Abstract
A 45 year-old man with history of nonischemic cardiomyopathy (NICM) and sustained ventricular tachycardia (VT), status-post implantable cardioverter defibrillator implantation, presents with ICD storm despite treatment with sotalol and is referred for catheter ablation. The case highlights unique features regarding identification, mapping, and ablation of arrhythmogenic substrate in NICM VT.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nonischemic cardiomyopathy
- Ventricular arrhythmias
- Catheter ablation
- Mid myocardial substrate
- Cardiac imaging
- Intracardiac echocardiography
- Adjunctive ablation techniques
1 Case Summary
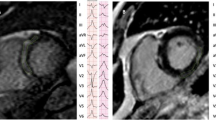
A 45 year-old man with history of nonischemic cardiomyopathy (NICM) and sustained ventricular tachycardia (VT), status-post implantable cardioverter defibrillator implantation, presents with ICD storm despite treatment with sotalol and is referred for catheter ablation. Shown in Fig. 111.1a–c are the pre-procedural cardiac magnetic resonance imaging (MRI), the endocardial left ventricular bipolar and unipolar voltage maps obtained in sinus rhythm, and the VT morphology produced with programmed stimulation. What should proceed next and why?
(a–c) (a) Shown are short-axis and four-chamber views of the patient’s cardiac magnetic resonance images showing a patchy distribution of late gadolinium enhancement (white arrows) involving predominantly the inferolateral base. (b) Electroanatomic left ventricular endocardial bipolar (left) and unipolar (right) voltage maps indicate absence of obvious abnormalities using standard cut-offs. (c) 12-lead morphology of the patient’s predominant ventricular tachycardia
2 Case Discussion
Unlike for post-infarction VT, arrhythmogenic substrate in the setting of NICM tends to be heterogeneous in distribution, is often less densely consolidated, and very commonly involves the epicardium or midmyocardium. Although NICM substrate tends to cluster in the peri-valvular regions, and most commonly the lateral mitral annulus, in approximately 12% of cases it is contained predominantly within the septum, in which case epicardial mapping is of limited to no utility [1]. Knowing where to direct efforts, and whether to proceed with epicardial access, versus more detailed mapping and ablation of the right ventricle (RV) and interventricular septum, is important in optimizing procedural efficacy and limiting attendant risks.
Clues about the presence of midmyocardial or epicardial substrate may be obtained from endocardial mapping in sinus rhythm, with analysis of unipolar voltage. In the left ventricle (LV), endocardial unipolar voltage <8.3 mV has been established as a threshold for abnormal [2]. However, absolute cut-off values may not apply in all patients, and adjusting the color scale may help to elicit abnormalities in unipolar voltage that may be obscured by higher average unipolar voltage in a given individual. In this patient’s case, only when investigating unipolar voltages with an upper limit of abnormal of 17.5 mV revealed a region of interest, which also correlated with the late gadolinium enhancement seen on pre-procedure cardiac MRI (Figs. 111.1a–c and 111.2a–c).
(a–c) (a) Modifiying the upper limit of abnormal left ventricular endocardial unipolar voltage in this patient, an area of inferolateral, peri-mitral abnormality is elicited, which correlates with pre-procedural late gadolinium enhancement seen on cardiac magnetic resonance imaging (Fig. 111.1a–c) but also (b) on real-timeintracardiac echocardiography (yellow arrows and green outline on integrated image within electroanatomic mapping system). The epicardial bipolar voltage map (c) demonstrates the fidelity of the surrogate markers for arrhythmogenic substrate, including low-bipolar voltage amplitude region containing fractionated and late potentials, and alternating pacemaps with pacing from the same site
In frequent instances in which cardiac MRI is unavailable or unable to be performed, other relatively easily acquired data can provide guidance. Spontaneously observed or induced VT morphologies with specific 12-lead ECG features may indicate possible epicardial substrate in NICM (Fig. 111.3) [3]. These criteria generally quantify the concept that VTs exiting from the epicardium engage the intrinsic His-Purkinje system later than those originating closer to the endocardium, given the more endocardial course of the conduction system; qualitatively, features that are most recognized include delayed, slurred upstroke of the QRS in VT, absence of Q waves in the inferior leads, or lead I. The predominant VT in this patient had many of these features.
Intracardiac echocardiography (ICE) is of great value in VT ablation procedures. In addition to real-time assessment of catheter positions, intracardiac anatomic structures (i.e. papillary muscles), and complications during ablation, arrhythmogenic substrate can be identified as areas of increased hyperechogenicity, often with associated wall motion abnormalities [4]. Observation of primarily midmyocardial or epicardial abnormalities on ICE, as was observed in this case (Fig. 111.2a–c), provides guidance early on that epicardial access is justified and should be pursued expeditiously.
Finally, and especially in NICM VT cases in which ablation attempting to target substrate may involve extensive ablation within normal local myocardium, limited activation and entrainment mapping during VT may be necessary in order to determine where to concentrate greatest efforts in order to also minimize potential collateral damage. Identification of mid-diastolic potentials (MDPs) during VT, especially if participation in the reentrant isthmus can be confirmed with entrainment, provides justification about concentrating ablating at a given site (Fig. 111.2a–c). In this patient’s case, additional mapping confirmed presence of epicardial arrhythmogenic substrate. Additionally, pacing within the same region of abnormality produced alternating exits (different paced QRS morphologies, Fig. 111.2a–c), indicating baseline properties favoring unidirectional block, even at relatively slow cycle lengths, and ability to participate in sustained reentry.
In some cases, adjunctive ablation techniques, including bipolar ablation, use of radiofrequency (RF) ablation electrode irrigant of low ionic concentration, or RF delivery using a needle electrode have been necessary to penetrate to sufficient depth to effectively and durably target critical elements of reentry [5]. Usual endpoints for ablation are utilized, including lack of VT inducibility following ablation, as well as demonstration of electrical homogenization of the region ablated, including entrance block (loss of late potentials) and exit block (lack of capture at high output in regions which previously captured). Hemodynamic stability of the patient must also be considered, particularly during prolonged procedures.
References
Haqqani HM, Tschabrunn CM, Tzou WS, Dixit S, Cooper JM, Riley MP, et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: Incidence, characterization, and implications. Heart Rhythm. 2011;8:1169–76.
Hutchinson MD, Gerstenfeld EP, Desjardins B, Bala R, Riley MP, Garcia FC, et al. Endocardial unipolar voltage mapping to detect epicardial VT substrate in patients with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:49–55.
Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:63–71.
Enriquez A, Saenz LC, Rosso R, Silvestry FE, Callans D, Marchlinski FE, et al. Use of intracardiac echocardiography in interventional cardiology: working with the anatomy rather than fighting it. Circulation. 2018;137(21):2278–94.
Nguyen DT, Tzou WS, Sandhu A, Gianni C, Anter E, Tung R, et al. Prospective multicenter experience with cooled radiofrequency ablation using high impedance irrigant to target deep myocardial substrate refractory to standard ablation. JACC Electrophysiol. 2018;4:1176–85.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tzou, W.S. (2020). Ventricular Tachycardia in Non-Ischemic Cardiomyopathy. In: Natale, A., Wang, P., Al-Ahmad, A., Estes, N. (eds) Cardiac Electrophysiology. Springer, Cham. https://doi.org/10.1007/978-3-030-28533-3_111
Download citation
DOI: https://doi.org/10.1007/978-3-030-28533-3_111
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28531-9
Online ISBN: 978-3-030-28533-3
eBook Packages: MedicineMedicine (R0)