Abstract
The first central stage of electrosensory processing in fish has proven to be a particularly useful model system for examining the general issue of how motor systems and behavior influence sensory processing. This chapter reviews this literature, focusing on a substantial body of work elucidating the synaptic, cellular, and circuit mechanisms for predicting and canceling self-generated sensory inputs. Some additional functions of motor corollary discharge signals in weakly electric mormyrid fish are also discussed along with the implications of studies on electrosensory systems for other sensory modalities and brain structures, including the auditory system and the cerebellum.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebellum
- Corollary discharge
- Dorsal cochlear nucleus
- Electric fish
- Electrosensory internal model
- Negative image
- Proprioception
- Reafference
- Synaptic plasticity
11.1 Introduction
Laboratory studies of sensory processing typically focus on characterizing neural responses evoked by sensory stimuli delivered to passive subjects (Churchland et al. 1994). However, under most natural circumstances, sensory information is acquired actively through movement and exploration. Movements allow animals to acquire more and better information about the world but also pose a fundamental challenge for the nervous system. Self-generated sensory inputs could interfere with the detection and processing of behaviorally relevant stimuli or trigger inappropriate motor behaviors. The fact that such difficulties seldom arise raises the question of how sensory-processing structures in the brain distinguish between patterns of sensory-receptor activation due to external events and those due to the animal’s own behavior. This so-called “reafference problem” has long been recognized and affects most, if not all, sensory systems (von Holst and Mittelstaedt 1950; Crapse and Sommer 2008).
Studies of electrosensory systems in fish have provided a detailed illustration of how this fundamental problem in neurobiology is solved. Neurons in the first stage of electrosensory processing generate specific predictions about the electrosensory consequences of the animal’s own behavior. Combined experimental and theoretical studies of electrosensory systems have provided an account of how these predictions, termed negative images, are formed at the level of synaptic plasticity, cells, and circuits. By canceling out the effects of self-generated inputs, negative images enhance detection and behavioral responses to external stimuli. This chapter reviews these studies and discusses their implications for other sensory systems and brain structures, including the mammalian auditory system and the cerebellum. This chapter also reviews some additional functions that have been identified for the prominent and well-studied electric organ corollary discharge (EOCD) system of weakly electric mormyrid fish.
11.2 Electrosensory Systems and the Problem of Reafference
Studies of both passive and active electrosensory systems have demonstrated that reafference, defined as sensory stimulation related to an animal’s own behavior, drives responses in electroreceptors. In elasmobranchs (the group that includes sharks, skates, and rays), ventilatory movements of the gills modulate the fish’s own standing bioelectric field, which, in turn, modulates the firing of afferents innervating exquisitely sensitive ampullary electroreceptors that serve passive electrolocation in these fish (Montgomery and Bodznick 1999). In weakly electric mormyrid fish, the electric organ discharge (EOD) pulses (serving active electrolocation) have been shown to strongly affect ampullary electroreceptors involved in passive electrolocation (Bell and Russell 1978). Studies of the passive electrosensory system of mormyrid fish provided the first evidence for negative images in an electrosensory system and is discussed in detail in Sect. 11.4. The EOD of weakly electric fish sets up a self-generated electric field that is modulated by objects in the environment and drives responses in afferents innervating mormyromast electroreceptors for active electrolocation. In these fish, swimming movements alter the position of the electric organ relative to electroreceptors on the skin, causing modulations in the fish’s self-generated electrical field as large or larger than those due to objects in the environment (Sawtell and Williams 2008; Fotowat et al. 2013). Some species of mormyrid fish have highly mobile chin appendages used for foraging (Amey-Ozel et al. 2015). Because this appendage is densely covered with electroreceptors, its rapid movement during foraging behavior is also likely to be a major source of reafference (Engelmann et al. 2009).
The manner in which the brain solves the reafference problem likely depends both on the nature of the self-generated signals and how they compare with the external signals that the system has evolved to process. If the animal’s own behavior results in patterns of receptor activation that are very different from those due to external events, invariant spatial or temporal filtering strategies may contribute to removing reafference. Along these lines, a “common-mode rejection” mechanism for suppressing spatially uniform ventilatory reafference has been described in elasmobranchs (Montgomery 1984). Whereas electroreceptor afferents are strongly and uniformly modulated by ventilatory reafference, second-order neurons in the hindbrain show much weaker responses (Montgomery and Bodznick 1999). This difference is due, in part, to a commissural GABAergic inhibitory pathway that suppresses activity patterns that are shared by electroreceptors located on the opposite side of the body (Duman and Bodznick 1996). In many cases, however, the characteristics of reafference are similar to those of behaviorally relevant signals, necessitating more complex solutions.
Sperry (1950) and von Holst and Mittelstaedt (1950) performed a pioneering series of behavioral experiments in fish and flies that suggested that ambiguity in the origin of sensory stimulation could be resolved at central processing stages by integrating sensory information with additional signals related to the animal’s own movements and behavior such as motor corollary discharge. A challenge for subsequent neurophysiological and neuroanatomical studies was to pinpoint such signals in the brain. In the case of vision, where the question of how visual perceptual stability is maintained in the face of rapid eye movements has been extensively studied, these were termed extraretinal signals (Grusser 1986). Roles for both motor corollary discharge signals related to eye movements and ocular proprioception have been identified in maintaining stable and accurate visual perception in primates (Sun and Goldberg 2016). However, due to the complexity of the neocortical structures involved, circuit-level questions regarding how visual and extraretinal signals are integrated have been difficult to address. The convergence of peripheral sensory input with multiple streams of information related to movements and behavior, including both corollary discharge and proprioception, is a prominent feature of the first central stage of electrosensory processing in the brains of fish. The relative simplicity of these circuits and their close proximity to the sensory periphery has made it possible to gain a detailed mechanistic understanding of how these circuits solve the reafference problem.
Finally, it should be noted that changes in sensory input due to behavior can also convey useful information (Gibson 1979). This is particularly clear in active sensory systems, such as the active electrosensory systems of weakly electric fish in which the animal generates signals used for sensing. A brief example of how motor corollary discharge signals in mormyrid fish may aid in the processing of information contained in the fish’s own EOD is given in Sect. 11.5.2. Although not reviewed in depth here, electric fish are also a useful model system to address the general question of how an animal’s motor behavior may enhance sensory processing and perception. For example, when encountering a novel object, weakly electric fish engage in stereotyped patterns of movement termed probing motor acts (Toerring and Moller 1984). It has also been suggested that mormyrids use self-motion-derived electrosensory cues (analogous to optic flow in the visual system) to judge the distance of objects (Hofmann et al. 2017). Clearly, some components of sensory reafference are not canceled out and may, in fact, play critical roles in perception.
11.3 Convergence of Electrosensory and Behavior-Related Signals in Cerebellum-Like Structures
The first central stage of electrosensory processing in the brains of fish occurs in hindbrain structures that share numerous similarities with the cerebellum in terms of their evolution, development, patterns of gene expression, and circuitry (Bell 2002; Bell et al. 2008). The so-called cerebellum-like electrosensory processing structures discussed in this review are the dorsal octavolateral nucleus (DON) in elasmobranchs and the electrosensory lobe (ELL) of weakly electric mormyrid and gymnotiform fish (Bell and Maler 2005). Although strikingly similar in numerous respects, the electrosensory systems of these three groups of fish appear to have evolved independently (Finger et al. 1986). Cerebellum-like sensory structures are also found in other vertebrate sensory systems and include the dorsal cochlear nucleus (DCN) in the mammalian auditory system, the medial octavolateral nucleus (MON) in the mechanosensory lateral line system of fish, and the optic tectum in the visual system of teleost fish (Fig. 11.1).
Local circuits of some cerebellum-like structures (A–D), the teleost cerebellum (E), and the mammalian cerebellum (F). All cerebellum-like structures and the cerebellum receive input from a granule cell-parallel fiber system in a molecular layer (blue). Cerebellum-like structures receive a separate input from peripheral sensory receptors (orange) and the cerebellum receives a separate climbing fiber input from the inferior olive in the brainstem (orange). For all of the circuits shown, parallel fibers convey signals, such as motor corollary discharge and proprioception, relevant for predicting components of the peripheral sensory or climbing fiber input that are self-generated. Green, GABAergic Purkinje-like cells of the mormyrid electrosensory lobe (ELL) and mammalian dorsal cochlear nucleus (DCN) as well as the Purkinje cells of the teleost and mammalian cerebellums; White, excitatory efferent cells; CN, cerebellar nucleus; DGR, dorsal granular ridge; DON, dorsal octavolateral nucleus; EGp, eminentia granularis posterior; GCD, granule cell domain; IC, inferior colliculus; PE, preeminential nucleus
Primary afferent fibers from electroreceptors terminate in the deep layers of the DON and ELL where they form a map of the sensory surface. Principal cells of these structures have basilar dendrites that are affected either directly by electroreceptor afferents or indirectly via interneurons. The spiny apical dendrites of principal cells receive numerous excitatory inputs from the thin, unmyelinated axons of granule cells that course long distances through a molecular layer, like the parallel fibers in the molecular layer of the cerebellar cortex. The molecular layer also contains GABAergic interneurons that receive parallel fiber input and provide feedforward inhibitory input to principal cells, similar to the molecular layer interneurons of the cerebellum.
In the elasmobranch DON and gymnotiform ELL, the main site of integration of electroreceptor and parallel fiber inputs are glutamatergic output neurons that project to higher stages of electrosensory processing in the midbrain. In the mormyrid ELL, such integration occurs in both glutamatergic output neurons as well as in a more numerous class of GABAergic neurons known as medium ganglion (MG) cells (Meek et al. 1996, 1999). MG cells inhibit the output cells and hence occupy a position in the circuitry of cerebellum-like structures that is similar to that of the Purkinje cells in the cerebellum. This similarity is particularly clear for the teleost cerebellum where cerebellar output neurons are located adjacent to the Purkinje cells (as in the mormyrid ELL) instead of in a separate deep cerebellar nucleus (as in the cerebellum of most other vertebrates).
Instead of being located in a layer beneath the Purkinje cells, as in most vertebrate cerebella, granule cells in cerebellum-like structures are typically found in external granule cell masses. The granule cells themselves are similar in size and morphology to cerebellar granule cells. These granule cell masses are similar to the cerebellar granular layer in that they contain large GABAergic Golgi cells and, in some cases, a specialized class of glutamatergic interneuron known as the unipolar brush cell (UBC; Campbell et al. 2007; Borges-Merjane and Trussell 2015). Mossy fiber inputs to granule cells arise from numerous brain regions and convey a variety of signals including motor corollary discharge signals related to the EOD and ventilation and swimming movements; proprioceptive signals related to the movements and position of the tail, trunk, and fins; electrosensory input from higher processing stages; and input from other sensory modalities such as the mechanosensory lateral line, (see Bell 2002; Bell et al. 2008 for original references).
11.4 Mechanisms for Predicting and Canceling Self-Generated Sensory Input
In vitro and in vivo electrophysiological studies and computational modeling of the DON, the mormyrid ELL, and the gymnotiform ELL all point to a common functional logic for this organization (Bell et al. 1997a; Bell 2001). Namely, the granule cell-parallel fiber system conveys signals that are used to cancel out predictable components of the electrosensory input to principal cells, including those due to the animal’s own movements and behavior.
11.4.1 Negative Images: Neural Correlates for Sensory Predictions
Direct evidence for the generation and subtraction of predictions of electrosensory input patterns has been obtained from in vivo recordings of principal cells in the DON of elasmobranchs and the ELL of both mormyrid and gymnotiform fish (see Bell et al. 2008 for original references). In each case, pairing artificial electrosensory stimuli with central predictive signals (a proprioceptive or motor corollary discharge signal in the case of the mormyrid ELL; a proprioceptive or electrosensory signal in the case of the gymnotiform ELL; and a proprioceptive, electrosensory, or motor corollary discharge signal in the case of the elasmobranch DON) results in a marked decline in the response to the paired stimulus over a timescale of ~5–10 min (Fig. 11.2). Such changes cannot be explained by adaptation of peripheral receptors or fatigue of postsynaptic responses because they are not observed when the same electrosensory stimuli are delivered unpaired to central signals. Strikingly, these experiments also reveal changes in the response to the predictive signals alone (after turning the stimulus off) that resemble a negative image of the response to the previously paired (and now predicted) stimulus. The negative images develop over the same timescale as the decline in the paired response and are specific to the sign as well as to the spatial and temporal patterns of principal cell activity evoked by the stimulus.
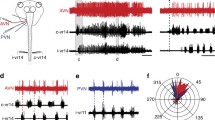
Formation of negative images of predicted sensory responses in three different cerebellum-like structures. A: raster display of the responses of a cell in the ampullary region of the mormyrid ELL. Each dot represents an action potential, and each row shows the spiking activity time-locked to each electric organ discharge (EOD) command (t0). At the beginning of the experiment (top), the command alone has no effect on the cell’s spiking activity. An electrosensory stimulus is then time-locked to the EOD command (vertical black line indicates onset), which evokes a pause-burst spiking response. After several minutes of pairing, the stimulus is turned off, and a response to command alone that was not present before the pairing and that is a negative image of the previously paired sensory response is revealed. By the end of the experiment (bottom), the cell no longer responds to command alone (as in the beginning of the experiment). From Bell (1986). B: raster display of spiking responses of a cell in the gymnotiform ELL. The tail is moved back and forth passively. Each row of dots shows the response to one cycle of movement. Initially, the tail bend has no effect on the cell. An electrosensory stimulus that evokes a burst-pause response is then delivered in phase with the movement. The electrosensory stimulus is turned off after several minutes of pairing, revealing a response to tail bending alone that was not present before the pairing and that is a negative image of the previously paired sensory response. From Bastian (1995). C: spiking responses of a cell in the elasmobranch DON. Each histogram shows the average response to one cycle of ventilation. Initially, the cell does not respond to the exhalation (Ex)-inhalation (In) ventilatory cycle of the fish (top histogram). An electrosensory stimulus that evokes a burst-pause is then delivered in phase with the ventilatory cycle. The response to ventilation plus the electrosensory stimulus decreases during 25 min of pairing. Turning off the electrosensory stimulus after pairing reveals the presence of a response to ventilation alone that was not present before and that is a negative image of the previously paired sensory response. From Montgomery and Bodznick (1994)
Further evidence that negative images reflect a memory-based process comes from studies of the passive electrosensory system of the mormyrid ELL. In the mormyrid ELL, negative images are induced by pairing the motor command to produce the EOD with an electrosensory stimulus; the emission of the actual EOD is blocked in these experiments by a paralytic agent. After turning the stimulus off, negative images decay on roughly the same timescale over which they are formed (5–10 min). However, if the EOD motor command is blocked by injection of the action potential blocker lidocaine into the EOD command nucleus, then negative images persist for at least 30 min. This finding suggests that the decay of negative images normally observed after pairing is not simply a passive “forgetting” but rather that negative images may be due to a persistent form of synaptic plasticity. Related experiments in the elasmobranch DON paired an electrosensory stimulus with brief bouts of passive fin movements, separated by longer periods of rest (Zhang and Bodznick 2008). Negative images formed under these conditions persisted after rest periods of up to 3 hours but were rapidly extinguished when passive movements were delivered without a stimulus. These experiments demonstrate how negative images could function in the context of episodic behaviors such as swimming.
The function of negative images is not restricted to canceling out self-generated electrosensory inputs. In the mormyrid and gymnotiform ELL, granule cells receive input from a higher stage of electrosensory processing, the midbrain preeminential nucleus (Bastian and Bratton 1990; von der Emde and Bell 1996). Such inputs allow for negative images to be formed based on electrosensory information. Experiments in both the gymnotiform ELL and the elasmobranch DON have shown that negative images are formed when strong, focal activation of principal cells is paired with a spatially diffuse electrosensory stimulus (Bodznick et al. 1999; Bastian et al. 2004). Studies of gymnotiform fish have suggested that such negative images serve to cancel out interference due to the EODs of other fish. More generally, negative images based on electrosensory information could serve to remove any persistent spatial or temporal correlations in electrosensory input.
The discovery of negative images in electrosensory systems provided a striking confirmation of the longstanding ideas of von Holst, Mittelstaedt, and others that central signals could be used to predict and cancel out reafference. These findings were of obvious functional importance because they provided a mechanism for selectively removing the effects of self-generated stimuli while maintaining sensitivity to external stimuli. As described in Sect. 11.4.2, a number of advantageous features of electrosensory systems, including their accessibility to detailed electrophysiological studies, have allowed for significant progress in understanding the mechanisms of negative image formation.
11.4.2 Sites and Synaptic Mechanisms of Negative Image Formation
Several lines of evidence indicate that the formation of negative images is due to plastic changes occurring within the cerebellum-like structures themselves. Pairing predictive signals with intracellular current injections in vivo results in the formation of negative images in principal cells in all three groups of fish, indicating that synaptic inputs to the recorded cell are plastic (Bell et al. 2008). Given the diversity of signals involved in negative image formation, synapses between parallel fibers and principal cells are the most natural candidate for the site of the plastic changes. Immunohistochemical studies have shown that N-methyl-d-aspartate (NMDA)-type glutamate receptors are present in the apical dendrites of principal cells in the DON of elasmobranchs and the ELL of both mormyrid and gymnotiform fish (Bell et al. 2008, Zhang and Bodznick 2010). NMDA receptors are known to play central roles in the induction of associative synaptic plasticity in many brain regions, including the hippocampus and cerebral cortex. Direct physiological evidence for plasticity at parallel fiber synapses with principal cells has been obtained in all three classes of fish (Bell et al. 2008; Harvey-Girard et al. 2010). In principal cells of the DON, pairing electrical stimulation of parallel fibers with an electrosensory stimulus results in depression of the response to parallel fiber stimulation alone. Pharmacological blockade of NMDA receptors disrupts negative image formation in vivo in both the elasmobranch DON and the mormyrid ELL (Zhang and Bodznick 2010; Enikolopov et al. 2018).
In vitro studies of the mormryid ELL provided evidence for the plasticity of parallel fiber synapses onto one class of principal cells, the MG cells. MG cells fire two types of action potentials, known as narrow and broad spikes (Grant et al. 1998). The narrow spikes occur at high rates and originate in the axon, whereas the broad spikes are infrequent, originate in the proximal dendrites, and backpropagate into the molecular layer. Hence, the two spike types in MG cells are similar in some respects to simple spikes and complex spikes in Purkinje cells (Sawtell et al. 2007). Repeated pairing of broad (but not narrow) spikes with electrical stimulation of parallel fibers results in persistent changes in the strength of parallel fiber-evoked excitatory postsynaptic potentials (EPSPs; Bell et al. 1997b). Critically, the polarity of the changes depends on the relative timing between the EPSP onset and the postsynaptic spike. EPSPs that preceded postsynaptic broad spikes by 50 ms or less are depressed, whereas those occurring at other delays are potentiated. The depression is dependent on postsynaptic calcium- and NMDA-type glutamate-receptor activation (Han et al. 2000). This was one of the first demonstrations of spike timing-dependent synaptic plasticity in the vertebrate brain (Markram et al. 2011).
A distinctive feature of the plasticity rule described in the mormyrid ELL is that presynaptic inputs that shortly precede, and hence could contribute to evoking a postsynaptic spike, are weakened. In more common Hebbian forms of plasticity, including those found in the hippocampus or neocortex, presynaptic inputs that precede a postsynaptic spike are strengthened. For this reason, plasticity in the ELL was referred to as anti-Hebbian. A similar form of anti-Hebbian plasticity occurs at parallel fiber synapses onto principal cells in the gymnotiform ELL (Harvey-Girard et al. 2010). In this case, brief bursts of pre- and postsynaptic spikes are required to induce synaptic depression and no potentiation is observed. It was immediately realized that such anti-Hebbian plasticity could potentially explain the negative image formation observed in principal cells in vivo. This intuition was formalized by computational models that showed how anti-Hebbian plasticity rules of the type demonstrated experimentally provide a simple and powerful mechanism for canceling principal cell responses that are predictable based on parallel fiber inputs (Nelson and Paulin 1995; Roberts and Bell 2000). Increases in principal cell firing that occur together with (i.e., can be predicted by) parallel fiber input are opposed by the weakening of parallel fiber synapses. Conversely, predictable decreases in principal cell firing are opposed by increases in parallel fiber synaptic strength.
11.4.3 Granule Cells Provide a Basis for Negative Image Formation
Modeling studies have highlighted the critical role of granule cells in providing the raw material out of which negative images are sculpted via parallel fiber synaptic plasticity. In the region of the mormyrid ELL involved in passive electrolocation, the ventrolateral zone (VLZ), negative images serve to cancel out responses evoked by the fish’s own EOD (Fig. 11.3). Although the EOD pulse itself is extremely brief (<0.5 ms), it evokes a bi- or triphasic pattern of activation in ampullary electroreceptors that lasts ~200 ms (Bell and Russell 1978). To cancel out the effects of the EOD, negative images in the VLZ must be temporally specific in relation to the fish’s EOD motor command. Numerous studies, including experiments in which an artificial electrosensory stimulus is delivered at different delays after the EOD command, have confirmed that this is indeed the case (Bell 1982; Bell et al. 1993). Negative images observed in such experiments are specific to the paired delay up to ~200 ms after the EOD command. Modeling studies explained these paradoxical findings based on anti-Hebbian plasticity acting on a set of granule cells, each of which is active at a different delay after the EOD command, forming a temporal delay line (Roberts and Bell 2000). In the model, these temporally specific responses provided a baseline of excitation out of which negative images could be sculpted via associative synaptic depression and nonassociative potentiation. This model raised the important questions of whether temporal representations actually exist in granule cells and, if so, how they are generated. Because the motor command to discharge the electric organ is a brief spike burst lasting just a few milliseconds, some cellular or circuit mechanism(s) would seemingly be required to generate the diversity of temporal response patterns required by the model.
Negative image formation in the mormyrid ELL. Medium ganglion (MG) cells (center, green) receive sensory input via electroreceptors (orange lines) along with motor corollary discharge input via granule cells (blue lines). To encode behaviorally relevant external events, the MG cell must cancel the sensory input due to the fish’s EOD (bottom left orange box). EOD command, time of the motor command that drives the EOD. Previous results have shown that this is accomplished by the generation and subtraction of a temporally specific negative image of the self-generated electrosensory input (bottom right green box). An experimentally observed form of spike timing-dependent plasticity (top right gray box) at synapses between granule cells and MG cells can explain the negative images if granule cells exhibit a diversity of temporal responses that span the 200 ms over which negative images can be formed (top left blue box). EPSP, excitatory postsynaptic potential
In vivo whole cell recordings from granule cells as well as additional cellular elements of the granule layer in mormyrid fish shed light on both of these questions (Kennedy et al. 2014). The recordings showed, as expected based on past results, that EOCD inputs to granule cells were highly stereotyped bursts restricted to short delays after the EOD command. In contrast, granule cell responses were more temporally diverse and delayed. A particular class of interneuron providing excitatory synaptic input to granule cells, the UBCs, appeared to be the source of the diverse and delayed responses. This is consistent with in vitro studies of UBCs in the mammalian cerebellum and cochlear nucleus that have found that UBCs possess a variety of synaptic and intrinsic properties capable of transforming brief synaptic inputs into more temporally diverse, sustained, or delayed output (Mugnaini et al. 2011). Importantly, granule cells do not form a perfect delay line like the one assumed in models. Instead, the majority show activity restricted to short delays, with a minority responding at longer delays. However, theoretical modeling shows that the anti-Hebbian synaptic plasticity rule measured in vitro acting on the granule cell responses recorded in vivo is indeed sufficient to explain the formation of temporally specific negative images to EOD-driven ampullary responses and can also account for previously unexplained features of negative images observed in in vivo recordings (Roberts 1999; Roberts and Bell 2000).
Recordings in the mormyrid ELL have also suggested that granule cells provide a higher dimensional recoding of their mossy fiber inputs, consistent with theories of cerebellar granule cell function (Litwin-Kumar et al. 2017). In addition to EOCD inputs described above, proprioceptive and skeletomotor corollary discharges reach the granule cell domain of ELL via mossy fibers originating in the spinal cord (Requarth and Sawtell 2014), similar to spinocerebellar pathways described in other vertebrates. Although each granule cell receives just a few (2–4) excitatory inputs from mossy fibers or UBCs, it was shown that these inputs may be of different types, e.g., a proprioceptive signal and an EOCD signal (Sawtell 2010). Such integration may allow granule cells to selectively encode specific combinations of events, such as a particular tail position and a particular time after the EOD command. Collectively, such granule cell representations may provide the raw material for forming more complex negative images, such as those that would be required to predict and cancel the electrosensory consequences of the rapid and intricate probing motor acts made by mormyrid fish when exploring a novel object (Toerring and Moller 1984).
11.4.4 Behavioral Significance of Negative Images
Although it was suggested at the time of their discovery that negative images serve to enhance the detection and processing of behaviorally relevant stimuli, an experimental demonstration of this was not provided until many years later (Enikolopov et al. 2018). Recordings of neural responses of ELL neurons to prey-like stimuli before, during, and after negative image formation directly demonstrated the time course and extent of improvements in the neural encoding of prey-like stimuli due to negative images. Weakly electric mormyrid fish increase their EOD rate when they detect a stimulus (Post and von der Emde 1999). This simple unconditioned behavior, known as the electromotor novelty response, was used to demonstrate improvements in the behavioral detection of prey during negative image formation. The time course of improvements in neural coding and behavioral detection both matched the time course of negative image formation. Finally, pharmacological manipulations of synaptic plasticity in ELL were shown to disrupt both the neural and behavioral detection of prey-like stimuli, providing a causal link between the mechanisms of negative image formation and behavioral function.
11.4.5 Questions for Future Research
There is much still to be learned about the mechanisms of negative image formation and sensory cancellation in electrosensory systems. Most previous experimental and theoretical work has focused on understanding how negative images and sensory cancellation can be explained by bidirectional plasticity of excitatory synapses between granule cells and principal cells. However, such single-neuron models are likely to be a gross oversimplification in that plasticity distributed across many neurons at multiple sites in the network are likely to underlie sensory cancellation. In this regard, a major remaining puzzle about the mormyrid ELL is the functional importance of the two different classes of principal cells on which electrosensory input and parallel fiber input converge: the Purkinje-like MG cells and the glutamatergic output cells. Although both cell types exhibit anti-Hebbian plasticity at their parallel fiber synapses, the functional logic of such an arrangement remains unclear. If anti-Hebbian plasticity at synapses between parallel fibers and MG cells cancels the effects of the fish’s own EOD, it would seem that the MG cells could play no role in canceling the effects of the EOD in the output cells that are their main synaptic targets. Interestingly, analogs of MG cells have not been described in the DON or the gymnotiform ELL but are present in the mammalian DCN, where they are known as cartwheel cells (Berrebi et al. 1990). The respective roles of plasticity of parallel fiber synapses onto cartwheel versus DCN output cells are similarly unknown (see Sect. 11.6.1).
Another question for future work relates to how negative images operate under more naturalistic circumstances in which electrosensory reafference is more varied and complex than in the limited experimental settings studied in the past, i.e., immobilized preparations. Under natural conditions, electrosensory reafference depends on potentially complex interactions between electromotor behavior (e.g., EOD pulse rate), the movements of the fish, and the nearby environment. For example, the same change in tail position relative to electroreceptors on the skin is expected to have different effects on the fish’s electrical field depending on whether the fish is in open water or hiding in a crevice (a nonconducting boundary; Pereira et al. 2005). In the simplest view, negative images could represent a prediction of the electrosensory consequences of behavior averaged over some relatively long timescale. Although compatible with what is known about ELL, such “average” predictions might have limited utility if the characteristics of reafference are highly dependent on behavioral context on much shorter timescales. The fact that granule cells receive many different streams of information, including extensive feedback from higher stages of electrosensory processing, suggests the possibility that negative images may possess specificity for certain contexts and/or the capacity to generalize appropriately from one context to another. Recordings from freely swimming fish may allow such questions to be addressed experimentally (Fotowat et al. 2013).
11.5 Additional Functions of Electric Organ Corollary Discharge in Mormyrid Fish
Corollary discharge pathways associated with the motor command to discharge the electric organ are particularly prominent and accessible to study in pulse-type mormyrid fish (Fig. 11.4A; Bell et al. 1983). The role of EOCD inputs in negative image formation and cancellation of reafference in ELL was discussed in Sect. 11.4. However, the role of EOCD signals are likely to be more diverse as evidenced by anatomical and electrophysiological data suggesting that they impact many regions of the mormyrid brain, including higher brain regions such as the telencephalon (Prechtl et al. 1998) and the hypertrophied cerebellum (Russell and Bell 1978). EOCD signals also likely impact the electromotor system that controls the rate and sequence of emission of the EOD (von der Emde et al. 2000; Carlson 2002). Unfortunately, very few studies have addressed these issues. As discussed in Sects. 11.5.1 and 11.5.2, additional functions of EOCD pathways have been identified in relation to the early processing stages of electrocommunication and active electrolocation in mormyrids.
Functions of electric organ corollary discharge (EOCD) in mormyrid fish. A: activation of the EOD command nucleus elicits an EOD. This reafferent stimulus evokes responses in afferents of all three electroreceptor types. Concurrently, activation of the EOD command nucleus (green lines) drives electric organ corollary discharge (EOCD) input (purple lines) to the first central processing stages associated with each type of electroreceptor. The role of EOCD input in cancellation (via modifiable synapses) of sensory reafference in the cerebellum-like circuits of mormyrid ELL is reviewed (along with examples from other cerebellum-like electrosensory structures) in Sect. 11.4. Section 11.5 reviews two additional functions accomplished by EOCD input in the nucleus of ELL (nELL) and the granular cell layer of ELL in mormyrid fish. Adapted from Bell (1989). B: knollenorgan afferents (green) form mixed chemical-electrical synapses on the output cells of nELL (yellow), which are inhibited by EOCD inputs precisely timed to gate out responses to the fish’s own EOD in electrocommunication. C: mormyromast afferents form mixed chemical-electrical synapses on the granular cells of the ELL (yellow) and elicit inhibition via GABAergic large multipolar interneurons (LMI; gray). In addition to afferent input, granular cells receive a precisely timed EOCD-driven spike that seems to function in enhancing and recoding sensory responses to the fish’s own EOD in active electrolocation
11.5.1 Inhibitory Gating of Self-Generated Input in the Electrocommunication System
In mormyrid fish, the detection and processing of EODs of other fish is mediated by a specialized class of electroreceptors known as knollenorgans, which terminate in a dedicated hindbrain nucleus, the nucleus of the ELL (nELL). The nELL is anatomically separate from the ELL, which is the first processing stage for active and passive electrolocation. Knollenorgans respond to the EODs of other fish by firing a single action potential. However, the fish’s own EOD is far above the threshold of activation of knollenorgans, which must be sensitive enough to detect EODs of other fish at some distance to be useful for communication. How does the fish distinguish its own pulses from those of other fish? Knollenorgan afferents form mixed chemical-electrical synapses onto nELL output cells. nELL output cells also receive input from GABAergic neurons of the ventral lemniscus (Fig. 11.4B; Bell et al. 1981; Mugnaini and Maler 1987). Intracellular recordings from nELL output cells reveal two types of synaptic events: (1) EPSPs driven by stimulation of knollenorgan receptors and (2) inhibitory postsynaptic potentials time locked to the EOD motor command, which are driven by EOCD inputs to the nELL (Bell and Grant 1989). The EOCD-evoked inhibition is brief, precisely timed, and appears to be entirely fixed or nonplastic. Critically, knollenorgan responses to reafferent input arrive during the peak of EOCD-evoked inhibition and hence fail to evoke an action potential in nELL neurons. Different nELL output neurons receive knollenorgan afferent input at slightly different latencies (although response latencies are fixed for each afferent) due to variable axonal lengths for receptors located on different regions of the body surface. Remarkably, a corresponding variation in the timing of EOCD-evoked inhibition is observed, which ensures a tight matching of the timing of the EOCD inhibition relative to reafferent input. Although remarkably simple, this corollary discharge gating strategy is extremely effective in nELL because of the brevity and fixed latency of the reafference.
11.5.2 Roles for Corollary Discharge in Active Electrosensory Processing in Mormyrid Fish
In contrast to the case of the passive electrosensory and the electrocommunication systems, the fish’s own EOD is the signal of interest for the active electrosensory system. Objects in the environment with conductivity different from the water alter patterns of EOD-induced current flowing through the fish’s skin. These changes cast electrical images on the skin that are encoded by a specialized class of tuberous electroreceptors known as mormyromasts. Mormyromasts typically fire one to four action potentials following each EOD with a precise latency that is a function of EOD amplitude at the receptor. Increases in EOD amplitude (as would be caused by a conducting object such as prey) cause decreases in spike latency, whereas decreases in EOD amplitude (as would be caused by a nonconducting object such as a rock) cause an increase in spike latency (Szabo and Hagiwara 1967). The timing of spikes is extremely precise such that submillisecond shifts in spike latency convey information about EOD amplitude (Sawtell and Williams 2008). Mormyromast afferents synapse onto a class of small, highly numerous interneurons in the deep layers of the ELL, known as granular cells (not to be confused with the granule cells that send parallel fibers to the principal cells discussed in Sect. 11.4.3; Fig. 11.4C; Meek et al. 1999; Zhang et al. 2007). Several lines of evidence indicate that the granular cells also receive precisely timed excitatory EOCD input (Bell 1990; Bell and von der Emde 1995). Rather than blocking reafferent input, as in the electrocommunication system, this input appears to enhance EOD-evoked afferent input. Evidence for this comes both from physiological studies showing that ELL principal cells respond much more strongly when stimuli are delivered around the time of the EOD command and from behavioral studies showing that fish more readily detect and respond to an electrosensory stimulus when it is delivered within ~12 ms of the EOD motor command (Hall et al. 1995). EOCD inputs to granular cells have also been hypothesized to play a role in “decoding” the tiny shifts in spike latency of mormyromast afferent input. Behavioral experiments have shown that fish can detect a 0.1-ms shift in the latency of an electrosensory stimulus (causing a shift in mormyromast spike latency) relative to the fish’s own EOD motor command (Hall et al. 1995). Recordings from ELL output cells show that information about object-induced changes in EOD amplitude is coded by changes in spike number (as well as timing; Sawtell and Williams 2008). Such a transformation could be achieved, at least in part, by integrating precisely timed afferent spikes with a precisely timed excitatory EOCD input, although confirmation of this awaits direct in vivo recordings from granular cells.
11.6 Implications for Other Systems
The studies of reafference cancellation in electrosensory systems described in Sect. 11.4 are relevant to a number of general issues in neuroscience. Predicting sensory events is a critical function of the nervous system as a whole and likely involves a diverse set of cellular and circuit mechanisms distributed across many brain regions. Studies of cerebellum-like structures provide a useful example of how the mechanisms of such functions may be elucidated. Although sensory systems are typically studied in isolation from one another and also in isolation from motor systems, this reflects a methodological convenience more than a biological reality. Cerebellum-like structures in fish offer a system in which interactions between peripheral sensory input and signals from other sensory modalities and motor signals are both prominent and well characterized. Finally, forging links between synaptic plasticity, well-defined neural circuits, and systems-level function is a primary, but rarely achieved, goal of neuroscience. Cerebellum-like circuits in fish provide a foremost example of a vertebrate system in which it has been possible to make such links. In addition to these broad implications, studies of cerebellum-like electrosensory structures in fish offer more specific insights into a number of similar structures including the cerebellum-like DCN of mammals and the cerebellum itself.
11.6.1 Reafference Cancellation in a Cerebellum-Like Circuit in the Auditory System
The DCN at the first stage of auditory processing in mammals is a cerebellum-like structure and shares many similarities with the cerebellum-like structures in fish discussed in Sects. 11.3 and 11.4 (Fig. 11.1D; Oertel and Young 2004; Bell et al. 2008). Fusiform cells are the major efferent cell type of the DCN (Cant 1992). Their basilar dendrites are contacted by primary afferent fibers from the cochlea, which form a tonotopic map in the deeper layers below the molecular layer. The fusiform cells extend their spine-covered apical dendrites up into the molecular layer where they are contacted by parallel fibers. The parallel fibers arise from granule cells located around the margins of the nucleus. The cartwheel cell is a second type of principal cell in the DCN. These cells are considered Purkinje-like in that they are GABAergic, have extensive spine-covered dendrites in the molecular layer, and share patterns of gene expression with Purkinje cells. Purkinje cells and cartwheel cells are similarly affected by genetic mutations in several mouse strains (Berrebi et al. 1990). Cartwheel cells inhibit the fusiform cells. Similarities between the local circuits of the mormyrid ELL and the teleost cerebellum were noted in Sect. 11.3. The DCN shares these similarities in that the parallel fibers of the DCN, like those of the mormyrid ELL and the teleost cerebellum, pass through and excite the dendrites of both efferent cells and Purkinje-like cells.
Movements of the animal’s pinna, head, or body have predictable effects on how the cochlea responds to an external sound source, and orofacial behaviors such as chewing, licking, and vocalization will also have predictable consequences on auditory input. The granule cells of the DCN receive various types of input that provide information about such behaviors (see Bell 2002; Oertel and Young 2004 for original references). Thus, the signals conveyed by parallel fibers in the DCN molecular layer could generate predictions about changes in afferent activity from the cochlea, as in other cerebellum-like structures. In vitro studies have revealed parallel fiber synaptic plasticity mechanisms remarkably similar to those found in cerebellum-like structures in fish (Tzounopoulos et al. 2004, 2007). For example, in both the mormyrid ELL and the DCN, plasticity at parallel fiber synapses onto Purkinje-like cells (MG cells in the ELL and cartwheel cells in the DCN) is anti-Hebbian, NMDA receptor-dependent, presynaptically expressed, and reversed by a timing-independent form of parallel fiber synaptic potentiation.
The numerous striking similarities between the circuitry and synaptic plasticity of DCN and cerebellum-like structures in fish suggest that they may perform similar functions. Evidence for this hypothesis was provided by a study in mice showing that self-generated sounds related to licking behavior drove much stronger responses in neurons of the ventral cochlear nucleus than in putative output neurons of the DCN despite both classes showing comparable sensitivity to external sounds, even during licking (Singla et al. 2017). Cancellation of reafference in this system depended, at least in part, on nonauditory signals conveyed by the parallel fiber system. Additionally, repeated pairing of an external sound at a fixed delay relative to licking led to a gradual reduction in the response to the paired sound, similar to cancellation of electrosensory stimuli paired with behavior-related signals in principal cells in fish. Although additional studies are needed, these results are consistent with a conserved reafference cancellation function for cerebellum-like structures in fish and mammals.
11.6.2 Implications for Cerebellar Function
The operation of cerebellum-like circuits associated with electrosensory processing in fish (and perhaps also those associated with mammalian auditory processing) appear similar in important respects to those in the cerebellum. Bidirectional plasticity at parallel fiber synapses has been linked to the formation of negative images of predicted sensory input in cerebellum-like structures and to motor learning in the mammalian cerebellum (Ito 1984). In both cases, plasticity acts to alter principal or Purkinje cell responses to parallel fiber input under the guidance of a separate nonplastic input. In the cerebellum, plasticity and learning are supervised by climbing fiber input from the inferior olive, whereas in cerebellum-like structures, the nonplastic signal is the peripheral sensory input itself. Anti-Hebbian plasticity of parallel fiber synapses in cerebellum-like structures generates negative images, which act within principal cells to oppose the effects of predictable electrosensory input. Plasticity of parallel fiber synapses onto Purkinje cells shapes what could be considered negative images of climbing fiber inputs. However, the main effect of such changes is not to directly cancel the effects of climbing fiber input within the Purkinje cells but instead to alter its simple spike firing patterns and thereby influence downstream neurons, i.e., those in the deep cerebellar or vestibular nuclei.
The similarities between cerebellar plasticity and negative image formation are nicely illustrated by the changes in Purkinje cell responses observed during delay eyelid conditioning. Delay eyelid conditioning is a classical conditioning paradigm in which a neutral stimulus, e.g., a tone, is paired with a periorbital shock or puff of air to the eye. Extensive past work has shown that information about the neutral stimulus, or conditioned stimulus (CS), is conveyed via the mossy fiber-granule cell system and information about the periorbital shock or puff of air, the unconditioned stimulus (US), is conveyed via climbing fibers (Medina et al. 2000b; Ohyama et al. 2003). After repeated CS-US pairings, animals blink their eyes in response to the CS alone, in anticipation of the US. The learning of this conditioned response is cerebellum dependent. Such pairing also leads to the emergence of pauses in Purkinje cells, the timing of which is matched to the CS-US delay (Jirenhed and Hesslow 2011; Halverson et al. 2015). Such pauses are believed to drive the conditioned response by releasing cerebellar nucleus neurons from inhibition. Leading models of eyelid conditioning suggest that such Purkinje cell pauses emerge as a result of bidirectional plasticity at parallel fiber synapses such that synapses immediately preceding the US-evoked climbing fiber are weakened, whereas others are strengthened (Medina et al. 2000a; Medina and Mauk 2000; but see Johansson et al. 2014). Work in the mormyrid ELL provides a mechanism for how a brief signal (like the CS) can be recoded to generate a diversity of responses to the CS. Hence models that explain temporally specific learning and Purkinje cell responses in the context of eyelid conditioning closely resemble models of temporally specific negative image formation in mormyrid fish.
These similarities also extend to Marr-Albus and adaptive filter models of the cerebellum (Fujita 1982). In a proposal inspired in part by negative images and sensory cancellation in electrosensory systems, it was suggested that anti-Hebbian plasticity could improve vestibular ocular reflex performance by removing correlations between mossy fiber inputs signaling eye movement motor commands and sensory error signals, i.e., retinal slip, conveyed by climbing fibers (Dean et al. 2002).
The function of some regions of the cerebellum may also be similar to that described for cerebellum-like structures in fish. A role for the cerebellum in the cancellation of self-generated sensory inputs has been demonstrated in regions of the primate cerebellum involved in processing vestibular information. Whereas primary vestibular afferents respond identically to passive and active, i.e., self-generated head, movements, neurons in the rostral fastigial nucleus have been found that respond selectively to passive head movements (Brooks and Cullen 2013). When the relationship between the intended head movement and its vestibular consequences was abruptly altered, cerebellar neurons showed sensitivity to the now unexpected sensory consequences of self-generated movements (Brooks et al. 2015). This sensitivity declined as the animal adapted to the new relationship between motor commands and head movements. In addition to processing vestibular sensory input, many sensory areas of the brain are interconnected with the cerebellum (Baumann et al. 2015). However, the functional role of the cerebellum in sensory processing remains unclear in most cases. Based on studies of cerebellum-like structures in fish, cancellation of predictable sensory inputs could be suggested as one such function.
In the context of motor control, a leading idea is that the cerebellum is involved in generating so-called forward models (Ebner and Pasalar 2008; Machado et al. 2015). In a forward model, copies of a motor command are conveyed to the cerebellum together with information about the current state of the system, such as positions and velocities of the limbs. The cerebellum then generates a prediction about the sensory consequences of the commanded motor act in the current context. Taking into account all that is known about the current state of the system, a forward model that predicts the sensory consequences of a motor command allows fast, coordinated movement sequences. Classical symptoms of cerebellar damage, such as decomposition of movement, slowness, and tremor, can all be understood as due to the absence of predictive forward models and reliance on peripheral feedback (Nixon and Passingham 2001; Bastian 2006). Quantitative effects of Purkinje cell degeneration in mice can be understood in terms of a failure of forward model predictions (Machado et al. 2015). Furthermore, electrophysiological studies in nonhuman primates suggest that the Purkinje cell output from large regions of the cerebellar hemispheres is indeed more tightly coupled to predictions about consequences of the movement than to the motor commands themselves (Pasalar et al. 2006). Although the forward model hypothesis seems plausible, what remains missing in the cerebellum is an understanding of how forward models are generated at the circuit level.
Examples of what are, in effect, forward models in the cerebellum-like structures of mormyrid and elasmobranch fish are described in Sect. 11.4. In these systems, corollary discharge signals come to elicit a prediction about the sensory input pattern that is expected to follow the motor command. As discussed, circuit mechanisms for generating such forward models are fairly well understood in cerebellum-like structures, the key ingredients being an appropriate plasticity rule acting on a sufficiently rich set of motor corollary discharge signals conveyed by granule cells. Hence, studies of electrosensory systems provide a proof of concept that forward models can indeed be generated within structures like the cerebellum.
11.7 Conclusion
Studies of cerebellum-like structures associated with electrosensory processing in fish have yielded unique insights into the fundamental question of how the nervous system distinguishes self-generated from external sensory input, including a relatively complete mechanistic account of how copies of motor commands are transformed into predictions of sensory events. These accounts are also notable in that they provide an understanding of how synaptic plasticity operating in a well-defined circuit performs a complex and behaviorally relevant computation. Insights from these studies are likely to extend to other cerebellum-like sensory structures, including those found in the mammalian auditory system, as well as to the cerebellum itself.
References
Amey-Ozel M, von der Emde G, Engelmann J, Grant K (2015) More a finger than a nose: the trigeminal motor and sensory innervation of the Schnauzenorgan in the elephant-nose fish Gnathonemus petersii. J Comp Neurol 523(5):769–789
Bastian J (1995) Pyramidal cell plasticity in weakly electric fish: a mechanism for attenuating responses to reafferent electrosensory inputs. J Comp Physiol A 176:63–78
Bastian AJ (2006) Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16(6):645–649
Bastian J, Bratton B (1990) Descending control of electroreception. I Properties of nucleus praeeminentialis neurons projecting indirectly to the electrosensory lateral line lobe. J Neurosci 10:1226–1240
Bastian J, Chacron MJ, Maler L (2004) Plastic and nonplastic pyramidal cells perform unique roles in a network capable of adaptive redundancy reduction. Neuron 41(5):767–779
Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M, Moulton EA, Paulin MG, Pavlova MA, Schmahmann JD, Sokolov AA (2015) Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum 14(2):197–220
Bell C, Bodznick D, Montgomery J, Bastian J (1997a) The generation and subtraction of sensory expectations within cerebellum-like structures. Brain Behav Evol 50:17–31
Bell C, von der Emde G (1995) Electric organ corollary discharge pathways in mormyrid fish. II The medial juxtalabar nucleus. J Comp Physiol A 177:463–479
Bell CC (1982) Properties of a modifiable efference copy in electric fish. J Neurophysiol 47:1043–1056
Bell CC (1986) Duration of plastic change in a modifiable efference copy. Brain Res 369:29–36
Bell CC (1989) Sensory coding and corollary discharge effects in mormyrid electric fish. J Exp Biol 146:229–253
Bell CC (1990) Mormyromast electroreceptor organs and their afferents in mormyrid electric fish: II. Intra-axonal recordings show initial stages of central processing. J Neurophysiol 63:303–318
Bell CC (2001) Memory-based expectations in electrosensory systems. Curr Opin Neurobiol 11:481–487
Bell CC (2002) Evolution of cerebellum-like structures. Brain Behav Evol 59:312–326
Bell CC, Caputi A, Grant K, Serrier J (1993) Storage of a sensory pattern by anti-Hebbian synaptic plasticity in an electric fish. Proc Nat Acad Sci 90:4650–4654
Bell CC, Finger TE, Russell CJ (1981) Central connections of the posterior lateral line lobe in mormyrid fish. Exp Brain Res 42:9–22
Bell CC, Grant K (1989) Corollary discharge inhibition and preservation of temporal information in a sensory nucleus of mormyrid electric fish. J Neurosci 9:1029–1044
Bell CC, Han V, Sawtell NB (2008) Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci 31:1–24
Bell CC, Han VZ, Sugawara S, Grant K (1997b) Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature 387:278–281
Bell CC, Libouban S, Szabo T (1983) Pathways of the electric organ discharge command and its corollary discharges in mormyrid fish. J Comp Neurol 216:327–338
Bell CC, Maler L (2005) Central neuroanatomy of electrosensory systems in fish. In: Bullock TH, Hopkins CD, Popper AN, Fay RR (eds) Electroreception. Springer, New York, pp 68–111
Bell CC, Russell CJ (1978) Effect of electric organ discharge on ampullary receptors in a mormyrid. Brain Res 145:85–96
Berrebi AS, Morgan JI, Mugnaini E (1990) The Purkinje cell class may extend beyond the cerebellum. J Neurocytol 19(5):643–654
Bodznick D, Montgomery JC, Carey M (1999) Adaptive mechanisms in the elasmobranch hindbrain. J Exp Biol 202:1357–1364
Borges-Merjane C, Trussell LO (2015) ON and OFF unipolar brush cells transform multisensory inputs to the auditory system. Neuron 85(5):1029–1042
Brooks JX, Carriot J, Cullen KE (2015) Learning to expect the unexpected: rapid updating in primate cerebellum during voluntary self-motion. Nat Neurosci 18(9):1310–1317
Brooks JX, Cullen KE (2013) The primate cerebellum selectively encodes unexpected self-motion. Curr Biol 23(11):947–955
Campbell HR, Meek J, Zhang J, Bell CC (2007) Anatomy of the posterior caudal lobe of the cerebellum and the eminentia granularis posterior in a mormyrid fish. J Comp Neurol 502(5):714–735
Cant NB (1992) The cochlear nucleus: neuronal types and their synaptic organization. In: Webster DB, Popper AN, Fay RR (eds) The mammalian auditory pathway: neuroanatomy. Springer, New York, pp 66–116
Carlson BA (2002) Neuroanatomy of the mormyrid electromotor control system. J Comp Neurol 454:440–455
Churchland PS, Ramachandran VS, Sejnowski TJ (1994) A critique of pure vision. In: Koch C, Davis JL (eds) Large-scale neuronal theories of the brain. MIT Press, Cambridge, pp 23–74
Crapse TB, Sommer MA (2008) Corollary discharge across the animal kingdom. Nat Rev Neurosci 9(8):587–600
Dean P, Porrill J, Stone JV (2002) Decorrelation control by the cerebellum achieves oculomotor plant compensation in simulated vestibulo-ocular reflex. Proc Biol Sci 269(1503):1895–1904
Duman CH, Bodznick D (1996) A role for GABAergic inhibition in electrosensory processing and common mode rejection in the dorsal nucleus of the little skate, Raja erinacea. J Comp Physiol A 179:797–807
Ebner TJ, Pasalar S (2008) Cerebellum predicts the future motor state. Cerebellum 7(4):583–588
Engelmann J, Nobel S, Rover T, Emde G (2009) The Schnauzenorgan-response of Gnathonemus petersii. Front Zool 6:21
Enikolopov AG, Abbott LF, Sawtell NB (2018) Internally generated predictions enhance neural and behavioral detection of sensory stimuli in an electric fish. Neuron 99(1):135–146
Finger TE, Bell CC, Carr C (1986) Comparisons among electroreceptive teleosts: why are the electrosensory systems so similar? In: Bullock TH, Heiligenberg W (eds) Electroreception. Wiley, New York, pp 465–481
Fotowat H, Harrison RR, Krahe R (2013) Statistics of the electrosensory input in the freely swimming weakly electric fish Apteronotus leptorhynchus. J Neurosci 33(34):13758–13772
Fujita M (1982) Adaptive filter model of the cerebellum. Biol Cybern 45(3):195–206
Gibson JJ (1979) The ecological approach to visual perception. Houghton Mifflin, Boston
Grant K, Sugawara S, Gomez L, Han VZ, Bell CC (1998) The Mormyrid electrosensory lobe in vitro: physiology and pharmacology of cells and circuits. J Neurosci 18:6009–6025
Grusser OJ (1986) Interaction of efferent and afferent signals in visual perception: a history of ideas and experimental paradigms. Acta Psychol 63:3–21
Hall JC, Bell C, Zelick R (1995) Behavioral evidence of a latency code for stimulus intensity in mormyrid electric fish. J Comp Physiol A 177:29–39
Halverson HE, Khilkevich A, Mauk MD (2015) Relating cerebellar purkinje cell activity to the timing and amplitude of conditioned eyelid responses. J Neurosci 35:7813–7832
Han VZ, Grant G, Bell CC (2000) Reversible associative depression and nonassociative potentiation at a parallel fiber synapse. Neuron 27:611–622
Harvey-Girard E, Lewis J, Maler L (2010) Burst-induced anti-Hebbian depression acts through short-term synaptic dynamics to cancel redundant sensory signals. J Neurosci 30:6152–6169
Hofmann V, Sanguinetti-Scheck JI, Gomez-Sena L, Engelmann J (2017) Sensory flow as a basis for a novel distance cue in freely behaving electric fish. J Neurosci 37:302–312
Ito M (1984) The cerebellum and neural control. Raven Press, New York
Jirenhed DA, Hesslow G (2011) Learning stimulus intervals--adaptive timing of conditioned purkinje cell responses. Cerebellum 10(3):523–535
Johansson F, Jirenhed DA, Rasmussen A, Zucca R, Hesslow G (2014) Memory trace and timing mechanism localized to cerebellar Purkinje cells. Proc Natl Acad Sci U S A 111(41):14930–14934
Kennedy A, Wayne G, Kaifosh P, Alvina K, Abbott LF, Sawtell NB (2014) A temporal basis for predicting the sensory consequences of motor commands in an electric fish. Nat Neurosci 17:416–422
Litwin-Kumar A, Harris KD, Axel R, Sompolinsky H, Abbott LF (2017) Optimal degrees of synaptic connectivity. Neuron 93:1153–1164
Machado AS, Darmohray DM, Fayad J, Marques HG, Carey MR (2015) A quantitative framework for whole-body coordination reveals specific deficits in freely walking ataxic mice. elife 4:e07892
Markram H, Gerstner W, Sjostrom PJ (2011) A history of spike-timing-dependent plasticity. Front Synaptic Neurosci 3:4
Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD (2000a) Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J Neurosci 20:5516–5525
Medina JF, Mauk MD (2000) Computer simulation of cerebellar information processing. Nat Neurosci 3:1205–1211
Medina JF, Nores WL, Ohyama T, Mauk MD (2000b) Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol 10:717–724
Meek J, Grant K, Bell C (1999) Structural organization of the mormyrid electrosensory lateral line lobe. J Exp Biol 202:1291–1300
Meek J, Grant K, Sugawara S, Hafmans TGM, Veron M, Denizot JP (1996) Interneurons of the ganglionic layer in the mormyrid electrosensory lateral line lobe: morphology, immunocytochemistry, and synaptology. J Comp Neurol 375:43–65
Montgomery JC (1984) Noise cancellation in the electrosensory system of the thornback ray; common mode rejection of input produced by the animal's own ventilatory movement. J Comp Physiol A 155:103–111
Montgomery JC, Bodznick D (1994) An adaptive filter that cancels self-induced noise in the electrosensory and lateral line mechanosensory systems of fish. Neurosci Lett 174:145–148
Montgomery JC, Bodznick D (1999) Signals and noise in the elasmobranch electrosensory system. J Exp Biol 202:1349–1355
Mugnaini E, Maler L (1987) Cytology and immunohistochemistry of the nucleus exterlateralis anterior of the mormyrid brain: possible role of GABAergic synapses in temporal analysis. Anat Embryol 176:313–336
Mugnaini E, Sekerkova G, Martina M (2011) The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev 66:220–245
Nelson ME, Paulin MG (1995) Neural simulations of adaptive reafference suppression in the elasmobranch electrosensory system. J Comp Physiol A 177:723–736
Nixon DP, Passingham RE (2001) Predicting sensory events: the role of the cerebellum in motor learning. Exp Brain Res 138:251–257
Oertel D, Young ED (2004) What's a cerebellar circuit doing in the auditory system? Trends Neurosci 27:104–110
Ohyama T, Nores WL, Murphy M, Mauk MD (2003) What the cerebellum computes. Trends Neurosci 26:222–227
Pasalar S, Roitman AV, Durfee WK, Ebner TJ (2006) Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9:1404–1411
Pereira AC, Centurion V, Caputi AA (2005) Contextual effects of small environments on the electric images of objects and their brain evoked responses in weakly electric fish. J Exp Biol 208:961–972
Post N, von der Emde G (1999) The ‘novelty response’ in an electric fish: response properties and habituation. Physiol Behav 68:115–128
Prechtl JC, von der Emde G, Wolfart J, Karamursel S, Akoev GN, Andrianov YN, Bullock TH (1998) Sensory processing in the pallium of a mormyrid fish. J Neurosci 18:7381–7393
Requarth T, Sawtell NB (2014) Plastic corollary discharge predicts sensory consequences of movements in a cerebellum-like circuit. Neuron 82(4):896–907
Roberts PD (1999) Computational consequences of temporally asymmetric learning rules: I. Differential hebbian learning. J Comp Neurosci 7:235–246
Roberts PD, Bell CC (2000) Computational consequences of temporally asymmetric learning rules: II. Sensory image cancellation. J Comput Neurosci 9(1):67–83
Russell CJ, Bell CC (1978) Neuronal responses to electrosensory input in the mormyrid valvula cerebelli. J Neurophysiol 41:1495–1510
Sawtell NB (2010) Multimodal integration in granule cells as a basis for associative plasticity and sensory prediction in a cerebellum-like circuit. Neuron 66(4):573–584
Sawtell NB, Williams A (2008) Transformations of electrosensory encoding associated with an adaptive filter. J Neurosci 28(7):1598–1612
Sawtell NB, Williams A, Bell CC (2007) Central control of dendritic spikes shapes the responses of Purkinje-like cells through spike timing-dependent synaptic plasticity. J Neurosci 27:1552–1565
Singla S, Dempsey C, Warren R, Enikolopov AG, Sawtell NB (2017) A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds. Nat Neurosci 20:943–950
Sperry RW (1950) Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43:482–489
Sun LD, Goldberg ME (2016) Corollary discharge and oculomotor proprioception: cortical mechanisms for spatially accurate vision. Annu Rev Vis Sci 2:61–84
Szabo T, Hagiwara S (1967) A latency-change mechanism involved in sensory coding of electric fish (mormyrids). Physiol Behav 2:331–335
Toerring MJ, Moller P (1984) Locomotor and electric displays associated with electrolocation during exploratory behavior in mormyrid fish. Behav Brain Res 12:291–306
Tzounopoulos T, Kim Y, Oertel D, Trussell LO (2004) Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7:719–725
Tzounopoulos T, Rubio ME, Keen JE, Trussell LO (2007) Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron 54:291–301
von der Emde G, Bell C (1996) Nucleus preeminentialis of mormyrid fish, a center for recurrent electrosensory feedback. I. Electrosensory and corollary discharge responses. J Neurophysiol 76:1581–1596
von der Emde G, Sena LG, Niso R, Grant K (2000) The midbrain precommand nucleus of the mormyrid electromotor network. J Neurosci 20:5483–5495
von Holst E, Mittelstaedt H (1950) The reafference principle. Naturwissenschaften 37:464–476
Zhang J, Han VZ, Meek J, Bell CC (2007) Granular cells of the mormyrid electrosensory lobe and postsynaptic control over presynaptic spike occurrence and amplitude through an electrical synapse. J Neurophysiol 9(3):2191–2203
Zhang Z, Bodznick D (2008) Plasticity in a cerebellar-like structure: suppressing reafference during episodic behaviors. J Exp Biol 211:3720–3728
Zhang Z, Bodznick D (2010) The importance of N-methyl-D-aspartate (NMDA) receptors in subtraction of electrosensory reafference in the dorsal nucleus of skates. J Exp Biol 213:2700–2709
Acknowledgments
This work was supported by grants from the National Science Foundation, the National Institutes of Health, and the Irma T. Hirschl Trust to Nathaniel B. Sawtell.
Compliance with Ethics Requirements
Krista Perks declares that she has no conflict of interest.
Nathaniel B. Sawtell declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Perks, K., Sawtell, N.B. (2019). Influences of Motor Systems on Electrosensory Processing. In: Carlson, B., Sisneros, J., Popper, A., Fay, R. (eds) Electroreception: Fundamental Insights from Comparative Approaches. Springer Handbook of Auditory Research, vol 70. Springer, Cham. https://doi.org/10.1007/978-3-030-29105-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-29105-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-29104-4
Online ISBN: 978-3-030-29105-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)