Abstract
Peritoneal carcinomatosis is a common progression of colorectal cancer with poor prognosis for many patients. Though the advent of modern chemotherapy and biologic treatments has resulted in improved survival for patients with advanced colorectal cancer, patients with peritoneal carcinomatosis have shown worse response to systemic chemotherapy and improvement in their overall and disease-free survival lags behind those with liver and lung metastases. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer represent an important regional approach adjuvant therapy. Though few randomized studies exist, case report and case-control studies continue to demonstrate better than expected survival in select patients with peritoneal carcinomatosis from colorectal cancer when compared to historical controls. Though morbidity and mortality of cytoreductive surgery and HIPEC for advanced colorectal cancer remain high, it is within a range established for other interventions for advanced cancers and has been shown to improve with increased surgeon and institution experience. Though HIPEC was initially introduced for the management of established peritoneal carcinomatosis, ongoing research into adjuvant HIPEC at the time of curative surgery in patients with locally advanced tumors and HIPEC paired with planned “second-look” surgery in high-risk patients with advanced minimally metastatic disease suggests HIPEC may have an expanded role in the management of advanced colorectal cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cytoreductive therapy (CRS)
- Intraperitoneal chemotherapy (IPC)
- Hyperthermic intraperitoneal chemotherapy (HIPEC)
- Early postoperative intraperitoneal chemotherapy (EPIC)
- Pressurized intraperitoneal aerosolized chemotherapy (PIPAC)
- Colorectal cancer (CRC)
- Peritoneal carcinomatosis (PC)
- Second-look surgery
Introduction
Peritoneal carcinomatosis (PC) is a common progression in the natural history of colorectal cancer (CRC). PC is present in 4–7% of patients at the time of initial diagnosis [1]. However, in patients who develop metachronous metastases, 20% will develop PC, and of these patients 25% of them will have metastases confined to the peritoneal cavity [2]. Historically, PC from colorectal cancer was associated with a dismal prognosis, 5.2–7 months median survival in the era of fluorouracil-only treatment with significant morbidity during that time [3].
Even in the era of modern chemotherapy, PC in patients with advanced colon cancer is associated with shorter overall survival. A 2016 pooled analysis of 10,533 pts. enrolled in phase III trials of systemic chemotherapy for metastatic colorectal cancer found isolated peritoneal carcinomatosis was associated with a worse median survival than isolated metastases to any other site (16.3 versus 20 months) and approximately the same median survival as multifocal nonperitoneal metastases (16.3 versus 15.7 months) [4]. Similar findings were reported in a second pooled analysis of patients in two phase III trials of systemic chemotherapy for patients with metastatic CRC. Overall survival was worse for patients with PC as compared to other metastatic disease, 12.7 months versus 17.6 months, and that peritoneal carcinomatosis was associated with also worse survival when stratified by chemotherapeutic regimen [5].
Data for Survival Benefit with HIPEC
When regional therapy with cytoreductive surgery (CRS) and intraperitoneal chemotherapy (IPC) for peritoneal carcinomatosis from colorectal cancer was first proposed in the 1990s, multiple small case series suggested prolonged survival with CRS and IPC or hyperthermic intraperitoneal chemotherapy (HIPEC) [6]. However, due to the novelty and complexity of the procedure, it was only performed at a small number of expert centers and recruitment of patients for controlled studies was challenging. In the past decade, as more centers have begun performing regional therapy, studies with internal controls and large-scale multi-institution studies have suggested improved outcomes with this approach.
To date, only two randomized controlled trials comparing systemic chemotherapy to CRS and IPC have been completed. Between 1998 and 2001, Verwaal et al. randomized 105 patients to either to receive cytoreductive therapy and mitomycin C HIPEC or to receive standard of care systemic chemotherapy (fluorouracil with leucovorin) with palliative surgery for bowel obstruction as indicated. Overall survival for the experimental arm was 22.3 months versus 12.6 months in the standard arm. Moreover, in patients without evidence of residual disease after surgery, median survival was 48 months [7].
One major criticism of the study is that the patients in the control arm received fluorouracil (5-FU) and leucovorin, standard of care at the time, but now no longer first-line chemotherapy. Advances in modern systemic chemotherapy have significantly improved the overall survival of patients with advanced CRC. A recent randomized controlled trial comparing surgery and IPC to modern systemic chemo alone recapitulated the findings of Verwaal et al., demonstrating better survival with surgery and IPC than systemic chemotherapy.
Forty-eight patients with confirmed CRC or appendiceal cancer and spread to two or more peritoneal sites without extra-abdominal metastases were randomized to receive resection and CRS followed by early postoperative intraperitoneal chemotherapy (EPIC) with 5-FU and leucovorin 3 hours postoperatively and then every 4–5 weeks for a total of six treatments over 6 months or to receive systemic chemotherapy with the FOLFOX (folinic acid, fluorouracil, and oxaliplatin) regimen for 6 months. The study ended prematurely due to slow accrual, but was sufficiently powered to draw significant conclusions. Survival was better in the surgery arm than in the systemic chemotherapy arm, 54% versus 38% 2-year survival, and 33% versus 4% 5-year survival. On multivariate analysis, it was found that surgical resectability was the only factor affecting survival, and the 5-year survival among patients with resectable disease median survival was 40 months with 5-year survival of 40% [8].
Two more recent nonrandomized studies compared outcomes in patients receiving CRS and HIPEC with those in patients receiving modern systemic chemotherapy alone and demonstrated a significant survival difference in patients who received CRS and HIPEC.
Elias et al. compared 48 prospectively evaluated patients with PC from CRC undergoing CRS and HIPEC with 48 retrospective matched controls who received only systemic chemotherapy. The experimental arm received induction therapy, complete resection (CCR0 or CCR1), and HIPEC with oxaliplatin with intravenous (IV) 5-FU potentiation. Both groups received a mean of 2.3 lines of modern chemotherapy. Median survival for the HIPEC group was significantly longer than for the group receiving systemic therapy alone, 62.7 versus 23.9 months. It is notable that these median survivals are quite long in comparison with other studies, and the patients in both groups were highly selected for age < 65, low tumor burden, and lack of symptoms [9].
In 2010, Franko et al. published a case-control study of 105 patients at University of Pittsburgh Medical Center. Sixty-seven patients in the experimental arm underwent CRS and HIPEC with mitomycin C at two closely associated facilities with the same physician team. These patients were matched with 38 controls with confirmed CRC peritoneal carcinomatosis who either refused CRS and HIPEC or were unable to receive it for logistical reasons. Patients in both arms received 5-FU and irinotecan. Median survival was longer for patients who underwent surgery and HIPEC, 34.7 months versus 16.8 months for those who received systemic chemotherapy only. Again, the groups were not analyzed based on completeness of resection, but six patients included in the analysis of the experimental arm were noted to have R2 resections [10].
Two large multi-institution prospective studies from France were performed in the mid-2000s. These large-scale multicenter studies represent wide range of techniques, intraperitoneal and systemic chemotherapeutic regimens, and institutional expertise.
In 2004, Glehen et al. published a “world tour” retrospective cohort study evaluating outcomes in 506 patients with PC from CRC and no extra-abdominal metastases in patients who underwent CRS and HIPEC or EPIC from 28 institutions on 4 continents between 1987 and 2002. The institutions represented a range of volume and experience, with over half contributing 25 or fewer cases to the study. Median overall survival was 19.2 months, and survival of patients with complete resection was 32.4 months, while that for patients in whom complete resection was not possible was 8.4 months [11]. Five years later, Elias et al. published another retrospective multi-institution cohort study of 523 patients with the same selection criteria as those who underwent CRS and perioperative intraperitoneal chemotherapy between 1990 and 2007. In this study, median overall survival was 30.1 months, and median survival for complete resection was 33 months versus 7 months in those patients for whom complete resection was not possible [12].
Morbidity and Mortality
Cytoreductive surgery with HIPEC is traditionally associated with a high morbidity and perioperative mortality. The extensive nature of the surgical cytoreduction and exposure of fresh surgical sites, including bowel anastomoses, to concentrated chemotherapeutic agents may make this a challenging procedure with high risk of complication. The most common complications include gastrointestinal fistula, anastomotic leak, and hematologic toxicity.
A 2006 systematic review of morbidity and mortality from 10 studies across nearly two decades with patient numbers ranging from 18 to 506 reported morbidity ranging from 23% to 44% and mortality from 0% to 12% [13]. A meta-analysis of 76 studies on HIPEC for CRC published between 1993 and 2016 found a mean morbidity of 25–34% and mean mortality of 2.8% [14].
In the two recent large multicenter studies out of France, mortality and morbidity were 3–4% and 23–31%, respectively. Elias et al. also found that high volume of treated patients had a lower rate of morbidity and mortality [11, 12].
It has been established that in some complex surgical procedures for advanced cancers, institution volume correlates strongly with morbidity and survival [15]; this may also be true for CRS and IPC for PC for CRC. Several single institution analyses of outcomes following CRS and HIPEC for peritoneal carcinomatosis of gastrointestinal (GI) origin have demonstrated lower complication rate and better survival with increasing experience. One study estimated the plateau of the learning curve at around 130 cases [16, 17].
Overall, while CRS and HIPEC have a high morbidity and mortality, it is comparable to other similarly extensive oncologic surgeries. Furthermore, complication rate and perioperative mortality appear to correlate with institutional experience, suggesting that as physicians and staff are more widely trained in cytoreductive therapy and intraperitoneal chemotherapy techniques, overall complication rates may further decline.
Data for Hyperthermia
The advantage of hyperthermia in intraperitoneal chemotherapy for colorectal cancer remains uncertain even in animal studies. Hyperthermia was added to intraperitoneal chemotherapy regimens based on animal studies suggesting improved tumor penetration of chemotherapeutic agents and adjuvant thermotoxicity [18,19,20]. More recent animal studies, however, have demonstrated no survival benefit from hyperthermia in addition to CRS or IPC [21].
No clinical studies directly examining the effect of hyperthermia have been completed. Several studies comparing HIPEC with other forms of intraperitoneal chemotherapy have been performed, which suggest no effect or minor advantage to hyperthermia; however, the differences in chemotherapeutic agent and technique between the hyperthermic and normothermic groups make strong conclusions difficult to draw (Table 9.1).
A retrospective cohort study by Cashin et al. examined 126 patients who were identified to have peritoneal disease from colorectal cancer. Of those, 69 underwent CRS and HIPEC with mitomycin C, oxaliplatin, or oxaliplatin and irinotecan, and 57 underwent CRS and sequential intraperitoneal chemotherapy with 5-FU. Ninety-day mortality was identical between groups. The HIPEC group demonstrated higher median overall survival and 5-year survival (34 months and 40%) compared to the sequential IPC group (25 months and 18%). Multivariate analysis revealed the type of IPC was an independent prognostic factor, with better outcomes in patients who received HIPEC. In patients with CC0 resections, however, no significant difference in median survival was noted between HIPEC and sequential IPC groups (39 months versus 32 months p = 0.3) [22].
Retrospective case-control study of 46 patients by Elias et al. matched 23 patients with CRC who underwent CRS and HIPEC with oxaliplatin and IV 5-FU with 23 CRC patients who underwent CRS with normothermic intraperitoneal (IP) mitomycin C and 5-FU EPIC on postoperative day 4. No statistically significant difference in mortality or survival was identified between the groups.
Peritoneal recurrence was significantly lower in the HIPEC group (26% versus 57% p = 0.03) [23]. In a follow-up multicenter study of 523 patients by Elias et al., no difference in survival was noted between patients who underwent HIPEC and those who underwent normothermic EPIC [12].
To evaluate concerns that hyperthermia may increase perioperative complications, Gremonprez et al. performed a recently published propensity-matched study comparing CRS followed by intraperitoneal oxaliplatin at either normothermia (38°) or hyperthermia (40°) in 146 patients and found no significant different in mortality, major morbidity, or anastomotic leakage [24].
Data for Selection of Intraperitoneal Chemotherapeutic Agent
To date, it is unknown which agent or combination of agents is optimal for intraperitoneal chemotherapy for CRC. Centers of expertise have published studies using mitomycin C, irinotecan, oxaliplatin, and doxorubicin with apparent efficacy. Choice of agent is directed by physician preference, cost, availability, and prior patient exposure but no randomized trials comparing regimens for CRC have been conducted.
Four nonrandomized studies have been performed comparing IPC with oxaliplatin and mitomycin C in patients with PC from CRC receiving CRS and HIPEC, without a clear consensus (Table 9.2). Two studies found no significant difference in disease-free or overall survival between groups, one study found better outcomes with mitomycin C in patients with low disease burden and favorable histology, and the final study found better survival with oxaliplatin [25,26,27].
The largest study was performed by the American Society of Peritoneal Surface Malignancies which evaluated outcomes of HIPEC with mitomycin C versus oxaliplatin in 539 patients who underwent complete cytoreduction for PC from CRC. Median survival was the same between both groups, but when stratified based on Peritoneal Surface Disease Severity Score (PSDSS), an evaluation of symptoms burden of disease and histology, patients with low severity scores (PSDSS I/II) were found to have better overall survival with mitomycin C versus oxaliplatin (54.3 versus 28.2 months p = 0.012) [28].
Two nonrandomized studies have been performed comparing oxaliplatin and irinotecan. Quenet et al. performed a prospective study on 146 patients undergoing CRS and HIPEC for CRC. Forty-three patients received oxaliplatin alone and 103 received oxaliplatin and irinotecan; treatment was otherwise the same. No difference was found in in-hospital mortality, disease-free survival, or overall survival; a significant difference in morbidity was noted in the group that received irinotecan as compared to oxaliplatin alone (52.4% versus 34.9% p = 0.05) [29]. Glockzin et al. compared outcomes between oxaliplatin and irinotecan in 32 patients with colorectal or appendiceal cancer who underwent CRS and HIPEC with CCR0/1 resections. There was no perioperative mortality and morbidity, and 3-year survival was not significantly different between groups [30]. These limited studies suggest that intensification of oxaliplatin HIPEC may increase complications without added benefit, but show little to recommend one agent over another.
Data for IPC in Addition to CRS in CRC
The rationale for IPC following resection of gross disease is that chemotherapy will address residual microscopic disease, reducing recurrence and improving survival. Most studies have demonstrated improved outcomes associated with the completeness of cytoreduction, without demonstrating an added benefit from intraperitoneal chemotherapy. A randomized study in rats has shown improved outcomes with IPC plus CRS versus CRS alone [21]. Two randomized controlled trials in humans have been performed, one ended prematurely due to poor accrual, and the other found the addition of HIPEC to CRS increased late postoperative complications without providing a survival benefit.
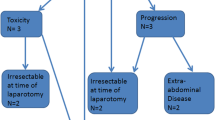
A randomized trial started by Elias et al. was stopped prematurely due to poor accrual and subject rejection of randomization into the non-IPC arm. Thirty-five of 90 patients were recruited, of whom 16 were randomized to receive CRS with immediate IP mitomycin C and postoperative IP 5-FU and 19 were randomized to receive CRS with systemic chemotherapy alone. Two-year survival was 60% in both groups; however, the EPIC group was notable for three postoperative deaths, more extensive peritoneal disease, and higher incidence of concomitant hepatectomy. In light of the limited size and premature conclusion, no definitive conclusions can be drawn from this study [31].
The PRODIGE 7 trial recruited patients who had CRC and PC with metastases limited to the abdomen. All recruited patients underwent CRS, and of the patients with resection with residual tumor ≤1 mm, 133 were randomized to receive HIPEC with oxaliplatin potentiated with 5-FU and 132 were randomized to receive no IPC. Postoperative mortality and 30-day morbidity were the same between groups, but 60-day morbidity was higher in the arm that received HIPEC compared to those undergoing CRS alone, 24.1 versus 13.6%. Overall survival and relapse-free survival were identical between groups [32] indicating that HIPEC with oxaliplatin is not an effective therapy for colorectal carcinomatosis; however, the median overall survival of almost 4 years in both arms suggests that surgical outcomes in selected patients are favorable.
Future Directions: Proactive HIPEC, Second-Look Surgery, and PIPAC
Even in patients with colorectal cancer who do not present with advanced disease, peritoneal carcinomatosis has been reported in 2–19% of patients following curative surgery and on autopsy in 36–40% of patients who received curative surgery and succumbed to their disease [33]. In patients who do have recurrence after curative surgery, peritoneal carcinomatosis is the only site of disease in up to 25% [34].
Many investigators hypothesize that CRS and HIPEC are most efficacious in patients with limited disease where complete resection is possible [35]. Limited peritoneal disease, as defined by PCI (peritoneal cancer index) score, is also associated with lower perioperative morbidity and mortality [12]. Therefore, early diagnosis and intervention for patients with peritoneal carcinomatosis may improve long-term outcomes, and novel locoregional therapies are needed for patients with advanced and unresectable disease. This has led investigators to consider adjuvant HIPEC at the time of surgery or planned “second-look” surgery with or without HIPEC for selected patients at high risk of peritoneal recurrences and to investigate pressurized intraperitoneal aerosolized chemotherapy (PIPAC) for patients with unresectable PC.
Identification of patients at high risk of developing PC after curative resection has been based on retrospective analysis of outcomes. In a retrospective analysis of 8044 patients who underwent resection of colorectal tumors, Segelmen found emergency surgery, non-R0 resection, and pT4 and pN2 with lymphadenectomy to be associated with increased risk of recurrence with PC [36]. A systematic review of recurrent PC after CRC resection was performed in 2013. All studies available had low-quality evidence, but 16 informative nonrandomized clinical studies consisting of a total of 598 patients were identified. Synchronous PC, synchronous isolated ovarian metastases, and perforated primary tumor were identified as probable risk factors for the development of PC, but no other significant conclusions were able to be drawn [37].
To assess the utility of adjuvant IPC, Noura et al. reported on a nonrandomized comparative study of 52 patients with positive cytology on peritoneal lavage but no macroscopic evidence of PC. Thirty-one of the 52 patients were administered intraperitoneal mitomycin C at the time of resection. Subjects receiving IPC had significantly better 5-year survival as compared to those who received conventional treatment (54.3% versus 9.5%) and significantly lower rates of peritoneal recurrence (12% versus 59.9%) [38].
Sammartino et al. also performed a nonrandomized study comparing outcomes in 25 patients with T3/T4 colon cancer without macroscopic evidence of PC who received adjuvant HIPEC with oxaliplatin during their initial resection with 50 well-matched controls who received only conventional therapy. They again found better overall survival and disease-free survival, and locoregional recurrence was significantly reduced (4% versus 28%) [39].
Based on the preliminary data from these limited comparative studies, two randomized clinical trials are currently underway to evaluate adjuvant HIPEC in patients at elevated risk of peritoneal recurrence. HIPECT4 has been registered in Spain, intending to recruit 200 patients with cT4NxM0 tumors of colorectal origin for intraoperative randomization to adjuvant HIPEC with mitomycin C or conventional therapy with systemic chemotherapy only. The primary outcome is locoregional control after 3 years of follow-up [40]. COLOPEC is a Dutch study planning to randomize 176 patients with T4 or intra-abdominally perforated colorectal cancer to receive adjuvant HIPEC with oxaliplatin and systemic chemotherapy or systemic chemotherapy alone. Patients will be followed for 18 months, at which point diagnostic laparoscopy will be performed to assess disease-free survival in each group [41].
An alternative adjuvant approach pairs HIPEC with planned second-look surgery in patients with risk factors for recurrence with peritoneal carcinomatosis on their initial operation. Preoperative computed tomography (CT) scans have shown poor detection of PC with large interobserver variation, particularly in PC with small tumor deposits [42], an observation which has been borne out in preliminary second-look surgery studies which have consistently found PC in >50% of high-risk patients without radiographic evidence of PC. This alternative opens the option for a prospective randomized trial of HIPEC for patients with high-risk features noted during resection not performed at a regional therapy center and may limit morbidity associated with HIPEC in patients who would not otherwise go on to develop PC.
Between 2007 and 2011, Delhorme et al. performed planned second-look surgery on 14 patients who had undergone a complete initial oncological resection of CRC with synchronous PC and/or ovarian metastasis with PC. Seventy-one percent of the patients were found to have PC on second look, with a median PCI of 10. All patients with PC received HIPEC with mitomycin C or oxaliplatin. Postoperative mortality was 0%, and Clavien-Dindo grade II–IV complications occurred in 7% of patients, much lower then in other reports of HIPEC for PC from CRC. The 2-year overall survival and disease-free survival rates were 91% and 38%, respectively. Radiographic peritoneal recurrence occurred in only 8% of patients who had undergone HIPEC at a second-look operation [43].
In 2011, Elias et al. performed a prospective study of “second-look” surgery on patients with resected CRC with risk factors for recurrence with PC. Forty-one patients who had undergone R0 resections for CRC and had no symptoms or radiographic findings consistent with PC but were considered high risk because of minimal synchronous PC, ovarian metastases, or perforation of the primary tumor during the initial surgery underwent “second-look” laparotomy approximately 1 year after surgery. Macroscopic PC was found in 56% of subjects; all subjects underwent HIPEC. Mortality was 2% and > grade II morbidity was 9.7%, again demonstrating lower complication rates than in CRS and HIPEC for patients with established PC. The 5-year overall survival was 90% [44].
This same group is continuing their work with a phase III study, ProphyloCHIP. In this study, 130 patients with resected CRC with high risk of peritoneal recurrence (limited peritoneal implants, ovarian metastases, or perforated tumor) will be randomized to either undergo laparotomy with HIPEC (intraperitoneal oxaliplatin and intravenous 5-FU) within 12 months of surgery or conventional follow-up, both groups receiving systemic adjuvant therapy. Recruitment and data collection are completed, and analysis was scheduled to be completed in June 2019 [45].
For patients with advanced or unresectable peritoneal carcinomatosis who would not be candidates for CRS and HIPEC, pressurized intraperitoneal aerosolized chemotherapy (PIPAC) has been suggested as an alternative treatment. Preclinical and animal studies of PIPAC, in which chemotherapeutic agents are applied as a pressurized aerosol to the peritoneal cavity, have suggested tissue penetration in PIPAC may be superior to HIPEC, allowing for the treatment or downstaging of bulky disease [46].
While many centers, primarily in Europe, have started offering PIPAC, evidence for the efficacy of the technique in CRC is limited. Several small studies in mixed tumor types including CRC have demonstrated the safety of PIPAC with histologic response ranging from 71% to 100% [47]. One study by Demtroder et al. in 2016 focused on PIPAC for PC from CRC exclusively. In this small retrospective study, 17 patients with PC from CRC ineligible for CRS and HIPEC underwent PIPAC with oxaliplatin. Median survival was 15.7 months and 71% of patients showed histologic tumor response [48]. While early trials have used low doses of oxaliplatin, a number of studies have begun testing escalating doses of oxaliplatin in PC from digestive cancers. One such study, the PIPOX trial, recently reported complete response in 3 of 10 patients during the phase I portion of the trial [49].
To further establish the role for PIPAC in treating PC, a number of phase II trials are underway. Public registries report 10 international clinical trials of PIPAC in gynecological and gastrointestinal malignancies [50].
Conclusion
As CRS and IPC for CRC with PC have become more widely practiced, data have accumulated to demonstrate its utility in selected patients. Ongoing research to optimize this technique may further improve outcomes. An abundance of case studies has demonstrated that survival with CRS and HIPEC appears better than historical controls treated with systemic chemotherapy. Two randomized controlled trials also demonstrated longer survival with CRS and IPC compared to systemic chemotherapy alone. More recent reports have demonstrated lower morbidity and perioperative mortality compared to initial studies. The incremental benefit of adding HIPEC to CRS remains unknown. Recent data demonstrated that HIPEC with oxaliplatin has no survival benefit over CRS alone. Nevertheless, there are ongoing investigations into the efficacy of adjuvant HIPEC with or without second-look surgery in patients at high risk of peritoneal recurrence from CRC. These trials may identify a new role for IPC in the coming years.
References
Jayne DG, Fook S, Loi C, Seow-Choen F. Br J Surg. 2002;89(12):1545–50.
van Gestel YR, Thomassen I, Lemmens VE, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40:963–9.
Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–63.
Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, Saltz L, Punt CJA, Koopman M, Tournigand C, Tebbutt NC, Diaz-Rubio E, Souglakos J, Falcone A, Chibaudel B, Heinemann V, Moen J, De Gramont A, Sargent DJ, Grothey A, Analysis and Research in Cancers of the Digestive System (ARCAD) Group. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trial from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–19.
Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263–7.
Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221(2):124–32.
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43.
Cashin PH, Mahteme H, Spång N, Syk I, Frödin JE, Torkzad M, Glimelius B, Graf W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: a randomised trial. Eur J Cancer. 2016;53:155–62.
Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D, Bonastre J. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27(5):681–5.
Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756–62.
Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AA, Lorimier G, Bernard JL, Bereder JM, Porcheron J, Gomez-Portilla A, Shen P, Deraco M, Rat P. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–92.
Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–8.
Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24(24):4011–9.
Huang CQ, Min Y, Wang SY, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget. 2017;8(33):55657–83.
Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–51.
Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94(11):1408–14.
Levine EA, Stewart JH, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204(5):943–53; discussion 953–5.
Los G, Smals OA, van Vugt MJ, van der Vlist M, den Engelse L, McVie JG, Pinedo HM. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity. Cancer Res. 1992;52(5):1252–8.
Shiu MH, Fortner JG. Intraperitoneal hyperthermic treatment of implanted peritoneal cancer in rats. Cancer Res. 1980;40(11):4081–4.
Los G, van Vugt MJ, Pinedo HM. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br J Cancer. 1994;69(2):235–41.
Klaver YL, Hendriks T, Lomme RM, et al. Hyperthermia and intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis: an experimental study. Ann Surg. 2011;254:125–30.
Cashin PH, Graf W, Nygren P, Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol. 2012;38(6):509–15.
Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2007;14(2):509–14.
Gremonprez F, Gossye H, Ceelen W. Use of hyperthermia versus normothermia during intraperitoneal chemoperfusion with oxaliplatin for colorectal peritoneal carcinomatosis: a propensity score matched analysis. Eur J Surg Oncol. 2019;45(3):366–70.
Hompes D, D’Hoore A, Wolthuis A, Fieuws S, Mirck B, Bruin S, Verwaal V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol. 2014;109(6):527–32.
Leung V, Huo YR, Liauw W, Morris DL. Oxaliplatin versus Mitomycin C for HIPEC in colorectal cancer peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43(1):144–9.
van Eden WJ, Kok NFM, Woensdregt K, Huitema ADR, Boot H, Aalbers AGJ. Safety of intraperitoneal Mitomycin C versus intraperitoneal oxaliplatin in patients with peritoneal carcinomatosis of colorectal cancer undergoing cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2018;44(2):220–7.
Prada-Villaverde A, Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, Flores-Ayala G, Glehen O, Gomez-Portilla A, González-Moreno S, Goodman M, Halkia E, Kusamura S, Moller M, Passot G, Pocard M, Salti G, Sardi A, Senthil M, Spiliotis J, Torres-Melero J, Turaga K, Trout R. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol. 2014;110(7):779–85.
Quenet F, Goéré D, Mehta SS, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254(2):294–301.
Glockzin G, Gerken M, Lang SA, Klinkhammer-Schalke M, Piso P, Schlitt HJ. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: a retrospective analysis. BMC Cancer. 2014;14:807.
Elias D, Delperro JR, Sideris L, et al. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11(5):518–21.
Quenet F, D Elias D, Roca L, Goere D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, De Forges H, Stanbury T, Paineau J, Glehen O. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. ASCO Annual Meeting. Chicago. 2018.
Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006;243:212–22.
Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy: a prospective study of prognostic factors. Cancer. 1989;63:364–7.
da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203:878–86.
Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Color Dis. 2014;16:359–67.
Honoré C, Goéré D, Souadka A, Dumont F, Elias D. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20(1):183–92.
Noura S, Ohue M, Shingai T, et al. Effects of intraperitoneal chemotherapy with mitomycin C on the prevention of peritoneal recurrence in colorectal cancer patients with positive peritoneal lavage cytology findings. Ann Surg Oncol. 2011;18(2):396–404.
Sammartino P, Sibio S, Biacchi D, et al. Long-term results after proactive management for locoregional control in patients with colonic cancer at high risk of peritoneal metastases. Int J Color Dis. 2014;29(9):1081–9.
Arjona-Sánchez A, Barrios P, Boldo-Roda E, et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer. 2018;18:183.
Klaver CEL, Musters GD, Bemelman WA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer. 2015;15:428.
de Bree E, Koops W, Kroger R. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol. 2004;86:64–73.
Delhorme JB, Romain B, Meyer N, Rohra S, Brigand C. Routine second-look after surgical treatment of colonic peritoneal carcinomatosis. J Visceral Surg. 2015;152(3):149–54.
Elias D, Honoré C, Dumont F, Ducreux M, Boige V, Malka D, Burtin P, Dromain C, Goéré D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254(2):289–93.
Pinto A, Eveno C, Pocard M. Update on clinical trials in colorectal cancer peritoneal metastasis. Int J Hyperth. 2017;33(5):543–7.
Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Habner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg. 2017;104(6):669–78.
Nowacki M, Alyami M, Villeneuve L, Mercier F, Hubner M, Willaert W, Ceelen W, Reymond M, Pezet D, Arvieux C, Khomyakov V, Lay L, Gianni S, Zegarski W, Bakrin N, Glehen O. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: an international survey study. Eur J Surg Oncol. 2018;44(7):991–6.
Demtroder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Color Dis. 2016;18(4):364–71.
Dumont F, Senellart H, Pein F, Campion L, Glehen O, Goere D, Pocard M, Thibaudeau E. Phase I/II study of oxaliplatin dose escalation via laparoscopic approach using pressurized aerosol intraperitoneal chemotherapy (PIPOX trial) for nonresectable peritoneal metastases of digestive cancers (stomach, small bowel, and colorectal): rationale and design. Pleura Peritoneum. 2018;3(3):20180120.
Tempfer C, Giger-Pabst U, Hilal Z, Dogan A, Rezniczek GA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch Gynecol Obstet. 2018;298(2):243–57.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Aveson, V., Nash, G.M. (2020). Data for HIPEC in Colorectal Cancer (T4 Lesions and Metastases). In: Fong, Y., Gamblin, T., Han, E., Lee, B., Zager, J. (eds) Cancer Regional Therapy. Springer, Cham. https://doi.org/10.1007/978-3-030-28891-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-28891-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28890-7
Online ISBN: 978-3-030-28891-4
eBook Packages: MedicineMedicine (R0)