Abstract
Since its introduction into clinical practice in the 1990s, thermal ablation has become an important tool in the treatment of patients with tumors of solid organs and soft tissue. In well-selected cases, ablation can provide long-term survival on par with resection; furthermore, the addition of intraoperative ablation has increased the number of patients amenable to curative resection. There are several different modalities commercially available, and in order to choose the optimal tool for a given situation, the user should have an understanding of both the mechanisms of action and limitations of each device. This chapter presents an overview of the different modalities followed by a discussion of their clinical applications in the liver, focusing on hepatocellular carcinoma and metastases from colon and breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ablation
- Radiofrequency ablation
- Microwave ablation
- Cryoablation
- Thermal ablation
- Chemical ablation
- Hepatocellular carcinoma
Introduction
Image-guided ablation is an accepted treatment for select benign and malignant tumors in multiple organs. Numerous ablation modalities are available, including but not limited to radiofrequency ablation, microwave ablation, cryoablation, laser ablation, irreversible electroporation, and chemical ablation. This chapter focuses on the principles behind commonly used ablation modalities. The number of available ablation modalities and the rapid changes in technology requires the user to understand the mechanisms behind each device, as that will allow the operators to choose the appropriate modality for a specific situation.
This chapter will summarize the mechanisms of action of the common ablation modalities with attention to the advantages, disadvantages, and limitations encountered in clinical practice. A basic understanding of the underlying physical processes is imperative to determining the modality to be used in any given clinical scenario. A summary of the clinical efficacy of the liver ablation is also included.
Chemical Ablation
Intratumoral administration of chemicals is the oldest available percutaneous ablation technique, particularly in the treatment of hepatocellular carcinoma (HCC) [1,2,3,4]. Advantages over other techniques include its low cost and relatively simple equipment. The challenge remains in achieving a homogenous distribution of the substance throughout the tumor and the ability to treat a margin of tissue surrounding the lesion. Therefore, chemical ablation has largely been replaced by energy-based techniques and is now reserved for tumors that are difficult to treat with other therapies (i.e., close proximity to critical structures) or just as a single technique when treating smaller lesions [5].
The principle behind ethanol injection relies on two mechanisms: (1) immediate dehydration of the cell cytoplasm and subsequent protein denaturation followed by coagulation necrosis and (2) ischemic necrosis while circulating in the tumoral blood vessels due to disruption of the vascular endothelium leading to platelet aggregation, thrombosis, and ischemia [6]. Ethanol is typically reserved for HCC because it is a soft tumor that usually occurs in the setting of cirrhosis. The cirrhotic liver act as a tumor pseudocapsule limiting the diffusion of the chemical out of the tumor into the parenchyma. Liver metastases, on the other hand, tend to be fibrotic and occur in relatively “soft” liver allowing for diffusion of an injectate such as ethanol in the normal tissue [7]. This is the main reason why other alternatives such as acetic acid have been used for chemical ablation. The necrosis mechanisms are essentially the same; however, acetic acid has better diffusion in fibrotic tissue, which theoretically brings advantages when treating metastatic disease [6, 7].
Energy-Based Thermal Ablation
Cryoablation (Hypothermic Ablation)
The Joule-Thomson effect is the principle behind cryoablation. This describes the change in temperature of a gas that occurs as a result of compression or expansion. Argon, the most common gas used in clinically available cryoablation systems, is a gas that cools during expansion in a chamber at the tip of the cryoprobe. With intermittent freezing and thawing (which may be passive or active using helium) to below 20 C, cell death is caused by formation of intracellular ice crystals that results in damage to plasma membranes and organelles [8]. The ice crystals continue to grow during thawing, maximizing cell death [9]. Tumor response depends on the rate of cooling, depth of hypothermia, rate of thawing, the number of freeze-thaw cycles, and delayed effects of post-thaw ischemia. Repeated freeze-thaw cycles can improve the efficacy.
Cryoablation is not commonly used in the liver because of the large diameter of currently available probes, frequent need to use multiple probes, and the risk of cryoshock, a clinical syndrome characterized by renal failure, disseminated intravascular coagulation, and adult respiratory distress syndrome [10].
Hyperthermic Ablation
It has been well described that irreversible cellular injury occurs when cells are heated to 46 °C for 60 minutes, and there is direct correlation between increasing temperature and cell death [11]. High temperatures can generate immediate damage by inducing coagulation of the cytosol, mitochondrial enzymes, and nucleic acid-histone protein complexes [12,13,14]. This reaction triggers cell death over the course of time. That is the main reason why histopathologic studies [12,13,14,15] have demonstrated that tissues treated with thermal ablation may still have viable morphology in the immediate postoperative period and that coagulative necrosis develops as a delayed consequence.
Radiofrequency Ablation
Radiofrequency utilizes an electrical current from a generator that oscillates between electrodes through the ion channels present in most biologic tissues. Tissues act as the resistive element leading to ionic friction and subsequent heat generation (Joule effect). Heating occurs rapidly in the areas closest to the electrode, and the more peripheral areas are heated passively from thermal conduction [16, 17].
Most RF ablation systems operate in a monopolar mode by using one electrode in the probe and dispersive electrodes on the skin surface (i.e., grounding pads). That way, the probe electrode delivers the energy generating heat and the grounding pad closes the electrical circuit decreasing the possibility of skin injury.
Internally cooled electrodes were developed to minimize char formation in tissue adjacent to the electrode. In these devices, fluid is circulated inside the electrode probe, to decrease temperatures at the electrode-tissue interface. This minimizes tissue charring and allows more power deposition thereby increasing the size of the ablation zone [18, 19]. Other designs included a probe with multiple tines in an attempt to distribute energy spatially [20] in order to generate a larger ablation zone [17,18,19].
Bipolar systems have also been developed, which allowed the current to oscillate between two probes eliminating the need of grounding pads [21, 22]. The bipolar approach restricts current flow to the area between the electrodes, decreasing cooling mediated by the perfusion of the area (heat-sink effect), resulting in faster and more focal heating between the electrodes. Therefore, in bipolar systems, the placement of the electrodes becomes critical to create a confluent zone of necrosis [22].
Microwave Ablation
Microwave ablation refers to electromagnetic energy operated at either 915 MHz or 2.45 GHz. The heating of tissues is produced as a result of the “dielectric hysteresis” phenomenon, which implies that when electromagnetic energy is applied to the tissue, it forces water molecules that have an intrinsic dipole moment to continuously rotate and realign. This continuous rotation of molecules increases kinetic energy and results in temperature rise [23, 24]. Microwaves can penetrate through biologic materials including tissues with low conductivity and are not affected by dehydration or charring producing extremely high temperatures (>150 °C) improving conduction into the surrounding tissue [25]. This makes microwave energy more efficient at heat generation. Microwave also does not require grounding pads and at the same time, multiple probes can be operated simultaneously [26] increasing the volume of tissue that can be ablated.
Modern microwave systems also contain a cooling jacket in order to prevent potential skin burns since the heat can be transmitted to the shaft of the probe and even the connections of the antenna [27]. The presence of cooling jackets also increases the amount of power that can safely be delivered to the tumor [25].
Laser Ablation
Laser sources emit approximately 600–1000 nm wavelength light energy [28] that induce electromagnetic heating. One of the primary advantages of using laser energy is its compatibility with magnetic resonance (MR) imaging. In addition, the relative lack of metal throughout the system and the small diameter of most applicators effectively eliminate imaging artifacts on CT and MR, allowing real-time monitoring of temperature maps [29].
Also, laser light can be a very precise energy for tissue heating. Since any tissue absorbs light very quickly, smaller ablation zones can be created (1–2-cm diameter) which make laser the perfect platform for delicate or small organs (brain, thyroid, prostate) [30]. Light does not penetrate through charred or desiccated tissues. Therefore, diffusers are used to improve the heating profiles, but when higher powers are used, the fiber needs to be cooled to avoid skin burns or probe failure [31]. Cooling mechanisms increase the diameter of the applicator, and when larger ablation zones are pursued, multiple can be used and operated independently or simultaneously [28].
Energy-Based Nonthermal Ablation
Irreversible Electroporation
Irreversible electroporation (IRE) uses pulsed electric fields to induce cell death. At a specific electric potential threshold, the cell membrane lipid bilayer becomes inundated with pores, a change that is reversible at low current but becomes permanent and results in cell death as the electric field strength is increased [32]. IRE devices can deliver up to 3000 V and 50 A through either unipolar or bipolar needle electrodes. Ablation zone size can be influenced by the number of electrodes, the length of the electrode tip, distance between electrodes, pulse number, duration of pulses, and voltage applied [33]. Electric fields are strongly influenced by the conductivity of the local environment, which depends on tissue heterogeneity and the presence of metal such as biliary stents. Since IRE does not depend on heating or cooling of target tissues, the technique is not limited by the heat-sink effect when performing ablation of tumors close to major blood vessels [34] and does not appear to have deleterious effects on adjacent normal tissues including nerves and bile ducts [35, 36].
Current IRE devices do have disadvantages, including generation of potentially dangerous electrical harmonics that can stimulate muscle contraction or cardiac arrhythmias. Therefore, the technique requires general anesthesia and paralytic induction. There is also a requirement for accurate placement of several needles to achieve even moderate-sized ablations. The lack of coagulation around the needle entry points can theoretically increase bleeding complication risks.
Clinical Applications in the Liver
When compared to surgical resection and radiation therapy, percutaneous ablation is a relatively new technology. As such, the indications are in evolution and vary from practice to practice. An appropriate tumor can be treated with ablation in a single outpatient session with fewer major complications than hepatic resection. Other advantages include the ability to perform additional ablations when needed to treat local recurrence or new lesions, that it is a parenchymal sparing technique, and finally that it typically does not require prolonged interruption of concurrent therapies. However, there are limitations based on size, geometry and location of the lesion in the liver (Fig. 4.1). It also requires, in most cases, sedation or anesthesia, and is more invasive than radiation therapy. Percutaneous ablation techniques can be used in many organs, from bone to thyroid, but the majority of data is for ablation of tumors in the liver.
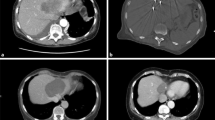
Hydrodissection to facilitate microwave ablation of solitary liver metastasis in a 69-year-old female with pancreatic cancer. (a) Portal venous phase CT shows a solitary metastasis in segment 6 (arrow). The ascending colon is immediately adjacent to the lesion (arrowhead). This was the only known site of metastatic disease for over 1 year. (b) A 20-gauge needle was advanced between the liver and colon, and dilute contrast was injected to “hydrodissect” the colon away from the liver to prevent thermal injury to the colon during ablation. (c) The lesion was then targeted with a microwave probe. (d) Follow-up CT performed 1 month later shows the ablation zone surrounding the treated tumor. There was no clinical or radiographic suggestion of injury to the colon
Primary Liver Tumors
The majority of patients that develop hepatocellular carcinoma (HCC) do so in the setting of a known risk factor, commonly viral hepatitis, and increasingly nonalcoholic fatty liver disease. Despite early projections, the incidence of HCC continues to increase in the United States and HCC is a leading cause of cancer-related deaths worldwide [37, 38]. To improve early detection, medical societies including the AASLD and the NCCN to name but a few have endorsed routine screening for at-risk patients to identify small tumors amenable to curative therapies [38].
Before thermal ablative techniques were widely available, chemical ablation was commonly used to treat small HCC. Most patients with HCC have cirrhosis, and HCC is a relatively soft tumor. Injection of a chemical directly into the tumor is therefore largely contained by the surrounding fibrotic parenchyma. Chemical ablation achieved by advancing a straight or multi-pronged needle into the tumor, and incrementally injecting small aliquots of ethanol or acetic acid under imaging guidance. Ethanol is of similar density to fat, and therefore CT is particularly useful if larger doses of injectate are planned because its distribution within the tumor (and not in other structures such as vascular structures) can be easily seen (Fig. 4.2). When ultrasound guidance is used, this is typically performed as a staged procedure using smaller doses of ethanol.
Ethanol ablation of a focus of recurrent hepatocellular carcinoma in a 62-year-old man. (a) Arterial phase CT shows a solitary 1.6 cm HCC (arrow) in the caudate lobe adjacent to the portal vein and bile duct. (b) A 20-gauge needle was advanced into the tumor and 5 cc ethanol injected. (c) After a total of 14 cc ethanol injected, the low-density ethanol is seen conforming to the tumor geometry. (d) Arterial phase CT 3 months later shows no residual hypervascular tumor and low density in the caudate consistent with complete tumor necrosis
Radiofrequency ablation (RFA) was introduced in the early 1990s, and in 1999 Livrhagi et al. published a prospective trial of HCC <= 3 cm that randomized to treatment with ethanol injection (PEIT) or RFA [39]. RFA was found to have a higher rate of complete necrosis and require fewer sessions than PEIT. A few years later, Lencioni et al. reported a prospective randomized comparison of RFA with PEIT in HCC patients within Milan criteria [40]. With a mean follow-up of nearly 2 years, the overall survival in the RFA group was 100% and 98% and complete necrosis was seen in 91% of tumors. This was significantly better than PEIT, and moreover, comparable to results seen after hepatic resection (HR). This made a compelling case for prospective randomized studies to directly compare outcomes of RFA and HR.
Between 2006 and 2017, there were four prospective randomized trials performed in China comparing RFA and HR for small HCC [41,42,43,44]. The inclusion criteria of each study are somewhat different, but all include lesions <= 5 cm in size. The data from these three trials are well summarized in a meta-analysis published in PlusOne in which the compiled data from prospective studies showed no difference in survival between patients undergoing RFA or HR at up to 4 years [45]. In the retrospective studies evaluated, however, there was a significant survival benefit of HR compared to RFA, likely reflecting selection bias with poor surgical candidates preferentially undergoing RFA.
Ablation for HCC may be performed in combination with arterially directed therapy. Elnekave et al. reported a retrospective case-controlled study of patients who underwent HR and combination embolization/ablation for solitary HCC <= 7 cm [46]. With a median follow-up of over 11 years, patients matched for Okuda stage who underwent HR or RFA had no significant difference in overall survival. Peng et al. randomized patients with recurrent HCC <= 5 cm to undergo RFA alone or in combination with chemoembolization (TACE) [47]. Overall survival at 5 years was better for patients who underwent combination treatment, but only for those with tumors that were 3.1–5 cm. Patients with tumors <= 3 cm did not benefit from the addition of TACE.
Three centimeters has been somewhat of a magic number for ablation, with tumors larger than 3 cm proving difficult to treat completely with ablation alone. This has long been thought to be related to the size of ablation that can be achieved with a single probe, with larger tumors requiring overlapping zones of ablation in order to achieve an adequate margin. Ideal positioning of multiple simultaneous or sequential probes is simple in theory but challenging to perform in practice.
Treating a margin of surrounding parenchyma is critical to ensure adequate treatment of microscopic tumor. In a study of 100 resected solitary HCC, Sasaki et al. found that of 100 resected tumors, 46—or almost half—had microsatellite of viable tumor detected by light microscopy [48]. When tumors less than 3 cm in size were found to have microsatellites, they were almost exclusively within a few mm of the index tumor. Tumors larger than 3 cm, however, had a significantly higher frequency and distance of microsatellites from the index tumor, with tumor cells found up to 3 cm away. Based on this data, one can easily see that ablation of a 3.5 cm tumor with the possibility of microsatellites up to 3 cm away tumor would be expected to have a high incidence of “local” recurrence.
The majority of prospective clinical trials studying percutaneous thermal ablation for HCC to date have looked at RFA. With new technologies, including microwave (MV) and irreversible electroporation (IRE), it is possible that larger tumors (MW) and tumors adjacent to structures susceptible to thermal damage (IRE) will increase the pool of candidates suitable for ablation.
Liver Metastases
The data for ablation of liver metastases is less robust than that for HCC.
Colon Cancer
Colon cancer is unusual among solid tumors in that when it metastasizes to liver, the liver is the only site of disease approximately half of the time. Although there are no prospective randomized trials to support metastasectomy, early surgical series suggested improved survival following resection of Colorectal liver metastasis (CRLM) [49, 50]. When ablative treatments became available, it stood to reason that if ablation were able to completely eradicate viable tumor, that this would also be an acceptable technique to treat eligible patients, particularly those with lesions in difficult locations for resection.
A systematic review of 75 retrospective studies found local recurrence rates of 12–39% with 1-, 3-, and 5-year survival of 84%, 37%, and 17%, better than that typically reported for systemic chemotherapy alone even though it included studies with high local recurrence rates [51]. It is likely that the high local recurrence rates in the early studies were largely due to undertreatment. As experience with ablation has matured, it has been demonstrated that achieving a margin of at least 5 mm and ideally 10 mm circumferentially around a CRLM tumor is critical to ensuring long-term treatment success [52] (Figs. 4.3 and 4.4).
Positron emission tomography (PET)-guided microwave ablation in a 54-year-old man with oligometastatic colorectal cancer to the liver. (a) Portal venous phase CT image demonstrates a solitary segment VII metastasis (arrow). (b) Intraprocedural planning PET/CT remonstrates solitary segment VII lesion, Fluorodeoxyglucose (FDG) avid (arrowhead). (c) Intraprocedural PET/CT shows microwave antenna positioned in the superior and lateral margin of the lesion (arrow). (d) Intraprocedural PET shows microwave antenna positioned in the inferior and medial margin of the lesion (arrow). (d) Immediate post-therapy PET/CT image demonstrates adequate margin and lack of metabolic activity within the ablation cavity (arrowheads). (e) CT abdomen with contrast in portal venous phase 1-month post-therapy shows complete response to therapy with adequate margins and no residual/recurrent disease (arrowheads)
Example of 3D assessment of ablation margins. (a) CT images with overlay of segmented tumor (yellow) and theoretical 5 mm and 10 mm margins shown in axial plane and sagittal and coronal reformats. (b) CT immediately after ablation shows segmented ablation zone. (c) Comparison with the initial segmentation shows insufficient treatment (residual tumor in red, 5 mm margin shown in purple contour and 10 mm margin in brown. (d) Axial images of follow-up CT obtained in arterial (top) and portal venous (bottom) phases show local tumor progression as hyperintense area on arterial phase and hypointense area on venous phase. Contours of ablation zone, and insufficiently covered volumes are overlaid showing spatial agreement between the location of progression and insufficiently covered regions of tumor. (Courtesy of Elena Kaye)
One of few prospective studies of ablation for CRLM was a phase II study that randomized patients with unresectable CRLM to systemic treatment with or without RFA [53]. At a median follow-up of 9.7 years, there was a significant benefit seen in the combination therapy arm with 35.9% alive at 8 years compared to 8.9% in patients who received systemic chemotherapy alone.
Unfortunately, our ability to predict which patients with metastatic CRLM are likely to benefit from local therapy is imperfect. A modified clinical risk score for ablation was described by Shady et al., based on the Fong Score used for surgical resection [54]. Based on a study of 162 patients, they describe 5 factors that were associated with poor survival: node-positive primary tumor, disease-free interval <12 months, multiple tumors, largest tumor >3 cm, and CEA > 30 ng/mL.
Another approach to determine to identify patients with good biology of disease is the “test of time approach” described by Livrhagi et al. [55]. This study followed 88 patients with resectable CRLM with RFA for a median follow-up of 28 months after ablation. Twenty-three (26%) patients remained disease-free and another 44 (50%) developed new lesions making them unsuitable for resection. In other words, 67 of 88 patients (76%) avoided surgery from which they were unlikely to benefit. The authors concluded ablation could reduce the number of resections without negatively impacting the clinical outcome.
Breast Cancer
Metastatic breast cancer is a systemic disease, but a subset of patients may have only oligometastatic disease for years, and these patients are good candidates for regional therapies including ablation, resection, and radiation therapy. Unfortunately, oligometastatic breast cancer has a heterogenous phenotype, and it is difficult to predict prospectively which subpopulation(s) will benefit from these treatments.
As with CRLM, the interest in local therapy of breast cancer liver metastases is based on reports in the surgical literature describing a survival advantage following hepatic metastasectomy [56, 57].
There are no prospective trials evaluating outcomes of ablation for Breast cancer liver metastasis (BCLM), and the systemic therapy options have changed dramatically in the time that ablation has been clinically available making measurable outcomes a moving target. The Mammary Cancer Microtherapy and Interventional Approaches (MAMMA MIA) study out of Germany retrospectively looked at 59 patients with breast cancer who underwent RFA, brachytherapy or radioembolization of BCLM to define characteristics of patients who benefited from local therapy [58]. Not surprisingly, they found that maximum tumor diameter <4 cm and history of <3 prior lines of systemic therapy were independent predictors of improved survival. This is supported by a retrospective study of Barral et al. who found that ablation provided effective local control and improved disease-free survival in patients with <4 cm lesions of oligometastatic breast cancer in lung and bone as well as BCLM [59].
Conclusion
Percutaneous ablation techniques are well recognized as a primary local control tool in the treatment of focal malignancies. In the last two decades, multiple studies have characterized the basic principles underlying ablative therapies. Understanding these basic principles allows the physician understanding patient/lesion selection as well as how to improve outcomes based on the advantage and disadvantages of each technique. It is also clear that the evidence is more robust for ablation of primary liver malignancies; however, in the metastatic disease scenario and taking into account lesion size, ablation has comparable performance when compared to metastasectomy. Combination therapies continue to be an evolving field, and it is very possible that ablation may have an expanded role outside the traditional size criteria.
Key Points
-
In general, we favor the use of high-powered microwave ablation for the treatment of tumors in the liver, and the operator must pursue tract cautery and avoid direct puncture of peripheral tumors.
-
RF and laser ablation have substantial physical limitations compared with microwave ablation for tissue heating and thus reserved for short/precise ablation zones.
-
Cryoablation should be used with caution in the liver, although it may be useful in the treatment of lesion close to critical structures.
-
IRE may have a role in the treatment of central tumors or in those near critical structures.
References
Taniguchi M, et al. Long-term outcome of percutaneous ethanol injection therapy for minimum-sized hepatocellular carcinoma. World J Gastroenterol. 2008;14(13):1997–2002.
Mazzanti R, et al. Survival and prognostic factors in patients with hepatocellular carcinoma treated by percutaneous ethanol injection: a 10-year experience. Can J Gastroenterol. 2004;18(10):611–8.
Andriulli A, et al. Survival of cirrhotic patients with early hepatocellular carcinoma treated by percutaneous ethanol injection or liver transplantation. Liver Transpl. 2004;10(11):1355–63.
Blendis L. Percutaneous ethanol ablation of small hepatocellular carcinomas: twenty years on. Gastroenterology. 2006;130(1):280–2.. discussion 282
Livraghi T, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197(1):101–8.
Kawano M. An experimental study of percutaneous absolute ethanol injection therapy for small hepatocellular carcinoma: effects of absolute ethanol on the healthy canine liver. Gastroenterol Jpn. 1989;24(6):663–9.
Giovannini M, Seitz JF. Ultrasound-guided percutaneous alcohol injection of small liver metastases. Results in 40 patients. Cancer. 1994;73(2):294–7.
Georgiades CS, et al. Safety and efficacy of CT-guided percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol. 2008;19(9):1302–10.
Permpongkosol S, et al. Differences in ablation size in porcine kidney, liver, and lung after cryoablation using the same ablation protocol. AJR Am J Roentgenol. 2007;188(4):1028–32.
Yu H, Burke CT. Comparison of percutaneous ablation technologies in the treatment of malignant liver tumors. Semin Intervent Radiol. 2014;31(2):129–37.
Andrasina T, et al. Interventional radiology therapies for liver cancer. Cas Lek Cesk. 2018;157(4):195–202.
Larson TR, Bostwick DG, Corica A. Temperature-correlated histopathologic changes following microwave thermoablation of obstructive tissue in patients with benign prostatic hyperplasia. Urology. 1996;47(4):463–9.
Goldberg SN, et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88(11):2452–63.
Zervas NT, Kuwayama A. Pathological characteristics of experimental thermal lesions. Comparison of induction heating and radiofrequency electrocoagulation. J Neurosurg. 1972;37(4):418–22.
Thomsen S. Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem Photobiol. 1991;53(6):825–35.
Goldberg SN, et al. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3(3):212–8.
Haemmerich D, Pilcher TA. Convective cooling affects cardiac catheter cryoablation and radiofrequency ablation in opposite directions. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1499–502.
Lorentzen T. The loop electrode: in vitro evaluation of a device for ultrasound-guided interstitial tissue ablation using radiofrequency electrosurgery. Acad Radiol. 1996;3(3):219–24.
Lencioni R, et al. Radio-frequency thermal ablation of liver metastases with a cooled-tip electrode needle: results of a pilot clinical trial. Eur Radiol. 1998;8(7):1205–11.
Haemmerich D, et al. Hepatic radiofrequency ablation with internally cooled probes: effect of coolant temperature on lesion size. IEEE Trans Biomed Eng. 2003;50(4):493–500.
McGahan JP, et al. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996;3(5):418–22.
Ritz JP, et al. Bipolar radiofrequency ablation of liver metastases during laparotomy. First clinical experiences with a new multipolar ablation concept. Int J Color Dis. 2006;21(1):25–32.
Wolf FJ, et al. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–9.
Wright AS, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236(1):132–9.
Yang D, et al. Measurement and analysis of tissue temperature during microwave liver ablation. IEEE Trans Biomed Eng. 2007;54(1):150–5.
Brace CL, et al. Microwave ablation with a single small-gauge triaxial antenna: in vivo porcine liver model. Radiology. 2007;242(2):435–40.
Wang Y, et al. Internally cooled antenna for microwave ablation: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2008;67(2):357–61.
Walser EM. Percutaneous laser ablation in the treatment of hepatocellular carcinoma with a tumor size of 4 cm or smaller: analysis of factors affecting the achievement of tumor necrosis. J Vasc Interv Radiol. 2005;16(11):1427–9.
Stollberger R, et al. Temperature monitoring of interstitial thermal tissue coagulation using MR phase images. J Magn Reson Imaging. 1998;8(1):188–96.
Skinner MG, et al. A theoretical comparison of energy sources—microwave, ultrasound and laser—for interstitial thermal therapy. Phys Med Biol. 1998;43(12):3535–47.
Heisterkamp J, et al. Heat-resistant cylindrical diffuser for interstitial laser coagulation: comparison with the bare-tip fiber in a porcine liver model. Lasers Surg Med. 1997;20(3):304–9.
Silk M, et al. The state of irreversible electroporation in interventional oncology. Semin Intervent Radiol. 2014;31(2):111–7.
Charpentier KP, et al. Irreversible electroporation of the liver and liver hilum in swine. HPB (Oxford). 2011;13(3):168–73.
Schoellnast H, et al. Acute and subacute effects of irreversible electroporation on nerves: experimental study in a pig model. Radiology. 2011;260(2):421–7.
Silk MT, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25(1):112–8.
Thomson KR, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22(5):611–21.
Benson AB 3rd, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Cancer Netw. 2017;15(5):563–73.
Bertuccio P, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–9.
Livraghi T, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210(3):655–61.
Lencioni RA, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235–40.
Chen MS, et al. Comparison of effects of percutaneous radiofrequency ablation and surgical resection on small hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi. 2005;85(2):80–3.
Feng K, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802.
Huang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–12.
Ng KKC, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104(13):1775–84.
Wang Y, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9(1):e84484.
Elnekave E, et al. Long-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC<7 cm. Ann Surg Oncol. 2013;20(9):2881–6.
Peng ZW, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012;262(2):689–700.
Sasaki A, et al. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103(2):299–306.
Pedersen IK, et al. Resection of liver metastases from colorectal cancer. Indications and results. Dis Colon Rectum. 1994;37(11):1078–82.
Steele G Jr, et al. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: gastrointestinal tumor study group protocol 6584. J Clin Oncol. 1991;9(7):1105–12.
Pathak S, et al. Ablative therapies for colorectal liver metastases: a systematic review. Color Dis. 2011;13(9):e252–65.
Wang X, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–75.
Ruers T, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9). https://doi.org/10.1093/jnci/djx015.
Shady W, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278(2):601–11.
Livraghi T, et al. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer. 2003;97(12):3027–35.
Fairhurst K, et al. The safety and effectiveness of liver resection for breast cancer liver metastases: a systematic review. Breast. 2016;30:175–84.
Margonis GA, et al. The role of liver-directed surgery in patients with hepatic metastasis from primary breast cancer: a multi-institutional analysis. HPB (Oxford). 2016;18(8):700–5.
Seidensticker M, et al. Locally ablative treatment of breast cancer liver metastases: identification of factors influencing survival (the Mammary Cancer Microtherapy and Interventional Approaches (MAMMA MIA) study). BMC Cancer. 2015;15:517.
Barral M, et al. Percutaneous thermal ablation of breast cancer metastases in oligometastatic patients. Cardiovasc Intervent Radiol. 2016;39(6):885–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Camacho, J.C., Covey, A.M. (2020). Principles of Percutaneous Ablation in the Liver. In: Fong, Y., Gamblin, T., Han, E., Lee, B., Zager, J. (eds) Cancer Regional Therapy. Springer, Cham. https://doi.org/10.1007/978-3-030-28891-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-28891-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28890-7
Online ISBN: 978-3-030-28891-4
eBook Packages: MedicineMedicine (R0)