Abstract
Renal neoplasms comprise a heterogeneous group of tumors with divergent clinicopathological and molecular characteristics as well as therapeutic options. Therefore, accurate diagnosis and classification of the different subtypes of renal cell carcinoma (RCC), and to verify the renal primary in the setting of metastatic disease are critical for patient management and determining prognosis. In the last three decades, more and more subtypes of RCC have been described and many of them have overlapping clinicomorphologic features. Incorporating diagnostic immunohistochemistry and molecular tests in diagnosis of renal tumors is a critical component of our daily surgical pathology practice. In this chapter, we will discuss the immunohistochemical markers that are commonly used in clinical laboratories. In addition, common diagnostic problems that deserve special attention in the differential diagnosis of major RCC subtypes will also be addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Kidney
- Renal cell carcinoma
- Differential diagnosis of renal carcinoma
- Classification of renal cell carcinoma
- Immunohistochemistry of renal cell carcinoma
Diagnosis and classification of renal tumors are usually straightforward based on gross and routine hematoxylin and eosin (H&E) microscopic examination of the biopsy and resection specimens. Immunohistochemistry (IHC), however, has been increasingly used in the workup of challenging cases [1,2,3,4,5,6,7,8]. IHC markers are used to verify histological subtypes, distinguish primary renal cell carcinomas (RCC) from other nonrenal cell tumor types that can occur in the kidney, or from the rare metastasis to the kidney. Metastatic RCCs to distant sites often require confirmation of its renal origin by IHC. Finally, needle biopsies with limited material often require IHC stains to establish diagnosis and classification [9, 10].
In this chapter, we will discuss immunophenotypes of major renal tumors and IHC markers that are commonly used in clinical laboratories. In addition, algorithms incorporating morphology and IHC profiles in the differential diagnosis of major RCC histological subtypes will also be discussed.
Immunohistochemical (IHC) Markers Commonly Used in the Diagnosis of Renal Tumors
Markers That Support the Renal Origin
These markers are expressed in the different parts of the nephron structures and majority of renal cell neoplasms, but infrequently in non-renal cell neoplasms. Because of their relative specificity in renal tumors, they are often used to distinguish renal and nonrenal cell neoplasms and to confirm the renal origin of metastatic RCC at distant sites. These markers include cytokeratins (CKs), vimentin, CD10, RCC marker (RCCMa), human kidney injury molecule-1 (hKIM-1), PAX2, and PAX8. It should be noted that several commonly used IHC markers are almost always negative in RCC. These include TTF-1, CDX2, P63, GATA3, NKX3.1, and PSA. Labeling any of these markers is a strong argument against the diagnosis of RCC. The International Society of Urological Pathology (ISUP) recommended that PAX8 is the most useful IHC marker for establishing the diagnosis of metastatic RCC. One can use IHC for other markers such as ER, CDX2, NKX3.1, PSA, TTF-1, GATA3, and P63 to help exclude other nonrenal origin carcinomas, including those that also label for PAX8. The other IHC markers in common practice are more valuable in RCC classification (see below), can be also supportive of metastatic RCC but usually not indicated or useful [6].
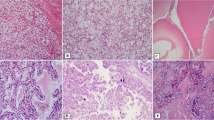
PAX2 and PAX8 are both nuclear transcriptional factors mediating embryonic development of the kidney, Mullerian and other organ systems [11, 12]. Their expression in human tissues is similar except PAX8 is also expressed in thyroid follicular cells while PAX2 is not. They are expressed diffusely in normal kidney with higher level in the distal tubules than the proximal tubules (Fig. 15.1a) and patchy and weakly in the urothelium of the collecting system (Fig. 15.1b). They have a similar expression profile and are found in approximately 90% of all the histological subtypes of renal cell neoplasms, including the high-grade sarcomatoid and metastatic RCCs (Fig. 15.1c, d). PAX2 and PAX8 are therefore considered the most useful markers to confirm a diagnosis of renal cell neoplasms both in the kidney and at distant sites due to their high sensitivity, high percentage of positive tumor cells in positive cases and discrete nuclear staining pattern. These two markers do have some differences. For example, some renal tumors that may be negative or infrequently positive for PAX2, including oncocytoma and chromophobe RCC (chRCC), are often positive for PAX8. Another diagnostic pitfall is occasional expression of PAX2 and PAX8 in other nonrenal neoplasms, including 10–15% of pelvic urothelial carcinoma, parathyroid tumor, and tumors derived from the Mullerian and Wolffian duct systems. PAX8 is also expressed in pancreatic well-differentiated neuroendocrine tumors, thyroid follicular cells, and thyroid neoplasms. However, PAX2 is usually negative in thyroid neoplasms, making itself a better marker to use in the distinction between RCC and thyroid carcinoma. Positive staining is also reported in neuroendocrine tumors and B-cell lymphoma due to antibody cross-reactivity with other members of the PAX gene family.
Expression of PAX8 in normal and neoplastic renal tissues. PAX8 is expressed throughout renal tubules, but more intensely in distal tubules and collecting ducts (a), and weakly in urothelial lining the renal papillae and minor calyx/renal pelvis (b). PAX8 expresses in the majority of renal cell neoplasms, including medullary RCC and metastatic RCCs (c, d), and therefore PAX8 is considered the most useful marker to confirm a diagnosis of renal cell origin
RCC Marker (RCC Ma)
RCC Ma is a monoclonal antibody raised against a glycoprotein on the brush border of proximal renal tubules. It is considered a “renal” marker as its expression is found in approximately 80% of renal cell neoplasms, present in almost all low-grade clear cell (cc) and papillary (p) RCC [13]. Its expression in other renal tumors is widely variable and the staining is often focal. It is absent in oncocytoma and collecting duct carcinoma (CDC). Its main disadvantage is the poor specificity with expression reported in many other nonrenal tumors, including neoplasms of parathyroid, salivary gland, breast, lung, colon, adrenal gland, testicular germ cell tumors, and mesothelioma. Its use to support the renal origin of a poorly differentiated tumor is now largely supplanted by other more sensitive and specific renal markers (i.e., PAX8 and PAX2).
CD10
CD10 is a cell-surface glycoprotein expressed on the proximal renal tubular epithelial cells and podocytes and many renal tumors with the expression pattern similar to that of RCC Ma. It was therefore considered a useful marker to support the renal origin of a poorly differentiated neoplasm. Almost all ccRCCs and pRCCs are positive for this marker while other types of renal cell neoplasms are negative. Unfortunately, CD10 is even less specific than RCC Ma. Its expression is reported in wide array of nonrenal tumors, including carcinomas of lung, colon, ovary, and urinary bladder, and mesenchymal tumors such as atypical fibroxanthoma, fibrous histiocytoma, endometrial stromal sarcoma, and lymphomas. CD10 has fallen out of favor with the advent of PAX8/PAX2.
Human Kidney Injury Molecule-1 (hKIM-1)
hKIM-1 is a type I transmembrane glycoprotein expressed in injured proximal renal tubules. Its expression is also detected in the majority of ccRCCs and pRCCs [14]. None or rare cases of chRCC and oncocytoma express this marker. It is therefore a relatively sensitive (80%) and specific (90%) marker for ccRCC and pRCC, and metastatic RCCs. However, its expression is also detected in the majority (93.8%) of ovarian clear cell carcinoma, one-third of endometrial clear cell carcinoma, and infrequently in colonic adenocarcinoma, limiting its use to narrow clinical circumstances.
Vimentin
Vimentin is found in majority of proximal origin RCCs, such as ccRCC and pRCC, but it is negative on oncocytoma and chRCC. It by itself is not a specific renal marker as it is a broad mesenchymal marker and its expression is found in wide range of neoplasms. Co-expression of vimentin and CK, however, is limited to RCC and a few other carcinomas including endometrioid carcinoma, thyroid carcinoma, and mesothelioma. Therefore, co-expression of vimentin and CK suggests RCC as one of the possible diagnoses.
Cytokeratins (CKs)
As an epithelial marker, pan-CK expressed in most RCCs while down expressed in high grade, sarcomatoid RCC, succinate dehydrogenase deficient (SDH) RCC and MiTF translocation RCCs. Differential CKs are mainly used to discriminate different types of renal neoplasms. The most widely expressed CK was CK7 that was present in 87% pRCCs, 73% of chRCCs, 83% of CDCs and almost 100% clear cell papillary (ccp) RCCs (Fig. 15.2a–c) and mucinous tubular and spindle cell carcinomas (MTSCCs). Lower expression of CK7 was found in ccRCC (20%) and tubulocystic RCC (33%). CK7 was negative or very focally/scattered positive in oncocytoma (Fig. 15.2d). CK5/6 were expressed in 75% of urothelial carcinoma, 17% CDC/medullary RCC, while negative in most other renal tumor types.
Markers That Are Differentially Expressed in Different RCC Subtypes
Different histological subtypes of RCC are postulated to be derived from, or differentiate toward, different parts of nephron units which have distinct immunoprofiles. Therefore, renal tumors may be classified based on their immunoprofiles that recapitulate those of the normal nephrons. For example, CD10 and RCC Ma are found on the proximal renal tubules and in ccRCC and pRCC that are derived from the proximal renal tubules. Kidney-specific cadherin (Ksp-cadherin), parvalbumin, claudins, and S100A are found on the distal nephrons and corresponding chRCC and oncocytomas. High molecular weight (HMW) CKs are detected on collecting ducts of Bellini and the namesake CDCs. However, caution is required, because morphology–-immunophenotype concordance is not necessarily perfect. Such discordance occurs as the result of heterogeneity in tumor biology and technicality of IHC. Furthermore, most published studies utilized morphologically straightforward cases but not genetically confirmed difficult cases with ambiguous morphology. One has also to bear in mind that the published immunoprofiles are generally derived from studies of renal tumors of typical morphology. A poorly differentiated often retains at least partially the characteristic immunoprofile of the renal tumors of the same histological class. However, significant deviation from the “typical” immunoprofile of a particular renal tumor type can occur and may impact the utility of these IHC markers in the classification of renal tumors. It should be emphasized that IHC plays a supportive, rather than primary and definitive, role in the histological classification of RCC, and is best applied in the context of differential diagnosis. Therefore, while a concordant immunoprofile supports classifying the tumor under study into the subtype with that immunoprofile, a lack of concordance does not invalidate that classification.
Carbonic Anhydrase IX (CA9)
CA9 is a transmembrane protein of the carbonic anhydrase family that regulates intracellular pH as well as the transfer of CO2 across the renal tubules. It is regulated by hypoxia inducible factor (HIF) and considered a marker for tissue hypoxia. CA9 is not expressed in healthy renal tissue as opposed to other carbonic anhydrase family members. It is instead expressed in most cc RCC through HIF-1α accumulation driven by hypoxia or inactivation of the VHL gene [11, 15]. The staining pattern in ccRCC is circumferential membranous, “box shape” and is usually diffusely positive in most or all tumor cells (Fig. 15.3a). Focal staining is seen in up to one-fourth of cases, typically in high-grade cancer. Its expression is also detected in ccpRCC with a unique “cup-like” pattern with staining decorating the basolateral, but not the apical portion of cells lining glandular and cystic spaces (Fig. 15.2b). Its expression may also be detected in other high-grade tumors in the kidney including CDC and pelvic urothelial carcinoma, and can be seen adjacent to tumor necrosis due to ischemia and hypoxia. CA9 is usually not expressed in chRCCs and oncocytomas.
CA9 expression is also seen in many non-renal tumors, including tumors of endometrium, stomach, cervix, breast, lung, liver, neuroendocrine tumors, mesotheliomas, and brain tumors. Therefore, CA9 has limited value in distinguishing renal versus nonrenal carcinomas. It is mainly used to confirm a diagnosis of ccRCC or ccpRCC.
α-Methylacyl Coenzyme A Racemase (AMACR)
AMACR is a mitochondrial enzyme involved in the oxidation of branched chain fatty acids and bile acid [16]. In the kidney, it is expressed in the proximal renal tubules. Majority of pRCC, both type 1 and 2, are positive for AMACR as granular cytoplasmic staining [17] (Fig. 15.4). Its expression is also found in MTSCCA, tubulocystic RCC, MiTF translocation RCC, but not in ccRCC, ccpRCC, oncocytomas, and chRCC. Therefore, a positive AMACR staining provides support for a morphological diagnosis of papillary RCC.
AMACR is found in a wide array of non-renal tumors, most commonly in prostate adenocarcinoma, rendering itself of little use in distinguishing renal from nonrenal tumors.
Parvalbumin
Parvalbumin is a calcium-binding protein involved in the intracellular calcium homeostasis. In the kidney, its expression is limited to the distal nephrons from which chRCC and oncocytomas are postulated to be derived. In support of such a histogenic derivation, parvalbumin expression is detected in these two subtypes of renal cell neoplasms, but is absent in other subtypes [18]. Therefore, parvalbumin immunostains may be used to differentiate oncocytoma and chRCC from other renal tumors with similar “oncocytic” cytoplasm.
E-Cadherin and Kidney-Specific Cadherin (Ksp-Cadherin)
E-cadherin is a calcium-dependent cell–cell adhesion glycoprotein. It is normally expressed in many cell types including renal tubular epithelial cells. Ksp-cadherin is an isoform of E-cadherin whose expression is exclusively found on the basolateral cell membranes of the distal convoluted tubules and collecting ducts [19]. Both E-cadherin and ksp-cadherin are expressed in almost all chRCC and oncocytomas, but variably in other subtypes, including CDC, MiTF translocation RCC, MTSCCA, and urothelial carcinoma. They are usually negative in ccRCC and pRCC. Therefore, E-cadherin and ksp-cadherin may be used to distinguish chRCC and oncocytoma from other renal tumors with “oncocytic cytoplasm.”
E-cadherin expression is commonly seen in other nonrenal tumors, often with positive staining in high percentage of tumor cells, including lung, breast, and bladder carcinomas, rendering it unsuitable for differentiating renal from nonrenal tumors.
CD117
CD117, or c-Kit, is a receptor tyrosine kinase that, upon binding to its ligands, phosphorylates and activates signal transduction molecules that propagate signals in cells and plays a critical role in cell survival, proliferation, and differentiation. Most chRCC and oncocytomas are positive for CD117 [20] (Fig. 15.5). However, no mutations were identified in exons 9 and 11 of c-Kit gene the presence of which corresponds to the therapeutic response to Gleevec in gastrointestinal stromal tumors. ccRCC and pRCC are in general negative for CD117. Its expression has also been described in sarcomatoid RCC [21] and a small portion of pelvic urothelial carcinomas.
S100A1
A member of S100 gene family, S100A1 is a calcium-binding protein and its expression is found in nephrons in the adult kidney. It is expressed in most oncocytomas, but in significantly lower percentage of chRCC cases. Such a differential expression pattern may aid the distinction of these two tumors. Its expression, however, is also found in majority of ccRCC and pRCC.
TFE3, TFEB, and Cathepsin K
TFE3 protein is encoded by TFE3 gene on chromosome Xp11.2, and TFEB protein is encoded by TFEB gene on chromosome 6p21. Both genes are members of “microphthalmia transcription factor/transcription factor E (MiTF/TFE)” gene family. RCCs harboring chromosomal translocations involving respective genes overexpresses TFE3 and TFEB proteins which can be detected by IHC [22,23,24]. Although molecular genetic analysis for the chromosomal translocation involving TFE3 and TFEB genes provides the most definitive evidence, IHC stains for TFE3 and TFEB proteins are simple, sensitive, specific, and highly correlate with the TFE3 and TFEB gene status in these tumors. TFE3 is undetectable in normal kidney tissues. TFE3 fusion protein, in contrast, is overexpressed in Xp11 translocation RCC and is detected in over 95% of Xp11.2 translocation RCC confirmed molecularly (Fig. 15.6). However, TFE3 immunostaining can rarely be seen in tumors other than Xp11.2 translocation RCC, as its expression is detected in many perivascular epithelioid cell tumors (PECOMA) of soft tissue and gynecological tract, a subset of which indeed harbors TFE3 gene alteration. Rarely, TFE3 immunostain is also detected in other tumors, including adrenal cortical carcinoma, granular cell tumor, bile duct carcinoma, and high-grade myxofibrosarcoma. The IHC stain for TFEB protein is both sensitive and specific for RCC associated with TFEB translocation, and is not detectable in other neoplasms. Weak nuclear staining for TFEB is rarely detected in scattered normal lymphocytes. The most significant issue with the IHC detection of TFE3 and TFEB proteins is that the staining is susceptible to tissue fixation. Inconsistent staining results are often encountered, especially when the staining is performed on an automatic stainer. Some staining protocols call for manual staining.
Cathepsin K is transcriptionally regulated by members of the MiTF/TFE gene family. Its overexpression is seen in all TFEB RCC and 60% of TFE3 RCC, but none of the other RCC subtypes [25, 26]. Its expression in nonrenal carcinomas is rare (2.7%), although very common in mesenchymal tumors (>50%). These findings suggest that cathepsin K may be used as a surrogate marker for TFE3 and TFEB overexpression and is a highly specific marker for translocation RCC.
Markers for Urothelial Lineage Differentiation
Markers for urothelial lineage differentiation, including p63, thrombomodulin, uroplakin and GATA3, are expressed in high percentage of urothelial carcinoma but not in RCC, and therefore, can be used in the diagnosis of a poorly differentiated carcinoma where the differential diagnosis is between a urothelial carcinoma and RCC [27]. One caveat is that some of these “urothelial” markers including uroplakin, GATA3, and p63 have found to be expressed in a small fraction of RCC, particularly CDC, but usually weak and focal.
Differential Cytokeratins (CKs)
Different types of CK are expressed in different renal tumors and can be taken advantage of for the purpose of differential diagnosis (also see above). For example, CK18, a low molecular weight cytokeratin expressed in simple epithelia, is detected while CK20 is virtually absent in all major renal tumors except the newly described eosinophilic solid cystic RCC [28, 29] CK7, a low molecular weight cytokeratin, is expressed in pRCC (predominantly type 1), ccpRCC, chRCC, CDC, and urothelial carcinoma. It is usually negative in oncocytoma and ccRCC. High molecular weight cytokeratins (HMWCKs), detected by antibody clone 34βE12 and CK5/6, in contrast, are expressed in the majority of CDC and almost all urothelial carcinoma and significant proportion of ccpRCC, but uncommonly in other RCC subtypes.
Clinically several CK monoclonal antibody clones are used, including AE1/3, CAM5.2, 34βE12, and CK5/6. AE1/3 is considered a pan-CK as it detects both LMW (CK7, 8, and 19) and HMW (CK10, 14–16) CKs, but it lacks reactivity to CK18, a CK almost ubiquitously present in simple epithelia, including renal tumors. Notably, AE1/3 is positive in only one-third ccRCC and one-fourth of translocation RCC. If one wishes to confirm the carcinomatous nature of a poorly differentiated tumor in the kidney, a panel of markers, including AE1/3, CAM5.2, and CK18, is recommended to be used.
Immunophenotype of Common Renal Tumors
Clear Cell Renal Cell Carcinomas (ccRCCs)
ccRCCs are commonly reactive for kidney-specific transcriptional factors including PAX2 and PAX8 (Fig. 15.1). Most also react with brush border antigens such as RCC Ma and CD10, low molecular weight cytokeratins (LMWCKs), epithelial membrane antigen (EMA), and vimentin. HMWCKs are rarely expressed. CA9, a downstream target gene of hypoxia inducible pathway, is expressed in the majority of cases (Fig. 15.3). CK7, CK20, CD117, epithelial cadherin, parvalbumin, and CD117 are usually negative. Some ccRCCs demonstrate a focal “pseudopapillary” growth pattern secondary to tumor cell dropout sparing the cells at the periphery of blood vessels. Unlike pRCC, histiocytes, and intracellular hemosiderin are usually absent from the papillae. Classic ccRCC foci are usually evident elsewhere, but if necessary, CK7, and CA9 may be useful as CK7 often is expressed in pRCC, but not by ccRCC and CA9 has a reverse expression pattern.
Multilocular Cystic Renal Cell Neoplasm of Low Malignant Potential
It has almost identical immunoprofiles with classic low-grade ccRCC. The clusters of epithelial cells within the septa react with antibodies to CKs, EMA, CK7, and CA9, but not histiocytic markers.
Clear Cell (Tubulo) Papillary Renal Cell Carcinomas (ccpRCCs)
ccpRCCs have a characteristic immunophenotype. Tumor cells are diffusely positive for CK7 (Fig. 15.2b), and positive for CA9 with a “cup-shaped” staining pattern (Fig. 15.3b), but negative for CD10, AMACR, and TFE-3. Due to the presence of papillary structures lined with clear cells, ccRCCs, and pRCCs often enter into the differential diagnosis for ccpRCC. However, ccRCC does not contain extensive papillary structures, and are positive for CD10 but negative for CK7. pRCC, on the other hand, does not harbor a prominent clear cell component, and is positive for CD10 and AMACR. RCC associated with Xp11.2/TFE3 translocation typically affects children and young adults. Papillae lined with tumor cells with abundant clear and granular cytoplasm are characteristic. However, tumor cells have high-grade nuclei and are positive for TFE-3 and negative for CKs.
Papillary Renal Cell Carcinomas (pRCCs)
pRCCs are usually strongly reactive with antibodies to panCKs and LMWCKs, but only rarely with antibodies to HMWCKs. CK7 expression is more frequent in type 1 (87%) than in type 2 (20%) (Fig. 15.2a). Reactivity for vimentin and EMA is variable and inconsistent. CD10, AMACR, RCC Ma, PAX2, and PAX8 are expressed in pRCC. Several other renal tumors should be differentiated from pRCC. Lesions 1.5 cm or less than 1.5 cm with low grade (Fuhrman grade 1 or 2) and lacking of distinct capsule have been designated papillary adenomas while those larger than 1.5 cm are classified as pRCCs. ccpRCCS are small tumors arising frequently in end-stage kidney disease with cystic and fibrotic background. ccpRCC characteristically has low-grade tumor cells and clear cytoplasm. Nuclei are characteristically linearly polarized away from the basement membrane toward the luminal surface (reverse polarization of nuclei). Tumor cells are diffusely positive for CK7, positive for CA9 with “cup-like” staining pattern, and negative for CD10. pRCC can be confused with ccRCC exhibiting a pseudopapillary growth pattern. Such pseudopapillae are typically devoid of the fibrovascular cores of pRCCs. ccRCC is also much less likely to show calcification, cytoplasmic hemosiderin and islands of foamy macrophages expanding papillae. Tumor cells of ccRCC express CA9, but lack CK7 and AMACR expression which is frequently seen in the pRCCs. CDC with papillary features can be distinguished from pRCC by its medullary location in the kidney, desmoplastic stromal reaction, its high-grade features, and intracytoplasmic, and luminal mucin. In contrast to CDC, pRCC rarely demonstrates a desmoplastic stroma. Immunohistochemically, unlike most pRCCs, CDC reacts with CEA, and HMWCKs. Metanephric adenoma is well circumscribed, but does not have a tumor capsule. Tumor cells have scant cytoplasm and uniform and round nuclei, and are negative for cytokeratin but positive for WT-1. WT-1 is frequently detectable in the nuclei of metanephric adenomas. The cells of metanephric adenoma are positive for PAX2, PAX8, and CD57, frequently negative for EMA, CK7, cytokeratin CK AE1/AE3, CD56, and AMACR. CD57 and WT1 are positive, and BRAF V600E is positive in 90% metanephic adenoma [30, 31].
Therefore, before to render the diagnosis of metanephric adenoma, pan-CK, and CK7 should be performed and if CK is positive, this tumor is most likely not metanephric adenoma.
Chromophobe Renal Cell Carcinoma (chRCC)
Antibody to cytokeratin 7 is useful in diagnosing chRCC. It typically reacts strongly with the great majority of cells and the reaction is accentuated at the cell membrane (Fig. 15.2c). E-cadherin, kidney-specific cadherin, parvalbumin, and CD117 are also diffusely positive in chRCCs (Fig. 15.5). Vimentin, CA9, and AMACR are negative. Classic chRCC including distinct plant-like cell membrane, raisinoid nuclei, and perinuclear clearing often can be diagnosed with confidence on hematoxylin and eosin sections. ccRCC occasionally enters into the differential diagnosis and the correct diagnosis is usually achieved by adequate sampling, diffusely positive CK7 and E-cadherin staining, and negative CA9 staining. Eosinophilic chRCC closely resembles renal oncocytoma. In oncocytomas, Hale’s colloidal iron stain is negative and only scattered cells or small clumps of cells react with antibody to cytokeratin 7.
MiT Family Translocation RCC
This tumor underexpresses epithelial markers such as CKs and EMA. CD10, RCC Ma, PAX2, and PAX8 are consistently expressed. Melanocytic markers such as HMB-45 and Melan A are positive in some tumors. Nuclear immunoreactivity for TFE3 gene product is confirmatory (Fig. 15.6). Cathepsin K, whose expression is modulated by MiTF and other members of MIT family including TFE3, is detected in 60% of Xp11.2/TFE3 translocation RCCs.
Collecting Duct Carcinoma (CDC) of Bellini
There is no specific immunoprofile for CDCs. Most cases stain with LMWCK and broad-spectrum CKs, CEA, peanut lectin agglutinin (PNA) and Ulex europaeus agglutinin (UEA). The majority expresses HMWCK 34βE12, CK7, PAX2, and PAX8. CD10 and P63 are usually negative. The differential diagnosis includes pRCC (discussed earlier), hereditary leiomyomatosis RCC syndrome, urothelial carcinoma with glandular differentiation, medullary carcinoma, and metastatic carcinoma. Urothelial carcinoma presents the most difficult challenge since it shares many features with CDC such as the propensity to form tubular glands, desmoplastic stroma, tumor cells in adjacent collecting tubules, and intracytoplasmic mucin. The finding of in situ urothelial carcinoma within adjacent calyces or the renal pelvis is supportive of the diagnosis of urothelial carcinoma. Therefore, generous sampling of renal pelvis and proximal ureter with careful microscopic examination is mandatory. Additionally, the immunohistochemical profile of urothelial carcinoma is PAX8-/P63+/GATA3+. Hereditary leiomyomatosis RCC syndrome typically affects young patients who may also be afflicted with multiple cutaneous leiomyomas and early-onset uterine fibroids. Tumor cells characteristically have very prominent nucleoli that resemble melanoma nuclei or cytomegalovirus inclusions.
Renal Medullary Carcinoma
The main differential diagnosis of renal medullary carcinoma includes CDC and high-grade invasive urothelial carcinoma. The tumor cells are positive for keratin AE1/AE3, PAX2, and PAX8, and variably for EMA and CEA. They are negative for HMWCK and immunohistochemical loss of expression of the nuclear transcriptional regulator SMARCB1 (INI1) is seen in all renal medullary carcinoma but not in other types of RCCs except a small portion of CDCs [32] (Fig. 15.7). OCT3/4 also stains medullary carcinoma, usually not for CDC or urothelial carcinomas [33].
Tubulocystic RCC
The immunoprofile is closely related to pRCC with diffuse positivity with AMACR, CD10, PAX2, and PAX8 staining. CK 7 staining is positive in all cases, but is often heterogeneous and weak.
Acquired Cystic Disease-Associated RCC
A consistent immunoprofile is not yet known, but the morphology is so distinct and in general, no immunostains are required. This tumor is known to express AE1/3, CD10, RCC Ma, and AMACR. CK7 is negative, or at most, focally positive.
Hereditary Leiomyomatosis RCC (HLRCC RCC)
The epithelial component of the tumor showed positive immunoreactivity for CK7, CAM 5.2, and CD-10, and negative for smooth muscle actin (SMA). The stromal component is positive for α-SMA and negative for HMB45, CD117, CKs, ER, and PR. Loss of fumarate hydratase (FH) is specific for HLRCC RCC. Loss of FH expression can be confirmed by immunohistochemistry if specific antibody is available. Recently, immunoexpression of S-(2-succinyl cysteine (2SC) has been shown to be of diagnostic utility for HLRCC RCC. 2SC results from the reaction of accumulate fumarate with the cysteine sulfhydryl group of proteins and shows strong nuclear and cytoplasmic expression in these tumors. Most of other types of RCCs are negative for 2SC, although some cytoplasmic staining can be seen in type 2 pRCCs. Together with the loss of FH and high expression of 2SC a very useful ancillary tool in the differentiation of HLRCC RCCs from other high-grade RCCs [34] (Fig. 15.8).
Succinate Dehydrogenase-Deficient Renal Cell Carcinoma (SDH-Deficient RCC)
The tumor cells show variable CK expression and are positive for PAX8 and kidney-specific cadherin. Loss of IHC staining for SDHB is considered a requirement for the diagnosis of SDH-deficient RCC. One should be cautious in interpreting SDHB immunohistochemistry in renal tumors and markedly decreased or weak SDHB staining should not be interpreted as SDHB-deficient RCC [35, 36].
Oncocytoma
Oncocytomas are immunoreactive with antibodies to EMA, most CKs, CD117, E-cadherin, and kidney-specific cadherin. Their CK7 reactivity is focal, decorating scattered single cells and small clusters of cells (Fig. 15.2d). Vimentin is invariably negative.
Metanephric Adenomas
WT-1 is frequently detectable in the nuclei of metanephric adenomas. The cells of metanephric adenoma are positive for PAX2, PAX8, and CD57, frequently negative for EMA, CK7, CK AE1/AE3, CD56, and AMACR. CD57 and WT1 are positive, and BRAF V600E is positive in 90% metanephic adenoma. It is recommended that all adult cases histologically resembling metanephric adenoma have WT1, CD57, CK7, and AMACR immunohistochemical staining performed. If the staining pattern is characteristic for metanephric adenoma (CK7-, AMACR-, WT1+, and CD57+, including membranous staining), then no other diagnostic tests are indicated. However, if there is a different immunostaining pattern, then we recommend FISH analysis [31].
Utility of Immunohistochemistry in Morphological Classification of Renal Tumors
With the exception of TFE3, TFEB, FH, 2-SC, and SDHB, none of the above-mentioned markers are specific for a specific type of renal tumors. Immunostains should then be used to corroborate, rather than to establish, the morphological classification. One should always carefully examine the H&E morphology of the tumor first to generate a differential diagnosis and then apply appropriate markers. A panel of markers is preferred to include markers that support the favored diagnosis and markers that rule out other diagnoses included in the differential diagnosis.
Renal Tumors with Predominantly Clear Cell Nests and Sheets
Besides, ccRCC, many other renal tumors have clear, or pale-staining, cytoplasm at least focally but often as the predominant morphological feature. Their characteristic morphological features should lead to the correct diagnosis, or at least narrow down the differential diagnosis in most cases. An initial panel of markers, including CK7, CA9, and ksp-cadherin (or CD117), is recommended for working up of difficult cases. Additional markers can be performed judiciously based on the differential diagnosis. For example, urothelial markers, including p63, GATA-3, and HMWCK can be stained if urothelial carcinoma is suspected. Adrenal cortical markers including inhibin, calretinin, and MelanA can be performed to rule out intrarenal adrenal cortical tissue. TFE3, TFEB, Cathepsin K, and melanoma-associated markers can be used for the diagnosis of MiTF translocation RCC. HMB45, MART-1 as well as cathepsin K are quite valuable in the diagnosis of epithelioid angiomyolipoma (AML) when initial CK is negative. One important clinical question frequently raised by clinicians is whether a poorly differentiated RCC is a ccRCC or a non-ccRCC for treatment purpose. The tumor should be extensively sampled to look for areas with classical ccRCC morphology. The latter may be minute, but if present and possessing a characteristic immunoprofile (CK7-, CA9+, ksp-cadherin-, p63-), supports the diagnosis of ccRCC.
Renal Tumors with “Oncocytic” Cytoplasm (Pink Cell Tumor)
Oncocytic cytoplasm can be seen in many renal tumors and may pose significant diagnostic challenges. In ccRCC, high-grade tumor cells tend to lose cytoplasmic clarity and acquire oncocytic cytoplasm. The initial panel to work up on a challenging tumor with oncocytic cytoplasm includes CK7, CA9, AMACR, and ksp-cadherin (or CD117). Additional markers can be added if other tumors are suspected, including melanocytic markers for oncocytic AML, and TFE3, TFEB, and cathepsin K for translocation RCC. One frequently faced diagnostic issue is the distinction between an oncocytoma and chRCC, eosinophilic variant. Oncocytomas are characteristically negative or positive in single or small clusters of cells for CK7, diffusely positive CD117, ksp-cadherin, and E-cadherin. ChRCC, on the other hand, is diffusely positive for CK7, CD117, ksp-cadherin, and E-cadherin. Deviation from these characteristic immunoprofiles may justify labeling the tumor as “oncocytic tumor” without further subclassification. For example, an oncocytoma with diffuse CK7 staining is not characteristic and may be labeled as “oncocytic tumor, not otherwise specified,” especially when other atypical features, such as diffuse nuclear atypia, are present. Recently, some new entities of RCCs with oncocytic/eosinophilic cytoplasm have been described and these include SDH-deficient RCC, FH-deficient RCC (HLRCC RCC), and eosinophilic cystic solid RCC (ESC). Li et al. reviewed 33 unclassified RCCs with predominantly eosinophilic cytoplasm in patients aged 35 years or younger and performed IHC for SDHB, FH, and CK20 (a marker of ESC RCC) on all cases. 30% cases were reclassified as ESC RCCs; 24% as SDH-deficient RCCs. 12% as FH-deficient RCCs; and 33% remained unclassified. The authors suggested that pathologists should have a low threshold for performing FH, SDHB, and CK20 IHC when confronted with unclassified eosinophilic RCC or “oncocytoma” in young patients [37].
Renal Tumors with Predominantly Papillary Components
Renal tumors with predominantly papillary components are pRCC, ccpRCC, and MiTF translocation RCCs, although focally papillary pattern is seen in many other renal tumors, especially in high-grade tumors [38]. The initial panel of markers should include CK7, AMACR, and CA9. A high-grade renal tumor with predominantly papillary architecture should elicit a differential diagnosis of type 2 pRCC, CDC, HLRCC RCC, and metastatic adenocarcinoma to the kidney. Except for lineage-specific markers (CDX2, TTF-1, etc.), other markers are considerably variable in their expression pattern in these tumors; therefore, offer little help in the classification of these tumors. Classification of these tumors therefore depends largely on morphology and clinical manifestation.
Renal Tumors with Papillae Covered with Clear Cells as Predominant Features
The differential diagnosis includes ccpRCC, pRCC, and translocation RCC. Characteristic morphological features and immunoprofiles can readily distinguish these three lesions. ccpRCC is a recently described new subtype which behaves in a benign or indolent fashion [39]. Therefore, it is important to distinguish it from ccRCC and pRCC. It has characteristic morphology and immunoprofile (CK7+, CD10-, CA9+ with “cup-shaped” staining pattern,” and AMACR-) [39].
Renal Tumors with Tubulopapillary Architecture in Children and Young Adults
If a renal tumor has tubulopapillary architecture in children and young adults, the differential diagnosis should include pRCC, metanephric adenoma, and epithelial predominant Wilms tumor. With appropriate clinical history and morphology, translocation RCC and metastatic adenocarcinoma may also be considered.
Renal Tumors with High-Grade Infiltrative Growth Pattern
Renal tumors with multinodular growth, desmoplastic stroma, and invasive borders (tumor cells infiltrating between renal tubules and glomeruli at the advancing front) are difficult to classify based on morphology alone. One has to first rule out a metastasis to the kidney. PAX8 and PAX2 are probably the most useful markers owing to their relatively high sensitivity and specificity. If a tumor is deemed likely to originate in the kidney, urothelial carcinoma should always be considered and ruled out as the management for urothelial carcinoma and RCC is drastically different. The presence of urothelial carcinoma in pelvic mucosa and typical staining pattern (CK7+, CK20+, PAX8-, HMWCK+, and p63+) supports a diagnosis of an urothelial carcinoma. Clinical history of hemoglobin disease and loss of INI1 make the diagnosis of medullary RCC. To make a diagnosis of CDC we need to rule out other possibilities, particularly urothelial carcinoma, HLRCC RCC (FH-deficient RCC) and metastatic tumors [32].
Use of Immunohistochemical Markers in the Interpretation of Needle Biopsies of Renal Masses
Needle biopsy of renal masses has recently become more popular in the management of patients with renal masses owing to several reasons. The biopsy aims to clarify at least three questions. (A) Is the renal mass a neoplasm? (B). Is it a primary RCC, or metastatic cancer/lymphoma? (C). What is the histological classification of a primary RCC?
The most significant limitation of renal mass needle biopsy is the small quantity of tissue procured which may limit the morphological evaluation of the renal mass lesion. Consequently, IHC is often employed to supplement the morphological evaluation. A recent study found that standard morphological evaluation and judicious use of five markers (CK7, CD10, CA9, AMACR, and CD117) yielded accurate diagnoses in >90% of cases in an ex vivo needle biopsy study after nephrectomy [9]. When using IHC to work-up a renal mass biopsy, one should use the same, if not more, due diligence as in the workup of nephrectomy specimens. Careful morphological examination should be performed first to generate a list of differential diagnoses. Appropriate markers are then applied and the results are used to corroborate, rather than to establish, the morphological diagnosis.
Prognostic and Predictive Markers
The roles of several genetic pathways, including mammalian target of rapamycin (mTOR) and hypoxia inducible factor (HIF), in renal carcinogenesis and progression have been increasingly elucidated. Key components of these pathways have been investigated for their prognostic and predictive value for targeted therapies. For example, von Hippel–Lindau gene (VHL), a tumor suppressor gene on chromosome 3p25–26, plays a crucial role in HIF pathway. In normal cells, VHL targets HIF for proteasome-mediated degradation and therefore keeps HIF at low level. When VHL gene is inactivated, by gene mutation or promoter hypermethylation, HIF accumulates and activates the downstream target genes, including vascular endothelial growth factor (VEGF) and CA9. Many of these molecules contribute to carcinogenesis in ccRCC. Functional loss of VHL is implicated in hereditary and sporadic ccRCC. However, studies have shown conflicting data on the prognostic value of VHL gene alteration. Loss of function mutation in VHL gene correlated with response to anti-VEGF therapy in some studies.
Several studies have found that the level of CA9 expression seems to have prognostic significance, with low expression (≤85% of tumor cells) correlated with worse overall survival in metastatic RCC, and high CA9 expression (>85%) was predictive of response to IL-2 [40, 41]. In addition, high CA9 expression (>85%) is associated with greater tumor shrinkage in response to sorafenib, a VEGF inhibitor, treatment [42]. However, more recent data from the TARGET study did not find CA9 expression status to be either predictive of clinical benefit for treatment with sorafenib or of prognostic value in patients with metastatic ccRCC following cytokine therapy [43].
Other molecules that have been investigated for their prognostic and predictive roles in RCC include key components of mTOR pathway, B7 family members that are coregulatory molecules inhibiting T-cell-mediated immunity, IMP3 which is a member of the insulin-like growth factor II mRNA-binding protein [44], p53, histone-modifying and chromatin-remodeling genes [45]. However, vast majority of published studies are of single-center research and comprise small number of cases. No marker has so far emerged as being reproducible and consistent across published studies. Therefore, no markers are ready to be recommended in routine clinical use for prognosis and prediction of therapy response. Large, multicenter prospective studies are needed to validate some promising markers. CA9 may be performed at clinician’s request and expression can be quantified as ≤85% or >85%.
Summary
Diagnosis and classification of renal cell neoplasms, based primarily on the routine H&E morphological features, are usually straightforward. IHC markers, however, play an important role in several clinical settings, including distinguishing renal from nonrenal tumors, subtyping of renal cell neoplasms and working up renal mass needle biopsy with limited tissue quantity. These markers include those whose expression supports a renal origin (PAX2/PAX8, RCC Ma, CD10, HKIM-1, and vimentin) and those with differential expression in different renal tumor subtypes (CA9, AMACR, parvalbumin, E-cadherin, ksp-cadherin, claudin 7/8, CD117, S100A1, TFE3, TFEB, cathepsin K, markers of urothelial differentiation, and various CKs). Each marker has its utility in a specific diagnostic setting. A panel of markers should be used to corroborate, but not to supplant, the morphological diagnosis and classification. So far, no markers have proven clinical utility in the prediction of clinical outcomes and response to novel targeted therapy.
References
Zhou M, Roma A, Magi-Galluzzi C. The usefulness of immunohistochemical markers in the differential diagnosis of renal neoplasms. Clin Lab Med. 2005;25(2):247–57.
Skinnider BF, Amin MA. An immunohistochemical approach to the differential diagnosis of renal tumors. Semin Diagn Pathol. 2005;22(1):51–68.
Hammerich KH, Ayala GE, Wheeler TM. Application of immunohistochemistry to the genitourinary system (prostate, urinary bladder, testis, and kidney). Arch Pathol Lab Med. 2008;132(3):432–40.
Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135(1):92–109.
Shen SS, Truong LD, Scarpelli M, Lopez-Beltran A. Role of immunohistochemistry in diagnosing renal neoplasms: when is it really useful? Arch Pathol Lab Med. 2012;136(4):410–7.
Reuter VE, Argani P, Zhou M, Delahunt B, et al. Best practices recommendations in the application of immunohistochemistry in the kidney tumors: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38(8):e35–49.
Zhou M, Deng FM. The utility of immunohistochemistry in the differential diagnosis of renal cell carcinoma. In: Magi-Galluzzi C, Przybycin G, editors. Genitourinary pathology: practical advances. New York: Springer Science; 2015. p. 383–99.
Deng FM, Zhou M. Molecular genetics and immunohistochemistry of renal tumours: translation into clinical practice. Diagn Histopathol. 2016;22(2):73–9.
Al-Ahmadie HA, Alden D, Fines SW, Gopalan A, Touijer KA, Russo P, et al. Role of immunohistochemistry in the evaluation of needle core biopsies in adult renal cortical tumors: an ex vivo study. Am J Surg Pathol. 2011;35(7):949–61.
Alderman MA, Daignault S, Wolf JS Jr, Palapattu GS, Weizer AZ, et al. Categorizing renal oncocytic neoplasms on core needle biopsy: a morphologic and immunophenotypic study of 144 cases with clinical follow-up. Hum Pathol. 2016;55:1–10.
Gupta R, Balzer B, Picken M, Osunkoya AO, Shet T, Alsabeh R, et al. Diagnostic implications of transcription factor Pax 2 protein and transmembrane enzyme complex carbonic anhydrase IX immunoreactivity in adult renal epithelial neoplasms. Am J Surg Pathol. 2009;33(2):241–7.
Ozcan A, de la Roza G, Ro JY, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Arch Pathol Lab Med. 2016;136(12):1541–51.
McGregor DK, Khurana KK, Cao C, Ayala G, Krishnan B, et al. Diagnosing primary and metastatic renal cell carcinoma: the use of the monoclonal antibody ‘Renal Cell Carcinoma Marker’. Am J Surg Pathol. 2001;25(12):1485–92.
Lin F, Zhang PL, Yang XJ, Shi J, Blasick T, Han WK, et al. Human kidney injury molecule-1 (hKIM-1): a useful immunohistochemical marker for diagnosing renal cell carcinoma and ovarian clear cell carcinoma. Am J Surg Pathol. 2007;31(3):371–81.
Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158(3):905–19.
Zhou M, Chinnaiyan AM, Kleer CG, Lucas PC, Rubin MA. Alpha-Methylacyl-CoA racemase: a novel tumor marker over-expressed in several human cancers and their precursor lesions. Am J Surg Pathol. 2002;26(7):926–31.
Molinie V, Balaton A, Rotman S, Mansouri D, De Pinieux I, Homsi T, et al. Alpha-methyl CoA racemase expression in renal cell carcinomas. Hum Pathol. 2006;37(6):698–703.
Young AN, de Oliveira Salles PG, Lim SD, Cohen C, Petros JA, Marshall FF, et al. Beta defensin-1, parvalbumin, and vimentin: a panel of diagnostic immunohistochemical markers for renal tumors derived from gene expression profiling studies using cDNA microarrays. Am J Surg Pathol. 2003;27(2):199–205.
Shen SS, Krishna B, Chirala R, Amato RJ, Truong LD. Kidney-specific cadherin, a specific marker for the distal portion of the nephron and related renal neoplasms. Mod Pathol. 2005;18(7):933–40.
Huo L, Sugimura J, Tretiakova MS, Patton KT, Gupta R, Popov B, et al. C-kit expression in renal oncocytomas and chromophobe renal cell carcinomas. Hum Pathol. 2005;36(3):262–8.
Castillo M, Petit A, Mellado B, Palacín A, Alcover JB, Mallofré C. C-kit expression in sarcomatoid renal cell carcinoma: potential therapy with imatinib. J Urol. 2004;171(6 Pt 1):2176–80.
Argani P, Lal P, Hutchinson B, Lui MY, Reuter VE, Ladanyi M. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol. 2003;27(6):750–61.
Argani P, Lae M, Hutchinson B, Reuter VE, Collins MH, Perentesis J, et al. Renal carcinomas with the t(6;11)(p21;q12): clinicopathologic features and demonstration of the specific alpha-TFEB gene fusion by immunohistochemistry, RT-PCR, and DNA PCR. Am J Surg Pathol. 2005;29(2):230–40.
Camparo P, Vasiliu V, Molinie V, Couturier J, Dykema KJ, Petillo D, et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am J Surg Pathol. 2008;32(5):656–70.
Martignoni G, Pea M, Gobbo S, Brunelli M, Bonetti F, Segala D, et al. Cathepsin-K immunoreactivity distinguishes MiTF/TFE family renal translocation carcinomas from other renal carcinomas. Mod Pathol. 2009;22(8):1016–22.
Martignoni G, Bonetti F, Chilosi M, Brunelli M, Segala D, Amin MB, et al. Cathepsin K expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Mod Pathol. 2012;25(1):100–11.
Albadine R, Schultz L, Illei P, Ertoy D, Hicks J, Sharma R, et al. PAX8 (+)/p63 (−) immunostaining pattern in renal collecting duct carcinoma (CDC): a useful immunoprofile in the differential diagnosis of CDC versus urothelial carcinoma of upper urinary tract. Am J Surg Pathol. 2010;34(7):965–9.
Trpkov K, Abou-Ouf H, Hes O, Lopez JI, Nesi G, Comperat E, et al. Eosinophilic Solid and Cystic Renal Cell Carcinoma (ESC RCC): further morphologic and molecular characterization of ESC RCC as a distinct entity. Am J Surg Pathol. 2017;41(10):1299–308.
Palsgrove DN, Li Y, Pratilas C, Lin MT, Pallavajjalla A, Gocke C, et al. Eosinophilic Solid and Cystic (ESC) renal cell carcinomas harbor TSC mutations: molecular analysis supports an expanding clinicopathologic spectrum. Am J Surg Pathol. 2018; https://doi.org/10.1097/PAS.0000000000001111.
Choueiri TK, Cheville J, Palescandolo E, Fay AP, Kantoff PW, Atkins MB, et al. BRAF mutations in metanephric adenoma of the kidney. Eur Urol. 2012;62(5):91722.
Kinney SN, Eble JN, Hes O, Williamson SR, Grignon DJ, Wang M, et al. Metanephric adenoma: the utility of immunohistochemical and cytogenetic analyses in differential diagnosis, including solid variant papillary renal cell carcinoma and epithelial-predominant nephroblastoma. Mod Pathol. 2015;28(9):1236–48.
Ohe C, Smith SC, Sirohi D, Divatia M, de Peralta-Venturina M, Paner GP, et al. Reappraisal of morphologic differences between renal medullary carcinoma, collecting duct carcinoma, and fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol. 2018;42(3):279–92.
Rao P, Tannir NM, Tamboli P. Expression of OCT3/4 in renal medullary carcinoma represents a potential diagnostic pitfall. Am J Surg Pathol. 2012;36(4):583–8.
Chen YB, Brannon AR, Toubaji A, Dudas ME, Won HH, Al-Ahmadie HA, et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol. 2014;38(5):627–37.
Gill AJ, Pachter NS, Clarkson A, Tucker KM, Winship IM, Benn DE, et al. Renal tumors and hereditary pheochromocytoma-paraganglioma syndrome type 4. N Engl J Med. 2011;364:885–6.
Gill AJ, Hes O, Papathomas T, Šedivcová M, Tan PH, Agaimy A, et al. Succinate dehydrogenase (SDH)-deficient renal carcinoma: a morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol. 2014;38(12):1588–602.
Li Y, Reuter VE, Matoso A, Netto GJ, Epstein JI, Argani P. Re-evaluation of 33 ‘unclassified’ eosinophilic renal cell carcinomas in young patients. Histopathology. 2018;72(4):588–600.
Deng FM, Kong M, Zhou M. Papillary or pseudopapillary tumors of the kidney. Semin Diagn Pathol. 2015;32:124–39.
Aydin H, Chen L, Cheng L, Vaziri S, He H, Ganapathi R, et al. Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol. 2010;34(11):1608–21.
Atkins M, Regan M, McDermott D, Mier J, Stanbridge E, Youmans A, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11(10):3714–21.
Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol. 2010;58(1):75–83.
Choueiri TK, Regan MM, Rosenberg JE, et al. Carbonic anhydrase IX and pathological features as predictors of outcome in patients with metastatic clear-cell renal cell carcinoma receiving vascular endothelial growth factor-targeted therapy. BJU Int. 2010;106:772–8.
TK C, Cheng S, Qu AQ, Pastorek J, Atkins MB, Signoretti S. Carbonic anhydrase IX as a potential biomarker of efficacy in metastatic clear-cell renal cell carcinoma patients receiving sorafenib or placebo: analysis from the treatment approaches in renal cancer global evaluation trial (TARGET). Urol Oncol. 2013;8:1788–93.
Jiang Z, Chu PG, Kang Y, Lee SS. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006;7(7):556–64.
Hakimi AA, Chen YB, Wren J, Gonen M, Abdel-Wahab O, Heguy A, et al. Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol. 2013;63(5):848–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Deng, FM., Zhai, Q.J. (2020). Application of Immunohistochemistry in Diagnosis of Renal Cell Neoplasms. In: Divatia, M., Ozcan, A., Guo, C., Ro, J. (eds) Kidney Cancer. Springer, Cham. https://doi.org/10.1007/978-3-030-28333-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-28333-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28332-2
Online ISBN: 978-3-030-28333-9
eBook Packages: MedicineMedicine (R0)