Abstract
There have been six independent origins of electric organs within extant vertebrates. In each lineage, the electric organs are derived from either skeletal muscle precursors or from fully differentiated skeletal muscles. Remarkably little is known about the mechanisms underlying this process. With recently acquired genomics datasets from a diverse array of electric fishes, however, this is beginning to change. These new data provide an opportune time for a comprehensive review of electric organ development. This chapter provides a brief introduction on the prospects, progress, and major obstacles to understanding electric organ development, followed by a brief overview of skeletal muscle development. This is followed by a consideration of data accumulated over the past 150 years on electric organ development, ranging from early histological observations to the characterization of novel microRNAs that regulate electric organ development to the first attempts at examining mechanisms of development in comparative genomics framework. The purposes of this chapter are to (1) synthesize a broad literature on electric organ development; (2) introduce the reader to more recent advances in understanding the molecular mechanisms of electric organ development that have occurred in the past 20-30 years; (3) consider these historical and more contemporary references in light of a new comparative study of gene expression across multiple lineages of electric fishes; (4) summarize the current broader themes in electric organ development; and (5) identify the needs for new research programs to answer lingering questions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Convergent evolution
- Development
- Electric organs
- Genomics
- Histology
- Muscle
- Myogenic regulatory factors
- RNA sequencing
- Stem cells
4.1 Introduction
Vertebrates have evolved a multitude of adaptive traits to exploit resources and habitats in the air, on the land, and in the water. Several studies have begun to elucidate the genetic and developmental processes underlying major vertebrate traits such as fins (Davis et al. 2007), limbs (Schneider et al. 2011), feathers (Harris et al. 2002), and teeth (McCollum and Sharpe 2001). Few of these structures have evolved repeatedly, particularly in extant lineages where molecular and developmental studies are possible. This prevents the analysis of molecular and developmental processes underlying novel traits in a comparative framework, limiting insights into the degree of constraint and repeatability of the evolutionary processes underlying novel vertebrate traits.
Of the few traits that have evolved multiple times in vertebrates, one of the most distinctive is the electric organ. These have evolved to produce electric fields for the purposes of communication, navigation, and, in extreme cases, predation and defense. In contrast with most other vertebrate traits, there have been six independent origins of electrogenesis (Fig. 4.1) within extant vertebrate lineages. The taxonomic diversity of electrogenic fishes is so broad that Darwin (1859) considered the multiple origins of electric organs difficult to reconcile with his theory of natural selection. Although it has been more than 150 years since the publication of The Origin of Species, remarkably little is known about the “steps by which these organs have been produced” despite their clear benefit as a model for understanding general principles of how complex vertebrate tissues may have evolved repeatedly.
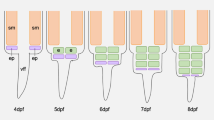
A: phylogenetic distribution of electrogenic lineages with the major events in the evolution of electroreception (see Baker, Chap. 2) and electrogenesis highlighted. Red, taxa that have independently evolved electric organs. Three right columns: representative sketches of species in each of the major electrogenic taxa, approximate location, and size of electric organs (gray). The electric organ (B) is composed of electrocyte cells (C). For each species, a schematic of the three-dimensional configuration of the electrocytes in the electric organ (B) and of the three-dimensional anatomy of an electrocyte (C) is shown. Note that these schematics are not to scale and mainly serve to orient the reader to the text in Sect. 4.3.
Because this chapter is aimed at the newcomer to electric fish, it is prudent to begin with a consideration of why electric organ development is of broad interest. First, the study of electric organs should appeal to students of evolution and development because systems that produce novel structures are not often biologically replicated in evolution. Electric organs have evolved multiple times (Fig. 4.1) and could therefore be tremendously informative in understanding the constraints that operate on the evolution of gene regulatory networks. Second, of interest to vertebrate biologists more generally is the role of gene duplication in the evolution of novel vertebrate structures. Electric organs have evolved in two lineages that predate and three lineages that follow the hypothesized teleost-specific whole genome duplication (see Fig. 4.1). In this sense, specific hypotheses about how whole genome duplication contributes to the evolution of novelty may be directly addressed by electric organ biology. As an example, Thompson et al. (2014, 2016), using electric fish as a model, have created a compelling hypothesis for how genes become “neofunctionalized” through the combined effects of dosage compensation and genetic drift. Finally, electric organs undergo a relatively “rare” developmental process, essentially transforming from one fully differentiated cell type to another (discussed in Sect. 4.2). Together with the well-known abilities of some electric fishes to completely regenerate their electric organs, characterizing electric organ development may one day have broad impacts in fields such as developmental biology, regenerative medicine, and even biological engineering. The ability to produce “biological batteries” from stem cells may one day inspire new classes of artificial biological devices with their own power supplies (Ozbolat and Hospodiuk 2016).
Although there have been several comprehensive reviews on the anatomy and physiology of electric organs (e.g., Bennett 1971; Bass 1986; Markham 2013), there has to date been no comparative reviews of electric organ development across the six lineages of electrogenic fishes. Therefore, the first purpose of this chapter is to provide a synthesis of the broad literature on electric organ development, which has been actively studied for over a period of about 150 years (see Sect. 4.3). The second purpose of this chapter is to introduce the reader to more recent advances in understanding the molecular mechanisms of electric organ development that have occurred in the past 20-30 years (see Sect. 4.4). The third purpose of this chapter is to consider these historical and more contemporary references in light of a new comparative study of gene expression across multiple lineages of electric fishes (see Sect. 4.4). The fourth purpose is to summarize the broader themes achieved thus far in the field (see Sect. 4.5). The final purpose of this chapter is to provide some sense of where the field is heading and identify important questions that should be addressed when considering new research on this exciting topic (see Sect. 4.5).
The study of electric organ development is a difficult task. Perhaps the most obvious and fundamental problem with understanding electric organ development is the paucity of embryological materials available for developmental work; electric fish species are not easily cultured in the laboratory. The majority of early developmental studies were based on the serendipitous availability of embryos and small specimens obtained from the field. Because of this problem, information about the most critical phase of development, the point at which electric organ tissue is specified, is often missed. Because of incomplete embryological series, key time points in the development of electric organs may also be missed. This can lead to misinterpretations about the development of electric organs, as was the case in the electric eel Electrophorus electricus (see Sect. 4.3). It was therefore a major breakthrough when Kirschbaum (1975), discovered the environmental factors necessary to promote gonadal maturation in some electric fish species under laboratory conditions, making it possible to breed two lineages of weakly electric fish, the Gymnotiformes and Mormyroidea.
Another means of circumventing this problem has been to consider the postembryonic development and regeneration (see Sect. 4.3). This form of development has been used by several researchers as a proxy for understanding the development of electric organs. This strategy was used to study the gymnotiformes Sternopygus (Patterson and Zakon 1997) and Eigenmannia (Baillet-Derbin 1978). Despite the success of these studies, it motivates essential questions about the similarity between embryonic and postembryonic developmental mechanisms (Schwassmann et al. 2014; see Sect. 4.3.4).
Another limitation of understanding electric organ development is the inconsistency in techniques applied to various electric fish species. Although the majority of species have been studied using light microscopy, some species have been investigated using electron microscopy, which grants considerable insights into the biochemical and structural properties of electric organs and their precursor cells. An even smaller number of electric fish species (see Sect. 4.4) have been studied using modern molecular biological techniques (e.g., in situ hybridization, immunohistochemistry, next-generation sequencing) that also serve to greatly enhance the conclusions about the developmental origins of these materials.
A final problem with the study of electric organ development is the relatively descriptive nature of the work, which is problematic for identifying general comparative themes in electric organ development. Although researchers clearly read each other’s work and communicate about their findings, studies of electric organ development lack clear hypothesis testing, particularly across lineages. This is further complicated by the incredible taxonomic diversity of electric fishes, which derive their electric organs from a variety of muscles and muscle precursors, even within the same taxonomic groups. One of the purposes of this review is to highlight the common “themes” in electric organ development studies, which will hopefully motivate clear hypotheses to test with newly available genomic data (see Sect. 4.4).
4.2 Electric Organ and Skeletal Muscle Development: A Primer
A more practical issue in approaching the literature on electric organ development is that of terminology. Because of the relatively wide time span over which the studies were performed, the breadth of researchers and disciplines involved, the varied techniques utilized, there are a large number of synonymous terms and potentially terms that are only used by one researcher. To remedy this issue, Sect. 4.2.1 begins with a brief overview of the organization, major structures, and development of teleost skeletal muscle, the tissue most closely related to electric organ tissue in every taxon that has evolved electric organs. This allows for a common conceptual framework in which to approach electric organ development as well as a standardized set of terms by which one can consider the development of electric organs. Wherever possible, attempts are made to use terminology common to muscle development to describe the major ontogenetic events in electric organs.
In Sect. 4.2.2, major structures and features common to all electric organs are considered. The references within these sections will provide a much larger degree of detail than can be provided. For a more in-depth review of the form and function of electric organs, please see Bennett (1971), Bass (1986), and Markham (2013) as well as Markham (Chap. 5).
4.2.1 Skeletal Muscle: Anatomy and Physiology
The lateral musculature in fishes is divided into segmentally arranged myotomes. In gnathostomes, myotomes have characteristic “W”-like shapes, whereas in more basal vertebrate lineages, the shape is simpler (Katz 2002). The myotomes form multiple nested “cones” that enable the force transmission necessary for the wave-like motions of the body used for swimming (Katz 2002). The myotomes themselves are made up of individual muscle cells (muscle fibers), and individual myotomes are separated by a collagenous sheet of tissue called a myoseptum.
Another widely recognized characteristic of fish muscle is the nearly complete separation of muscle fiber types at the anatomical level. Oxidative (slow-twitch) muscle fibers, deeply red in color and used in long duration, low-intensity activity, are located deep and close to the midline, whereas the remaining volume of muscle is glycolytic (fast-twitch) muscle fibers used in high-intensity movements (Bone 1978; Ochi and Westerfield 2007). The relative proportions of the two muscle types vary dramatically, as any sushi aficionado may appreciate.
Unlike many other cells, muscle cells are highly enriched in mitochondria and are multinucleated, partially as a consequence of their unique development. Muscle cells consist of multiple bundles of myofibril proteins surrounded by a specialized membrane called the sarcolemma. Bundles of myofibril proteins inside muscle cells are arranged in a highly regular fashion, which consists of repeating sections of sarcomeres appearing as alternating light and dark striations, giving muscle its characteristic appearance. Sarcomeres consist of many long filamentous proteins; chief among these are myosin, actin, troponin, and tropomyosin.
Innervation of muscle cells occurs in specific locations, named the neuromuscular junction, which consist of a highly folded sarcolemma enriched for acetylcholine receptors. On stimulation with acetylcholine, these receptors open, allowing for the rapid influx of sodium. Like neurons, the sarcolemma propagates action potentials using voltage-gated sodium channels (typically using NaV1.4; see Zakon et al. 2006; Arnegard et al. 2010). Unlike neurons, however, the sarcolemma propagates action potentials through an elaborate network of transverse tubules (T-tubules), allowing action potentials to propagate not only along the cell but deeply into the cell. The action potentials propagate toward intracellular calcium stores in the sarcoplasmic reticulum. Specialized extensions of the sarcoplasmic reticulum called terminal cisternae meet the T-tubule network such that they are closely apposed in an arrangement known as a triad.
As action potentials propagate via the T-tubule network, this leads to stimulation of L-type Ca2+ dihydropyridine receptors (DHPRs) in the T-tubules, which, in turn, physically interact with ryanodine receptors located in the terminal cisternae (Franzini-Armstrong and Protasi 1997). As ryanodine receptors open, Ca2+ is released into the intracellular space. Ca2+ binds to troponin, unmasking myosin binding sites on the actin molecule. In the absence of ATP, actin and myosin remain bound (the source of rigor mortis on an animal’s death), whereas in the presence of ATP, myosin undergoes a conformational change that causes both the myosin head to move and then detach from the actin molecule. Due to the conformational change of the myosin head, the result is a ratcheting motion of myosin along the actin molecule, causing the two filaments to slide past one another and the physical contraction of the cell (Rome 2001).
4.2.2 Skeletal Muscle Development
In all vertebrates, muscle cells originate from paraxial mesoderm, tissue immediately adjacent to the developing neural tube of vertebrates. In fishes, unlike other amniotes, the paraxial mesoderm is specified by the combinatorial actions of fibroblast growth factor (FGF) signals and two T-box domain-containing proteins, spadetail and floating head (Watabe 2001; Bentzinger et al. 2012). These combined signals activate the expression of the early myogenic regulatory factors (MRFs) Myod and Myf5, the earliest recognizable markers of commitment to the myogenic fate. In contrast with other vertebrates, fish myogenic precursor cells express Myod much earlier in development, after gastrulation but before the formation of somites and segmentation (Ochi and Westerfield 2007). Initially appearing as two triangular fields flanking the developing notochord, this population of cells extends into a single layer of cuboidal cells, called adaxial cells, immediately adjacent to the notochord (Currie and Ingham 2001; Ochi and Westerfield 2007). Adaxial cells are morphologically distinct from the surrounding paraxial mesoderm cells, are molecularly distinct, and are characterized by the expression of engrailed 1 and 2 (Ochi and Westerfield 2007).
A subset of adaxial cells migrate to the lateral edge of the developing somite, forming the superficial “slow-twitch” muscle cells (i.e., express “slow” myosin heavy chain isoforms), whereas another subset of nonmigratory adaxial cells, termed the “muscle pioneer” cells, remain medial (Devoto et al. 1996; Ochi and Westerfield 2007). Muscle pioneer cells are among the first to elongate and differentiate into striated, multinucleated myotubes. Because of their early differentiation, they are thought to serve a role as intermediate targets for early motor neuron growth cones and to facilitate the formation of myosepta between adjacent myotomes, thus instructive in the formation of myotomes. The remaining cells between the lateral edge of the somite and the pioneer cells ultimately become “fast-twitch” muscle fibers (i.e., express “fast” myosin heavy chain isoforms). The decision between fast and slow fiber types is mediated partly by the positional gradients of hedgehog, FGF8, and retinoic acid signals (Ochi and Westerfield 2007).
Cells committed to differentiating into mature muscle cells express the early MRFs Myod and Myf5 and are referred to as myoblasts. Myoblasts, in contrast with their mature progeny, are spherical, consist of a single nucleus, and are proliferative. After receiving the appropriate developmental signal, myoblasts cease proliferation and begin to modify their extracellular matrices and cell-adhesive properties to facilitate alignment into long chains. Next, the cells fuse into large, multinucleated cells, termed myotubes, through the expression of a family of proteins called meltrins that physically act to fuse cell membranes (Gilbert and Barresi 2016). It is at this point that the MRFs myogenin and mef2 become active, upregulating sarcomeric and other muscle-specific proteins. As muscle-specific protein expression continues, the myotubes “mature” into muscle fibers, recruiting additional myoblasts to fuse with the growing myotube, eventually increasing the size of the mature myofiber (Gilbert and Barresi 2016).
It is important to note that a subset of paraxial mesoderm cells, although somewhat committed to the myogenic fate by the early expression of myod, remain relatively undifferentiated through development. These cells support one of the most important features of muscle, its regenerative properties, well-known by even the most modest athlete. These cells express a combination of pax3 and pax7 and microRNAs (miRNAs) that are thought to inhibit muscle differentiation (Bentzinger et al. 2012). These cells can divide asynchronously to produce satellite cells and stem cells that can replenish the pool of satellite cells (Fauconneau and Paboeuf 2001). Satellite cells can proliferate and differentiate in response to stress and injury and either can be incorporated into existing muscle fibers (muscle hypertrophy) or form new muscle fibers (Fauconneau and Paboeuf 2001; Bentzinger et al. 2012).
4.3 Electric Organ Development
4.3.1 Electric Organs: General Features and Themes
With these general principles of skeletal muscle development in mind, the stage is set for considering electric organ development. Darwin (1859) observed that electrogenic fishes are “remote in their affinities.” Indeed, the independent origins of electrogenesis appear to span vertebrates, with two lineages of elasmobranch fishes (members of the order Torpediniformes and the family Rajidae) and four lineages of teleost fishes (the superorder Mormyroidea, the order Gymnotiformes, the family Malapturidae, and the genus Astroscopus). In all cases, development has been at least superficially studied using light microscopy; in many cases, using electron microscopy; and in some systems, using modern molecular biology approaches.
In all cases, myogenic electric organs are composed of individual cells, termed electrocytes (synonymous with electroplax). Following conventions established in muscle development as well as in some electric organ development literature, this chapter refers to fully differentiated electric organ cells as electrocytes and their precursor cells as electroblasts. In general, electrocytes tend to (1) be much larger than skeletal muscle cells; (2) have fewer and/or poorly organized myofibril proteins; (3) have disrupted coupling between excitation of the cell membrane and contraction of any remaining myofibril proteins; (4) exhibit strong cellular polarity, with a single innervated face and an uninnervated face characterized by elaborate folds (canniculi); and (5) have organized connective tissue septa to “‘direct” the flow of current through the organ. Evidence for each of these features for each taxon of electric fish is considered in Sect. 4.3.
The mechanisms underlying the development of the electric organs seem to differ considerably between taxa, both in terms of the embryological origin of the tissue and in whether the nascent organ is induced by the presence of neuronal tissue or autonomous of it. Depending on the taxon under consideration, fully differentiated electrocytes resemble the structure of skeletal muscle more or less closely. This is in large part due to the developmental history of the precursor cells. As exemplified by Electrophorus electricus (see Sect. 4.3.4), electric organ cells can derive directly from mesodermal precursor cells, but in most lineages, electrocytes develop from fully differentiated skeletal muscle fibers. This mode of development, called transdifferentation (Patterson and Zakon 1997), is a relatively rare mode of development in vertebrates but occurs most often in myogenically derived cells (Patapoutian et al. 1995). In this mode of development, electrocytes can develop from extraocular muscles (i.e., Astroscopus) or from axial muscles in other lineages (e.g., Mormyroidea). In addition, electric organ development may be conditional on the presence of innervation (e.g., Sternopygus; see Sect. 4.3.4) or may, as in many lineages, be autonomous (e.g. Mormyroidea and Torpedo; see Sects. 4.3.2 and 4.3.3), where electrocytes are well differentiated before innervation is even present.
Finally, electric organs change throughout postembryonic growth. In at least two lineages, the Mormyriformes of Africa and the Gymnotiformes of South America, the fully differentiated adult electric organ is preceded by the development of a distinct larval electric organ (see Sects. 4.3.3 and 4.3.4). The electrocytes of the larval organ tend to resemble adult electrocytes but generally lack the anatomical specializations of the adult organ. Electrocytes and the electric organs they comprise, like muscles, must also grow with the organism and be repaired when damaged. Although not extensively characterized in all electrogenic lineages, it appears that, like muscle cells, satellite cells are involved in this process (see Sect. 4.3.4).
The ensuing subsections consider each of the major taxa of electrogenic fishes in turn, first describing the anatomy of the electric organ and then considering its development, bearing in mind the themes enumerated in this section. For the convenience of the reader, Fig. 4.1 illustrates both the three-dimensional organization of electrocytes inside the electric organ and the overall shape and major features of individual electrocytes in each lineage.
4.3.2 Rajiformes
Skates in the family Rajidae are a group of approximately 200 species distributed across approximately 20 genera (Eschmeyer and Fong 2018). These saltwater skates are not well-known for their electrogenic abilities, possibly due to the fact that the fishes make weak discharges somewhat infrequently (Bennett 1971); however, all are characterized by a weak electric organ located in the tail.
Skate electrocytes are located medially in the tail between longitudinally running muscle fibers (Bennett 1971). The organs are spindle shaped (Ewart 1889a) and run most of the length of the tail. The individual electrocytes comprising the organ are oriented anterior-posterior (Fig. 4.1), innervated on the anterior face, and bounded by connective tissue septa (Ewart 1889a; Bennett 1971). In large skates, there can be more than 10,000 electrocytes per organ, and each electrocyte can have a surface area of 2 square millimeters (Ewart 1892). The anterior face is directly innervated by the electromotor nerves.
There are two alternative morphological configurations of electrocytes present in skates: cup-shaped and disc-shaped cells. Cup-shaped cells are moderately convex and relatively smooth on both faces, with a slightly greater number of canniculi on the posterior (uninnervated) face (Bennett 1971). Disc-shaped cells, in contrast, have more elaborate canniculi (Ewart 1889a) but lack the convex bending. Both classes of cells contain a layer of striated, filamentous material between the two faces (Ewart 1889a, b).
Ewart (1889a, b, 1892) published a series of studies on the development of electric organs from three species of Raja using observations from light microscopy. Based on a preliminary survey of eight species, Ewart (1889a) concluded that the disc-shaped electrocytes of Rajidae are the derived condition and the cup-like electrocytes are likely ancestral.
In young embryos, the tail is composed of fully differentiated muscle fibers. From these muscle fibers, electrocytes begin to form in embryos as small as 7 centimeters. In these specimens, muscle fibers closest to the notochord develop a “club”-like morphology and are surrounded by diffuse connective tissue. As these electroblasts age, the “head” of the club enlarges, flattens, and forms a shallow cup, which Ewart (1889a) posits is facilitated by the activity of myofilaments still present in the anterior part of the cell. The posterior face simultaneously develops canniculi that increase in their size and fuse with each other. Ewart (1889a) notes the presence of a “prong-like” backward extension of the original muscle fiber that is retained through development, much longer than the developing electroblast. This long process, a clear remnant of the muscular origin of these cells, eventually atrophies as the skate ages beyond 60 centimeters (Ewart 1889a).
4.3.3 Torpediniformes
The electric rays in the order Torpediniformes are a group of approximately 70 species distributed across approximately 4 families (Nelson 1994). All species are well-known for their ability to produce strong electric discharges from two large organs located in the head.
The electric organs of Torpedo and Narcine are kidney shaped, dorsoventrally flattened, and bilaterally located lateral to the eyes. Each organ is made up of 500-1,000 closely packed columns, each consisting of approximately 1,000 dorsoventrally flattened electrocytes as large as 5-7 millimeters in diameter and stacked like coins (Bennett 1971). The ventral surface is innervated, with the nerve fibers entering the space between electrocytes, and the dorsal face is uninnervated, consisting of many canniculi (Fig. 4.1).
The electric organ forms in the segments containing the first four branchial arches (Mellinger et al. 1978) from sheets of cells organized in columns of dorsal and ventral plates. The cells are observed initially to contain single nuclei containing myofibrils and then fuse into multinucleated myotubes with recognizable sarcomeric structures, including both thick and thin filaments. These cells are comparatively similar to surrounding muscle, aside from their distinct anatomical arrangement (Fox and Richardson 1978).
When the larvae are approximately 40 millimeters in length, the muscle cells that comprise the future electric organ appear to rotate approximately 90° with respect to the body axis. This is caused by a profound change in cell shape from a myotube to a rounded myotube and then eventually to horizontally flattened electrocytes (Fox and Richardson 1978). During this change in shape, nuclei become repositioned on the equatorial plane of the rounding cell. As development proceeds, the adjacent electroblasts interdigitate as they expand horizontally, stacking to form columns. The myofibrils contained within each electrocyte become contorted and disorganized, breaking into isolated components. Finally, disassembly of the myofibrils begins, with a longitudinal splitting followed by loss of A-bands, which results in the isolation of Z-bands with thin filaments attached, beginning at the ventral pole and then spreading to the dorsal pole, completing the morphological transformation into an electrocyte.
The dorsal and ventral poles of each cell begin to diverge in their appearance. The ventral, innervated surface is characterized by smooth secretory vesicles that secrete an amorphous, unknown substance, which is apparently missing from the dorsal surface (Fox and Richardson 1979). In contrast, the dorsal surface begins to contain more cellular organelles, including a high concentration of heterogeneously sized vacuoles. These vacuoles fuse with the dorsal surface, creating pseudopodia that “loop” back to the membrane, creating the canniculi that characterize the dorsal surface (Fox and Richardson 1979).
Satellite cells are observed to be concentrated on the dorsal (uninnervated) surface, diagnosed by their round shape and mononucleated appearance. As the electric organ grows, these mononucleated cells are “enveloped” by the dorsal canniculi, and, eventually, the membranes fuse, increasing the number of nuclei present in the growing electrocyte (Fox and Richardson 1979).
The parallel efforts of Fox and Richardson (1978, 1979) and Mellinger (1978) are largely in agreement, with the exception of the role of innervation. Fox and Richardson (1979) report, based on both light microscopy and electron microscopy, that although the nerve is present through most of the developmental stages of the electric organ, no synaptic contacts between the tissues are present; only after the organ has formed do neurites penetrate the interelectrocyte space and establish synaptic contact. In contrast, Mellinger et al. (1978) suggest an inductive role for the nerve, although the observations that support this are restricted to light microscopy. Gautron (1974) reported that surgical denervation of adult electric organs leads to slow degeneration of electrocytes, including the reappearance of myofibril bundles in the cytoplasm. This finding suggests that although innervation may not play a role in the early development, it may serve a role in the terminal differentiation of electrocytes and perhaps maintenance of their phenotype.
4.3.4 Mormyroidea
The monotypic Gymnarchidae and the more than 200 species of Mormyridae all have weakly electric, myogenic electric organs (Sullivan et al. 2000). After hatching, mormyrids develop a distinct larval electric organ that completely degenerates by the time the larvae have become approximately 25 millimeters long. The organ is “replaced” by an anatomically and biochemically distinct adult electric organ, which develops after hatching but is not active until the larvae have become approximately 15 millimeters long. Gymnarchus niloticus maintains a single electric organ from hatching to adulthood. This electric organ closely resembles the structure of the larval organ in mormyrids. This, together with the phylogenetic relationship between the Gymnarchidae and Mormyridae (Sullivan et al. 2000), supports the hypothesis that these structures are homologous and suggests that the adult mormyrid organ is a derived structure among the Mormyridae (Hopkins 1999).
4.3.4.1 The Larval Mormyrid Organ/Gymnarchus Organ
The larval electrocytes are characterized by extensive membrane invaginations on both faces. The cytoplasm contains few organelles but many vesicles, which open into the invaginations of the plasma membrane, as well as irregularly shaped vacuoles containing an unknown “coating material” (Denizot et al. 1978). The faces are characterized by the presence of many nuclei and mitochondria as well as other cytoplasmic organelles. Another striking feature of the larval organs is the presence of abundant myofibrils. Rather than the parallel arrangement of muscle fibers found in muscles, myofibrils are arranged orthogonally such that muscle fiber bundles run in different directions, although sarcomeric structures (Z-lines and H-zones) are visible (Denizot et al. 1978). Myofibrils apparently do not extend into the stalk. The larval organ extends from the edge of the skull to the end of the dorsal fin and consists of four tubes of electrocytes, two dorsal and two ventral, all of which are located medially within the lateral muscle. The electrocytes are distinct from muscle fibers in that they are barrel shaped and oriented approximately 45° to the anterior-posterior axis (Denizot et al. 1978).
In Gymnarchus, the electrocytes are arranged in eight long tubes (referred to in earlier literature as “spindles”), four on each side of the body, located medially within the lateral muscle but extending to the tip of the tail (Dahlgren 1914; Srivastava and Szabo 1972). As in the larval mormyrid organ, the electrocytes are barrel shaped and innervated on the posterior face, although there is no stalk present (Fig. 4.1). Although both faces are “moderately convoluted” (Srivastava and Szabo 1972), the anterior (uninnervated) face reportedly has many small canniculi absent on the relatively smooth innervated face.
The electrocytes that comprise the larval electric organ in mormyrids are arranged in parallel on myotomes, each myotome contributing both muscle cells and electrocytes (Denizot et al. 1978). The outer portion of the myotome is devoted chiefly to the muscle cells and the inner portion is devoted to the larval electric organ, although the boundary between the muscle cells and electrocytes within a myotome is ambiguous and “intermediate” stages can be observed (Denizot et al. 1978). Very little else is known about the development of the larval organs in mormyrids. Detailed studies have been performed on Gymnarchus niloticus (Dahlgren 1914; Srivastava and Szabo 1972). Development of the Gymnarchus electric organ occurs between the 9th and 15th day of embryonic life (Dahlgren 1914). Dahlgren (1914) reports that the columns of electrocytes develop at different rates, a fact leveraged to observe different points of development within the same juvenile individuals. A subsequent study (Srivastava and Szabo 1972) examined a series of embryological material.
Electrocytes derive from two differentiated muscle fibers on the inner edge of myotome, which detach from the main myotome to form an electroblast (Dahlgren 1914; Srivastava and Szabo 1972). Later in development, muscle cells intermediately situated between the myotome and electroblast degenerate (Srivastava and Szabo 1972). The electroblast initially appears elongate, syncytial, and multinucleated with a visible pair of two distinct bundles of myofibrils. As the primordium increases in length and width, the myofibril bundles begin to fuse, still showing transverse striations. As the electroblasts continue to grow, the myofibrils begin to occupy a central position. Throughout the process, the number of nuclei increase and additional myofibril bundles appear in addition to the central bundle, which join the central bundle and add to its thickness; transverse striations are still visible (Srivastava and Szabo 1972).
As these electroblasts continue to grow, the anterior ends remain pointed and the posterior ends become thick and round as the middle portion of the primordium becomes wider and more barrel shaped, likely due to the appearance of vesicles secreting an “amorphous substance” (Srivastava and Szabo 1972). As these vacuoles increase in number and size, the myofibril bundle disintegrates into filaments and transverse striations disappear.
As the transverse striations disappear, the nerve endings make contact with the posterior face of the electrocyte (Srivastava and Szabo 1972). A second vacuole type, unassociated with the nucleus, begins to appear on the posterior face. As these vacuoles increase in number, they fuse with the membrane, leaving canniculi on the posterior face.
As the primordium grows, the electromotor nerve approaches the posterior end, increasing the number of vacuoles in the central portion of the electrocyte as the myofibril bundle continues to disintegrate and striations disappear; only after this does synaptogenesis begin (Srivastava and Szabo 1972).
4.3.4.2 The Mormyrid Adult Organ
4.3.4.2.1 Anatomy
The adult electric organ consists of 4 columns of electrocytes, 2 on each side of the body surrounding the spinal cord, each composed of approximately 100 electrocytes (Bennett 1971; Bass 1986). Each electrocyte is flattened in the anterior-posterior dimension, consisting of anterior and posterior faces approximately 0.5 millimeter in diameter (Bass 1986). Typically, the posterior face is characterized by finger-like invaginations (Bass 1986) that fuse to form a stalk structure that is innervated by electromotor neurons. This innervation occurs away from the electrocyte on either the anterior or posterior side. In some cases, where innervation occurs on the anterior face, the electrocyte face is penetrated by the stalk system, which has consequences for the electric signal it produces (see Fig. 4.1; Bennett 1971; Gallant et al. 2011; also see Markham, Chap. 5). Penetrations are apparently unique to mormyrid electrocytes. Each electrocyte is bounded by a connective tissue septum, and the entire organ is surrounded by a connective tissue sheath. The anterior and posterior faces are characterized by numerous canniculi (Bass et al. 1986). Unlike many other electric fish species, myofibril bundles, complete with sarcomeric structures, are retained in the center of the electrocyte parallel to the two faces (Bass et al. 1986).
4.3.4.2.2 Development
Early studies of juvenile Mormyrus rume obtained from the field (Szabo 1960) indicate that the anterior aspect of the organ develops earlier than the posterior aspect. Szabo (1960) describes the most anterior cells as still attached to the myoseptum, suggesting that electrocytes originate from already differentiated muscle fibers. A subsequent study on Pollimyrus drew on observations from a developmental series of individuals bred under laboratory conditions and utilized light microscopy and electron microcopy observations (Denizot et al. 1982). This study does not describe these earliest stages of development, commenting only that electrocytes initially derive from 7 to 10 myotomes from tissue “that resembles myoblast tissue” (Denizot et al. 1982). More studies of the early development of adult electric organs are needed to clarify if adult electric organs, like their larval counterparts, arise from differentiated myotubes or from presomitic mesodermal precursor cells.
The first recognizable, differentiating electroblasts are found in 10- to 12-millimeters specimens (Denizot et al. 1982). These cells initially retain a myotome-like arrangement at approximately 45° angles to the anterior-posterior axis and are bounded by loose connective tissue. At this early stage, electroblasts already possess stalks, but there is no indication of penetrations (Denizot et al. 1982). Electrocytes also possess many myofilaments, similar to those of the larval electrocytes, which are retained into adulthood (Denizot et al. 1982). By time the larvae have reached 13 millimeters in length, the electrocytes have begun to lose their myotome-like arrangement and by 15.5 millimeters in larval length, the electrocytes are regularly arranged. The amount of muscle lateral to the developing electric organ is substantially decreased as the electrocytes increase in size. By 16 millimeters in larval length, the electrocytes begin to flatten and widen and nuclei of the stalk system become less heavily stained. Satellite cells are observed on the posterior face surrounding stalks and electrocytes, which appear to “facilitate” the formation of penetrations (Denizot et al. 1982). Penetrations begin to appear when the larvae reach 19 millimeters in length and increase in number as the fish grows beyond 33 millimeters.
No synapses are observed when the larvae are 10-12 millimeters long, and the adult electric organ is incapable of discharge at this time. Electric organ discharges are first observed in larvae that are 15.5 millimeters in length (Denizot et al. 1982). Denervation of the electric organ by Szabo and Kirschbaum (1983) revealed that disrupted innervation does not appear to affect differentiation of the electrocytes.
4.3.5 Gymnotiformes
Gymnotiformes are a diverse group of about 200 species (Albert and Crampton 2006), all of which are considered to have myogenic electric organs (with the exception of Apteronotus; but see Kirschbaum 1983). The diversity of electric organs among Gymnotiformes is considerable. Electrophorus is well-known for its ability to produce strong and weak electric discharges using three electric organs: the main organ, the organ of Sachs (Sachs 1877), and Hunter’s organ (Hunter 1775). Although Electrophorus is singular in this group for its strong discharge abilities, several other species are known to have multiple “accessory organs” (Bennett 1971; Stoddard 2002). A large group of species can continuously discharge their electric organ, leading to a quasi-sinusoidal electric organ discharge, the so-called wave-type discharging fishes, whereas another large group produces intermittent electric organ discharges, the so-called pulse-type discharging fishes (see Fig. 4.2). In addition, many wave-discharging species develop distinct larval and adult organs, but all pulse-type fishes (families Gymnotidae, Hypopomidae, and Ramphicthydiae) retain their “larval” organ through adulthood (Franchina 1997; Albert and Crampton 2006; Pereira et al. 2007; Schwassmann et al. 2014). Some species (e.g., Sternopygus) have well-characterized abilities to regenerate large portions of their electric organs after loss due to predation (Dunlap et al. 2016).
A: condensed phylogeny of the major families of Gymnotiformes (after Crampton and Albert 2006). According to this phylogeny, pulse-type fishes are ancestral and wave-type discharges are derived. Type A and type B electrocytes (following the classification of Kirschbaum and Schwassmann 2008) show that type B electrocytes are ancestral and typical of pulse-type fish, whereas type A electrocytes are derived and characteristic of wave-type species. B: organization of musculature in a schematic cross section of a gymnotiform shows the location of epaxial and hypaxial musculature, highlighting the distinct origins of type A and type B electrocytes. C: distinct developmental mechanisms appear to underlie the difference between type A and type B electrocytes. See Fig. 4.3 for more details.
A: comparison of canonical muscle development (top) to two distinct modes of electrocyte development. The first, exemplified by Electrophorus, where electrocytes differentiate directly from presomitic precursor cells (direct development, center). The second, exemplified by most other species of electric fish, whereby cells develop like normal muscle and then transdifferentiate into electrocytes (bottom). B: major transcription factors and myogenic regulatory factor (MRF) profiles at the various stages of muscle and electric organ development (Bentzinger et al. 2012; Gallant et al. 2014). Note that there are no data for gene expression in electroblasts at present (dotted lines). The major stages in development are highlighted (gray boxes).
Despite the considerable diversity of Gymnotiformes, there is a relative paucity of developmental material available for analysis. The majority of developmental materials are obtained from field-captured specimens, although there have been successes in breeding Gymnotiformes in captivity (Kirschbaum 1975; Franchina 1997). This problem has been circumvented by some researchers by drawing on the regenerative capacity of Gymnotiformes. Unlike other taxa of electric fish, some Gymnotiformes are capable of regenerating the posterior (i.e., tail) portion of their bodies, possibly in response to intense predation (Dunlap et al. 2016). Thus, in both Sternopygus and Eigenmannia, surgical removal of the electric organ prompts regeneration of the electric organ, enabling an entire body of studies on the developmental mechanisms in these genera without the need for embryonic materials (Baillet-Derbin 1978; Zakon and Unguez 1999).
The Gymnotiformes vary widely in terms of the organization of their electric organs. In pulse-type Gymnotiformes, electrocytes are cylindrical in shape, about 300 microns in diameter, and about 200 microns thick (Bennett 1971; Bass 1986). Stalklike processes extend from the electrocyte, which receives the innervation and may be on the anterior or posterior face (Bennett 1971; Bass 1986). Electrophorus has posteriorly located, short stalks where innervation terminates (Bass 1986). The uninnervated anterior face is characterized by the presence of canniculi (Bennett 1971; Bass 1986). The electrocytes of Gymnotus are exceptional among pulse-type fishes, because the electrocyte faces are smooth and are innervated directly on the posterior side without stalklike process (Fig. 4.1). Gymnotus lack formal accessory organs but have groups of cells that discharge asynchronously, serving an analogous physiological function (Castello et al. 2009; Rodriguez-Cattaneo and Caputi 2009; Crampton et al. 2013; Rodriguez-Cattaneo et al. 2013).
Wave-type discharging fish (i.e., Eigenmannia and Sternopygus) have more “cigar-shaped” electrocytes (about 1-2 millimeters long and 200 microns in diameter; Bennett 1971; Bass 1986) and are loosely arranged in multiple columns of electrocytes that parallel the body axis. Innervation is typically on the posterior face, and extensive canniculi characterize the uninnervated face (Bennett 1971; Bass 1986). Stalks are not typically found in wave-type species (Fig. 4.1).
The developmental origins of electrocytes in Gymnotiformes have been a subject of debate in the literature; this is partially motivated by the sheer diversity of species considered together with a paucity of developmental materials for study. Early studies of Electrophorus suggested that electrocytes originated from skeletal muscle precursors (e.g., Fritsch 1883), whereas others claimed that electrocytes originated from undifferentiated presomitic mesodermal precursor cells in a defined germative zone (e.g., Keynes 1961).
Evidence from numerous developmental studies (Wachtel 1964; Szabo 1966; Esquibel et al. 1971) seem to support the former hypothesis, and Szabo (1966) attempted to reconcile the two by accepting the position of the germative zone postulated by Keynes (1961) but claiming that the early electroblasts passed through an intermediate skeletal muscle phase, in-line with histological evidence that he obtained. Schwassmann et al. (2014), after obtaining a much larger sample of field-captured embryological materials, demonstrated unambiguously that electrocytes originated from a group of metamerically organized, undifferentiated embryonic trunk mesoderm cells, supporting the original hypothesis of Keynes (1961).
These most recent results are in apparent contrast to findings in Sternopygus by Patterson and Zakon (1996, 1997) and in Eigenmannia by Baillet-Derbin (1978) who both studied the regeneration of the electric organ as a proxy for understanding the development of the electric organ. Baillet-Derbin (1978) and Patterson and Zakon (1997) demonstrated that electrocytes derived from striated muscle cells, complete with sarcomeric structures. Using sophisticated cell labeling and electron microcopy and light-microscopy observations, Patterson and Zakon (1993) was able to unambiguously show that the source of these muscle fibers were satellite cells near the wound, which first differentiated into muscle and then into mature electrocytes.
The apparent contradiction of these results could be resolved by a comparative synthesis by Kirschbaum and Schwassmann (2008). By examining embryonic materials from eight species representing each of the major families of Gymnotiformes, it becomes evident that there are two electrocyte types present in Gymnotiformes. Type A electrocytes, characteristic of wave-type Gymnotiformes (i.e., Eigenmannia, Sternopygus, and Apteronotus), originate inside both hypaxial and epaxial myomeres. Type B electrocytes, characteristic of the pulse-type species (i.e., Electrophorus, Gymnotus, Rhamphythis, and Brachyhypopomus), originate from a distinctive germative zone below hypaxial muscle. Intriguingly, Gymnotiformes with type B electrocytes lack distinct larval and adult electric organs (Albert and Crampton 2006), whereas Gymnotiformes with type A electrocytes develop distinct larval and adult organs (see Fig. 4.2).
The apparent contradiction of results on the origins of electrocytes in Gymnotiformes may therefore be partly explained by two distinct developmental mechanisms: that type A electrocytes originate between muscle fibers and retain muscle fiber-like morphology for several weeks and that type B electrocytes differentiate directly from a mesodermal precursor cell into electrocytes with no intermediate stage resembling skeletal muscle. This is a satisfying explanation for a standing enigma in Gymnotiformes, but additional studies are necessary to explore this concept further. One key question without an answer was raised by Schwassmann et al. (2014): how closely related are the developmental mechanisms regulating postembryonic/regenerative properties to embryonic mechanisms? A second question is the apparent discordance between the observations of several authors’ study of Electrophorus development that seem to support development from skeletal muscle rather than from undifferentiated presomitic mesoderm. One possible explanation is the considerable postembryonic development, noted by Szabo (1960), that likely occurs as the animal grows. It is conceivable that mechanisms of “growth” of the electric organ and embryonic development may be difficult to differentiate in juvenile specimens.
4.3.5.1 Type A Electrocytes
No studies have described the embryonic development of myogenic electric organs in wave-type discharging electric fish. Instead, Baillet-Derbin (1978) and Patterson and Zakon (1997) leveraged the regenerative properties of Gymnotiformes (see Sect. 4.3.5) to examine the development of the electric organ. In both cases, (1) a blastema forms at the wound site following surgical amputation of the tail; (2) blastemal cells cluster to form fully differentiated, multinucleated muscle cells (i.e., expressing normal sarcomeric proteins); (3) the earliest recognizable electroblasts contain myofilaments and even sarcomeric structures but are much larger in cross-sectional area than muscle; and (4) these myofilaments quickly disassemble, followed by the invagination of the posterior (innervated) face to form canniculi that coincides with the appearance of innervation in Eigenmannia (Baillet-Derbin 1978; Patterson and Zakon 1997).
Drawing on multiple lines of evidence, Patterson and Zakon (1993) concluded that the cells that form the blastema after amputation are satellite cells, including the fact that the cells express Pax7, which is characteristic of stem cells (Weber et al. 2012; see Sect. 4.2.2). Unguez and Zakon (1998a) delineated fast and slow muscle isoforms and discovered that following differentiation, centrally located fast muscle fibers fuse to form electrocytes, whereas more peripherally located slow muscle fibers do not.
In a follow-up experimental study, Unguez and Zakon (1998b) examined the changes in protein expression and electrocyte morphology in Sternopygus. Most surprisingly, they found that although muscle cells did not change their biochemical profile, electrocytes began to express sarcomeric proteins, myosin heavy chain, and tropomyosin within weeks of spinal transection. Striking electron micrographs reveal the formation of new sarcomeric structures in denervated electrocytes, suggesting an “inhibitory” role of innervation on the maintenance of the electrocyte phenotype.
4.3.5.2 Type B Electrocytes
Light-microscopy observations with immunohistochemistry revealed that vertically aligned electrocytes in Brachyhypopomus gauderio form near the ventral boundary of the ventral mass of hypaxial musculature, which appear in specimens about 6 days old (Franchina 1997). Electrocytes take on a cylindrical shape with tapered ends and lack stalks. In later stages, the electric organ extends rostrally and caudally as the electrocytes begin to flatten, separate into rows, and develop stalks on the posterior face.
In Electrophorus electricus, electrocytes originate from a “germinal zone” in the ventral tip of the myotome (Szabo 1960; Keynes 1961; Schwassmann et al. 2014) and appear to differentiate from the anterior portion of the animal such that the posteriormost electrocytes are the most developed (Szabo 1960; Schwassmann et al. 2014). Sach’s organ is the first to develop, followed by the main organ, and eventually Hunter’s organ (Szabo 1960). These cells lose their cell membrane and are described as “mere nuclei” (Schwassmann et al. 2014) before rapidly producing electroplasm, aligning, and forming a new syncytial membrane. Myofilaments appear near several of the electroblast nuclei, although they are relatively sparsely distributed without sarcomeric arrangement. As development continues, electrocyte nuclei become more numerous at the posterior (innervated face) of the electrocytes, which are characterized by newly forming canniculi. At this phase, the electromotor neurons are making synaptic contacts with the posterior face (Schwassmann et al. 2014).
4.3.6 Siluriformes
The family Malapteruridae consists of 2 genera and approximately 20 species, all of which are electrogenic. Best known among these is Malapterurus electricus, well-known for its strong electric discharges. The first histological analyses concluded that the electric organ originated from the glandular portions of the epidermis, an idea attributed by Johnels (1956) to Fritsch (1883). A single study on the development of Malapterurus was performed by Johnels (1956).
The electric organ of Malapterurus lies in the skin and surrounds the body over most of its length. This electric organ is unusual among electric fish in that the millions of constituent “lily pad”-like electrocytes are irregularly organized, superficially located, and surround the entire body near the skin much like a jacket. The electric organ is innervated by two nerves originating from the first spinal segment and branching to innervate the electrocytes. The cells are disc shaped, about 1 millimeter in diameter, and 20-40 microns thick. A conical region in the center of the caudal face produces a stalk that protrudes and is innervated by a motorneuron (Fig. 4.1). The electrocytes are flanked on both sides by a layer of connective tissue isolating the electric organ from the skin and the body.
Analysis of the electric organ in 11.4- and 12.7-millimeters specimens revealed small but adultlike electrocytes in the vast majority of the body. Despite this, a distinct germinal zone was located in the rostral portion of the electric organ, dorsal to the pectoral fin near the shoulder girdle. Here, the interior and exterior connective tissue layers surrounding the electric organ meet and attach to the shoulder girdle. A few researchers noted a small “deficiency” in the muscle wall in this region of adult Malapterurus that is more pronounced in the juvenile fishes at the point at which the electric nerve enters the organ. Here, electrocytes can be observed forming, although there are few histological details about these cells to permit further interpretation.
4.3.7 Euteleostei
A single genus of marine perciformes, Astroscopus, is known to be electric. These unusual and enigmatic fishes have been poorly studied compared with many of the other species considered in this chapter.
The electric organ in Astroscopus is located just behind the eye (Bennett 1971) and consists of two irregular vertical columns that surround slender extraocular muscles (White 1918; Bennett 1971). Each column is composed of approximately 200 parallel plates, separated by connective tissues and consisting of approximately 20 electrocytes laying side by side (White 1918; Dahlgren 1927; Bennett 1971). The electrocytes are flattened horizontally and are densely innervated on the dorsal surface. The ventral surface has many short papillae and canniculi that increase the surface area (Fig. 4.1). Only the innervated face is active during discharges, created by postsynaptic potentials in the dorsal surface (Dahlgren 1927; Bennett 1971).
There is only one study on the development of Astroscopus, performed by White (1918), that found that the electric organ derives from muscle cells comprising four of the six extraocular muscles of each eye. The earliest stages of electric organ development were observed in embryos of 4-14 millimeters in length. Future electrocyte cells absorb stain more darkly and are smaller than other muscle cells that comprise the four extraocular muscles. Electric organs derive from the lateral edge of the four muscles farthest from the eye (White 1918).
By the time the larvae have become about 14 millimeters in length, these cells have increased to about 6× times the diameter of normal surrounding muscle cells and their nuclei have moved close to the cell membrane. At this stage, the cytoplasm contains numerous vacuoles that form the papillae of the ventral face. By the time the larvae have become about 33-35 millimeters, the 4 electric organs are well developed and separate from the eye muscles, and the electrocytes begin to assume their flattened shape, broadening laterally without deepening in the dorsal-ventral aspect. The dorsal face changes in structure to a flat smooth structure, whereas the ventral surface retains it numerous papillae (White 1918; Dahlgren 1927). Similar to other electric fishes, the Astroscopus electrocyte is polarized (White 1918; Bennett 1971) along the dorsal-ventral body axis.
4.4 Comparative Genomics
The earliest studies concerning the molecular biology of electric organs employed a variety of candidate gene approaches, examining the expression patterns of mRNAs and proteins identified to be important in developmental processes, homologous to those found in canonical model systems. These studies were followed by next-generation sequencing approaches in the past decade, which has led to a proliferation of genomics and RNA sequencing and proteomic datasets for electric organs, motivating “unbiased” surveys of both gene identity and gene expression. For a summary of these techniques, see Pitchers et al. (2016).
From a molecular biology perspective, the most comprehensively surveyed group of electric fish are the Gymnotiformes, chiefly the species Sternopygus macrurus (Kim et al. 2009; Güeth et al. 2013; Pinch et al. 2016) and Electrophorus electricus, which were the first species of electric fish to have a completed genome, along with full somatic mRNA and miRNA transcriptomes and an annotated proteome (Gallant et al. 2014; Traeger et al. 2015, 2017). Several studies have focused on the proteomics and transcriptomics of the neuromuscular junction in Narcine (Nazarian et al. 2007, 2011; Mate et al. 2011) and may provide datasets that have relevance to development in future analyses. Relative latecomers to molecular techniques are the mormyrid electric fish (Gallant et al. 2012; Lamanna et al. 2014, 2015), which now also have a completely sequenced genome and somatic transcriptome (Gallant et al. 2017).
Although comparative biology is in the “DNA” of the electric fish research community, efforts to compare molecular mechanisms between lineages have only just begun in the past few years. The first of these studies assembled the genome and somatic transcriptomes of Electrophorus and the electric organ and skeletal muscle transcriptomes of several other species (the gymnotiforms Eigenmannia and Sternopygus, the siluriform Malapterurus, and the mormyroid Brienomyrus), representing three independent origins of electroreception (Gallant et al. 2014). This study identified orthologous genes between each of these lineages and compared expression in adult electric organs across these taxa, with a particular focus on genes with well-annotated functions in vertebrates. The overall result of this study was the discovery of several groups of genes, with known biological functions, that shared highly similar patterns of gene expression across each of the independently evolved electric organs.
A key limitation to the study by Gallant et al. (2014) and to nearly every other dataset on the molecular biology of electric organs is that they are based on adult tissue samples. As such, they give only a snapshot of one point in time, namely, after the electric organ has already formed, and therefore miss the period of embryonic electric organ development. This thereby limits the ability to interpret the role of particular genes in the evolution and development of electric organs; however, there is still great value in these studies. First, they give a biochemical “inventory” of electrocytes across many species. Second, they describe patterns of gene expression that are the consequence of embryonic developmental processes and reflect signatures of ongoing postembryonic development/growth. Much in the way that examining cosmic background radiation may give insights to how the “big bang” may have unfolded, so too might these studies provide insights into the mechanisms of development of electric organs.
In efforts to integrate these comparative results with previous molecular studies, this section has been organized by the themes discussed in Sect. 4.3.1. Section 4.5 considers where new data are needed, which may identify opportunities for future studies and the application of new techniques.
4.4.1 What Are the Common Features of Electric Organs Across Lineages?
Analyses of the transcriptome of the electric organ of Electrophorus (Gallant et al. 2014; Traeger et al. 2015) revealed strong upregulation of many genes associated with ion transport, including voltage-gated ion channels and transporters, acetylcholine receptor activity, and Ca2+ binding, although these were not explicitly examined in other species. One notable example is the Na+/K+-ATPase α-subunit, of which there are several paralogues in fishes, where it appears that species have evolved the use of different subunits for membrane repolarization.
Expression analysis of the scn4aa gene (Zakon et al. 2006; Arnegard et al. 2010) reveals that weakly electric fish have convergently neofunctionalized the voltage-gated sodium channel NaV1.4a, normally expressed in muscle, to generate action potentials in electrocytes. A more detailed review of these studies is provided by Markham (Chap. 5). This robust result has been confirmed in many studies (e.g., Gallant et al. 2014; Lamanna et al. 2014).
The abundance of sarcomeric proteins appears to be lower than in skeletal muscle for all electric organs studied thus far, which has been a robust finding in both ultrastructural studies (discussed in Sect. 4.3) and numerous molecular studies (Mate et al. 2011; Gallant et al. 2012; Lamanna et al. 2015). Intriguingly, the relative amount of sarcomeric proteins between electric fish lineages is more variable; mormyrids have a much higher amount of sarcomeric proteins (Gallant et al. 2012; Lamanna et al. 2014, 2015) than Gymnotiformes (Cuellar et al. 2006) and Torpediniformes (Mate et al. 2011), which has been noted by ultrastructural studies (see Sect. 4.3). Although most electric fish species seem to achieve a low level of sarcomeric proteins by repressing mRNA expression, the Gymnotiformes Sternopygus seems to be exceptional in this regard. Several studies have indicated that Sternopygus electrocytes express a full complement of sarcomeric mRNAs, essentially at the same levels found in skeletal muscle (Gallant et al. 2014; Pinch et al. 2016) but lack their proteins (Cuellar et al. 2006), which has implicated a presently unknown mechanism of posttranscriptional repression. This issue is revisited in Sect. 4.4.2. Despite the heterogeneity in the amount of sarcomeric protein and the mechanism by which this paucity arises, the genes smyd1a, smyd1b, and hsb11, all implicated in the assembly and maintenance of sarcomeric integrity, are highly downregulated in all electric fish lineages examined (Gallant et al. 2014).
As reviewed in Sect. 4.2, muscle cells translate action potentials into the release of Ca2+ from the sarcoplasmic reticulum, which causes the sarcomeres to contract. A sudden change in the shape of the electrocytes would have deleterious effects on the strength of electric signals, thereby affecting the efficiency of electric signaling. Because no electrocytes are known to be contractile, it would appear that the excitability of electrocytes and the ability to contract has been decoupled. Gallant et al. (2014) noted that a consistent feature of electric organs appears to be the downregulation of the DHPR cacna1sa. In skeletal muscle, this would have the result of preventing the release of Ca2+ from the sarcoplasmic reticulum on depolarizing the plasma membrane, regardless of how much sarcomeric protein is present.
To maximize the strength of electric fields, current dissipation must be minimized and the current must be conducted unidirectionally through the electric organ. This is partially achieved through the uniform orientation of individual electrocytes (Bennett 1971). An additional property that may facilitate this is a key structural similarity of all electric organs outlined above: connective tissue septa forming the boundaries of individual electrocytes and surrounding the electric organ, which may further prevent the dissipation of current throughout the body. Although the biochemistry of these septa has not been explicitly examined, Gallant et al. (2014) noted the expression of two collagen genes, col6a6 and col141a1, a glycosyltransferase (gyltl1b), and dystrophin (mutations of which cause muscular dystrophy). These proteins may act in concert to form the collagenous sheaths that facilitate current flow through the electric organ.
4.4.1.1 Cell Size
Another convergent feature of electrocytes, as described in Sect. 4.3, is that electrocytes are much larger than muscle fibers. The mechanisms by which electrocytes achieve this remarkable cell size is presently unknown, although it may involve a combination of both embryonic and postembryonic mechanisms. Gallant et al. (2014) note the upregulation of several members of the insulin-like growth factor-signaling pathway genes, including ligands (e.g., gill), effectors (e.g., pik3r3b), and proregulatory factors (e.g., net-37) within this pathway, as well as profound downregulation of negative inhibitors (e.g., fbxo40). Because insulin-like growth factor-signaling pathways have been implicated in organism size polymorphisms as well as in changes in individual tissue size, these may be good candidate genes for the regulation of cell size in electric organs as well (Gallant et al. 2014).
4.4.2 What Is the Role of Postembryonic Growth in the Development of the Electric Organ?
Denervation studies of Sternopygus and Torpedo electrocytes (Gautron 1974; Unguez and Zakon 1998b) have led to the hypothesis that the electromotor neurons may have an inductive role in the development of electrocytes. The results obtained in Sternopygus in turn led to the hypothesis that electromotor neurons may posttranscriptionally regulate protein expression (Zakon and Unguez 1999). Strong evidence for this hypothesis was provided by Cuellar et al. (2006), demonstrating that mRNAs for sarcomeric proteins in electric organs are expressed at comparable levels in muscle but sarcomeric proteins are not. The potential mechanisms that underlie the regulation of proteins are currently unidentified, but the potential mechanisms in the light of current evidence are reviewed by Güeth et al. (2013). To contrast with these results, denervation studies in mormyrids (Szabo and Kirschbaum 1983) demonstrate that the electric organ persists without neuronal input. In many of the electric fish lineages described in Sect. 4.3, differentiation of the electrocyte is well underway before synaptogenesis has begun. The role of innervation in the development and maintenance of electrocytes is an area that needs more careful and thorough study.
4.4.3 Do Electrocytes Arise from Fully Differentiated Muscles or Mesodermal Precursors?
Histological studies on electric organ development considered in Sect. 4.3 have motivated at least two distinct pathways by which electric fish appear to achieve fully differentiated electrocytes (Fig. 4.3). Efforts to characterize the molecular basis of the developmental mechanisms regulating the progression of electrocytes through these pathways have been largely fruitless. One potentially useful piece of data is the varying degrees of sarcomeric protein expression as well as the timing of their expression during development. These data support at least two distinct pathways to an electrocyte: one that relies on fully differentiated muscles transdifferentiating into electrocytes and another direct pathway from mesodermal precursors (Fig. 4.3A).
A potentially important source of information in understanding the mechanisms underlying these various developmental mechanisms are the transcription factors (Fig. 4.3B). Transcription factors have been a favorite subject of examination in the molecular biology of electric organs, particularly the myogenic regulatory factors (MRFs). To date, MRFs have been identified in Torpediniformes, Mormyriformes, and Gymnotiformes through a variety of approaches. As described in Sect. 4.2, MRFs act in a hierarchical fashion to specify, commit, and eventually cause differentiation of skeletal muscle cells by working to activate muscle-specific genes. MRFs are activated by transcription factors that pattern the early presomitic mesoderm.
Three transcription factors, six2a, hey1a, and hey1b, are normally expressed at low levels in differentiated skeletal muscle (Fig. 4.3B). However, in all lineages of electric fish examined to date, they are highly abundant (Gallant et al. 2014; Lamanna et al. 2015). The downstream targets of these genes are the MRFs myod, myogenin, and six4b. The expression of myod has been observed in both Sternopygus (Kim et al. 2004, 2009; Pinch et al. 2016) and Torpedo (Neville and Schmidt 1992; Asher et al. 1994) to be at comparable levels to those of skeletal muscle. In contrast, myogenin and six4b are expressed at very low levels in nearly every electric fish lineage, with the exception of Sternopygus (Kim et al. 2004, 2009; Gallant et al. 2014; Pinch et al. 2016). This is consistent with the relatively high levels of sarcomeric mRNAs characteristic of this lineage.
Mormyrid electric organs all highly express erh, mef2aa, and mef2b, all transcription factors downstream of or parallel to myogenin (Gallant et al. 2014, 2017; Lamanna et al. 2015). A recent study demonstrated that mef2aa is among the 50 most abundant genes expressed in the electric organ (Gallant et al. 2017). This is in stark contrast to the electric organ of Sternopygus, which expresses a variety of MGFs, including mrf4 and mef2, at comparable or slightly higher levels in electrocytes than in skeletal muscle (Kim et al. 2004, 2009; Pinch et al. 2016).
4.5 Summary
The independent origins of electrogenesis span vertebrates (Fig. 4.1), represented by two lineages of elasmobranch fishes and four lineages of teleost fishes. Despite the considerable diversity of taxa represented by the term “electric fish,” electric organs share many aspects of their form and physiological function. These similarities are likely the result of two major modes of development (see Fig. 4.3) originating from the same developmental precursor, namely, presomitic mesoderm, which also ultimately forms muscle. Although the majority of electric organs are the result of transdifferentation of skeletal muscle, it would appear that pulse-type Gymnotiformes in particular (e.g., Electrophorus; see Sect. 4.3.4.2) may derive their organs directly from undifferentiated myoblasts or somatic mesoderm. Throughout this process, the electromotor nerves may or may not play an inductive role. In mormyrids, Malapterurus, Rajidae, and Astroscopus, electrocytes seem to form without synaptic contact, whereas in some Gymnotiformes as well as in Torpedo, denervation studies have revealed a role in the maintenance of the organ.
Synthesizing information from what is known about skeletal muscle development (see Sect. 4.2) with observations of electric organ development (see Sect. 4.3) and available molecular data (see Sect. 4.4), we are left with glimmers of insight as to key molecular mechanisms that may regulate the development of electric organs. First, the MRF myogenin is almost universally repressed in electric fish, potentially by “upregulated” the transcription factors six2a, hey1a, and hey1b. Second, mRNA for sarcomeric proteins seems to be tightly coupled to myogenin expression. In Sternopygus, myogenin and sarcomeric transcripts are comparable to muscle, and in all other electric fish lineages, both myogenin and sarcomeric mRNAs are low in abundance among electrocytes compared with skeletal muscle. Mormyrids, despite relatively low levels of myogenin expression, seem to have relatively high levels of sarcomeric proteins compared with electrocytes in other electric fish lineages. This may correspond to relatively high levels of transcription factors downstream of myogenin (i.e., mef2a).
A striking outcome of this comparative treatment of electric organ development is the uniqueness of the Sternopygus electric organ compared with nearly every other electric fish species examined thus far. Sternopygus has been observed to contain both type A and type B electrocytes within the same individual, suggesting that Sternopygus may represent a “more primitive or earlier, evolutionary pathway” (Schwassmann et al. 2014). This pathway may well be the pathway that is recapitulated by regeneration in the wave-type electric fishes Eigenmannia and Sternopygus. Alternatively, Sternopygus could represent a distinct developmental mechanism from those in all other electric fishes. Until detailed studies of the embryonic development of Sternopygus are performed, this will remain a mystery. Regardless, this highlights the importance of comparative approaches in attempting to understand the “general principles” in development. Given the volume of studies on Sternopygus, one must be careful not to assume that the mechanisms at play in Sternopygus are necessarily representative of other electric fish.
4.5.1 Need for New Data
There are several obvious places for additional data to understand electric organ development. First, more developmental studies need to be performed on a series of well-fixed embryological materials for a variety of species, particularly Astroscopus, Rajidae, and Malapteruridae.
Second, given the proliferation of genomics data, new developmental studies should be considered in the context where transcriptomics and genomics can be maximally utilized. A comparative, developmental series of gene expression will likely provide the greatest insight into understanding the mechanisms of gene expression. These studies should also consider the spatial patterns (i.e., in situ hybridization) in addition to the temporal patterns of gene expression. In species where developmental series are difficult or impossible to obtain, any transcriptomic and genomic data would be of great importance. Along these lines, additional data on the development and developmental genetics of larval organs would also be of great importance.
Third is the need for a hypothesis testing framework in studies of electric organ development. This is particularly important concerning many of the genes identified by next-generation sequencing screens and will require the construction of new tools (e.g., CRISPR/Cas9 gene editing, antisense RNA interference, viral-mediated gene transfer) to evaluate the hypothetical roles of particular genes in developmental contexts. Thankfully, these techniques and tools are readily applied in “noncanonical” model systems, and the prospects for applying these techniques in electric fish look bright (Pitchers et al. 2016).
Other hypotheses about electric organ development may be tested without the application of sophisticated gene manipulation techniques. Although a handful of studies have examined the role of denervation of the electric organ, this has not been systematically evaluated. Establishing the role that electromotor neurons play in both inducing electric organ development and maintaining its phenotype should be straightforward experiments, but they have not yet been universally applied to representative species for all independent origins of electric organs.
Fourth is the need for a cleaner separation between embryonic development and postembryonic development/growth. Vertebrate embryos are typically born with a set number of muscles. Throughout life,. the number and size of individual fibers may change, largely through exercise and potentially injury. The developmental mechanisms that underlie the embryonic development of muscle and these postembryonic mechanisms are distinguishable. Electric organs face similar problems of damage and the need to increase in size as the animal grows. It is likely that histologists, in their reliance on juvenile animals, may have conflated these two processes, particularly given the conflicting accounts of Electrophorus development described in Sect. 4.3 and the studies of regeneration in Sternopygus and Eigenmannia. How do electric organs grow and repair to meet the demands of living fish? Are these mechanisms like those of muscle or distinct in different lineages? Are there distinct populations of electrocyte stem cells?
Finally, an area of great importance in electric organ form and function is the development of highly polarized cells from symmetrical precursors. Despite the nearly universal description of vacuoles creating canniculi in the uninnervated face of electrocytes, there are hardly any insights into the mechanisms underlying this process. Although the innervated face is characterized in some species by the proliferation of folds, the answers to the developmental mechanisms underlying this likely lie in the profound literature on the development of the neuromuscular junction. In contrast, the canniculi of the various taxa seem to occur around the time that innervation develops but on the side opposite the innervation. Schwartz et al. (1975) hypothesized, based on ultrastructural analyses in several species, that these canniculi may be homologous to the T-tubules in skeletal muscle, but this hypothesis remains essentially untested.
4.5.2 New Techniques and Approaches
New insights into the evolution and development of electric organs will also be motivated by the application of new techniques. One particularly exciting area is that of “regulomics”: how the protein profile of a cell is modified by networks of gene regulation but also by phosphorylation and small molecules such as miRNAs. There have been a few of these studies, mainly in Electrophorus, that have identified the mechanisms of regulation that have not widely been considered in other electric fish lineages. For instance, Traeger et al. (2015) discovered 18 novel miRNAs in Electrophorus electricus and characterized 294 miRNAs that are conserved in other species. Of these, 18 were differentially expressed between adult muscle and the electric organ (Traeger et al. 2015). Several of these miRNAs play inhibitory roles (i.e., miR-193, -218, and -365) in muscle development. One very highly expressed miRNA, referred to as mir-11054, was found to be exclusively expressed in the electric organ and is apparently novel to Electrophorus. This mRNA is expressed 30-fold higher in electric organs versus that in smooth muscle, is not expressed in other somatic tissues, and derives from the intron of the inward-rectifier K+-channel gene kcnj12b, which is highly abundant in electrocytes. The functional role of this “electromiR” is presently unknown.
A second area of regulation is phosphorylation, an important posttranslational cell regulatory mechanism, which has been poorly studied in electric fish and may have very important implications for development. Recently, Traeger et al. (2017) constructed an improved genomic assembly and annotation of Electrophorus and performed a comprehensive analysis of the proteome using cutting-edge isotope-assisted quantitative mass spectrometry. This analysis revealed numerous known and previously uncharacterized phosphorylation sites in a variety of transcription factors and membrane-bound proteins and revealed specific differences in the abundance of phosphoproteins between each of the three organs of Electrophorus. The differences in the abundance of phosphoproteins and cellular regulators of phosphorylation will undoubtedly be an important area of research in trying to understand how electric organs develop and function.
4.5.3 Concluding Remarks
Efforts to understand the evolution of electric organ development has been an ongoing enterprise in biology as old as The Origin of Species. Histological techniques, applied in every major lineage of electrogenic fishes, have set the stage for a new generation of genomics studies. Although there are still many unanswered questions, the answers have far-ranging implications for essential questions in evolutionary biology, physiology, and developmental biology. The arrival of new datasets and a growing set of new tools make the prospect of identifying the “steps by which these wondrous organs have been produced” (Darwin 1859) ever brighter.
References
Albert JS, Crampton WGR (2006) Electroreception and electrogenesis. In: Evans DH, Claiborne JB (eds) The physiology of fishes, 3rd edn. Taylor & Francis, Boca Raton
Arnegard ME, Zwickl DJ, Lu Y, Zakon HH (2010) Old gene duplication facilitates origin and diversification of an innovative communication system – twice. Proc Natl Acad Sci U S A 107(51):22172–22177
Asher O, Fuchs S, Souroujon MC (1994) Acetylcholine receptor and myogenic factor gene expression in Torpedo embryonic development. Neuroreport 5(13):1581–1584
Baillet-Derbin C (1978) Cytodifferentiation of the regenerating electrocyte in an electric fish. Biol Cell 33:15–24
Bass AH (1986) Electric organs revisited. In: Bullock TH, Heiligenberg W (eds) Electroreception. Wiley, New York, pp 13–70
Bass AH, Denizot JP, Marchaterre MA (1986) Ultrastructural features and hormone-dependent sex differences of mormyrid electric organs. J Comp Neurol 254(4):511–528
Bennett MVL (1971) Electric organs. In: Randall DJ (ed) Fish physiology, vol 5. Academic, New York, pp 347–491
Bentzinger CF, Wang YX, Rudnicki MA (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4(2)
Bone Q (1978) Locomotor muscle. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 7. Academic, New York, pp 361–424
Castello ME, Rodriguez-Cattaneo A, Aguilera PA, Iribarne L, Pereira AC, Caputi AA (2009) Waveform generation in the weakly electric fish Gymnotus coropinae (Hoedeman): the electric organ and the electric organ discharge. J Exp Biol 212(Pt 9):1351–1364
Crampton W, Albert J (2006) Evolution of electric signal diversity in gymnotiform fishes. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes. Science Publishers, Enfield, pp 647–696
Crampton WG, Rodriguez-Cattaneo A, Lovejoy NR, Caputi AA (2013) Proximate and ultimate causes of signal diversity in the electric fish Gymnotus. J Exp Biol 216(Pt 13):2523–2541
Cuellar H, Kim JA, Unguez GA (2006) Evidence of post-transcriptional regulation in the maintenance of a partial muscle phenotype by electrogenic cells of S. macrurus. FASEB J 20(14):2540
Currie PD, Ingham PW (2001) 1 - Induction and patterning of embryonic skeletal muscle cells in the zebrafish. In: Johnston IA (ed) Fish physiology, vol 18. Academic Press, Cambridge, MA, pp 1–17
Dahlgren U (1914) Origin of the electric tissues of Gymnarchus niloticus. Carnegie Institution of Washington, Washington, D.C.. Tortugas Laboratory Papers
Dahlgren U (1927) The life history of the fish Astroscopus (the “Stargazer”). Sci Mon 24:348–365
Darwin C (1859) On the origin of species by means of natural selection. J. Murray, London
Davis M, Dahn R, Shubin N (2007) An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature 24:473–476
Denizot JP, Kirschbaum F, Westby GWM, Tsuji S (1978) Larval electric organ of weakly electric fish Pollimyrus (Marcusenius) Isidori (Mormyridae, Teleostei). J Neurocytol 7(2):165–181
Denizot JP, Kirschbaum F, Westby GWM, Tsuji S (1982) On the development of the adult electric organ in the Mormyrid fish Pollimyrus-Isidori (with special focus on the innervation). J Neurocytol 11(6):913–934
Devoto SH, Melancon E, Eisen JS, Westerfield M (1996) Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 122(11):3371–3380
Dunlap KD, Tran A, Ragazzi MA, Krahe R, Salazar VL (2016) Predators inhibit brain cell proliferation in natural populations of electric fish, Brachyhypopomus occidentalis. Proc Biol Sci 283(1824)
Eschmeyer WN, Fong JDS (2018) Species by family/subfamily. Available at http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp. Accessed 1 Jan 2018
Esquibel MA, Alonso I, Meyer H, Chagas C, de Castro G (1971) Some aspects of the histogenesis and ontogenesis of electric organs in Electrophorus electricus (L.). C R Acad Sci Hebd Seances Acad Sci D 273(2):196–199
Ewart JC (1889a) The electric organ of the skate: on the development of the electric organ of Raia batis. Harrison and Sons, London
Ewart JC (1889b) The electric organ of the skate: the electric organ of Raia radiata. Harrison and Sons, London
Ewart JC (1892) The electric organ of the skate: observations on the structure, relations, progressive development, and growth of the electric organ of the skate. Harrison and Sons, London
Fauconneau B, Paboeuf G (2001) 4 - Muscle satellite cells in fish. In: Johnston IA (ed) Fish physiology, vol 18. Academic Press, Cambridge, MA, pp 73–101
Fox GQ, Richardson GP (1978) Developmental morphology of Torpedo-Marmorata - electric organ - myogenic phase. J Comp Neurol 179(3)
Fox GQ, Richardson GP (1979) Developmental morphology of Torpedo-Marmorata - electric organ electrogenic phase. J Comp Neurol 185(2):293–315
Franchina CR (1997) Ontogeny of the electric organ discharge and the electric organ in the weakly electric pulse fish Brachyhypopomus pinnicaudatus (Hypopomidae, Gymnotiformes). J Comp Physiol A 181(2):111–119
Franzini-Armstrong C, Protasi F (1997) Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev 77(3):699–729
Fritsch G (1883) Die elektrischen Fische im Lichte der Deszendenzlehre. In: Virchow R, von Holtzendorff FR (eds) Sammlung gemeinverständlicher wissenschaftlicher Vorträge, vol 835–898. Habel, Berlin
Gallant JR, Arnegard ME, Sullivan JP, Carlson BA, Hopkins CD (2011) Signal variation and its morphological correlates in Paramormyrops kingsleyae provide insight into the evolution of electrogenic signal diversity in mormyrid electric fish. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197(8):799–817
Gallant JR, Hopkins CD, Deitcher DL (2012) Differential expression of genes and proteins between electric organ and skeletal muscle in the mormyrid electric fish Brienomyrus brachyistius. J Exp Biol 215(Pt 14):2479–2494
Gallant JR, Traeger LL, Volkening JD, Moffett H, Chen PH, Novina CD, Phillips GN, Anand R, Wells GB, Pinch M, Güth R, Unguez GA, Albert JS, Zakon HH, Samanta MP, Sussman MR (2014) Genomic basis for the convergent evolution of electric organs. Science 344(6191):1522–1525
Gallant JR, Losilla M, Tomlinson C, Warren WC (2017) The genome and adult somatic transcriptome of the mormyrid electric fish Paramormyrops kingsleyae. Genome Biol Evol 9(12):3525–3530
Gautron J (1974) Effet de la section de nerf electrique sur la structure des electroplaques de la Torpille. Journal de micorscopie 21:85–92
Gilbert SF, Barresi MJF (2016) Developmental biology, 11th edn. Sinauer Associates Inc. Publishers, Sunderland, MA
Güeth R, Pinch M, Unguez GA (2013) Mechanisms of muscle gene regulation in the electric organ of Sternopygus macrurus. J Exp Biol 216(Pt 13):2469–2477
Harris M, Fallon J, Prum R (2002) Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool 294:160–176
Hopkins CD (1999) Design features for electric communication. J Exp Biol 202(Pt 10):1217–1228
Hunter J (1775) XXXIX. An account of the Gymnotus Electricus. Philos Trans 65:395–407
Johnels AG (1956) On the origin of the electric organ in Malapterurus-Electricus. Q J Microsc Sci 97(3):455
Katz SL (2002) Design of heterothermic muscle in fish. J Exp Biol 205(15):2251–2266
Keynes RD (1961) The development of the electric organ in Electrophorus electricus L. In: Chagas C, Paes de Carvalho A (eds) Bioelectrogenesis. Elsevier, Amsterdam, pp 14–19
Kim JA, Jonsson CB, Calderone T, Unguez GA (2004) Transcription of MyoD and myogenin in the non-contractile electrogenic cells of the weakly electric fish, Sternopygus macrurus. Dev Genes Evol 214(8):380–392
Kim HJ, Guth R, Jonsson CB, Unguez GA (2009) S. macrurus myogenic regulatory factors (MRFs) induce mammalian skeletal muscle differentiation; evidence for functional conservation of MRFs. Int J Dev Biol 53(7):993–1002
Kirschbaum F (1975) Environmental factors control periodical reproduction of tropical electric fish. Experientia 31(10):1159–1160
Kirschbaum F (1983) Myogenic electric organ precedes the neurogenic organ in Apteronotid fish. Naturwissenschaften 70(4):205–207
Kirschbaum F, Schwassmann HO (2008) Ontogeny and evolution of electric organs in gymnotiform fish. J Physiol Paris 102(4-6):347–356
Lamanna F, Kirschbaum F, Tiedemann R (2014) De novo assembly and characterization of the skeletal muscle and electric organ transcriptomes of the African weakly electric fish Campylomormyrus compressirostris (Mormyridae, Teleostei). Mol Ecol Resour 14(6):1222–1230
Lamanna F, Kirschbaum F, Waurick I, Dieterich C, Tiedemann R (2015) Cross-tissue and cross-species analysis of gene expression in skeletal muscle and electric organ of African weakly-electric fish (Teleostei; Mormyridae). BMC Genomics 16:668
Markham MR (2013) Electrocyte physiology: 50 years later. J Exp Biol 216(13):2451–2458
Mate S, Brown K, Hoffman E (2011) Integrated genomics and proteomics of the Torpedo californica electric organ: concordance with the mammalian neuromuscular junction. Skelet Muscle 1:20
McCollum M, Sharpe P (2001) Evolution and development of teeth. J Anat 199:153–159
Mellinger J, Belbenoit P, Ravaille M, Szabo T (1978) Electric organ development in Torpedo-Marmorata, chondrichthyes. Dev Biol 67(1):167–188
Nazarian J, Hathout Y, Vertes A, Hoffman EP (2007) The proteome survey of an electricity-generating organ (Torpedo californica electric organ). Proteomics 7(4):617–627
Nazarian J, Berry DL, Sanjari S, Razvi M, Brown K, Hathout Y, Vertes A, Dadgar S, Hoffman EP (2011) Evolution and comparative genomics of subcellular specializations: EST sequencing of Torpedo electric organ. Mar Genomics 4(1):33–40
Nelson JS (1994) Fishes of the world. Wiley, New York
Neville CM, Schmidt J (1992) Expression of myogenic factors in skeletal-muscle and electric organ of Torpedo-Californica. FEBS Lett 305(1):23–26
Ochi H, Westerfield M (2007) Signaling networks that regulate muscle development: lessons from zebrafish. Develop Growth Differ 49(1):1–11
Ozbolat IT, Hospodiuk M (2016) Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76:321–343
Patapoutian A, Would BJ, Wagner RA (1995) Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science 270(5243):1818–1821
Patterson JM, Zakon HH (1993) Bromodeoxyuridine labeling reveals a class of satellite-like cells within the electric organ. J Neurobiol 24(5):660–674
Patterson JM, Zakon HH (1996) Differential expression of proteins in muscle and electric organ, a muscle derivative. J Comp Neurol 370(3):367–376
Patterson JM, Zakon HH (1997) Transdifferentiation of muscle to electric organ: regulation of muscle-specific proteins is independent of patterned nerve activity. Dev Biol 186(1):115–126
Pereira AC, Rodriguez-Cattaneo A, Castello ME, Caputi AA (2007) Post-natal development of the electromotor system in a pulse gymnotid electric fish. J Exp Biol 210(Pt 5):800–814
Pinch M, Guth R, Samanta MP, Chaidez A, Unguez GA (2016) The myogenic electric organ of Sternopygus macrurus: a non-contractile tissue with a skeletal muscle transcriptome. PeerJ 4:e1828
Pitchers WR, Constantinou SJ, Losilla M, Gallant JR (2016) Electric fish genomics: progress, prospects, and new tools for neuroethology. J Physiol Paris 110(3 Pt B):259–272
Rodriguez-Cattaneo A, Caputi AA (2009) Waveform diversity of electric organ discharges: the role of electric organ auto-excitability in Gymnotus spp. J Exp Biol 212(Pt 21):3478–3489
Rodriguez-Cattaneo A, Aguilera P, Cilleruelo E, Crampton WG, Caputi AA (2013) Electric organ discharge diversity in the genus Gymnotus: anatomo-functional groups and electrogenic mechanisms. J Exp Biol 216(Pt 8):1501–1515
Rome LC (2001) Comparative vertebrate muscle physiology eLS. Wiley, Hoboken, NJ
Sachs C (1877) Beobachtungen und Versuche am sudamerikanis- chen Zitteraale (Gymnotus electricus). Arch Physiol
Schneider I, Aneas I, Gehrke A, Dahn R, Nobrega M, Shubin N (2011) Appendage expression driven by the Hoxd Global Control Region is an ancient gnathostome feature. Proc Natl Acad Sci U S A 108:12782–12786
Schwartz IR, Pappas GD, Bennett MV (1975) The fine structure of electrocytes in weakly electric teleosts. J Neurocytol 4(1):87–114
Schwassmann HO, Assuncao MIS, Kirschbaum F (2014) Ontogeny of the electric organs in the electric eel, Electrophorus electricus: physiological, histological, and fine structural investigations. Brain Behav Evol 84(4):288–302
Srivastava CB, Szabo T (1972) Development of electric organs of Gymnarchus-Niloticus (Fam-Gymnarchidae). 1. Origin and histogenesis of electroplates. J Morphol 138(3):375. +
Stoddard PK (2002) The evolutionary origins of electric signal complexity. J Physiol Paris 96(5-6):485–491
Sullivan JP, Lavoué S, Hopkins CD (2000) Molecular systematics of the African electric fishes (Mormyroidea: teleostei) and a model for the evolution of their electric organs. J Exp Biol 203(Pt 4):665–683
Szabo T (1960) Development of the electric organ of Mormyridae. Nature 188(4752):760–762
Szabo T (1966) Origin of electric organs of Electrophorus Electricus. Anat Rec 155(1):103–110
Szabo T, Kirschbaum F (1983) On the differentiation of electric organs in the absence of central connections or peripheral innervation. In: Grinnell AD, Moody WJ Jr (eds) The physiology of excitable cells. Alan R. Liss, New York, pp 451–460
Thompson A, Vo D, Comfort C, Zakon HH (2014) Expression evolution facilitated the convergent neofunctionalization of a sodium channel gene. Mol Biol Evol 31(8):1941–1955
Thompson A, Zakon HH, Kirkpatrick M (2016) Compensatory drift and the evolutionary dynamics of dosage-sensitive duplicate genes. Genetics 202(2):765–774
Traeger LL, Volkening JD, Moffett H, Gallant JR, Chen PH, Novina CD, Phillips GN, Anand R, Wells GB, Pinch M, Guth R, Unguez GA, Albert JS, Zakon H, Sussman MR, Samanta MP (2015) Unique patterns of transcript and miRNA expression in the South American strong voltage electric eel (Electrophorus electricus). BMC Genomics 16:243
Traeger LL, Sabat G, Barrett-Wilt GA, Wells GB, Sussman MR (2017) A tail of two voltages: proteomic comparison of the three electric organs of the electric eel. Sci Adv 3(7):e1700523
Unguez GA, Zakon IH (1998a) Phenotypic conversion of distinct muscle fiber populations to electrocytes in a weakly electric fish. J Comp Neurol 399(1):20–34
Unguez GA, Zakon HH (1998b) Reexpression of myogenic proteins in mature electric organ after removal of neural input. J Neurosci 18(23):9924–9935
Wachtel AW (1964) The ultrastructural relationships of electric organs and muscle. I. Filamentous systems. J Morphol 114(2):325–359
Watabe S (2001) 2 - Myogenic regulatory factors. In: Johnston IA (ed) Fish physiology, vol 18. Academic Press, Cambridge, MA, pp 19–41
Weber CM, Martindale MQ, Tapscott SJ, Unguez GA (2012) Activation of Pax7-positive cells in a non-contractile tissue contributes to regeneration of myogenic tissues in the electric fish S. macrurus. Plos One 7(5):e36819
White EG (1918) The origin of the electric organs in Astroscopus guttatus. Carnegie Institution of Washington, Washington, D.C.
Zakon HH, Unguez GA (1999) Development and regeneration of the electric organ. J Exp Biol 202(10):1427–1434
Zakon HH, Lu Y, Zwickl DJ, Hillis DM (2006) Sodium channel genes and the evolution of diversity in communication signals of electric fishes: convergent molecular evolution. Proc Natl Acad Sci U S A 103(10):3675–3680
Acknowledgments
I thank B. Carlson, J. Sisneros, and A. Popper for the opportunity to write this review and for their helpful conceptual and editorial comments. This work was supported by National Science Foundation Grants 1455405, 1557657, and 1644965.
Compliance with Ethics Requirements
Jason R. Gallant declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gallant, J.R. (2019). The Evolution and Development of Electric Organs. In: Carlson, B., Sisneros, J., Popper, A., Fay, R. (eds) Electroreception: Fundamental Insights from Comparative Approaches. Springer Handbook of Auditory Research, vol 70. Springer, Cham. https://doi.org/10.1007/978-3-030-29105-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-29105-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29104-4
Online ISBN: 978-3-030-29105-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)