Abstract
The electric sensory and communication signals of electric fish show remarkable diversity in their waveforms, and this diversity is driven by selective pressures related to reproduction, sensory ecology, predation avoidance, and the metabolic costs of signaling. These electric signals are generated by electrocytes, electrically excitable cells that comprise the electric organ. Although the signaling rate is controlled by a brainstem pacemaker or command nucleus that coordinates the discharge of electrocytes within the electric organ, waveform diversity arises primarily from the underlying biophysics of electrocytes, including their passive electrical properties, morphology, voltage-gated ion channels, and regulatory pathways that modify electrocyte function. Electrocyte morphology and innervation patterns are a major source of signal diversity in the African mormyrid electric fishes, whereas diversity of ion-channel expression patterns has a strong influence on waveform diversity in the South American gymnotiforms. Convergent evolution of ion channels in both clades further contributes to signal diversity. Little is known about the ionic mechanisms of signal diversity in mormyrids. Additionally, asynchronous activation of distinct electric organ regions with different electrocyte properties enhances waveform complexity in some gymnotiforms. Signal diversity associated with development and sexual dimorphism arises from the effects of steroid hormones on electrocyte ion channel kinetics, and the rapid changes in signal waveform are mediated by the effects of peptide hormones on electrocyte action potentials and ion channel function. These processes have been investigated primarily in a small number of gymnotiforms, highlighting a great need for broader comparative studies across gymnotiform species and between mormyrids and gymnotiforms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Action potential
- Electric organ
- Electric organ discharge
- Electrocyte
- Ion channels
- Melanocortin hormones
- Sexual dimorphism
- Steroid hormones
5.1 Introduction: The Dimensions of Electric Signal Diversity
The signals generated by electric fish are extremely diverse in some ways that are obvious to any casual observer and also in many ways that are not as readily apparent. Electric signal diversity is most apparent in the hundreds of freshwater electric fish species, where signals vary across multiple dimensions and timescales. These fishes are distributed broadly across African (Roberts 1975) and South American (Albert and Crampton 2005) waters. The independent origin of electrogenesis in the African mormyrids and the South American gymnotiforms provides a rare opportunity for comparative analyses of signal diversity mechanisms across two independent evolutionary replicates on two different continents (Lavoué et al. 2012). Understanding the mechanisms of this signal diversity requires integrative and comparative studies across levels of biological organization from genes to molecules, cells, organisms, social networks, and ecology. The end result of such comparative approaches to understanding the biophysical mechanisms of electric signal diversity is the promise of discovering both the general principles of signal production as well as the mechanisms of convergence and divergence in signal biophysics.
5.1.1 Strong Versus Weak Electric Signals; Fast Versus Slow Electric Signals
Fish generate electric signals known as electric organ discharges (EODs). One obvious difference among these electric signals is in the amplitude of the EOD, which would occur to anyone brave enough to put their arm first into an aquarium housing a weakly electric gymnotiform or mormyrid fish and then into an aquarium housing an electric catfish or eel. In the first case, the electric signal of the gymnotid or mormyrid is so weak as to be imperceptible, whereas in the latter instance, the electric signal of the catfish or eel is strong enough to cause extreme pain (Catania 2017).
Another rather obvious difference among electric signals is the EOD rate that can be easily appreciated by listening to EODs transduced to sound with an inexpensive audio amplifier. Some electric fish species generate EODs at very low rates with long, irregular intervals between EODs, sounding like a Geiger counter or a stuttering gas lawn mower. Other species produce regular, high-frequency EODs (about 100–2000 Hz) that sound like pure tones from the middle musical octaves. This difference is the basis for one of the most fundamental distinctions in electric signal diversity, the difference between pulse fish and wave fish.
5.1.2 Diversity of Electric Signal Waveforms
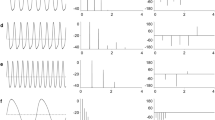
The true breadth of signal diversity begins to appear when comparing the time-voltage waveforms of individual EODs. It is this dimension of signal diversity that is the focus of this chapter. The EOD is typically recorded “head to tail” with two differential electrodes located in the longitudinal axis more than a body length from the fish. EODs recorded in this manner reveal vast differences in the waveforms between species (Fig. 5.1). These far-field EODs are species specific, showing variation in signal duration, the number of positive and negative phases, inflection points within a phase, and the order of positive and negative phases. In some species, EOD waveforms are also sexually dimorphic and can even be individually specific in some cases (McGregor and Westby 1992). The far-field EODs likely are not useful for electrosensing but are potentially effective as communication signals at this distance (Aguilera et al. 2001).
Diversity of electric signal waveform in pulse-type mormyrid (left) and gymnotiform (right) fish. Solid lines, electric organ discharges (EODs) with head-positive potentials plotted upward; dashed lines, 0 V. Amplitudes are scaled to similar peak-to-peak amplitudes. Scale bar, 1 ms for all recordings except for the mormyrid Campylomormyrus numenius (bottom left; scale bar, 10 ms). The EODs from mormyrid species span families within the mormyrid clade, whereas the gymnotiform waveforms show the immense diversity within the genus Gymnotus. Signal diversity in Gymnotus is representative of the diversity seen across the other pulse-type gymnotiform families. Mormyrid EODs adapted from Arnegard et al. (2010b); gymnotiform EODs adapted from Crampton et al. (2013)
When the EOD is recorded from gymnotiforms using electrodes at various locations within a body length of the fish, these near-field waveforms show remarkable spatiotemporal variation (Assad et al. 1999; Caputi 1999). The near-field waveforms often bear little to no resemblance to the far-field signal, but the near-field signals likely are crucial for electrolocation and communication. The significance and mechanisms of near-field signal diversity are detailed in an excellent earlier review (Caputi 1999) and in a recent study (Waddell et al. 2016).
5.1.3 Diversity in the Spectral Content of Electric Signals
An important feature of electric signals is the power spectrum of the signal that represents the relative power in the signal across a range of frequencies ranging from 0 Hz DC to 10 kHz or higher. Although the power spectrum is determined exclusively by the time-voltage waveform of the EOD, differences in the power spectra are often not readily apparent by examining the differences in time-voltage waveforms (Fig. 5.2). Accordingly, electric signals that seem quite similar when presented as time-voltage recordings can have very different power spectra, with important consequences for both the communication and sensory functions of the signal. Low-frequency components of electric signals (approximately 0–50 Hz) activate ampullary electroreceptors and have important communication functions, whereas higher frequency components of the signal (approximately 100 Hz to 10 kHz) are detected by tuberous electroreceptors and serve both sensory and communication functions (see also Baker, Chap. 2; Leitch and Julius, Chap. 3).
Power spectra of diverse signal waveforms. EOD waveforms for mormyrid (left) and gymnotiform (right) fish are shown next to their power spectra. Note that the mormyrid species designated as Brienomyrus brachyistius have since been revised. Signals for which the time-voltage waveform is symmetrical around zero show spectral suppression of energy in the low-frequency range of ampullary electroreceptors. Continuous waveforms from wave-type fish have narrow spectra consisting primarily of the EOD frequency and its harmonics. Pulse fish have a much broader spectral content. Mormyrid waveforms and spectra adapted from Hopkins (1980); gymnotiform waveforms and spectra adapted from Stoddard and Markham (2008)
For pulse fish, monophasic signals with a baseline at or near 0 V carry the majority of their energy in the low-frequency range, whereas the addition of one or more additional phases that make the signal symmetrical about 0 V greatly suppresses low-frequency energy while maintaining the high-frequency components. In wave fish, the power spectrum includes peaks at the fish’s EOD frequency as well as at higher harmonics of the fundamental frequency. If the baseline of the signal is near 0 V, then the signal also carries significant energy in the low-frequency range (with a peak at 0 Hz). However, most wave fish offset the EOD baseline below 0 V or their discrete EODs are symmetrical about 0 V, thereby suppressing the low-frequency energy in the signal (Fig. 5.2).
5.1.4 Plasticity of Electric Signal Properties
Finally, the diversity of EOD waveforms in some species also extends to waveform variations that occur over timescales ranging from minutes to months, including developmental changes during maturation, seasonal variation, circadian changes in the waveform, and rapid waveform changes in response to stress and social encounters (Fig. 5.3). These waveform modulations also produce corresponding changes in the power spectrum of the signal. Most commonly, electric signal plasticity involves changes to the amplitude and/or the duration of the signal, under the control of multiple hormonal axes.
Circadian and developmental changes in signal waveforms and power spectra. a: Day-to-night changes in EOD waveform for the pulse gymnotiform Brachyhypopomus gauderio (top) and the wave gymnotiform Eigenmannia virescens (bottom). Brachyhypopomus gauderio waveforms show enhanced amplitude and extension of the second phase at night, whereas Eigenmannia virescens waveforms show only increased signal amplitude. Power spectra (right) show that increasing the head-negative second phase of the EOD (P2) duration in Brachyhypopomus gauderio produces a marked increase in low-frequency content of the signal (left), whereas signal amplitude enhancement in Eigenmannia virescens changes the spectral power but not the frequency content. b: Development of EOD waveform in Brachyhypopomus gauderio. A series of EODs were recorded at progressive ages (days postfertilization). Waveforms have been rescaled to standardize the amplitude of the first phase. Young juveniles produce a monophasic signal (17 days) with maximal power in the spectral range of the ampullary electroreceptors (red box). Over the course of ~3 months, the signal becomes increasingly biphasic with the addition and subsequent enhancement of the negative second phase of the signal. At 110 days, the signal is nearly symmetric around 0 V, thereby significantly suppressing energy in the low-frequency range of the ampullary electroreceptors. Adapted from Stoddard and Markham (2008)
5.2 Why Are Electric Signals Diverse?
Given this broad diversity in electric signal characteristics, a fundamental question is why these signals are so diverse. Ultimately, electric signal waveforms are determined by evolutionary forces, as is true for any animal signal. Electric signals, however, are distinctive among animal signals because they are used both for communication and for the basis of an active electrosensory process that yields electric “images” of the animal’s environment (see Jung and Engelmann, Chap. 12). As a result, these electric signals are shaped by a combination of evolutionary forces that act on communication signals and selective pressures that act on active sensory signals. In some cases, these selective forces can exert opposite pressures on a signal. For example, sexual selection might favor high-amplitude signals while predator avoidance instead favors low-amplitude signals. These competing forces often lead to tradeoffs in signal characteristics or to behavioral or life-history adaptations that balance signal costs and benefits. Most importantly, the sometimes peculiar biophysical mechanisms of electric signal production are made far more understandable with an appreciation of the sometimes conflicting evolutionary forces that act on them. A comprehensive review of the evolutionary influences on signal diversity is provided by Krahe (Chap. 7), but it will be useful here to survey a subset of these selective factors to provide context for appreciating the many proximal mechanisms of signal diversity.
5.2.1 Predation Risk
Most animal signals are detectable by one or more sympatric predators, exposing the signaler to potential injury or death. Such predation costs would generally be expected to favor signal characteristics that reduce the salience of the signal to predators. In the case of electric signals in fish, the primary predation risk arises from electroreceptive predators that possess ampullary electroreceptors. This situation would likely favor electric signals of lower amplitude as well as signals where the power spectrum has reduced energy in the lower frequency range of ampullary electroreceptors (0–50 Hz). This is not a universal solution, however, because some large piscivorous weakly electric fish species can use their tuberous electroreceptors to detect and consume their smaller weakly electric cousins based on high-frequency signal components.
5.2.2 Metabolic Cost
In addition to predation costs, electric signals also have metabolic costs. Any animal signal incurs some degree of metabolic cost, and energy devoted to signaling is not available for other essential physiological functions such as locomotion, body maintenance, or immune function. Accordingly, with all other factors being equal, signals that require less metabolic investment would be preferable, thereby favoring low-amplitude signals, adaptations that increase the efficiency of signal production, and adaptations that maximize power transfer from the fish to the surrounding water. Despite these pressures, recent findings suggest that the metabolic cost of electric signal production can be quite high, consuming from 10 to 30% or more of the animal’s daily energy budget (Salazar and Stoddard 2008; Salazar et al. 2013).
5.2.3 Sexual Selection
Sexual selection can exert a strong evolutionary influence on animal signals when female sensory biases result in the exaggeration of certain signal characteristics in males. This process generally favors male signals that are more conspicuous, and for electric fish, this translates into higher amplitude signals with enhanced low-frequency spectral content, a situation that increases both the predation risk and the metabolic cost of the signal. Sexual selection is also likely to be the driving force behind the sexual dimorphism in electric signals observed in many mormyrid and gymnotiform species, and evidence suggests that sexual selection for signal diversification has driven speciation in some instances (Arnegard et al. 2010a).
5.2.4 Reproductive Isolation
In many locations in both Africa and South America, multispecies assemblages of closely related species are sympatric. These conditions increase the risk of costly reproductive interference through mismating between heterospecifics when species recognition signals are not sufficiently different to distinguish between species. Another cause of reproductive interference is masking interference in which the communication signals of two species are sufficiently similar in their spectral characteristics to disrupt communication within each species. In such situations, selective forces tend to promote and maintain diverse signal characteristics, a phenomenon known as reproductive character displacement that has been clearly documented in gymnotiforms (Crampton et al. 2011) and may contribute to signal diversity in some mormyrid clades (Arnegard et al. 2010a).
5.3 Physiological Mechanisms of Electric Signal Production
In all electric fish, the electric signal originates from postsynaptic potentials and/or action potentials (APs) generated by electrocytes in the electric organ. The timing and pattern of electrocyte activation is regulated by a brainstem pacemaker nucleus in gymnotiforms and the mormyroid wave species Gymnarchus niloticus (“aba knife”). In mormyrids, electrocyte activity is controlled by a medullary command nucleus. The resulting simultaneous or near-simultaneous potentials generated by hundreds to thousands of electrocytes sum to produce the EOD (Fig. 5.4). Variations in EOD rate and timing carry important social information, and the central circuits controlling EOD timing have been well characterized in both gymnotiforms and mormyrids (Caputi et al. 2005).
General schematic of EOD production. a: The electric signal, an EOD, is produced by the near-simultaneous action potentials (APs) of electrocytes in the electric organ (EO). A medullary pacemaker nucleus initiates the electrocyte APs via spinal electromotor neurons that innervate the electrocytes, forming a broad cholinergic synapse. b: Simplified electrocyte schematic. Electrocytes are large cells, often greater than 1 millimeter in length, innervated on the posterior end of the cell. Activation of the cholinergic synapse initiates the AP when sodium enters the cell via voltage-gated sodium channels. The electrocyte geometry and localization of Na+ channels to the posterior region causes the Na+ current to move along the rostral-caudal body axis. c: A section of the EO from the tail of Eigenmannia virescens, with skin removed to expose the electrocytes that are densely packed within the EO. One electrocyte is outlined in black. d: The near-simultaneous APs of all electrocytes in the EO sum to generate current that moves forward toward the head, then follows a return path (black line) through the water to the tail. By convention, the current moving toward the head is measured as positive (upward). e: A single EOD is a monophasic pulse. f: EOD waveform from a fish with an EOD frequency of ≈500 Hz. Adapted from Ban et al. (2015)
Beyond the differences in signal amplitude and rate or timing, the biophysical mechanisms that shape electric signal diversity center primarily on the biophysical properties of electrocytes, with contributions also from their innervation patterns and their arrangement within the electric organ. The electrocytes are a central link in the electrosensory and electrocommunication systems of electric fish. They are the target of central control by the pacemaker/command nucleus, their function is regulated by multiple hormonal axes, and they are the cellular source of the electric signal that is the primary information carrier in the environment and the input to the electrosensory system. Accordingly, the focus for the remainder of this chapter will be on the cellular biophysics of electrocyte function that give rise to the broad diversity of electric signal waveforms in mormyrid and gymnotiform fishes.
5.3.1 General Biophysics of Excitable Cells
Electrocytes are electrically excitable cells, and, as such, they share certain biophysical features in common with all electrically excitable cells including neurons and myocytes. The key features of excitable cells are their passive membrane resistance and capacitance, the maintenance of ionic gradients across the cell membrane, and the presence of synaptic receptors and voltage-gated ion channels that regulate ionic currents across the membrane. These properties together give rise to the postsynaptic potentials and APs generated by excitable cells.
The most fundamental biophysical properties of any excitable cell are its membrane resistance and membrane capacitance. The membrane resistance is determined by the number of ion channels in a conductive state at any given moment, with resistance increasing as the number of conducting channels decreases and vice versa. The membrane resistance of the cell determines the magnitude of membrane voltage changes in accordance with Ohm’s law (voltage = current × resistance). For a given input current (say a postsynaptic current), the magnitude of the resulting membrane voltage response is a linear function of the membrane resistance. Higher resistances will produce larger voltage responses and vice versa.
The cell membrane also acts as a capacitor, which is to say that the membrane is able to both store and release the electrical charge on its surface. Membrane capacitance is a linear function of the total membrane area of the cell, and it is important because any current delivered to the cell will first charge the membrane capacitance before any ionic current begins to cross the membrane to change the membrane voltage. Once the input current terminates, the charge stored on the membrane will be released. Cells with greater surface area (and therefore greater capacitance) can store and release more charge. The rate at which the membrane stores and releases charge is affected by the membrane resistance. At higher resistances, the charging and discharging of the membrane capacitance are slower, as is the rate of any changes in membrane voltage. The opposite is also true: at low resistances, charge movement on and off of the membrane is faster and the corresponding changes in membrane voltage are also faster.
All excitable cells also maintain concentration gradients of several key ion species across the cell membrane. These gradients are maintained by active transport mechanisms, typically transmembrane enzymes known as transporters or pumps, that require energy to transport ions against their concentration gradients. These include transporters for calcium (Ca2+), chloride (Cl−), sodium (Na+), and potassium (K+). The most important transporter for the present discussion is Na+/K+-ATPase, also known as the sodium-potassium pump. For every catalytic cycle, this transmembrane protein hydrolyzes one ATP in order to transport three sodium ions to the extracellular space and two potassium ions to the intracellular space. As a result, most excitable cells maintain Na+ gradients with much higher concentrations outside the cell than inside and K+ gradients with much higher concentrations inside the cell than outside.
These concentration gradients give rise to equilibrium potentials for each ion species such that when the membrane becomes more permeable to a particular ion, the membrane potential will move toward the equilibrium potential of that ion. In the case of most excitable cells, the equilibrium potential for sodium (ENa) is approximately 60 mV, whereas the equilibrium potential for potassium (EK) is approximately −90 mV for neurons and −100 mV for skeletal muscle. These equilibrium potentials set the limits on membrane voltage during an AP, with the minimum being the EK and the maximum being the ENa. As a result, the maximum voltage excursion during the AP is approximately 150 mV.
The activation of ionotropic receptors and voltage-gated ion channels allows ionic current to flow across the membrane and is thereby responsible for the membrane voltage changes characteristic of electrically excitable cells. The synaptic activation of ionotropic receptors or the activation of voltage-gated ion channels can have depolarizing or hyperpolarizing effects on the cell depending on the ion selectivity of the channel. The canonical AP begins from the resting potential that is near the EK due to the resting membrane being predominantly permeable to K+. The AP is initiated by synaptic stimulation of excitatory receptors permeable primarily to Na+ that depolarize the membrane. The ensuing rapid activation of voltage-gated Na+ channels produces an inward current of Na+ that depolarizes the membrane toward the ENa. Subsequently, Na+-channel inactivation terminates the inward Na+ current, and activation of voltage-gated K+ channels produces an outward K+ current that repolarizes the membrane toward the EK. Following each AP, the Na+/K+-ATPases restore the proper ionic gradients.
5.3.2 The Special Case of Electrocytes
Electrocytes operate by the same functional principles as any excitable cell but are unique excitable cells in many ways. The sheer size of an electrocyte is one of the most notable characteristics. They are multinucleated cells that can be up to two millimeters in length and/or width. With such a large membrane surface area, electrocytes have extremely large membrane capacitances, on the order of tens to hundreds of nanofarads, orders of magnitude larger than observed in neurons where membrane capacitances are on the order of picofarads. Electrocytes also are notable for very low membrane resistances, usually well below 1 MΩ and as low as 10 kΩ in most cases (Bennett 1961; Markham and Stoddard 2013), compared with values for neurons and myocytes that fall in the range of tens to hundreds of megaohms. The high membrane capacitance and low resistance have important consequences for the electrical properties of the electrocyte. By virtue of the large capacitance, the electrocyte membrane can store and release a great deal of electrical charge. Furthermore, the low resting membrane resistance allows this charge to be stored and released very quickly but also means that the cell requires very large input currents to change the membrane voltage.
Beyond the sheer size of electrocytes, their morphologies are fascinating. In all but one clade of weakly electric fish, adult electrocytes are derived from skeletal muscle. The South American genus Apteronotus is the sole exception because the adult electric organ is of neural origin (Kirschbaum 1983). The electrocytes in apteronotids themselves are enlarged terminals of the spinal motor neurons. Myogenic electrocytes are generally cylindrical cells with flattened areas (faces) of electrically excitable membrane oriented such that the membrane currents across these areas are directed along the rostral-caudal body axis (Figs. 5.5 and 5.6). Electrocytes can be elongated cigar-shaped cells such as for the wave-type gymnotiform Eigenmannia virescens (“glass knifefish”; Fig. 5.6b) or the wave-type mormyroid Gymnarchus niloticus. For pulse-type gymnotiforms and mormyrids, electrocytes are flattened and disc-like, with widths being very narrow relative to the diameter (Fig. 5.6a). The disc-like electrocytes sometimes also feature stalks that protrude from the flattened membrane surfaces (Figs. 5.5, bottom, and 5.6a).
Mechanisms of EOD generation and EOD waveform diversity in gymnotiform and mormyrid fish. Line drawings are cross sections of electrocytes from representative species and the Fig 5.5 (continued) corresponding EOD waveforms are below the outlines. In the schematic representations of electrocyte function, active synaptic inputs are represented by black triangles and inactive synaptic inputs are represented by gray triangles. Solid dashed lines, activated excitable membrane; arrows. direction of membrane current flow. Top: monophasic EODs in both gymnotiform and mormyroid fish are produced by electrocytes innervated on the posterior membrane where only the innervated membrane is active. These generate a single AP on the posterior membrane following synaptic activation, which produces headward current flow and a monophasic head-positive EOD pulse. Center: biphasic EODs in gymnotiforms and mormyrids are typically produced by disk-shaped electrocytes where both the anterior and posterior membranes are electrically active. Synaptic activation elicits an AP on the posterior membrane that creates headward current flow and the head-positive EOD phase (P1). A subsequent AP on the noninnervated anterior membrane produces the P2. Bottom: multiphasic EOD waveforms are produced by different mechanisms in the gymnotiform Gymnotus carapo and the mormyrid Brevimyrus niger. In Gymnotus carapo, the EOD is a multiphasic waveform with two initial head-negative components (a and b), followed by a head-positive phase (c), and then a final head-negative phase (d). This waveform is produced by the asynchronous activation of three distinct electric organ regions populated by three types of electrocytes (blue, green, and red). Two populations are innervated on both faces, and the third population is innervated only on the posterior face (Macadar et al. 1989a). In one type of doubly innervated electrocytes, synaptic activation elicits an AP on both the anterior and posterior membranes (blue area). In the second type of doubly innervated electrocyte, activation of the anterior synapse produces only a postsynaptic potential and activation of the posterior synapse produces an AP (green area). Electrocytes innervated only on the posterior membrane (red area) produce an AP on the posterior membrane followed by an AP on the anterior membrane. The spatiotemporal activation pattern of these three electrocyte populations produces the complex multiphasic EOD (Caputi 1999). In the mormyrid Brevimyrus niger, the EOD is a multiphasic waveform that begins with a head-negative component (a), followed by a head-positive phase (b), and then a final head-negative phase (c). The Brevimyrus EOD is generated by the near-simultaneous activation of a single population of electrocytes. The electrocytes are innervated from the anterior side on a stalk that then penetrates through the electrocyte to join the posterior membrane. Synaptic activation initiates an AP in the stalk, and the propagation of this AP along the stalk through the electrocyte penetration produces the initial head-negative phase (a). The subsequent initiation of an AP on the posterior membrane face produces the head-positive second phase (b), and the resulting AP on the anterior membrane face produces the final head-negative phase (c). Adapted from Markham (2013)
Electrocyte morphology. a: Confocal 3-D projections of the anterior and posterior face of a single electrocyte from Paramormyrops kingsleyae showing the innervating nerve, stalks, and sites of stalk penetrations through the electrocyte. Electrocyte thickness is 60 μm. Image courtesy of Jason Gallant; annotations adapted from Gallant et al. (2011). b: 3-D reconstruction from serial confocal scanning through a live Eigenmannia virescens electrocyte injected with rhodamine B dextran (10,000 MW). Arrow, site of dextran injection; arrowheads, horizontal and vertical lines that are artifacts caused by the image tile overlap. The posterior face contains deep invaginations that dramatically increase the surface area of the cell, whereas the anterior face features large lobes penetrated by capillaries. Image adapted from Ban et al. (2015)
Finally, electrocytes are also unique in the magnitude of the ionic currents that generate their postsynaptic potentials and APs. Although the whole cell ionic currents in neurons and myocytes are at most a few nanoamperes, ionic currents in electrocytes typically are on the order of 1 μA, and in some cases, they exceed 10 μA in magnitude (Sierra et al. 2007; Markham et al. 2013). An important consequence of such large ionic currents is the metabolic demand incurred by the Na+/K+-ATPases that restore the ionic gradients after each AP at a cost of one ATP for every three Na+ ions that enter the cell during the AP. The resulting metabolic costs associated with electric signal production are significant and can consume up to 30% of the animals’ daily energy budget (Salazar et al. 2013; Lewis et al. 2014).
5.4 Physiological Mechanisms of Signal Diversity
The simplest EOD waveform in both gymnotiforms and mormyrids is a monophasic pulse produced by electrocytes where only one face is electrically excitable while the other is electrically passive. Synaptic input at large cholinergic synapses innervated by the spinal motor neurons initiates an AP on the excitable face where Na+ current enters the cell via voltage-gated Na+ channels, creating an axial current within the electric organ. If, for example, the active face is the posterior electrocyte membrane, the net positive current is directed headward. Subsequent repolarization by voltage-gated K+ channels terminates the AP. The result is a monophasic head-positive pulse shaped by the depolarization and repolarization of the innervated posterior membrane (Fig. 5.5, top).
Species with monophasic EODs include both pulse and wave fish. In the case of wave fish, the interval between EODs is approximately the same as the EOD duration, creating a sinusoidal signal, whereas in monophasic pulse fish, the EODs are separated by long intervals. Across monophasic species, EOD duration varies over a wide range, from a few hundred microseconds to tens of milliseconds or more. And within wave species, frequency differences among conspecifics are associated with different EOD durations, with higher frequency individuals having shorter duration EODs and vice versa.
In species with more complex biphasic EOD waveforms, at least some of the electrocytes in the electric organ have two electrically excitable faces. An AP is initiated first on the posterior innervated face (AP1), followed closely by an AP on the opposite face (AP2; Fig. 5.5, center). The two successive APs with their ionic currents directed in opposite directions create a biphasic electrocyte discharge that is shaped both by the the waveforms of the two APs and the delay between the APs (Bennett 1961; Markham and Zakon 2014). For gymnotiform species with complex multiphasic EOD waveforms, the mechanisms of signal complexity are best understood in Gymnotus carapo (“banded knifefish”). Waveform complexity in Gymnotus carapo arises from the asynchronous recruitment of several electrocyte populations with different discharge characteristics (Lorenzo et al. 1988; Caputi 1999). Some electrocytes in Gymnotus carapo are innervated on both the anterior and posterior faces, and both faces produce APs as they are activated asynchronously. Another population of doubly innervated electrocytes produce a synaptic potential just on the anterior face, whereas activation of the posterior face elicits an AP. A third population of electrocytes is innervated only on the posterior face, and synaptic activation elicits an AP on the posterior face followed by an AP on the anterior face. The spatiotemporal pattern of activation across these electrocyte populations produces the multiphasic EOD waveform measured head to tail (Fig. 5.5, bottom) as well as producing a large spatial variation in EOD waveforms measured at different locations near the body (Caputi 1999).
Many mormyrid species produce multiphasic EODs with complexity comparable to Gymnotus carapo, but the underlying waveform complexity mechanisms are quite different. Mormyrid electric organs consist of a single, relatively homogeneous population of electroctyes. It is the particular innervation pattern and morphological complexity of these electrocytes that produces the complex multiphasic EODs. In mormyrids with complex EOD waveforms, electrically excitable stalks penetrate the electrocyte (Fig. 5.5, bottom) that contribute to waveform complexity when the APs are propagated along the stalks toward the electrocyte body (Fig. 5.5, bottom; Bennett and Grundfest 1961). The morphological complexity in mormyrid electrocytes yields diversity and complexity of EOD waveforms that rival or exceed EOD diversity and complexity in gymnotiforms (cf. Crampton and Albert 2006; Arnegard et al. 2010a).
5.5 Cellular Biophysics of Signal Diversity
5.5.1 Expression Patterns of Diverse Ion Channels
Electrocyte morphology is clearly important for shaping the EOD waveform in both gymnotiform and mormyrid fishes, but morphology alone cannot account for the vast differences in signal durations and waveforms. The particular complement of ion channels expressed by the electroctye and their kinetics and voltage dependence as well as their localization on the membrane plays a central role in determining the EOD waveform.
Patch- and voltage-clamp recordings from electrocytes across several genera and species have revealed that electrocytes in different species express a remarkably diverse complement of ionic currents. Patch-clamp studies of Electrophorus electricus (electric eel) electrocytes (Shenkel and Sigworth 1991) showed that the dominant ionic currents of the electrocyte were voltage-gated Na+ currents and inward rectifier K+ currents. In the monophasic wave-type gymnotiform Sternopygus macrurus (“longtail knifefish”), electrocytes express voltage-gated Na+ currents, inward rectifier K+ currents, and delayed rectifier K+ currents (Ferrari and Zakon 1993). For both Electrophorus and Sternopygus, the EOD and electrocyte APs are monophasic and relatively long duration, generally several milliseconds or more. In Eigenmannia virescens, a high-frequency wave fish with brief EODs approximately 1 ms long, electrocytes express an inward rectifier K+ current and a transient Na+ current and, surprisingly, the predominant repolarizing ion current is not a voltage-gated K+ current but is instead a Na+-activated K+ current (Markham et al. 2013; Ban et al. 2015).
Electrocytes that produce brief, biphasic discharges apparently recruit a much broader complement of ionic conductances. The biphasic electrocytes of Steatogenys elegans (“barred knifefish”) express an inward rectifier K+ current, two distinct transient Na+ currents, a delayed rectifier K+ current, and an inactivating A-type K+ current (Markham and Zakon 2014). Gymnotus carapo electrocytes express two functionally distinct inward rectifiers, a transient Na+ current, a delayed rectifier K+ current, an inactivating A-type K+ current, and a persistent Na+ plateau current (Sierra et al. 2005, 2007).
Data for electrocyte ionic currents are available for just this small subset from the 200+ gymnotiform species but shows a striking diversity of ion-channel mechanisms that contribute to signal diversity. This suggests that recruitment of different ion-channel mechanisms is a major driver of signal diversity in this clade. Unfortunately, very little electrophysiological data on the ionic currents expressed in mormyrid electrocytes are currently available. The complex, multiphasic EOD waveforms in many mormyrid species are likely attributable to electrocyte morphology and multiple patterns of stalk penetration (Alves-Gomes and Hopkins 1997; Gallant et al. 2011). This suggests the interpretation that selective pressures for signal diversity resulted in the recruitment of diverse ion-channel combinations in gymnotiform electrocytes, whereas in mormyrids, signal diversity arose by increased variability in electrocyte morphologies and membrane properties (Bass et al. 1986). However, the extreme diversity of EOD durations in mormyrids, ranging from hundreds of microseconds to more than 10 ms (Hopkins 1999), is also likely a function of the particular ion channels expressed in mormyrid electrocytes and their particular kinetics. This highlights a clear need for both molecular and electrophysiogical data regarding the ionic currents expressed in mormyrid electroctyes.
5.5.2 Ionic Mechanisms of Signal Diversity
Signal diversity between species, as outlined in Sects. 5.4 and 5.5.1, arises from the morphology and ionic conductances of electrocytes together with their innervation pattern. Given the apparently broad diversity of ion channels expressed by the electrocytes of different clades, how does the particular complement of ion channels expressed by electrocytes ultimately determine signal waveform?
5.5.2.1 Signal Duration in Monophasic Signals
The underlying mechanisms regulating EOD duration are differences in the electrocyte AP duration, which are associated with differences in the kinetics of the underlying voltage-gated Na+ and K+ channels. Slower channel kinetics are associated with longer duration electrocyte APs and vice versa (Fig. 5.7). The relationship between ion-channel kinetics and signal duration has been most thoroughly investigated in the wave fish Sternopygus macrurus, where EOD frequencies range from ~70 to 150 Hz and EOD durations vary over a fourfold range between individuals (from ~3 to ~12 ms). In this case, the kinetics of both the voltage-gated Na+ channels and the voltage-gated K+ channels are tightly coregulated across electrocytes from fish with different EOD durations (McAnelly and Zakon 2000). Increased EOD duration (lower EOD frequency) is associated with the slower kinetics of both currents, and the Na+- and K+-current kinetics are tightly correlated (Fig. 5.8).
Variation in Na+-current kinetics across gymnotiform species for which data are available. Shown are families of Na+ currents elicited by increasingly depolarizing voltage steps under two-electrode voltage clamp (Gymnotus carapo, Brachyhypopomus bennetti, Eigenmannia virescens) or single-electrode patch clamp (Steatogenys elegans, Brachyhypopomus gauderio). In all species, Na+ currents are the product of NaV1.4 voltage-gated Na+ channels, yet the activation and inactivation kinetics are vastly different across species, with current durations ranging from ~0.5 to more than 3 ms. Data for Steatogenys elegans from Markham and Zakon (2014), for Gymnotus carapo adapted from Sierra et al. (2005), for Brachyhypopomus gauderio adapted from Markham (2013), for Brachyhypopomus bennetti from David Saenz (with permission), and for Eigenmannia virescens from Markham et al. (2013)
Coregulation of Na+- and K+-current kinetics in Sternopygus macrurus. a: Current families for voltage-dependent K+ currents (left) and voltage-dependent Na+ currents (right) in an electrocyte from a high-frequency (freq.) fish (131 Hz) and an electrocyte from a low-frequency fish (55 Hz). Activation of the K+ current is much faster for the high-frequency fish and activation/inactivation of the Na+ current is also faster in the high-frequency fish. b: K+-current activation time constants and Na+-current inactivation time constants are tightly correlated across electrocytes from fish with different EOD frequencies. Adapted from McAnelly and Zakon (2000)
It would be possible to shape AP duration by regulating the kinetics of either the depolarizing Na+ current or the repolarizing K+ current in isolation, raising the question of why both currents are regulated in tandem. One likely explanation is that coregulating Na+- and K+-channel kinetics minimizes the energetically wasteful overlap of Na+ and K+ currents during the AP (Alle et al. 2009; Carter and Bean 2009). That is, when both the Na+ and K+ conductances are simultaneously active, the inward Na+ current is offset by the outward K+ current and does not contribute to changes in membrane potential, yet the Na+ must still be returned to the extracellular space with the associated metabolic costs incurred by Na+/K+-ATPase. Accordingly, the coordinated regulation of kinetics in two molecularly distinct ion channels is likely driven by pressures to reduce the metabolic costs of EOD production.
In the high-frequency wave fish Eigenmannia virescens, EOD frequencies range from about 200–600 Hz (Scheich 1977), with EOD durations of about 1–2 ms. Sustaining such high firing rates presents two related challenges for electrocytes. The first is the generation of very brief APs, and the second is minimizing the refractory period following each AP. In Eigenmannia virescens electrocytes, the voltage-gated Na+ currents show extremely fast activation/inactivation kinetics and a time constant for recovery from inactivation of about 300 μs, with complete recovery from inactivation in less than 1 ms (Markham et al. 2013). Thus, Na+-channel kinetics in Eigenmannia virescens electrocytes are well suited to both brief and high-frequency APs.
Electrocytes of Eigenmannia virescens appear to be unique in that they repolarize the AP with Na+-activated K+ (KNa) channels (Markham et al. 2013) rather than voltage-gated K+ channels in other species where data on electrocyte ionic currents are available (Ferrari and Zakon 1993; Markham 2013). As electrophysiological data become available for a broader range of species, the expression of KNa channels in electrocytes may turn out to be more widespread than expected. However, given the transition from voltage-gated K+ channels in Sternopygus macrurus electrocytes to the molecularly distinct class of KNa channels in Eigenmannia virescens electrocytes, the question arises as to what functional adaptation the KNa channels might serve in Eigenmannia virescens. Computational simulations suggest that repolarizing the electrocyte AP with KNa channels might serve to further minimize the wasteful overlap of Na+ and K+ currents in electrocytes with brief APs (Markham et al. 2013), thereby improving the energy efficiency of EOD production for high-frequency wave-type fish. Subsequent findings, however, suggest that KNa channels serve a different purpose in Eigenmannia virescens because the KNa channels in these electrocytes are found on the opposite end of the electrocyte, more than 1 mm from the voltage-gated Na+ channels (Fig. 5.9; Ban et al. 2015). This arrangement is especially puzzling because, in other systems, micrometer-scale colocalization of Na+ and KNa channels is necessary for KNa-channel activation (Budelli et al. 2009; Hage and Salkoff 2012).
Expression patterns of voltage-gated Na+ channels (NaV) and sodium-activated K+ channels (KNa) in an electrocyte from Eigenmannia virescens. Cells were immunolabeled with antibodies for nerve (3A10), NaV channels, and KNa channels. 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) labels nuclei that reside just under the membrane in these multinucleated electrocytes, thereby providing a general outline of the cell morphology. A1: only the posterior region (right) is innervated, with axons of innervating spinal motor neurons labeled with 3A10 (green). B1: KNa channels are expressed only on the anterior face (left). A2 and B2: enlarged areas from white-line boxes. Adapted from Ban et al. (2015)
5.5.2.2 Signal Waveform in Multiphasic Signals
Electrocytes that produce biphasic discharges do so by the sequential generation of APs on two distinct regions of excitable membrane as described in Sect. 5.4 (Fig. 5.5, center). The AP1-AP2 delay in this type of electrocyte must be very tightly regulated to maintain the electrocyte discharge waveform. Where precise data are available, the AP1-AP2 delay ranges from approximately 30–100 ms (Markham and Stoddard 2005; Markham and Zakon 2014), and very small differences in this delay, even a few microseconds, can significantly distort the resulting biphasic electrocyte discharge (μEOD; Markham and Zakon 2014).
Several mechanisms are possible for maintaining the AP1-AP2 delay in biphasic electrocytes. In some cases, both electrocyte faces are innervated so the timing of the respective APs is controlled by the spinal motor neurons (Macadar et al. 1989b; Caputi et al. 1994). The maintenance of a precise AP1-AP2 delay in biphasic electrocytes where only the posterior membrane is innervated is less easily understood. An intuitive assumption is that the initiation of the AP1 on the posterior membrane depolarizes the anterior membrane and initiates the AP2 with some propagation delay. This mechanism, however, would be insufficient to maintain an extremely precise delay. The earliest hypothesis for the reliable AP1-AP2 delay in electrocytes was that differences in passive properties between the two excitable membranes controlled the order and timing of the two APs (Bennett 1961). For example, a higher resistance or lower capacitance on the innervated membrane would result in a larger and/or faster depolarization of that membrane and earlier initiation of the AP1.
In the one case where the mechanisms of the AP1-AP2 delay have been investigated, it is active ionic mechanisms that regulate the AP1-AP2 delay. The biphasic electrocytes from Steatogenys elegans maintain a delay of ~30 μs between the two APs. The innervated posterior face generates the AP1 followed by the AP2 on the noninnervated anterior face, and this delay is rigidly maintained within ±3 μs across a variety of stimulus conditions that would be expected to disrupt the timing of the 2 APs and the resulting μEOD waveform. Patch-clamp recordings of voltage-gated Na+ currents on each electrocyte face revealed that the activation voltage of the Na+ channels on the posterior face was approximately 10 mV lower than for the Na+ channels on the anterior face. Computational simulations support the conclusion that this difference alone is sufficient to account for the precise AP timing observed in Steatogenys elegans electrocytes (Markham and Zakon 2014). This, of course, does not rule out different mechanisms for regulating AP timing in other species but simply provides evidence of potentially varied biophysical mechanisms for achieving a particular signal characteristic.
The two APs generated by biphasic electrocytes have different durations, with the AP2 typically being longer than the AP1 (Bennett 1970, 1961). In the two multiphasic gymnotiforms where the electrocyte ionic currents have been characterized (Gymnotus carapo and Steatogenys elegans), electrocytes express inactivating A-type K+ channels (KA) in addition to the classical delayed rectifier K+ channels found in electrocytes of monophasic wave fish (Sierra et al. 2007; Markham and Zakon 2014). The activation kinetics of these KA channels are extremely rapid, and in Steatogenys elegans they are the dominant repolarizing current for both AP1 and AP2, with computational simulations suggesting that a higher density of KA channels on the innervated posterior membrane is responsible for shortening the AP1 duration relative to the AP2 duration (Markham and Zakon 2014), and the same mechanism seems likely in Gymnotus carapo electrocytes. An important and unanswered comparative question is what biophysical mechanisms regulate the AP duration in biphasic electrocytes of mormyrids in which 1 or more phases of the complex signal waveform can exceed 20 ms (Hopkins 1999), far longer than any multiphasic discharge observed in gymnotiforms, which are at most a few milliseconds in duration (Crampton and Albert 2006).
5.5.2.3 Biophysical Mechanisms of Signal Cloaking
Almost all weakly electric fish have developed mechanisms for centering the EOD energy on 0 V DC, thereby “cloaking” the signal from electroreceptive predators sensitive to low frequencies (Stoddard and Markham 2008). In wave-type fish with monophasic head-positive EODs, the noninnervated anterior faces on the electrocytes generate a head-negative DC current that sums with the head-positive APs (Bennett 1961) to center the EOD energy around 0 V. This occurs in Gymnarchus niloticus through the passive discharge of the sizable capacitance of the anterior membrane. In wave-type gymnotiforms with myogenic electric organs, such as Eigenmannia and Sternopygus, the cellular mechanism underlying this head-negative DC component remains unknown. A persistent active process seems likely because this DC potential decays over the course of 10–15 ms after electrocyte discharges are silenced (Bennett 1961). Solving this puzzle would provide an intriguing comparative perspective concerning the parallel or convergent evolution of similar mechanisms for reducing predation risk.
The remaining gymnotiform and mormyrid species also reduce low-frequency spectral energy in the EOD but accomplish this with a different mechanism at the level of individual electrocytes. By generating biphasic or multiphasic signals with roughly equal head-positive and head-negative phases, the signal has approximately as much energy above 0 V DC as below, which nulls the DC component of the signal and attenuates the low-frequency energy. This enhancement of signal complexity likely serves to make the signal less conspicuous to electroreceptive predators (Stoddard 1999; see Krahe Chap. 7).
5.5.3 Molecular Evolution of Ion Channels Contributes to Signal Diversity
At the root of the ionic mechanisms of signal diversity is the molecular evolution of electrocyte ion channels. The whole genome duplication that preceded the radiation of teleosts (Hurley et al. 2007) provided these fishes with two paralogs of every gene. In both gymnotiforms and mormyrids, the presence of a second gene for each ion channel allowed the exclusive expression of one paralog in electrocytes where functionally significant modifications that might otherwise be crippling or fatal might instead produce adaptive diversity. The best example is the NaV1.4 sodium-channel gene that is expressed in vertebrate skeletal muscle. In both gymnotiforms and mormyrids, one NaV1.4 paralog (NaV1.4a) is expressed only in electrocytes, whereas its paralog NaV1.4b is expressed in both muscle and electrocytes (Zakon et al. 2006; Arnegard et al. 2010b).
The NaV1.4a channels expressed only in gymnotiform and mormyrid electrocytes have rapidly accumulated mutations at locations in the channel gene known to affect channel kinetics (Zakon et al. 2006; Arnegard et al. 2010b), likely because this gene was released from purifying selection pressures in skeletal muscle and subject to positive selection on EOD waveform divergence. Interestingly, some of the mutations that presumably drive signal diversity in the electric signals are associated with disease states when they occur in human sodium channels (Zakon et al. 2006). The rapid evolution of sodium-channel genes in electrocytes has likely been accompanied by a similarly rapid evolution of other key ion channels that accompanied the broad divergence of EOD waveforms and waveform regulation mechanisms.
More recent results have shown that the molecular evolution of electrocyte voltage-gated K+ channels also plays an important role in shaping EOD waveforms (Swapna et al. 2018). The wave-type mormyroid Gymnarchus niloticus generates electrocyte APs and EODs that are more than 1 ms in duration, whereas the pulse-type mormyrid Brienomyrus brachyistius (“baby whale”) produces much shorter electrocyte APs and EODs (approximately 200 ms long). Transcriptomic analyses showed that the same voltage-gated K+ channel, KV1.7a, is expressed at high levels in the electrocytes of both species and that this K+ channel had undergone rapid molecular evolution in mormyrids compared with the more basal Gymnarchus. Electrophysiological analysis of these KV1.7a channels expressed in Xenopus oocytes showed that Brienomyrus KV1.7a activates at more hyperpolarized membrane potentials than Gymnarchus KV1.7a because of the insertion of a patch of negative amino acids near the voltage-sensing element of Brienomyrus KV1.7a. By activating at lower membrane potentials, the Brienomyrus channel activates much sooner after the initiation of the AP, thereby terminating the AP more rapidly than the Gymnarchus channel that would activate much later after AP onset. This change appears to be sufficient to account for the different durations of Gymnarchus and Brienomyrus EODs, demonstrating that relatively small molecular changes can have profound impacts on electric communication signals.
Taken together, these findings from just two electrocyte ion channels emphasize that further identification and characterization of additional signaling mechanisms subject to rapid evolution in electrocytes is a key area for future investigation. Given the apparently broad range of ion channels responsible for shaping the electrocyte AP across species, it seems highly likely that molecular tuning of ion-channel function across multiple ion-channel families is a major contributing factor underlying electric signal diversity.
5.6 Mechanisms of Signal Development and Plasticity
5.6.1 Developmental Changes
The ontogenic development of electric organs is an area of intense investigation, now aided by recent genomic advances (see Gallant, Chap. 4). Larval electric organs are replaced during development by adult electric organs in many mormyrid and gymnotiform species (Franchina 1997; Kirschbaum and Schwassmann 2008). The neurogenic organs of apteronotids also arise after the development and loss of a myogenic larval organ (Kirschbaum 1983). In many mormyrids where adult electrocytes show complex patterns of innervation and stalk morphology, the adult electric organ is preceded by a larval organ in which the electrocytes resemble (and may be homologous to) the structurally simpler electrocytes of Gymnarchus niloticus (Westby and Kirschbaum 1977, 1978). In some gymnotiform species, the larval electric organ is not replaced by a distinct adult organ, but instead, electrocytes are transformed during development into adult electrocytes (Franchina 1997; Kirschbaum and Schwassmann 2008). Brachyhypopomus pinnicaudatus (“feathertail knifefish”; now Brachyhypopomus gauderio) is one species where larval electrocytes are transformed into adult electrocytes over the course of approximately 3 mo. The larval electrocytes are elongated cylindrical cells resembling the electrocytes of monophasic wave fish such as Eigenmannia virescens and they produce a head-positive monophasic signal. During development, these larval electrocytes gradually compress in length, becoming increasingly box-like, and these changes are accompanied by the gradual addition of a second head-negative phase (Fig. 5.3; Franchina 1997). A fascinating yet unanswered question is what changes in the ion-channel expression and localization of the electrocyte accompany the developmental transformation from a monophasic electrocyte to a biphasic electrocyte.
5.6.2 Steroid Hormones and Sexual Dimorphism of Electric Signals
A comprehensive review covering the central and peripheral hormonal regulation of electric signaling behavior is offered by Silva (Chap. 6). The focus here is on the biophysical mechanisms by which these hormones regulate and modulate electric signal production. Sexual dimorphism in electric signals was first described in the gymnotiform Sternopygus macrurus (Hopkins 1972), and subsequently, sexually dimorphic EOD waveforms were reported in many gymnotiform (Hagedorn and Carr 1985; Hopkins et al. 1990) and mormyrid (Hopkins 1980, 1981) species. These sex differences are regulated by steroid hormones. Experimental administration of exogenous androgens increases EOD duration and enhances low-frequency spectral content (“masculinizes” the EOD) of juvenile and female fish by altering the AP waveforms of the electrocytes in both mormyrid (Bass and Hopkins 1985; Bass and Volman 1987) and gymnotiform (Hagedorn and Carr 1985; Mills and Zakon 1991) species. In mormyrids, estrogens also increase EOD duration (Bass and Hopkins 1985), whereas estrogens have the opposite effect of reducing EOD duration in gymnotiforms (Dunlap et al. 1997). The ionic mechanisms by which steroid hormones modify electrocyte APs have been investigated in several gymnotiform species, but no comparable data are yet available for mormyrids.
5.6.2.1 Steroid Hormone Regulation of Voltage-Gated Ion-Channel Kinetics
In the monophasic wave fish Sternopygus macrurus, exogenous androgens increase electrocyte AP duration by slowing the kinetics of the voltage-gated Na+ current of the electrocyte (Ferrari et al. 1995) and estrogen treatment shortens the electrocyte AP waveform by speeding up the inactivation kinetics of the voltage-gated Na+ current of the electrocyte (Dunlap et al. 1997). A similar pattern was found for the kinetics of the voltage-gated K+ currents of the electrocytes (McAnelly and Zakon 2007). The molecular mechanisms behind these steroid-induced effects are complex but fascinating.
Sodium channels consist of a single α-subunit that by itself can form a functional channel. The addition of accessory β-subunits often alters the functional properties of these channels. Sternopygus electrocytes express two different α-subunits, NaV1.4a and NaV1.4b, and the NaV1.4b gene is expressed as both long (NaV1.4bL) and short (NaV1.4bS) splice variants. NaV1.4b is found in both skeletal muscle and in electrocytes, whereas NaV1.4a is expressed only in electrocytes (Zakon et al. 2006). Expression levels of NaV1.4a in electrocytes is constant regardless of EOD duration, but expression levels of NaV1.4bL are correlated with shorter EOD durations. Androgen treatment suppresses NaV1.4bL expression levels in electrocytes, which is the likely mechanism behind longer duration male EODs. Expression levels of a sodium-channel β1-subunit expressed in electrocytes ares also correlated with shorter EODs and are also suppressed by androgen treatment (Liu et al. 2007). The role of both NaV1.4bL and the β1-subunits in accelerating Na+-channel inactivation (thereby shortening electrocyte APs) has been confirmed in heterologous expression systems, providing strong evidence that regulation of their expression levels in electrocytes is the mechanism through which androgens control EOD duration at the level of electrocytes (Liu et al. 2007, 2008).
A similar picture emerges for the voltage-gated K+ channels in Sternopygus electroctyes, which express three different K+-channel genes from the KV1 family. One of these genes, KV1.2b, shows no difference in expression levels across individuals, whereas expression levels of KV1.1a and KV1.2a are correlated with shorter EOD durations. Treatment with steroid hormones that change EOD duration produce corresponding changes in the expression levels of these genes (Few and Zakon 2007). Voltage-gated K+ channels differ from voltage-gated Na+ channels because they are formed as tetramers of channel subunits, either homotetramers of a single subunit variety or heterotetramers of different subunits from the same family. Heterotetrameric channels typically exhibit functional properties intermediate between the properties of the various subunits. In Sternopygus, changing the relative representation of KV1.1a and KV1.2a subunits in the voltage-gated K+ channels of the electrocyte is likely the mechanism underlying the effects of steroid hormones on K+-channel kinetics and electrocyte AP duration.
5.6.2.2 Regulation of Multiphasic Signal Waveforms
Sexual dimorphism of biphasic electric signals is observed in a subset of both mormyrid and gymnotiform species, with the predominant sex difference being that males exaggerate the duration of one or more of the phases of the signal (Hopkins et al. 1990; Hopkins 1999). Regulation of this sexual dimorphism is mediated by steroid hormones, and where experimental evidence is available, the signal regulation occurs at the level of the electrocytes in both mormyrids and gymnotiforms (Hagedorn and Carr 1985; Bass and Volman 1987). In the biphasic gymnotiform Brachyhypopomus occidentalis, males show a prolonged extension of the negative second phase of the signal. Hagedorn and Carr (1985) found that this results from the selective broadening of the electrocyte AP2 in males, whereas the width of AP1 remains relatively constant. A similar mechanism is at work in the related Brachyhypopomus gauderio (Markham and Stoddard 2013).
5.6.3 Temperature-Dependent Signal Changes
In some temperate-zone gymnotiforms, the signal waveform shows temperature-sensitive changes. In both Brachyhypopomus pinnicaudatus and Gymnotus carapo, fish that are not in the reproductive condition decrease the amplitude of the EOD head-negative second phase when the water temperature increases from ~20 °C to above 28 °C (Caputi et al. 1998; Ardanaz et al. 2001). One would suspect that this is a function of temperature-induced accelerations in biophysical kinetics, but these changes occur much more slowly than the change in water temperature and the signal modulations are often transient, indicating that these temperature effects are actively initiated and not simply a matter of temperature-dependent kinetics. Additionally, this is a steroid-dependent effect because sexually mature fish in the breeding condition and nondifferentiated fish given testosterone implants do not exhibit temperature-related changes in signal waveform (Silva et al. 1999; Quintana et al. 2004). Analyzing the underlying cellular mechanisms of this temperature sensitivity and the role of androgen regulation is an important area for future experimental work, especially given the potential implications for reproduction given the imminent thermal disruptions from climate change.
5.6.4 Metabolic Stress and Signal Plasticity
In at least some species, signal characteristics are modulated in response to metabolic stress induced by hypoxia or food restriction. The wave-type gymnotiforms Eigenmannia virescens and Apteronotus leptorhynchus (“brown ghost knifefish”) reduce the signal amplitude within minutes of exposure to hypoxic conditions while the signal frequency remains constant (Reardon et al. 2011). This response to metabolic stress likely serves to reduce the metabolic costs of EOD production, which are known to be extremely high for Eigenmannia (Lewis et al. 2014), whereas no data for signal costs are yet available for Apteronotus. These hypoxia-induced reductions in signal amplitude might result from an absolute energy shortfall in the electric organ or might instead be a proactive physiological mechanism for conserving energy in hypoxic conditions.
Under metabolic stress caused by one or more days of food restriction, Eigenmannia virescens reduces the signal amplitude but not the signal frequency as it does under hypoxia. However, these reductions in amplitude occur over the course of hours to days, much more slowly than hypoxia-induced changes. Reduced signal amplitude during food restriction does not reflect an absolute energetic limitation in the electric organ because full signal amplitude rapidly recovers during social encounters. Instead, the reduction in signal amplitude is a proactive response mediated by the levels of the peptide hormone leptin (Sinnett and Markham 2015). Leptin could be acting via a central endocrine pathway to regulate signal amplitude, it could be acting directly on electrocytes, or both mechanisms could be present.
Interestingly, the pulse-type gymnotiform Brachyhypopomus gauderio does not reduce the signal amplitude during food deprivation. Instead, males increase their signaling effort in social contexts, perhaps a terminal investment in reproduction (Gavassa and Stoddard 2012). Different signaling strategies under food deprivation between Eigenmannia virescens and Brachyhypopomus gauderio could be a function of their different reproductive life-histories. Brachyhypopomus gauderio are semelparous single-season breeders that rarely survive to a second reproductive season. In contrast, Eigenmannia virescens are iteroparous breeders that live for many years.
5.6.5 Biophysical Mechanisms of Rapid Signal Plasticity
In addition to developmental changes in signal waveforms and the sexual differentiation of signal waveforms over the course of weeks to months, signal waveforms in some gymnotiform species also vary on shorter timescales of minutes to hours in response to environmental conditions and social encounters. These rapid signal modulations occur in both monophasic wave fish where waveform modulations are primarily in signal amplitude (Markham et al. 2009b; Sinnett and Markham 2015) and biphasic pulse fish where changes are found in the amplitude of both phases and the duration of the second phase (Fig. 5.3; Franchina and Stoddard 1998; Franchina et al. 2001). These observations suggest that these rapid signal modulations are produced by moment-to-moment modulations in the underlying biophysics of the electrocytes.
Research on the rapid neuroendocrine regulation of the EOD waveform in the monophasic wave fish Sternopygus macrurus (Markham et al. 2009b) and in the biphasic pulse fish Brachyhypopomus gauderio (Stoddard et al. 2003; Markham et al. 2009a) led to identification of melanocortin peptide hormones as factors that act directly on electrocytes to produce rapid changes in the signal waveform. These melanocortin hormones, such as adrenocorticotropic hormone or α-melanocyte-stimulating hormone, bind to G-protein-coupled receptors in the electrocyte membrane and activate an intracellular cAMP/protein kinase A (PKA) pathway that then modulates the electrocyte biophysics.
In Sternopygus macrurus, rapid signal modulations include rapid increases in signal amplitude within minutes of social encounters (Fig. 5.10a) and circadian increases in signal amplitude at night when the fish are active (Fig. 5.10b). These modulations of signal amplitude in Sternopygus are mediated by circulating melanocortin peptides that activate the cAMP/PKA pathway where PKA upregulates the trafficking of preformed voltage-gated Na+ channels and inward rectifier K+ channels into the electrocyte membrane, increasing signal amplitude by up to 40% within a matter of minutes (Fig. 5.10c; Markham et al. 2009b). This process is remarkable for its speed and raises the question of why such a large pool of ion channels would be available but not inserted in the membrane at all times. A likely explanation is that the ability to reduce signal amplitude during periods of rest or low social interaction confers significant metabolic savings by reducing the Na+ influx during the electrocyte AP. With a ready pool of preformed ion channels at the ready, signal amplitude can be returned to maximum on demand without the significant delays associated with protein synthesis and processing.
Rapid signal amplitude modulations in Sternopygus macrurus are caused by hormonally regulated trafficking of voltage-gated ion channels into the electrocyte membrane. a: EOD amplitudes of 4 representative fish (black lines) recorded approximately every 60 s over 2 days. A second fish added to the recording tank for 1 hour on the second day (arrowheads) caused rapid and transient increases in EOD amplitudes of all fish. b: EOD amplitudes of a representative fish (black dots) recorded approximately every 60 s over 3 days. The signal amplitude increases at night during darkness (gray areas) and decreases during the day with the lights on (white areas). Inset: superimposed EOD waveforms from the same fish taken at nighttime maximum and daytime minimum. c: Schematic diagram of the cellular mechanisms underlying rapid signal amplitude changes in Sternopygus macrurus electrocytes. Ion channels are synthesized in the endoplasmic reticulum, then processed and packaged into vesicles in the Golgi apparatus. Delayed rectifier potassium channels undergo exocytosis to the cell surface and are stable there. Inward rectifier K+ channels and voltage-gated Na+ channels are constitutively cycled into and out of the membrane. This process is modulated when the melanocortin peptide hormone ACTH activates a G-protein-coupled melanocortin receptor that elevates cAMP and activates protein kinase A (PKA). PKA accelerates only the exocytosis of channels into the membrane, thereby increasing the density of Na+ and inward rectifier K+ channels in the electrocyte membrane, producing a higher magnitude of both conductances. Adapted from Markham et al. (2009a)
In the biphasic gymnotiform Brachyhypopomus gauderio, rapid waveform modulations include changes in overall signal amplitude as well as changes in the duration of the P2 of the signal. These signal modulations increase the active range of the signal as well as significantly increase the low-frequency content of the signal (Fig. 5.3). As with Sternopygus, these modulations occur on a circadian rhythm where amplitude and P2 duration increase at night when fish are active and then decrease to a minimum during daytime hours (Stoddard et al. 2007). Rapid increases in signal amplitude and P2 duration also accompany social challenges. These signal modulations are regulated by melanocortin hormones that activate an intracellular cAMP/PKA pathway in Brachyhypopomus electrocytes as is the case in Sternopygus. The cellular mechanisms, however, are quite different.
The biphasic electrocyte discharge (μEOD) from Brachyhypopomus gauderio electrocytes is produced by a sequence of two APs; AP1 initiates first on the innervated posterior membrane followed approximately 75 μs later by initiation of AP2 on the noninnervated posterior membrane, with AP2 being a broader spike than AP1 (Fig. 5.11a). Application of the melanocortin peptide ACTH changes the μEOD waveform in the same manner as the electric signal in vivo: the amplitude of the head-positive EOD phase (P1) and P2 are increased as well as the duration of P2 (Fig. 5.11b, c). The increased P2 amplitude and duration are both a function of the selective broadening of AP2, whereas AP1 width is unchanged. Increases in P1 amplitude, interestingly, do not arise from changes in AP1 or AP2 amplitude because both remain constant. Instead, the AP1-AP2 delay increases by ~35 μs, allowing an increased influence of AP1 on P1 amplitude (Markham and Stoddard 2005).
Mechanisms of rapid signal waveform modulation in Brachyhypopomus gauderio. a: A biphasic electrocyte discharge (μEOD) is produced by two action potentials (APs) generated in close succession. The innervated posterior membrane fires first (AP1; red) followed approximately 75 microseconds later by an AP on the noninnervated anterior membrane (AP2; blue). AP2 is inverted in this figure to reflect the fact that the ionic currents producing AP2 are directed in the direction opposite of the ionic currents that produce AP1. These two APs sum to produce the biphasic μEOD. Adapted from Markham and Stoddard (2013). b: Rapid μEOD waveform changes are initiated by the melanocortin peptide adrenocorticotropic hormone (ACTH). Solid lines, baseline recordings; dashed lines, recordings after 30 min of exposure to ACTH in vitro. ACTH causes increased P1 and P2 amplitude in the μEOD as well as a pronounced increase in the duration of P2. The amplitudes of AP1 (red) and AP2 (blue) do not change, and AP2 is broadened while AP1 duration is constant. c: Increased amplitude and duration of μEOD P2 is produced by the broadening of AP2 (blue), whereas the increased amplitude of μEOD P1 results from an increase of ~35 μs in the delay between AP1 and AP2 (red). Because AP1 and AP2 partially overlap, increasing the AP1-AP2 delay increases the μEOD P1 amplitude by “unmasking” the effects of AP1. Data from Markham and Stoddard (2005)
A number of studies have now shown that steroid and peptide hormones have interactive effects on electrocyte discharge waveform and the resulting signal waveform. In addition to regulating sex differences in baseline EOD characteristics, steroid hormones also regulate the extent and nature of signal waveform changes in response to social interactions and injections of melanocortin hormones where androgens enhance the responsiveness of the signal to melanocortin hormones (Allee et al. 2009; Goldina et al. 2011). These findings provide compelling evidence that electrocytes are the cell-autonomous point of convergence where long-term effects of steroid hormones shape the nature of short-term signal modulation by peptide hormones. The mechanisms by which steroid and peptide hormones interact to coregulate the electrocyte discharge waveform, however, remain unknown and a fertile area for further investigation.
5.7 Summary: Current State of Knowledge, Critical Gaps, and Prospects for the Future
From the early work by Bennett (1961) on the cellular basis of electric signal diversity and continuing through the most recent advances in understanding electrocyte biophysics, the picture that emerges is one of a system that is ripe for comparative analyses that promise important discoveries about the evolutionary shaping of the physiology and biophysics of signal production. The present survey of the known mechanisms of signal diversity shows a remarkable variety of morphological, biophysical, and endocrine mechanisms that shape signal diversity across timescales ranging from evolutionary time to the microsecond-scale timing of bioelectrical events. The mechanisms of diversity across species are themselves remarkable, made all the more interesting by the fairly recent discoveries concerning the mechanisms by which excitable cells such as electrocytes modify their molecular-level functional properties with surprising speed.
The current state of knowledge, however, must be recognized as far from complete. Perhaps the most glaring gap at the moment is the lack of data regarding the ionic mechanisms of signal diversity in mormyrids. Related to this, knowledge about the ionic mechanisms of signal diversity in gymnotiforms is limited to just a handful of species, with no data available for the entire apteronotid family. Heterologous expression of electrocyte ion channels based on genomic data from apteronotids (Thompson et al. 2018) and mormyrids (Nagel et al. 2017; Swapna et al. 2018) will help to fill these gaps, but the need remains for data on electrocyte ionic currents in situ. A broader comparative dataset across continents and across species on each continent, coupled with ecological and life-history data would enable a better understanding of how and why evolutionary- and life-history forces have shaped the particular biophysical mechanisms that shape signal waveforms.
Even for the species where data are available for the ionic mechanisms of signal production, key questions remain about the mechanisms that regulate the signal waveform. For example, in all known cases where EOD waveforms are rapidly modulated by stress, social encounters, or circadian cues, activation of PKA is the key intracellular factor mediating changes in electrocyte excitability (McAnelly et al. 2003; Markham and Stoddard 2005). The exact phosphorylation events that regulate electrocyte biophysics, however, are unknown. Possibilities include phosphorylation of ion channels, vesicular trafficking components, or other regulators of ion-channel function.
It is also important to note that both mormyrid and gymnotiform fishes show sexual dimorphism in their EOD waveforms, regulated by steroid hormones. However, only a subset of gymnotiform species exhibit rapid circadian and socially induced EOD modulations, and no mormyrid species observed to date exhibit rapid EOD waveform modulation. Broad comparative assays for rapid signal modulation across species could determine if this pattern is reliable and begin to address the question of what conditions supported the emergence (and possible loss) of social and circadian EOD modulations in gymnotiforms and why rapid EOD waveform plasticity is not found in mormyrids.
Given that electrocyte morphology plays such a large role in signal diversity both across species and developmentally within species, investigating the mechanisms that determine electrocyte morphology will be essential for a full understanding of signal diversity. The ongoing revolution in genomics and genetic manipulation techniques should enable progress on this front at a rate that would have been considered impossible just a few years ago. This, coupled with ongoing progress in understanding the genetic mechanisms guiding the developmental origin of electrocytes (Gallant et al. 2014; Pinch et al. 2016), will be essential for a full understanding of the cellular mechanisms of signal diversity. Alongside efforts to better understand morphology, continued investigation of the molecular evolution in molecules key to electrical excitability are necessary for a complete understanding of what exactly makes the electrocytes of one species produce a signal so different from even closely related sympatric species. To answer this question is ultimately to understand the biophysical basis of signal diversity in electric fish.
References
Aguilera PA, Castello ME, Caputi AA (2001) Electroreception in Gymnotus carapo: differences between self-generated and conspecific-generated signal carriers. J Exp Biol 204(Pt 2):185–198
Albert JS, Crampton WGR (2005) Diversity and phylogeny of neotropical electric fishes. In: Bullock TH, Hopkins CD, Popper AN, Fay RR (eds) Electroreception. Springer, New York, pp 360–409
Alle H, Roth A, Geiger JRP (2009) Energy-efficient action potentials in hippocampal mossy fibers. Science 325(5946):1405–1408. https://doi.org/10.1126/science.1174331
Allee SJ, Markham MR, Stoddard PK (2009) Androgens enhance plasticity of an electric communication signal in female knifefish, Brachyhypopomus pinnicaudatus. Horm Behav 56(2):264–273. https://doi.org/10.1016/j.yhbeh.2009.05.005
Alves-Gomes J, Hopkins CD (1997) Molecular insights into the phylogeny of mormyriform fishes and the evolution of their electric organs. Brain Behav Evol 49(6):324–350
Ardanaz JL, Silva A, Macadar O (2001) Temperature sensitivity of the electric organ discharge waveform in Gymnotus carapo. J Comp Physiol A 187(11):853–864
Arnegard ME, McIntyre PB, Harmon LJ, Zelditch ML, Crampton WG, Davis JK, Sullivan JP, Lavoue S, Hopkins CD (2010a) Sexual signal evolution outpaces ecological divergence during electric fish species radiation. Am Nat 176(3):335–356. https://doi.org/10.1086/655221
Arnegard ME, Zwickl DJ, Lu Y, Zakon HH (2010b) Old gene duplication facilitates origin and diversification of an innovative communication system--twice. Proc Natl Acad Sci U S A 107(51):22172–22177. https://doi.org/10.1073/pnas.1011803107
Assad C, Rasnow B, Stoddard PK (1999) Electric organ discharges and electric images during electrolocation. J Exp Biol 202(Pt 10):1185–1193
Ban Y, Smith BE, Markham MR (2015) A highly polarized excitable cell separates sodium channels from sodium-activated potassium channels by more than a millimeter. J Neurophysiol 114(1):520–530
Bass AH, Hopkins CD (1985) Hormonal control of sex differences in the electric organ discharge (EOD) of mormyrid fishes. J Comp Physiol A 156(5):587–604
Bass AH, Volman SF (1987) From behavior to membranes: testosterone-induced changes in action potential duration in electric organs. Proc Natl Acad Sci U S A 84(24):9295–9298
Bass AH, Denizot JP, Marchaterre MA (1986) Ultrastructural features and hormone-dependent sex differences of mormyrid electric organs. J Comp Neurol 254(4):511–528. https://doi.org/10.1002/cne.902540405
Bennett MVL (1961) Modes of operation of electric organs. Ann N Y Acad Sci 94:458–509
Bennett MVL (1970) Comparative physiology: electric organs. Annu Rev Physiol 32:471–528
Bennett MVL, Grundfest H (1961) Studies on the morphology and electrophysiology of electric organs III: electrophysiology of electric organs in mormyrids. In: Chagas C, APd C (eds) Bioelectrogenesis: a comparative survey of its mechanisms with particular emphasis on electric fishes. Elsevier Pub. Co., Amsterdam, New York, pp 113–135
Budelli G, Hage TA, Wei A, Rojas P, Jong YJ, O’Malley K, Salkoff L (2009) Na+−activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat Neurosci 12(6):745–750. https://doi.org/10.1038/nn.2313
Caputi AA (1999) The electric organ discharge of pulse gymnotiforms: the transformation of a simple impulse into a complex spatio-temporal electromotor pattern. J Exp Biol 202:1229–1241
Caputi AA, Macadar O, Trujillo-Cenóz O (1994) Waveform generation in Rhamphichthys rostratus (L) (Teleostei, Gymnotiformes). J Comp Physiol A 174(5):633–642
Caputi AA, Silva AC, Macadar O (1998) The electric organ discharge of Brachyhypopomus pinnicaudatus. The effects of environmental variables on waveform generation. Brain Behav Evol 52(3):148–158
Caputi AA, Carlson BA, Macadar O (2005) Electric organs and their control. In: Bullock TH, Hopkins CD, Popper AN, Fay RR (eds) Electroreception. Springer, New York, pp 410–451
Carter BC, Bean BP (2009) Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron 64(6):898–909. https://doi.org/10.1016/j.neuron.2009.12.011
Catania KC (2017) Power transfer to a human during an eectric eel’s shocking leap. Curr Biol 27(18):2887–2891. e2882. https://doi.org/10.1016/j.cub.2017.08.034
Crampton WG, Albert JS (2006) Evolution of electric signal diversity in gymnotiform fishes. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes. Science Publishers, Enfield, pp 647–731
Crampton WG, Lovejoy NR, Waddell JC (2011) Reproductive character displacement and signal ontogeny in a sympatric assemblage of electric fish. Evolution 65(6):1650–1666. https://doi.org/10.1111/j.1558-5646.2011.01245.x
Crampton WGR, Rodríguez-Cattáneo A, Lovejoy NR, Caputi AA (2013) Proximate and ultimate causes of signal diversity in the electric fish Gymnotus. J Exp Biol 216(13):2523–2541. https://doi.org/10.1242/jeb.083261
Dunlap KD, McAnelly ML, Zakon HH (1997) Estrogen modifies an electrocommunication signal by altering the electrocyte sodium current in an electric fish, Sternopygus. J Neurosci 17(8):2869–2875
Ferrari MB, Zakon HH (1993) Conductances contributing to the action potential of Sternopygus electrocytes. J Comp Physiol A 173(3):281–292
Ferrari MB, McAnelly ML, Zakon HH (1995) Individual variation in and androgen-modulation of the sodium current in electric organ. J Neurosci 15(5 Pt 2):4023–4032
Few WP, Zakon HH (2007) Sex differences in and hormonal regulation of Kv1 potassium channel gene expression in the electric organ: molecular control of a social signal. Dev Neurobiol 67(5):535–549. https://doi.org/10.1002/dneu.20305
Franchina CR (1997) Ontogeny of the electric organ discharge and the electric organ in the weakly electric pulse fish Brachyhypopomus pinnicaudatus (Hypopomidae, Gymnotiformes). J Comp Physiol A 181(2):111–119
Franchina CR, Stoddard PK (1998) Plasticity of the electric organ discharge waveform of the electric fish Brachyhypopomus pinnicaudatus. I Quantification of day-night changes. J Comp Physiol, A 183(6):759–768
Franchina CR, Salazar VL, Volmar CH, Stoddard PK (2001) Plasticity of the electric organ discharge waveform of male Brachyhypopomus pinnicaudatus. II. Social effects. J Comp Physiol, A 187(1):45–52
Gallant JR, Arnegard ME, Sullivan JP, Carlson BA, Hopkins CD (2011) Signal variation and its morphological correlates in Paramormyrops kingsleyae provide insight into the evolution of electrogenic signal diversity in mormyrid electric fish. J Comp Physiol A 197(8):799–817. https://doi.org/10.1007/s00359-011-0643-8
Gallant JR, Traeger LL, Volkening JD, Moffett H, Chen P-H, Novina CD, Phillips GN, Anand R, Wells GB, Pinch M, Güth R, Unguez GA, Albert JS, Zakon HH, Samanta MP, Sussman MR (2014) Genomic basis for the convergent evolution of electric organs. Science 344(6191):1522–1525. https://doi.org/10.1126/science.1254432
Gavassa S, Stoddard PK (2012) Food restriction promotes signaling effort in response to social challenge in a short-lived electric fish. Horm Behav 62(4):381–388. https://doi.org/10.1016/j.yhbeh.2012.07.003
Goldina A, Gavassa S, Stoddard PK (2011) Testosterone and 11-ketotestosterone have different regulatory effects on electric communication signals of male Brachyhypopomus gauderio. Horm Behav 60(2):139–147. https://doi.org/10.1016/j.yhbeh.2011.03.014
Hage TA, Salkoff L (2012) Sodium-activated potassium channels are functionally coupled to persistent sodium currents. J Neurosci 32(8):2714–2721. https://doi.org/10.1523/JNEUROSCI.5088-11.2012
Hagedorn M, Carr C (1985) Single electrocytes produce a sexually dimorphic signal in South American electric fish, Hypopomus occidentalis (Gymnotiformes, Hypopomidae). J Comp Physiol A 156:511–523
Hopkins CD (1972) Sex differences in electric signaling in an electric fish. Science 176(4038):1035–1037. https://doi.org/10.1126/science.176.4038.1035
Hopkins CD (1980) Evolution of electric communication channels of mormyrids. Behav Ecol Sociobiol 7(1):1–13
Hopkins CD (1981) On the diversity of eectric signals in a community of mormyrid electric fish in West Africa. Am Zool 21(1):211–222
Hopkins CD (1999) Design features for electric communication. J Exp Biol 202(Pt 10):1217–1228
Hopkins CD, Comfort NC, Bastian J, Bass AH (1990) Functional analysis of sexual dimorphism in an electric fish, Hypopomus pinnicaudatus, order Gymnotiformes. Brain Behav Evol 35(6):350–367
Hurley IA, Mueller RL, Dunn KA, Schmidt EJ, Friedman M, Ho RK, Prince VE, Yang Z, Thomas MG, Coates MI (2007) A new time-scale for ray-finned fish evolution. Proc Biol Sci 274(1609):489–498
Kirschbaum F (1983) Myogenic electric organ precedes the neurogenic organ in apteronotid fish. Naturwissenschaften 70(4):205–207
Kirschbaum F, Schwassmann HO (2008) Ontogeny and evolution of electric organs in gymnotiform fish. J Physiol Paris 102(4–6):347–356. https://doi.org/10.1016/j.jphysparis.2008.10.008
Lavoué S, Miya M, Arnegard ME, Sullivan JP, Hopkins CD, Nishida M (2012) Comparable ages for the independent origins of electrogenesis in African and South American weakly electric fishes. PLoS One 7(5):e36287. https://doi.org/10.1371/journal.pone.0036287
Lewis JE, Gilmour KM, Moorhead MJ, Perry SF, Markham MR (2014) Action potential energetics at the organismal level reveal a trade-off in efficiency at high firing rates. J Neurosci 34(1):197–201. https://doi.org/10.1523/jneurosci.3180-13.2014
Liu H, Wu MM, Zakon HH (2007) Individual variation and hormonal modulation of a sodium channel beta subunit in the electric organ correlate with variation in a social signal. Dev Neurobiol 67(10):1289–1304. https://doi.org/10.1002/dneu.20404
Liu H, Wu MM, Zakon HH (2008) A novel Na+ channel splice form contributes to the regulation of an androgen-dependent social signal. J Neurosci 28(37):9173–9182. https://doi.org/10.1523/JNEUROSCI.2783-08.2008
Lorenzo D, Velluti JC, Macadar O (1988) Electrophysiological properties of abdominal electrocytes in the weakly electric fish Gymnotus carapo. J Comp Physiol A 162(1):141–144
Macadar O, Lorenzo D, Velluti JC (1989a) Waveform generation of the electric organ discharge in Gymnotus carapo. J Comp Physiol A 165(3):353–360
Macadar O, Lorenzo D, Velluti JC (1989b) Waveform generation of the electric organ discharge in Gymnotus carapo 2. Electrophysiological properties of single electrocytes. J Comp Physiol A 165(3):353–360. https://doi.org/10.1007/Bf00619354
Markham MR (2013) Electrocyte physiology: 50 years later. J Exp Biol 216(13):2451–2458. https://doi.org/10.1242/jeb.082628
Markham MR, Stoddard PK (2005) Adrenocorticotropic hormone enhances the masculinity of an electric communication signal by modulating the waveform and timing of action potentials within individual cells. J Neurosci 25(38):8746–8754
Markham MR, Stoddard PK (2013) Cellular mechanisms of developmental and sex differences in the rapid hormonal modulation of a social communication signal. Horm Behav 63(4):586–597. https://doi.org/10.1016/j.yhbeh.2013.02.004
Markham MR, Zakon HH (2014) Ionic mechanisms of microsecond-scale spike timing in single cells. J Neurosci 34(19):6668–6678. https://doi.org/10.1523/jneurosci.0615-14.2014
Markham MR, Allee SJ, Goldina A, Stoddard PK (2009a) Melanocortins regulate the electric waveforms of gymnotiform electric fish. Horm Behav 55(2):306–313. https://doi.org/10.1016/j.yhbeh.2008.11.002
Markham MR, McAnelly ML, Stoddard PK, Zakon HH (2009b) Circadian and social cues regulate ion channel trafficking. PLoS Biol 7(9):e1000203
Markham MR, Kaczmarek LK, Zakon HH (2013) A sodium-activated potassium channel supports high-frequency firing and reduces energetic costs during rapid modulations of action potential amplitude. J Neurophysiol 109(7):1713–1723. https://doi.org/10.1152/jn.00875.2012
McAnelly ML, Zakon HH (2000) Coregulation of voltage-dependent kinetics of Na(+) and K(+) currents in electric organ. J Neurosci 20(9):3408–3414
McAnelly ML, Zakon HH (2007) Androgen modulates the kinetics of the delayed rectifying K+ current in the electric organ of a weakly electric fish. Dev Neurobiol 67(12):1589–1597. https://doi.org/10.1002/dneu.20530
McAnelly L, Silva A, Zakon HH (2003) Cyclic AMP modulates electrical signaling in a weakly electric fish. J Comp Physiol A 189(4):273–282. https://doi.org/10.1007/s00359-003-0400-8
McGregor PK, Westby GWM (1992) Discrimination of individually characteristic electric organ discharges by a weakly electric fish. Anim Behav 43(Part 6):977–986
Mills A, Zakon HH (1991) Chronic androgen treatment increases action potential duration in the electric organ of Sternopygus. J Neurosci 11(8):2349–2361
Nagel R, Kirschbaum F, Tiedemann R (2017) Electric organ discharge diversification in mormyrid weakly electric fish is associated with differential expression of voltage-gated ion channel genes. J Comp Physiol A 203(3):183–195. https://doi.org/10.1007/s00359-017-1151-2
Pinch M, Guth R, Samanta MP, Chaidez A, Unguez GA (2016) The myogenic electric organ of Sternopygus macrurus: a non-contractile tissue with a skeletal muscle transcriptome. PeerJ 4:e1828. https://doi.org/10.7717/peerj.1828
Quintana L, Silva A, Berois N, Macadar O (2004) Temperature induces gonadal maturation and affects electrophysiological sexual maturity indicators in Brachyhypopomus pinnicaudatus from a temperate climate. J Exp Biol 207(11):1843–1853
Reardon EE, Parisi A, Krahe R, Chapman LJ (2011) Energetic constraints on electric signalling in wave-type weakly electric fishes. J Exp Biol 214(Pt 24):4141–4150. https://doi.org/10.1242/jeb.059444
Roberts TR (1975) Geographical distribution of african freshwater fishes. Zool J Linn Soc 57(4):249–319. https://doi.org/10.1111/j.1096-3642.1975.tb01893.x
Salazar VL, Stoddard PK (2008) Sex differences in energetic costs explain sexual dimorphism in the circadian rhythm modulation of the electrocommunication signal of the gymnotiform fish Brachyhypopomus pinnicaudatus. J Exp Biol 211(Pt 6):1012–1020. https://doi.org/10.1242/jeb.014795
Salazar VL, Krahe R, Lewis JE (2013) The energetics of electric organ discharge generation in gymnotiform weakly electric fish. J Exp Biol 216(Pt 13):2459–2468. https://doi.org/10.1242/jeb.082735
Scheich H (1977) Neural basis of communication in the high frequency electric fish, Eigenmannia virescens (jamming avoidance response). J Comp Physiol A 113(2):181–206
Shenkel S, Sigworth FJ (1991) Patch recordings from the electrocytes of Electrophorus electricus. Na currents and PNa/PK variability. J Gen Physiol 97(5):1013–1041. https://doi.org/10.1085/jgp.97.5.1013
Sierra F, Comas V, Buno W, Macadar O (2005) Sodium-dependent plateau potentials in electrocytes of the electric fish Gymnotus carapo. J Comp Physiol, A 191(1):1
Sierra F, Comas V, Buno W, Macadar O (2007) Voltage-gated potassium conductances in Gymnotus electrocytes. Neuroscience 145(2):453–463. https://doi.org/10.1016/j.neuroscience.2006.12.002
Silva A, Quintana L, Galeano M, Errandonea P, Macadar O (1999) Water temperature sensitivity of EOD waveform in Brachyhypopomus pinnicaudatus. J Comp Physiol A 185(2):187–197
Sinnett PM, Markham MR (2015) Food deprivation reduces and leptin increases the amplitude of an active sensory and communication signal in a weakly electric fish. Horm Behav 71:31–40. https://doi.org/10.1016/j.yhbeh.2015.03.010
Stoddard PK (1999) Predation enhances complexity in the evolution of electric fish signals. Nature 400(6741):254–256
Stoddard PK, Markham MR (2008) Signal cloaking by electric fish. Bioscience 58(5):415–425
Stoddard PK, Markham MR, Salazar VL (2003) Serotonin modulates the electric waveform of the gymnotiform electric fish Brachyhypopomus pinnicaudatus. J Exp Biol 206(Pt 8):1353–1362
Stoddard PK, Markham MR, Salazar VL, Allee S (2007) Circadian rhythms in electric waveform structure and rate in the electric fish Brachyhypopomus pinnicaudatus. Physiol Behav 90(1):11–20
Swapna I, Ghezzi A, York JM, Markham MR, Halling DB, Lu Y, Gallant JR, Zakon HH (2018) Electrostatic tuning of a potassium channel in electric fish. Curr Biol 28(13):2094–2102.e2095. https://doi.org/10.1016/j.cub.2018.05.012
Thompson A, Infield DT, Smith AR, Smith GT, Ahern CA, Zakon HH (2018) Rapid evolution of a voltage-gated sodium channel gene in a lineage of electric fish leads to a persistent sodium current. PLoS Biol 16(3):e2004892. https://doi.org/10.1371/journal.pbio.2004892
Waddell JC, Rodriguez-Cattaneo A, Caputi AA, Crampton WGR (2016) Electric organ discharges and near-field spatiotemporal patterns of the electromotive force in a sympatric assemblage of Neotropical electric knifefish. J Physiol Paris 110(3 Pt B):164–181. https://doi.org/10.1016/j.jphysparis.2016.10.004
Westby GWM, Kirschbaum F (1977) Emergence and development of electric organ discharge in mormyrid fish, Pollimyrus isidori. 1. Larval discharge. J Comp Physiol 122(2):251–271
Westby GWM, Kirschbaum F (1978) Emergence and development of electric organ discharge in mormyrid fish, Pollimyrus isidori. 2. Replacement of larval by adult discharge. J Comp Physiol 127(1):45–59
Zakon HH, Lu Y, Zwickl DJ, Hillis DM (2006) Sodium channel genes and the evolution of diversity in communication signals of electric fishes: convergent molecular evolution. Proc Natl Acad Sci U S A 103(10):3675–3680. https://doi.org/10.1073/pnas.0600160103
Acknowledgments
Preparation of this chapter was supported by Grants IOS1644965, IOS1350753, and IOS1257580 from the National Science Foundation and by the Case-Hooper endowment, funded through a gift from Dr. and Mrs. Robert Case to The University of Oklahoma, Norman.
Compliance with Ethics Requirements
Michael R. Markham declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Markham, M.R. (2019). Biophysical Basis of Electric Signal Diversity. In: Carlson, B., Sisneros, J., Popper, A., Fay, R. (eds) Electroreception: Fundamental Insights from Comparative Approaches. Springer Handbook of Auditory Research, vol 70. Springer, Cham. https://doi.org/10.1007/978-3-030-29105-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-29105-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29104-4
Online ISBN: 978-3-030-29105-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)