Abstract
This chapter presents an overview of the emerging research on spatial learning in weakly electric fish. In the first part, mechanisms by which active electrolocation can provide spatial information are summarized. This includes research on the intricate dynamics of sensorimotor behaviors that enable weakly electric fish to actively generate electrosensory flow. Starting from a summary of spatial learning mechanisms in nonelectric teleost fish, behavioral studies that have begun to investigate spatial learning in weakly electric fish are presented. The behavioral data are then connected with what is known about the neuronal substrate of spatial cognition in teleost fish in general, with a particular focus on the involvement of the dorsal telencephalon. Based on this, the final section summarizes the current data on the telencephalic networks of weakly electric fish. Comparative studies have led to partially novel and hypothetical views that posit similarities between forebrain networks of weakly electric fish and mammalian cortical and thalamocortical networks. Although being a newly emerging line of research, the sensory specialties of the active sensory system of weakly electric fish clearly offer a chance to widen research on the spatial cognition of teleosts by providing novel insights through comparative approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Active electrolocation
- Allocentric
- Egocentric
- Electric image
- Electrosensory flow
- Navigation
- Path integration
- Pattern completion
- Pattern separation
- Spatial learning
- Telencephalic networks
12.1 Introduction
An animal’s behavior may be understood as a compromise between the costs of sensory information acquisition and the accuracy of the information obtained. In this view, sensing behaviors are the overt result of decision-making processes (Wolpert and Landy 2012). How animals optimize their motor behavior can reveal what kind of information they are seeking (Gordon et al. 2011; Hofmann et al. 2013b). Active sensory systems are particularly amenable to such studies because the process of sensory acquisition can be well quantified in time and space. This is especially true in weakly electric fish in which the near-range nature of electric sensing makes the analysis of what an animal is attending to comparatively easy. Furthermore, the energetic constraints of active electrolocation require weakly electric fish to finely regulate their sampling behavior (Salazar et al. 2013), and it has been suggested that this regulation is an overt expression of volition and attention (Jun et al. 2014).
Spatial memories can be regarded as one way to deal with sensory limitations. Instead of navigating based solely on sensory input, internalized information can replace elevated sensory sampling once animals are familiar with their environment (Monaco et al. 2014; Jun et al. 2016). This chapter addresses how weakly electric fish may form spatial memories using their electric sense to aid in navigation. The ease of measuring the sensory input and the animals’ attention, connected with the comparatively simple structure of the teleost forebrain, make weakly electric fish a very interesting model in which to study general aspects of memory and learning in the future.
12.1.1 Electrolocation
Seminal studies in the 1950s provided evidence that weakly electric fish can detect and analyze nearby objects by means of their active electric sense (Lissmann 1951; Lissmann and Machin 1958). For this, they rely on information embedded in the modulation of the self-emitted electric signal. This signal is built up through the synchronous discharge of the electric organ (see Gallant, Chap. 4; Markham, Chap. 5) and is referred to as the electric organ discharge (EOD; see Table 12.1 for a complete list of abbreviations used in this chapter). The potential at the skin of the fish depends on this self-generated field, and objects in the environment can distort this basal electric field (Fig. 12.1A). This results in modulations of the potential at the skin, termed the electric image (EI) of an object (Fig. 12.1A, right). The electroreceptors embedded in the skin thus provide a two-dimensional representation of the three-dimensional world to the brain (see Leitch and Julius, Chap. 2). Because the amplitude of electric images decays steeply with distance (proportional to 1/d4; Chen et al. 2005), the working range of electrolocation is limited to a volume of about one body length (Lissmann 1958; Nelson and MacIver 1999). However, the three-dimensional electric field and the wide distribution of electroreceptors enable electric fish to sense in an omnidirectional manner (see Fig. 12.1B; Snyder et al. 2007).
The electric image (EI) of objects within the sensory volume depends on the distance and position. A: top view of the electric field modeled for the elephantnose fish (Gnathonemus petersii). Left: effect of a conductive sphere on the isopotential lines (orange colors show positive and blue colors show negative isopotential lines) and the electric field (black lines). The fields are shown for the positive peak of the electric organ discharge (EOD). Center: difference between the electric field with and without the object present is shown as a normalized color map (the equivalent perturbing field) with the field lines superimposed in black. Right: three-dimensional view showing the EI calculated based on the perturbing field. B: artistic representation of the sensory volume. Note that the electric sense, albeit limited in range, is omnidirectional. Black bar, theoretically predicted acuity for separating two objects; red bar, density of electroreceptors. C: schematic summarizing the effects of distance and size of objects on the EI. Left: effect of distance for a large (blue) and small (orange) conductive object. The calculated two-dimensional images are shown above the fish. Note the ambiguous effect of object distance and size. Right: effect of lateral distance of a conductive object on the position of the EI on the skin of the fish. Note that the shift of the EI depends on the lateral distance such that it moves more caudal with increasing distance. Data for A kindly provided by F. Pedraja; B modified from Snyder et al. (2007)
Despite the physical limitations briefly outlined here, weakly electric fish can perform amazingly well based on their electric sense. While an in-depth review of their capabilities is beyond the scope of this chapter, it is important to note that they can determine the size, distance, resistance, capacitance, and shape of objects within their sensory volume (von der Emde et al. 2010). Here, we show that this depends on physical as well as anatomical and behavioral specializations.
Contrary to vision, EIs become wider and decrease in intensity with the increasing distance of an object (Fig. 12.1C; Rasnow 1996; Caputi and Budelli 2006). Two objects of different size and distance can have EIs of similar amplitude, causing a size-distance ambiguity (Fig. 12.1C, left). Nonetheless, weakly electric fish are able to estimate distances (Heiligenberg 1973a, b), for which they rely on the blurriness of the images (Rasnow 1996; von der Emde et al. 1998). Behavioral evidence for this was provided by von der Emde et al. (1998). These authors specifically proposed that fish estimate distance based on the maximum slope-to-amplitude ratio of the EI, a mechanism comparable to estimating the half-maximum width of EIs (Fig. 12.1C; Rasnow 1996; Lewis and Maler 2001).
Fish must also determine the rostrocaudal (x, y) position of objects. This probably is based on the location of the peak of the EI on the skin (Rasnow 1996). This position should be readily available via somatotopically organized neuronal maps through the computation of a population vector (Lewis and Maler 2001). However, as first shown by Rasnow (1996), there is a systematic distance-dependent offset of the rostrocaudal position of the EI (Fig. 12.1C, right). As discussed in Sect. 12.1.2, this effect can be used to obtain a dynamic distance cue. Despite the ambiguities inherent to the electric images, the precision of the prey-capture behavior reported in black ghost knifefish (Apteronotus albifrons; Postlethwaite et al. 2009) suggests that position (x, y, and z) as well as movement of the prey (or objects) are accurately tracked by the animals (MacIver et al. 2001).
Due to the superposition effect of EIs, the acuity of separating two nearby objects is moderate. At a 1-cm distance, two Daphnia have been modeled to be separable if the interprey spacing is about 10 mm (Babineau et al. 2007). Electroacuity thus is comparable to that of human touch, where the 2-point discrimination ability ranges from 5 to 50 mm (Rowin and Meriggioli 2007). The coherence of the electric field lines is highest at the midbody (Figs. 12.1A and 12.2A), suggesting that electroacuity should be best at the trunk. However, the density of electroreceptors is highest at the head (Fig. 12.1B), potentially resulting in a more balanced acuity.
A: top view of the calculated electric field of Gnathonemus petersii. Red, positive normalized voltage; blue, negative normalized voltage; black lines, electric field lines. R-C, rostrocaudal; M-L, mediolateral. B: perturbation of the field due to a metal sphere placed at two different rostrocaudal locations and a fixed lateral distance. The colors represent the voltage difference between the electric field with and without the object present: red, positive normalized voltage; blue, negative normalized voltage. White circles, position and size of the sphere; white arrows, polarization gradient of the electric field due to the object differs by almost 90° between the rostral and the caudal objects; black circles, spheres at a closer distance; black arrows, corresponding gradient. The angular difference of the polarization gradients is smaller for the closer object. C: modeled EIs for the object position shown in B for increasing lateral distances (for color coded distance, see color bar). Note that the maxima of the EIs (black circles) gradually shift to more medial positions on the animal’s trunk for further distances. D: schematic of the EIs of a sphere moving at a fixed distance along the trunk. Top: schematic shows the sphere (orange circle) moving closer to the animal’s skin; bottom: schematic shows the sphere (blue circle) moving at a further distance from the animal’s skin. Although the object moves over an identical distance, the image speed (and hence distance) that the EI travels on the surface of the fish decreases with lateral distance. E: Δimage-to-Δobject ratio (IOR) decreases with distance in the three electric fish species that were tested (orange, Apteronotus albifrons; black, Gnathonemus petersii; blue, Eigenmannia virescens). Data modified from Pedraja et al. (2018)
Details of the electric field not only depend on the environment but also on the animals’ body. Their low internal resistance funnels the current toward the head region. This is the source of the asymmetry of the dipole field seen in Fig. 12.1A. A direct consequence of this asymmetry is that the EI of an object depends on the rostrocaudal position of the object (see Fig. 12.2). Fish thus may be able to obtain different viewpoints of an object by changing their position with respect to an object (Hofmann et al. 2017; Pedraja et al. 2018) or by actively moving their electric organ (Sim and Kim 2011). Analyzing the electric scene from different viewpoints has also been proposed as a mechanism to deal with the superposition nature of EIs (Migliaro et al. 2005; Babineau et al. 2007). Although the asymmetry of the electric field may be used to actively extract spatial information, passive (prereceptor) mechanisms can further affect the properties of the EIs. For example, the epithelia containing the electroreceptors have a high resistivity that improves their sensitivity (von der Emde and Schwarz 2002; Migliaro et al. 2005). The density of the electroreceptors is typically highest at the head (Castelló et al. 2000; Bacelo et al. 2008), forming a short-range but high-resolution fovea, whereas the trunk may be preferentially used to detect objects with high sensitivity.
12.1.2 Sensory Flow and Electrolocation
The ability to localize minute prey items and the concomitant occurrence of stereotyped back-and-forth scanning motor patterns (“va-et-vient” movements) in prey-catching behavior have led to the hypothesis that spatiotemporal sensory patterns generated by these motor patterns can aid in electrolocation (for details on the tight relationship between electroreception and the motor system, see Perks and Sawtell, Chap. 11). Indeed, many of the well-known stereotyped probing motor acts may induce relative motion cues and hence are regarded as an active-sensing strategy to enhance electroreception (Hofmann et al. 2013b). Modeling the sensory input associated with the back-and-forth scanning movements revealed that in the absence of movements, the superposition nature of EIs should make prey detection very difficult. However, relative movement was shown to result in time-varying local EIs that let the prey stand out (Babineau et al. 2007). Such time-varying signals do contain distance information that may be extracted, similar to the slope-to-amplitude ratio discussed in Sect. 12.1.2 (Hofmann et al. 2013a).
But are these motion-induced signals actually used? A behavioral study of the electroacuity of the elephantnose fish (Gnathonemus petersii) found that they are surprisingly good at discriminating gapped from solid objects (Fechler et al. 2012). This was suggested to indicate that the fish exploit the spatiotemporal sequence of different electric viewpoints. Importantly, the gap-detection experiments provide evidence for an enhancement of electroacuity in the presence of moving backgrounds, fitting to the predictions of Babineau (2007). This agrees well with a study on the same species that found that the natural dynamics of electrosensory information (electrosensory flow) lead to the emergence of a highly reliable distance estimate (Hofmann et al. 2013a).
Are stereotyped motor patterns and, in particular, the back-and-forth scanning movements performed in a purposeful manner to shape the sensory flow? Such back-and-forth movements are suggestive of peering movements used by insects to exploit and generate a visual parallax for a visual depth estimation (Poteser and Kral 1995). Indeed, the asymmetrical electric field provides a similar depth cue through its nonlinear distortion of the field lines (Pedraja et al. 2018). The direction of the field lines (Fig. 12.2A) determine where an EI is created on the sensory surface of the fish. The dipole moment of the perturbation due to an object is parallel to this electric field as shown in Fig. 12.2B by the polarization gradient. Hence, as schematically shown in Fig. 12.2C, D, the EI of an object that recedes laterally from the fish not only becomes blurry but is also projected more toward the midbody. When the fish swims along a stationary object, the EI travels a fixed distance (△image) over the skin. The △image decreases with the lateral distance of the object, whereas the distance moved by the fish is constant (△object; Fig. 12.2D). The apparent speed of the EI thus decreases with the distance of the object (image-to-object ratio; Fig. 12.2E). Relative motion between object and animal thus gives rise to a parallax-like distance cue that fish may exploit. This was tested using the well-established shelter-tracking behavior in which weakly electric fish maintain a centered position within a moving shelter. When the sides moved at different speeds, the fish shifted toward the slower side (Fig. 12.3A). As predicted by the motion parallax hypothesis, the magnitude of this shift increased with the speed difference (Fig. 12.3B). Sensory flow is exploited to estimate depth, strongly supporting the idea that back-and-forth scanning behavior is a strategy to actively acquire depth information (see Stamper, Madhav, Cowan, and Fortune, Chap. 8).
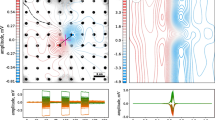
A: top view of a fish positioned between moving shuttle walls (gray) that contain an embedded metal stripe (black). In the control condition, both walls move back and forth at 2 cm/s (blue arrows); in parallax conditions, one wall moves at 1.8 (90%; orange) or 1.4 (70%; black) cm/s. B: exemplary normalized distributions of a single fish’s position during centering behavior. For illustrative purposes, the position data were adjusted to represent the parallax condition on the right while the experimental conditions were tested on either side at random. With a stronger parallax cue (black > orange > blue), the skewness of the distributions increased as quantified by the 90% quantiles (arrows). There was a significant shift toward the slower side, as predicted from the electric field study (Fig. 12.2). C: top: schematic of the paradigm used by Heiligenberg (1973b). When fish (Eigenmannia virescens) station between two rods (gray) that oscillate transversally (blue arrows), they maintain a preferred distance with respect to the closest rod of approximately 13.7 mm. Bottom: simulating this in a physiological experiment shows that this distance conveys optimal electrosensory information and is precisely the distance where ON- and OFF-cells of the electrosensory lateral line lobe (ELL) transition from tonic to burst-firing mode in brown ghost knifefish (Apteronotus leptorhynchus). Data is pooled for ON-and OFF-cell responses to looming metal and plastic spheres, respectively. Right: ON- and OFF-cell pair response to the looming and receding movement. Note that both types respond to the same change of local sensory contrast in a manner that suggests sensitivity to the temporal derivative of contrast. The colored background for both types shows regions of bursting. Encoding of motion requires combining ON- and OFF-population, whereas directionality is encoded in the balance between both populations. A and B modified from Pedraja et al. (2018); C modified from Clarke and Maler (2017)
Significant progress toward understanding how weakly electric fish can encode the distance to moving objects has been made for looming and receding motions. Glass knifefish (Eigenmannia virescens) maintain a preferred distance when positioning between two swinging rods (Fig. 12.3C, top). This preferred distance is the distance where the ON- and OFF-cell populations of the electrosensory lateral line lobe (ELL) report the presence of a looming object optimally (Clarke et al. 2015). At this distance, these neurons transition from a tonic to a burst-firing mode (Fig. 12.3C, bottom) and the distance where this occurs is invariant of object size and speed (Clarke et al. 2015). This requires a dynamic control of the bursting activity that was shown to depend on sophisticated mid- and hindbrain feedback loops (Clarke and Maler 2017). Keeping bursting ON- and OFF-cells in balance will enable the animal to reliably track objects. Furthermore, a switch in the ratio of bursting ON-cells to OFF-cells provides information about the direction of the movement of the object (Fig. 12.3C, right). This is a compelling example that shows how neural coding and motor output together influence information transfer in active sensing.
12.1.3 Electrolocation and Multimodal Integration
The ability to integrate multimodal information is crucial to build up reliable sensory representations. Multimodal integration can lead to multisensory enhancement when stimuli of different modalities match temporally and spatially (Meredith et al. 1987; Meredith and Stein 1996). The magnitude of this multimodal enhancement is inversely proportional to the effectiveness of the single sensory component (Stanford et al. 2005), while the weighting of a single modality is inversely proportional to the variance of the stimulus (Ernst and Banks 2002).
Weakly electric fish also rely on different sensory modalities (von der Emde and Bleckmann 1998; Moller 2003). Synergistic effects between the active electric sense and vision (Rojas and Moller 2002) as well as passive electroreception and the mechanosensory lateral line (Pluta and Kawasaki 2008) have been found in electric fish, suggesting that multimodal enhancement may aid in electrolocation. Furthermore, studies on Gnathonemus petersii have shown that weakly electric fish are capable of spontaneous cross-modal object recognition (Schumacher et al. 2016, 2017). After being trained to discriminate between objects with either vision or the active electric sense only, fish were able to discriminate the objects correctly using the untrained modality alone. The different sensory modalities are weighted according to their reliability in a manner consistent with the notion of a division between far- and near-range sensory systems.
12.2 Spatial Learning
Aquatic environments are highly variable and offer ample potential cues that fish may use to orient. Two broad distinctions are commonly made: self- and world-centered orientation. The first is referred to as egocentric navigation, the latter as allocentric navigation.
Egocentric navigation is possible in the absence of any external sensory information, relying on self-generated (idiothetic) information from the proprioceptive and vestibular systems (Etienne 2004). As such, egocentric navigation depends on active movement (Mittelstaedt and Mittelstaedt 1982). Through integration of the current direction and velocity with knowledge of the positions visited, it theoretically is possible to determine the current position, and a vector to any memorized place along a trajectory can be calculated. Because this ability is frequently tested by forcing an animal to navigate to its home, this vector is called the homing vector. Behaviors in which the relationships between landmarks are used to navigate are called allocentric because the animal references its position in space with respect to external (allothetic) information. With allocentric input, animals can navigate to new locations, provided they have a cognitive representation of space. To form such a representation, however, path integration is initially required because it provides the knowledge of the distance and location of external cues. This may be seen as the conundrum of navigation: egocentric metrics must be acquired by active movements to establish an allocentric map (McNaughton et al. 2006; Buzsáki and Moser 2013). Cue learning can be considered as a special case in ego- and allocentric navigation, where a salient feature of the environment is used as a beacon to guide an animal’s behavior.
12.2.1 Spatial Learning Strategies Used in Fish
Studies of spatial navigation and memory range from field observations to laboratory studies (Salas et al. 2006). A simple example illustrating the ability of fish to form spatial representations is the work on the frillfin goby (Bathygobius soporator; Aronson 1971). These fish live in tidal pools and accurately jump from their home pool to adjacent pools to evade predation. Testing this behavior in the laboratory, Aronson found that it depends on the presence of landmarks outside the pools (Fig. 12.4). On the one hand, this exemplifies the need for fish to deal with variable environments (tide); on the other hand, it shows that they can do so by formation of a spatial representation of their environments (landmarks). Note that the term cue is used when a single feature in the environment provides information about a goal, whereas the term landmark is used when the constellation of several cues is constant and therefore can be used in spatial learning.
Spatial learning in Bathygobius soporator depends on the availability of landmarks. The fish were put in an artificial tidal pool-like environment from where they try to escape by jumping toward the neighboring basin. This basin cannot be seen from the tidal pool. Precision of the evasive jumps was scored (dashed-line arrows) in presence (right) and absence (left) of visual landmarks. The behavior was significantly more accurate (directed) in the presence of the landmarks (center). Modified from Aronson (1971)
As discussed here, exemplary studies on goldfish (Carassius auratus) are presented that bridge a behavioral account with the neuronal structures underlying navigation in fish (Odling-Smee et al. 2006; Rodriguez et al. 2006). In a seminal study, Carassius auratus were trained in a maze to either perform a fixed turning behavior or swim to a location defined through a set of stable visual landmarks external to the maze (Fig. 12.5; Rodriguez et al. 1994). In the first task, fish could rely on egocentric cues, whereas the second task required them to use external references. In test trials, fish relied on the previously trained strategy (Fig. 12.5). Note that the external landmarks were available to both groups, suggesting that the fish adjusted their strategies depending on requirements or context. Although experiments as the one discussed here focus on the navigation in two dimensions, work on pelagic fish has shown that fish also navigate in three dimensions (Burt de Perera et al. 2016).
Evidence for ego- and allocentric navigation strategies in Carassius auratus. Left column: in the egocentric task, Carassius auratus were required to perform a fixed turn strategy to receive a reward (asterisks), whereas they were required to navigate to a location in the maze that was only identifiable through the external visual landmarks (symbols outside the maze) in the allocentric group. Note that in this condition, the arm opposite to the starting position of the fish was closed. Center column: to test which strategy fish had learned to navigate the maze, fish were released from a new global (room centered) position. In these test trials, all arms of the maze were opened. Fish in the egocentric group preferred the turn strategy (right), whereas the allocentric group preferentially swam to the position corresponding to the place where the reward was presented in the learning trials. Numbers, percentage of trials that fish choose the arm. Right column: in a second test, the whole maze was rotated such that one arm of the maze was at the global position that was rewarded in the learning trials (gray area shows the previous location of the maze). Again, an egocentric strategy was preferred by fish trained for the turn strategy, whereas the allocentrically trained fish preferred to swim to the global position. Lesions to the ventral division (Dlv) and posterior division (Dld) of the dorsolateral telencephalon (gray areas) prevented fish from using an allocentric strategy (Salas et al. 1996a; Rodríguez et al. 2002). Dm, medial division of the dorsolateral telencephalon; Dc, central division of the dorsolateral telencephalon; Dp, posterior division of the dorsolateral telencephalon. Data redrawn from Rodriguez et al. (1994); schematic brain section redrawn from Wullimann and Mueller (2004)
12.2.2 The Role of Active Electroreception during Spatial Learning
Gymnotiform and mormyrid weakly electric fish perform seasonal migrations as well as foraging excursions in the dark (Corbet 1961; Moller et al. 1979). Their nocturnal lifestyle suggests that olfactory as well as electro- and mechanosensory cues are essential in these behaviors. The near-range nature of these modalities suggests that local sensory input has to be stitched together to be transformed into an allocentric representation of space.
A study by Graff (2004) demonstrated that gymnotiform and mormyrid weakly electric fish are capable of electric-pattern recognition. In this study Gnathonemus petersii and Sternopygus macrurus (longtail knifefish) were trained to orient in a Y-maze according to the similarity of a local electric cue with a cue experienced at the start of a trial. The design of the object was such that the spatial patterns of the objects (Fig. 12.6A) were not globally available to the fish. Hence the fish had to sample the electrodes locally to generate a spatial representation of the object. Furthermore, the fish needed to compare the similarity of this object with the one in their home compartment. This demonstrates that weakly electric fish are able to attend to spatially segregated information in a manner similar to pattern separation and completion.
A: summary of experiments addressing the ability of Gnathonemus petersii and Sternopygus macrurus to learn and generalize electric patterns like “vertical” and “horizontal” and to generalize these features when only partial sensory information is provided. Yellow areas are an artistic representation of locally increased conductivity that can be created through short circuiting between the connected electrodes (red circles). In these experiments, fish were able to correctly generalize. Due to the limited range of electroreception, the fish needed to sample the electric pattern sequentially and connect this locally acquired information to form a representation of the global pattern. To correctly solve the task, the test tube that had the same global configuration as the home tube had to be chosen (left) and generalization of the pattern was tested by presenting reduced patterns (right). Note that the pattern in the home tube against which fish had to compare the two patterns in either arm of the Y maze was turned off at the beginning of each trial. B: overview of an experiment in which Mormyrus rume proboscirostris were trained to navigate a maze in the presence of visual external landmarks (red wall segments). The thin lines in the circular arena indicate the partitions of the maze that were removed in probe trials after the fish had learned to successfully navigate through the maze. Left: during acquisition, the maze either contained no additional local electric landmarks or fish were trained with an “electrically enhanced” maze that contained conductive (solid squares) as well as nonconductive objects (gray squares). Test trials were performed in the absence of the maze (with/without local landmarks; center) and in the absence of the maze and local cues with fish starting from a novel release point (right). The main navigational strategies are summarized by the differently colored trajectories. Recall in the absence of local cues resulted in a targeted approach to the goal. The outbound trajectory of fish trained in presence of local cues and tested in presence of those was initially oriented toward the first cue, from where a more directed approach to the goal was found. This is consistent with an egocentric navigation strategy that relies on sensory-guided updating at a local landmark with path integration. If the fish were rotated during recall, they consistently showed a purely egocentric strategy. A modified from Graff et al. (2004); B modified from Walton and Moller (2010)
How these abilities are used in navigation was initially addressed in a paradigm where Gnathonemus petersii had to learn to shuttle between two compartments of an arena that were connected by an elevated opening in either the presence or absence of a local electric cue (Cain 1995; Cain and Malwal 2002). Acquisition of this task depended on the active electric sense, but silencing the electric organ after the fish had acquired the task did not impair their performance. This suggests that the fish used idiothetic information to follow a previously acquired route. In acquiring this route, the local electrosensory cue was also probably used as a beacon. This interpretation gains support from the finding that when the hydrostatic pressure in the tank was changed after the fish had acquired the task, they again began to attend to the local electric cue. Contrary to this, fish trained in the absence of the electric cue searched for the opening above the actual aperture (i.e., they changed their behavior in accordance with hydrostatic pressure).
The results show that local electrosensory information can be used to calibrate navigation but did not test for allo- and egocentric strategies. To do this, Walton and Moller (2010) trained Mormyrus rume proboscirostris to navigate a maze with and without visual input and with and without local electrosensory cues (Fig. 12.6B, red lines and squares, respectively). The electric cues were objects of different electrical properties but of identical visual appearance that were positioned at the turning points of the maze. Thus, they could be used to learn in which direction to turn and are thus referred to as local electrosensory cues. In the recall phase, the maze was removed and the fish were released from their original entry to the maze or from an entry not used during the training. Fish trained in the absence of local electric cues consistently headed in the direction opposite their release site (Fig. 12.6B, center and right), as would be expected if they relied on an egocentric behavior. Even fish trained in the presence of external visual landmarks showed this behavior, showing that the external visual landmarks were not used. When trained with both visual and electric cues, fish veered toward the position of the first local cue when released from their initial position during recall. This indicates that local electric cues are attended to during the learning phase. When these fish were released from a novel site in the absence of the local cues, no veering in the direction of the previous local cue occurred and the fish again swam straight (Fig. 12.6B, right). Hence, in the absence of local cues, weakly electric fish followed an egocentric strategy that they could combine with a cue-based strategy if local electric cues were present. As the first cue is outside the electrolocation range when the fish leave the start box, this may suggest that they had formed some internal representation of the location of this electric cue with regard to their start box and therefore headed in this direction during recall. However, this requires further research to be confirmed. Integrating the local cue as part of a learned motor routine can help in correcting for errors accumulated during path integration to the cue, a mechanism known from both mammals and ants (Etienne 2004; Knaden 2006). The notion that weakly electric fish seem to preferably follow egocentric strategies in recalling routes over short distances was further confirmed in two studies with Gnathonemus petersii (Schumacher et al. 2017; Jung et al. 2019).
With the exception of the studies by Cain (Cain 1995; Cain and Malwal 2002), which documented a tight link between familiarity of an environment and the EOD frequency, the electric sampling behavior during spatial learning has not been studied in the work discussed thus far. This is surprising because the electric sampling can be directly linked to motor planning and knowledge of an environment. Pulse-type weakly electric fish are known to show electromotor orienting responses to changes in their environment (Sokolov 1990), the so-called novelty response (Post and von der Emde 1999; Caputi et al. 2003). Novelty responses are considered an economic strategy to update the memory of the recent sensory past whenever it deviates from the current sensory state (Heiligenberg 1980; Caputi et al. 2003). A similar link exists between active sensing and motor planning in the banded knifefish (Gymnotus sp.). When Gymnotus initiate movement in the absence of an observable change of their sensory input, this is preceded by an increase in EOD frequency, a so-called up state (Jun et al. 2016). Up states as well as novelty responses are overt electrical behaviors that reflect top-down control. Consequently, the study on Gymnotus sp. (Jun et al. 2016) started to focus on sampling density (number of EODs per distance traveled) in the acquisition and recall of spatial memory tasks. In this study, the fish had to find food within a large circular arena. Because the experiments were performed in darkness, the fish had to rely on idiothetic cues and near-range sensory information, including the electric sense. Food was presented in a fixed spatial relationship to four different local electric landmarks (see Fig. 12.7A), and the ability to navigate to the food was investigated.
Spatial learning and active sampling behaviors. A: overview of the arena in which Gymnotus sp. were tested with four stable landmarks (gray squares) and a food-baited goal location (red circle). B: overview of the change in parameters observed near the landmarks at the early and late phase of learning and the probe trials. Search time (solid black lines and arrows), sampling density (solid orange lines and arrows) and back-scan probability all decline while the fish acquired the task (compare early and late data). Sampling density and B-scans are again elevated in the probe trials (green arrows). C: schematic showing the visit density distribution in probe trials for fish trained in the absence and presence of landmarks. Note that the fish are more accurate and precise in targeting the goal if they were trained with landmarks. Redrawn after Jun et al. (2016)
In agreement with the data of Cain et al. (1994), the local electric landmarks improved performance. Fish trained in the absence of landmarks followed a random search strategy, taking longer trajectories to locate the food than fish trained with stable landmarks. After having acquired the task, the fish spent significantly less time at the landmarks (Fig. 12.7B), sampled them less (Fig. 12.7B), and showed fewer stereotyped back-and-forth motor patterns at the landmarks (Fig. 12.7B; as introduced in Sect. 12.1, the back-and-forth movements generate sensory flow that produces a distance metric). In probe trials without food being present, the fish increased these motor patterns and the sampling density at the landmarks again (Fig. 12.7B).
The changes in sampling behavior observed by Jun et al. (2016) are consistent with the interpretation that on encountering a salient cue in unexplored terrain, electric fish perform place learning and associate this landmark with their idiothetic information of the cue’s position. Afterward, the landmarks are sampled less and may be used to compensate for errors in path navigation. Once the fish encounter a change in the pattern of local landmarks (including the food location in these experiments), they resample them, probably with the aim to recalibrate. This is supported by the finding that the fish were able to reduce their heading error over the course of the probe trials. A second series of experiments add support to the importance of landmarks in which fish were trained either with stable landmarks or in the absence of landmarks (Fig. 12.7C). Only the stable group shifted their mean sampling density distribution toward the true goal (Fig. 12.7C, right) and thus had a higher accuracy and precision. This shows that fish acquired and used relational information they obtained electrically to guide their behavior. Hence, contrary to the experiments reported thus far, this shows that they are capable of forming some form of global representation based on the highly localized sensory input.
This suggest that weakly electric fish incorporate relational knowledge of electric landmarks acquired through local active electric sampling to support idiothetic navigation. How egocentric and sensory information can be transformed to relational allocentric representations of space is currently unknown. A study in Apteronotus leptorhynchus recorded from the preglomerular complex (PG), an analog of the mammalian thalamus that provides visual and electrosensory input to the dorsolateral pallium (see Sect. 12.3.1; Wallach et al. 2018). Neurons in the PG were found to transform the topographic visual information into temporal information about time between object encounters. When combined with information on swim speed, the distance between objects could be computed. In this way, sequential exploration of different landmarks could provide egocentric distance measures from which allocentric spatial maps may later be computed.
In summary, all available data consistently demonstrate that weakly electric fish can use egocentric strategies in spatial learning. These strategies were shown to benefit from the presence of local cues and landmarks. With increasing familiarity, the electric sampling of these decreases. This suggests that fish tend to rely more on path integration mechanisms in familiar terrain, but active electric sampling of cues is increased when such internalized strategies fail. Relying on path integration whenever possible and updating the path integration mechanisms only by reorientation toward electrically detectable local landmarks seems to be an efficient strategy in the absence of far-range sensory information. Hence, weakly electric fish are highly suitable to study the contribution of active exploratory behaviors for the computation and acquisition of spatial relationships. In a broader context, active movements are the key element to disambiguate an animal’s environment and offer a direct link between overt behaviors and internal processes like spatial learning and memory.
12.3 Neural Substrate of Spatial Learning in Teleost Fish
12.3.1 Primer on the Teleost Telencephalon
The telencephalon is crucial for spatial learning in fish, and hence a coarse anatomical overview is presented in this section (for a comprehensive overview, see Meek and Nieuwenhuys 1998). The telencephalon is separated into the dorsal pallium (area dorsalis) and the ventral subpallium (area ventralis). The relationship between the teleost and tetrapod dorsal pallia has stirred a veritable scientific debate (e.g., Mueller et al. 2011; Yamamoto et al. 2017). In part, the difficulty of establishing homologies is based on the different morphogenesis of the dorsal telencephalon, which in actinopterygian fish develops though eversion of the forebrain anlage, whereas in all nonactinopterygians, it is characterized by an evagination process (Fig. 12.8). The details of this process have not yet been resolved, but it is evident that evagination will lead to considerably different topologies of potentially homologous brain areas (for reviews summarizing the different interpretations, please refer to Northcutt 2008; Braford 2009; Nieuwenhuys 2009). Nieuwenhuys’ (1963) topology-based nomenclature in describing the forebrain is used throughout the chapter. Accordingly, the dorsal telencephalon is separated into a medial division (Dm), dorsal division (Dd), central division (Dc), lateral division (Dl), and posterior division (Dp; see Fig. 12.8C). Except for two areas, no consensus on homologies has been reached. Hodological criteria, expression studies, and functional studies make it likely that the Dl is a homologue to the amniote hippocampus (medial pallium; Fig. 12.8), whereas similar arguments have led to the view that the Dm is the homologue of the amniote amygdala (ventral pallium; Fig. 12.8; Portavella et al. 2004; Vargas et al. 2009).
A: vertebrate cladogram with key brain innovations (modified after Murray et al. 2017). Where lesions of the medial pallium have been tested (blue species), spatial navigation consistently was impaired. B: schematic comparing the development of the actinopterygian telencephalon and nonactinopterygian telencephalon. Assuming that four pallial zones are present in the early stage, the bottom row exemplifies the process of evagination resulting in the hippocampus being internalized by the growth of the isocortex. Because of this process, the four zones maintain their original order. The eversion characteristic for actinopterygian fish results in a rearrangement of the pallium. The details of this rearrangement are presently unresolved. The brain schematics show a zebrafish (top) and mouse brain (bottom) with the medial pallium areas implicated in navigation depicted in blue. MEC, medial enthorhinal cortex; Hip, hippocampus. C: schematic half-cross sections comparing teleost (top) and mammalian forebrain (bottom). Most authors see sufficient support to consider the Dm of teleosts (red striped area) as a homologue of the lateral pallium (LP, amygdala) and the lateral division of the dorsolateral pallium (Dl; blue striped area) as a homologue of the medial pallium (MP, hippocampal formation). Dd, dorsal division of the dorsolateral pallium; Vc, central division of the ventral pallium; Vd, dorsal division of the ventral pallium; Vl, lateral division of the ventral pallium; Vv, ventral division of the ventral pallium. B and C modified from Meek and Nieuwenhuys (1998), Mueller et al. (2011), and Yamamoto et al. (2017)
The ventral part of the telencephalon is divided into a dorsal division (Vd), central division (Vc), lateral division (Vl), and ventral division (Vv; see Fig. 12.8C). Again, no clear comparative pattern has emerged yet, but in toto the ventral pallium may be considered similar to the basal ganglia of nonacinopterygians (Mueller and Wullimann 2009).
12.3.2 Experiments Addressing Sites of Spatial Learning
To navigate, motor output needs to be calibrated. The mammalian cerebellum contributes to this in two ways. It computes idiothetic self-motion information (vestibular, proprioceptive, motor-command efference copy, and, potentially, optic flow information) that is integrated into an allocentric frame of reference in the hippocampus. The computation of self-motion cues is instrumental for path integration (Wallace et al. 2002). In return, the cerebellum uses this reference frame to coordinate actions (Rochefort et al. 2013).
In teleosts, evidence for a direct cerebellar input to the telencephalon is limited (Vonderschen et al. 2002; Ikenaga et al. 2006). In mormyrid fish, this includes a direct cerebellotelencephalic projection from the valvula cerebelli to the Dd (Wullimann and Rooney 1990). This projection may provide a pathway for electrosensory information (for a review of the neuroanatomy in electric fish, see Bell and Maler 2005). Goldfish with lesions to the corpus of the cerebellum took longer to learn to navigate in a hole-board task (Fig. 12.9) and performed less well than sham-operated fish (Durán et al. 2014). All fish were impaired when landmarks were removed or rearranged. However, the selective effects of lesions were found when only the nearest landmark (i.e., a beacon) to the goal was removed (Fig. 12.9, red arrows). The effect was as strong as removing all proximal landmarks.
Effect of cerebellar lesion on spatial learning. Left: schematic of the hole-board arena. Circles, the 25 holes in the bottom of the tank; red circle, the goal. Star and plusses, six landmarks (objects distributed in the arena). Right: effect on the accuracy of the localization of the goal for different conditions in recall. Blue, data from sham-operated fish; red, data from cerebellum-operated fish. Cerebellum-lesioned fish had significantly reduced performance in the absence of proximal cues, specifically the cue closest to the goal (orange star). Modified from Durán et al. (2014)
The teleost optic tectum (homologue of the superior colliculus) contains sensory topographic maps as well as a motor map. It thus provides a body-centered site for sensory-motor transformation and is the major motor output center of teleosts (Isa and Sasaki 2002; Torres et al. 2005). In weakly electric fish, this includes electromotor output (Wullimann and Northcutt 1990; Carlson 2002). However, no studies have addressed how the motor output of the tectum contributes to spatial navigation or learning.
Allocentric navigation in mammals depends on the place cell system of the hippocampal formation (O’Keefe and Dostrovsky 1971; O’Keefe and Nadel 1979), including the medial enthorhinal cortex (MEC) as a key element. It integrates idiothetic input and provides metrical relationships like head direction, speed of movement, or distance to the hippocampus. From this, an allocentric representation of space is created in the hippocampus (Hafting et al. 2005; Fyhn et al. 2007). The MEC is considered as the site where path integration occurs. Furthermore, it may also be elemental in resetting the path integrator because head direction cells of the MEC were shown to change their tuning with respect to allocentric cues (Taube 2007).
Similarities of self-referenced egocentric navigation and episodic memory on the one hand and allocentric navigation and semantic memory on the other hand have led to the hypothesis that episodic and semantic memories have evolved from navigation circuits (Buzsáki 2005; Eichenbaum and Cohen 2014). This evolutionary perspective suggests that the fundamental principles of cortical memory formation and recall can be studied in much simpler brains, including the brains of teleost fish. There is general agreement that the medial pallium of early vertebrates likely evolved as a general memory navigation system (Murray and Wise 2004). However, homologies between forebrain structures of teleost fish and amniotes are currently not fully resolved (Fig. 12.8). This paragraph summarizes key findings, mainly in Carassius auratus, which led to the view that the dorsolateral pallium of teleosts is the homologue of the mammalian hippocampus. This view is largely based on lesion studies (Fig. 12.8) that revealed that allocentric strategies are selectively impaired by lesions of the dorsolateral telencephalon (ventral division [Dlv] and posterior division [Dld]; see Fig. 12.5; Rodríguez et al. 2002; Salas et al. 2003; Broglio et al. 2010). These lesions did not affect egocentric or cued learning (Salas et al. 1996b). The view that the Dl may be the homologue of the mammalian hippocampus is further supported through gene expression studies (see, e.g., Ganz et al. 2014), the report of place cells in the dorsolateral pallium of Carassius auratus (Canfield and Mizumori 2004), and the finding that neurons in the goldfish Dl have elevated neuronal activity during spatial learning (Broglio et al. 2010; Ocaña et al. 2017). In summary, the lateral pallium in teleosts is considered to be the homologue of the mammalian hippocampus and was shown to enable relational allocentric learning.
12.3.3 Forebrain Circuitry in Weakly Electric Fish and Their Implications in Spatial Learning
The following overview of the dorsal forebrain of weakly electric fish is based mainly on an excellent series of papers on the telencephalon of Apteronotus leptorhynchus and Gymnotus sp. Where available, information for the mormyrid Gnathonemus petersii is included. Rostral to the anterior commissure, the dorsal pallium of Apteronotus leptorhynchus consists of a large Dl and a distinct small Dlv. The Dlv as well as the Dp are the major recipients of olfactory input (Sas et al. 1993), which in Gnathonemus petersii is confined to the Dp (see Fig. 12.10B; Rooney et al. 1989). In both species, Dl is the major recipient for extratelencephalic input from PG (Fig. 12.11): In Apteronotus leptorhynchus, PG receives electrosensory input through the midbrain torus semicircularis and the optic tectum. In Gnathonemus petersii, Dl receives input via the ventral PG that obtains sensory input from the medial ventral nucleus of the torus semicircularis (von der Emde and Prechtl 1999). Additional electrosensory input is provided to the posterior division of Dl (Dlp) of mormyrids through a cerebellotelencephalic projection from the valvula (Wullimann and Rooney 1990).
Major dorsal forebrain areas of electric fish. A: transverse sections though the forebrain of Apteronotus leptorhynchus at a level rostrocaudal to the anterior commissure. Bottom left, schematic sagittal section of the forebrain. B: transverse sections of the forebrain of Gnathonemus petersii. Bottom left: side view of the brain. Dotted-line arrows, identified subareas of the four major dorsal divisions. Dla, anterior division of the Dl; DLp, posterior division of the Dl; Dm1 and Dm2, parts 1 and 2, respectively, of the Dm; Ob, olfactory bulb. Scale bars, 500 μm. A based on photomicrographs from Trinh et al. (2016) and Giassi et al. (2012b); B based on photomicrographs from Rooney et al. (1989) and Meek and Josten (1993)
A: reduced overview of the afferent and efferent connections of the dorsal telencephalon in weakly electric fish (Gymnotus sp. and Apteronotus leptorhynchus). The figure focuses on the hippocampus-like network and connections that are relevant in the context of spatial learning. Hy, hypothalamus. With the exception of the somatosensory input through the thalamic nucleus to Dc (Corrêa et al. 1998; Meek and Nieuwenhuys, 1998), the data are based on the work of Giassi et al. (2012a, b) and the references cited therein. B: schematic overview of the Dl-Dd network. Presumed functional mammalian homologues are indicated in blue font. Although Dl together with the rostral endopeduncular nucleus (Er) shares several aspects of the gyrus dentatus, the Dd and magnocellular division of the Dd (Ddmg) are akin to the CA3 region. The projection to the intermediate division of Dd (Ddi) is similar to the mossy fiber pathway. Dl is further reciprocally connected with Ddmg and Er. Ddi is strongly recursive and projects to Ddmg. This projection is similar to the back-projection from the CA3 region to the DG, suggesting a functional equivalence of Ddi and the CA3 region. Ddmg is comparable to the mossy cells (MC) of the gyrus dentatus with prominent commissural projections and connects with Er. This is equivalent to DG → hilar interneurons that, in turn, project diffusely to the gyrus dentatus, as does Er to Dl. The colors are used to bind functionally coherent divisions (Dl versus Dd and Er) together. Data based on Elliot et al. (2017). C: schematic representation of pattern separation and pattern completion in relation to the connectivity pattern of Dl → Dd → Dl. Red line, hypothetical response of neurons performing pattern separation; yellow line, hypothetical response of neurons performing pattern completion. Separation is characterized through strong nonlinearity that makes the output of the Dl more dissimilar from the input. Patterns a and b are two very similar electric patterns that a fish may be required to separate. Completion as found in mammalian CA3 neurons would, in turn, result in a unified output for diverging input (yellow area below the dotted unity line). The network hypothesis in B suggests that Dl and Dd perform pattern separation and completion, respectively. Modified from Yassa and Stark (2011)
The Dl borders on Dd. Dd can be identified in Apteronotus leptorhynchus through its lack of expression of CaMKIIα and extrinsic connectivity. Although Dd appears to be prominent in Gnathonemus petersii, no details are available yet. Notably, Dd may be absent in zebrafish (Danio rerio; Mueller et al. 2011). Dm borders on Dd and is divided into a rostral (Dm1) and a caudal (Dm2) part in Apteronotus leptorhynchus. No subdivisions of Dm have been reported for Gnathonemus petersii, where Dm is considerably larger, but modality-specific areas were reported based on physiological investigations (Prechtl et al. 1998). In both species, Dm receives input from the PG (von der Emde and Prechtl 1999; Giassi et al. 2012a). The large core of the pallium is formed by Dc. Dc is the major output region of the dorsal pallium (Fig. 12.11) and receives input from PG in Apteronotus leptorhynchus and Gnathonemus petersii.
As discussed in Sect. 12.3.2, Dl is considered as the teleost homologue of the hippocampus. Fitting to this view, the Dl of Apteronotus leptorhynchus was shown to express FoxO3, a hippocampal marker (Harvey-Girard et al. 2012). A study on Danio rerio (Ganz et al. 2014) reported selective expression of Prox1, a marker of the gyrus dentatus in mammals and birds, in the Dld. This has led to the hypothesis that this part of the Dl might be homologous to the gyrus dentatus (Ganz et al. 2014), a view supported by the pattern of neurogenesis and cell migration of interneurons (Grandel et al. 2006). However, the expression of Prox1 could not be confirmed in the Dl of Apteronotus leptorhynchus. In this species, it was shown that the Dl connects to the intermediate division of the Dd (Ddi; Fig. 12.11B), a highly recurrent area that projects to the magnocellular division of the Dd (Ddmg) and the subpallium. If the Dl, or parts of Dl, truly resembles the gyrus dentatus, this puts Ddi in a position comparable to area 3 of the cornu ammonis (CA3) field of the hippocampus (see Fig. 12.11B; Elliott et al. 2017). In their interpretation, Elliott et al. (2017) further consider the subpallial GABAergic cells of the entopeduncular nucleus (Er; Fig. 12.11B) to be comparable to pallial hilar GABAergic interneurons (the Er, at least functionally, forms a unit with the Dd despite being of subpallial origin; Fig. 12.11B, gray lines in yellow area). The Dl together with the Ddi, Ddmg, and Er may form a network comparable to the connections between the dentate gyrus (DG) and the CA3 (DG → CA3 pathway, including the back projection of the CA3 [Ddi] via mossy cells [Ddmg] to the Dl). This is intriguing because it suggests that canonical cortical circuits may exist and hence can be investigated in less complex teleost brains. However, this hypothesis requires more comparative work.
Results obtained in gymnotiform fish offer yet an alternative hypothesis that extends the thalamocortical loop hypothesis (visual input → thalamus → layer IV → layers V/VI → superior colliculus) of Ito and Yamamoto (2009) to include the sensory-recipient pallial areas. Being the major recipient of PG places Dl in similar positon as cortical layer IV (Ito and Yamamoto 2009), whereas PG resembles the thalamus (see Ishikawa et al. 2007). The Dl of Apteronotus leptorhynchus has a laminar and recurrent columnar organization, which, while not a defining feature of cortical layer IV, stresses the organizational resemblance of the preglomerular-telencephalic loop with the organization of mammalian sensory cortices (Trinh et al. 2016). The Dc is the major output of the dorsal telencephalon projecting to PG, the deep layer of the teleost optic tectum (homologue of the superior colliculus and a key motor output center of teleosts), and the hypothalamus as well as to other sensory midbrain areas (see Fig. 12.11A; Giassi et al. 2012a). Furthermore, most Dc neurons in Apteronotus leptorhynchus are spiny glutamatergic neurons (Giassi et al. 2012c) and express FoxP2 and OTX1. Altogether, this makes the Dc comparable to sensory cortex layers V/VI in mammals (Harvey-Girard et al. 2012).
The reciprocal connectivity between the Dc and the optic tectum has been implicated in establishing the relevance of visually and/or electrically sensed features and the consecutive choice of an accurate behavior (Fig. 12.11; Giassi et al. 2012a). The saliency of behaviorally relevant electrosensory information (e.g., prey) typically is low, leading to the hypothesis that behavioral relevance of such signals should be determined through top-down processes that establish a weighted priority of attention for signals of low saliency. In mammals, this is achieved in the superior colliculus (Fecteau and Munoz 2006). The teleost homologue of the superior colliculus, the optic tectum, represents multimodal and abstracted electrosensory features (Bastian 1982) and is reciprocally connected with Dc.
Efficient learning, including spatial learning, requires storing different items in a manner that separates them well. Accordingly, representations of different items should be uncorrelated, a process considered to be accomplished through pattern separation in the hippocampus. Similarly, retrieving memories must be stable despite incomplete sensory input and therefore should rely on pattern completion. As initially shown by Marr (1969), recurrent networks with sparse representations can perform such tasks. Detailed theoretical work that is largely supported by physiology and anatomy indicates that the strongly recurrent CA3 region is the site where pattern completion is achieved, whereas pattern separation takes place in the gyrus dentatus (for a review, see Rolls 2016).
In the context of spatial learning in electric fish, pattern separation is required in the acquisition of information regarding local electrosensory cues. The ability to memorize and compare spatial electrosensory patterns requires sensory input that is gathered in temporal contiguity to be bound together and separated from information gathered at a different place and time (Sect. 12.2.2). The hypothesis that compares the Dl → Dd with the mammalian DG → CA3 network (Fig. 12.11B) posits that Dl and Dd are the key sites for pattern separation and completion, respectively. This leads to predictions that should be tested in future behavioral and neurophysiological research of these parts of the dorsal pallium of weakly electric fish.
12.4 Chapter Summary
Current research postulates that hippocampal-like circuits of navigation are a shared characteristic of vertebrates. Because hippocampal circuits also form the basis for declarative and episodic memories in mammals (Buzsáki and Moser 2013), understanding the core circuits of spatial navigation in teleosts is likely to reveal core circuits shared by vertebrates in general. However, to date, the neural basis of navigation in teleosts remains ill defined. Future research should provide anatomical, neurophysiological, and behavioral data to generate an evolutionary and holistic perspective on navigation as a specific form of cognition.
The work summarized here shows that weakly electric fish rely on spatial memories in a variety of behaviors and that dedicated neuronal networks of their dorsal telencephalon are involved in their spatial cognition capabilities. Where researchers have started to tackle the neuronal substrate of these networks, striking similarities to cortical and thalamocortical networks of mammals have emerged. Weakly electric fish are of particular interest because a wealth of anatomical, physiological, and behavioral data is available that should ease unraveling to what extent the telencephalon of fish can serve as a blueprint of the intricate cortical networks of cognition in mammals. The near-range sensing strategy of weakly electric fish, the ease at which this behavior can be measured and quantified, and the tight link between their overt electric sampling behavior and spatial attention and learning make them uniquely suited for the comparative study of neural mechanisms mediating navigation.
References
Aronson LR (1971) Further studies on orientation and jumping behavior in the gobid fish, Bathygobius soporator. Ann N Y Acad Sci 188:378–392. https://doi.org/10.1111/j.1749-6632.1971.tb13110.x
Babineau D, Lewis JE, Longtin A (2007) Spatial acuity and prey detection in weakly electric fish. PLoS Comput Biol 3(3):e38. https://doi.org/10.1371/journal.pcbi.0030038
Bacelo J, Engelmann J, Hollmann M et al (2008) Functional foveae in an electrosensory system. J Comp Neurol 511(3):342–359. https://doi.org/10.1002/cne.21843
Bastian J (1982) Vision and electroreception: Integration of sensory information in the optic tectum of the weakly electric fish Apteronotus albifrons. J Comp Physiol A 147(3):287–297. https://doi.org/10.1007/BF00609662
Bell CC, Maler L (2005) Central neuroanatomy of electrosensory systems in fish. In: Bullock TH, Hopkins CD, Popper AN, Fay RR (eds) Electroreception. Springer, New York, pp 68–111. https://doi.org/10.1007/0-387-28275-0_4
Braford MR (2009) Stalking the everted telencephalon: comparisons of forebrain organization in basal ray-finned fishes and teleosts. Brain Behav Evol 74(1):56–76. https://doi.org/10.1159/000229013
Broglio C, Rodríguez F, Gómez A et al (2010) Selective involvement of the goldfish lateral pallium in spatial memory. Behav Brain Res 210(2):480–487. https://doi.org/10.1016/j.bbr.2010.06.010
Burt de Perera T, Holbrook RI, Davis V (2016) The representation of three-dimensional space in fish. Front Behav Neurosci 10:40. https://doi.org/10.3389/fnbeh.2016.00040
Buzsáki G (2005) Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15(7):827–840. https://doi.org/10.1002/hipo.20113
Buzsáki G, Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci 16(2):130–138. https://doi.org/10.1038/nn.3304
Cain P (1995) Navigation in familiar environments by the weakly electric elephantnose fish, Gnathonemus petersii L. (Mormyriformes, Teleostei). Ethology 99(4):332–349. https://doi.org/10.1111/j.1439-0310.1995.tb00907.x
Cain P, Malwal S (2002) Landmark use and development of navigation behaviour in the weakly electric fish Gnathonemus petersii (Mormyridae; Teleostei). J Exp Biol 205:3915–3923
Cain P, Gerin W, Moller P (1994) Short-range navigation of the weakly electric fish, Gnathonemus petersii L. (Mormyridae, Teleostei), in novel and familiar environments. Ethology 96(1):33–45. https://doi.org/10.1111/j.1439-0310.1994.tb00879.x
Canfield JG, Mizumori SJY (2004) Methods for chronic neural recording in the telencephalon of freely behaving fish. J Neurosci Methods 133:127–134. https://doi.org/10.1016/j.jneumeth.2003.10.011
Caputi AA, Budelli R (2006) Peripheral electrosensory imaging by weakly electric fish. J Comp Physiol A 192(6):587–600. https://doi.org/10.1007/s00359-006-0100-2
Caputi AA, Aguilera PA, Castelló ME (2003) Probability and amplitude of novelty responses as a function of the change in contrast of the reafferent image in G. carapo. J Exp Biol 206:999–1010. https://doi.org/10.1242/jeb.00199
Carlson BA (2002) Neuroanatomy of the mormyrid electromotor control system. J Comp Neurol 454(4):440–455. https://doi.org/10.1002/cne.10462
Castelló ME, Aguilera PA, Trujillo-Cenóz O et al (2000) Electroreception in Gymnotus carapo: pre-receptor processing and the distribution of electroreceptor types. J Exp Biol 203:3279–3287
Chen L, House JL, Krahe R et al (2005) Modeling signal and background components of electrosensory scenes. J Comp Physiol A 191(4):331–345. https://doi.org/10.1007/s00359-004-0587-3
Clarke SE, Maler L (2017) Feedback synthesizes neural codes for motion. Curr Biol 27(9):1356–1361. https://doi.org/10.1016/j.cub.2017.03.068
Clarke SE, Longtin A, Maler L (2015) The neural dynamics of sensory focus. Nat Commun 6:8764. https://doi.org/10.1038/ncomms9764
Corbet PS (1961) The food of non-cichlid fishes in the Lake Victoria Basin, with remarks on their evolution and adaptation to lacustrine conditions. Proc Zool Soc London 136(1):1–101. https://doi.org/10.1111/j.1469-7998.1961.tb06080.x
Corrêa SA, Grant K, Hoffmann A (1998) Afferent and efferent connections of the dorsocentral telencephalon in an electrosensory teleost, Gymnotus carapo. Brain Behav Evol 52(2):81–98. https://doi.org/10.1159/000006554
Durán E, Ocaña FM, Martín-Monzón I et al (2014) Cerebellum and spatial cognition in goldfish. Behav Brain Res 259:1–8. https://doi.org/10.1016/j.bbr.2013.10.039
Eichenbaum H, Cohen NJ (2014) Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron 83(4):764–770. https://doi.org/10.1016/j.neuron.2014.07.032
Elliott SB, Harvey-Girard E, Giassi ACC et al (2017) Hippocampal-like circuitry in the pallium of an electric fish: possible substrates for recursive pattern separation and completion. J Comp Neurol 525(1):8–46. https://doi.org/10.1002/cne.24060
Ernst MO, Banks MS (2002) Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415(6870):429–433. https://doi.org/10.1038/415429a
Etienne AS (2004) Resetting the path integrator: a basic condition for route-based navigation. J Exp Biol 207(9):1491–1508. https://doi.org/10.1242/jeb.00906
Fechler K, Holtkamp D, Neusel G et al (2012) Mind the gap: the minimal detectable separation distance between two objects during active electrolocation. J Fish Biol 81(7):2255–2276. https://doi.org/10.1111/j.1095-8649.2012.03438.x
Fecteau JH, Munoz DP (2006) Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci 10(8):382–390. https://doi.org/10.1016/j.tics.2006.06.011
Fyhn M, Hafting T, Treves A et al (2007) Hippocampal remapping and grid realignment in entorhinal cortex. Nature 446(7132):190–194. https://doi.org/10.1038/nature05601
Ganz J, Kroehne V, Freudenreich D et al (2014) Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Res (3):1–20. https://doi.org/10.12688/f1000research.5595.1
Giassi ACC, Duarte TT, Ellis W et al (2012a) Organization of the gymnotiform fish pallium in relation to learning and memory: II. Extrinsic connections. J Comp Neurol 520(15):3338–3368. https://doi.org/10.1002/cne.23109
Giassi ACC, Ellis W, Maler L (2012b) Organization of the gymnotiform fish pallium in relation to learning and memory: III. Intrinsic connections. J Comp Neurol 520(15):3369–3394. https://doi.org/10.1002/cne.23108
Giassi ACC, Harvey-Girard E, Valsamis B et al (2012c) Organization of the gymnotiform fish pallium in relation to learning and memory: I. Cytoarchitectonics and cellular morphology. J Comp Neurol 520(15):3314–3337. https://doi.org/10.1002/cne.23097
Gordon G, Kaplan DM, Lankow B et al (2011) Toward an integrated approach to perception and action: conference report and future directions. Front Syst Neurosci 5(20):1–6. https://doi.org/10.3389/fnsys.2011.00020
Graff C, Kaminski G, Gresty M et al (2004) Fish perform spatial pattern recognition and abstraction by exclusive use of active electrolocation. Curr Biol 14(9):818–823. https://doi.org/10.1016/j.cub.2004.04.039
Grandel H, Kaslin J, Ganz J et al (2006) Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol 295(1):263–277. https://doi.org/10.1016/j.ydbio.2006.03.040
Hafting T, Fyhn M, Molden S et al (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436(7052):801–806. https://doi.org/10.1038/nature03721
Harvey-Girard E, Giassi ACC, Ellis W et al (2012) Organization of the gymnotiform fish pallium in relation to learning and memory: IV. Expression of conserved transcription factors and implications for the evolution of dorsal telencephalon. J Comp Neurol 520(15):3395–3413. https://doi.org/10.1002/cne.23107
Heiligenberg W (1973a) Electrolocation of objects in the electric fish Eigenmannia (Rhamphichthyidae, Gymnotoidei). J Comp Physiol 87(2):137–164. https://doi.org/10.1007/BF01352158
Heiligenberg W (1973b) “Electromotor” response in the electric fish Eigenmannia (Rhamphichthyidae, Gymnotoidei). Nature 243(5405):301–302. https://doi.org/10.1038/243301a0
Heiligenberg W (1980) The evaluation of electroreceptive feedback in a gymnotoid fish with pulse-type electric organ discharges. J Comp Physiol – A 138(2):173–185. https://doi.org/10.1007/BF00680441
Hofmann V, Sanguinetti-Scheck JI, Gómez-Sena L et al (2013a) From static electric images to electric flow: towards dynamic perceptual cues in active electroreception. J Physiol Paris 107(1–2):95–106. https://doi.org/10.1016/j.jphysparis.2012.06.003
Hofmann V, Sanguinetti-Scheck JI, Kunzel S et al (2013b) Sensory flow shaped by active sensing: sensorimotor strategies in electric fish. J Exp Biol 216(13):2487–2500. https://doi.org/10.1242/jeb.082420
Hofmann V, Sanguinetti-Scheck JI, Gómez-Sena L et al (2017) Sensory flow as a basis for a novel distance cue in freely behaving electric fish. J Neurosci 37(2):302–312. https://doi.org/10.1523/JNEUROSCI.1361-16.2017
Ikenaga T, Yoshida M, Uematsu K (2006) Cerebellar efferent neurons in teleost fish. Cerebellum 5(4):268–274. https://doi.org/10.1080/14734220600930588
Isa T, Sasaki S (2002) Brainstem control of head movements during orienting; Organization of the premotor circuits. Prog Neurobiol l66:205–241. https://doi.org/10.1016/S0301-0082(02)00006-0
Ishikawa Y, Yamamoto N, Yoshimoto M et al (2007) Developmental origin of diencephalic sensory relay nuclei in teleosts. Brain Behav Evol 69(2):87–95. https://doi.org/10.1159/000095197
Ito H, Yamamoto N (2009) Non-laminar cerebral cortex in teleost fishes? Biol Lett 5(1):117–121. https://doi.org/10.1098/rsbl.2008.0397
Jun JJ, Longtin A, Maler L (2014) Enhanced sensory sampling precedes self-initiated locomotion in an electric fish. J Exp Biol 217(20):3615–3628. https://doi.org/10.1242/jeb.105502
Jun JJ, Longtin A, Maler L (2016) Active sensing associated with spatial learning reveals memory-based attention in an electric fish. J Neurophysiol 115(5):2577–2592. https://doi.org/10.1152/jn.00979.2015
Jung SN, Künzel S, Engelmann J (2019) Spatial learning through active electroreception in Gnathonemus petersii. Anim Behav 156:1–10. https://doi.org/10.1016/j.anbehav.2019.06.029
Knaden M (2006) Ant navigation: resetting the path integrator. J Exp Biol 209(1):26–31. https://doi.org/10.1242/jeb.01976
Lewis JE, Maler L (2001) Neuronal population codes and the perception of object distance in weakly electric fish. J Neurosci 21(8):2842–2850
Lissmann HW (1951) Continuous electrical signals from the tail of a fish, Gymnarchus niloticus Cuv. Nature 167(4240):201–202. https://doi.org/10.1038/167201a0
Lissmann HW (1958) On the function and evolution of electric organs in fish. J Exp Biol 35(1):156–191
Lissmann HW, Machin KE (1958) The mechanism of object location in Gymnarchus niloticus and similar fish. J Exp Biol 35:451–486
MacIver MA, Sharabash NM, Nelson ME (2001) Prey-capture behavior in gymnotid electric fish: motion analysis and effects of water conductivity. J Exp Biol 204(3):543–557
Marr D (1969) A theory of cerebellar cortex. J Physiol 202(2):437–470. https://doi.org/10.1113/jphysiol.1969.sp008820
McNaughton BL, Battaglia FP, Jensen O et al (2006) Path integration and the neural basis of the “cognitive map”. Nat Rev Neurosci 7(8):663–678. https://doi.org/10.1038/nrn1932
Meek J, Joosten HW (1993) Tyrosine hydroxylase-immunoreactive cell groups in the brain of the teleost fish Gnathonemus petersii. J Chem Neuroanat 6(6):431–446
Meek J, Nieuwenhuys R (1998) Holosteans and teleosts. In: Nieuwenhuys R, Donkelaar, H J ten, Nicholson, C (eds) The central nervous system of vertebrates. Springer, Berlin, Heidelberg, p 759–937. doi:https://doi.org/10.1007/978-3-642-18262-4_15
Meredith MA, Stein BE (1996) Spatial determinants of multisensory integration in cat superior colliculus neurons. J Neurophysiol 75(5):1843–1857. https://doi.org/10.1152/jn.1996.75.5.1843
Meredith MA, Nemitz JW, Stein BE (1987) Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors. J Neurosci 7(10):3215–3229
Migliaro A, Caputi AA, Budelli R (2005) Theoretical analysis of pre-receptor image conditioning in weakly electric fish. PLoS Comput Biol 1(2):e16. https://doi.org/10.1371/journal.pcbi.0010016
Mittelstaedt H, Mittelstaedt ML (1982) Homing by path integration. In: Papi F, Wallraff HG (eds) Avian navigation. Springer, Berlin, Heidelberg, pp 290–297. https://doi.org/10.1007/978-3-642-68616-0_29
Moller P (2003) Multimodal sensory integration in weakly electric fish: a behavioral account. J Physiol Paris 96(5–6):547–556. https://doi.org/10.1016/S0928-4257(03)00010-X
Moller P, Serrier J, Belbenoit P et al (1979) Notes on ethology and ecology of the Swashi River mormyrids (Lake Kainji, Nigeria). Behav Ecol Sociobiol 4:357–368
Monaco JD, Rao G, Roth ED et al (2014) Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat Neurosci 17(5):725–731. https://doi.org/10.1038/nn.3687
Mueller T, Wullimann MF (2009) An evolutionary interpretation of teleostean forebrain anatomy. Brain Behav Evol 74(1):30–42. https://doi.org/10.1159/000229011
Mueller T, Dong Z, Berberoglu M et al (2011) The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res 49:95–105. https://doi.org/10.1016/j.brainres.2010.12.089
Murray EA, Wise SP (2004) What, if anything, is the medial temporal lobe, and how can the amygdala be part of it if there is no such thing? Neurobiol Learn Mem 82(3):178–198. https://doi.org/10.1016/j.nlm.2004.05.005
Murray EA, Wise SP, Graham KS (2017) Representational specializations of the hippocampus in phylogenetic perspective. Neurosci Lett 680:4–12. https://doi.org/10.1016/j.neulet.2017.04.065
Nelson ME, MacIver MA (1999) Prey capture in the weakly electric fish Apteronotus albifrons: sensory acquisition strategies and electrosensory consequences. J Exp Biol 202(10):1195–1203
Nieuwenhuys R (1963) The comparative anatomy of the actinopterygian forebrain. J Hirnforsch 6:171–192
Nieuwenhuys R (2009) The forebrain of actinopterygians revisited. Brain Behav Evol 73(4):229–252. https://doi.org/10.1159/000225622
Northcutt RG (2008) Forebrain evolution in bony fishes. Brain Res Bull 75(2–4):191–205. https://doi.org/10.1016/j.brainresbull.2007.10.058
O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34(1):171–175. https://doi.org/10.1016/0006-8993(71)90358-1
O’Keefe J, Nadel L (1979) The Hippocampus as a cognitive map. Clarendon Press, Oxford
Ocaña FM, Uceda S, Arias JL et al (2017) Dynamics of goldfish subregional hippocampal pallium activity throughout spatial memory formation. Brain Behav Evol 90(2):154–170. https://doi.org/10.1159/000478843
Odling-Smee L, Simpson SD, Braithwaite VA (2006) The role of learning in fish orientation. In: Brown C, Laland KN, Krause J (eds) Fish cognition and behavior. Blackwell Publishing Ltd., pp 119–137
Pedraja F, Hofmann V, Lucas K et al (2018) Motion parallax in electric sensing. PNAS 115(3):573–577. https://doi.org/10.1073/pnas.1712380115
Pluta SR, Kawasaki M (2008) Multisensory enhancement of electromotor responses to a single moving object. J Exp Biol 211(Pt 18):2919–2930. https://doi.org/10.1242/jeb.016154
Portavella M, Torres B, Salas C et al (2004) Lesions of the medial pallium, but not of the lateral pallium, disrupt spaced-trial avoidance learning in goldfish (Carassius auratus). Neurosci Lett 362(2):75–78. https://doi.org/10.1016/j.neulet.2004.01.083
Post N, von der Emde G (1999) The “novelty response” in an electric fish: response properties and habituation. Physiol Behav 68(1–2):115–128
Postlethwaite CM, Psemeneki TM, Selimkhanov J et al (2009) Optimal movement in the prey strikes of weakly electric fish: a case study of the interplay of body plan and movement capability. J Royal Soc Interface 6(34):417–433. https://doi.org/10.1098/rsif.2008.0286
Poteser M, Kral K (1995) Visual distance discrimination between stationary targets in praying-mantis - an index of the use of motion parallax. J Exp Biol 198(10):2127–2137
Prechtl JC, von der Emde G, Wolfart J et al (1998) Sensory processing in the pallium of a mormyrid fish. J Neurosci 18(18):7381–7393
Rasnow B (1996) The effects of simple objects on the electric field of Apteronotus. J Comp Physiol A 78(3):397–411. https://doi.org/10.1007/BF00193977
Rochefort C, Lefort J, Rondi-Reig L (2013) The cerebellum: a new key structure in the navigation system. Front Neural Circuits 7:1–12. https://doi.org/10.3389/fncir.2013.00035
Rodriguez F, Durán E, Vargas JP et al (1994) Performance of goldfish trained in allocentric and egocentric maze procedures suggests the presence of a cognitive mapping system in fishes. Anim Learn Behav 22(4):409–420. https://doi.org/10.3758/BF03209160
Rodríguez F, López JC, Vargas JP et al (2002) Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J Neurosci 22(7):2894–2903
Rodriguez F, Broglio C, Durán E et al (2006) Neural mechanisms of learning in teleost fish. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior. Blackwell Publishing Ltd., pp 243–277
Rojas R, Moller P (2002) Multisensory contributions to the shelter-seeking behavior of a mormyrid fish, Gnathonemus petersii Günther (mormyridae, teleostei): the role of vision, and the passive and active electrosenses. Brain Behav Evol 59(4):211–221. https://doi.org/10.1159/000064908
Rolls ET (2016) Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol Learn Mem 129:4–28. https://doi.org/10.1016/j.nlm.2015.07.008
Rooney DJ, New JG, Szabo T et al (1989) Central connections of the olfactory bulb in the weakly electric fish, Gnathonemus petersii. Cell Tissue Res 257(2):423–436. https://doi.org/10.1007/BF00261845
Rowin J, Meriggioli MN (2007) Proprioception, touch, and vibratory sensation. In: Textbook of clinical neurology. Elsevier, pp 343–361. https://doi.org/10.1016/B978-141603618-0.10019-0
Salas C, Broglio C, Rodríguez F et al (1996a) Telencephalic ablation in goldfish impairs performance in a “spatial constancy” problem but not in a cued one. Behav Brain Res 79(1–2):193–200
Salas C, Rodríguez F, Vargas JP et al (1996b) Spatial learning and memory deficits after telencephalic ablation in goldfish trained in place and turn maze procedures. Behav Neurosci 110(5):965–980. https://doi.org/10.1037/0735-7044.110.5.965
Salas C, Broglio C, Rodríguez F (2003) Evolution of forebrain and spatial cognition in vertebrates: conservation across diversity. Brain Behav Evol 62(2):72–82. https://doi.org/10.1159/000072438
Salas C, Broglio C, Durán E et al (2006) Neuropsychology of learning and memory in teleost fish. Zebrafish 3(2):157–171. https://doi.org/10.1089/zeb.2006.3.157
Salazar VL, Krahe R, Lewis JE (2013) The energetics of electric organ discharge generation in gymnotiform weakly electric fish. J Exp Biol 216(13):2459–2468. https://doi.org/10.1242/jeb.082735
Sas E, Maler L, Weld M (1993) Connections of the olfactory bulb in the gymnotiform fish, Apteronotus leptorhynchus. J Comp Neurol 335(4):486–507. https://doi.org/10.1002/cne.903350403
Schumacher S, Burt de Perera T, Thenert J et al (2016) Cross-modal object recognition and dynamic weighting of sensory inputs in a fish. PNAS 113(27):7638–7643. https://doi.org/10.1073/pnas.1603120113
Schumacher S, von der Emde G, Burt de Perera T (2017) Sensory influence on navigation in the weakly electric fish Gnathonemus petersii. Anim Behav 132:1–12. https://doi.org/10.1016/j.anbehav.2017.07.016
Sim M, Kim D (2011) Electrolocation based on tail-bending movements in weakly electric fish. J Exp Biol 214(Pt 14):2443–2450. https://doi.org/10.1242/jeb.052308
Snyder JB, Nelson ME, Burdick JW et al (2007) Omnidirectional sensory and motor volumes in electric fish. PLoS Biol 5(11):e301. https://doi.org/10.1371/journal.pbio.0050301
Sokolov EN (1990) The orienting response, and future directions of its development. Pavlov J Biol Sci 25(3):142–150
Stanford TR, Quessy S, Stein BE (2005) Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci 13(25):6499–6508. https://doi.org/10.1523/JNEUROSCI.5095-04.2005
Taube JS (2007) The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30(1):181–207. https://doi.org/10.1146/annurev.neuro.29.051605.112854
Torres B, Luque MA, Pérez-Pérez MP et al (2005) Visual orienting response in goldfish: a multidisciplinary study. Brain Res Bull 66(4–6):376–380. https://doi.org/10.1016/j.brainresbull.2005.02.002
Trinh AT, Harvey-Girard E, Teixeira F et al (2016) Cryptic laminar and columnar organization in the dorsolateral pallium of a weakly electric fish. J Comp Neurol 524(2):408–428. https://doi.org/10.1002/cne.23874
Vargas JP, López JC, Portavella M (2009) What are the functions of fish brain pallium? Brain Res Bull 79(6):436–440. https://doi.org/10.1016/j.brainresbull.2009.05.008
von der Emde G, Bleckmann H (1998) Finding food: senses involved in foraging for insect larvae in the electric fish Gnathonemus petersii. J Exp Biol 201(1):969–980
von der Emde G, Prechtl JC (1999) Anatomical connections of auditory and lateral line areas of the dorsal telencephalon (Dm) in the osteoglossomorph teleost, Gnathonemus petersii. Brain Res 818(2):355–367
von der Emde G, Schwarz S (2002) Imaging of objects through active electrolocation in Gnathonemus petersii. J Physiol Paris 96(5–6):431–444. https://doi.org/10.1016/S0928-4257(03)00021-4
von der Emde G, Schwarz S, Gomez L et al (1998) Electric fish measure distance in the dark. Nature 395(6705):890–894. https://doi.org/10.1038/27655
von der Emde G, Behr K, Bouton B et al (2010) 3-dimensional scene perception during active electrolocation in a weakly electric pulse fish. Front Behav Neurosci 4(26):1–13. https://doi.org/10.3389/fnbeh.2010.00026
Vonderschen K, Bleckmann H, Hofmann MH (2002) A direct projection from the cerebellum to the telencephalon in the goldfish, Carassius auratus. Neurosci Lett 320(1–2):37–40. https://doi.org/10.1016/S0304-3940(02)00022-8
Wallace DG, Hines DJ, Pellis SM et al (2002) Vestibular information is required for dead reckoning in the rat. J Neurosci 22(22):10009–10017
Wallach A, Harvey-Girard E, Jun JJ et al (2018) A time-stamp mechanism may provide temporal information necessary for egocentric to allocentric spatial transformations. elife;7:e36769. https://doi.org/10.7554/eLife.36769
Walton AG, Moller P (2010) Maze learning and recall in a weakly electric fish, Mormyrus rume proboscirostris Boulenger (Mormyridae, Teleostei). Ethology 116(10):904–919. https://doi.org/10.1111/j.1439-0310.2010.01807.x
Wolpert DM, Landy MS (2012) Motor control is decision-making. Curr Opin Neurobiol 22(6):996–1003. https://doi.org/10.1016/j.conb.2012.05.003
Wullimann MF, Mueller T (2004) Teleostean and mammalian forebrains contrasted: evidence from genes to behavior. J Comp Neurol 475(2):143–162. https://doi.org/10.1002/cne.20183
Wullimann MF, Northcutt RG (1990) Visual and electrosensory circuits of the diencephalon in mormyrids: an evolutionary perspective. J Comp Neurol 297(4):537–552. https://doi.org/10.1002/cne.902970407
Wullimann MF, Rooney DJ (1990) A direct cerebello-telencephalic projection in an electrosensory mormyrid fish. Brain Res 520(1–2):354–357. https://doi.org/10.1016/0006-8993(90)91730-5
Yamamoto K, Bloch S, Vernier P (2017) New perspective on the regionalization of the anterior forebrain in Osteichthyes. Develop Growth Differ 59(4):175–187. https://doi.org/10.1111/dgd.12348
Yassa MA, Stark CEL (2011) Pattern separation in the hippocampus. Trends Neurosci 34(10):515–525. https://doi.org/10.1016/j.tins.2011.06.006
Compliance with Ethics Requirements
Sarah Nicola Jung declares that she has no conflict of interest.
Jacob Engelmann declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jung, S.N., Engelmann, J. (2019). Active Electrolocation and Spatial Learning. In: Carlson, B., Sisneros, J., Popper, A., Fay, R. (eds) Electroreception: Fundamental Insights from Comparative Approaches. Springer Handbook of Auditory Research, vol 70. Springer, Cham. https://doi.org/10.1007/978-3-030-29105-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-29105-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29104-4
Online ISBN: 978-3-030-29105-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)