Abstract
Brain cancer is regarded as the most widespread cancer of the central nervous system. The complexity of the glial cell tumor renders the survival and prognosis of glioma difficult, even after conventional chemotherapy, radiotherapy, and surgery treatments. The major concern is the formidable blood–brain barrier (BBB) that guards the entry of all exogenous moieties into the brain. This chapter addresses in brief the various approaches to bypass the BBB. Among different strategies, receptor-oriented drug delivery takes advantage of receptors on the BBB to traverse into the brain and if appropriately designed can enter the cancer cells through receptors overexpressed on their surface. This chapter will also summarize the various receptors, their physiology, ligands for the receptor, and drug-delivery strategies that could improve brain cancer therapy.
Rijo John and Heero Vaswani are equally contributed to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Brain cancers, the uncontrolled growth of cells in brain, are among the most feared malignancies owing to their fast development and poor diagnosis. These are either primary or secondary metastasized. Primary brain cancers arise from a single brain cell type and most common are gliomas, meningiomas, medulloblastomas, and primary central nervous system (CNS) lymphomas [1]. Metastatic brain cancers originate from cancerous cells that are transported through the blood stream from distant areas located in other regions of the body. Although efforts in drug discovery have resulted in newer drugs for glioma, crossing the blood–brain barrier (BBB) constitutes the major limiting factor in the effective treatment of brain cancers. This section describes the BBB and discusses briefly the different opportunities of crossing the same. A specific focus is the endocytic pathway through the receptors on the BBB and the various strategies for targeting drugs and drug-loaded nanocarriers into the brain by receptor-mediated endocytosis.
2 Blood–Brain Barrier
The blood–brain barrier is a discriminating barrier endowed with the ability to block about 98% of molecules with low molecular weight and almost 100% of larger molecules [2]. The endothelial cells of brain capillary with the interspersed tight junctions, supported with basal lamina, pericytes, and astrocytes regulate the transport of all molecules across the brain [3]. This ensures adequate delivery of nutrients and other endogenous moieties while restricting delivery of foreign substances. The interface between blood and brain constitutes the brain endothelium that is interspersed with tight junctions formed by intimate connection of the endothelial cells and astrocytes. .These tight junctions serve as gatekeepers for the paracellular pathways through the BBB. The endothelial lining consists of two discrete sides, the abluminal (brain side) and luminal (blood side). Abluminal side is supported by pericytes which are attached at irregular intervals and is surrounded by the basal lamina (30–40 nm) comprising proteoglycans, type IV collagen, fibronectin, laminin, heparin sulfate, and other matrix proteins which are extracellular. The basal lamina is closely linked with the astrocytes end feet. These collectively constitute the protective BBB which prevents the transport of molecules from the luminal portion to the abluminal portion and into the brain [4]. The astrocytes end feet play a crucial role in regulating the BBB permeability. However, there are a number of pathways through the BBB that allow vital compounds to be transported into the brain [5]. These are discussed in brief and schematically depicted in Fig. 2.1.
2.1 Transport Mechanisms across Across the Blood–Brain Barrier
2.1.1 Transport of Water, Ions, and Nutrients
The movement of water in the brain is regulated through Aquaporin-4 (AQP4) membrane channels which help in osmotic regulation. The transport of ions across the BBB is generally through ion channels. Optimal ion concentrations are important to maintain proper environment for synaptic and neural function. Astrocytes play a significant role in maintaining the blood–brain barrier (BBB) by ensuring ion homeostasis and monitoring metabolism of the amino acid neurotransmitters, as well as assisting neurons with their energy and nutrient requirements [6].
2.1.2 Paracellular Transport
Paracellular transport is a mechanism by which transport of small molecules occurs across the endothelial cell. By this way, it bypasses a group of substrate-specific transport systems that are designed to mediate transport across the BBB [7]. The transport of gaseous molecules like O2 and CO2 along with some smaller molecules having sufficient hydrophilicity and lipophilicity such as ethanol and caffeine can occur via paracellular pathway. The entry of larger molecules is limited by this mechanism [8]. Some strategies reported to enhance the paracellular transport of the drug molecule include chemical or physical modification, alteration of physicochemical properties, and the use of compounds modulating tight junctions such as surfactants and chelating agents [9].
2.1.3 Transcellular Diffusion
The transport of drugs or endogenous molecules by transcellular diffusion is a process dependent on concentration gradient, allowing a wide range of low molecular weight lipophilic molecules to cross the BBB based on Lipinski’s rule [10]. Two important criteria governing this transport are the lipophilicity (Log P between −0.2 and 1.3) and molecular mass (<400 Da) of the compound. Lipophilicity is directly proportional while molecular weight is inversely proportional to the transport of drug across BBB [11]. Other factors that can influence the diffusion of drug across the BBB are the ionization, shape, protein complexation, and hydrogen bonding [12]. Significant limitations of transcellular diffusion have demanded alternative strategies for transport of drugs across the BBB.
2.1.4 Carrier-Mediated Transport
Carrier-mediated efflux is critical for exchange of nutrients, neurotransmitters, and metabolites crossing the BBB. These carriers belong to the Solute Carrier (SLC) superfamily of transporters which are accountable for the transport of specific substrates. Some carriers are very selective in their stereochemical substrate requirements, such as glucose transporter type 1 for glucose, monocarboxylic acid transporter 1 for monocarboxylic acids, and for large neutral and cationic amino acids, the large neutral amino acid transporter I and cationic amino acid transporter I, respectively. The highest carrying capacity is shown by hexose and large neutral amino acid carriers which can serve as effective transporters of cargo to the brain [13].
2.1.5 Adsorptive Endocytosis
Adsorptive endocytosis involves the interaction of the ligand with the cell surface and is activated by electrostatic interaction with micromolecules or positively charged proteins with the negatively charged plasma membrane. Adsorptive endocytosis is nonspecific, occurs via clathrin-mediated mode, and provides the opportunity for movement of molecules across the BBB via endothelial cytoplasm [14]. Cell-penetrating peptides and cationic proteins exhibit adsorptive endocytic uptake. The method adopts the brain endothelial cell technique for binding and absorbing cationic molecules from the blood vessels into the brain via molecular exocytosis [15].
2.1.6 Receptor-Mediated Endocytosis
Receptor-mediated endocytosis (RME) involves binding of transmembrane receptors with ligands. These receptors are expressed on the apical plasma membrane of the endothelial cells. Receptors on the BBB are proteins residing on the membrane of postsynaptic neurons receiving neurochemicals released by brain cells from synapses. Receptors bind with neurotransmitters using a lock and key metaphor, and can be classified based on their location as transmembrane receptors and intracellular receptors [16]. Cells with numerous receptors bind with ligands to activate or inhibit a specific receptor-mediated biochemical pathway. Endocytosis can occur by one or more of the mechanisms detailed in Chap. 1. While clathrin-mediated endocytosis is the major pathway, receptors depending on their constitution may exhibit other pathways of internalization. The brain uptake of nutrients such as leptin [17], iron, and insulin [18] occurs by receptor-mediated transport mechanism. Other receptors present on the BBB are generally exploited for transport across BBB for chemotherapy of brain tumors [19]. Receptor-mediated endocytosis relies on specific and selective affinity of receptors for certain ligands which can be effectively utilized to target therapeutics into the brain through the BBB [20]. Nanocarriers have been successfully delivered using such strategies.
Once delivered inside the brain by one or more of the above strategies, nanocarriers can diffuse out through leaky blood vessels in tumors enabling delivery and concentration buildup through enhanced permeability and retention (EPR) effect [21]. This enables a second stage of RME, wherein nanocarriers are internalized into brain cancer cells through overexpressed receptors. EPR relies on pathophysiological characteristics of tumor tissue, wherein the fenestrated vasculature permits extravasation of materials of nanometer size. This supported by the lack of lymphatic drainage aids in the enhanced and effective accumulation of nanocarriers in tumors [22].
3 Targeting Receptors for Brain Cancer
The BBB has many receptors which can be exploited for drugs to enter the brain. Figure 2.2 depicts the receptors on the BBB and those overexpressed in brain cancers. Interestingly, most of these receptors are overexpressed in brain cancers with the exception of diphtheria toxin receptor and the tumor necrosis factor receptor. The cascade receptor-targeting strategy is therefore appropriate for the therapy of brain cancers, wherein a single ligand can be used for targeting. In the first stage, the ligand would ensure receptor-mediated transport of drug-loaded nanocarriers across the BBB, and in the second stage serve as ligand for receptors, thereby boosting drug concentration to enhance efficacy.
Among the receptors that are majorly studied for targeted drug delivery, this chapter focuses on low-density lipoprotein receptor, integrin receptor, interleukin receptor, and lactoferrin receptor. Other receptors are explicitly discussed in other chapters of book and therefore not dealt with here. The ephrin, immunoglobulin, and insulin receptors are minimally studied and hence not considered under the purview of this chapter.
3.1 Low-Density Lipoprotein Receptors in Brain Cancer
3.1.1 Occurrence of Low-Density Lipoprotein Receptors
Low-density lipoprotein receptors (LDLR) along with their allied proteins 1 (LRP 1) and 2 (LRP 2) mediate the movement of lipoproteins and ligands across the blood–brain barrier by receptor-mediated endocytosis. These receptors are densely localized in specific areas with 80% covering only 2% of the cell surface. LDLs such as cholesterol, tocopherol, and apolipoprotein can bind to the LDLR, located in the caveolae membrane fraction and go across the BBB to reach inside brain tissue through caveolin-mediated endocytosis. Apolipoproteins (Apo B and Apo E) [23] mediate particle interaction with lipoprotein receptors including LRP1, LRP2 and LDLR [24]. The significantly high amount of LDLR on BBB and glioma cells [25] is exploited to carry anticancer drugs through BBB using nanocarriers as vehicles.
3.1.2 Structure and Signal Transduction of LDLR
Low-density lipoprotein receptor consists of spherical particles of 220 Å in diameter having a nonpolar core of approximately 1500 molecules of cholesterol that are esterified by means of fatty acids like oleate and linoleate. LDLR is a transmembrane glycoprotein with single chain [26]. It has five distinct domains, including a ligand-binding domain, EGF precursor-homology domain, O-linked sugar domain, membrane-spanning domain, and cytoplasmic tail [27]. Receptors contain different endocytosis signals that mediate their interactions with the endocytic machinery, and these receptor cytoplasmic tails appear to bind many cytosolic adapters and scaffold proteins that add to signal transduction [28].
3.1.3 Recognition Domain of Low-Density Lipoprotein Receptor
The region of LDLR binding to ligand includes cysteine-rich repeats of 40 amino acids which are seven in number followed by EGF precursor-homology domain consisting of three units of EGF-like repeats of 40 amino acids. The third region, O-linked sugar domain, is enriched with residues of inserin as well as threonine that act as acceptors for O-linked sugars. A hydrophobic 24 amino acids membrane-spanning domain is responsible for the attachment of LDLR to the lipid bilayer. The cytoplasmic tail regulates the intracellular transport and endocytosis across the LDLR. The cysteine units located at amino terminus of receptor mediate the folding of each repetition into a compact structure by forming three disulfide bridges with the bunch of negatively loaded residues with tripeptide surface. The linkers play a significant role by offering the domain with flexibility to accommodate distinct size lipoprotein ligands. LDL particles constitute an outer phospholipid surface layer covered by single protein apoB-100 [29]. The surface exposing the proteins allows recognition of receptor by the amino acid residues and serves as LDLR binding domain. Intercalation of free cholesterol with chains of phospholipid fatty acids imparts some amount of stiffness to the outer monolayer of LDL [30].
3.1.4 Binding of Ligand and Receptor Pathway
The internalization of LDL that binds to LDLR occurs via clathrin-dependent pathway. However, internalization of LDL particles can occur by receptor-mediated and receptor-independent processes. On an average, 30–40% of complete LDL plasma pool is cleaned from body every day, with about two-thirds of the circulating LDL pool removed by receptor-mediated uptake [31]. The receptor-binding sequences present on apoB-100 are highly cationic, while on the cell surface, complementary anionic sequences are present. Electrostatic interactions between them enable binding of LDL particles to LDLR implanted in clathrin-coated pits on the cell membrane [27]. The ligand-binding sites in LDLR are contained in clusters, suggesting a functional duplication within receptor and high-affinity binding of most ligands needs multiple ligand-binding repeats. Some ligands can sequentially identify distinct combinations of these repeats, while others seem to identify repeats from different clusters [32]. The LDL receptor carries particular macromolecules, primarily cholesterol-rich lipoprotein LDL, through receptor-mediated endocytosis into cells.
3.1.5 Ligands for LDLR
LDL mainly contains cholesterol (approximately 50%) in free and esterified form, 25% proteins, 22% phospholipids, and 5% triacylglycerol [33]. Native LDL exhibits high affinity for LDLR; nevertheless, difficulty in isolation on large scale and high variability in composition and size and difficulty in purification limit its use. Studies on several ligands and their use to transport nanocarriers, antibodies, and drugs through BBB are reported [34]. Aprotinin, angiopep-2, ApoE3 mimetic, and p97 can be used to target LDL receptor family [35]. Targeting must preferably not affect receptor function, as this may result in toxicity or other side effects and ligands should also not be immunogenic [36]. Thus, synthetic LDL analogs prepared as replacements of native LDL could have better applications.
3.2 Integrin Receptors in Brain Cancer
3.2.1 Occurrence of Integrin Receptors
Integrins are powerful heterodimeric transmembrane receptors on the cell surface which play a significant part in cell-to-stroma interaction. They are expressed in significant numbers in tumor vasculature, angiogenic endothelial cells, and tumor cells in gliomas. It is proposed that integrins play a major role in initiation with advancement in cancer [37]. In various cancers, integrins αvβ3 and αvβ5 are reported to be essential for angiogenesis caused by fibroblast growth factor and tumor necrosis factor specifically in malignant gliomas. Integrin αvβ3 is expressed in angiogenic endothelial cells [38]. Immunohistochemistry validation by molecular imaging using tracer [18F]Galacto-RGD confirmed that αvβ3 integrin expression was primarily restricted to the tumor region and was absent in ordinary tissues [39]. Integrin plays a significant role in encouraging glioma development, invasion, and angiogenesis, and hence can be used as a target for improving cancer therapy.
3.2.2 Structure and Signal Transduction by Integrin Receptor
Integrins are 18α and 8β subunits that are assembled into receptors. Twenty-four integrin receptors are recognized at different locations on nucleated cells with separate features. Each α/β subunit comprises 1000 amino acids with a single membrane-spreading helix and 750 amino acids with a short cytoplasmic tail [40].
Integrin alpha (α) subunit is formed by seven-bladed β-propellers linked to a thigh. The leg structures formed by a calf-1 and a calf-2 domain support the integrin head domain. The end three or four blades of β-propeller contain EF hand domain which connects calcium on the lower side facing away from the ligand-binding surface. Ca2+ binding to these sites allosterically affects ligand binding [41].
The integrin beta (β) subunit includes four domains, a β1 domain, a plexin semaphorin integrin domain (PSI), a hybrid domain and a β tail domain with four EGF repeats which are cysteine-rich [42]. The β1 domain also shows the presence of a Mg2+-coordinating MIDAS (Metal-ion-dependent adhesion site) and an adjacent MIDAS site (ADMIDAS) that binds a Ca2+ ion inhibitor. Binding of ADMIDAS site to Mg2+ ion enables integrins in the active form [41]. Integrin receptors are enzymatically inactive and require linkage with certain components of cell to generate signal transduction. The phenomena known as “outside-in signaling” is associated with integrins, for transducing signals within the cells after binding to extracellular matrix. This signaling is necessary for polymerization of actin cytoskeleton during cell linkage and for controlling cell migration, division, and survival. Phosphorylation of proteins is the first event detected in response to integrins stimulation. Other responses consist of cytosolic kinases induction, phosphoinositides metabolism stimulation, Ras/MAPK activation, and protein kinase C (PKC) pathway as well as regulation of Ras homologous (Rho) GTPases. Integrins have an impact on cell survival as it regulates programmed cell death, which is a tyrosine phosphorylation-dependent response. The signaling pathways of integrins synergize with pathways of other receptors to elevate or decline signals produced by each of the receptors [43]. Thus, a challenging aspect here is the identification of signaling molecules that arbitrate cross talk between the different receptor pathways.
3.2.3 Recognition Domain of Integrin Receptor
The RGD tripeptide in ligands serves as the identification site for different integrins such as αv integrins, β1 integrins α5 and α8, and αIIbβ3. Some of crystal structures complexed with RGD ligands disclosed the same atomic essentials for interaction [44]. RGD shows binding on the α and β subunit interface domain where basic residues fit the cleft in the b-propeller module subunit, while cation-coordinated acid residue shows binding to b-I domain [45]. RGD-binding integrins are most promising among the receptor family, particularly binding β3 integrins to large amounts of extracellular matrix and soluble vascular ligands. While subsets of integrins share many ligands, the affinity order of ligand differs, probably reflecting accuracy of ligand RGD fit with particular α,β active site pockets.
3.2.4 Binding of Ligand and Integrin Receptor Pathway
Integrins are known to follow multiple internalization pathways including clathrin-mediated endocytosis, caveolae-mediated endocytosis, and sometimes by both clathrin- and caveolae-mediated endocytosis. These pathways occur depending on factors such as the cell environment, cell condition, and cell type [46]. However, adhesion is extremely dynamic, with cells continually sampling their pericellular environment and reacting by quickly altering their position and differentiation status, offering an extremely responsive mechanism for receptor activation. The formation of explained complexes is achieved in two ways, by clustering receptors, which increases molecular interactions resulting in increased binding rate of effector molecules, and by inducing conformational modification in receptors that create or expose binding sites. Conformational regulation is generally the main mode for integrin regulation to affinity [47]. Thus, suitable mechanisms to convey conformational changes over a comparatively large distance between cytoplasmic tails and ligand-binding head domain (20 nm) are important [48].
3.2.5 Ligands for Integrin Receptors
The various ligands with their respective partner integrins include the following: bone sialoprotein that interacts with αvβ3 and αvβ5; collagens that interact with α1β1, α2β1, α11β1, and αIbβ3; fibronectin that interacts with α2β1, α3β1, α4β1, α4β7, α5β1, α8β1, αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, and αIIbβ3; matrix metalloproteinase-2 that interacts with αvβ3; and plasminogen that interacts with αIIbβ3 [49].
3.3 Interleukin Receptors in Brain Cancer
3.3.1 Occurrence of Interleukin Receptors
Interleukin is a proinflammatory cytokine that promotes malignant actions by activating different cells to upregulate major oncogenic event molecules. Upregulation of cytokines IL-1b, IL-4, IL-6, and IL-8 are majorly observed in patient samples and glioma cell lines. Cell line and xenography studies hypothesize that these inflammatory mediators may be valuable in brain tumor treatment. Interleukin 13 receptor has a key role in immune response regulation coupled with immune microenvironment modulation. IL13Rα2 set a well-defined, fresh glioma therapy. Even before the receptor was characterized, the therapeutic advantage of IL-3-truncated PE fusion chimera protein (IL13PE38QQR) was identified in gliomas [50].
3.3.2 Structure and Signal Transduction by Interleukin Receptor
The receptor consists of 2 distinct chains IL-13Rα1 and IL-13Rα2 which are paired with heterodimer complex IL-4Rα and IL-4Rβ forming the main IL-4 binding protein. This complex acts as a transcription factor activator. Binding triggers cell signals which lead to activation of transcription factors. Cytoplasmic areas of both type II receptors IL-13Rα1 and IL-4Rα chains communicate with different tyrosine kinases, which eventually interact with PI3 kinase and STAT6. Endocytosis is, however, triggered only by additional binding of IL-13 to the IL-13Rα2 chain, found only in type IL-13R. Studies showing high levels of IL-13Rα2 discovered within the intracellular vesicles confirmed that this form IL-13R is in endocytic conduct. In addition, affinity of IL-13 for IL-13Rα2 is higher when compared to IL-13Rα1. IL-13Rα1 activates intracellular signaling in tumor cells, endothelial cells, fibroblasts, and immune cells via Janus-activated kinase/signal transducers and transcription pathway activators. In addition, these ILs are powerful signaling pathway activators that control cell survival and encourage chemotherapy resistance. Therefore, in conjunction with cytotoxic agents, targeting inflammatory cytokines in GBM microenvironment with kinase inhibitors could bring therapeutic benefits to patients with persistent GBM [51].
3.3.3 Recognition Domain of Interleukin Receptor
The extracellular portions of IL-13Rα1 and IL-13Rα2 are composed of three fibronectin type III domains D1, D2, and D3. Each domain includes 100 amino acid residues, developing a β sandwich structure. Cysteine residues in fibronectin domain form two disulfide bonds that play a major role in positioning and binding of ligands to receptor. Domains D2 and D3 comprise the cytokine receptor homology module, a common structure of cytokine receptor family. However, there has been no information about the role of D1 domain in IL-13Rα1 and IL-13Rα2 binding to IL-13 and critical amino acids of cytokine receptor homology modules of IL-13Rα1 and IL13Rα2 [52]. IL13Rα2 gene encodes for a 380-amino-acid protein that consist of a 26-amino-acid signaling sequence and a short 17-amino-acid intracellular domain. This expresses about 30,000 binding sites for IL-13 in brain cancer.
3.3.4 Binding of Ligand with Interleukin Receptor Pathway
The IL-13 ligand binds at diverse cytokine receptor homology sites in IL13Rα1 compared with IL13Rα2; and furthermore, IL13Rα1 binding requires heterodimerization with IL4Rα for a high-affinity bond. A definite sequence between the glutamic acid at position 9 of IL-4 and that at position 13 of IL-13 is believed to be involved in the binding of IL-13 to IL4Rα. IL-13 first gets bound to IL-13Rα1 chain through interactions involving amino acid residues namely, arginine, lysine, and glutamic acid on IL-13 αD helix; this interaction generates an IL-4Rα chain to bind to IL-13/IL-13Rα1 complex at residues on IL-13 helices completing type II IL-13R formation. Once IL-13 is linked to equal receptor chains, its affinity improves dramatically, suggesting binding. Internalization occurs through a distinctive clathrin-independent endocytosis pathway. IL-13Rα2 is a membrane-bound protein with high affinity to IL-13. Furthermore, it was found that only interactions involving IL-13 and IL-13R could cause internalization owing to existence of the distinctive IL-13Rα2 chain of receptors [53].
3.3.5 Ligand for Interleukin Receptors
IL-13 receptors are recognized as a prospective target for glioblastoma of high grade. IL-13 ligands have four alpha helices of which helix D is mainly accountable for its IL-13Rα2 interaction. Additional mutations of arginine to aspartic acid, serine to aspartic acid, and lysine to arginine at various positions enhance specificity of IL-13 ligand to IL-13RA2.
3.4 Lactoferrin Receptors in Brain Cancer
3.4.1 Occurrence of Lactoferrin Receptors
Lactoferrin (Lf) plays numerous physiological roles especially in host defenses against infections and serious inflammation. This wide range of biological activities is based on Lf’s interaction with a large number of cells, which mediates the absorption of Lf into cells. It occurs not only in distinct species on the BBB, but also on glioma cell surface, making it a prospective cascade-targeting ligand for brain cancer. Lf’s adsorption is controlled by LDL, which are excessively expressed in glioma cells, allowing Lf to mediate transcytosis of carriers into glioma cells.
3.4.2 Structure of Lactoferrin Receptor
Lactoferrin (Lf) is a transferrin (Tf) family mammalian cationic iron-binding glycoprotein. It shows the presence of two identical lobes that are bound to each other by an extended helical loop that is a highly sensitive site for protease cleavage. Lactoferrin constitutes a 25 amino acid peptide that includes two cysteine residues connected by a disulfide bridge and also contains positively charged hydrophobic residues. Lf exists in iron-free form in biological fluids making them susceptible to proteolysis [54].
3.4.3 Binding of Ligand and Lactoferrin Receptor Pathway
The cerebral lactoferrin receptor has two types of binding sites [55]. The endogenous Lf is lower than the receptor’s peak binding constant, and hence cannot bind in sizeable amount to LfR receptor. It is unable therefore to competitively inhibit exogenous lactoferrin. LRP 1 receptor, belonging to the LDL receptor family engaged in internalizing and subsequent degradation of remaining chylomicron particles, mainly internalizes Lf. It is found that Lf is physically associated with cluster of differentiation 14 (CD14), lipopolysaccharide (LPS), and lipopolysaccharide-binding protein (LBP), which signifies interaction of Lf with accessory molecules involved in the Toll-like receptor (TLR4) pathway [56]. LfR located on the plasma membrane is responsible for the uptake of Lf through clathrin-mediated and caveolae-mediated endocytosis [57].
3.4.4 Ligand for Lactoferrin Receptors
The major ligand binding to LfR is lactoferrin. Thus, the different ligands of Lf are categorized based on the different species in which they bind to different tissues. These include human Lf (hLf), bovine Lf (bLf), mouse Lf (mLf), and piglet Lf (pLf) [54].
4 Receptor-Mediated Targeting Strategies
Developments in chemotherapy, radiotherapy, and surgery have resulted in several benefits for brain tumor treatment. While they have demonstrated significant advantages beneficial for primary cancers, brain cancers of metastatic origin demand newer therapeutic options to improve survival rates and quality of life. Novel promising approaches intended for brain cancer therapy should focus on increasing drug accumulation at the tumor site while ensuring minimal toxic effects to other parts of the brain. The overexpression of different receptors as discussed above with continuous changes in tumor microenvironment and vascular characteristics are features that can drive such developments. Among many approaches, receptor-mediated endocytosis which is the focus of this chapter is defined as a mature strategy for targeted brain delivery [58] with high specificity, affinity, and selectivity. Nanotechnology strategies in particular elicit great promise for receptor-mediated delivery [59]. The different targeting approaches are detailed in the following text.
4.1 Prodrugs
Prodrugs are inactive molecules consisting of inert moieties generally lipophilic, such as fatty acids, glycerides, or phospholipids which are covalently bonded to the parent drug moiety. This renders the molecule the desired lipophilicity to cross the BBB. On reaching the brain through blood, the active agent is released by reaction with enzymes present on the surface of blood–brain barrier [60]. The prodrug approach enables delivery of hydrophilic drugs to CNS without modification in their pharmacological activity [61]. A lactoferrin-modified pH-sensitive prodrug comprising hyaluronic acid-doxorubicin exhibited highest cytotoxicity to C6 cells and great promise in C6 glioma-bearing nude mice model.
Different techniques are employed for prodrug targeting such as prodrugs selective for hypoxia, antibody-directed enzyme prodrug therapy (ADEPT) , gene-directed enzyme prodrug therapy (GDEPT), and virus-directed enzyme prodrug therapy (VDEPT). All these strategies can be used for prodrug targeting with optimized treatment of brain tumor. For greater details, readers are directed to the following review [62].
4.2 Antibody–Drug Conjugates
Antibody–drug conjugates (ADC) are basically antibodies linked with a cytotoxic drug, which allows immediate delivery of the payload to tumor cells based on the specificity of the targeted antibody. The critical factors in development of antibody–drug conjugate involve the antibody, the cytotoxic drug, and linker that joins the other two moieties to form the antibody–drug conjugate. Antibody attached must be specific for an antigen which is overexpressed selectively on cancer cells in comparison to normal cells [63]. The conjugate must exhibit adequate stability in the blood to enable delivery at the target site [64]. Conjugates can be classified as either cleavable, where the chemical bond between drug and linking site on antibody is cleaved intracellularly [63] or noncleavable where the conjugate itself is released inside cells, and the drug released by proteolytic degradation within the cell lysosome [65]. The selection of optimal concentration of antibody, ideal linker, and optimal ratio of drug linked to the antibody are key factors to maximize efficacy of the conjugates [66]. Immunotoxins have generally been delivered as ADCs. The toxins evaluated include bacterial toxins such as diphtheria toxin, and Pseudomonas aeruginosa exotoxin A (PE) which are uptaken by receptor-mediated endocytosis. Inside the lysosomal compartments, these immunotoxins get degraded, causing the toxin payload to be released [67]. Studies have revealed that systemically administered drugs are delivered in higher concentrations by the anti-EGFR ADCs in glioma. Advancement in ADC technology has increased their demand in the treatment of patients with glioblastoma [68]. Nevertheless, because of their elevated specificity, resistance to ADC is likely to occur as a consequence of tumor heterogeneity, which needs to be addressed by including other strategies simultaneously in glioma treatment. The major benefit with ADC is their ability to cross the BBB which is a key requirement for promising therapeutic outcomes.
4.3 Nanocarriers for Brain Targeting
Nano-drug delivery systems have great potential for targeting drugs to brain by crossing BBB [69]. Various nanosystems explored are liposomes, nanoparticles, nanosuspensions, nanoemulsions and microemulsions, micelles, and others [70]. Such systems when designed to have sizes <100 nm or are surface-modified to escape detection by the reticuloendothelial system (RES) can reach the BBB in adequate concentration for delivery into the brain. The structure and characteristics of nanoparticles can be modified to carry active therapeutic molecules of different physicochemical properties that can control release pattern of drugs in the tumor cell [71].
4.3.1 Liposomes
Liposomes are described as a potential system for better therapeutic impacts in glioma therapy. Their distinctive physicochemical features and high level of safety have resulted in their investigation as carriers for glioma therapy. Daunomycin on conjugation with monoclonal OX26 antibody-loaded PEG sterically stabilized liposome had a prominent effect on the brain targeting [72]. A significant rise in distribution volume at a steady state was associated with high brain tissue accumulation of OX26 immunoliposomes. Liposome with topotecan modified using Tamoxifen and wheat germ agglutinin designed for dual-targeting strategy showed a substantial enhancement in brain tumor-bearing rats’ general survival relative to free liposomes [73]. A concise summary of the liposome-based receptor-targeted therapies for brain cancer using other ligands is described in Table 2.1.
4.3.2 Polymeric Nanoparticles
Salient features of nanoparticles include better solubility, nanosize, greater opportunities for surface modification, and multifunctionality which could increase capability of particles to interact with cellular functions in new ways [86]. Nanoparticles for brain delivery have been designed to target various receptors on the BBB. In most cases, the cascade receptor strategy has been relied on. Table 2.2 depicts studies on ligand-mediated targeted delivery.
4.3.3 Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
Solid lipid nanoparticles comprise solid lipids and are particularly efficient in carrying hydrophobic drugs. When modified with liquid lipids to enhance drug loading and improve stability, they are called as nanostructured lipid carriers. Such lipidic nanocarriers demonstrate a greater capacity to be trapped by the reticuloendothelial system (RES). However, modifying specific primary features including size and surface can impart stealth feature to enable brain targeting and enhanced therapeutic efficacy. Size preferably less than 200 nm, sphericity, and appropriate deformability are essential characteristics to guarantee escape from primary RES organs like the liver and spleen. The surface coating of developed SLN with specific proteins like Apo E, Apo C-II, immunoglobulin, and albumin can prevent opsonization and hence play a critical role in site-specific targeting to brain. Several studies confirmed that SLN can penetrate more efficiently into the cytoplasm of cancer cells with in vitro evaluations on respective U87 human glioblastoma and U373 human astrocytoma cell lines [102]. Angiopep-2, an LRP 1 receptor ligand overexpressed in endothelial cells of both brain and glioma, was grafted to SLN surface for improved delivery of docetaxel. In vivo studies of SLN in glioblastoma-induced C57BL/6 mice model exhibited a considerable targeting effect [103]. A brief summary of different SLN/NLC used for receptor-mediated targeting is provided in Table 2.3.
4.3.4 Polymeric Micelles
Polymer micelles, core–shell nanoparticles formed by the self-assembly of block copolymers, are broadly accepted as promising nanocarriers for targeted brain cancer therapy. Studies have shown that anti-TF antibody fragment-conjugated micelles containing epirubicin and DACHPt have been effectively internalized by TF-overexpressing cancer cells and have shown higher in vitro and in vivo antitumor activity [115]. Antibody and its fragments are beneficial as targetable ligands for active targeting of micelles. Immunomicelles conjugated with antibodies were developed for targeting EGFR receptors [116]. A brief summary of various polymeric micelles used for receptor-based targeted delivery for brain cancer is given in Table 2.4.
4.3.5 Microemulsions and Nanoemulsions
Microemulsions and nanoemulsions are characterized by small globule size (<100 nm). While microemulsions are thermodynamically stable and spontaneously forming, nanoemulsions require energy input and exhibit kinetic stability. Functionalization of microemulsions and nanoemulsions with ligands for specific receptors can aid in targeted delivery as confirmed by targeting studies. Microemulsions and nanoemulsions coated with apolipoprotein E as ligand on surface would be taken into cells through receptor-mediated endocytosis and release active agent targeting tumor cells. The high concentration of DHA in capillary endothelium of brain indicates that DHA is absorbed from diet by DHA transporters including particular fatty acid-binding lipoprotein transporters. Therefore, DHA introduced the option of overcoming the BBB through receptor-mediated endocytosis (RME). Microemulsions with DHA-rich oil hence could be a constructive vehicle for the solubilization, increased bioavailability, and improved drug delivery to the brain. Curcumin microemulsion displayed enhanced targeting efficiency to brain due to DHA-mediated transport overcoming BBB with prolonged drug retention in the brain [127]. A novel microemulsion formulation was developed using folate ligand for tumor targeted therapy with antibiotic aclacinomycin [128]. The study proved that folate alteration on emulsions is an efficient way to target tumor cells in the brain. A brief summary of microemulsions/nanoemulsions used for receptor-based targeted delivery for brain cancer is given in Table 2.5.
4.4 Dendrimers
Dendrimers are an evolving class of hyper-branched macromolecules that confer distinctive characteristics such as important molecular size control, elevated branching density, nanoscale size, and elevated surface functionality. HAIYPRH is a peptide having high affinity for Tf receptor and has been functionalized with PEGylated PAMAM dendrimers loaded with doxorubicin [133]. Compared to free drug, the complex exhibited elevated internalization indicating targeting of brain tumor cells. A brief summary of various dendrimers used for receptor-based targeted delivery for brain cancer is given in Table 2.6.
4.5 Aptamers
AS1411 aptamer and a phage-displayed TGN peptide, that are particular BBB and cancer cell ligands, were respectively combined with nanoparticles to develop a cascade delivery system (AsTNP) for brain glioma. In vivo imaging showed maximum tumor distribution was achieved by AsTNP [144]. Two ssDNA aptamers, GBM 128 and GBM 131, exhibited selective binding to U118-MG glioma cells, and in segments of human tissues, these aptamers revealed binding only to glioma tissue and not to regular brain tissue, suggesting possible diagnostic applications of these aptamers [145]. Nanoparticles decorated with AS1411 (Ap) exhibited high binding to nucleolin highly expressed in plasma membrane of tumor cells, to facilitate delivery of paclitaxel (PTX). The Ap-PTX-NP displayed longer circulation time and increased accumulation of PTX at tumor site, which eventually resulted in considerably prominent tumor inhibition in mice carrying C6 glioma xenografts and prolonged survival rates in rats carrying intracranial C6 gliomas compared to PTX-NP and Taxol [146].
4.6 Carbon Nanotubes and Carbon Dots
Carbon nanotubes and carbon dots are promising method for selective delivery of therapeutic moiety at the tumor cells. Multiwalled PEGylated carbon nanotubes integrated with angiopep-2 as multitargeting delivery system for therapy of brain glioma proved that DOX accumulation in glioma for DOX-ANG was more than control DOX at 2 h postinjection and maintained up to 24 h postinjection [147]. Multiwalled carbon nanotubes with iron oxide magnetic nanoparticles and loaded with doxorubicin exhibited a significant decrease in IC50 compared to that of plain DOX [148]. Studies on carbon dots suggest its ability to cross the BBB when modified with transferrin, showing that the transport occurs through transferrin receptor-mediated transcytosis which was confirmed by the zebrafish model [149]. Further conjugates of carbon dots loaded with doxorubicin were functionalized using transferrin for delivery of doxorubicin to treat pediatric brain tumors. The conjugates show significantly more cytotoxic effect on CHLA-266, SJGBM2, and CHLA-200 brain tumor cell lines as compared to doxorubicin alone [150].
4.7 Diagnostics and Theranostics
4.7.1 Diagnostics
The utilization of macromolecular agents focused on dendrigraft poly-L-lysines (DGLs) with chlorotoxin (CTx) as ligand proved promising results in field of clinical diagnosis of brain tumors. Results revealed that mice treated with CTx-modified contrast had signal enhancement which reached peak level at 5 min for glioma, considerably higher than control [151]. Study on transferrin-conjugated superparamagnetic iron oxide nanoparticles proved considerable contrast enhancement of brain glioma following 48 h postinjection, suggesting Tf-SPIONs as a prospective targeting MR contrast agent for brain glioma [152]. Another work on lactoferrin-conjugated superparamagnetic iron oxide nanoparticles revealed appropriate diagnosis of brain tumors with enhanced signal intensity [153]. Nanoparticles attached with multiple imaging/targeting agents for optical imaging enables higher sensitivity, quicker acquisation time and lesser running cost than MRI. G5 dendrimer labelled with angiopep-2 through a polyethylene glycol linker showed a higher fluorescence intensity and significantly high T/N (tumor/normal tissue) ratio of nanoprobes in brain [154]. RGD peptide-labelled quantum dots administered in glioma bearing mice reported to have contrast enhancement and higher T2 relaxivity signifying integrin targeted optical imaging and cancer detection [155].
4.7.2 Theranostics
Theranostics is a combined form of imaging and therapy where imaging will not only suggest the possibility to noninvasively detect tumor, but also provide quantitative approach to assess the delivery of therapy in tumor cells. A study on PEG-free porphyrin-mimicking lipoproteins demonstrated the possibility for intraoperative fluorescence-guided surgery and tumor-specific PDT [156]. Dual-targeted (EGFpep+Tfpep)-AuNPs-Pc 4 nanoparticles with a photosensitizer phthalocyanine 4 revealed high cellular association and increased cytotoxicity with in vivo studies proving accumulation of nanoparticles in tumor regions [157]. ApoE3-dependent release of porphyrin from lipid nanoparticles in orthotopic U87-GFP tumor-bearing animals revealed reduction in glioblastoma with selective uptake of porphyrin in malignant tissue [158]. Combination treatment of CTX-NP-siMGMT with chlorotoxin as ligand resulted in reduction of tumor growth that was detected by MRI [159]. SLNs conjugated with c(RGDyK) was developed as carriers to enhance the targeted delivery of IR-780 to the tumors. In vitro assays and in vivo PTT treatment defined the eradication of tumor by applying SLN under laser irradiation [160]. Quantum dots (QDs) and apomorphine were incorporated into liposomes to improve brain targeting. Higher fluorescence intensity was observed in mouse brains treated with liposomes compared to that of free QDs with 2.4-fold improved accumulation [161].
5 Receptor-Mediated Delivery in Clinical Trials
The primary therapy for high-grade brain cancer patients is multimodal, including tumor removal followed by radiation and chemotherapy. Research on novel molecularly targeted therapies reflects an improvement in treatment of patients with brain cancer. Targeted chemotherapeutic agent treatment in conjunction with carrier ligands has progressed with some conjugates reaching clinical trials for treating malignant brain tumors. Receptor-mediated delivery of drugs targeting brain cancer in clinical trials is depicted in Table 2.7.
6 Advantages and Limitations
Brain cancer continues to be a deadly disease. Nevertheless, the challenges in the treatment of brain cancer through chemotherapy can find viable solutions through receptor-mediated targeting approaches. The multiple receptors and variety of ligand possibilities coupled with advancements in nanotechnology can harness existing drugs for significantly improved therapy. Nevertheless, directing drugs to the brain in high concentration must be handled with caution. A sensitive organ like the brain may be deleteriously affected if drug concentrations are not titrated to remain at safe concentration. Such off-site brain toxicity is to be handled with caution.
7 Conclusion and Critical Comments
Receptor-mediated endocytosis is a promising approach for improved therapy of brain cancer, provided toxicity concerns are appropriately addressed.
Abbreviations
- ADC:
-
Antibody–drug conjugate
- ADMIDAS:
-
Adjacent to MIDAS
- AP1:
-
CRKRLDRNC peptide
- Apo B:
-
Apolipoprotein B
- Apo E:
-
Apolipoprotein E
- AQP4:
-
Aquaporin-4
- BBB:
-
Blood-brain barrier
- BSA:
-
Bovine serum albumin
- CNS:
-
Central nervous system
- DHA:
-
Docosahexaenoic acid
- DNA:
-
Deoxyribonucleic acid
- DOX:
-
Doxorubicin
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epithelial growth factor receptor
- EPR:
-
Enhanced permeability and retention
- GBM:
-
Glioblastoma
- IC50:
-
Half-maximal inhibitory concentration
- IL:
-
Interleukin
- IL-13Rα2:
-
Interleukin-13 receptor subunit alpha-2
- IL-2Rγc:
-
Interleukin-2 receptor common gamma chain
- Kd:
-
Dissociation constant
- LDH:
-
Lactate dehydrogenase
- LDL:
-
Low-density lipoprotein
- LDLR:
-
Low-density lipoprotein receptor
- Lf:
-
Lactoferrin
- LfR:
-
Lactoferrin receptor
- LRP:
-
Low-density lipoprotein receptor-related protein
- MIDAS:
-
Metal ion-dependent adhesion Site
- mPEG:
-
Methoxy polyethylene glycol
- MTT:
-
(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NPs:
-
Nanoparticles
- PE:
-
Paired end
- PEG:
-
Polyethylene glycol
- PI3 kinase:
-
Phosphoinositide-3-kinase
- PLA:
-
Poly lactic acid
- PTX:
-
Paclitaxel
- RAP:
-
Receptor-associated protein
- RGD:
-
Arginine–glycine–aspartic acid
- RNA:
-
Ribonucleic acid
- STAT 6:
-
Signal transducer and activator of transcription 6
- Tf:
-
Transferrin
- TPGS:
-
D-ɑ-tocopheryl polyethylene glycol succinate
- VLDLR:
-
Very low-density lipoprotein receptor
References
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro Oncol. 2002;4(4):278–99.
Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–74.
Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72.
Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481–93.
Lalatsa A, Butt AM. Physiology of the blood–brain barrier and mechanisms of transport across the BBB. In: Nanotechnology-based targeted drug delivery systems for brain tumors. Academic Press, USA. 2018;49–74.
Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129(4):877–96.
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7.
Laksitorini M, Prasasty VD, Kiptoo PK, Siahaan TJ. Pathways and progress in improving drug delivery through the intestinal mucosa and blood–brain barriers. Ther Deliv. 2014;5(10):1143–63.
Salamat-Miller N, Johnston TP. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int J Pharm. 2005;294(1–2):201–16.
Shinde RL, Jindal AB, Devarajan PV. Microemulsions and nanoemulsions for targeted drug delivery to the brain. Curr Nanosci. 2011;7(1):119–33.
Karanth H, Murthy RS. Nanotechnology in brain targeting. Int. J. Pharm. Sci. Nanotechnol. 2008;1:10–24.
Bellettato CM, Scarpa M. Possible strategies to cross the blood–brain barrier. Ital J Pediatr. 2018;44(2):131.
Liu F, Li X, Zhang L-Y, Song Q-R, Zhang M, Zhao C-X, et al. Stimuli-responsive Nanocarriers for drug delivery to the central nervous system. CNANO. 2015;12(1):4–17.
Hervé F, Ghinea N, Scherrmann J-M. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10(3):455–72.
Xiao G, Gan L-S. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int J Cell Biol. 2013;2013:1–14.
Gabathuler R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol Dis. 2010;37(1):48–57.
Golden PL, Maccagnan TJ, Pardridge WM. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Investig. 1997;99(1):14–8.
Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420(1):32–8.
Akhtar MJ, Ahamed M, Alhadlaq HA, Alrokayan SA, Kumar S. Targeted anticancer therapy: overexpressed receptors and nanotechnology. Clin Chim Acta. 2014;436:78–92.
Wei X, Chen X, Ying M, Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm Sin B. 2014;4(3):193–201.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–84.
Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010;21(5):797–802.
Wang Y-Y, Lui PC, Li JY. Receptor-mediated therapeutic transport across the blood–brain barrier. Immunotherapy. 2009;1(6):983–93.
Lajoie JM, Shusta EV. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev Pharmacol Toxicol. 2015;55(1):613–31.
Zhang B, Sun X, Mei H, Wang Y, Liao Z, Chen J, et al. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials. 2013;34(36):9171–82.
Xu J, Potenza MN, Calhoun VD. Spatial ICA reveals functional activity hidden from traditional fMRI GLM-based analyses. Front Neurosci. 2013;7:154.
Brown M, Goldstein J. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47.
Li Y, Cam J, Bu G. Low-density lipoprotein receptor family. Mol Neurobiol. 2001;23:15.
Prassl R, Laggner P. Molecular structure of low density lipoprotein: current status and future challenges. Eur Biophys J. 2009;38(2):145–58.
Nikanjam M, Blakely EA, Bjornstad KA, Shu X, Budinger TF, Forte TM. Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme. Int J Pharm. 2007;328(1):86–94.
Cassidy SM, Strobel FW, Wasan KM. Plasma lipoprotein distribution of liposomal nystatin is influenced by protein content of high-density lipoproteins. Antimicrob Agents Chemother. 1998;42(8):1878–88.
Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai C-M, et al. Impact of EGFR inhibitor in non–small cell lung Cancer on progression-free and overall survival: a meta-analysis. JNCI J Nat Cancer Inst. 2013;105(9):595–605.
Orlova EV, Sherman MB, Chiu W, Mowri H, Smith LC, Gotto AM. Three-dimensional structure of low density lipoproteins by electron cryomicroscopy. Proc Natl Acad Sci. 1999;96(15):8420–5.
Wang Y-Y, Lui PC, Li JY. Receptor-mediated therapeutic transport across the blood–brain barrier. Immunotherapy. 2009;1(6):983–93.
Papademetriou IT, Porter T. Promising approaches to circumvent the blood–brain barrier: progress, pitfalls and clinical prospects in brain cancer. Ther Deliv. 2015;6(8):989–1016.
Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J Control Release. 2012;164(2):125–37.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22.
Taga T, Suzuki A, Gonzalez-Gomez I, Gilles FH, Stins M, Shimada H, et al. alpha v-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer. 2002;98(5):690–7.
Beer AJ. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin v 3 expression in man. Clin Cancer Res. 2006;12(13):3942–9.
Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–80.
Humphries M. Mapping functional residues onto integrin crystal structures. Curr Opin Struct Biol. 2003;13(2):236–43.
Lee J-O, Bankston LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3(12):1333–40.
Clark E, Brugge J. Integrins and signal transduction pathways: the road taken. Science. 1995;268(5208):233–9.
Arnaout MA, Goodman SL, Xiong J-P. Coming to grips with integrin binding to ligands. Curr Opin Cell Biol. 2002;14(5):641–52.
Xiao X. Modeling gross primary production of temperate deciduous broadleaf forest using satellite images and climate data. Remote Sens Environ. 2004;91(2):256–70.
Shin S, Wolgamott L, Yoon S-O. Integrin trafficking and tumor progression. Int J Cell Biol. 2012;2012:1–7.
van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta Mol Cell Res. 2001;1538(2–3):99–117.
Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275(29):21785–8.
Humphries JD. Integrin ligands at a glance. J Cell Sci. 2006;119(19):3901–3.
Mintz A, Gibo DM, Slagle-Webb B, Christensent ND, Debinski W. IL-13Rα2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4(5):388–99.
Kosmopoulos M, Christofides A, Drekolias D, Zavras PD, Gargalionis AN, Piperi C. Critical role of IL-8 targeting in gliomas. Curr Med Chem. 2018;25(17):1954–67.
Arima K, Sato K, Tanaka G, Kanaji S, Terada T, Honjo E, et al. Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J Biol Chem. 2005;280(26):24915–22.
Joshi BH, Kawakami K, Leland P, Puri RK. Heterogeneity in interleukin-13 receptor expression and subunit structure in squamous cell carcinoma of head and neck: differential sensitivity to chimeric fusion proteins comprised of interleukin-13 and a mutated form of pseudomonas exotoxin. Clin Cancer Res. 2002;8(6):1948–56.
Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. In: Bösze Z, editor. Bioactive components of milk. 2008. p. 163–94.
Suzuki YA, Lopez V, Lönnerdal B. Lactoferrin: mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci. 2005;62(22):2560–75.
Curran CS, Demick KP, Mansfield JM. Lactoferrin activates macrophages via TLR4-dependent and -independent signaling pathways. Cell Immunol. 2006;242(1):23–30.
Jiang R, Lopez V, Kelleher SL, Lönnerdal B. Apo- and holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in caco-2 cells. J Cell Physiol. 2011;226(11):3022–31.
Sharma P, Debinski W. Receptor-targeted glial brain tumor therapies. Int J Mol Sci. 2018;19(11):3326.
Large DE, Soucy JR, Hebert J, Auguste DT. Advances in receptor-mediated, tumor-targeted drug delivery. Adv Ther. 2019;2(1):1800091.
Kratz F, Müller IA, Ryppa C, Warnecke A. Prodrug strategies in anticancer chemotherapy. ChemMedChem. 2008;3(1):20–53.
Rautio J, Laine K, Gynther M, Savolainen J. Prodrug approaches for CNS delivery. AAPS J. 2008;10(1):92–102.
Xu G, McLeod HL. Strategies for enzyme/prodrug Cancer therapy. Clin Cancer Res. 2001;7(11):3314–24.
Chari RVJ, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed. 2014;53(15):3796–827.
Lambert JM, Morris CQ. Antibody–drug conjugates (ADCs) for personalized treatment of solid tumors: a review. Adv Ther. 2017;34(5):1015–35.
Erickson HK, Widdison WC, Mayo MF, Whiteman K, Audette C, Wilhelm SD, et al. Tumor delivery and in vivo processing of disulfide-linked and thioether-linked antibody−maytansinoid conjugates. Bioconjug Chem. 2009;21(1):84–92.
Sharkey RM, Goldenberg DM. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv Drug Deliv Rev. 2008;60(12):1407–20.
Parakh S, Parslow AC, Gan HK, Scott AM. Antibody-mediated delivery of therapeutics for cancer therapy. Expert Opin Drug Deliv. 2016;13(3):401–19.
Razpotnik R, Novak N, Čurin Šerbec V, Rajcevic U. Targeting malignant brain tumors with antibodies. Front Immunol. 2017;8:1181.
Muntoni E, Martina K, Marini E, Giorgis M, Lazzarato L, Salaroglio I, et al. Methotrexate-loaded solid lipid nanoparticles: protein functionalization to improve brain biodistribution. Pharmaceutics. 2019;11(2):65.
Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C. 2016;60:569–78.
Chen Y, Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 2012;64(7):640–65.
Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci. 1996;93(24):14164–9.
Du J, Lu W-L, Ying X, Liu Y, Du P, Tian W, et al. Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood−brain barrier and survival of brain tumor-bearing animals. Mol Pharm. 2009;6(3):905–17.
Re F, Cambianica I, Zona C, Sesana S, Gregori M, Rigolio R, et al. Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood-brain barrier model. Nanomedicine. 2011;7(5):551–9.
Pinzón-Daza M, Garzón R, Couraud P, Romero I, Weksler B, Ghigo D, et al. The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood-brain barrier cells: new strategy for drug delivery into brain tumours. Br J Pharmacol. 2012;167(7):1431–47.
Chen H, Qin Y, Zhang Q, Jiang W, Tang L, Liu J, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 2011;44(1–2):164–73.
Huang F-Y, Chen W-J, Lee W-Y, Lo S-T, Lee T-W, Lo J-M. In vitro and in vivo evaluation of lactoferrin-conjugated liposomes as a novel carrier to improve the brain delivery. IJMS. 2013;14(2):2862–74.
Madhankumar AB, Slagle-Webb B, Wang X, Yang QX, Antonetti DA, Miller PA, et al. Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther. 2009;8(3):648–54.
Shi K, Long Y, Xu C, Wang Y, Qiu Y, Yu Q, et al. Liposomes combined an integrin α v β 3 -specific vector with pH-responsible cell-penetrating property for highly effective Antiglioma therapy through the blood–brain barrier. ACS Appl Mater Interfaces. 2015;7(38):21442–54.
Qin L, Wang C-Z, Fan H-J, Zhang C-J, Zhang H-W, Lv M-H, et al. A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncol Lett. 2014;8(5):2000–6.
Lv Q, Li L-M, Han M, Tang X-J, Yao J-N, Ying X-Y, et al. Characteristics of sequential targeting of brain glioma for transferrin-modified cisplatin liposome. Int J Pharm. 2013;444(1–2):1–9.
Song X, Liu S, Jiang Y, Gu L, Xiao Y, Wang X, et al. Targeting vincristine plus tetrandrine liposomes modified with DSPE-PEG 2000 -transferrin in treatment of brain glioma. Eur J Pharm Sci. 2017;96:129–40.
Ying X, Wen H, Lu W-L, Du J, Guo J, Tian W, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141(2):183–92.
Gao J-Q, Lv Q, Li L-M, Tang X-J, Li F-Z, Hu Y-L, et al. Glioma targeting and blood–brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34(22):5628–39.
McNeeley KM, Annapragada A, Bellamkonda RV. Decreased circulation time offsets increased efficacy of PEGylated nanocarriers targeting folate receptors of glioma. Nanotechnology. 2007;18(38):385101.
Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40(5):385–403.
Zhang B, Sun X, Mei H, Wang Y, Liao Z, Chen J, et al. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials. 2013;34(36):9171–82.
Wang C-X, Huang L-S, Hou L-B, Jiang L, Yan Z-T, Wang Y-L, et al. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009;1261:91–9.
Jose S, Sowmya S, Cinu TA, Aleykutty NA, Thomas S, Souto EB. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur J Pharm Sci. 2014;63:29–35.
Wagner S, Zensi A, Wien SL, Tschickardt SE, Maier W, Vogel T, et al. Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. Deli MA, editor. PLoS One. 2012;7(3):e32568.
Su Z, Xing L, Chen Y, Xu Y, Yang F, Zhang C, et al. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol Pharm. 2014;11(6):1823–34.
Xu Y, Asghar S, Yang L, Li H, Wang Z, Ping Q, et al. Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr Polym. 2017;157:419–28.
Shi K, Zhou J, Zhang Q, Gao H, Liu Y, Zong T, et al. Arginine-glycine-aspartic acid-modified lipid-polymer hybrid nanoparticles for docetaxel delivery in glioblastoma multiforme. J Biomed Nanotechnol. 2015;11(3):382–91.
Wang S. Antitumoral cascade-targeting ligand for IL-6 receptor-mediated gene delivery to glioma. Mol Ther. 2017;25(7):1556–66.
Gao H, Xiong Y, Zhang S, Yang Z, Cao S, Jiang X. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and Neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm. 2014;11(3):1042–52.
Jain A, Jain A, Garg NK, Tyagi RK, Singh B, Katare OP, Webster TJ, Soni V. Surface engineered polymeric nanocarriers mediate the delivery of transferrin–methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater. 2015;24:140–51.
Jain A, Chasoo G, Singh SK, Saxena AK, Jain SK. Transferrin-appended PEGylated nanoparticles for temozolomide delivery to brain: in vitro characterisation. J Microencapsul. 2011;28(1):21–8.
Cui Y, Xu Q, Chow PK-H, Wang D, Wang C-H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34(33):8511–20.
Ghadiri M, Vasheghani-Farahani E, Atyabi F, Kobarfard F, Mohamadyar-Toupkanlou F, Hosseinkhani H. Transferrin-conjugated magnetic dextran-spermine nanoparticles for targeted drug transport across blood-brain barrier: transferrin-conjugated magnetic dextran-spermine nanoparticles. J Biomed Mater Res A. 2017;105(10):2851–64.
Yan F, Wang Y, He S, Ku S, Gu W, Ye L. Transferrin-conjugated, fluorescein-loaded magnetic nanoparticles for targeted delivery across the blood–brain barrier. J Mater Sci Mater Med. 2013;24(10):2371–9.
Chang J, Paillard A, Passirani C, Morille M, Benoit J-P, Betbeder D, et al. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res. 2012;29(6):1495–505.
Brioschi AM, Calderoni S, Zara GP, Priano L, Gasco MR, Mauro A. Solid lipid nanoparticles for brain tumors therapy: state of the art and novel challenges. In: Progress in brain research. 2009;180:193–223.
Kadari A, Pooja D, Gora RH, Gudem S, Kolapalli VRM, Kulhari H, et al. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur J Pharm Biopharm. 2018;132:168–79.
Kadari A, Pooja D, Gora RH, Gudem S, Kolapalli VRM, Kulhari H, et al. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur J Pharm Biopharm. 2018;132:168–79.
Neves AR, Queiroz JF, Lima SAC, Reis S. Apo E-functionalization of solid lipid nanoparticles enhances brain drug delivery: uptake mechanism and transport pathways. Bioconjug Chem. 2017;28(4):995–1004.
Kuo Y-C, Lee I-H. Delivery of doxorubicin to glioblastoma multiforme in vitro using solid lipid nanoparticles with surface aprotinin and melanotransferrin antibody for enhanced chemotherapy. J Taiwan Inst Chem Eng. 2016;61:32–45.
Kuo Y-C, Wang I-H. Enhanced delivery of etoposide across the blood–brain barrier to restrain brain tumor growth using melanotransferrin antibody- and tamoxifen-conjugated solid lipid nanoparticles. J Drug Target. 2016;24(7):645–54.
Jain A, Singhai P, Gurnany E, Updhayay S, Mody N. Transferrin-tailored solid lipid nanoparticles as vectors for site-specific delivery of temozolomide to brain. J Nanopart Res. 2013;15(3):1518.
Kuo Y-C, Liang C-T. Catanionic solid lipid nanoparticles carrying doxorubicin for inhibiting the growth of U87MG cells. Colloids Surf B: Biointerfaces. 2011;85(2):131–7.
Emami J, Yousefian H, Sadeghi H. Targeted nanostructured lipid carrier for brain delivery of artemisinin: design, preparation, characterization, optimization and cell toxicity. J Pharm Pharm Sci. 2018;21(1s):225s–41s.
Emami J, Rezazadeh M, Sadeghi H, Khadivar K. Development and optimization of transferrin-conjugated nanostructured lipid carriers for brain delivery of paclitaxel using box–Behnken design. Pharm Dev Technol. 2017;22(3):370–82.
Meng F, Asghar S, Xu Y, Wang J, Jin X, Wang Z, et al. Design and evaluation of lipoprotein resembling curcumin-encapsulated protein-free nanostructured lipid carrier for brain targeting. Int J Pharm. 2016;506(1–2):46–56.
Song S, Mao G, Du J, Zhu X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016;23(4):1404–8.
Zhang J, Xiao X, Zhu J, Gao Z, Lai X, Zhu X, et al. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int J Nanomedicine. 2018;13:3039.
Ahn J, Miura Y, Yamada N, Chida T, Liu X, Kim A, et al. Antibody fragment-conjugated polymeric micelles incorporating platinum drugs for targeted therapy of pancreatic cancer. Biomaterials. 2015;39:23–30.
Noh T, Kook YH, Park C, Youn H, Kim H, Oh ET, et al. Block copolymer micelles conjugated with anti-EGFR antibody for targeted delivery of anticancer drug. J Polym Sci A Polym Chem. 2008;46(22):7321–31.
Zhan C, Li B, Hu L, Wei X, Feng L, Fu W, et al. Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand. Angew Chem Int Ed. 2011;50(24):5482–5.
Shen J, Zhan C, Xie C, Meng Q, Gu B, Li C, et al. Poly(ethylene glycol)-block-poly(d, l -lactide acid) micelles anchored with angiopep-2 for brain-targeting delivery. J Drug Target. 2011;19(3):197–203.
Li A-J, Zheng Y-H, Liu G-D, Liu W-S, Cao P-C, Bu Z-F. Efficient delivery of docetaxel for the treatment of brain tumors by cyclic RGD-tagged polymeric micelles. Mol Med Rep. 2015;11(4):3078–86.
Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143(1):136–42.
Huang Y, Liu W, Gao F, Fang X, Chen Y. c(RGDyK)-decorated pluronic micelles for enhanced doxorubicin and paclitaxel delivery to brain glioma. Int J Nanomed. 2016;11:1629.
Zhang P, Hu L, Yin Q, Feng L, Li Y. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Mol Pharm. 2012;9(6):1590–8.
Zhang P, Hu L, Yin Q, Zhang Z, Feng L, Li Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: synthesis, preparation and in vivo evaluation. J Control Release. 2012;159(3):429–34.
Agrawal P, Sonali, Singh RP, Sharma G, Mehata AK, Singh S, et al. Bioadhesive micelles of d -α-tocopherol polyethylene glycol succinate 1000: synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf B Biointerfaces. 2017;152:277–288.
Ren W, Chang J, Yan C, Qian X, Long L, He B, et al. Development of transferrin functionalized poly(ethylene glycol)/poly(lactic acid) amphiphilic block copolymeric micelles as a potential delivery system targeting brain glioma. J Mater Sci Mater Med. 2010;21(9):2673–81.
Niu J, Wang A, Ke Z, Zheng Z. Glucose transporter and folic acid receptor-mediated Pluronic P105 polymeric micelles loaded with doxorubicin for brain tumor treating. J Drug Target. 2014;22(8):712–23.
Shinde RL, Devarajan PV. Docosahexaenoic acid–mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2017;24(1):152–61.
Muzaffar F, Singh UK, Chauhan L. Review on microemulsion as futuristic drug delivery. Int J Pharm Pharm Sci. 2013;5(3):39–53.
Etman SM, Elnaggar YSR, Abdelmonsif DA, Abdallah OY. Oral brain-targeted microemulsion for enhanced Piperine delivery in Alzheimer’s disease therapy: in vitro appraisal, in vivo activity, and nanotoxicity. AAPS PharmSciTech. 2018;19(8):3698–711.
Harun S, Amin Nordin S, Abd Gani SS, Shamsuddin AF, Basri M, Bin BH. Development of nanoemulsion for efficient brain parenteral delivery of cefuroxime: designs, characterizations, and pharmacokinetics. Int J Nanomedicine. 2018;13:2571.
Prabhakar K, Afzal SM, Surender G, Kishan V. Tween 80 containing lipid nanoemulsions for delivery of indinavir to brain. Acta Pharm Sin B. 2013;3(5):345–53.
Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347(1–2):93–101.
Han L, Huang R, Liu S, Huang S, Jiang C. Peptide-conjugated PAMAM for targeted doxorubicin delivery to transferrin receptor overexpressed tumors. Mol Pharm. 2010;7(6):2156–65.
Hao B, Gao S, Li J, Jiang C, Hong B. Plasmid pORF-hTRAIL targeting to glioma using transferrin-modified polyamidoamine dendrimer. Drug Des Devel Ther. 2016;10:1.
He H, Li Y, Jia X-R, Du J, Ying X, Lu W-L, et al. PEGylated poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32(2):478–87.
Huang R-Q, Qu Y-H, Ke W-L, Zhu J-H, Pei Y-Y, Jiang C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007;21(4):1117–25.
Li Y, He H, Jia X, Lu W-L, Lou J, Wei Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33(15):3899–908.
Somani S, Blatchford DR, Millington O, Stevenson ML, Dufès C. Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J Control Release. 2014;188:78–86.
Yuan Q, Fu Y, Kao WJ, Janigro D, Yang H. Transbuccal delivery of CNS therapeutic nanoparticles: synthesis, characterization, and in vitro permeation studies. ACS Chem Neurosci. 2011;2(11):676–83.
Sun T, Wu H, Li Y, Huang Y, Yao L, Chen X, et al. Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy. Oncotarget. 2017;8(43):74451.
Ke W, Shao K, Huang R, Han L, Liu Y, Li J, et al. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials. 2009;30(36):6976–85.
Somani S, Robb G, Pickard BS, Dufès C. Enhanced gene expression in the brain following intravenous administration of lactoferrin-bearing polypropylenimine dendriplex. J Control Release. 2015;217:235–42.
Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30(25):4195–202.
Gao H, Qian J, Cao S, Yang Z, Pang Z, Pan S, et al. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials. 2012;33(20):5115–23.
Delač M, Motaln H, Ulrich H, Lah TT. Aptamer for imaging and therapeutic targeting of brain tumor glioblastoma: aptamers in glioblastoma. Cytometry. 2015;87(9):806–16.
Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, et al. Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32(31):8010–20.
Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33(11):3324–33.
Lu Y-J, Wei K-C, Ma C-CM, Yang S-Y, Chen J-P. Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf B: Biointerfaces. 2012;89:1–9.
Li S, Peng Z, Dallman J, Baker J, Othman AM, Blackwelder PL, et al. Crossing the blood–brain–barrier with transferrin conjugated carbon dots: a zebrafish model study. Colloids Surf B: Biointerfaces. 2016;145:251–6.
Li S, Amat D, Peng Z, Vanni S, Raskin S, De Angulo G, et al. Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells. Nanoscale. 2016;8(37):16662–9.
Huang R, Han L, Li J, Liu S, Shao K, Kuang Y, et al. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials. 2011;32(22):5177–86.
Jiang W, Xie H, Ghoorah D, Shang Y, Shi H, Liu F, et al. Conjugation of functionalized SPIONs with transferrin for targeting and imaging brain glial tumors in rat model. Brechbiel MW, editor. PLoS One. 2012;7(5):e37376.
Xie H, Zhu Y, Jiang W, Zhou Q, Yang H, Gu N, et al. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials. 2011;32(2):495–502.
Yan H, Wang J, Yi P, Lei H, Zhan C, Xie C, et al. Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood-brain barrier. Chem Commun. 2011;47(28):8130–2.
Cai W, Shin D-W, Chen K, Gheysens O, Cao Q, Wang SX, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6(4):669–76.
Cui L, Lin Q, Jin CS, Jiang W, Huang H, Ding L, et al. A PEGylation-free biomimetic porphyrin nanoplatform for personalized cancer theranostics. ACS Nano. 2015;9(4):4484–95.
Dixit S, Miller K, Zhu Y, McKinnon E, Novak T, Kenney ME, et al. Dual receptor-targeted theranostic nanoparticles for localized delivery and activation of photodynamic therapy drug in glioblastomas. Mol Pharm. 2015;12(9):3250–60.
Rajora MA, Ding L, Valic M, Jiang W, Overchuk M, Chen J, et al. Tailored theranostic apolipoprotein E3 porphyrin-lipid nanoparticles target glioblastoma. Chem Sci. 2017;8(8):5371–84.
Yoo B, Ifediba MA, Ghosh S, Medarova Z, Moore A. Combination treatment with theranostic nanoparticles for glioblastoma sensitization to TMZ. Mol Imaging Biol. 2014;16(5):680–9.
Kuang Y, Zhang K, Cao Y, Chen X, Wang K, Liu M, et al. Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl Mater Interfaces. 2017;9(14):12217–26.
Fang J-Y, Wen C-J, Zhang LW, Al-Suwayeh SA, Yen T-C. Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int J Nanomed. 2012;7:1599.
Cytotoxic T cells and interleukin-2 in treating adult patients with recurrent brain tumors – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT00002572.
ANG1005 in breast cancer patients with recurrent brain metastases – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02048059.
Cellular immunotherapy study for brain cancer – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT01144247.
IL-4(38-37)-PE38KDEL immunotoxin in treating patients with recurrent malignant astrocytoma – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT00003842.
Study of therapy with TransMID™ compared to best standard of care in patients with glioblastoma multiforme – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT00083447.
T cells expressing HER2-specific chimeric antigen receptors(CAR) for patients with HER2-positive CNS tumors – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02442297.
Chemotherapy and vaccine therapy followed by bone marrow or peripheral stem cell transplantation and interleukin-2 in treating patients with recurrent or refractory brain cancer – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT00014573.
EGFRBi-armed autologous T cells in treating patients with recurrent or refractory glioblastoma – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02521090.
A study of ABT-414 in subjects with newly diagnosed glioblastoma (GBM) with epidermal growth factor receptor (EGFR) amplification – full text view – ClinicalTrials.gov [Internet]. [cited 2019 Jun 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02573324.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 American Association of Pharmaceutical Scientists
About this chapter
Cite this chapter
John, R., Vaswani, H., Dandekar, P., Devarajan, P.V. (2019). Brain Cancer Receptors and Targeting Strategies. In: Devarajan, P., Dandekar, P., D'Souza, A. (eds) Targeted Intracellular Drug Delivery by Receptor Mediated Endocytosis. AAPS Advances in the Pharmaceutical Sciences Series, vol 39. Springer, Cham. https://doi.org/10.1007/978-3-030-29168-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-29168-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29167-9
Online ISBN: 978-3-030-29168-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)


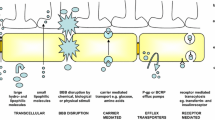
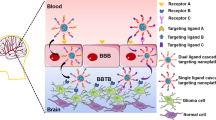


 ) and on the BBB and brain cancer cells (
) and on the BBB and brain cancer cells ( )
)