Abstract
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is a syndrome with different causes, characterised by clinical evidence of myocardial infarction with normal or near-normal coronary arteries on angiography. Its prevalence ranges between 5% and 25% of all myocardial infarction. The prognosis is extremely variable, depending on the cause of MINOCA. The key principle in the management of this syndrome is to clarify the underlying individual mechanisms to achieve patient-specific treatments. Clinical history, electrocardiogram, cardiac enzymes, echocardiography, coronary angiography and left ventricular angiography represent the first level diagnostic investigations to identify the causes of MINOCA.
Regional wall motion abnormalities at left ventricular angiography limited to a single epicardial coronary artery territory identify an ‘epicardial pattern’whereas regional wall motion abnormalities extended beyond a single epicardial coronary artery territory identify a ‘microvascular pattern’. The most common causes of MINOCA are represented by coronary plaque disease, coronary dissection, coronary artery spasm, coronary microvascular spasm, Takotsubo cardiomyopathy, myocarditis, coronary thromboembolism, other forms of type 2 myocardial infarction and MINOCA of uncertain aetiology. This review aims at summarising the diagnosis and management of MINOCA, according to the underlying physiopathology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Clinical Case
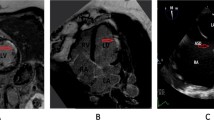
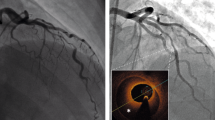
A 65 years old female patient with systolic hypertension, dyslipidemia, history of transient ischemic attack and diagnosis of Patent Foramen Ovale (PFO) subjected to a percutaneous closure procedure with Amplatzer device 1 year ago, was referred to our Hospital for No-ST segment elevation myocardial infarction (NSTEMI). The electrocardiogram ( ECG) showed diffuse repolarization abnormalities (Fig. 7.1), and the peak of high sensible (HS) Troponine T was 0.168 ng/mL. Trans-thoracic echocardiogram revealed a slightly hypertrophic left ventricle (LV), with normal volumes and preserved global and regional function, and Amplatzer device localized in the atrial septum. The contrast echocardiography did not show passage of microbubbles from the right to the left atrium both at rest and after the Valsalva maneuver, rulling out the hypothesis of acute myocardial infarction (AMI) caused by embolization. Coronary angiography did not point out the presence of significant coronary stenosis, while intra-coronary ergonovine administration (up to 50 μg) caused diffuse ST-segment depression at ECG and symptoms, in absence of epicardic coronary spasm (Fig. 7.2, panel A and B). For this reason, the diagnosis of MI and non-obstructive coronary arteries ( MINOCA) due to coronary microvascular spasm (CMS) was done. The medical therapy was optimized with the add of calcium blockers and the patient was discharged without symptoms 7 day after. She remained asymptomatic until 4 years later, when she was admitted at our Hospital for sinus nodal disease and underwent to the procedure of pacemaker implantation.
Introduction
MINOCA is a syndrome with different causes, characterized by clinical evidence of MI with normal or near-normal coronary arteries on angiography [1, 2] (Table 7.1). Data from large MI registries suggest a prevalence between 5% and 25% [3], but the most recent study, in a contemporary cohort of patients, reported a prevalence of 8.8%, which appears to reflect daily clinical experience [4]. Of note, the prognosis of MINOCA is not as benign as reported by early cohort studies and as commonly assumed by physicians [4]. Moreover, a recent retrospective analysis of patients enrolled in the ACUITY trial showed that, compared with NSTEMI patients and obstructive coronary arteries disease (CAD), patients with MINOCA had a higher adjusted risk of mortality at 1 year, driven by a greater non-cardiac mortality [4]. Recently, compared to patients with obstructive CAD, those with MINOCA showed both physical and mental distress from 6 weeks to 3 months after the acute event and, in some perspectives, even lower scores especially in the mental component of quality of life [5]. MINOCA patients represent a conundrum given the very many possible aetiologies and pathogenic mechanisms associated with this syndrome [6]. For this reason, the key principle in the management of this syndrome is clarify the underlying individual mechanisms to achieve patient-specific treatments.
Clinical history, ECG, cardiac enzymes, echocardiography, coronary angiography and left ventricular ( LV) angiography, represent the first level diagnostic investigations to identify the causes of MINOCA. In particular, regional wall motion abnormalities at LV angiography limited to a single epicardial coronary artery territory identify an “epicardial pattern”, whereas regional wall motion abnormalities extended beyond a single epicardial coronary artery territory identifiy a “microvascular pattern” [1]. In this context, the most common causes of MINOCA that the clinicians must consider are: coronary plaque disease, coronary dissection, coronary artery spasm (CAS), CMS, Takotsubo Syndrome ( TTS), myocarditis, coronary thromboembolism, other forms of type-2 MI and MINOCA of uncertain aetiology [7] (Fig. 7.3 and Table 7.2).
Clinical history, ECG, cardiac enzymes, echocardiography, coronary angiography and left ventricular (LV) angiography, represent the first level diagnostic investigations to identify the causes of myocardial infarction without obstructive coronary artery obstruction (MINOCA). In particular, regional wall motion abnormalities at LV angiography limited to a single epicardial coronary artery territory identify an “epicardial pattern”, whereas regional wall motion abnormalities extended beyond a single epicardial coronary artery territory identify a “microvascular pattern”. The most common epicardial causes of MINOCA are represented by: coronary plaque disease, coronary dissection and coronary artery spasm (CAS). The principal microvascular causes of MINOCA are: coronary microvascular spasm (CMS), Takotsubo Syndrome, myocarditis, coronary embolism
Epicardial Causes of MINOCA
Coronary Plaque Disease
Plaque rupture (PR) and erosion ( PE) are comprised within type-1 AMI in the Universal Definition of MI, even when no thrombus can be found [8]. Of note, MINOCA comprises 5–20% of all type-1 AMI cases [7].
Two independent studies using intravascular ultrasound ( IVUS) identified PR and PE in 40% of patients with MINOCA [7, 8]. Higher resolution intracoronary images, like optical coherence tomography (OCT), would likely show an even higher prevalence of PR and PE but this technique has not been routinely applied in controlled studies within the MINOCA population. Calcified nodule with thrombus has also been suggested as a cause of AMI on intracoronary imaging [9]. PR or PE may occur in areas of the vessel appearing normal on conventional angiography or presenting some degree of atherosclerosis, even if minimal.
Myonecrosis in MINOCA with PR and PE is mediated by thrombosis, thromboembolism, superimposed vasospasm, or a combination of these processes. The theory of spontaneous thrombolysis or autolysis of a coronary thrombosis has been proposed. Spontaneous thrombolysis is thought to be an endogenous protective mechanism against thrombus formation even in the presence of a PR [10]. In this context, cardiac magnetic resonance (CMR) imaging may show large areas of myocardial oedema with or without small areas of necrosis among patients with MINOCA and plaque disruption, suggesting that flow was compromised transiently in a larger vessel. However, the theory that spontaneous coronary thrombolysis rather than vasospasm leads to this appearance can neither be dismissed nor proved, and both may play a role. CMR imaging can shows a smaller, well-defined area of late gadolinium enhancement ( LGE), subtended by a smaller vessel, suggesting that embolization of athero-thrombotic debris from the disruption site is the most likely mechanism of myonecrosis [11].
Thrombosis and/or thromboembolism almost certainly have a major role in pathogenesis of MINOCA with coronary plaque disease. Considering the limits of coronary angiography, the use of intra-vascular imaging (e.g. OCT and IVUS) seems mandatory.
From the prognostic point of view, the finding of PR on OCT was associated with major adverse cardiac events (MACEs) in a cohort of patients undergoing OCT for acute coronary syndrome (ACS) [1]. Overall, the risk of recurrent MI or death in MINOCA patients is about 2% up to 12 months [12, 13].
Dual antiplatelet therapy is recommended for 1 year followed by lifetime single antiplatelet therapy for patients with suspected or confirmed plaque disruption and MINOCA [14]. Because disruption occurs on a background of non-obstructive CAD, statin therapy is also recommended, even if only a minor degree of atherosclerosis is found.
Coronary Dissection
Spontaneous coronary dissection (SCAD) typically causes an AMI via luminal obstruction, although this may not always be apparent on coronary angiography, prompting a diagnosis of MINOCA [14]. Intramural haematoma of the coronary arteries without intimal tear presents similarly. Intracoronary imaging is crucial in making this diagnosis [14].
Findings can be graded into three types: (1) the classic description is of a longitudinal filling defect, representing the radiolucent intimal flap, there is often contrast staining of the arterial wall with appearance of a double lumen; (2) diffuse long smooth tubular lesions (due to intramural haematoma) with no visible dissection plane that can result in complete vessel occlusion, lesions are typically >30 mm in length with an abrupt change in vessel diameter between normal and diseased segments, there is no response to intracoronary nitrates and there are no atherosclerotic lesions in other coronary segments; (3) multiple focal tubular lesions due to intramural haematoma that mimic atherosclerosis.
The condition is more common among women. Indeed, it is estimated to be responsible for up to 25% of all ACS cases in women<50 years of age [15]. The reasons for the occurrence of coronary dissection are still unclear. However, in the majority of cases when screening is performed, fibromuscular dysplasia is present in other vascular beds [16]. Changes in the intima-media composition due to hormones, pregnancy and delivery have also been implicated. Regarding the prognosis, the in-hospital ad long-term survival has been shown to be excellent. However, the risk of recurrence of acute event has been reported to be high (27% at 5 years) [17]. At present, there is no effective treatment to reduce the long-term risk. A conservative approach is advocated because coronary intervention and stenting tend to cause propagation of the dissection and outcomes are acceptable with medical management [18]. Despite the lack of evidence, beta-blockers and single antiplatelet therapy are considered a cornerstone of medical treatment.
Recently, the SCAD registry reported that most patients (84.3%) received conservative treatment, others underwent percutaneous coronary intervention (PCI) (14.1%) and a minority had coronary artery bypass surgery (0.7%). The in-hospital major adverse event rate was 8.8%, including cardiac arrest (3.9%), cardiogenic shock (2.0%), recurrent MI (4.0%) and unplanned revascularisation (2.5%). Importantly, the 34 patients (4.5%) with peri-partum SCAD had higher in-hospital major adverse events. The incidence of MACE at one month was 8.8%, consisting primarily of recurrent MI (6.1%), stroke/transient ischaemic attack (1.2%) and unplanned revascularisation (2.7%). Peri-partum SCAD and connective tissue disorder were independent predictors of 30-day MACE. Finally, acute in-hospital and one-month survival was good, with only one death (0.1%) reported [19].
Coronary Artery Spasm
The prevalence of CAS ranges between 3 and 95% of MINOCA patients; this wide difference depends on the stimuli used to trigger spasm, definitions of spasm and ethnic reasons [11]. In particular, in a recent study, provocative tests were positive in 46% of patients with MINOCA [20].
CAS usually occurs at a localized segment of an epicardial artery, but sometimes involves 2 or more segments of the same (multifocal spasm) or of different (multivessel spasm) coronary arteries, or may involve diffusely one or multiple coronary branches. CAS results from interaction of 2 components: (1) an usually localized, but sometimes diffuse, hyperreactivity of vascular smooth muscle cells (VSMCs), and (2) a transient vasoconstrictor stimulus acting on the hyperreactive VSMCs. The main cause of VSMCs hyperreactivity seems to be enhanced Rho kinase activity [7]. CAS can occur as vascular smooth muscle hyper-reactivity to endogenous vasospastic substances (as in vasospastic angina) but may also occur in the context of exogenous vasospastic agents (e.g. cocaine or metamphetamines) [21]. Patients with CAS typically refer angina at rest, during the night or early in the morning, associated with a transient ST segment elevation. In absence of ECG documentation, the diagnosis is based on an intracororonary (IC) provocative test, whereas CAS is generally defined as reduction of at least 75% of the vessel caliber together with symptoms/signs of myocardial ischaemia [22]. The diagnostic test with IC ergonovine and Acetylcholine ( Ach) have been shown to be safe and its positive result portends a worse prognosis with regard to both hard clinical endpoints ( death from any cause, cardiac death, readmission for recurrent ACS) and quality of life (worse angina status). The negative prognostic value of positive provocative tests seems mainly related to the induction of epicardial spasm. Accordingly, a calcium antagonist dose reduction or discontinuation was associated with mortality, supporting the crucial role of epicardial spasm in the occurrence of fatal events in our patients. While the IC egonovine test is a well standardized procedure, the provocative test with IC Ach is performed in different ways in different countries [22,23,24] (Table 7.3).
The prognosis is variable. Apart from multivessel CAS, other independent predictors of cardiovascular outcome emerged from studies on the Japanese population: history of out-of-hospital cardiac arrest, smoking, angina at rest alone, organic coronary stenosis, ST-segment elevation during angina, and beta-blockers use [11, 25]. However, it is difficult to extrapolate these findings to Caucasian populations; indeed, while the prevalence of CAS is higher in the Japanese population, its outcome is better in the Caucasian population.
Non-specific vasodilators such nitrates and calcium channel blockers constitute the standard treatment. In case of refractory vasospastic angina (ranging from 10% to 20% of cases), fasudil has been found effective in Japanese patients, although these positive findings cannot be directly extrapolated to Caucasian patients. In selected cases, stent implantation or partial sympathetic denervation [25] can be employed. Utilization of implantable cardiac defibrillators are needed in patients at high risk of spasm-related cardiac death.
Microvascular Causes of MINOCA
Coronary Microvascular Spasm
CMS is characterized by transient myocardial ischaemia, as indicated by ST segment changes and angina, in the presence of non-obstructive coronary arteries. It may be considered the unstable counterpart of chronic microvascular angina [26]. Previous studies showed that about 25% of patients with MINOCA have evidence of CMS [26]. The diagnosis can be made when ergonovine or Ach test reproduces the symptoms usually experienced by the patients and triggered ischemic ECG changes (i.e. ST-segment depression or ST segment elevation of ≥0.1 mV or T-wave peaking in at least 2 contigous leads), in absence of epicardial spasm (>90% diameter reduction) [26].
Although previous studies pointed out excellent results with regard to mortality, angina seems to persist (ranging about 36%) in many patients even on calcium channel blockers [27]. In this case, Fasudil may be considered a possible alternative treatment. Finally, Arrebola-Moreno and Crea proposed that CMS may be able to cause perfusion and contractile abnormalities and cardiac troponin elevations, and therefore to have the potential to lead to adverse clinical outcomes during long-term follow up [28, 29].
Takotsubo Syndrome
TTS is estimated to represent approximately 1–3% of all and 5–6% of female patients presenting with suspected STEMI [30]. Recurrence rate of TTS is estimated to be 1.8% per-patient year [31] About 90% of TTS patients have been shown to be women with a mean age of 67–70 years and around 80% are older than 50 years. Women older than 55 years have a fivefold greater risk of developing TTS than women younger than 55 years and a tenfold greater risk than men. With growing awareness of TTS, male patients are diagnosed more often, especially after a physical triggering event [31]. Moreover, it has been reported that TTS seems to be uncommon in African–Americans and Hispanics while most of the cases reported in the United States have been Caucasians. Furthermore, patients of African-American descent seem to have more in-hospital complications such as respiratory failure, stroke and require more frequently mechanical ventilation compared to Caucasians and Hispanics [32]. With regard to ECG differences, it has been shown that QT prolongation as well as T-wave inversion are more often reported in African- American women with TTS [33]. Of note, regarding gender differences the TTS prevalence in men appears to be higher in Japan. The prevalence of TTS appears to be higher in patients with non-emotional triggers admitted to intensive care units. Moreover, it is likely that subclinical TTS cases remain undetected, especially in non-PCI [34].
TTS often presents as an ACS with ST segment changes. The transient nature of LV dysfunction has puzzled physicians worldwide. Clinical presentation is characterized by acute, reversible heart failure associated with myocardial stunning, in the absence of occlusive CAD.
The revised Mayo clinic diagnostic criteria include: (1) transient hypokinesis, akinesis, or dyskinesis of the LV mid segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often, but not always present. (2) The absence of obstructive CAD or angiographic evidence of acute plaque rupture (though it is recognized that obstructive CAD may pre-date the Takotsubo event in some cases). (3) New electrocardiographic abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin. (4) The absence of pheochromocytoma and myocarditis [35]. The Mayo Clinic Diagnostic Criteria are the most widely known, but exceptions to the rule [e.g. the presence of CAD] are poorly appreciated among physicians and cardiologists. More recently, other research groups have proposed slightly different criteria for TTS, i.e. the Japanese Guidelines, the Gothenburg criteria, the Johns Hopkins criteria, the Tako-tsubo Italian Network proposal, the criteria of the Heart Failure Association (HFA) TTS Taskforce of the European Society of Cardiology (ESC), as well as the criteria recommended by Madias [35]. Thus, there is a lack of a worldwide consensus. Based on current knowledge, new international diagnostic criteria ( InterTAK Diagnostic Criteria, Table 7.4) have been recently developed for the diagnosis of TTS that may help to improve identification and stratification of TTS [36].
The pathophysiological mechanisms responsible for TTS are complex and may vary between patients. Although several mechanisms have been proposed (e.g. multivessel epicardial spasm, catecholamine-induced myocardial stunning, spontaneous coronary thrombus lysis, and acute microvascular spasm) the causes still remain debated. A previous study demonstrated that, irrespectively of its etiology, reversible coronary microvascular dysfunction is a common pathophysiological determinant of TTS [37]. Indeed, the extent of myocardial hypoperfusion at myocardial contrast echocardiography (MCE) was similar in patients with TTS and in patients with ST elevation MI, whereas a transient significant improvement of myocardial perfusion and of LV function during adenosine infusion was observed in the former only.
Left ventriculography after documentation of MINOCA allows the diagnosis of TTS. Although several anatomical TTS variants have been described, four major types can be differentiated based on the distribution of regional wall motion abnormalities (Fig. 7.4) [38]. The most common TTS type and widely recognized form is the (1) apical ballooning type, also known as the typical TTS form, which occurs in the majority of cases [38]. Over the past years, atypical TTS types have been increasingly recognized [38]. These include the (2) midventricular, (3) basal, and (4) focal wall motion patterns. Recently, it has been demonstrated that patients suffering from atypical TTS have a different clinical phenotype [38]. These patients are younger, more often with neurologic comorbidities, lower brain natriuretic peptide values, a less impaired LVEF, and more frequent ST-segment depression compared to typical TTS [38]. In-hospital complication rate is similar between typical and atypical types, while 1-year mortality is higher in typical TTS [38]. After adjustment for confounders, LVEF <45%, atrial fibrillation, neurologic disorders but not TTS phenotype were independent predictors of death. Beyond 1-year, long-term mortality is similar in typical and atypical TTS phenotypes, therefore, patients should be equally monitored and treated [38]. The basal phenotype has been reported to be associated with the presence of pheochromocytoma, epinephrine-induced TTS or subarachnoid haemorrhage consequently, these conditions should be considered in this particular setting [39]. Besides the four major TTS types, other morphological variants have been described including the biventricular (apical type and right ventricular involvement), isolated right ventricular, and global form [40]. Global hypokinesia as a manifestation of TTS is difficult to prove, given the very broad differential diagnoses including conditions such as tachycardia-induced cardiomyopathy. Right ventricular involvement is present in about one-third of TTS patients and may be a predictor for worse outcome [41]. The true prevalence of the isolated right ventricular form is unknown since little attention is paid to the right ventricle in daily clinical echo routine. Patients with recurrent TTS can demonstrate different wall motion patterns at each event suggesting that LV adrenergic receptor distribution does not explain different TTS types.
MCE with adenosine may confirm the diagnosis by showing reversible coronary microvascular constriction [42]. CMR with contrast medium shows the typical LV dysfunction associated with a hyperintense signal on T2 sequences without detectable myocardial necrosis after gadolinium administration [43].
Finally, F-18 fluorodeoxyglucose positron emission is providing encouraging results in the diagnosis of TTS [44].
Although TTS is generally considered a benign disease, contemporary observations show that rates of cardiogenic shock and death are comparable to ACS patients treated according to current guidelines.
While TTS is a reversible condition, hemodynamic and electrical instability during the acute phase expose patients to the risk of serious adverse in-hospital events which occur in approximately one-fifth of TTS patients. This substantial incidence of life-threatening complications requires close monitoring and early intervention in unstable TTS patients with risk stratification at diagnosis allowing triage to appropriate care. Parameters predicting adverse in-hospital outcome include: physical trigger, acute neurologic or psychiatric diseases, initial troponin >10 upper reference limit, and admission LVEF <45%. Furthermore, male patients have an up to three-fold increased rate of death and major adverse cardiac and cerebrovascular events (MACCE) and more often had an underlying critical illness, further contributing to the higher mortality [45]. Sobue et al. [46] demonstrated that physical triggers and male gender represent independent risk factors of in-hospital mortality in TTS. Data from the Tokyo Coronary Care Unit Network revealed that high values of BNP and white blood cell counts were also linked to higher rates of in-hospital complications [47]. Complications included cardiac death, pump failure (Killip grade ≥II), sustained ventricular tachycardia or ventricular fibrillation, and advanced atrio ventricular block. In the study by Takashio et al. [48] the magnitude and extent of ST-segment elevation with ECG were found to be independent predictors of in-hospital adverse events. However, those findings were not confirmed by others. Common in-hospital complications include cardiac arrhythmias, left ventricular outflow tract obstruction (LVOTO), cardiogenic shock, ventricular thrombus, pulmonary oedema, ventricular septal defect, and free wall rupture. In addition, to the demographic parameter of age ≥75, echocardiographic parameters that predict adverse in-hospital outcome ( acute heart failure, cardiogenic shock, and in-hospital mortality) include LVEF, E/e’ ratio, and reversible moderate to severe mitral regurgitation (MR). However, only reversible moderate to severe MR was an independent predictor when considering cardiogenic shock and death as the composite outcome in this study, in addition to heart rate [49]. Moreover, it has been demonstrated that high heart rate and low systolic blood pressure are associated with increased mortality in TTS [50]. Along with the Charlson comorbidity index and systolic pulmonary artery pressure, right ventricular involvement is an independent predictor of acute heart failure and of a composite endpoint including adverse events, such as acute heart failure, cardiogenic shock, and in-hospital mortality [50].
Data on long-term survival are scarce. In 2007, Elesber et al. [51] reported that long-term mortality did not differ between a TTS population and an age-, gender-, birth-, year-, and race-matched population.
While Sharkey et al. [52] found that all-cause mortality during follow up exceeded a matched general population with most deaths occurring in the first year. More recently, it has been reported that long-term mortality of patients with TTS is similar to patients with CAD [53]. TTS patient data from the Swedish Angiography and Angioplasty Registry (SCAAR) from 2009 to 2013 were compared to data from patients with and without CAD, and demonstrated that mortality rates for TTS were worse than in patients without CAD and comparable to those of patients with CAD [54]. In the largest TTS registry to date, death rates are estimated to be 5.6% and rate of MACCE 9.9% per-patient year, suggesting that TTS is not a benign disease. A recent study found that patients with the typical TTS type have a comparable outcome to patients presenting with the atypical type even after adjustment for confounders, suggesting that both patient groups should be equally monitored in the long-term [38]. On the other hand, 1-year mortality differs between the two groups, as it is driven by clinical factors including atrial fibrillation, LVEF on admission <45%, and neurologic disorders, rather than by TTS type [38]. In a smaller study, predictive factors of long-term mortality in TTS were male sex, Killip class III/IV, and diabetes mellitus [54].
The prognostic role of diabetes mellitus is controversial, as it is postulated that it may exert a protective effect in TTS, given that the prevalence of diabetes mellitus in TTS is lower than expected for an age and sex-matched population.
Patients who survive the initial event have a second event in approximately 5% of cases, mostly occurring 3 weeks to 3.8 years after the first event. Recurrent TTS afflicts men and women and may occur at any age including in childhood [36].
Some have postulated that an index TTS event may protect the affected LV regions from recurrent involvement through a mechanism akin to ischaemic ‘pre-conditioning’ [38]. However, detailed review of published cases and clinical experience suggest that there are frequent examples of recurrence in which the ballooning pattern is similar between episodes, thereby making this hypothesis unlikely.
Guidelines regarding TTS management are lacking as no prospective randomized clinical trials have been performed in this patient population. Therapeutic strategies are therefore based on clinical experience and expert consensus (evidence level C).
As TTS is clinically difficult to distinguish from ACS, upon first presentation patients should be transferred to a cardiology unit with imaging capabilities and a cardiac catheterization laboratory and receive guideline based treatment of ACS [38] in particular aspirin, heparin, and if required morphine and oxygen. Patients with cardiogenic shock or post cardiac arrest require intensive care. Electrocardiogram monitoring is essential as a prolonged QT-interval may trigger malignant ventricular arrhythmias (torsades de pointes) and AV-block may occur.
Takotsubo syndrome patients with cardiogenic shock, in particular those with apical ballooning should be promptly evaluated for the presence of LVOTO, which occurs in about 20% of cases. This should be performed during angiography with LV pressure recording during careful retraction of the pigtail catheter from the LV apex beyond the aortic valve. Similarly, a pressure gradient can be detected and quantified using Doppler echocardiography using continuous wave Doppler. Particularly, when using catecholamines serial Doppler studies should be considered to detect an evolving pressure gradient. In TTS patients treated with catecholamine drugs a 20% mortality has been reported; although this may represent a selection bias due to the initial presentation of the patients. Recently, it has been suggested that the Ca2þ-sensitizer levosimendan could be used safely and effectively in TTS as an alternative inotrope to catecholamine agents [38]. Furthermore, beta-blockers may improve LVOTO, but are contraindicated in acute and severe heart failure with low LVEF, hypotension, and in those with bradycardia. Although evidence is unproven, TTS patients with LVOTO may benefit from the If channel inhibitor ivabradine. As catecholamine levels are elevated in TTS, beta-blockers seem to be reasonable until full recovery of LVEF, but trials supporting this hypothesis are lacking. Animal experiments have shown that apical ballooning is attenuated after administration of drugs with both alpha and beta-adrenoceptor blocking properties. In an animal model, intravenous metoprolol improved epinephrine-induced apical ballooning [55]. However, due to the potential risk of pause-dependent torsades de pointes, beta-blockers should be used cautiously, especially in patients with bradycardia and QTc >500 ms. Angiotensinconverting-enzyme inhibitors (ACEi) or angiotensin II receptor blockers ( ARB) may potentially facilitate LV recovery. Diuretics are recommended in patients with pulmonary oedema. In addition, nitroglycerin is useful to reduce LV and right ventricular filling pressures and afterload in the case of acute heart failure; however, the administration of nitroglycerin in the presence of LVOTO has been found to worsen the pressure gradient and therefore should be avoided in this scenario.
QT-interval prolonging drugs should be used cautiously in the acute phase because of the risk to induce torsades de pointes or ventricular tachycardia and fibrillation. Severe LV dysfunction with extended apical ballooning entails the risk of an LV thrombus and subsequent systemic embolism. Although evidence is lacking, anticoagulation with intravenous/subcutaneous heparin would appear to be appropriate in such patients and post-discharge oral anticoagulation or antiplatelet therapy may be considered on an individual, per-patient basis. As LV dysfunction and ECG abnormalities are reversible, an implantable cardioverter defibrillator for primary or secondary prevention is of uncertain value in TTS patients experiencing malignant ventricular arrhythmias.
In case of excessive prolongation of the QT interval or life-threatening ventricular arrhythmias a wearable defibrillator could be considered [38]. The residual risk of malignant arrhythmic events after recovery from TTS is unknown. A temporary trans-venous pacemaker is appropriate for those with haemodynamically significant bradycardia.
The use of ACEi or ARB was associated with improved survival at 1-year follow-up even after propensity matching. In contrast, there was no evidence of any survival benefit for the use of beta-blockers.
Moreover, one-third of patients experienced a TTS recurrence during beta-blockade suggesting that other receptors such as alpha-receptors, that are more prevalent in the coronary microcirculation, might be involved.
The prevalence of recurrent TTS is relatively low, consequently conducting randomised trials of pharmacological agents to prevent recurrence is challenging. Beta-blocker therapy after hospital discharge does not appear to prevent recurrence [31], whereas ACEi or ARB are associated with a lower prevalence of recurrence. The significance of this observation remains uncertain and requires validation in other cohorts.
If concomitant coronary atherosclerosis is present, aspirin and statins are appropriate. As TTS mainly occurs in postmenopausal women, oestrogen supplementation in those with recurrence is questionable.
Myocarditis
Myocarditis has a variable presentation including an ACS-like presentation in the presence/absence of ventricular dysfunction and without obstructive CAD. In patients with a classical myocarditis presentation, the specific diagnosis of myocarditis should be made before or at coronary angiography, but in many cases the diagnosis will not be clinically apparent and the working diagnosis of MINOCA should be made until specific testing is performed.
The prevalence of myocarditis among patients with a clinical diagnosis of MINOCA varies based on the populations studied, with a prevalence of 33% in a recent meta-analysis [56]. The most common cause of biopsy-proven myocarditis is viral infection, confirmed with polymerase chain reaction (PCR) assay of the pathogen DNA/RNA on endomyocardial biopsy (EMB). Adenoviruses, parvovirus B19 (PVB19), human herpesvirus 6 and Coxsackie virus are considered the most common causes of viral myocarditis [57]. Previous studies suggested that the clinical presentation is related to the type of virus [57]. In particular, PVB19 myocarditis may mimick MINOCA.
Endothelial cells represent PVB19-specific targets in PVB19-associated myocarditis, probably through blood group P antigen [58]. Thus, symptoms of chest pain and ST segment elevation at ECG in patients with viral myocarditis but no obstructive CAD, may be caused by intense coronary microvascular constriction, as a result of myocardial inflammation and/or PVB19 infection of vascular endothelial cells and microvascular dysfunction. Accordingly, Yilmaz et al. [58] demonstrated that, after administration of Ach, patients with myocarditis mimicking MINOCA showed a CAS at the distal segment of epicardial vessel, with probable extension at the microvascular level. In conclusion, the infection of coronary endothelial cells with PVB19 may cause a kind of “coronary vasculitis” which may constitute a major determinant of the clinical course and the myocardial spread of inflammation.
Other causes of myocarditis are immunomediated diseases, endocrine diseases, drugs and toxins [59].
Autoimmune myocarditis may occur with exclusive cardiac involvement or in the context of systemic autoimmune disorders, e.g. systemic lupus erythematosus and is infection-negative by PCR on EMB [59].
The initial investigation of suspected myocarditis should include CMR imaging. Although this non-invasive investigation compares favourably with the gold-standard technique of EMB [60], only EMB provides the opportunity of identifying the underlying cause for the myocarditis. CMR has been reported to detect 79% of EMB-confirmed myocarditis [61]. Furthermore, in the new ESC guidelines on Pericardial disease CMR is recommended for the confirmation of myocardial involvement (myocarditis) as a Class I recommendation [61].
The importance of diagnosing myocarditis in patients with MINOCA relates to its prognosis and treatment. Myocarditis resolve over a 2–4 weeks period in 50% of patients, but 12–25% may acutely deteriorate and either succumb to fulminant heart failure or progress onto end-stage dilated cardiomyopathy requiring heart transplantation [62]. Giant cell myocarditis is particularly associated with a poor prognosis [62]. Thus patients with myocarditis may require intravenous inotropic agents and/or mechanical circulatory support as a bridge to recovery or transplantation, and do not require anti-ischaemic therapies utilized in other causes of MINOCA.
The diagnosis of biopsy-proven infection-negative myocarditis is the basis for safe immunosuppression, that is indicated in specific autoimmune forms, such as in giant cell myocarditis, which is associated with a poor prognosis, cardiac sarcoidosis, eosinophilic myocarditis, as well as in lymphocitic forms refractory to standard therapy [62]. EMB also provides differential diagnosis with other causes of MINOCA, including Takotsubo cardiomyopathy.
Treatment of myocarditis mimicking MI and characterized by LV dysfunction is based on the use of beta-blockers and ACEI. In the last years, many trials have been designed in order to detect further therapeutic approach, with controversial results. A recent study demonstrated that, in the enteroviral and adenoviral myocarditis characterized by LV dysfunction, virus clearance obtained with the interferon-beta administration, seems be associated with a more favourable prognosis compared to those with virus persistence [63]. Actually, there are no effective treatments for PVB19 myocarditis.
Coronary Thromboembolism
Coronary embolism is included in microvascular causes of MINOCA as it usually involves microcirculation, although an angiographically visible embolization of epicardial coronary artery branches may occur. Of note, in this latter case, the coronary arteries are obviously not normal due to the evidence of either an abrupt vessel stump or thrombotic material inside epicardial coronary artery. Coronary thrombosis may arise from hereditary or acquired thrombotic disorders and coronary emboli may occur from coronary or systemic arterial thrombi.
Hereditary thrombophilia disorders include Factor V Leiden thrombophilia, Protein S and C deficiencies. Thrombophilia screening studies in patients with MINOCA have reported a 14% prevalence of these inherited disorders [64]. Acquired thrombophilia disorders should also be considered such as the antiphospholipid syndrome and myeloproliferative disorders, although these have not been systematically investigated in MINOCA.
Paradoxical embolism due to right-left shunts might be a rare cause of MINOCA. It can be related to a patent foramen ovale (PFO), a large atrial septal defect or a coronary arteriovenous fistula [65]. Of note, paradoxical embolism has been described relatively often as cause of systemic embolization, especially for cryptogenic stroke [65]. Coronary emboli may also occur in the context of the above thrombophilia disorders or other predisposing hypercoagulable states such as atrial fibrillation and valvular heart disease. Emboli may arise from non-thrombotic sources also including valvular vegetations, cardiac tumours (e.g. myxoma and papillary fibroelastoma), calcified valves, and iatrogenic air emboli.
The criteria for paradoxical embolism diagnosis include: evidence of arterial embolism in absence of a source in the left heart, source of embolism in the venous system, and communication between venous and arterial circulation [65]. Transthoracic, transesophageal, and bubble contrast- echocardiography are the cornerstone methods for detection of cardiac sources of embolism as causes of MINOCA. Moreover, Wöhrle et al. [66] demonstrated subclinical MI in 10.8% of patients with PFO undergoing CMR after a first cryptogenic cerebral ischemic event. Importantly, in patients in whom paradoxical embolism is suspected, coronary angiography needs to be carefully analyzed for the identification of amputation of distal coronary branches. Finally, left-side origin of coronary embolism should be also excluded.
Prognostic data of patients with paroxysmal embolism and MINOCA derive mostly from case reports and is mainly determined by the underlying cause which needs to be carefully identified as well as for cases caused by thrombus formation on left-side structures.
The standard treatment remains individualized and mostly focused on multiple factors including patient characteristics, time of presentation, and presence or absence of other embolic sites. Regarding atrial septal defect, paroxysmal embolism requires trans-catheter device closure or surgical repair [67, 68]. The options for secondary prevention of PFO induced cryptogenic embolism consist in the administration of antithrombotic medications or in percutaneous closure [67, 68]. Of note, the last studies showed that, among patients who had had a recent cryptogenic stroke attributed to PFO, the rate of stroke recurrence was lower among those assigned to PFO closure combined with antiplatelet therapy than among those assigned to antiplatelet therapy alone. However, PFO closure was associated with higher rates of device complications and atrial fibrillation [68].
Anticoagulant therapy may finally be appropriate for the prevention of embolic events in left-side origin coronary embolism.
Miscellanea
Other Forms of Type-2 MI
Type 2 AMI is defined as myocardial cell necrosis due to supply–demand mismatch, characterized by significant increase and/or decrease in troponins with at least one value above the 99th percentile of a normal reference population in the absence of evidence for coronary plaque rupture in addition to at least one of the other criteria for AMI [8]. Among patients with non-obstructive CAD, a profound supply–demand mismatch should be present to consider type-2 AMI. Therapeutically, the condition underlying the oxygen supply–demand mismatch is to be reversed if possible. Furthermore, aspirin and b-blockers may be useful and application of specific secondary prevention measures must be considered in the context of the specific insult.
MINOCA with Uncertain Aetiology
CMR imaging is a useful tool in MINOCA patients because it provides insights into potential causes and confirmation of the diagnosis of AMI. In particular, the presence and pattern of any LGE may point towards a vascular or non-vascular cause. However, 8–67% of patients with MINOCA have no evidence of LGE, myocardial oedema, or wall motion abnormalities on CMR [69].
A possible explanation could be that some patients with normal CMR may have too little myonecrosis to be detected. Alternatively, the normal CMR appearance might be the result of a broader spatial distribution of myonecrosis. That is, necrotic myocytes may be distributed over a larger area with no contiguous island of cell death of sufficient size to be detected by LGE imaging.
When CMR is normal and diagnostic evaluation as recommended herein does not reveal the mechanism of AMI, there is a diagnostic and therapeutic dilemma for clinicians. From first principles, vasospastic angina, coronary plaque disease, or thromboembolism may all potentially cause MINOCA with normal CMR imaging. In a series of patients with MINOCA who underwent both CMR and IVUS imaging, a subset of those with plaque disruption had a normal CMR (25%). If intracoronary imaging had not been performed during cardiac catheterization, this diagnosis would have been missed. Furthermore, MINOCA studies undertaking provocative spasm testing or assessing microvascular dysfunction have not routinely performed before CMR. However, epicardial CAS may produce transient trans-mural myocardial ischaemia that is associated with a small troponin rise [70]. An alternative consideration is that the troponin rise is due to other causes such as pulmonary embolism or myocarditis.
Regarding the treatment, aspirin, statins and, in cases of vasospasm, calcium channel blockers as routine treatments could be proposed, since these would be of benefit for the potential underlying mechanisms of coronary plaque disease, coronary spasm, and thromboembolism.
Conclusions
MINOCA patients represent a conundrum given the very many possible aetiologies and pathogenic mechanisms associated with this syndrome. Clarify the underlying individual mechanisms is crucial to achieve patient-specific treatments.
References
Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475–81.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, ESC Scientific Document Group, ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–77.
Gehrie ER, Reynolds HR, Chen AY, Neelon BH, Roe MT, Gibler WB, Ohman EM, Newby LK, Peterson ED, Hochman JS. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. 2009;158:688–94.
Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, Stone GW. Prognosis of patients with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity-matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy Trial. Circ Cardiovasc Interv. 2014;7:285–93.
Daniel M, Agewall S, Caidahl K, Collste O, Ekenbäck C, Frick M, Y-Hassan S, Henareh L, Jernberg T, Malmqvist K, Schenck-Gustafsson K, Sörensson P, Sundin Ö, Hofman-Bang C, Tornvall P. Effect of myocardial infarction with nonobstructive coronary arteries on physical capacity and quality-of-life. Am J Cardiol. 2017;120:341–6.
Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–70.
Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P, WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–53.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, ESC Committee for Practice Guidelines (CPG). Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–67.
Ouldzein H, Elbaz M, Roncalli J, Cagnac R, Carrié D, Puel J, Alibelli-Chemarin MJ. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol. 2012;61:20–6.
Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–58.
Iqbal SN, Feit F, Mancini GB, Wood D, Patel R, Pena-Sing I, Attubato M, Yatskar L, Slater JN, Hochman JS, Reynolds HR. Characteristics of plaque disruption by intravascularultrasound in women presenting with myocardial infarction without obstructive coronary artery disease. Am Heart J. 2014;167:715–22.
Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25.
Patel MR, Chen AY, Peterson ED, Newby LK, Pollack CV Jr, Brindis RG, Gibson CM, Kleiman NS, Saucedo JF, Bhatt DL, Gibler WB, Ohman EM, Harrington RA, Roe MT. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152:641–7.
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315.
Antonsen L, Thayssen P, Jensen LO. Large coronary intramural hematomas: a case series and focused literature review. Cardiovasc Revasc Med. 2015;16:116–23.
Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, Aymong E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87:E54–61.
Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–9.
Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–88.
Jacqueline Saw Canadian SCAD Study ESC congress; 2018.
Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–8.
Kaski JC, Crea F, Meran D, Rodriguez L, Araujo L, Chierchia S, Davies G, Maseri A. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation. 1986;74:1255–65.
Sueda S, Oshita A, Nomoto T, Izoe Y, Kohno H, Fukuda H, Mineoi K, Ochi T, Uraoka T. Recommendations for performing acetylcholine tests safely: STOP dangerous complications induced by acetylcholine tests (STOP DCIAT). J Cardiol. 2008;51:131–4.
Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schäufele T, Mahrholdt H, Kaski JC, Sechtem U. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–30.
Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol Intv. 2012;5:646–53.
Lanza GA, Sestito A, Sgueglia GA, Infusino F, Manolfi M, Crea F, Maseri A. Current clinical features, diagnostic assessment and prognostic determinants of patients with variant angina. Int J Cardiol. 2007;118:41–7.
Mohri M, Koyanagi M, Egashira K, Tagawa H, Ichiki T, Shimokawa H, Takeshita A. Angina pectoris caused by coronary microvascular spasm. Lancet. 1998;351:1165–9.
Masumoto A, Mohri M, Takeshita A. Three-year follow-up of the Japanese patients with microvascular angina attributable to coronary microvascular spasm. Int J Cardiol. 2001;81:151–6.
Arrebola-Moreno AL, Arrebola JP, Moral-Ruiz A, Ramirez-Hernandez JA, Melgares-Moreno R, Kaski JC. Coronary microvascular spasm triggers transient ischemic left ventricular diastolic abnormalities in patients with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2014;236:207–14.
Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG, Coronary Vasomotion Disorders International Study Group (COVADIS). The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38:473–7.
Redfors B, Vedad R, Angeras O, Ramunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D, Libungan B, Shao Y, Albertsson P, Stone GW, Omerovic E. Mortality in Takotsubo syndrome is similar to mortality in myocardial infarction—a report from the SWEDEHEART registry. Int J Cardiol. 2015;185:282–9.
Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H-P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K-H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–38.
Franco E, Dias A, Koshkelashvili N, Pressman GS, Hebert K, Figueredo VM. Distinctive electrocardiographic features in African Americans diagnosed with Takotsubo cardiomyopathy. Ann Noninvasive Electrocardiol. 2016;21:486–92.
Qaqa A, Daoko J, Jallad N, Aburomeh O, Goldfarb I, Shamoon F. Takotsubo syndrome in African American vs. non-African American women. West J Emerg Med. 2011;12:218–23.
Park JH, Kang SJ, Song JK, Kim HK, Lim CM, Kang DH, Koh Y. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128:296–302.
Madias JE. Why the current diagnostic criteria of Takotsubo syndrome are outmoded: a proposal for new criteria. Int J Cardiol. 2014;174:468–70.
Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–46.
Napp LC, Ghadri JR, Bauersachs J, Templin C. Acute coronary syndrome or Takotsubo cardiomyopathy: the suspect may not always be the culprit. Int J Cardiol. 2015;187:116–9.
Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Luscher TF, Templin C, International Takotsubo Registry. Differences in the clinical profile and outcomes of typical and atypical Takotsubo syndrome: data from the International Takotsubo Registry. JAMA Cardiol. 2016;1:335–40.
Y-Hassan S. Clinical features and outcome of pheochromocytoma-induced Takotsubo syndrome: analysis of 80 published cases. Am J Cardiol. 2016;117:1836–44.
Elikowski W, Malek M, Lanocha M, Wroblewski D, Angerer D, Kurosz J, Rachuta K. [Reversible dilated cardiomyopathy as an atypical form of Takotsubo cardiomyopathy]. Pol Merkur Lekarski. 2013;34:219–23.
Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277–86.
Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, Rebuzzi AG, Crea F. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in apical ballooning or Takotsubo syndrome. Eur Heart J. 2010;31:1319–27.
Collste O, Sörensson P, Frick M, Agewall S, Daniel M, Henareh L, Ekenbäck C, Eurenius L, Guiron C, Jernberg T, Hofman-Bang C, Malmqvist K, Nagy E, Arheden H, Tornvall P. Myocardial infarction with normal coronary arteries is common and associated with normal findings on cardiovascular magnetic resonance imaging: results from the Stockholm Myocardial Infarction with Normal Coronaries study. J Intern Med. 2013;273:189–96.
Cacciotti L, Passaseo I, Marazzi G, Camastra G, Campolongo G, Beni S, Lupparelli F, Ansalone G. Observational study on Takotsubo-like cardiomyopathy: clinical features, diagnosis, prognosis and follow-up. BMJ Open. 2012;5:e001165.
Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with Takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164:215–21.
Sobue Y, Watanabe E, Ichikawa T, Koshikawa M, Yamamoto M, Harada M, Ozaki Y. Physically triggered Takotsubo cardiomyopathy has a higher in-hospital mortality rate. Int J Cardiol. 2017;235:87–93.
Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Konishi Y, Sakata K, Nagao K, Yamamoto T, Takayama M, Committee CCUNS. Characterization of predictors of in-hospital cardiac complications of Takotsubo cardiomyopathy: multi-center registry from Tokyo CCU Network. J Cardiol. 2014;63:269–73.
Takashio S, Yamamuro M, Kojima S, Izumiya Y, Kaikita K, Hokimoto S, Sugiyama S, Tsunoda R, Nakao K, Ogawa H. Usefulness of SUM of ST-segment elevation on electrocardiograms (limb leads) for predicting in-hospital complications in patients with stress (Takotsubo) cardiomyopathy. Am J Cardiol. 2012;109:1651–6.
Böhm M, Cammann VL, Ghadri JR, Ukena C, Gili S, Di Vece D, Kato K, Ding KJ, Szawan KA, Micek J, Jurisic S, D’Ascenzo F, Frangieh AH, Rechsteiner D, Seifert B, Ruschitzka F, Lüscher T, Templin C, InterTAK Collaborators. Interaction of systolic blood pressure and resting heart rate with clinical outcomes in Takotsubo syndrome: insights from the International Takotsubo Registry. Eur J Heart Fail. 2018;20:1021.
Citro R, Bossone E, Parodi G, Rigo F, Nardi F, Provenza G, Zito C, Novo G, Vitale G, Prota C, Silverio A, Vriz O, D’Andrea A, Antonini-Canterin F, Salerno-Uriarte J, Piscione F, Tako-tsubo Italian Network Investigators. Independent impact of RV involvement on in-hospital outcome of patients with Takotsubo syndrome. JACC Cardiovasc Imaging. 2016;9:894–5.
Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448–52.
Sharkey SW, Pink VR, Lesser JR, Garberich RF, Maron MS, Maron BJ. Clinical profile of patients with high-risk Tako-Tsubo cardiomyopathy. Am J Cardiol. 2015;116:765–72.
Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, Vonthein R, Schuler G, Thiele H, Eitel I. Long-term excess mortality in Takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail. 2016;18:650–6.
Tornvall P, Collste O, Ehrenborg E, Jarnbert-Petterson H. A case-control study of risk markers and mortality in Takotsubo stress cardiomyopathy. J Am Coll Cardiol. 2016;67:1931–6.
Izumi Y, Okatani H, Shiota M, Nakao T, Ise R, Kito G, Miura K, Iwao H. Effects of metoprolol on epinephrine-induced Takotsubo-like left ventricular dysfunction in non-human primates. Hypertens Res. 2009;32:339–46.
Tornvall P, Gerbaud E, Behaghel A, Chopard R, Collste O, Laraudogoitia E, Leurent G, Meneveau N, Montaudon M, Perez-David E, Sörensson P, Agewall S. A meta-analysis of individual data regarding prevalence and risk markers for myocarditis and infarction determined by cardiac magnetic resonance imaging in myocardial infarction with non-obstructive coronary artery disease. Atherosclerosis. 2015;241:87–91.
Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90.
Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, Kispert EM, Deluigi C, Baccouche H, Spodarev E, Klingel K, Kandolf R, Sechtem U. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart. 2008;94:1456–63.
Caforio AL, Marcolongo R, Jahns R, Fu M, Felix SB, Iliceto S. Immune-mediated and autoimmune myocarditis: clinical presentation, diagnosis and management. Heart Fail Rev. 2013;18:715–32.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management and therapy of myocarditis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48.
Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d’Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:245–74.
Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–48.
Kühl U, Lassner D, von Schlippenbach J, Poller W, Schultheiss HP. Interferon-beta improves survival in enterovirus-associated cardiomyopathy. J Am Coll Cardiol. 2012;60:1295–6.
Sastry S, Riding G, Morris J, Taberner D, Cherry N, Heagerty A, McCollum C. Young Adult Myocardial Infarction and Ischemic Stroke: the role of paradoxical embolism and thrombophilia (The YAMIS Study). J Am Coll Cardiol. 2006;48:686–91.
Srivastava TN, Payment MF. Images in clinical medicine. Paradoxical embolism thrombus in transit through a patent foramen ovale. N Engl J Med. 1997;337:681.
Wöhrle J, Kochs M, Hombach V, Merkle N. Prevalence of myocardial scar in patients with cryptogenic cerebral ischemic events and patent foramen ovale. JACC Cardiovasc Imaging. 2010;3:833–9.
Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Jüni P, PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–91.
Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L, Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–42.
Baccouche H, Mahrholdt H, Meinhardt G, Merher R, Voehringer M, Hill S, Klingel K, Kandolf R, Sechtem U, Yilmaz A. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J. 2009;30:2869–79.
Leurent G, Langella B, Fougerou C, Lentz PA, Larralde A, Bedossa M, Boulmier D, Le Breton H. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch Cardiovasc Dis. 2011;104:161–70.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Niccoli, G., Scalone, G., Crea, F. (2020). Myocardial Infarction with Non-obstructive Coronary Artery Disease. In: Dorobantu, M., Badimon, L. (eds) Microcirculation. Springer, Cham. https://doi.org/10.1007/978-3-030-28199-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-28199-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28198-4
Online ISBN: 978-3-030-28199-1
eBook Packages: MedicineMedicine (R0)