 9.3) (Lee et al. 2010). Thrombin aptamers were functionalized on quantum dots, and the complementary nucleotide strand had a quencher at the end. In the presence of thrombin in the test sample, the single-strand aptamer bound to thrombin and the complementary DNA-containing quencher were released that lead to fluorescence of quantum dot (Levy et al. 2005). In advancement of the above method, Choi et al. (2006) modified the sensor where interaction of thrombin with aptamers brought them close to quantum dots that resulted in selective quenching of fluorescence due to the charge transfer occurring from thrombin to the quantum dots with a limit of detection of 1 mM (Choi et al. 2006).
Aptamer-quantum dot-based fluorescent sensors. (a) Thrombin aptamer is conjugated onto quantum dot and hybridized to a complementary DNA with a quencher. Fluorescence of quantum dot is quenched due to energy transfer from the quantum dot to the quencher. The complementary DNA containing the quencher can be released after introduction of thrombin, inducing recovery of the fluorescence from quantum dot. (b) Thrombin aptamers are conjugated to quantum dots. The fluorescence of quantum dots can be quenched as thrombin binds to aptamer due to the charge transfer from thrombin to quantum dot. [Adapted from Lee, Yigit (Lee et al. 2010) with permission from Elsevier]. (QD-quantum dot)
Meng et al. (Meng et al. 2016) devised a FRET-based biosensor for sensitive determination of adenosine triphosphate (ATP) using aptamers. In this setup, 5′-carboxyfluorescein (5′-FAM), gold nanoparticles were used as energy donor and acceptor, respectively, and were conjugated with complementary single-stranded DNA. This aptasensor was developed by hybridization between the complementary DNA strands of both donor and acceptor molecules. The setup could detect ATP in the linear range of 0.1–100 μM with limit of detection 15.2 nM. In the presence of specific ATP as the target, the FRET frequency of this detection system was gradually increased while nonspecific targets like uridine. Cytidine and guanosine triphosphates had no such significant changes in frequency (Meng et al. 2016). In order to reduce background fluorescence, enhance regeneration and long-term storage, the fluorophore-labeled DNA could be immobilized on a solid surface.
While quantum dots conjugation with aptamers has been used for fluorescence based sensors, metallic nanoparticles have been conjugated with aptamers for developing colorimetric and electrochemical based diagnosis methods discussed in the following sections.
9.2.5.2 Colorimetric Sensor
Colorimetric properties of nanoparticles are a function of their size and complexity. The most commonly used nanoparticles for colorimetric detection are gold and silver nanoparticles. Gold nanoparticles have a very high extinction coefficient. As discussed earlier, dispersed gold nanoparticles show reddish color while aggregated show blue color due to surface plasmon resonance shift to a higher wavelength (Mirkin et al. 1996). Chang et al. (2013) demonstrated the detection of platelet-derived growth factor (PDGF) using gold nanoparticles-aptamer bioconjugate by colorimetric detection (Fig. 9.4). The detection was based on base stacking effect coupled with unmodified gold nanoparticles as indicator. In the presence of target protein which binds to aptamer probe, followed by base stacking effect that resulted in a favourable and stable interaction between the aptamer and the capture probes. Further, capture probes dissociation from the gold nanoparticles surface induced their aggregation, while in the absence of PDGF, both the aptamer and the capture probe coexisted in solution because the complementary sequences were short (8 bp only). This label-free sensitive colorimetric sensor can detect up to 6 nM (Chang et al. 2013). Colorimetric sensing being more sensitive, many research groups focused on the development of nanoparticle-aptamer conjugate for the detection of biomarkers for a disease or disease-causing organism or even whole cell of infection agent. More recently, our group has developed a “turn-on” aptasensor based on the peroxidase-like activity of gold nanoparticles (regarded as NanoZyme) for the detection of small molecules like kanamycin and acetamiprid, a neurotoxic pesticide (Weerathunge et al. 2014; Sharma et al. 2014). This approach gives a highly specific colorimetric output owing to the specific interaction of aptamer to its cognate target. This strategy yields highly sensitive detection down to 0.1 ppm acetamiprid and ~1.5 nM kanamycin (Sharma et al. 2014). Being a generic approach, this strategy may also be used for the detection of other complex targets like whole cells.
(a) Schematic of the PDGF-BB detection design. (b) Absorption spectra for the aptasensor in the presence of specific and nonspecific proteins in 1X phosphate buffer saline. The concentrations of all proteins were 50 nM. Inset: Photographic images of the corresponding solutions. [Adapted from Chang, Wei (Chang et al. 2013) with permission from Elsevier]. (PDGF platelet-derived growth factor, BB aptamer probe, IFN-γ interferon γ, BSA bovine serum albumin, NaCl sodium chloride, Abs absorbance)
Optical sensors exhibit high sensitivity and specificity, but their sensitivity is reduced in presence of a colored sample which interferes with the detection mechanism. The diagnostic tool often employed for detection of analytes in colored samples such as blood is based on electrochemical sensing discussed as follows.
9.2.5.3 Electrochemical Sensor
For colored samples, electrochemical sensors are indispensable. Blood sample being colored can be easily monitored using electrochemical sensors. Lai et al. (2007) reported the detection of PDGF using an electrochemical, aptamer-based sensor directly in unmodified blood serum. They claimed that the sensor being very sensitive and highly selective could “detect the BB variant of PDGF at 1 nM directly in undiluted, unmodified blood serum and at 50 pM (1.25 ng/mL) in serum-diluted 2-fold with aqueous buffer” (Lai et al. 2007). Compared to optical detection methods, this approach has improved the detection limit by four orders of magnitude. Further, the aptamer-based sensing method is reusable, label-free, and electronic. These features encourage the implementation of electrochemical sensors in portable microdevices to be used on-site use for detection of protein and small molecules in clinical samples.
In a very interesting work, Shukoor et al. (2012) developed Boolean logic operations for the detection of PDGF and vascular endothelial growth factor (VEGF) (Fig. 9.5). “In this work, gold nanoparticles perform Boolean logic operations in response to two proangiogenic targets important in cancer diagnosis and treatment: PDGF and VEGF. In the absence of protein target, gold nanoparticles are initially dispersed as a red solution; the addition of target proteins causes nanoparticle aggregation, turning the solution blue, as well as the release of dye-labeled aptamer probes, which causes an increase in fluorescence. These outputs constitute an AND or OR gate for simultaneous protein detection. We believe this logic-gate-based detection system will become the basis for novel rapid, cheap, and reliable sensors for diagnostic applications” (quoted with permission from ACS). The developed sensor could spectrophotometrically detect protein concentrations as low as 1 nM; however, a clear visible color change from red to purple was observed at a protein concentration of 25 nM (Shukoor et al. 2012). The logic-based detection method due to its high specificity and sensitivity presents itself as a promising diagnostic tool.
(a) Scheme 1. (Top) Two Types of Nanoparticles That Make up the Nanoparticle Logic Gate with No Target in the System and Each Aptamer (Purple and Orange) Protecting Its Complementary DNA. (Bottom) Components Used in the System. (b) Scheme 2. Schematic of Gold Nanoparticle-Based OR (Top) and AND (Bottom) Logic Gate Designs for Fluorescence Detection and Colorimetric Output Mode, Respectively. [Adapted from Shukoor, Altman (Shukoor et al. 2012) with permission from American Chemical Society (ACS)]
Thus, the conjugation of nanostructures with aptamers has been utilized for development of sensors with fluorescence, optical, and electrochemical detection signals. Aptamer-nanoparticles-based sensing method is sensitive, highly specific, reusable, and label-free. It presents itself as a promising diagnostic tool for the future. Apart from being wonderful sensors, nanoparticles have been successfully used to assist diagnostic imaging done by sophisticated techniques including magnetic resonance imaging, computed tomography, ultrasound, optical imaging, and photoacoustic imaging, as well as positron emission tomography and single photon emission computed tomography as pointed out in the subsequent section.
9.2.6 Nanostructures in Imaging
The unique properties of materials at the nanoscale make them suitable candidates for being explored to assist noninvasive imaging techniques such as magnetic resonance imaging, computed tomography, ultrasound, optical imaging, and photoacoustic imaging, as well as positron emission tomography and single photon emission computed tomography. The requisite properties of nanoparticles to make them suitable as contrast enhancement agents include high site specificity and appropriate blood circulation half-lives. While particles <5 nm have a very short circulation half-life due to uptake and clearance by the reticuloendothelial system, those >1 μm might have a very large circulation half-life due to no clearance or uptake by reticuloendothelial system increasing the background signal (Kiessling et al. 2014). Among the wide variety of nanomaterials available, iron oxide nanoparticles have been used as contrast agents in magnetic resonance imaging to either monitor gene expression or to detect cancer, inflammation, arthritis, or atherosclerotic plaques (Moghimi et al. 2005). Also, quantum dots have been employed for antigen, receptor, and enzyme imaging along with tracking of metastatic tumor cell extravasation (Akerman et al. 2002; Voura et al. 2004; Dahan et al. 2003; Wu et al. 2002).
Nanoparticle-aptamer conjugates have also been used in imaging methods such as magnetic resonance imaging and surface-enhanced Raman scattering (SERS). SERS imaging is based on “Raman scattering” which can be defined as the inelastic scattering of a photon by an excited molecule. Raman scattering can be amplified by employing SERS phenomenon. Wang et al. (2007) have shown the detection of α-thrombin by SERS. Two aptamers were selected for two binding sites of thrombin. Here in this work, they first immobilized aptamer 1 on a substrate and then treated with thrombin followed by gold nanoparticles functionalized with aptamer 2 and SERS reporter. The authors claimed the detection limit to be around 0.5 nM. Advantage of SERS is that it can be used for in vivo imaging also. Polyethylene glycol-modified nanoparticle-aptamer bioconjugate can be used for targeted imaging of tumors (Qian et al. 2007; Keren et al. 2008). Table 9.1 summarizes the use of different nanostructures for a wide variety of noninvasive imaging techniques used for diagnosing different pathophysiologies.
Therefore, nanostructures have improved medical diagnosis by providing inexpensive, reproducible, sensitive, and highly specific methods for disease diagnosis either in terms of sensors or as imaging agents. While a large variety of products have already reached market (Table 9.2), many are in different phases of clinical trials (Caster et al. 2017) or are being developed at laboratory scale to provide different tools and hence help understand the mechanisms in which normal and diseased cells differ, along with detecting disease-specific analytes. The magnificent properties of nanostructures have not only advanced nanodiagnostics, but the treatment of diseases has been explicitly enhanced by nanostructure-based delivery agents described in the rest part of the chapter.
9.3 Nanostructures for Drug and Gene Delivery
Nanostructures offer several advantages over conventional drug therapies, making them suitable candidates for drug and gene delivery applications. The small size of nanostructures, 5–100 nm, makes them suitable for staying in circulation for a longer time and increasing cellular uptake by various cells, thus increasing the probability of reaching the target site. Moreover, the size of nanostructures also makes them suitable candidates for enhanced permeability and retention effect which is helpful in treating cancer due to the leaky tumor vasculature (Kumar 2012; Greish 2010). The higher surface area to volume ratio of nanostructures increases drug dissolution, resulting in the drugs being effective at low dosage. Moreover, the bioavailability of drugs is increased by their association with nanostructures as they are protected against degradation while in circulation. Nanostructures also help in improving the pharmacokinetics of poorly water-soluble or insoluble drugs (Dreaden et al. 2012). Since conventional drug therapies involve systemic application of drugs, they do not just damage the diseased cells but even harm the healthy ones. The abovementioned issues can be circumvented by using different nanostructures such as micelles or liposomes and nanoparticles made of polymers, lipids, or inorganic material for drug or gene delivery. Targeted delivery of the therapeutic molecule by attaching antibodies and aptamers helps in specific and selective delivery, increasing the effectiveness of the drug. With targeted drug delivery, the concentration of the therapeutic molecule increases at the desired sites of action while other tissues remain unaffected. The following sections discuss applications of different nanostructures as antimicrobials for treatment of cancer, pain, asthma, multiple sclerosis, and kidney diseases.
9.3.1 Nanostructures for Cancer Treatment
Conventional therapies to treat cancer generally involve use of radiation and chemotherapy which cause significant damage to healthy cells as well. Nanostructures in the form of polymeric, lipid, inorganic, or magnetic nanoparticles are being extensively employed to treat cancer by active or passive targeting. The first liposomal formulation encapsulating anticancer drug Doxil was approved by Food and Drug Administration in 1995. Since then, a plethora of drugs including amphotericin B, daunorubicin, and morphine have been encapsulated in liposomes and are being marketed as commercial products (Table 9.3). Encapsulation of commonly used anticancer drug paclitaxel in albumin nanoparticles (130 nm) resulted in reducing the side effects (Micha et al. 2006) of the drug administered systematically along with higher drug dosing at tumor site (Ibrahim et al. 2002). Nanostructures have also been used for multiple drug therapy, for example, polymer poly-(lactic-co-glycolic) acid (PLGA) was used to encapsulate drug doxorubicin (chemotherapeutic) in liposomes composed of phospholipids conjugated with polyethylene glycol and drug combretastatin (antiangiogenic) (Sengupta et al. 2005). The synthesized nanostructures, 80–100 nm in diameter, assist control release due to slow degradation of PLGA along with increased residence time due to polyethylene glycol conjugation (Harris and Chess 2003), thus increasing the effectiveness of drugs by enhanced permeation and retention effect. Recently, Hrkach and Von Hoff (2012) reported synthesis of polylactic acid-PLGA nanoparticles containing anticancer drug docetaxel, which were targeted to prostate-specific membrane antigen. The synthesized nanoparticles were tested for their efficacy in mouse tumor models, and their pharmacokinetics was tested in mice, rats, monkeys, and humans.
Cationic liposomes, solid lipid nanoparticles are also being used to deliver nucleic acid-based therapeutics including small interfering RNA, antisense oligonucleotides, and aptamers (Farokhzad and Langer 2006). Active targeting of different nanostructures often involves attaching receptor molecules such as antibodies and aptamers on their surface that bind with antigens/molecules expressed on cancer cells (Kumar 2012). Different nanostructures being marketed for use in cancer treatment are summarized in Table 9.3 and those approved for different clinical phase trials in Tables 9.4 and 9.5. While cancer is the major target of most of the nanostructures being developed, nanostructures are also being engineered for treatment of diseases caused by microbial infections.
9.3.2 Nanostructures as Antimicrobials
Nanostructures not only act as delivery vehicles for delivery of different antimicrobials, but polymeric and metallic nanoparticles have themselves demonstrated antimicrobial activity (Caster et al. 2017; Sharma et al. 2012a, b). The renal and neurological toxicity of antibiotic aminoglycoside, a drug useful for treating multidrug-resistant tuberculosis and gram-negative bacteria, was prevented by encapsulating the drug in a liposomal formulation, which also increased the circulation time of the drug (Caminero et al. 2010; Canton et al. 2005; Meers et al. 2008). Inhalable liposomal formulation for drug-resistant pseudomonas infections is under clinical trials to treat patients with cystic fibrosis. Amphotericin B, an antifungal agent, is also being marketed as a liposomal formulation (Larson et al. 2000). Various dendrimeric and polymeric formulations containing antibiotic, antibacterial, and antifungal drugs are being developed to prevent these infections. Different polymeric and metallic nanoparticles are themselves being used as antimicrobials. While quaternary ammonium polyethyleneimine, a highly charged polymer molecule, has been employed to disrupt the membrane of gram-positive and gram-negative bacteria (Ortega et al. 2015), nano silver has been used to induce toxic effects. Nano silver kills bacteria by release of ions that can easily penetrate the organism. In a recent study by Sharma and Sapra (Sharma et al. 2012b), silver nanoparticles were functionalized in a single step with an antibacterial peptide, enterocin, from a food-grade lactic acid bacterium. The synthesized nanoparticles exhibited broad-spectrum inhibition without any detectable toxicity to red blood cells. Inhibition in the growth of Staphylococcus aureus and Escherichia coli was determined using scanning electron microscope and has been depicted in Fig. 9.6 (Sharma et al. 2012b).
Interaction of enterocin-capped silver nanoparticles with bacteria as observed through scanning electron microscope. Upper panel, Staphylococcus aureus (a) untreated, (b) treated with citrate-capped silver nanoparticles, and (c) treated with enterocin-capped silver nanoparticles. Lower panel, Escherichia coli (d) untreated, (e) treated with citrate-capped silver nanoparticles, and (f) treated with enterocin-capped silver nanoparticles (Sharma et al. 2012b)
Protein-based nanoparticles containing respiratory syncytial virus fusion protein have been developed to treat respiratory syncytial virus. Different nanostructures being marketed for use in treatment of infectious diseases are summarized in Table 9.3 and those approved for different clinical phase trials in Tables 9.4 and 9.5.
9.3.3 Nanostructures for Other Diseases
Nanostructures are also being explored for increasing the efficacy of drugs associated with ophthalmic conditions, pain management, psychological illness, metabolic disorders, as well as neurological, cardiovascular, respiratory, and autoimmune diseases. These have been discussed briefly in the subsequent paragraphs.
Blood-brain barrier is one of the major turning stones in treating diseases associated with central nervous system. Blood-brain barrier protects the brain cells by not allowing foreign substances to enter and thus prevents therapeutic molecules to enter. As a consequence, the amount of drug administered is very high, resulting in adverse side effects. However, nanoparticles have demonstrated the ability to cross the blood-brain barrier and effectively deliver the drugs to brain cells. Doxorubicin is unable to cross the blood-brain barrier when given directly; however, its association with polysorbate 80-modified polybutylcyanoacrylate nanoparticles facilitates its delivery to the brain (Gulyaev et al. 1999). An analgesic drug dalargin was delivered to the central nervous system by adsorbing onto poly(butyl cyanoacrylate) (PBCA) nanoparticles (Kreuter et al. 2003). PBCA has also been used to treat Alzheimer’s disease (Siegemund et al. 2006). Other polymers that have been employed include poly(hexadecyl cyanoacrylate) with polyethylene glycol, poly(propylene oxide), and P-85 polymer. Polyethylene glycol-conjugated liposomes have also demonstrated their potential to deliver drugs across the blood-brain barrier. Prednisolone, a drug used to treat multiple sclerosis, was delivered using polyethylene glycol-conjugated liposomes 90–100 nm in diameter (Schmidt et al. 2003). Nucleic acid-based therapeutics have also been developed using antibodies conjugated to liposomes which have demonstrated their potential in rat models (Shi et al. 2001).
Asthma, a very common allergic respiratory disease, is caused by drop in production of interferon-γ (IFN-γ), which results in the patient being susceptible to inflammation of the airways. Kumar et al. (Kumar et al. 2003) developed a polymer-drug conjugate comprising of chitosan and IFN-γ pDNA which was delivered intranasally to increase the production of IFN-γ and was reported to reduce inflammation. Liposome-based 73 nm nanostructures have also been developed for treatment of allergic asthma (John et al. 2003). However, the development of these nanostructures is still in inception and would need clinical trials to be brought to human use.
Nanostructures have also found applications in treating human immunodeficiency virus (HIV), acquired immunodeficiency syndrome, and different ophthalmic conditions. HIV-1 protease inhibitor delivery is being investigated by Allemann et al. using commercially available polymer Eudragit (De Jaeghere et al. 2000). Solid lipid nanoparticles prepared using cationic lipids and surfactants have been employed to adsorb HIV-1 Tat protein and DNA on the surface of nanoparticles (Rudolph et al. 2004). Treatment of ophthalmic conditions is often associated with the ability of the drug to get entrapped in the ocular mucus layer that protects the epithelial layer of cornea for a long duration. Nonsteroidal anti-inflammatory drugs flurbiprofen and ibuprofen have been delivered to rabbit eyes employing nanoparticles ̴ 100 nm in size using polymers such as poly(ethyl acrylate), poly(methyl methacrylate), poly(chlorotrimethyl-aminoethyl-methacrylate), and commercially available Eudragit (Pignatello et al. 2002a, b). These polymers assist controlled release due to their properties of being insoluble and capability of swelling under physiological conditions.
Nanostructures including solid dispersions, liposomes, nanoemulsions, self-emulsifying drug delivery systems, nanostructured lipid carriers, cyclodextrins, and nanocapsules have been employed for delivery of coenzyme Q10. Coenzyme Q10 is an antioxidant and essential molecule required for growth of every cell in the body. Deficiency of Coenzyme Q results in disorders including neurological degeneration, reproductive disorders, aging, and cancer. The use of nanostructures for delivery of coenzyme Q10 has resulted in reduced oxidative stress, increased bioavailability, controlled release, and increased dissolution rates (Paroha et al. 2018).
In comparison to cancer and infectious diseases, not many products have reached the nanomedicine market for treatment of ophthalmic conditions, pain management, psychological illness, metabolic disorders, as well as neurological, cardiovascular, respiratory, and autoimmune diseases. Table 9.3 summarizes the approved products available in market for treatment of the mentioned diseases. However, many nanostructures are under clinical trials (Tables 9.4 and 9.5) and in developmental stages to be used as effective drug and gene delivery vehicles. Nanostructures have also been conjugated to aptamers in order to enhance delivery efficacy. Aptamer-nanostructure conjugates discussed below, due to their high binding affinity and specificity, low immunogenicity, and versatile synthetic accessibility, offer themselves as promising therapeutic agents.
9.3.4 Aptamer-Nanoparticle Conjugates for Therapeutic Purposes
Nanoparticle-aptamer biconjugates along with varied diagnostic applications are used for therapeutic purposes as well (Ghosh et al. 2013; Lambadi et al. 2015). Aptamers with ease of selection and synthesis; high binding affinity and specificity; low immunogenicity; and versatile synthetic accessibility are good candidates for drug delivery in vivo. Different chemotherapy drugs like doxorubicin, docetaxel, daunorubicin, cisplatin, toxins such as gelonin along with various photodynamic therapy agents and small interfering RNAs can be delivered in the host body in a programmed manner using nanoparticle-aptamer bioconjugates.
In one report, the authors used nanoparticle-aptamer conjugate which encapsulated docetaxel. It was found that after endocytosis, the nanoparticle-aptamer-docetaxel conjugate bound to prostate-specific membrane antigen on the cancer cell surface caused cellular toxicity while the control nanoparticle-docetaxel did not cause toxicity to the cancer cell (Farokhzad et al. 2006; Bleickardt et al. 2002; Miller and Kris 2000). Similarly, cisplatin, an anticancer drug, was delivered into cancer cells (Dhar et al. 2008). In another work by Bagalkot et al. (Bagalkot et al. 2007), smart quantum dot-aptamer conjugate was designed which served both as a fluorescence imager and a drug delivery vehicle. Different components were quantum dots, prostate cancer cell-specific RNA aptamer, and doxorubicin. In the presence of cancer cells, quantum dot-aptamer (doxorubicin) system gradually released doxorubicin induced by the binding of target molecule onto RNA aptamer. This Dox release recovered the fluorescence of the quantum dot. So, the quantum dot-aptamer (doxorubicin) system allows both targeted drug delivery and imaging target cells (Bagalkot et al. 2007).
9.4 Further Applications of Nanostructures as Nanomedicine
Nanomedicine not only includes the fields of therapeutics and diagnostics but also involves development of implantable materials and devices. Implantable materials basically include materials for tissue repair/regeneration, bone implants, and implant coatings. Surgical aids, implantable sensors, and sensory aids fall under the category of implantable devices (Agrawal 2016; Lambadi et al. 2015).
Success of an artificial bone implant lies on its integration with the human body which involves generation of osteoblasts on the implant, resulting in minimum chances of rejection. If implant surfaces are left smooth, the surfaces get covered by fibrous tissue resulting in loosening of implant and inflammation. Thus, covering the implant surfaces with nanostructures made of polymers (nano-hydroxyapatite), ceramics, and metals results in efficient integration of osteoblasts to the implant. Coating of nanostructures has given rise to development of long-lasting and durable implants (Sato et al. 2008). In order to improve scratch resistance of human teeth, an artificial hybrid of poly(methyl methacrylate) copolymer and 15–18 nm ceramic nanoparticles is available in market (de la Isla et al. 2003). Also, spherical, functionalized magnetic nanoparticles have been used for cell separation and probing (Pankhurst et al. 2003). Table 9.6 summarizes different nanostructure products available in market as bone substitutes, dental composites, device coatings, medical dressings, dialysis filter, and tissue scaffolds. A wide variety are still under development and would benefit the nanomedicine market in near future.
Despite the innumerable advantages of nanostructures in the field of nanomedicine, only a handful of products have been able to reach the market due to several disadvantages that these magic bullets are associated with. However, maintenance of a balance between the advantages and disadvantages would definitely open up avenues for personalized medicine through therapeutics, diagnostics, and theranostics.
9.5 Darker Side of Nanomedicine and Future Perspective
Applications of nanostructures in the field of diagnostics, therapeutics, and development of implantable materials have helped the medicine industry because of several advantages that these nanostructures offer. Nanomedicine has resulted in higher specificity, increased sensitivity, and faster response time at a much lower price as compared to conventional drug therapies. Nanobiosensors help in detection of very low concentrations of analyte under consideration and have also assisted targeted delivery (Sharma et al. 2015). Nanomedicine has helped treat a wide range of illnesses including cancer, pain, asthma, multiple sclerosis, and kidney diseases (Manoharan et al. 2018). While nanomedicine is becoming an important part of modern medicine, toxicity of the developed nanostructures to humans and the environment is a major concern. Disposal of nanostructures used for diagnosis of infectious diseases is a serious environmental concern. Nanostructures developed for therapeutic purposes are in very preliminary stages, and the long-term effects on human health require further investigation. While toxicity is a major concern, studies are being done to reduce the toxicity of the synthesized nanostructures weighing the benefits provided by them. For example, toxic CdSe quantum dots have been coated with ZnS/polyethylene glycol to reduce their toxicity (Ballou et al. 2004). Naturally available polymers and lipids that are biocompatible and biodegradable are being employed to address the toxicity issues. Coating of nanostructures with different natural polymers such polyethylene glycol or proteins has been demonstrated to reduce toxicity to great extent (Goodman et al. 2004; Abraham et al. 2018). However, with risks associated with production, handling, and storage of these nanostructures in terms of loss of efficiency, toxicity needs to be understood further. Nanostructures possess the ability to integrate diagnostic and therapeutic applications in a single nanoparticulate formulation, making them promising candidates for theranostic applications which would help in personalizing nanomedicine-based treatment. Thus, in summary, nanostructures have shown great potential to play an important role in future development of diagnostics, therapeutics, and theranostic applications contributing to personalized medicine—the need of hour of modern medicine.
Abbreviations
- ATP:
-
adenosine triphosphate
- bp:
-
base pairs
- DNA:
-
deoxyribonucleic acid
- FRET:
-
Fluorescence Resonance Energy Transfer
- H2O2:
-
hydrogen peroxide
- HIV:
-
human immunodeficiency virus
- IFN-γ:
-
interferon-γ
- PBCA:
-
poly(butyl cyanoacrylate)
- PDGF:
-
platelet-derived growth factor
- PLGA:
-
poly-(lactic-co-glycolic) acid
- QD:
-
quantum dot
- RNA:
-
ribonucleic acid
- SERS:
-
surface-enhanced Raman scattering
- VEGF:
-
vascular endothelial growth factor
References
Abraham AN et al (2018) Phytochemicals as dynamic surface ligands to control nanoparticle–protein interactions. ACS Omega 3(2):2220–2229. https://doi.org/10.1021/acsomega.7b01878
Agrawal P (2016) Potential prospects of future medicine: nano medicine. J Pharm 4(1):1000–1149. https://doi.org/10.4172/2329-6887.1000e149
Akerman ME et al (2002) Nanocrystal targeting in vivo. Proc Natl Acad Sci U S A 99(20):12617–12621. https://doi.org/10.1073/pnas.152463399
Atanasijevic T et al (2006) Calcium-sensitive MRI contrast agents based on superparamagnetic iron oxide nanoparticles and calmodulin. Proc Natl Acad Sci U S A 103(40):14707–14712. https://doi.org/10.1073/pnas.0606749103
Baetke SC, Lammers T, Kiessling F (2015) Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol 88(1054):20150207. https://doi.org/10.1259/bjr.20150207
Bagalkot V et al (2007) Quantum dot−aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett 7(10):3065–3070. https://doi.org/10.1021/nl071546n
Ballou B et al (2004) Noninvasive imaging of quantum dots in mice. Bioconjug Chem 15(1):79–86. https://doi.org/10.1021/bc034153y
Baptista PV et al (2006) Gold-nanoparticle-probe–based assay for rapid and direct detection of mycobacterium tuberculosis DNA in clinical samples. Clin Chem 52(7):1433. https://doi.org/10.1373/clinchem.2005.065391
Bentzen EL et al (2005) Progression of respiratory syncytial virus infection monitored by fluorescent quantum dot probes. Nano Lett 5(4):591–595. https://doi.org/10.1021/nl048073u
Bleickardt E et al (2002) Phase I dose escalation trial of weekly docetaxel plus irinotecan in patients with advanced cancer. Cancer Biol Ther 1(6):646–651. https://doi.org/10.4161/cbt.314
Boisselier E, Astruc D (2009) Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 38(6):1759–1782. https://doi.org/10.1039/B806051G
Bruchez M et al (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281(5385):2013–2016. https://doi.org/10.1126/science.281.5385.2013
Caminero JA et al (2010) Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 10(9):621–629. https://doi.org/10.1016/s1473-3099(10)70139-0
Canton R et al (2005) Antimicrobial therapy for pulmonary pathogenic colonisation and infection by Pseudomonas aeruginosa in cystic fibrosis patients. Clin Microbiol Infect 11(9):690–703. https://doi.org/10.1111/j.1469-0691.2005.01217.x
Cao S et al (2013) Electrochemistry of cholesterol biosensor based on a novel Pt–Pd bimetallic nanoparticle decorated graphene catalyst. Talanta 109:167–172. https://doi.org/10.1016/j.talanta.2013.02.002
Caster JM et al (2017) Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol 9(1). https://doi.org/10.1002/wnan.1416
Chan WCW, Nie S (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281(5385):2016–2018. https://doi.org/10.1126/science.281.5385.2016
Chang CC et al (2013) Aptamer-based colorimetric detection of platelet-derived growth factor using unmodified gold nanoparticles. Biosens Bioelectron 42:119–123. https://doi.org/10.1016/j.bios.2012.10.072
Che X et al (2009) Hydrogen peroxide sensor based on horseradish peroxidase immobilized on an electrode modified with DNA-L-cysteine-gold-platinum nanoparticles in polypyrrole film. Microchimica Acta 167(3):159. https://doi.org/10.1007/s00604-009-0237-0
Choi JH, Chen KH, Strano MS (2006) Aptamer-capped nanocrystal quantum dots: a new method for label-free protein detection. J Am Chem Soc 128(49):15584–15585. https://doi.org/10.1021/ja066506k
Chopra A, Shukla R, Sharma TK (2014) Aptamers as an emerging player in biology. Aptamer Synth Antibodies 1:1–11
Dahan M et al (2003) Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science 302(5644):442–445. https://doi.org/10.1126/science.1088525
De Jaeghere F et al (2000) Oral bioavailability of a poorly water soluble HIV-1 protease inhibitor incorporated into pH-sensitive particles: effect of the particle size and nutritional state. J Control Release 68(2):291–298. https://doi.org/10.1016/S0168-3659(00)00272-8
de la Isla A et al (2003) Nanohybrid scratch resistant coatings for teeth and bone viscoelasticity manifested in tribology. Mater Res Innov 7(2):110–114. https://doi.org/10.1007/s10019-003-0236-4
Dhar S et al (2008) Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc Natl Acad Sci 105(45):17356–17361. https://doi.org/10.1073/pnas.0809154105
Dhiman A et al (2017) Aptamer-based point-of-care diagnostic platforms. Sensors Actuators B Chem 246:535–553. https://doi.org/10.1016/j.snb.2017.02.060
Dreaden EC et al (2012) Size matters: gold nanoparticles in targeted cancer drug delivery. Ther Deliv 3(4):457–478. https://doi.org/10.4155/tde.12.21
Edelstein RL et al (2000) The BARC biosensor applied to the detection of biological warfare agents. Biosens Bioelectron 14(10):805–813. https://doi.org/10.1016/S0956-5663(99)00054-8
Eghtedari M et al (2009) Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast Cancer cells. Nano Lett 9(1):287–291. https://doi.org/10.1021/nl802915q
Etheridge ML et al (2013) The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine 9(1):1–14. https://doi.org/10.1016/j.nano.2012.05.013
Farokhzad OC, Langer R (2006) Nanomedicine: developing smarter therapeutic and diagnostic modalities. Adv Drug Deliv Rev 58(14):1456–1459. https://doi.org/10.1016/j.addr.2006.09.011
Farokhzad OC et al (2006) Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A 103(16):6315–6320. https://doi.org/10.1073/pnas.0601755103
Gelderblom H et al (2001) Cremophor EL. Eur J Cancer 37(13):1590–1598. https://doi.org/10.1016/S0959-8049(01)00171-X
Ghosh IN et al (2013) Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: a case for judicious use of silver nanoparticles for antibacterial applications. Int J Nanomedicine 8:4721–4731. https://doi.org/10.2147/IJN.S49649
Goodman CM et al (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 15(4):897–900. https://doi.org/10.1021/bc049951i
Greish K (2010) Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol 624:25–37. https://doi.org/10.1007/978-1-60761-609-2_3
Gulyaev AE et al (1999) Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res 16(10):1564–1569. https://doi.org/10.1023/A:1018983904537
Harris JM, Chess RB (2003) Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2(3):214–221. https://doi.org/10.1038/nrd1033
Hobbs SK et al (1998) Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A 95(8):4607–4612. https://doi.org/10.1073/pnas.95.8.4607
Hrkach J et al (2012) Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med 4(128):128ra39. https://doi.org/10.1126/scitranslmed.3003651
Huang S-H (2007) Gold nanoparticle-based immunochromatographic assay for the detection of Staphylococcus aureus. Sensors Actuators B Chem 127(2):335–340. https://doi.org/10.1016/j.snb.2007.04.027
Ibrahim NK et al (2002) Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res 8(5):1038–1044
Jain KK (2007) Applications of nanobiotechnology in clinical diagnostics. Clin Chem 53(11):2002–2009. https://doi.org/10.1373/clinchem.2007.090795
Jayasena SD (1999) Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem 45(9):1628–1650
Jhaveri SD et al (2000) Designed signaling aptamers that transduce molecular recognition to changes in fluorescence intensity. J Am Chem Soc 122(11):2469–2473. https://doi.org/10.1021/ja992393b
John AE et al (2003) Discovery of a potent nanoparticle P-selectin antagonist with anti-inflammatory effects in allergic airway disease. FASEB J 17(15):2296–2298. https://doi.org/10.1096/fj.03-0166fje
Jyoti A, Tomar RS (2017) Detection of pathogenic bacteria using nanobiosensors. Environ Chem Lett 15(1):1–6. https://doi.org/10.1007/s10311-016-0594-y
Kalra P et al (2018) Simple methods and rational design for enhancing aptamer sensitivity and specificity. Front Mol Biosci 5(41):1–16. https://doi.org/10.3389/fmolb.2018.00041
Kaur H et al (2018) Aptamers in the therapeutics and diagnostics pipelines. Theranostics 8(15):4016–4032. https://doi.org/10.7150/thno.25958
Keren S et al (2008) Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc Natl Acad Sci U S A 105(15):5844–5849. https://doi.org/10.1073/pnas.0710575105
Kiessling F et al (2014) Nanoparticles for imaging: top or flop? Radiology 273(1):10–28. https://doi.org/10.1148/radiol.14131520
Kreuter J et al (2003) Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res 20(3):409–416. https://doi.org/10.1023/A:1022604120952
Kumar KV (2012) Targeted delivery of nanomedicines. ISRN Pharmacol 2012:571394. https://doi.org/10.5402/2012/571394
Kumar M et al (2003) Chitosan IFN-gamma-pDNA nanoparticle (CIN) therapy for allergic asthma. Genet Vaccines Ther 1(1):3. https://doi.org/10.1186/1479-0556-1-3
Lai RY, Plaxco KW, Heeger AJ (2007) Aptamer-based electrochemical detection of picomolar platelet-derived growth factor directly in blood serum. Anal Chem 79(1):229–233. https://doi.org/10.1021/ac061592s
Lambadi PR et al (2015) Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int J Nanomedicine 10:2155–2171. https://doi.org/10.2147/IJN.S72923
Larson JL et al (2000) The reproductive and developmental toxicity of the antifungal drug Nyotran (liposomal nystatin) in rats and rabbits. Toxicol Sci 53(2):421–429. https://doi.org/10.1093/toxsci/53.2.421
Lee JH et al (2010) Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv Drug Deliv Rev 62(6):592–605. https://doi.org/10.1016/j.addr.2010.03.003
Levy M, Cater SF, Ellington AD (2005) Quantum-dot aptamer beacons for the detection of proteins. Chembiochem 6(12):2163–2166. https://doi.org/10.1002/cbic.200500218
Li J, Lu Y (2000) A highly sensitive and selective catalytic DNA biosensor for lead ions. J Am Chem Soc 122(42):10466–10467. https://doi.org/10.1021/ja0021316
Ma J et al (2003) Biomimetic processing of nanocrystallite bioactive apatite coating on titanium. Nanotechnology 14(6):619. https://doi.org/10.1088/0957-4484/14/6/310
Mahmoudian M et al (2014) Synthesis of polypyrrole coated silver nanostrip bundles and their application for detection of hydrogen peroxide. J Electrochem Soc 161(9):H487–H492. https://doi.org/10.1149/2.0571409jes
Manoharan K, Saha A, Bhattacharya S (2018) Nanoparticles-based diagnostics. In: Environmental, chemical and medical sensors: energy, environment, and sustainability. Springer, Singapore, pp 253–269. https://doi.org/10.1007/978-981-10-7751-7_11
Mazeiko V et al (2013) Gold nanoparticle and conducting polymer-polyaniline-based nanocomposites for glucose biosensor design. Sensors Actuators B Chem 189:187–193. https://doi.org/10.1016/j.snb.2013.03.140
Meers P et al (2008) Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother 61(4):859–868. https://doi.org/10.1093/jac/dkn059
Meng C et al (2016) Selective and sensitive fluorescence aptamer biosensors of adenosine triphosphate. Nanomater Nanotechnol 6:33. https://doi.org/10.5772/63985
Micha JP et al (2006) Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol 100(2):437–438. https://doi.org/10.1016/j.ygyno.2005.09.012
Miller VA, Kris MG (2000) Docetaxel (Taxotere) as a single agent and in combination chemotherapy for the treatment of patients with advanced non-small cell lung cancer. Semin Oncol 27(2 Suppl 3):3–10
Min Y et al (2015) Clinical translation of nanomedicine. Chem Rev 115(19):11147–11190. https://doi.org/10.1021/acs.chemrev.5b00116
Mirkin CA et al (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382:607. https://doi.org/10.1038/382607a0
Moghimi SM, Hunter AC, Murray JC (2005) Nanomedicine: current status and future prospects. FASEB J 19(3):311–330. https://doi.org/10.1096/fj.04-2747rev
Navani NK, Li Y (2006) Nucleic acid aptamers and enzymes as sensors. Curr Opin Chem Biol 10(3):272–281. https://doi.org/10.1016/j.cbpa.2006.04.003
Ortega A et al (2015) Antimicrobial evaluation of quaternary ammonium polyethyleneimine nanoparticles against clinical isolates of pathogenic bacteria. IET Nanobiotechnol 9(6):342–348. https://doi.org/10.1049/iet-nbt.2014.0078
Pankhurst QA et al (2003) Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36(13):R167. https://doi.org/10.1088/0022-3727/36/13/201
Park C, Lee C, Kwon O (2016) Conducting polymer based nanobiosensors. Polymers 8(7):249. https://doi.org/10.3390/polym8070249
Paroha S, Chandel AKS, Dubey RD (2018) Nanosystems for drug delivery of coenzyme Q10. Environ Chem Lett 16(1):71–77. https://doi.org/10.1007/s10311-017-0664-9
Pignatello R et al (2002a) Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials 23(15):3247–3255. https://doi.org/10.1016/S0142-9612(02)00080-7
Pignatello R et al (2002b) Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci 16(1–2):53–61. https://doi.org/10.1016/S0928-0987(02)00057-X
Qian X et al (2007) In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol 26:83. https://doi.org/10.1038/nbt1377
Rudolph C et al (2004) Application of novel solid lipid nanoparticle (SLN)-gene vector formulations based on a dimeric HIV-1 TAT-peptide in vitro and in vivo. Pharm Res 21(9):1662–1669. https://doi.org/10.1023/B:PHAM.0000041463.56768.ec
Safavi A, Farjami F (2011) Electrodeposition of gold–platinum alloy nanoparticles on ionic liquid–chitosan composite film and its application in fabricating an amperometric cholesterol biosensor. Biosens Bioelectron 26(5):2547–2552. https://doi.org/10.1016/j.bios.2010.11.002
Sato M et al (2008) Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J Biomed Mater Res A 84(1):265–272. https://doi.org/10.1002/jbm.a.31469
Schmidt J et al (2003) Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain 126(Pt 8):1895–1904. https://doi.org/10.1093/brain/awg176
Sengupta S et al (2005) Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 436:568. https://doi.org/10.1038/nature03794
Sharma TK, Shukla R (2014) Nucleic acid aptamers as an emerging diagnostic tool for animal pathogens. Adv Anim Vet Sci 2(1):50–55. https://doi.org/10.14737/journal.aavs/2014.2.1.50.55
Sharma TK et al (2012a) Green synthesis and antimicrobial potential of silver nanoparticles. Int J Green Nanotechnol 4(1):1–16. https://doi.org/10.1080/19430892.2012.656040
Sharma TK et al (2012b) Interaction of bacteriocin-capped silver nanoparticles with food pathogens and their antibacterial effect. Int J Green Nanotechnol 4(2):93–110. https://doi.org/10.1080/19430892.2012.678757
Sharma TK et al (2014) Aptamer-mediated ‘turn-off/turn-on’ nanozyme activity of gold nanoparticles for kanamycin detection. Chem Commun 50(100):15856–15859. https://doi.org/10.1039/C4CC07275H
Sharma TK, Ramanathan R, Rakwal R, Agrawal GK, Bansal V (2015) Moving forward in plant food safety and security through nanoBioSensors: adopt or adapt biomedical technologies? Proteomics 15(10):1680–1692. https://doi.org/10.1002/pmic.201400503
Sharma TK, Bruno JG, Cho WC (2016) The point behind translation of aptamers for point of care diagnostics. Aptamers Synth Antibodies 2(2):36–42
Sharma TK, Bruno JG, Dhiman A (2017) ABCs of DNA aptamer and related assay development. Biotechnol Adv 35(2):275–301. https://doi.org/10.1016/j.biotechadv.2017.01.003
Shi N, Boado RJ, Pardridge WM (2001) Receptor-mediated gene targeting to tissues in vivo following intravenous administration of pegylated immunoliposomes. Pharm Res 18(8):1091–1095. https://doi.org/10.1023/a:1010910523202
Shinkai M et al (1999) Intracellular hyperthermia for cancer using magnetite cationic liposomes. J Magn Magn Mater 194(1):176–184. https://doi.org/10.1016/S0304-8853(98)00586-1
Shukoor MI et al (2012) Aptamer-nanoparticle assembly for logic-based detection. ACS Appl Mater Interfaces 4(6):3007–3011. https://doi.org/10.1021/am300374q
Siegemund T et al (2006) Thioflavins released from nanoparticles target fibrillar amyloid beta in the hippocampus of APP/PS1 transgenic mice. Int J Dev Neurosci 24(2–3):195–201. https://doi.org/10.1016/j.ijdevneu.2005.11.012
Song HS et al (2013) Human taste receptor-functionalized field effect transistor as a human-like nanobioelectronic tongue. Nano Lett 13(1):172–178. https://doi.org/10.1021/nl3038147
Voura EB et al (2004) Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med 10:993. https://doi.org/10.1038/nm1096
Wagner V et al (2006) The emerging nanomedicine landscape. Nat Biotechnol 24:1211. https://doi.org/10.1038/nbt1006-1211
Wang Y et al (2007) SERS opens a new way in aptasensor for protein recognition with high sensitivity and selectivity. Chem Commun 48:5220–5222. https://doi.org/10.1039/B709492B
Weerathunge P et al (2014) Aptamer-controlled reversible inhibition of gold nanozyme activity for pesticide sensing. Anal Chem 86(24):11937–11941. https://doi.org/10.1021/ac5028726
Weissleder R et al (1990) Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology 175(2):489–493. https://doi.org/10.1148/radiology.175.2.2326474
Wu X et al (2002) Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 21:41. https://doi.org/10.1038/nbt764
Yanyan Y et al (2011) Size-controllable gold–platinum alloy nanoparticles on nine functionalized ionic-liquid surfaces and their application as electrocatalysts for hydrogen peroxide reduction. Chem Eur J 17(40):11314–11323. https://doi.org/10.1002/chem.201100010
Yi L (2002) New transition-metal-dependent DNAzymes as efficient endonucleases and as selective metal biosensors. Chem Eur J 8(20):4588–4596. https://doi.org/10.1002/1521-3765(20021018)8:20<4588::AID-CHEM4588>3.0.CO;2-Q
Zhang X et al (2016) Sensitive colorimetric detection of glucose and cholesterol by using Au@Ag core-shell nanoparticles. RSC Adv 6(41):35001–35007. https://doi.org/10.1039/C6RA04976A
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bansal, M., Kumar, A., Malinee, M., Sharma, T.K. (2020). Nanomedicine: Diagnosis, Treatment, and Potential Prospects. In: Daima, H., PN, N., Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Nanoscience in Medicine Vol. 1. Environmental Chemistry for a Sustainable World, vol 39. Springer, Cham. https://doi.org/10.1007/978-3-030-29207-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-29207-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29206-5
Online ISBN: 978-3-030-29207-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)