Abstract
Ischemic stroke is a medical emergency associated with significant morbidity and mortality. Over the past 25 years, the treatment of acute ischemic stroke has dramatically evolved. Currently, ischemic brain reperfusion is possible with pharmacologic, endovascular, and combined treatments. More recently, the use of advanced imaging allows identifying patients with salvageable ischemic brain tissue beyond the classic time-based window (tissue-based window) who benefit from reperfusion treatments. Stroke patients also benefit from monitoring in dedicated stroke units. This specialized environment is ideal for the detection and treatment of neurological deterioration and potential complications, and the timely institution of secondary prevention treatments and rehabilitation. This chapter aims to provide an updated and practical evidence-based guidance to the initial evaluation and treatment of acute stroke patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Diagnostic Keys-
Assessment of time of symptom onset and stroke severity.
-
Exclusion of brain hemorrhage and assessment of irreversible brain damage with emergency CT scan.
-
CT angiography to identify candidates for endovascular treatment in the early time window (6 hours).CT perfusion (or MRI diffusion/perfusion) to identify candidates for endovascular treatment in the extended time window (6–24 hours).
-
Rapid reperfusion of the ischemic brain tissue.
-
Prevention of secondary brain injury and early diagnosis of potential complications.
-
Early initiation of the appropriate secondary prevention treatment.
-
Stroke is a leading cause of death and disability worldwide.
-
Prognosis mainly depends on timely reperfusion of the ischemic brain.
-
Prevention of secondary brain injury and early stroke recurrence in a dedicated stroke unit also improve outcomes.
Introduction
Since the approval of intravenous thrombolysis (IVT) in 1995, the treatment of acute ischemic stroke has dramatically changed. More recently, the development of more effective endovascular reperfusion devices has allowed to effectively treat patients with occlusions of proximal intracranial vessels. Advanced imaging (CT perfusion and MR diffusion/perfusion) allows to identify who may benefit from reperfusion therapies up to 24 hours after symptoms onset. Following treatment with reperfusion therapies, patients should be carefully monitored in a dedicated stroke unit for the prevention, early detection and treatment of potential complications. After the hyperacute phase, the identification of the underlying stroke mechanism should guide the institution of an appropriate secondary stroke prevention regimen. Modern evaluation and management of stroke patients have become a complex task that requires a multidisciplinary team needs under constant training. In this chapter, we provide an evidence-based, yet practical approach, to the initial evaluation and treatment of acute stroke patients.
Pathophysiology of Acute Stroke
The understanding of three basic pathophysiologic concepts is essential to optimize the evaluation and treatment of patients with acute ischemic stroke.
Ischemic Core and Penumbra
The immediate consequences of an arterial occlusion in the downstream brain tissue are heterogeneous. There is an area of irreversible brain damage (core) surrounded by hypoperfused and non-functioning but salvageable tissue (penumbra). Advanced brain imaging (CTP perfusion or MRI diffusion/perfusion) can delineate core and penumbra. The penumbra has a variable outcome; transition to irreversible brain damage is mainly determined by the severity and duration of ischemia and the quality of collateral blood flow [1]. Thus, early recanalization and enhancement of collateral circulation are high priorities of acute stroke treatment.
Collateral Circulation
Collateral circulation is a subsidiary network of vascular channels that may provide enough flow to the affected tissue to prevent critical ischemia when the main artery is compromised [2]. In other words, collateral flow supports the viability of the ischemic penumbra. Collateral status differs across patients; it is often tenuous and can sustain brain viability only for a limited period of time. Collateral flow can be supported by avoiding blood pressure drops, and possibly, by administering intravenous fluids. Hemodynamic augmentation with vasopressors may be beneficial in well-selected cases (such as patients with cervical internal carotid artery occlusion without tandem intracranial occlusion), but the safety and efficacy of this strategy remain largely unknown [3]. Keeping the head of the bed flat in an attempt to improve collateral flow and patients’ outcomes was not beneficial in the head position in Stroke Trial (HeadPoST) [4] and this intervention may increase the risk of aspiration, especially in patients with decreased level of consciousness and dysphagia. Yet, lowering the head of the bed may be helpful as a bridging alternative in selected patients with critical cerebral ischemia.
Secondary Brain Damage
Several neuroprotective treatments have failed to improve outcomes in acute stroke patients. However, secondary brain insults such as hypoglycemia, hypoxemia, and fever worsen stroke patients’ outcomes by exacerbating the biochemical disturbances of the ischemic brain. Hence, avoiding them can be considered neuroprotection. Hypoglycemia exacerbates energy failure and should be avoided. Hyperglycemia also worsens stroke patients’ outcomes. Current guidelines recommend to maintain a glycemia between 140 and 180 mg/dL [5]. Tighter glycemic control (80–130 mg/dL) proved ineffective to improve functional outcomes in the Stroke Hyperglycemia Insulin Network Effort (SHINE) trial [6]. Fever is also associated with poorer outcomes. Underlying mechanisms include increased brain metabolic demand, excitotoxicity, free-radical production, blood–brain barrier breakdown, and proteolysis. The routine administration of paracetamol to acute stroke patients does not improve outcomes, but treating fevers showed some benefits in the Paracetamol (Acetaminophen) Stroke (PAIS) trial [7]. A trial evaluating a higher dose of paracetamol is ongoing. The rate of oxygen extraction is increased in the area of penumbra, thus, providing adequate oxygen to avoid hypoxemia is recommended.
Evaluation in the Emergency Department
Sudden onset focal neurological symptoms should be regarded as secondary to ischemic stroke until proven otherwise. This situation should be considered a “brain code” and patients must be evaluated in the emergency department with maximal priority. After ruling out hemorrhage with neuroimaging, establishing the time of symptoms onset allows to decide whether the patient is a candidate for any reperfusion treatment: IVT, mechanical thrombectomy (typically with a retrievable stent) or both.
The effectiveness of reperfusion treatments decrease and the risk of serious side effects increase over time. Thus, a speedy response of the stroke team is crucial. The main metrics are the door-to-needle time for IVT and door-to-groin time for endovascular procedures. IVT within 60 minutes of arrival is associated with lower in-hospital mortality and lower rates of symptomatic intracranial hemorrhage. For every 15-minute reduction in door-to-needle time, there is a 5% reduction in in-hospital mortality [8].
Practical Steps for Initial Evaluation
Secure Airway, Breathing, and Circulation (ABC)
-
Insert two peripheral lines. At least one 16G line is recommended for IV contrast studies.
-
Supplementary oxygen to ensure an oxygen saturation >94%.
-
Cardiac and blood pressure monitoring. Blood pressure should be stable below 185/110 mm/Hg in order to start IVT. Avoid hypotension.
A Brief Questionnaire Focusing on
-
Time of symptoms onset. If the exact time is not known, the last known normal time should be recorded.
-
Past medical history of potential stroke mimickers, e.g., epilepsy, migraine with aura, use of insulin or oral hypoglycemic drugs, psychiatric diseases, brain tumors, etc.
-
Vascular risk factors : when present they increase the likelihood of stroke as a cause of symptoms.
-
Current medications with especial focus on anticoagulants. If the patient was on an anticoagulant, the dose, indication, and time of last dose should be recorded.
-
Contraindications for intravenous thrombolysis (Table 9.1). The exclusion criteria originally used in the NINDS trial have changed over time in light of safety data from stroke registries which demonstrated the safety of alteplase (recombinant tissue plasminogen activator or rtPA) in patients originally excluded from clinical trials. Currently, there are only a few absolute contraindications. The decision of giving alteplase should be based on the risk/benefit equation in individualized patients.
Physical Examination
The examination should be rapid and focused. The National Institute of Health Stroke Scale (NIHSS) is a validated tool developed for this purpose (http://www.nihstrokescale.org). Beyond calculating a number, the neurologist should decide if the symptoms are disabling for the individual patient. A rapid general examination is useful to find signs of head trauma, infectious endocarditis and coagulopathies, especially in those cases in which no reliable history can be obtained.
Additional Tests
After ruling out a history of coagulopathy with the anamnesis, hypoglycemia and hemorrhagic stroke on brain CT, the initiation of IV alteplase should not be delayed while waiting for the result of additional testing.
-
Brain CT: Non-contrast brain CT is necessary to differentiate between ischemic and hemorrhagic stroke. Once hemorrhage has been excluded, this scan also allows to identify patients with a low chance for recovery and a high chance for hemorrhagic complications with IVT (e.g., hypodensity of more than one-third of the MCA) and to find alternative diagnoses (e.g., brain tumor). In patients with a NIHSS of 6 or greater (or a high suspicion for proximal artery occlusion), the intracranial arteries should be studied with CT angiogram, MR angiogram, or catheter angiography in order to select candidates for mechanical thrombectomy. Early ischemic changes can be quantified using the Alberta Stroke Program Early CT Score (ASPECTS) [9]. An ASPECTS of 10 corresponds to a normal CT scan. In patients with acute stroke, 1 point should be subtracted for each one of 10 predefined regions when early ischemic change exists (Fig. 9.1). Lower ASPECTS correlates with larger ischemic damage.
-
Capillary glycemia: Hypoglycemia is a potential stroke mimicker and should be corrected before deciding to proceed with any reperfusion therapy.
-
Coagulation and platelets: It is highly unlikely to find abnormal laboratory tests values in patients with no history of hemorrhagic diathesis. Thus, if the past medical history is negative for coagulation disorders, the initiation of IV alteplase should not be delayed while waiting laboratory results. Yet, it is prudent to wait for these results in those patients in whom a history cannot be appropriately obtained. Patients receiving warfarin can receive IV rtPA within 3 hours of symptom onset if the international normalized ratio (INR) is 1.7 or less. Per current guidelines, IV alteplase should be held in patients receiving warfarin who present within the 3–4.5 hours window. Patients with platelet count lower than 100,000 per mm3 should not receive alteplase. Neither adequate safety data nor reliable laboratory studies to quantify the degree of anticoagulation are available for patients on direct oral anticoagulants (DOACs, dabigatran, rivaroxaban, apixaban, and edoxaban). Thus, it is most prudent to withhold thrombolysis in these patients. However, anticoagulated patients with proximal intracranial artery occlusion may benefit from mechanical thrombectomy regardless of whether they are on warfarin or DOACs.
With all these data the clinician should be able to decide on the most appropriate reperfusion therapy. Figure 9.2 provides an algorithm for guidance.
Intravenous Thrombolysis
In 1995, the NINDS study showed that IV alteplase administered within 3 hours of stroke symptom onset increases the chances of functional independence at 3 months by one-third [10]. A second landmark was the publication of the ECASS 3 trial in 2009 [11]. With its results, the therapeutic window for IV alteplase was expanded up to 4.5 hours. The number necessary to treat to help one more patient achieve functional independence increases from 3.6 within the first 90 minutes to 4.3 between 91 and 180 minutes and to 5.9 from 181 to 270 minutes, highlighting the time-dependent benefit of brain reperfusion (“time is brain”) [12] Thereafter, numerous data from stroke registries have expanded our knowledge on the efficacy and safety profile of IV alteplase.
Patients with Mild or Improving Symptoms and Relative Contraindications: A Judgment Call
Deciding whether to administer IVT in patients with mild or improving symptoms and those with relative contraindications is challenging. Observational data have shown that one-third of patients with mild or rapidly improving symptoms in whom thrombolysis was held were eventually disabled at 3 months [13]. Recently, the Effect of Alteplase vs Aspirin on Functional Outcome for Patients With Acute Ischemic Stroke and Minor Nondisabling Neurologic Deficits (PRISMS) trial randomized 313 patients with nondisabling acute ischemic stroke to receive alteplase vs. aspirin. There was no increase in the likelihood of a favorable functional outcome at 90 days with IVT. However, the study was stopped early because of slow recruitment and this precludes any definitive conclusions [14]. Until more conclusive data are available, the decision of whether to proceed with IVT in these situations should be individualized. Yet, if symptoms are causing disability at the time of the evaluation in the emergency department, we favor treatment with IV alteplase even if the symptoms are relatively mild or appear to be getting better.
IV Alteplase Administration
The total dose of IV alteplase is 0.9 mg/kg (do not exceed 90 mg); 10% as a 1-minute bolus and 90% as a 60-minute infusion. Subsequently, the patient should be monitored in a stroke unit or neuroscience ICU for at least 24 hours. IVT should not be delayed until the patient is transferred to such environments. Moreover, hospitals with no stroke capabilities can start the treatment while transfering the patient to a primary or comprehensive stroke center with stroke capabilities (drip-and-ship strategy). When not available, a stroke neurologist can assist with the therapeutic decision via telemedicine.
A lower dose of IV alteplase (0.6 mg/kg) administered within 4.5 hours of stroke onset did not meet the endpoint of non-inferiority when compared to standard dose alteplase in the ENCHANTED trial [15]. Tenecteplase has a longer life than alteplase which allows its administration as a single bolus. Tenecteplase at a dose of 0.4 mg/kg failed to prove superiority over alteplase 0.9 mg/kg in patients with mild stroke, though both drugs had a similar safety profile [16]. Yet, the efficacy and safety profile of tenecteplase still needs to be confirmed in patients with mild stroke and better studied in patients with more severe strokes.
Complications of IV Alteplase Infusion
The most feared complication of IVT is intracranial hemorrhage. The original studies that supported the approval of this therapy showed a total rate of intracranial hemorrhage of 6%, being symptomatic (causing deterioration of at least 4 points in the NIHSS) in about 4% [10]. Data from stroke registries and newer clinical trials showed that this number is probably lower (as low as 1.9% in some studies, depending on the definition of symptomatic ICH) [17].
When sudden neurologic decline, severe hypertension or vomiting occurs during alteplase infusion, the infusion should be immediately stopped and a CT scan should be emergently obtained. The treatment of symptomatic intracranial hemorrhage post IVT consists of control of hypertension (systolic target 140–160 mm Hg) and reversal of the fibrinolytic effect (Fig. 9.3). Orolingual angioedema occurs in 1.3–5% of patients who receive alteplase. It usually presents as a transient and self-limited swelling of the lips and tongue contralateral to the stroke. It is more common in patients with strokes affecting the insula and in those taking angiotensin-converting enzyme inhibitors. The most severe cases can compromise airway patency; thus, careful monitoring is indispensable. Treatment consists of a combination of diphenhydramine (50 mg IV), ranitidine (50 mg IV), and dexamethasone (10 mg IV). Emergent intubation and epinephrine may be needed in serious cases.
Mechanical Thrombectomy
Intravenous thrombolysis is poorly effective to open proximal occlusions of the major intracranial arteries and carotid bifurcation by large clots. These cases represent up to one-third of anterior circulation strokes and are associated with a poor prognosis. Detailed meta-analysis of multiple randomized controlled trials demonstrate that mechanical opening of the occluded vessel, generally with stent retrievers and in addition to IVT when indicated, allow 46% of treated patients to regain functional independence (relative benefit of 19%; odds ratio 2.35 [95% confidence intervals 1.85–2.98]; number necessary to treat of 2.8–3) [18]. The clinical trials that demonstrated the effectiveness of mechanical thrombectomy used different inclusion criteria and have been extensively reviewed elsewhere, but most of the patients enrolled in the initial trials of endovascular stroke therapy were treated within 6 hours of symptom onset [3]. Selection criteria proposed by the American Heart Association guidelines are presented in (Table 9.2) [5].
The fact that a patient is a candidate for endovascular treatment should not delay the infusion of alteplase when there are no contraindications. In one trial of patients with stroke symptoms from proximal artery occlusions, tenecteplase (0.25 mg/kg) was superior to alteplase in achieving recanalization of the occluded vessel and it was also associated with better functional outcomes at 3 months [19]. Yet, further studies are necessary to support the use of tenecteplase rather than alteplase in these cases before a change in practice can be broadly recommended.
Patients with basilar artery occlusion have been systematically excluded from randomized controlled trials testing IVT or mechanical thrombectomy. However, given the very poor prognosis of this type of stroke when reperfusion is not achieved, IVT and mechanical thrombectomy should be strongly considered.
Patients with Unknown Time of Onset and Extended Window Beyond 6 Hours
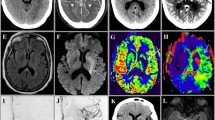
Two clinical trials have demonstrated that a subgroup of patients with proximal intracranial artery occlusion with unknown time of onset or symptoms for more than 6 hours may benefit from mechanical thrombectomy when carefully selected by advanced imaging (see Fig. 9.2 for specific selection criteria). An illustrative example of CT perfusion is displayed in Fig. 9.4.
Patient who awoke with aphasia and right hemiparesis. CT perfusion showed a mismatch between (a) the area of reduced cerebral blood flow (less than 30% than the contralateral region representing the ischemic core) and (b) the area with delayed arrival of contrast (time to maximum of the residue function [Tmax] greater than 6 seconds representing the region affected by hypoperfusion) as illustrated by the combined map (c) were the ischemic core is depicted in red and hypoperfusion is shown in yellow
The DAWN trial enrolled patients with terminal carotid or proximal MCA occlusion and a mismatch between clinical deficits and brain infarct (assessed by DWI or CT perfusion) who were last known normal 6–24 hours before presentation. The rate of functional independence at 90 days among patients randomized to mechanical thrombectomy was 49% compared to 13% in the standard medical therapy alone [20].
Similarly, the DEFUSE 3 trial randomized patients presenting between 6 and 16 hours from symptom onset (or last known normal time) with proximal intracranial artery occlusion and salvageable brain tissue assessed by CT perfusion or MRI DWI/perfusion to undergo mechanical thrombectomy vs. standard therapy alone. Patients in the mechanical thrombectomy group were more likely to be functionally independent at 90 days (45% vs. 17%) and also had a trend toward a lower mortality (14% vs. 26%) [21].
Intravenous alteplase may also have a role in patients with unknown time of onset. The MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset (WAKE UP) trial randomized patients who had an unknown time of stroke onset with an ischemic lesion visible on DWI but no parenchymal hyperintensity on FLAIR to receive alteplase or placebo. Those patients who received alteplase were more likely to have no disability at 90 days compared to those who did not (53.3% vs. 41.8%). There were no statistical differences in the rate of symptomatic ICH or death between the two groups [22].
More recently, the EXTEND trial randomized patients with salvageable brain tissue between 4.5 and 9 hours after stroke symptoms onset or wake-up stroke (if within 9 hours from the midpoint of sleep) to receive alteplase or placebo. Salvageable brain tissue could be measured by DWI-weighted MRI-MR perfusion or CT perfusion (Fig. 9.2). Additional inclusion criteria were minimal previous disability (mRS <2) and a stroke severity between 4 and 26 points in the NIHSS. The trial was terminated with 72% of the planned recruitment because of a loss of equipoise after the publication of the WAKE-UP trial. The primary outcome (no disability with or without residual symptoms [mRS 0–1] at 90 days) occurred in 35.4% and 29.5% of patients in the alteplase and placebo groups, respectively. Symptomatic ICH was more frequent in the alteplase group (6.2% vs 0.9%) [23]. These results, along with those of the ECASS4 and EPITHET trials, were included in a meta-analysis of individual patient data. Again, patients who received alteplase were more likely to have no disability (mRS 0–1) at 90 days (36% vs 29%) with an excess of symptomatic ICH (5% vs <1%). Yet, this excess did not negate the benefit of IVT [24].
Care After Reperfusion Therapy
-
Admission to a stroke unit reduces mortality by 13%, mortality or institutionalization by 22% and mortality or dependency by 21%, without increasing hospital length of stay [25].
-
Continuous cardiac monitoring allows to diagnose paroxysmal atrial fibrillation and other arrhythmic complications. Atrial fibrillation is a leading cause of stroke, especially in older patients.
-
Keeping the head of the bed flat may improve collateral flow in selected patients as explained above. Patients with dysphagia, decreased level of consciousness or early brain edema may benefit with the head of the bed at 30° to prevent aspiration and intracranial hypertension.
-
Avoid unnecessary urinary and nasogastric catheters, at least for the first few hours, if the patient received IV alteplase.
-
Serial neurological examination and blood pressure checks every 15 minutes during alteplase infusion and for 2 hours after its finalization. Later, measurements can be spaced out as per guidelines. To avoid unwanted hypotension, it is prudent to hold antihypertensive drugs the patient was previously taking. However, in order to avoid rebound tachycardia, home beta-blockers should be cut down in half rather than fully stopped. If blood pressure increases beyond safe limits it should be treated with IV drugs. Hypovolemia should be strictly avoided by providing IV saline in patients unable to tolerate oral intake.
-
Avoid hyperthermia. A third of patients with acute stroke have body temperatures greater than 37.5 °C within the first hours after onset and this is associated with poorer outcomes. Treating fever may be beneficial. [7]
-
Correct electrolyte disturbances, especially hyponatremia in patients with large strokes at risk of brain edema.
-
Prevention of deep venous thrombosis. Prophylaxis with heparin or low molecular weight heparin should be held in the first 24 hours after the reperfusion attempt due to the potential stroke hemorrhagic transformation. Sequential compression devices should be used during this timeframe.
-
Swallowing assessment to prevent aspiration pneumonia. This is necessary in patients with dysarthria, aphasia, and facial paresis.
-
Control neuroimaging within 24–36 hours of reperfusion therapy. It allows to assess the location and extension of the established stroke and to rule out hemorrhagic transformation. This will guide the timing of DVT prophylaxis and secondary stroke prevention strategy initiation. While a CT scan is enough in most cases, in selected patients, a brain MRI may be useful to clarify the mechanism of the stroke and to evaluate the long-term risk of bleeding on antithrombotics, which is higher in patients with cerebral micro-bleeds.
Transient Ischemic Attack, Stroke with Transient Symptoms, and Stroke with no Reperfusion Treatment
Many patients with transient symptoms, especially those lasting more than 60 minutes have an established infarct when assessed with MRI. These patients and those with transient ischemic attacks (symptoms lasting <24 hours without infarcts in DWI) [26] may benefit from hospital admission for an expedited work-up in order to diagnose stroke etiologies with a high risk of recurrence (e.g., carotid artery stenosis >70%) [27].
Patients who are not candidates for reperfusion therapies because symptoms are nondisabling, they have an established stroke and are beyond the time window for reperfusion or have other contraindications for reperfusion therapies, should be admitted for stroke work-up and receive the above-mentioned care. This will help to sustain the ischemic penumbra, prevent medical complications, diagnose the underlying stroke mechanism, and start rehabilitation. All stroke patients should receive treatment with antiplatelets and statins within the first 48 hours unless they have contraindications for those medications. Dual antiplatelet drugs for 3 weeks help to prevent early recurrences among patients presenting with mild strokes or transient ischemic attack [28]. In cases with a demonstrated cardioembolic source, anticoagulation should be started as soon as deemed safe. The size of the stroke is the main consideration when deciding on the timing of initiation of anticoagulation.
Classification and Secondary Prevention
After the hyperacute phase, all stroke patients should be evaluated to find the underlying mechanism of the ischemia. This will allow to tailor the most appropriate secondary prevention regimen. Classifying the mechanism of the stroke also has prognostic implications. There are many classification systems but we continue to prefer the classic TOAST system because it is practical and has been broadly validated [29].
Large Vessel Disease
Large vessel disease strokes are caused by an atheromatous obstruction greater that 50% of the cervical or intracranial artery perfusing the infarcted area. Symptoms at presentation generally produce cortical dysfunction (e.g., aphasia, neglect, hemianopia, etc.) but also can cause brainstem and cerebellar dysfunction. A careful questioning may reveal multiple TIAs in the affected vascular territory. Intermittent claudication, diminished peripheral pulses, and carotid bruits are additional findings of systemic atheromatosis. Brain imaging usually shows cortical or cerebellar infarcts. When producing subcortical infarcts, they are greater than 1.5 cm (macrolacunes); the greater size differentiates them from strokes caused by small vessel disease. Patients with ipsilateral carotid stenosis greater than 70% have the highest recurrence rate of ischemic stroke, and most recurrences occur within 2 weeks of the initial event. In these cases, the treatment choice is carotid revascularization: endarterectomy, or stenting. When the stenosis ranges from 50 to 70% the benefit of revascularization is less clear. Patients with a good functional status and more than 5 years of expected life may benefit from surgical intervention. Patients with intracranial stenosis were included in the SAMMPRIS trial that compared medical treatment (dual antiplatelet regimen with aspirin and clopidogrel for 3 months, high-dose statin, and strict blood pressure control) with a surgical approach (stenting). Patients in the medical treatment arm had fewer stroke recurrences [30].
Small Vessel Disease
Small vessel disease is also known as lacunar infarcts or lacunes. These patients present with lacunar syndromes (pure motor, pure sensory, motor-sensory, dysarthria clumsy hand, and ataxia-hemiparesis). Brain imaging show infarcts in the deep white or grey matter (including brainstem) smaller than 1.5 cm. However, in this context, it is prudent to exclude large artery disease and cardiac embolism. These patients will benefit from control of vascular risk factors, antiplatelets, and stains.
Cardiac Embolism
Clinical and imaging findings may be similar to large vessel disease stroke because they share the embolic mechanism. Strokes affecting multiple vascular territories are highly suggestive of a cardioembolic mechanism. Additionally, these patients may have a past medical history of systemic embolism. To be classified as cardioembolic, a cardioembolic source should be conclusively identified, and large vessel stenosis ruled out. The most common cause of cardioembolic stroke by far is atrial fibrillation. The treatment choice is anticoagulation. Other rarer cardioembolic sources (e.g., cardiac myxoma) may need specific treatments.
Other Causes
Cervical dissections are especially frequent in young patients, causing approximately 20% of ischemic strokes in this age group. The culprit mechanism is a tear in the intimal layer of the vessel, which creates a false lumen and subsequently stenosis and thrombosis with a high-risk of distal embolism. It should be suspected in patients with a history of trauma, which can be mild (e.g., massage or chiropractic maneuvers in the neck), who present with headache and neck pain. Patent foramen ovale (PFO) is the persistence of the embrionary foramen ovale in adulthood. This foramen communicates right and left atria, and may allow the passage of venous thrombi to the arterial circulation or produce local thrombosis due to turbulent flow. A PFO is present in 25% of the general population and 40% of patients with cryptogenic strokes. The ROPE score is a tool that can help to differentiate whether the finding of a PFO is pathogenic or an incidental finding [31]. Recent trials have shown that closing the PFO in selected patients decreases the rate of recurrent stroke [32]. This category also includes unusual etiologies such as hypercoagulable states and vasculitis. Given that this group is heterogeneous, clinical presentation and imaging findings vary. It should be suspected in young patients, without classic vascular risk factors, signs, and symptoms of systemic affection and signs of systemic inflammation (such as sedimentation rate or C reactive protein).
Undetermined
A patient can be classified in this group when:
-
There are two or more probable causes.
-
Negative work up.
-
Incomplete work up.
When patients have undetermined strokes due to a negative workup, antiplatelets are the mainstay for secondary prevention. If the infarct has an embolic appearance and clinical suspicion is high, covert atrial fibrillation should be ruled out with serial Holter monitoring or implantable recording devices. Vulnerable carotid plaques (i.e., with signs of inflammation, intramural hemorrhage, ulcerations) may be the culprit mechanism in some of these patients even if they do not produce major luminal stenosis. In such cases, control of vascular risk factors, statins, and antiplatelets should be optimized.
Cardinal Messages
-
The ischemic penumbra is an area of potentially salvageable brain that benefits from maximal therapeutic efforts.
-
Arterial recanalization, optimization of collateral flow, and avoidance of secondary brain injury determine the outcome of the ischemic penumbra.
-
Arterial recanalization can be achieved by intravenous thrombolysis and/or mechanical thrombectomy.
-
The severity of initial deficits and timely reperfusion are the main determinants of acute stroke outcome.
-
A minority of patients with proximal artery occlusion, small core, and large penumbra benefit from mechanical thrombectomy up to 24 hours from symptoms onset.
-
Organized inpatient care in a dedicated stroke unit improves mortality and functional outcomes.
-
Secondary stoke prevention treatments should be individually tailored based on the mechanism of ischemia.
References
Kaufmann AM, Firlik AD, Fukui MB, Wechsler LR, Jungries CA, Yonas H. Ischemic core and penumbra in human stroke. Stroke. 1999;30:93–9.
Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke. Stroke [online serial]; 2015;46:3302–3309.
Rabinstein AA. Treatment of acute ischemic stroke. Continuum (Minneap Minn). 2017;23:62–81.
Anderson CS, Arima H, Lavados P, et al. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med [online serial]. 2017;376:2437–47.
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke [online serial]. 2018;49:e46–110. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/29367334.
Johnston KC, Bruno A, Pauls Q, et al Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients With Acute Ischemic Stroke The SHINE RandomizedClinical TrialJAMA. 2019;322(4):326–335.
den Hertog HM, van der Worp HB, van Gemert HMA, et al. The Paracetamol (Acetaminophen) In Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol. 2009;8:434–40.
Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association’s target: stroke initiative. Stroke. 2011;42:2983–9.
Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet [online serial]. 2000;355:1670–4. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/10905241.
The National Institute Of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7.
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med [online serial]. 2008;359:1317–1329. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/18815396. Accessed 4 April 2016.
Lansberg MG, Schrooten M, Bluhmki E, Thijs VN, Saver JL. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified Rankin Scale. Stroke. 2009;40:2079–84.
Khatri P, Conaway MR, Johnston KC. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. 2012;43:560–2.
Khatri P, Kleindorfer DO, Devlin T, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: the PRISMS randomized clinical trial. JAMA. 2018;320:156–66.
Anderson CS, Robinson T, Lindley RI, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374:2313–23.
Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16:781–8.
Seet RCS, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis [online serial]. 2012;34:106–114. Accessed at: https://www.karger.com/Article/FullText/339675.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31.
Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med [online serial]. 2018;378:1573–82.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med [online serial]. Massachusetts Medical Society; 2017;378:11–21.
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med [online serial]. Massachusetts Medical Society; 2018;378:708–18.
Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med [online serial]. Massachusetts Medical Society; 2018;379:611–22.
Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–803.
Campbell BCV, Ma H, Ringleb PA, et al. Extending thrombolysis to 4.5–9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019;394(10193):139–47.
Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;(9):CD000197.
Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardio. Stroke [online serial]. 2009;40:2276–93.
Lavallee PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–60.
Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med [online serial]. Massachusetts Medical Society; 2018;379:215–25.
Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke [online serial]. 1993;24:35–41. Accessed at: http://www.ncbi.nlm.nih.gov/pubmed/7678184. Accessed 10 June 2016.
Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003.
Thaler DE, Ruthazer R, Weimar C, et al. Recurrent stroke predictors differ in medically treated patients with pathogenic vs. other PFOs. Neurology. 2014;83:221–6.
Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med [online serial]. Massachusetts Medical Society; 2017;377:1033–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hawkes, M.A., Rabinstein, A.A. (2020). Acute Ischemic Stroke. In: Rabinstein, A. (eds) Neurological Emergencies. Springer, Cham. https://doi.org/10.1007/978-3-030-28072-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-28072-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28071-0
Online ISBN: 978-3-030-28072-7
eBook Packages: MedicineMedicine (R0)