Abstract
Extremity fasciotomy is a procedure that may save a life or limb when performed correctly. When fasciotomy to treat acute compartment syndrome (ACS) is delayed or done incorrectly, permanent disability, limb loss, organ failure, or even death is possible. Injury to the limb is a common predisposing factor among patients requiring fasciotomy making it an important skill for surgeons caring for trauma patients to do competently. Identifying patients that require fasciotomy can be a challenge. Patients with patterns of injuries likely to result in raised tissue pressures are at risk of developing compartment syndrome. Clinical symptoms though present may be masked by other injuries, medications, peripheral blocks, or mental status changes. In these cases, laboratory studies and compartment pressure measurements added to clinical findings improve early recognition. Surgeons need to be familiar with the location of compartments in the extremity in order assure full decompression during fasciotomy. This chapter will describe location and content of the compartments. Landmarks used to accurately position incisions will also be described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fasciotomy
- Acute compartment syndrome
- Intracompartmental pressure
- Pain out of proportion to injury
- Fascia
- Myonecrosis
- Rhabdomyolysis
- Decompression
Introduction of the Problem
Richard von Volkmann in 1881 described the sequelae of applying overly restrictive dressings or casts to an injured limb–Volkmann’s contracture. In 1906 Hildebrand related Volkmann’s ischemic contracture to elevated tissue pressure. Murphy described the technique of fasciotomy for the treatment of elevated intracompartmental pressure (ICP) in 1910 [1]. Elevated ICP impairs the microcirculation of the involved compartment. The elevated pressure produces ischemia that affects muscles and nerves leading to muscle and nerve dysfunction [2].
The most common indication for fasciotomy is for the diagnosis of acute compartment syndrome (ACS). ACS is caused by raised ICP within a closed osteo-fascial or myofascial space. The overall incidence of compartment syndrome is estimated to be 0.7 per 100,000 in women and 7.3 per 100,000 in men [3]. The true incidence is not known because of variations in clinical presentation. Vigilance is therefore necessary to avoid the consequences of delayed treatment. Trauma is the most common etiology for extremity compartment syndrome. A 1.3% prevalence was found among trauma admissions at a level 1 trauma center [4]. Familiarity with the trauma-related etiologies helps to identify the patient populations at risk. Extremity ACS following trauma occurs most commonly after high-energy injury to the limbs. Fractures account for up to 75% of cases of ACS [2]. It is important to keep in mind that ACS can follow both open and closed fractures. It should not be assumed that decompression of the compartment has occurred because of the small fascial tears that accompany open fractures. It is estimated that 5.9% of open and 2.2% of closed tibial fractures result in ACS [5]. About 30% of extremity ACS occurs following soft tissue injury without associated fracture. Patients younger than 35 years are at higher risk of developing ACS and males at higher risk than females, perhaps because of increased muscle bulk. Older patients are at lower risk and patients with diastolic hypertension are at lower risk [6]. The site of fasciotomy varies as well. Sixty-eight percent involve the leg, 14% the forearm, and 9% the thigh. Gluteal, foot, and hand ACS occur less frequently [5].
Acute Compartment Syndrome
In addition to fracture and soft tissue injury, ACS may occur following compression with tight bandages or casts, vascular injury especially combined venous and arterial, reperfusion after prolonged ischemia, crush injuries, gunshot wounds, circumferential or electrical burns, extensive venous thrombosis, and prolonged immobilization (as can occur with alcohol or drug intoxication, or prolonged operative cases). Less common causes include envenomation and hematomas resulting from trauma, and/or coagulopathy, either congenital or acquired. Fasciotomy is sometimes performed in patients at high risk for the development of ACS before overt signs develop. Candidates for this approach have had vascular injury with shunting or repair after prolonged ischemia, have combined venous and arterial vascular injury, or have undergone venous ligation. Combined venous and arterial injury increases the incidence of compartment syndrome by 41.8% [5]. ACS can also be a sequela of soft tissue infection. Fasciotomy along with drainage and debridement is necessary to treat the soft tissue infection. Examples include pyomyositis and necrotizing fasciitis. Box 21.1 lists indications for fasciotomy.
Box 21.1: Indications for Fasciotomy
-
Acute compartment syndrome
-
High-energy blunt trauma to extremity with or without fracture
-
Penetrating wound to the extremity
-
Blast injury
-
Reperfusion following ischemia
-
Crush injury
-
Prolonged immobilization with intoxication or drug overdose
-
Prolonged operation
-
Prolonged lithotomy or Trendelenburg positioning
-
Envenomation
-
Infection (necrotizing soft tissue infections, severe pyomyositis, or cellulitis)
-
Circumferential burns or electrical burns
-
Vascular injury (especially combined arterial and venous)
-
Deep venous thrombosis
-
IV infiltration with extravasation of fluids or medicines
-
Vigorous crystalloid resuscitation
-
Coagulopathy
-
Tight casts or bandages
-
Strenuous exercise
-
High-pressure injections
-
-
Prophylaxis against development of compartment syndrome
-
Prolonged ischemia
-
Exploration for infection
Fasciotomy is performed to decrease the elevated ICP restoring perfusion. Unrelieved elevation of the pressure within a compartment above a threshold for a prolonged period can result in painful tissue ischemia, venous congestion, and neuropraxia. Irreversible ischemic damage to multiple compartments may make amputation necessary. The best strategy available to avoid delayed treatment of ACS is to recognize the patient at risk, closely monitor them for evolution of their exam, and to initiate surgical treatment when recognized. Box 21.2 lists factors that increase risk of ACS among trauma patients.
Box 21.2: Factors that Increase Risk of ACS in Trauma Patients
-
Demographics
-
Young age < 35 years [17]
-
Male sex
-
Injury patterns
-
Gunshot wounds
-
Blast injuries
-
Crush injuries
-
Combined arterial and venous injuries
-
Major vascular injury below the aortic bifurcation
-
Tibial fractures
-
Open fractures
-
Joint dislocations
-
-
Systemic factors
-
Need for massive transfusion
-
Large-volume crystalloid resuscitation
-
Admission hypotension
-
High injury severity score (ISS)
-
ACS Pathophysiology
Perfusion of a tissue compartment is related to the arteriolar and capillary perfusion gradients. Normal ICP is 0–8 mm Hg [7]. When pressures exceed capillary filling pressure, nutrient tissue perfusion is prevented resulting in tissue ischemia. At the same time, venous and lymphatic outflow are also compromised, producing further pressure buildup within the compartment. Blood flow at the arteriolar level is compromised by the higher compartmental pressure, and further ischemia results. Perfusion pressure is related to blood pressure. In experimental models, muscle ischemia results when intracompartmental pressure is within 10 mm Hg of the diastolic blood pressure (BP). The ischemia triggers the release of vasoactive chemicals and cytokines. The endothelium becomes permeable causing more tissue edema compounding the ICP increase. Muscle cells release myoglobin causing rhabdomyolysis. Rhabdomyolysis may accompany ACS in up to 23% of cases [3]. High circulating levels of myoglobin cause renal tubular obstruction and acute kidney injury. Ultimately, cell death occurs, and substances are released that amplify the production of edema within the compartment. Figure 21.1 illustrates the cycle that without timely interruption can result in tissue loss. Sustained ICP elevation above 30 mm Hg for 4–8 hours leads to irreversible tissue injury. Complete recovery of function occurs in nearly all patients undergoing fasciotomy within 6 hours, but only in 68% of patients when within 12 hours, and only 8% recover after 12 hours [8].
ACS pathophysiology. Constriction, tissue trauma, edema, or bleeding causes increased pressure within a myo- or osteo-fascial compartment. As the intracompartmental pressure increases, the venous capillary pressure rises leading to hypoperfusion (red arrows). Intracellular metabolism converts from aerobic to anaerobic. The cell becomes acidotic and oxidants accumulate. The cell swells as the Na/K pump fails. The cell membrane is destroyed and intracellular contents enter the interstitium resulting in more edema. When perfusion is decreased below critical thresholds for extended time, cell death and necrosis result
ACS Diagnosis
The diagnosis is heavily dependent on clinical evaluation . Swelling of the extremity will be present. Though swelling following injury is common, the swelling associated with compartment syndrome is tense, and the skin may be shiny and taut. Pain is the earliest and most sensitive–though least specific–indication of compartment syndrome. Trauma patients are expected to have pain, but the pain associated with compartment syndrome is often described as a change in intensity or to exceed that which would be expected for the injury. In addition, pain with passive stretch of the muscles within the compartment will produce excruciating pain. The six clinical signs of ischemia (6 Ps) are often associated with the diagnosis of compartment syndrome (Box 21.3). Since compartment syndrome results from changes in arteriolar and capillary perfusion gradients, ACS occurs even with palpable extremity pulses and without pallor or capillary refill delays. Muscle paralysis is also a late indication of compartment syndrome . Moreover, muscle strength is often difficult to elicit in an injured limb. However, a change in clinical examination would be an important clue. If fasciotomy is performed once all 6 Ps are present, functional recovery is unlikely. Patients must be alert and awake in order to elicit the most useful clinical findings. When the prognostic value of pain, pain on passive stretch, paresthesia, and paralysis (4 Ps) are compared, they individually have a prognostic value between 13 and 19%. When pain and pain on passive stretch are both present, the prognostic value rises to 68%. The addition of paresthesias and paralysis increases the probability to 93% and 98%, respectively. However, it has been reported that when foot drop is present at the time of diagnosis, only 13% of patients recover function following fasciotomy [9]. Though major sensory and motor deficits are late signs of ACS, diminished two-point discrimination and loss of vibratory sense are considered early markers of ACS [8].
Box 21.3: Signs of Vascular Ischemia
-
Pain
-
Paresthesias
-
Pallor
-
Poikilothermia
-
Paralysis
-
Pulselessness
The diagnosis can be difficult to make even in patients that are awake and alert. Patients receiving regional block and continuous analgesic drips and with altered mental status lack even the usual nonspecific physical findings. Repeated assessments continue to be essential to trigger the timely and accurate diagnosis of ACS. A warm extremity with palpable peripheral pulses is reassuring but does not exclude the diagnosis of ACS, particularly when worsening pain and paresthesias are also present.
Objective measures are added when the diagnosis is unclear. Unfortunately, there is no pathopneumonic diagnostic laboratory test . Myoglobin is released as cell death occurs, so elevation of serum myoglobin is associated with ACS. Myoglobin has a short half-life. Creatine kinase (CK), aldolase, and lactate dehydrogenase (LDH) are also released. No specific threshold is recognized especially in trauma patients who may have sustained direct muscle injury. CK and myoglobin levels should be serially measured though. CK levels >2000 U/L may be associated with compartment syndrome in the absence of fracture and >4000 when fracture is present [10]. A rising level is concerning. CK levels remain elevated for 1–3 days [11]. Urine myoglobin is also frequently measured especially in cases of crush or prolonged immobilization. Acute kidney injury (AKI) requiring dialysis results from precipitation of myoglobin in the renal tubules. Hydration, alkalinization of the urine, and early continuous renal replacement therapy are strategies to minimize renal damage.
ICP measurements supplement the physical examination. Measurements can be repeated to identify progression. This test is particularly useful in patients who are comatose, paralyzed, anesthetized, or under the effect of nerve blocks. Whiteside described a technique to measure ICP. His technique utilized a pressure manometer, syringe, two stopcocks, and a needle. Using his technique, saline is aspirated into an extension that is connected to a stopcock through a needle, taking care not to aspirate air. Another extension tube connects to a stopcock and to a manometer and the saline-filled extension tube. A syringe with 15 cc of air is connected to the unused stopcock port. The needle is advanced into the compartment, and the plunger of the air-filled syringe is depressed. When the saline meniscus changes from convex to flat, the manometer reading indicates the compartmental pressure [6]. Figure 21.2 illustrates the setup. Commercial devices are also available that utilize a similar principle. The most commonly used is made by Stryker (Kalamazoo, MI, USA) and is shown in Fig. 21.3a, b. Arterial pressure monitoring devices can be adapted to measure compartment pressures using appropriate needles or catheters, stopcocks, and extension tubing. Attach an 18 gauge needle to arterial line tubing. Flush and zero the setup. Advance the needle into the desired compartment, and note the mean arterial pressure (MAP). The MAP represents the ICP. Side port and slit catheters improve the accuracy over straight needles [12]. Several principles are important to remember. Pressures are higher within 5 cm of a fracture site and at the center of a compartment. Compartment pressures are not necessarily uniform throughout an extremity [2]. No precise best location to measure ICP has been determined. Multiple sites should be tested [13]. Serial measurements may be required to make the diagnosis. Continuous measurements are advocated by some to assure prompt diagnosis. An absolute measurement of 30–45 mm Hg is frequently used to diagnose compartment syndrome in the appropriate clinical setting. Some recognize the relationship between systemic blood pressure and ICP and prefer to use a calculated delta p. This is derived from subtracting the measured pressure from either the MAP or diastolic BP. Differences of less than 30 mm Hg indicate ACS [14]. Useful measurements are dependent on correct placement within the affected compartments.
Whitesides’ compartment pressure setup. Saline is introduced into intravenous tubing connected to a needle. A stopcock with a 20 ml syringe filled with air and a manometer connect to the tubing. The needle is introduced into the compartment. The plunger on the air-filled syringe is depressed. When the saline meniscus changes from convex to flat, the manometer reading will indicate the compartment pressure. The needles indicate the access points to anterior, lateral, superficial, and deep posterior compartments of the leg
ACS Treatment
General
Remove any constricting bandages or casts. Do not elevate the limb. Maintain it at the level of the heart except in cases of phlegmasia. Resuscitate to assure euvolemia. ACS is a surgical emergency. Operation should commence rapidly after diagnosis. Hyperbaric oxygen therapy may be a useful adjunct to fasciotomy to treat ACS and tissue ischemia.
Fasciotomy
Technique
General Principles
Several principles apply generally to fasciotomy regardless of the site involved. They include the following:
-
Make longitudinal incision over the entirety of the investing fascia of each compartment. The skin overlying the affected compartment should be completely opened.
-
Release the compartment contents.
-
Protect critical neurovascular structures.
-
Carefully evaluate the muscle for viability, remove frankly necrotic tissue, and reevaluate ischemic tissue with potential for reversibility.
-
Delayed closure.
Table 21.1 outlines the compartments, contents along with clinical symptoms observed, and outcome of delayed treatment.
Table 21.2 outlines the compartments and incisions used to approach them.
Buttock
Trauma is not a common indication for buttock fasciotomy. Prolonged immobilization following alcohol or drug intoxication, intramuscular injection, or prolonged operative procedures are indications that are more common. Case reports have described the condition following trauma associated with gluteal artery bleeding from aneurysmal rupture or injury and high-energy direct trauma to the buttock. Trauma-associated etiologies include contusion, gluteal artery injury, hip dislocation, acetabular fracture, pelvic fracture, and following vascular procedures. Infection and spontaneous hemorrhage are other potential etiologies [15]. Patients report buttock pain and will avoid putting pressure on the affected side. The hip is often held flexed and extension is avoided. Knee extension will elicit severe discomfort. Tense swelling is apparent on physical examination. Buttock numbness and sciatic nerve compression symptoms are often present. The large muscle burden of the area combined with the usual delay in presentation make rhabdomyolysis likely. Renal function, electrolytes, CK, and myoglobin levels should be followed. Should compartment pressure measurement be necessary, place the needle to minimize the risk of neurovascular injury. To measure pressure in the gluteus maximus, identify the proximal inner quadrant of the buttock 2 cm inferior and lateral to the posterior superior iliac spine (PSIS) . To measure pressure in the gluteus medius and minimus, insert the needle 2 cm below the iliac crest over the middle third of the iliac wing. The tensor compartment is accessed 2 cm anterior to and 3 cm distal to the greater trochanter [16]. The pressure associated with ACS of the buttock is not precisely known, and the numbers utilized for the lower and upper extremity are adopted for this region. Normal pressures are thought to range from 13 to 14 mm Hg [17].
Three compartments are recognized in the buttock. These include the tensor, the gluteus medius and minimus muscle, and the gluteus maximus muscle. The sciatic nerve does not lie within the gluteal compartments, but it is frequently compressed by swelling of the gluteus maximus above it [17].
To perform a buttock fasciotomy, position the patient in the lateral decubitus position with the affected side up. The hip is flexed. Prone positioning is also possible, particularly if there is bilateral involvement. Mark an incision that extends from just below the iliac crest lateral to the posterior superior iliac spine to the greater trochanter and below (posterior or Kocher-Langenbeck incision). The incision can be extended down the femur should there be a need to decompress the thigh. Incise the skin and deepen the incision to the gluteal fascia. Separate the superolateral edge of the gluteus maximus from the iliotibial tract [18]. The fascia is incised and the muscle split to assure decompression. Avoid injury to the gluteal artery. Split the gluteus maximus muscle in the direction of its fibers. The gluteus medius is exposed with superior retraction of the gluteus maximus, and the fascia is incised. The gluteus minimus is located deep to the medius. The muscles are inspected and evaluated for consistency, contractility, color, and perfusion. Any hematoma identified is evacuated, and frankly dead tissue is removed. Non-contractile muscle that is perfused is preserved as function may return after decompression. If arterial injury caused the compartment syndrome, ligation or embolization to control further bleeding will be necessary [16].
Leave the wound open. It can be dressed with moist gauze dressings. Negative pressure wound therapy (NPWT) is another option to manage the wound. Reassess the tissues in 24–48 hours.
Lower Extremity
Thigh
Due to the large volume spaces in the compartments, thigh compartment syndrome is not a common finding. However, patients at risk for developing it include those with femur/pelvic fractures and ligation of major venous structures such as the inferior vena cava (IVC) and those who have suffered blast injuries.
There are three compartments in the thigh (Fig. 21.4). These include the anterior comprising the quadriceps muscles, the posterior comprising the hamstring muscles, and the medial comprising the adductor muscles. In most cases, effective decompression of the anterior and posterior compartments is performed through a single laterally placed incision. To open the quadriceps compartment, an anterolateral longitudinal skin incision is created along the iliotibial tract, extending from the lateral condyle to the intertrochanteric space. A long skin incision along the lateral thigh is used and extended to the fascia lata. Incision of the fascia lata decompresses the anterior thigh. The fascia over the vastus lateralis is then opened to decompress the compartment. The vastus lateralis can be retracted anteriorly to enter the posterior compartment dividing the posterior intermuscular septum [16]. The medial compartment is rarely subject to compartment syndrome, and decompression is generally not specifically performed. However, if needed, a separate medial incision should be included.
Thigh compartments. The anterior (shaded in purple) including the rectus femoris, vastus lateralis, vastus intermedius, vastus medialis, and sartorius muscles. The posterior (shaded in green) including the biceps femoris and semimembranosus and semitendinosus. These compartments are decompressed through a lateral incision. The medial compartment includes the adductor longus, magnus, and gracilis and are decompressed through a separate medial incision when necessary
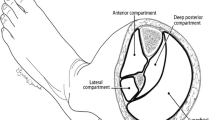
Leg
The leg has four compartments. They include the following:
-
Anterior compartment contains the anterior tibial, extensor digitorum longus, extensor hallucis longus, and peroneus tertius muscles and the anterior tibial artery and vein.
-
The lateral compartment contains the peroneus longus and brevis muscles and the superficial peroneal nerve.
-
The superficial posterior compartment contains the gastrocnemius and the soleus muscles and the posterior saphenous vein and the sural nerve.
-
The deep posterior compartment contains the tibialis posterior, the flexor hallucis longus and the flexor digitorum longus. The posterior tibial artery, vein, and nerve are also in this compartment.
The most common surgical approach to lower extremity fasciotomy is through two incisions to decompress the four compartments. Position the patient supine. A triangle placed behind the knee supporting the leg is useful to improve visualization. Important landmarks to identify include the patella, the tibial spine medial and lateral malleolus, the medial border of the tibia, and the head of the fibula. The anterolateral incision, which will decompress the anterior and lateral compartments, is made in a longitudinal line 1 fingerbreadth or about 2 cm anterior to the edge of the fibula. This may not be easily palpable and may be identified by drawing a line from the fibular head to the lateral malleolus, marking the course of the fibula. When the fascia is identified, try to find the intermuscular septum. Perforating vessels mark the location. Palpate the tibial spine; the anterior compartment will be directly adjacent to it. A horizontal incision is made across the septum. Then insert scissors with closed tips facing away from the septum. This avoids injury to the peroneal nerve. Insert the scissors through the other limb of the “H” to complete the decompression. Be particularly cautious in opening the fascia over the distal third of the lateral compartment. The superficial peroneal nerve exits the fascia and continues subcutaneously. Injury to the superficial peroneal nerve can lead to foot drop [19]. Figure 21.5 illustrates the lateral incision and decompression of the anterior and lateral compartments.
Decompression of the anterior and lateral compartments. The intermuscular septum separates the anterior and lateral compartments. An H-shaped incision is made to assure decompression of both compartments. The superficial peroneal nerve runs deep to the intermuscular septum and should be avoided when incising the fascia
The medial longitudinal skin incision is made 2 fingerbreadths below the tibial plateau, to 2 cm proximal to the medial malleolus, and the tibial spine is a midpoint reference between the lateral and medial skin incisions. There should be 7 cm between the medial and lateral incisions anteriorly [18]. This avoids skin compromise that could expose the tibia. Try to preserve the saphenous vein and accompanying nerve when opening the skin and subcutaneous tissues [20]. Perforating veins should be ligated, as they can cause profuse bleeding. The fascia over the superficial posterior compartment is opened 2 cm posterior to the edge of the tibia for the length of the skin incision. To open the deep compartment, detach the soleus from the tibia. Visualization of the posterior tibial neurovascular bundle assures the deep posterior compartment has been reached [21]. Figure 21.6 illustrates the medial fasciotomy incision to decompress the superficial and deep compartments. The plane between the soleus and gastrocnemius is sometimes mistaken for deep compartment. There is a high risk of compartmental hypertension in this area, which can affect the viability and function of the foot significantly as it contains major structures: the tibial nerve and the posterior tibial and peroneal arteries [22]. The use of generous incisions is important, as the skin envelope can be a limiting factor [23].
Medial incision lower leg fasciotomy . The fascia overlying the gastrocnemius and soleus is incised to release the superficial compartment. The soleus fibers are separated from the tibia to access the deep compartment. Visualizing the posterior tibial neurovascular bundle assures the deep compartment has been decompressed
Foot
Foot compartment syndrome (FCS) is uncommon and accounts for <5% of limb compartment syndromes, and isolated foot injuries result in compartment syndrome only 2% of the time. The most common cause of FCS is high-energy trauma producing fracture of the calcaneus. FCS occurs in 10% of these cases [24].
The foot contains nine compartments. Three run longitudinally the length of the foot: the medial, lateral, and superficial. The four interosseous and adductor compartments are within the forefoot, and the deep or calcaneal compartment is in the hindfoot and communicates with the deep posterior compartment of the leg. This is important as blood or infection can readily spread through these compartments. Because of this communication, tibial fracture can produce foot compartment syndrome [1]. The deep compartment contains the posterior tibial nerve, artery, and vein. It also contains the lateral plantar nerve artery and vein and the quadratus plantae muscle. There are four interosseous compartments that contain the plantar and dorsal interosseous muscles. The adductor compartment contains the adductor muscles. The medial compartment contains the flexor and abductor hallucis muscles. The lateral compartment contains abductor digiti minimi and the flexor digiti minimi brevis muscles. The superficial compartment contains the flexor digitorum brevis muscle and the flexor digitorum longus tendons [24].
Foot pain is a common complaint with foot ACS and generally expected based on the mechanism of injury. The pain is not improved with anticipated doses of analgesics. Dorsiflexion of the toes leads to stretch of the intrinsic muscles. One series found 86% of patients with ACS had this finding. Serial sensory examinations are considered to be more sensitive than a single examination [1]. The ability to discriminate two points on the plantar aspect of the foot is more sensitive than pinprick assessment. Motor deficits in the foot are difficult to assess and found late in the course of ACS, and as previously mentioned, pulselessness is a very late finding [1].
Pressure measurements are helpful in detecting ACS that is not clinically obvious. The deep (calcaneal) compartment has higher ICP than other foot compartments. Thresholds based on leg and forearm pressures are used to diagnose ACS. Pressures are measured in the superficial compartment by inserting the needle in the arch of foot entering the flexor digitorum brevis. The medial and deep compartments are measured through an approach 4 cm inferior to the medial malleolus. The lateral compartment is reached through a needle inserted below the 5th metatarsal, and the interosseous compartments are accessed dorsally between the 1st and 2nd metatarsals, and the needles are deepened to enter the adductor compartment [25].
Orthopedic specialists most often perform foot fasciotomy. The landmarks for decompression are as follows:
1. Make dorsal incisions to approach the interossei and adductor compartments medial to the 2nd metatarsal and lateral to the 4th metatarsal (MT). Dissection of the muscle medially from the 2nd MT exposes the adductor fascia that is incised. The incision lateral to the 4th MT allows access to the interossei on either side [26]. Figure 21.7 illustrates the incisions and routes to the various compartments.
2. Extend the medial plantar incision for 6 cm beginning 4 cm from the posterior aspect of the heel and 3 cm superior to the plantar surface paralleling the plantar surface. The abductor hallucis is retracted superiorly, and the intermuscular septum is incised bluntly medially avoiding injury to the lateral plantar neurovascular bundle to enter the deep compartment. The fascia is opened throughout the length of skin incision taking care to avoid injury to the medial plantar nerve at the distal extent of the opening. The quadratus should bulge through the incision indicating decompression of the deep compartment. Entry into the superficial and lateral compartments requires retraction of the medial compartment superiorly, and the superficial compartment is opened. The flexor digitorum brevis is released and retracted inferiorly to reach the lateral compartment [23]. The septum is opened posterior to anterior with sharp dissection; visualization of the muscles of the compartment assures decompression [1].
3. Wounds are left open and gauze or NPWT is used.
Upper Extremity
Arm
The upper arm has three compartments. The anterior compartment includes the biceps, the brachialis, and the coracobrachialis muscles. Several nerves including the median, ulnar, radial, medial, and lateral brachial cutaneous nerves pass through this compartment distally. The posterior compartment contains the triceps and anconeus muscles. The posterior antebrachial cutaneous nerve and nerve to the anconeus track through the posterior compartment. The third compartment includes the deltoid muscle [2].
Make a lateral skin incision that extends from the insertion of the deltoid on the humerus to the lateral epicondyle. This incision can be extended proximally to release the deltoid if it is involved. The incision is deepened to the fascia. Open the fascia over the anterior and posterior compartment using longitudinal incisions. A medial incision can be used when access to the brachial artery is needed. This incision can be easily extended to the forearm when necessary [27].
Forearm
There are three compartments in the forearm. The volar and mobile wad are decompressed through a volar skin incision. The volar compartment contains the wrist and finger flexors. The mobile wad contains the brachioradialis and the extensor carpi radialis longus and brevis muscles. The radial nerve and radial artery are between the mobile wad and the volar compartment. The volar compartment contains the anterior interosseous artery and branches of the radial artery, the median nerve, and branches of the ulnar artery [6]. Begin the volar incision medial to the biceps tendon, and angle it distally across the flexor crease, and then continue onto the forearm extending to the palm to include release of the carpal tunnel. Always cross the flexor crease at the wrist at an angle. Figure 21.8 illustrates the incision. At the elbow, divide the lacertus fibrosus (the bicipital aponneurosis) to prevent constriction of the brachial artery. The superficial muscles of the volar compartment are released individually. Retracting the flexor carpi ulnaris (FCU) and the ulnar neurovascular bundle medially and the flexor digitorum superficialis (FDS) and median nerve laterally exposes the flexor digitorum profundus (FDP). The FDP fascia is then released [28]. The superficial and deep volar muscles are released with these maneuvers. To release the mobile wad, incise the fascia overlying it, and free the three muscles in the compartment. Distally the carpal tunnel is released by dividing the transverse carpal ligament medial to palmaris longus. Look for the median nerve just proximal to the wrist [29].
Forearm fasciotomy . The upper panel illustrates the volar incision. It should extend to the elbow and can be extended proximally should vascular control be necessary. Distally it extends onto the hand to allow for decompression of the carpal tunnel. The lower panel illustrates the dorsal incisions. The incision extends from distal to the lateral condyle to the midline of the wrist. Incision over the hand is placed over the 2nd and 4th metacarpals. Thenar and hypothenar incisions are made at the junction of the palmar and dorsal skin when needed
The dorsal compartment contains the extensor muscles of the wrist and fingers and the posterior interosseous artery. Extensor muscle release is not always needed because release of the volar compartments may decompress the extensor muscles. If there is an ongoing concern, the dorsal incision and release is added [30]. The dorsal compartment is decompressed through an incision on the dorsal forearm placed between the ulna and radius. The incision begins distal to the lateral epicondyle and extends to the midline of the wrist (Thompson’s approach). Incise the fascia longitudinally and free the muscles individually [8]. Figure 21.7 illustrates the volar incision.
When decompression of the hand is required, two dorsal incisions are made. They are positioned over the 2nd and 4th metacarpals (MC). Incise the fascia over the dorsal interosseous muscle. Blunt dissection along the ulnar side of the metacarpal (MC) allows decompression of the volar interosseous muscles and the adductor pollicis. Add incisions over the thenar and hypothenar compartments at the junction of palmar and dorsal skin [31]. Figure 21.8 illustrates the placement of incision to decompress the hand. Figure 21.9 shows a cross section of the forearm and hand with the compartments and muscles labeled.
Cross section of the hand and forearm with the compartments labeled. Top: Cross section of the hand. The interosseous compartment is accessed through incisions over the 2nd and 4th metacarpals (MC). The adductor compartment can be reached through the incision at the 2nd MC. The midpalmar space is reached through the incision at the 4th MC. Thenar and hypothenar incisions are made to decompress those compartments. Bottom: Cross section of the forearm with the three compartments and their contents labeled. The volar compartment includes FCU (flexor carpi ulnaris), FDS (flexor digitorum superficialis), FPL (flexor pollicis longus), FCR (flexor carpi radialis), and (FDP) flexor digitorum profundus. The mobile wad includes BR (brachioradialis), ECRL (extensor carpi radialis longus), and ECRB (extensor carpi radialis brevis). The dorsal compartment includes the EPB (extensor pollicis brevis), ECU (extensor carpi ulnaris), ED minimi (extensor digiti minimi), ED communis (extensor digiti communis), and APL (abductor pollicis longus)
After freeing the muscles, determine the need for muscle debridement. Obviously nonviable tissue should be removed at once but questionable tissue left and reassessed at the next procedure [1]. Leave the incisions open. NPWT or moist gauze dressings are applied. Reassess the wounds in 24–48 hours.
Wound Management and Closure
Initially all fasciotomy wounds are left open. As mentioned previously, options for wound management are NPWT (negative pressure wound therapy) and moist gauze dressings. There are advantages to each, and circumstances might make one option more desirable than another. Wounds in which hemostasis has been challenging because of coagulopathy or inflammation are better suited to gauze dressings. These dressings are relatively inexpensive and easier to change at the bedside. Dressing changes at the bedside will identify evolution of muscle necrosis or bleeding. Adding a non-adherent contact layer will prevent further tissue trauma with dressing changes. Non-adherent contact dressings are either silicone based or impregnated with petroleum ointment. Examples include Mepitel® (Molnylcke Norcross, GA, USA), open mesh petroleum emulsion impregnated cellulose acetate fiber dressing (Covidien™/Curity™, USA, non-adherent dressing), or Restore® contact layer flex dressing (Hollister, Libertyville, IL, USA). Dressings containing hemostatic agents are useful in patients that have persistent coagulopathy and nonsurgical bleeding. QuickClot® (Z-MEDICA, LLC, Wallingford, CT, USA) and ChitoGauze® Pro (Portland, OR, USA) hemostatic dressings are available in a variety of forms including gauze pads, Z-fold, rolled gauze, and trauma pads.
NPWT uses a porous foam or gauze dressing combined with continuous negative pressure. Fluid is removed and can be quantified. There is a theoretic potential for improved tissue blood flow and removal of harmful cytokines as well. The negative pressure can amplify bleeding when hemostasis is inadequate and can potentially desiccate tissue when the vacuum seal is incomplete.
The wound is reevaluated in the operating room at 24–48 hours. Any necrotic tissue is removed. The skin edges will retract. Tension applied to the skin edges will prevent extensive retraction that could make primary closure impossible. Homemade or commercial devices are effective for preventing skin retraction. Homemade options include the so-called “Jacob’s ladder ” or shoelace configuration composed of skin staples and heavy crisscrossing silicon vessel loops knotted at one end and stapled to the skin. Staple the vessel loop to one side of the wound and advance distally, and staple the vessel loop to the other side of the wound. Multiple vessel loops are necessary to completely encompass the wound. The loops can be tightened on return to the operating room or at the bedside until the skin edges are approximated closely enough to allow for delayed primary closure (DPC). Another option is interrupted pulley sutures. These are triple mattress sutures that disperse tension across the wound. Place the far suture 2 cm from the edge, the middle 1 cm from the edge, and near a few millimeters for the edge [32]. These sutures can be progressively placed and/or tightened as the tissue edema resolves. DermaClose® (Synovis, Birmingham, AL, USA) is a commercial device utilizing a similar Jacob’s ladder configuration with hooks and heavy suture attached to a spring-loaded device (Fig. 21.10). In either case, too much tension results in skin necrosis and loss. When the wounds have stabilized, there is no further tissue necrosis and the edema has resolved closure is possible. When dual incisions are used and closure of only one incision is possible, choose to close over exposed bone. When primary closure is impossible, split-thickness skin grafting is used. In cases where there has been extensive muscle debridement, flap coverage could be needed. Closure may be possible as soon as 5–10 days following the initial procedure.
Complications
The consequences of failure to perform a necessary fasciotomy include limb loss or dysfunction, acute renal dysfunction, infection, and death. Although fasciotomy is performed to prevent morbidity, the procedure is not risk-free. Early complications include soft tissue infections, pain, and deep venous thrombosis. Long-term wound complications include dry scaly skin, itching, tethered scars or tendons, excessive scarring, extremity swelling, poor wound healing, osteomyelitis, and recurrent or chronic wounds. Complications and their relative frequency are listed in Table 21.3. Incomplete fasciotomy is also possible and can result in nerve damage and muscle loss [8]. In the lower extremity, the compartments that are most often incompletely decompressed or missed are the anterior and the deep posterior. Placing the lateral leg incision too far posteriorly can result in failing to decompress the anterior compartment. Use the tibial spine to aid proper orientation. The deep posterior compartment is sometimes confused with the superficial. Releasing the soleus from the tibia and visualizing the posterior tibial neurovascular bundle avoids this confusion. In the forearm, the connective tissue surrounding each muscle bundle can cause constriction. Thorough inspection of each muscle and release of the connective tissue surrounding individual muscles will assure adequate decompression. A military review found patients with missed or incomplete compartment release more frequently required muscle excision, were twice as likely to require amputation, and had increased mortality [29]. It is estimated that 80–95% of all patients undergoing fasciotomy experience long-term complications [2].
Outcome
Prognosis depends on several factors that include:
-
Extent of injury
-
Duration of inadequate perfusion
-
Comorbidities and functional status
-
Time to fasciotomy
The goal of fasciotomy is to avoid permanent muscle and nerve injury. Amputation is therefore one of the most severe complications. The need is based on damaged caused by prolonged ACS rather than the fasciotomy procedure. Amputation is required in 6–13% of those with extremity ACS. Factors that increase that risk are male gender, vascular injury, and diagnostic delay. Persistent disabilities occur even when amputation is not required. Chronic pain, sensory abnormalities, decreased range of motion, and foot drop are not uncommon following fasciotomy. Pain with activity and unsatisfactory scar appearance affect quality of life. One series reported nearly 70% did not return to work [33].
Summary
Fasciotomy is indicated to treat acute compartment syndrome and to prevent development of compartment syndrome in selected high-risk patients. When ACS is diagnosed, fasciotomy should follow quickly to minimize permanent muscle and nerve injury. Physical examination is important even though the findings are fairly nonspecific. Tense swelling, unexpectedly severe pain, and pain with passive stretch of compartmental muscles are the most reliable signs to look for. Vigilance is required to assure ACS is diagnosed early. Patients who cannot be evaluated clinically or those with equivocal exams may benefit from ICP measurements. It is therefore important the trauma surgeon is familiar with the anatomy of and approaches to the compartments of the upper and lower extremity. Table 21.2 contains a summary of the compartments and approaches to them. Full incisions with compete compartment release should be undertaken within 6 hours of symptom onset. Well-performed procedures may still result in wound complications but are preferable to the morbidity associated with missed compartment syndrome or incomplete compartment release.
Abbreviations
- 6 Ps:
-
Pain, pallor, paresthesias, paralysis, poikilothermia, pulselessness
- ACS:
-
Acute compartment syndrome
- APL:
-
Abductor pollicis longus
- BR:
-
Brachioradialis
- CK:
-
Creatine kinase
- DPC:
-
Delayed primary closure
- ECRB:
-
Extensor carpi radialis brevis
- ECRL:
-
Extensor carpi radialis longus
- ECU:
-
Extensor carpi ulnaris
- ED:
-
Extensor digiti
- EDC:
-
Extensor digitorum communis
- EPB:
-
Extensor pollicis brevis
- EPL:
-
Extensor pollicis longus
- FCS:
-
Foot compartment syndrome
- FCU:
-
Flexor carpi ulnaris
- FDP:
-
Flexor digitorum profundus
- FDS:
-
Flexor digitorum superficialis
- Hg:
-
Mercury
- ICP:
-
Intracompartmental pressure
- ISS:
-
Injury severity score
- IV:
-
Intravenous
- IVC:
-
Inferior vena cava
- L:
-
Liter
- LDH:
-
Lactate dehydrogenase
- MC:
-
Metacarpal
- Mm:
-
Millimeters
- MT:
-
Metatarsal
- N:
-
Nerve
- NPWT:
-
Negative pressure wound therapy
- U:
-
Units
References
Fulkerson E, Razi A, Tejwani N. Review: acute compartment syndrome of the foot. Foot Ankle Int. 2003;24(4):180–7.
Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625–32.
McQueen MM, Gatson P, Court-Brown CM. Acute compartment syndrome: who is at risk. J Bone Joint Surg Br. 2000;82(2):200–3.
Zuchelli D, Divaris N, McCormac JE, Huang EC, Chaudhary ND, Vosswinkel JA, et al. Extremity compartment syndrome following blunt trauma: a level I trauma center’s 5-year experience. J Surg Res. 2017;217:131–6.
Branco BC, Inaba K, Barmparas G, Schnüriger B, Lustenberger T, Talving P, et al. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10 year review in a mature level I trauma center. Injury. 2011;42:1157–63.
Whitesides TE Jr, Heckman MM. Acute compartment syndrome: update on diagnosis and treatment. J Am Acad Orthop Surg. 1996;4(4):209–18.
Seiler JG III, Womack S, De L’Aune WR, Whitesides TE, Hutton WC. Intracompartmental pressure measurements in the normal forearm. J Orthop Trauma. 1993;7:414–6.
Donaldson J, Haddad B, Khan WS. The pathophysiology, diagnosis and current management of acute compartment syndrome. Open Orthop J. 2014;8(Suppl 1:M8):185–93.
Bradley EL III. The anterior compartment syndrome. Surg Gynecol Obstet. 1973;136:289–97.
Valdez C, Schroeder E, Amdur R, Pasual J, Sarani B. Serum creatinine kinase levels are associated with extremity compartment syndrome. J Trauma. 2013;74(2):441–5.
Walls MH, Landing T. Compartment syndrome: an orthopedic emergency. J Emerg Nurs. 2017;43(4):303–7.
Boody AR, Wonworawat MD. Accuracy in the measurement of compartment pressures: a comparison of three commonly used devices. J Bone Joint Surg Am. 2005;84(11):2415–22.
Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures: the relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76:1285–92.
McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99–104.
Hayden G, Leung M, Leong J. Gluteal compartment syndrome. ANZ J Surg. 2006;76:668–70.
MacLean J, Wustrack R, Kandemir U. Gluteal compartment syndrome. Tech Orthop. 2012;27:43–6.
Rocos B, Ward A. Gluteal compartment syndrome with sciatic nerve palsy caused by traumatic rupture of the inferior gluteal artery: a successful surgical treatment. BMJ Case Rep. 2017;2017:bcr2016216709.
Hessman MH, Ingelfinger P, Rommens PM. Compartment syndrome of the lower extremity. Eur J Trauma Emerg Surg. 2007;33:589–99.
Bowyer M. Lower extremity fasciotomy: indications and technique. Curr Trauma Rep. 2014;1:35–44.
Owen C, Cavalcanti A, Molina V, Honore C. Decompressive fasciotomy for acute compartment syndrome of the leg. J Visc Surg. 2016;153:293–6.
Injuries to the extremities: compartment syndrome and fasciotomy. ASSET American College of Surgeons Committee on Trauma; 2010. p. 28–40.
Burns JB, Frykberg E. Management of extremity compartment syndrome. In: Cameron JL, editor. Current surgical therapy. 10th ed. Philadelphia: Elsevier; 2011. p. 1028–32.
Papachristos IV, Giannoudis PV. Acute compartment syndrome of the extremities: an update. Orthop Trauma. 2018;32(4):223–8.
Dodd A, Le I. Foot compartment syndrome: diagnosis and management. J Am Acad Ortho Surg. 2013;21:657–64.
Towater LJ, Heron S. Foot compartment syndrome: a rare presentation to the emergency department. J Emerg Med. 2013;44(2):e235–8.
Yoon P. Compartment syndrome of the foot. Tech Ortho. 2012;27:58–61.
Maeckelbergh F, Colen S, Ludwig A. Upper arm compartment syndrome: a case report and review of the literature. Ortho Surg. 2013;5(3):229–32.
Ronel DN, Mtui E, Nolan WB. Forearm compartment syndrome : anatomical analysis of surgical approaches to the deep space. Plast Reconstr Surg. 2004;114(3):697–705.
Schellenberg M, Chong V, Cone J, Keeley J, Inaba K. Extremity compartment syndrome. Curr Probl Surg. 2018;55:256–73.
Burgess AR, Aziz A. Fasciotomy. In: Dua A, Desai SS, Holcomb JB, Burgess AR, Frieschlag JA, editors. Clinical review of vascular trauma. New York: Springer; 2014. p. 65–7.
Prasam ML, Ouellette EA. Acute compartment syndrome of the upper extremity. J Am Acad Ortho Surg. 2011;19(1):49–58.
Rhee P, Dubose J. Soft tissue wounds and fasciotomy. In: Martin M, Beekley A, editors. Front line surgery: a practical approach. New York: Springer; 2011. p. 239–67.
Lolla I, Grabinsky A. Clinical and functional outcomes of acute lower extremity compartment syndrome at a major trauma hospital. Int J Crit Illn Inj Sci. 2015;6:133–42.
Fitzgerald AM, Gaston P, Wilson Y, Quaba A, McQueen MM. Long-term sequelae of fasciotomy wounds. Br J Plast Surg. 2000;53(8):690–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Henry, S.M., Park, H. (2021). Extremity Fasciotomies. In: Scalea, T.M. (eds) The Shock Trauma Manual of Operative Techniques. Springer, Cham. https://doi.org/10.1007/978-3-030-27596-9_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-27596-9_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27595-2
Online ISBN: 978-3-030-27596-9
eBook Packages: MedicineMedicine (R0)