Abstract
Food emulsions are complex formulations developed with many success criteria, including stability, safety, taste, and process feasibility. A key component of all these attributes is the correct choice of one, or multiple, emulsifiers that are compatible with all ingredients, food regulations, and the above criteria. The physical chemistry of emulsifiers in foods determines their efficiency and performance in a given formulation, and this chapter provides an overview of the surface properties of these materials and their phase behavior. Examples are provided for the main classes of food emulsifiers, enabling researchers and practitioners in food research, development, and processing to choose and apply these materials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adsorption
- Emulsion
- Surfactant
- Emulsifier
- Phospholipid
- Micelle
- Droplet
- Liquid crystal
- Stability
- Viscosity
- Lecithin
- Gel
- Tween
- Span
- Interfacial tension
- Shear
6.1 Introduction
The characteristic property of all emulsifiers is their surface activity. Surface activity is the ability to form a surface excess at interfaces. The formation of adsorbed emulsifier layers at interfaces is displayed in a change of a range of easily observable and technically important properties:
-
1.
The surface tension is reduced.
-
2.
The lifetimes of bubbles are increased. Only very pure water displays a very short lifetime, a few seconds, of bubbles created by shaking. Normal water, even double distilled, usually displays a bubble lifetime of about 20–30 s.
-
3.
The emulsifiability of oils in water is enhanced. Smaller drops with a longer lifetime are formed with less stirring.
-
4.
The aggregation rate of dispersed particles is changed. Surface-active additives may induce or prevent flocculation of dispersions.
-
5.
The sediment volume of settling particles is influenced. Surface additives inducing adhesion may create a loose or compact sediment.
-
6.
Crystallization properties are changed. This may include crystallization rate and crystal shape.
This chapter aims to discuss the principal physical origin of the various functions of typical lipid food emulsifiers. Emulsifier function under very different conditions in various foods will be discussed. The chapter will try to show how to select emulsifiers on the basis of their fundamental properties.
6.2 Surface Activity
When an additive is added to a solution, the gain of entropy is very large at low concentrations. If the additive displays surface activity and adsorbs at an interface, the system loses entropy, which has to be balanced by a gain in free energy due to the adsorption. At very low concentrations the solubility always prevails, but when the concentration is increased, more and more of the available surfaces will be covered by the adsorbed molecules. To display surface activity, an emulsifier needs to have certain properties:
-
1.
It has to produce a non-crystalline form1 in contact with water.
-
2.
It should have a low solubility in water due to a large hydrophobic part.
-
3.
It has to interact with water through polar interactions.
-
4.
It should have a significant molecular weight to reduce the effect of the decreased entropy when it adsorbs.
-
5.
It has to have a reduced solubility in an oil environment due to large size and the presence of polar groups at the interface.
High-melting emulsifiers do not display surface activity when dispersed in water until a critical temperature, the Krafft temperature, has been reached. At this temperature the emulsifier solubility in the solution has reached a sufficient concentration to allow for a significant formation of adsorbed layers at the interfaces.
The presence of hydrophobic parts of the molecules increases the energy gain due to adsorption. In aqueous environments most emulsifiers tend to aggregate in micelles above a key concentration, CMC (critical micelle concentration), or to precipitate as liquid crystals. Above the aggregation concentration all properties depending on the chemical potential, for instance the interfacial tension, are more or less constant. The aggregation is mainly driven by the presence of the hydrophobic parts of the molecules (Tanford 1973).
A polar part of the molecule is necessary to avoid the formation of a separate oil phase. The type of aggregates formed during the adsorption will reflect the balance between the polar part and the hydrophobic part of the molecule.
The free-energy gain at adsorption is mainly proportional to the molecular weight, while the entropy loss due to the demixing is independent of molecular weight. Hence, small molecules like lower alcohols do not form adsorbed layers at hydrophobic surfaces in contact with water solutions, while pronounced layers are formed with additives of higher molecular weights, for instance monoglycerides. Proteins display a much higher surface activity than protein hydrolysates.
In an oil environment, solvophobic effects are absent and the adsorption has to be generated by polar interactions between the second phase and the surface-active molecule.
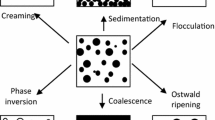
The interaction between droplets is influenced when an adsorbed layer of an emulsifier covers the droplets. The change in the interaction strongly influences the macroscopic properties of the dispersions. Table 6.1 indicates the effects of droplet interactions on droplet aggregation state and the resulting sediment volumes. Flocculated droplets form open, porous aggregates that do not pack as tightly as stable particle dispersions during sedimentation, producing larger sediment volumes.
The solution properties of emulsifiers are determined for the surface activity of the emulsifiers. In addition, the ability to generate repulsive interactions is also reflected in the solution properties of emulsifiers.
6.3 Solution Properties of Emulsifiers
When water is added to a surfactant system, the solution state of the system may in principle pass through a series of aggregation structures and phases in a particular sequence. Depending on the emulsifier structure, some phases may be omitted. The sequence is: reversed, water-in-oil, micelles → reversed hexagonal phase → cubic phase → lamellar phase → hexagonal phase → normal, oil-in-water, micellar solution → molecular solution (Fontell 1978) (Fig. 6.1). The presence of certain phases like lamellar and hexagonal phase can be detected using polarized light microscopy to match the birefringent textures formed by these phases (Rosevear 1968; Laughlin 1994).
A typical sequence of liquid-crystalline phases and solution phases formed in an aqueous emulsifier system. (Modified from Fontell 1978)
The free energy of solubilization, ΔGsolubilization, can be described as a sum of free energy contributions in the process by the expression: emulsifier phase + water → more solubilized phase:
where ΔGmixing is negative when changing from large aggregates to small aggregates like micelles and molecular solutions.
ΔGhydrophobic is positive and equal to the product of the area per molecule at the interface, Ahydrocarbon/water, and the oil-water interfacial tension, ǖFE;hydrocarbon/water. The hydrophobic effect is the driving force for the aggregation and gives the upper limit of the molecular solubility for amphiphilic molecules: the CMC or critical micelle concentration.
ΔGpolar group/water is negative. This term consists mainly of the work released when more water allows a larger separation between repelling aggregates or molecules:
where l is the average distance between the polar groups, also called head groups, and F(l) is the interaction force.
The area per molecule in the aggregates is found from the balance between the interfacial tension of the oil/water interface and of the space needed for the polar group itself in addition to any space generated by repulsive interactions between the emulsifier head groups at the interface.
The area per molecule expands in the series Areversed micelles < Areversed hexagonal < Acubic < Alamellar < Ahexagonal < Amicelles.
At a specific ratio of water and emulsifier, the system’s tendency is to obtain aggregates as small as possible to maximize the ΔGmixing and the ΔGpolar group/water. The lower limit in aggregate size is given by the onset of increased hydrophobic contact with the exposed hydrocarbon/water interface.
The interesting result of this exercise is that the interfacial area per molecule is to a large extent a measure of the ability to generate repulsive interactions.
In the solubilization sequence, reversed aggregates → lamellar phase → hexagonal phase → micellar solution → molecular solution, the area per molecule of the surfactant/water interface increases. Depending on the packing constraints given by the hydrophobic moiety in the aggregates, the range of the repulsive interaction on the polar side of the molecule, and the molecular weight, this process has to proceed more rapidly or more slowly (Israelachvili et al. 1976, 1977). Hence, the packing constraints of the hydrocarbon chain are an important link between proper- ties and aggregation.
The ratio of the actual area A, as it is created by the repulsive interactions, to the theoretical area of a saturated hydrocarbon chain, A0 = 23 Å2 enforces different aggregate geometries (Israelachvili et al. 1976, 1977) based on the most efficient packing of a given molecule into an aggregate and can be described using the different ratios of volume to area for common aggregates, as shown in Table 6.2. Spherical micelles form when the polar group area dominates molecular geometry, as conical shapes pack well into a sphere. Similarly, increasingly cylindrical geometries pack into flatter structures as the packing parameter approaches unity.
The successive solvation of surfactants in Table 6.2 corresponds to a successive change into aggregates because of more long-range interactions. If there is an upper limit for the repulsion, the solvation series is terminated at that stage. Hence, the maximum solvated aggregate formed in a surplus of water is a measure of the ability of the emulsifier to generate repulsive interactions.
The area of the molecule is a measure of the interaction when water is present, and may be generalized as the hydrophilicity of the molecule. The spatial requirement of the hydrophobic part of the molecule is of course a measure of the hydrophobicity of the molecule. Consequently, there is a close link with the classical view of emulsifiers as molecules with a balance between the hydrophobic and the hydrophilic properties, as they are expressed in the HLB numbers, proposed by Griffin (1949, 1979).
6.4 The Use of Phase Diagrams to Understand Emulsifier Properties
Friberg and coworkers (Wilton and Friberg 1971; Friberg and Mandell 1970a; b; Friberg and Rydhag 1971; Friberg and Wilton 1970; Rydhag 1979; Rydhag and Wilton 1981; Friberg et al. 1969; Friberg 1971) have investigated phase diagrams and emulsion stability extensively. They concluded that the optimum composition for a stable emulsion should be that at which the lamellar phase, the oil phase, and the water phase are in equilibrium in the corresponding phase diagram (Fig. 6.2).
Schematic drawing of the ternary structure map for an emulsion system with an emulsifier forming lamellar liquid-crystalline phase. A coating of the droplets can form at higher emulsifier levels, improving stability. (Modified from Friberg 2006)
The relation between the formation of lamellar phases and emulsion stability is basically of an empirical nature. The emulsifiability is enhanced at certain compositions (Friberg and Mandell 1970b; Friberg and Rydhag 1971; Friberg and Wilton 1970), and the formation of crystalline phases corresponds to an observed destabilization (Wilton and Friberg 1971). The formation of multilayers around the emulsion droplets under certain conditions has also been shown (Friberg 1990).
It was suggested that the formation of a multilayer of a lamellar liquid-crystalline phase coating the droplet surface reduces the van der Waal’s attraction and that this was an important contribution to the observed effects in the emulsification experiments (Friberg 1971). However, this explanation is not a useful general explanation since the emulsifier concentration in optimized food emulsions rarely is high enough to allow for multilayer adsorption (Walstra 1988; Dickinson 1986). Obviously, this observation is contradictive to the need for a separate phase of liquid-crystalline material around the droplet. However, a correlation between the presence of, or the possibility to form, liquid-crystalline phases and emulsion stability is still experimentally observed in several systems. More generally, to stabilize a dispersion, the emulsifier should:
-
1.
Contribute to the repulsive interactions between the droplets
-
2.
Contribute to the interfacial viscosity
-
3.
Be well anchored to the interface
These properties are reflected in the formation of various liquid-crystalline phases, such as lamellar bilayers (Table 6.3). These aspects are illustrated by a few examples.
There is also utility to the formation of cubic phases (Rodriguez et al. 2000) as their solid-like rheology prevents emulsion coalescence and sedimentation by gelling the emulsion, but little work has been done to study their performance at much lower phase volumes.
6.5 Examples of the Relation Between Phase Diagrams and Emulsion Stability
6.5.1 Monoglycerides
A technical monoglyceride at room temperature remains in a nonhydrated crystalline phase, or β phase, in equilibrium with a surplus of water. Above 40 °C, the monoglyceride takes up water and a lamellar phase is formed (Wilton and Friberg 1971). The lamellar phase coexists with a surplus of water (no micelles are formed). When the lamellar phase is cooled, a semicrystalline phase, termed “α phase,” is formed. This phase is metastable below 30°C and converts only slowly into an aqueous and a β phase.
The swelling of the lamellar and α/gel phases indicates the existence of a strong repulsive hydration force. This force has been measured by the osmotic stress technique (Fig. 6.3). In contrast, no hydration force strong enough to separate the bilayers is present in the β phase. The hydration force between emulsion droplets coated with this emulsifier depends on the liquid-crystalline state of the adsorbed emulsifier film in the same way. This explains why monoglycerides appearing in the β form are inactive as emulsifiers, and why a monoglyceride-stabilized emulsion rapidly destabilizes when the monoglyceride converts from lamellar or α into β phase (Wilton and Friberg 1971). In technical systems, it is important that the conversion of α phase into β phase is delayed. An α phase can be stabilized by the presence of ionic charges, as with soap (Larsson and Krog 1973), or by a wide distribution of the fatty acid-chain lengths. The solution properties of a range of food emulsifiers are summarized in Table 6.4.
The hydration repulsion between bilayers of monopalmitin in the liquid-crystalline and gel states. (Redrawn from Pezron et al. 1991)
6.5.2 Lecithins
Lecithin is one of the most commonly used food emulsifiers, and its popularity is expected given its natural origin. Technical lecithins, usually soybean lecithin, are always natural mixtures of various phospholipids. The most frequent one is phosphatidylcholine, PC. The second is phosphatidylethanolamine, PE. Phosphatidylinositol, PI, and phosphatidic acid, PA, are usually present at intermediate levels, and phosphatidyl serine, PS, lysophosphatides, LPC and LPE, etc., at low levels. Nonphosphatides such as steroids, vitamin E, and free fatty acids are usually also present in technical products. The properties of lecithins reflect some type of average properties of the mixture. This section will first describe the characteristic properties of the most common phosphatides and then discuss the properties of various mixtures.
6.5.3 Phosphatidylcholine
The phase diagram of a typical unsaturated phosphatidylcholine is displayed in Fig. 6.4. The phase diagram is characterized by a large swelling lamellar phase. Saturated phosphatidylcholines have a phase transition temperature up to about 40 °C, whereas the corresponding temperature for unsaturated lecithins is well below 0 °C. The phase diagram of soybean PC is described in Bergenståhl and Fontell (1983) and is rather similar to the phase diagram of dioleoyl PC.
The Phase diagram of water and dioleoylphosphatidylcholine (From Bergenståhl and Stenius 1987)
6.5.4 Phosphatidylethanolamine
Phosphatidylethanolamine is less hydrophilic than PC. The saturated ethanolamines form lamellar phases that swell less than the corresponding PC species. The phase transition temperature is about 10–40 °C above the corresponding temperature of the phosphatidylcholine (Fig. 6.5). The more limited ability of the molecule to create long-range repulsive interactions, and thereby to occupy a large interfacial area, is displayed in the tendency to form reversed hexagonal phase with unsaturated PE species, as shown in Table 6.5.
The main transition temperature for phosphatidylcholine (open square) and phosphatidylethanolamine (filled) as a function of chain length. Sources in Table 6.4
6.5.5 Phosphatidylinositol
The phase diagram of soybean PI and water has been determined by Bergenståhl (1991) and by Söderberg (1990). The diagram is characterized by a large lamellar phase with an unlimited swelling. The liquid-crystalline phase is formed below room temperature.
6.5.6 Phosphatidic Acid
The phase diagram of the sodium salt of dioleoylphosphatidic acid has been determined by Lindblom et al. (1991). The phase diagram is characterized by a lamellar phase that transforms to a reversed hexagonal phase at about 30% of water. This transformation occurs although there is an ionic charge on the molecules and despite their small head group. A possible explanation, supported by evidence from NMR measurements, is that this is due to ion condensation.
6.5.7 Lysophosphatides
The phase diagrams of a series of different lysophosphatides have been investigated by Arvidsson et al. (1985). Lysophosphatidylcholine has the same hydrophilic polar group as the ordinary PC but only one of the two fatty acids. This reduces the volume demand of the aggregate, and the packing parameter allows for the formation of micelles and hexagonal phases.
6.5.8 The Properties of Mixtures of Phosphatides
Technical phosphatides are always mixtures. Their properties reflect some type of average that the mixture develops. One way to investigate this is to determine the type of liquid-crystalline phase that develops when different phosphatides are allowed to interact together with water. Figure 6.6 shows the phase diagram of dioleoyl PC and dioleoyl PE in 40% water (Eriksson et al. 1985). The figure shows that a lamellar phase is formed when the system contains mainly PC, but that around 60% PE non-lamellar, hexagonal and cubic phases start to form. This change is enhanced at high temperatures.
The phase diagram of dioleoyl PC and dioleoyl PE with 40% water. (Redrawn from Eriksson et al. 1985)
The more highly unsaturated soybean PE and soybean PC display a similar aggregation pattern, but the temperature at which the system changes from lamellar to non-lamellar phases is lower (Fig. 6.7), and the phase diagram is dominated by the hydrophobic properties of the PE up to fairly high concentrations of PC.A mixture of PI and PC displays the extreme swelling properties of ionically charged emulsifiers at an early stage. This was indeed also expected since a similar pattern was observed when a small amount of ionically charged detergents was added to the lamellar phase formed by monoglycerides (Larsson and Krog 1973). When PI and PE are mixed, the properties of the mixture are dominated by the hydrophilic PI up to quite a high PE:PI ratio.
The phase diagram of soybean PC, soybean PE, and water; of soybean PC, soybean PI, and water; and of soybean PI, soybean PE, and water. (Redrawn from Bergenståhl 1991) The cubic phase was not included in the original drawing, but it is a possible interpretation of the x-ray peaks included in the paper. It is also supported by the data from the study by Eriksson et al. (1985)
A preliminary conclusion from this work is that the properties of phosphatide mixtures are determined by the ratio of anionic (particularly PI) phosphatides to PE rather than by the PC:PE ratio.
Technical soybean lecithin contains a mixture of different phospholipids (Rydhag 1979). In most cases, the weakly hydrophilic phosphatidylethanolamine dominates, and this type of lecithin is suitable for inverse emulsions such as in margarine. More hydrophilic soybean lecithins suitable for oil-in-water emulsions are obtained by partial hydrolysis to form lysolecithins. It is also possible to increase the effective hydrophilicity of the PE by making the polar head group larger through acetylation.
6.6 Some Ways to Classify Emulsifiers
A common problem in industrial development work is the choice of suitable surfactants to obtain the desired results. In the literature a number of different methods of making a fast preliminary selection of suitable emulsifiers have been proposed. The most common methods and concepts are discussed here and are compared with the function of the emulsifier in the emulsion.
6.6.1 The Solubility Concept
One of the first ideas, proposed by Bancroft (1913), was that the solubility of the emulsifier determines the type of emulsion that is formed. An oil-soluble emulsifier will create an oil-continuous emulsion, and a water-soluble emulsifier will turn the emulsion into a water-continuous one. This is true for low molecular weight emulsifiers with a high solubility, usually in micellar aggregates, but it is also valid for polymers. However, most likely, the concept can also, to some extent, be expanded to include emulsifiers with just a dispersibility in either one of the phases (for instance lecithin). Experience in this direction is exemplified in Table 6.6. However, the Bancroft rule provides us with the only very general directions. To proceed further we need to rank emulsifiers more quantitatively.
6.6.2 The Phase Inversion Concept
Ethoxylated surfactants have a tendency toward declining hydrophilicity with increasing temperature. This leads to a change from water solubility at low temperatures to oil solubility at higher temperatures. According to the Bancroft rule, this will result in a given system switching from being water-continuous to being oil-continuous. The hydrophilicity can be viewed as a property that is gradually lost with increasing temperature. The distance from the break even point, the phase-inversion temperature, or PIT, is then a measure of the strength of the hydrophilicity. Shinoda claims that the best stability of such an oil-in-water emulsion is obtained at 30 °C below the PIT and for a water-in-oil emulsion at about 20 °C above the PIT. However, the lowest interfacial tension, and the smallest droplet volume is obtained directly after homogenization by shaking at the PIT. Consequently, Shinoda suggests that the emulsifier should be chosen so that the emulsification can be performed at a PIT about 20–30 °C above the final storage temperature (Shinoda and Saito 1968). The emulsion can then be cooled and the increased interfacial tension stabilizes the small sizes obtained earlier.
Shinoda and coworkers (Shinoda and Saito 1968; Shinoda and Kunieda 1983; Kunieda and Ishikawa 1985, reviewed in Shinoda and Friberg 1986) have worked according to this concept and characterized a number of different ethoxylated emulsifiers in combination with various solvents. They then found that the PIT depends not only on the number of ethoxylate groups but also on the oil phase, indicating the importance of the solubility properties for the stability.
Emulsification experiments performed with a range of different oil-to-water ratios show that the emulsion type is determined mainly by the emulsifier properties and is for many systems, with pure solvents, very insensitive to the phase ratio (Shinoda and Friberg 1986).
It is obvious that this says a lot about the properties of ethoxylated surfactants but its applicability to food emulsions is very limited for two main reasons:
-
1.
The concept is based on strongly temperature-dependent properties of the emulsifiers. This excludes ionic emulsifiers (less important for the food industry), and it also excludes the most commonly used polyhydroxy and nonionic zwitterionic emulsifiers as they display a very weak temperature dependence of their hydrophilicity.
-
2.
The solvent properties are important in the PIT concept. However, food emulsions are made almost solely from triglyceride oils and water that will behave differently due to the large molecular weight of the oil molecules.
6.6.3 The HLB (Hydrophilic/Lipophilic Balance) Concept
Emulsifiers are molecules with a duality in their properties. The balance between the hydrophobic and hydrophilic properties of the molecules should then determine the performance and the type of emulsion formed. If the emulsifier is changed from hydrophobic to hydrophilic, the emulsion formed changes from oil-continuous to water-continuous. The balance of the emulsifier is recorded as a number, the HLB value. When this concept was introduced by Griffin (1949), the HLB value of unknown emulsifiers was determined by comparing the emulsification properties in a predetermined system of a mixture of hydrophobic and hydrophilic emulsifiers with a predefined HLB number.
The important development of the HLB system came when the group contribution system was constructed by Davies (1957), and it became possible to estimate an HLB value of an unknown emulsifier from the molecular formula (Table 6.7).
The advantage of the HLB concept is that it makes it possible to characterize numerous emulsifiers and emulsifier blends. It is usually assumed that it is possible to calculate an average HLB value from the w/w composition. Large tables of data for commercial emulsifiers are also available.
The limitation of the HLB value is that it provides a rather one-dimensional description of the properties, omitting molecular weight and temperature dependence. It is also difficult to calculate useful HLB values for several important food emulsifiers, for instance phospholipids. The HLB values also do not include the important crystallization properties of monoglycerides and modified monoglycerides.
6.6.4 A Comparison Between the HLB and the Geometry of the Molecule
There is an obvious analogy between the idea of a hydrophilic lipophilic balance and that of the balance in the molecules appearing in the packing parameters of different association structures (Fig. 6.8). Griffin (1979) has also suggested a relationship between various solution properties and the HLB number. Transforming these descriptions into various aggregation structures, a clear relation between the molecular packing and the HLB value is obtained.
A comparison between molecular aggregation, solution characteristics, A/A0, and the packing parameter. (Modified from Bergenståhl and Claesson 1990)
This result shows that the ability to form liquid-crystalline phases corresponds to the traditional HLB characterization of the emulsifiers.
6.6.5 The Role of the Emulsifier in Homogenization
The discussion so far has been dealing mainly with the situation when droplets are protected by a layer of emulsifier. However, the emulsifiers also have a crucial role during the emulsification that usually is included in all empirical tests that are the bases for the rules.
When an emulsion is created from a large and homogeneous oil phase, the emulsifier should support two different processes: the formation of new droplets and protection against recoalescence. The emulsifier acts according to both static and dynamic, diffusion-limited, interactions (Walstra 1983) (Table 6.8).
The principal role of the interfacial tension is obvious. The presence of emulsifiers lowers the interfacial tension from about 30 mN/m for a triglyceride/water system to between 1 and 10 mN/m. Nonionic emulsifiers close to the PIT create densely packed interfaces with very low interfacial tensions. However, the effects of the interfacial tension itself are not very large. Walstra (1983) has shown that the droplet size is only weakly dependent on the interfacial tension.
During the homogenization, new interfaces are formed. The emulsifiers have to diffuse to the interfaces to lower the interfacial tension during the events when the droplets are formed. This process must be rapid to be successful, as rapid as the time scale for the formation of the droplets. For geometrical reasons, the diffusion from the surrounding phase of the droplet is much more rapid than the diffusion from the internal liquid. This is one important contribution to the validity of the solution rules (Bancroft, PIT, HLB, and phase diagrams).
During the homogenization, the water-soluble substances in the oil phase diffuse over to the water phase. These types of diffusion across the interfaces create disturbances that contribute to the emulsification. In many systems, this effect gives an increased efficiency if the emulsifier is added to the oil phase before the emulsification. For dispersible emulsifiers (phospholipids) there are also other reasons why it is more efficient to add the emulsifier to the oil phase instead of the water phase. During the homogenization, phospholipids tend to form stable liposomal dispersion in competition with the emulsification of the oil phase. Westesen has indeed observed that a significant fraction of the phospholipids in a commercial phospholipid emulsion for paranteral use is lost in liposomal aggregates (Westesen and Wehler 1992).
Emulsification involves an intensive application of fluid shear stress. The shear stress by itself causes a high frequency of recoalescence events. If the emulsification is to be successful the formed droplets have to be protected from recoalescing. The repulsive interactions generated by adsorbed emulsifiers create a static protection.
Hydrodynamic interactions are crucial to the result of a collision due to shear. Hydrodynamic interactions depend on the existence of an interfacial viscosity and elasticity. During the collision event, the interface close to the approaching droplet is depleted of emulsifiers due to the local flow. The surfactant-depleted zone will then have a higher interfacial tension than the surrounding emulsifier-covered droplet surface. This leads to surface diffusion in the direction opposite to the liquid flow and produces a hydrodynamic resistance. If the emulsifier is oil-soluble, emulsifier from the internal part of the droplet will diffuse to the depleted area and thereby reduce the hydrodynamic protection of the droplet.
The discussion in this section has been very qualitative, but an important point is that the emulsifiers contribute to the emulsification as well as to the stabilization. The role of the emulsifier for the stabilization is usually difficult to identify in the simple type of shaking experiments that are the main background to the HLB, the PIT, and the phase diagram concepts. This type of simple, and thereby efficient, experiment provides information about both the emulsifiability and the stability with a certain emulsifier.
6.7 The Emulsifier Surface
The ability of various food emulsifiers to generate adsorbed layers influencing the interparticle interactions has been discussed. The type and magnitude depend on the composition of the surface generated from the adsorption process. Foods usually are complex mixtures. They may contain both low molecular weight surface-active lipids and a versatile range of more or less surface-active proteins and polysaccharides. The actual chemical composition of the emulsion droplet surface is then the key factor that determines most of the surface interactions.
In systems containing several surface-active components, three types of adsorbed layers can be identified based on how the layers are formed. In reality, the differences between the three adsorption structures discussed below are not sharp, but this simplified description can provide a basis for further discussion of the properties of complex systems.
-
1.
Competitive adsorption . A monolayer containing one predominant type of molecule at the interface builds up through competition with other less surface-active components that may be replaced in the interface.
-
2.
Associative adsorption . An adsorbed layer containing a mixture of several different surface-active components is formed.
-
3.
Layer adsorption . One component adsorbs on top of another or itself.
6.7.1 Competitive Adsorption
In a system with several surface-active components, a homogeneous monolayer is formed by the most surface-active component. The adsorption depends on the driving force for adsorption, mainly the hydrophobic interaction. Hence, from a mixture of two emulsifiers, the most hydrophobic emulsifier will have the strongest affinity for the interface. A consequence is that under competitive adsorption the component with the lowest water solubility, e.g., the lowest critical micelle concentration (Kronberg 1983), will dominate the interface.
The character of the adsorbed layer, for instance its ability to generate repulsive interactions, is determined by the dominating compound. The structure of the layer depends on the geometrical shape of the molecules and on lateral interactions between the molecules in the layer. Nonionic surfactants may form very dense layers due to head-group attraction. Ionic surfactants are able to form extremely loose layers due to inter-head-group repulsion.
An interesting experimental observation in agreement with this relation is that the concentration of emulsifier necessary to obtain an emulsion is much lower for ionic emulsifiers than for nonionic emulsifiers.
In a series of emulsions, we have studied the efficiency of the emulsification (Östberg et al. 1995) by droplet size measurements after homogenization. The results show that for several emulsifiers very small droplets are obtained, about 0.2–0.4 μm. The size obtained depends on the concentration of emulsifier. The nonionic emulsifiers lead to a constant droplet size down to a critical concentration, below which the ability to form emulsions is strongly reduced. The critical concentration can be compared with the thickness of the emulsifier layer on the emulsion droplet. The apparent thickness of the emulsifier layer can be estimated from the droplet size and the concentration of emulsifier on the dispersed phase, if we assume that all emulsifier is adsorbed to the interface. The apparent thickness gives the upper limit for the absorbed layer rather than the correct value:
where ccm is the emulsifier concentration (v/v) in the dispersed phase. The critical thickness, the thickness of the emulsifier layer at the critical concentration, can be compared with the size of the molecule. The results show a thickness of about 60% of the theoretical length of the molecule for nonionic emulsifiers. Hydrophobic emulsifiers are less efficient during the emulsification and give very high values of the apparent thickness. The properties of the ionic emulsifiers are different as they are able to form emulsions down to extremely low concentrations corresponding to very low surface concentrations and very thin layers.
6.7.2 Associative Adsorption
In associative adsorption, a mixed adsorption layer is formed at an interface. The interfacial properties displayed are then some sort of average properties.
A typical associative system may be a long alcohol, e.g., decanol and charged surfactants like soaps. The alcohol acts as a spacer between the charged groups, which decreases the head-group repulsion within the layer and reduces the surface energy. This increases the adsorption and enhances the surface activity. Similarly, a lamellar phase is formed in the corresponding three-component phase diagram: water/sodium caprylate/decanol (Fontell et al. 1968). Mixed layers are commonly formed due to associative adsorption with natural and technical emulsifier blends. This is also a necessary requirement of the common assumption that an average HLB value should describe the properties of an emulsifier blend (Davies 1957). A common system assumed to act in this way is a mixture of sorbitan esters and ethoxylated sorbitan esters where the smaller sorbitan esters can use the space between the bulky ethoxylated esters (Boyd et al. 1976).
In the case of associative adsorption, both components are expected to be present in the surface. If this situation is to be stable, the adsorption of the second component should be either enhanced by the presence of the first component or at least not influenced by it.
6.7.3 Layered Adsorption
Adsorption in layers is possible when different classes of surface-active components are present in a mixture. See Table 6.9. The two components must be very different in character to give a structure with a layered character rather than a mixed layer. The second component adsorbs to a droplet displaying the characteristic properties of the primary adsorbing emulsifier. This usually means a more hydrophilic surface, which can be expected to reduce the adsorbed amount. However, in some cases, the presence of certain groups increases the adsorption of specific substances. For example, the effects of emulsifiers on protein adsorption is essential in most food applications.
Ethoxylated surfactants usually give a strong reduction of protein adsorption. Courthaudon et al. (1991b) have shown C12EO8 totally displaces all adsorbed β-casein from an emulsion system. Similar effects have also been obtained with emulsions formed with polysorbates (Dickinson and Tanai 1992) and with monoglycerides (Hall and Pethica 1967). On the other hand, egg yolk PC did not reduce the adsorbed amount of β-casein more than about 20% (Courthaudon et al. 1991a).
The adsorption of a range of plasma proteins at various phospholipid surfaces has been characterized using ellipsometry (Malmsten 1995). A large variation of the adsorbed amount was obtained, depending on the combination of protein and phospholipid. Purified PC and PE gave low adsorbed amounts, while phosphatidic acid enhanced the adsorption of fibrinogen by a factor of 5 compared to a bare hydrophobic surface.
6.8 Conclusions
Emulsifiers form the basis for a wide range of essential stability, quality, and efficiency functions in food formulations and remain the preferred choice in most cases because of their simplicity and safety. Complex physicochemical emulsification phenomena occur in formulations and it is important to understand the interactions of emulsifiers and other species using the above information. Complementary reviews on polymeric and steric stabilizers (Heertje 2014) as well as other types of emulsifiers like proteins (Kravlova and Sjöblom 2009) are also recommended.
References
Arvidsson G et al (1985). Eur J Biochem 152:753–759
Bancroft WD (1913). J Phys Chem 17:501
Bergenståhl B (1991) In: Dickinson E (ed) Food polymers, gels, and colloids. Royal Society of Chemistry, London, pp 123–131
Bergenståhl B, Claesson PM (1990) In: Larsson K, Friberg S (eds) Food emulsions. Marcel Dekker, New York
Bergenståhl B, Fontell K (1983). Prog Coll Pol Sci 68:48–52
Bergenståhl B, Stenius PJ (1987). Phys Chem 91:5944–5948
Boyd JV et al (1976) In: Smith AL (ed) Theory and practice of emulsion technology. Academic Press, London
Caffrey M (1985). Biochemistry 24:4826–4844
Courthaudon JL et al (1991a). J Agr Food Chem 39:1365
Courthaudon JL et al (1991b). J Colloid Interface Sci 145:390
Darling D, Birkett RJ (1987) In: Dickinson E (ed) Food emulsions and foams. Royal Society of Chemistry, London
Davies JT (1957) Proc. Intern. Congr. Surf. Activity, vol 1. 2nd edn. Butterworth, London, p 426
Dickinson E (1986). Food Hydrocoll 1:3
Dickinson E, Tanai S (1992). Food Hydrocoll 6:163–171
Dickinson E et al (1991). Food Hydrocoll 4:403–414
Eriksson PO et al (1985). Chem Phys Lipids 37:357–371
Eriksson PO et al (1987). Phys Chem 91:846–863
Fontell K (1978). Progr Chem Fats Other Lipids 16:145–162
Fontell K et al (1968) Acta Polytechnica Scandinavica, Chapter 2, Chemistry Series III, 74, 2
Friberg S (1971). J Colloid Interface Sci 37:291
Friberg S (1990) In: Larsson K, Friberg S (eds) Food emulsions. Marcel Dekker, New York
Friberg SE (2006). Colloids Surf A 282-283:369
Friberg S, Mandell L (1970a). J Assoc Off Chem Soc 47:149
Friberg S, Mandell L (1970b). J Pharm Sci 59:1001–1004
Friberg S, Rydhag L (1971). Kolloid Z u Polymere 244:233–239
Friberg S, Wilton I (1970) Liquid crystals—the formula for emulsions. Am Parf Cosm 85:27–30
Friberg S et al (1969). J Colloid Interface Sci 29:155–156
Gawrish K et al (1992). Biochemistry 31:2856–2864
Griffin WC (1949). J Soc Cosmetic Chemists:311–326
Griffin WC (1979) Kirk-Othmer encyclopedia of chemical technology, vol 8. Wiley, New York
Hall DG, Pethica DA (1967) In: Schick MJ (ed) Nonionic surfactants. Marcel Dekker, New York, p 516
Heertje I (2014). Food Struct 1:3–23
Inoko Y, Mitsui TJ (1978). Phys Soc Jap 44:1918
Israelachvili J (1992) Intermolecular and surface forces. Academic Press, London
Israelachvili J et al (1976). J Chem Soc Faraday Transactions 11(72):1525
Israelachvili J et al (1977). Biochim Biophys Acta 470:185–201
Janiak MJ et al (1979). J Biol Chem 254:6068–6078
Kravlova I, Sjöblom J (2009). J Disp Sci Tech 30:1363–1383
Krog N (1990) In: Larsson K, Friberg S (eds) Food emulsion. Marcel Dekker, New York, p 127
Kronberg B (1983). J Colloid Interface Sci 96:55–68
Kunieda H, Ishikawa N (1985). J Colloid Interface Sci 107:122–128
Kunieda H, Shinoda K (1985). J Colloid Interface Sci 107:107–121
Larsson K, Krog N (1973). Chem Phys Lipids 10:177
Laughlin RG (1994) The aqueous phase behavior of surfactants. Academic Press, London
Lindblom G et al (1991). Biochemistry 30:10938–10948
Malmsten M (1995). J Colloid Interface Sci 172:106–115
Östberg G et al (1995). Colloid Surf A Physichem Eng Asp 94:161–171
Pezron I et al (1991). J Colloid Interface Sci 144:449–457
Rodriguez C, Shigeta K, Kunieda H (2000). J Colloid Int Sci 223:197–204
Rosevear F (1968). J Am Oil Chem Soc 31:628–639
Rydhag L (1979). Fette Seifen Anstrichm 81:168–173
Rydhag L, Wilton I (1981). J Assoc Off Chem Soc 58:830–837
Shinoda K, Friberg S (1986) Emulsions and solubilization. Wiley, New York
Shinoda K, Kunieda H (1983) In: Becher P (ed) Encyclopedia of emulsion technology, vol 1. Marcel Dekker, New York
Shinoda K, Saito H (1968). J Colloid Interface Sci 30:258–263
Small DM (1986) Handbook of lipid research: physical chemistry of lipids. Plenum, New York
Söderberg I (1990) Structural properties of monoglycerides. Phospholipids and fats in aqueous systems, PhD Thesis, University of Lund, Sweden
Tanford C (1973) The hydrophobic effect. Wiley, New York
Walstra P (1983) In: Becher P (ed) Encyclopedia of emulsion technology, vol 1. Marcel Dekker, New York, p 57
Walstra P (1988) In: Phillips GO et al (eds) Gums and stabilizers for the food industry, vol 4. IRL Press, Oxford, pp 233–336
Westesen K, Wehler T (1992). J Pharm Sci 81:777
Wilton I, Friberg S (1971) Influence of temperature-induced phase transition in fat emulsions. J Assoc Off Chem Soc 48:771–774
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bergenståhl, B., Spicer, P.T. (2019). Physicochemical Aspects of an Emulsifier Function. In: Hasenhuettl, G., Hartel, R. (eds) Food Emulsifiers and Their Applications. Springer, Cham. https://doi.org/10.1007/978-3-030-29187-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-29187-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29185-3
Online ISBN: 978-3-030-29187-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)