Abstract
First developed and introduced by Dr. Donald Morton and colleagues in the early 1990s, sentinel lymph node biopsy (SLNB) is a technique to evaluate the pathologic status of regional lymph nodes (LN) in patients who present with clinically node-negative melanoma. The evidence for performing SLNB is strongest for patients diagnosed with intermediate-thickness melanomas (>1.0–4.0 mm in Breslow thickness). Additionally, SLNB may be considered for patients with higher risk thin (<0.8 mm with high-risk features, or ≥0.8–1.0 mm) and thick (>4.0 mm) melanomas. Lymphoscintigraphy with radiolabeled colloid should be performed preoperatively in order to accurately identify the regional draining nodal basin. Intradermal injection of blue dye may be additionally used intraoperatively to assist with SLN identification. For thorough pathologic evaluation, SLNs should be bivalved and serially sectioned. A combination of hematoxylin-eosin staining and immunohistochemistry (S-100, HMB-45, and MART-1) is typically utilized to identify LN metastases. SLNB specimens should be evaluated for the location of the tumor deposit within the LN (subcapsular, trabecular or intraparenchymal), presence or absence of extracapsular invasion, and size of the tumor deposit.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Melanoma
- Sentinel lymph node biopsy
- Lymphoscintigraphy
- Radiolabeled colloid
- Isosulfan blue
- Lymph node sectioning
- Histopathologic evaluation
Introduction
The incidence of malignant melanoma has increased over the past few decades, and melanoma now represents the fifth most common cancer in the United States [1]. Prognosis following diagnosis is highly dependent on disease stage, as determined by Breslow thickness, primary tumor ulceration, and the presence of regional lymph node (LN), satellite/in-transit, or distant metastases [2]. In patients with clinically localized melanoma, sentinel lymph node biopsy (SLNB) is an important staging and prognostic tool used to evaluated the pathologic status of the regional LN basin.
History
First introduced to the surgical community by Morton et al. in the early 1990s, SLNB quickly replaced elective LN dissection (ELND) in determining whether tumor cells have spread beyond the primary site to the regional nodal basin [3]. Routine ELND in patients with early-stage melanoma was controversial for several reasons. Large multi-institutional prospective studies failed to demonstrate a significant survival benefit of ELND compared to nodal observation except in certain subgroups of patients [4, 5]. Clinically occult LN metastases were histologically identified in only about 15–20% of patients who underwent ELND, while patients were exposed to the potentially significant morbidity associated with ELND without a definite clinical benefit [3, 5].
In the initial report of SLNB published by Morton et al., SLNs were successfully identified in 194 (82%) of 237 specimens, ranging from 81% for cervical basins to 89% for the groin [3]. Among the 259 SLNs from the 194 specimens, 18% harbored microscopic melanoma metastases. In contrast, only 0.06% of non-sentinel nodes were found to be tumor-bearing (false-negative rate 1%), corroborating the notion that the SLNs were the initial sites of regional LN spread and confirming the high sensitivity of the technique [3].
The role of SLNB in the management of clinically localized melanoma was further assessed prospectively through a large randomized trial, the Multicenter Selective Lymphadenectomy Trial-1 (MSLT-1), which was initiated by Morton et al. in 1994 [6]. Ten-year survival outcomes were published in 2014 (Table 7.1) [7]. The phase III trial included 2001 patients diagnosed with localized cutaneous melanoma of Breslow thickness ≥1.2 mm. Patients were randomized to undergo wide local excision (WLE) of the primary tumor with SLNB, followed by immediate completion lymphadenectomy (CLND) for those with a positive SLN, versus wide excision of primary alone with nodal observation and therapeutic lymphadenectomy at time of nodal recurrence. While the trial found no significant difference between randomized groups for the primary study endpoint (melanoma-specific survival), SLNB was associated with improved 10-year disease-free survival in patients with intermediate-thickness melanomas, defined as 1.2–3.5 mm in Breslow thickness (SLNB vs. observation, Hazard Ratio [HR] 0.76, P = 0.01), and thick melanomas, or >3.5 mm (HR 0.70, P = 0.03). This was driven largely by higher regional recurrence rates in the observation arm of the trial. The trial reaffirmed the strong prognostic value of the SLN; nodal metastasis was associated with decreased melanoma-specific survival (intermediate-thickness, HR 3.09, P < 0.001; thick, HR 1.75, P = 0.03). Furthermore, earlier intervention with SLNB and immediate CLND, compared to therapeutic lymphadenectomy after nodal recurrence, appeared to be associated with improved melanoma-specific survival in the subgroup of patients with intermediate-thickness melanomas with nodal disease (HR 0.56, P = 0.006). A similar treatment-related response with early nodal intervention was not observed among patients with thick melanomas and LN metastases (HR 0.92, P = 0.78).
The important prognostic information provided by SLNB led to the incorporation of regional nodal micrometastases in the sixth edition of the American Joint Committee on Cancer staging system for melanoma in 2001 [8]. Historically, the distinction between clinical and pathologic staging was not emphasized. With the widespread use of SLNB, and increased upstaging of clinically node-negative patients, clinical and pathologic staging led to distinct populations of patients with disparate survival outcomes. The difference in survival conferred by the pathologic nodal status was most pronounced for clinically node-negative patients with melanomas >1.0–4.0 mm in Breslow thickness (P < 0.0001) [8].
Patient Selection for Sentinel Lymph Node Biopsy
Guideline Recommendations
SLNB is recommended for certain populations of patients presenting with clinically node-negative invasive melanoma with appreciable risk of regional nodal metastasis. It is not recommended for patients diagnosed with melanoma in situ or those with clinically-evident nodal disease (for which nodal microstaging is unnecessary). Clinical guidelines continue to evolve over time with respect to precise patient selection criteria, but generally are concordant in recommending SLNB for patients with intermediate-thickness melanomas >1.0–4.0 mm in Breslow thickness.
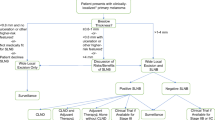
Current guidelines set forth by the American Society of Clinical Oncology (ASCO) and the Society of Surgical Oncology (SSO) recommend the performance of SLNB in patients with intermediate-thickness melanomas (>1.0–4.0 mm in Breslow thickness) (Fig. 7.1a) [9]. Furthermore, a SLNB may be considered after a thorough discussion of potential benefits and risks in patients with T1b melanomas (<0.8 mm in Breslow thickness with ulceration, or 0.8–1.0 mm in thickness irrespective of ulceration status). Similarly, it may be considered in patients with thick melanomas (>4.0 mm in thickness), who harbor a significant risk of regional LN metastasis. SLNB is not routinely recommended for patients with thin, non-ulcerated tumors <0.8 mm.
National guidelines for patient selection for sentinel lymph node biopsy. (a) American Society of Clinical Oncology (ASC) and Society of Surgical Oncology (SSO) guidelines [9]. (b) National Comprehensive Cancer Network (NCCN) guidelines [10]. aSentinel lymph node biopsy may be considered if other high-risk features are present, such as a very high mitotic rate (≥2 per mm2), especially in a young patient, lymphovascular invasion, or a combination
Guidelines from National Comprehensive Cancer Network (NCCN) recommend offering SLNB for patients with a risk of positive SLN of 10% or higher [10]. This would include patients with melanomas ≥1.0 mm in Breslow thickness, regardless of ulceration status. Unlike the ASCO/SSO guidelines, the NCCN guidelines do not differ in their recommendations for intermediate-thickness and thick melanomas. SLNB should be considered in those with 5–10% risk, such as patients with melanomas <0.8 mm with high-risk features (ulceration, mitotic rate ≥2 per mm2 [particularly in patients of young age], lymphovascular invasion, or a combination) or 0.8–1.0 mm in thickness. The guidelines further state that, among patients for whom SLNB should be considered or offered, individual clinical decisions depend on patient comorbidities, patient preferences, and other factors. SLNB is not recommended for those with <5% risk, such as patients with melanomas <0.8 mm in Breslow thickness without ulceration or other high-risk features. Additionally, the presence of microsatellitosis or in-transit disease at initial melanoma presentation already defines stage III disease, and while SLN status does have prognostic value, the importance of SLNB in this patient population has not been clearly defined [10, 11].
Evidence for Intermediate-Thickness and Thick Melanomas
Guideline recommendations for SLNB are based in part on the results from MSLT-1 and several other retrospective studies. Similar to MSLT-1, many retrospective studies demonstrated an improvement in disease-free survival, but not melanoma-specific survival, in patients with intermediate-thickness who underwent SLNB (Table 7.2) [12,13,14]. One retrospective study using data from the Surveillance Epidemiology and End Results (SEER) demonstrated worse melanoma-specific survival in patients with intermediate-thickness melanomas who underwent nodal observation compared to SLNB (HR 1.18, 95% Confidence Interval [CI] 1.04–1.34, P = 0.009) [15]. However, the authors noted that the absolute difference in survival was small (1.7%). Retrospective studies have identified increasing Breslow thickness, ulceration, mitoses, and lymphovascular invasion to be associated with SLN positivity in patients with intermediate-thickness melanomas (Table 7.3) [16,17,18].
Unlike for intermediate-thickness melanomas, the MSLT-1 did not demonstrate that early nodal intervention among patients with thick melanomas and nodal metastases was associated with improved melanoma-specific survival (SLNB positive vs. observation with nodal recurrence, HR 0.92, P = 0.78) [7]. Similar to MSLT-1, multiple retrospective studies have not demonstrated an improvement in melanoma-specific survival with receipt of SLNB in patients with thick melanomas [12, 14, 19, 20], where the frequency of occult systemic metastases may be appreciable [21]. SLN positivity rates for thick melanomas are quite high, reported as 32.9% in MSLT-1 [7] and ranging from 30% to 51.2% in retrospective series [12, 19, 21,22,23,24,25,26]. However, even despite the lack of any demonstrable survival benefit of the SLN procedure in this high risk population, retrospective studies have found the SLN status to be prognostic, with SLN positive patients experiencing worse disease-free [19, 20, 22, 23, 25], distant disease-free [24], melanoma-specific [19, 20, 25], and overall survival [22, 24,25,26] (Table 7.4). Reported factors associated with decreased likelihood of SLN positivity in patients with thick melanomas have included identified head/neck location, desmoplastic histology, and absence of satellitosis (Table 7.3) [25]. Other studies found the presence of ulceration [22, 24] and lymphovascular invasion [24] to be associated with SLN positivity by univariable analysis.
Evidence for Thin Melanomas
Evidence supporting SLNB in patients with thin melanomas are limited to retrospective studies as there are no randomized trials comparing SLNB to nodal observation for this lower risk patient population. Using data from SEER, Sperry et al. demonstrated an improvement in disease-free survival for patients with high-risk, thin (≥0.76–1.00 mm with ulceration or ≥1 mitoses per mm2) melanomas of the head and neck who underwent SLNB compared to observation, similar to the findings for patients with intermediate-thickness and thick melanomas (Table 7.2) [14]. In a two-center study of patients with thin melanoma, receipt of SLNB was associated with improved survival outcomes (5-year melanoma-specific survival 88% vs. 72%, P < 0.0001) in patients with identified SLN metastases compared to those who developed clinical nodal disease [27]. Further prospective study would be needed to delineate the influence of patient selection and other potential biases in these observed results. Among patients with thin melanomas, increasing Breslow thickness is a strong risk factor for SLN positivity, with most studies using a depth of 0.75 or 0.80 mm as the cutpoint for comparison [28,29,30,31] (Table 7.3). Other primary tumor factors, such as the presence of ulceration [28,29,30,31,32], mitoses [28,29,30, 32], and lymphovascular invasion [28], have also been associated with increased risk for SLN positivity, supporting the consideration of SLNB in patients with thin melanomas and these high-risk features.
In addition to tumor factors, patient age appears to be associated with SLN status (Table 7.3). Multiple studies have demonstrated lower rates of positive SLNs in older patients, regardless of other clinicopathologic features [16,17,18, 28, 29, 33, 34]. Paradoxically, however, older age is also associated with decreased melanoma-specific survival [33]. However, patient age is not included as a factor for consideration in current clinical practice guidelines, which focus on tumor factors.
Technical Performance
Lymphoscintigraphy and Tracer Injection
First developed in 1977, preoperative lymphoscintigraphy is the commonly accepted technique for identifying the regional draining LN basin in anatomic areas with variable drainage patterns, such as the head, neck, and trunk (Fig. 7.2) [35]. In truncal melanomas, for example, contralateral rather than ipsilateral nodal basins may be involved, and in head and neck melanomas, pre-auricular, parotid, or suboccipital sites rather than the cervical chain or supraclavicular nodes may serve as the primary draining basin. Information from lymphoscintigraphy helps to guide the biopsy of all involved regional LN basins. While it is typically performed on the day of surgery just prior to SLNB, surgery can be performed up to 24 h later without significant dissipation of radiolabeled colloid [36].
Lymphoscintigraphy typically begins with a four-point intradermal injection of 0.05–1 mCi of technetium 99-labeled sulfur colloid just adjacent to the primary melanoma biopsy site or clinical residual lesion [37]. It should be injected in wheels, 0.1 mL per aliquot, with a 25- to 27-gauge needle. Drainage to the nodal basin is usually brisk, within 10–30 min. Inadequate tissue tension in the wheel can lead to delayed drainage, and injected volumes larger than 0.1 mL risk obstructing dermal lymphatics [9, 38]. Also, increased pressure from the wheel can cause leakage when the needle is removed, leading to interference on gamma imaging. In some areas, such as the head and neck, the caudal injection is held as it may interfere with imaging of the nodal basin. Subcutaneous injection should be avoided, as drainage from subcutaneous lymphatics may not represent lymphatic drainage from the cutaneous melanoma. The radiation dose from a SLNB to the surgeon and other personnel is minimal. It is estimated that the radioactive dose from a single biopsy is one-thirtieth of the annual whole-body absorbed dose from background radiation [38].
Most centers implement planar gamma camera imaging following radiocolloid injection to identify the appropriate nodal basins and sentinel nodes. Some centers implement dynamic imaging to visualize nodes close to the injection site that receive direct lymphatic drainage. This technique captures images immediately after injection at 30 s per frame for 2–30 min. It is recommended that head and neck melanomas be evaluated by single-photon emission computed tomography (SPECT-CT) in addition to planar lymphoscintigraphy when available, as it has been shown to find an additional nodal basin in 38% of patients, increase the yield of positive SLNs, decrease local recurrence rates, and alter surgical approach in 20–50% of cases (Fig. 7.3) [39,40,41,42]. Other techniques used to assist with node localization include the use of a cobalt-57 flood source or other hot source to trace the outline of the patient. Furthermore, some centers perform skin markings over identified nodes in the appropriate operative position, occasionally from both the anterior and lateral views.
After lymphoscintigraphy, the patient can proceed to the operating room. Additional SLN localization may be performed by injection of blue dye with the identification of any blue-colored LNs as SLNs. Prior to injection of the blue dye, it is important to outline the margin for the WLE, as the dye may obscure a small biopsy scar. A four-point intradermal injection with up to 1–2 mL of blue dye is performed at the primary melanoma site. Five to ten minutes are needed for the blue dye to reach the nodal basin. Commercially available dyes include isosulfan blue and methylene blue. Both dyes are effectively taken up by the dermal lymphatics, but have different side effect profiles. In one study, 1.5% of patients had an adverse reaction to isosulfan blue, including a significant rate of anaphylaxis in 0.75% of patients [43]. Methylene blue has been associated with tissue necrosis, so care must be taken in anatomic regions where the dye might not be fully resected, such as the ankles, wrists, and face. Small amounts of blue dye left at the excision site may rarely result in permanent tattoo.
Two other SLN tracers used in the care of patients with melanoma include indocyanine green and tilmanocept (Lymphoseek®). Indocyanine green is used in conjunction with infrared fluorescence for detection of SLNs. While studies have shown that indocyanine green detects SLNs more efficiently than traditional methods, there is no long-term evidence that suggests its use improves outcomes [44]. The use of tilmanocept, a molecule specifically engineered as an ideal radiotracer for SLN detection with binding capacity to CD206 receptors on the surface of macrophages and dendritic cells, has been promising. In a clinical trial involving patients with clinically node negative melanoma, tilmanocept was found to have increased sensitivity compared to conventional SLN dyes [45].
Performance of Sentinel Lymph Node Biopsy
A gamma probe is placed in a sterile sleeve and used to identify areas of radiotracer uptake in nodal basins identified on preoperative lymphoscintigraphy. If there is significant radiotracer interference from the primary melanoma injection site, WLE of the primary tumor can be performed first to decrease interference. Otherwise, SLNB is usually performed prior to the excision of the primary site, to prevent potential cross-contamination and allow for more time for lymphatic drainage to the nodal basin. A small incision is made across the nodal basin and the dissection is carried down using instrument dissection and electrocautery. The incision is typically made such that it can be extended should a CLND ultimately be performed. Blue-stained lymphatics and the gamma probe are used to direct the dissection towards the SLN(s). Small lymphatics or vessels entering the node are ligated or clipped as necessary. Care is taken not to disrupt the capsule of the SLN using instruments or electrocautery, as it can affect pathological assessment [46]. In general, additional dissection should be avoided in the nodal basin other than that required to remove the SLNs.
All blue nodes, grossly abnormal nodes, or nodes with at least 10% of the ex vivo maximum radiotracer count of the hottest node are removed. This recommendation extends from a study from McMasters et al. that found that in 13.1% of positive nodal basins, the most radioactive SLN was negative for tumor, while another less radioactive LN was positive for tumor [47]. Furthermore, in 50% of those cases, the radioactive count of the positive node was ≤50% of the radioactive count of the hottest node. Approximately one to three SLNs are typically identified per dissection following these criteria.
In most cases, WLE and SLNB are completed during the same operation. However, some patients are referred for SLNB only after their WLE has been completed. A series of publications have evaluated the feasibility and accuracy of lymphoscintigraphy and SLNB in this setting [48,49,50,51]. In a large study of this type by Gannon et al., lymphatic mapping and SLNB were successful in 103 of 104 patients who had WLE prior to SLNB [50]. A comparison to a cohort of over 1000 patients who underwent concomitant WLE and SLNB at the same institution revealed no significant differences in the SLN identification rate, incidence of a positive SLN, or number of SLNs identified. Interestingly, more patients with axial primaries who underwent prior WLE were found to have multiple LN basin drainage, but this did not reach statistical significance (P = 0.07). Due to these findings, it is recommended that patients undergo concomitant WLE and SLNB whenever possible to provide patients with a single operation, lower costs, and avoid the risk and morbidity of a potentially larger second operation to accomplish accurate staging.
Further studies are needed to fully validate the accuracy of SLN mapping by tracking long-term false negative recurrences. The overall accuracy of SLNB depends on anatomic location, with likely increased accuracy for truncal and extremity locations where lymphatic drainage is more predictable, and a higher false negative rate in head and neck locations where drainage is more complex [52, 53].
Specimen Handling
SLN specimens should be intact with ideally a rim of adjacent adipose tissue present and without crush deformities or diathermic injury [46, 54]. Once removed, the length, width and height of the LN are measured and an ex vivo maximum radiotracer count is obtained by scanning the node with the gamma probe. Additionally, it is important to note the presence or absence of blue dye discoloration and additional markings, including collections of melanin and carbon pigment.
The method of choice for tissue preservation is routine processing with fixation in 4–10% buffered formalin [54,55,56,57,58,59,60,61]. Frozen sectioning is not preferred as it provides suboptimal morphology, has poor sensitivity, and does not adequately incorporate the subcapsular region of the LN, a site of frequent micrometastases [54,55,56,57,58,59,60,61]. When fixing tissue from the SLN in buffered formalin, the solution should be allowed to sit at room temperature for at least 12 h, although some institutions have advocated for 48 h of incubation [54, 59]. This allows the technetium-labeled sulfur colloid in the radiotracer to decay [59].
Pathologic Assessment of the Sentinel Lymph Node
Specimen Sectioning
Pathologic investigation of the SLNs, and the identification of micrometastases, is critical to the accurate staging of cutaneous melanoma, and ultimately, the determination of treatment options and prognosis. Following fixation, the specimen is dissected in order to embed in paraffin. Two methods have been proposed for the dissection of the SLN: bivalve and bread-loafing dissection. Bivalve bisection cuts the LN longitudinally along its longest axis and bread-loafing dissection slices the node perpendicular to the longitudinal axis. Of these techniques, bivalve dissection is considered to be the standard of specimen sectioning among institutions [54,55,56,57, 60, 61]. Bisection along the longitudinal axis allows the specimen to be transected through the hilum. By bisecting at the level of the hilum, a large number of lymphatic vessels, including efferent lymphatic vessels, and the subcapsular region are exposed. This increases the rate of detection of micrometastases in the SLN [54,55,56, 60].
Additional sectioning of the LN has been a topic of debate among institutions. At this time, there is no consensus on the number of sections or levels necessary for SLN analysis [54]. The majority of institutions employ sectioning into 2–4 mm slices with each block of tissue being further sectioned into 1–3 levels to be analyzed by hematoxylin-eosin (H&E) staining [58,59,60]. More levels are necessary for immunohistochemistry (IHC) analysis. Some institutions have advocated for utilizing serial-sectioning of samples to obtain more level as this has the potential for revealing occult metastases with minimal additive labor or cost [57].
Specimen Staining and Tumor Burden Assessment
Sections obtained from SLN specimens are analyzed histologically using H&E and IHC (Fig. 7.4). It is sometimes difficult to accurately interpret histology shown on H&E staining alone due to hypercellularity within the LN and similarities in morphology between melanoma and normal nodal cells. As many as 12% of metastases will be missed in the absence of IHC [62, 63]. Traditionally, S-100, a marker for metastatic melanoma, has been the primary target for staining in SLN specimens due to its high sensitivity (95–100%) [59, 60]. Additionally, HMB-45, a target of the antigen gp100, and MART-1, a melanoma-associated antigen recognized by T cells, are often used for staining. HMB-45 is reactive with 50–80% of metastatic melanoma cells, but often negative in an intracapsular nevus, which makes it useful in distinguishing intracapsular nevi from melanoma [59, 60, 62]. Tyrosinase, a marker specific for melanocytic differentiation, has a similar sensitivity and specificity profile as these other markers, and is useful in detecting false negatives following HMB45 and MART-1 staining. An antibody combination of these three markers, HMB-45, MART-1, and tyrosinase, is currently in circulated use with increased sensitivity compared to each antibody alone [60]. Lastly, SOX10, a transcription factor in neural crest cells, has, in limited studies, been shown to be sensitive and specific to melanoma metastases, but is not widely used at this time.
Representative photomicroscopy of melanoma sentinel lymph node. (a) Histology (H&E stain). Rare melanoma cells in the subcapsular area of this sentinel node. (b) S-100 stain. Rare subcapsular melanoma cells are positive for S-100. (c) HMB-45 stain. Rare subcapsular melanoma cells are positive for HMB-45. Bar indicates 80 μm. Arrows point to the melanoma cells
There is little consensus on a specimen protocol for SLNs for melanoma, but several institutions and organizations are in support of their own single-site protocols. Cochran et al. was first to propose a protocol where SLNs are bisected and sectioned serially into ten sections; four discontinuous sections are stained with H&E, one section is stained with S-100, and one section is stained with HMB45 [55, 64]. Moffitt Cancer Center sections the node in 2–3 mm intervals and forms section blocks at one to three levels; one section is used for H&E and one section is used for S-100 [64]. At the Massachusetts General Hospital, three serial sections are taken from the specimen block at three different levels measuring 80–100 μm apart and 1–2 mm in thickness: (1) the second, fifth, and eighth levels are stained with H&E, (2) the third, sixth, and ninth levels are stained with S-100 and HMB-45, and (3) the first, fourth, and seventh levels are stained with NK1C3, a protein present on activated granulocytes, and MART-1 [64]. At the Hospital of the University of Pennsylvania, sections are taken from the specimen at four different levels. The first and fourth levels are used for histology and the second and third levels are stained with S-100 and HMB-45. The European Organization for Research and Treatment of Cancer protocol, modified from the Cook et al. protocol, involves an initial full-face section, similar to the Cochran bivalving technique, followed by five step sections 50 μm apart with staining of the subsections with H&E, S-100, and HMB-45, respectively [54, 65]. While there are minute differences between various pathologic protocols, all protocols share a common understanding that bivalving of the node, in order to evaluate the subcapsular sinus, in combination with serial sectioning leads to the best positive predictive value for the identification of melanoma micrometastases [64].
Following section preparation, specimens are examined with particular attention to the subcapsular sinus region [54]. Positive SLNs are identified in the subcapsular region 86% of the time, so it is critical to preserve and examine this section pathologically [66]. Higher power magnification (400×) is typically utilized to confirm findings noted on low magnification. Melanoma cells can, at times, be difficult to differentiate from underlying cells present in LNs, including macrophages, dendritic cells, and nevus cells. All of these cells are positive for S-100, but can be distinguished based on size, nuclear and cytoplasmic characteristics, and distribution within the node. Nevus cells, benign and small nevomelanocytic cells, are usually negative for HMB45 and Ki67, and are typically intracapsular or trabecular [54, 66]. Melanoma cells are larger than nevus cells and contain larger nucleoli with a higher nuclear to cytoplasmic ratio [54, 63, 66]. Macrophages can be differentiated by noting the coarse melanin granules in contrast to the fine melanin granules of melanoma cells [54, 63].
When evaluating sections, there is limited consensus on a single scoring algorithm, but there is consensus on the assessment parameters for the SLNs. All specimens should be evaluated for the location of the tumor deposit within the LN (whether this be subcapsular, intraparenchymal, or trabecular), the presence or absence of extracapsular invasion, and the size of the tumor deposit [54, 67]. Extracapsular invasion should be documented as this has been associated with poor prognosis [63]. Extension of tumor cells into the central portion of the SLN indicates a worse prognosis, as location within the non-subcapsular location is sensitive for additional non-sentinel nodal metastases during complete LN dissection [54, 63, 66]. In fact, micrometastases within the subcapsular region only have a non-sentinel lymph node positivity rate of 2% and a melanoma-specific survival rate of 95%, making this biology more akin to negative SLNs and clinically insignificant [68]. The Rotterdam criteria suggests that tumor burden within the SLN <0.1 mm, particularly within the subcapsular region, may predict very low likelihood of additional non-sentinel LN disease in the nodal basin [68].
Reverse transcriptase polymerase chain reaction (RT-PCR) for molecular detection of melanoma tumor markers has been evaluated as a method for identifying positive SLNs [69]. However, RT-PCR status of histologically negative SLNs has not been associated with statistically different disease recurrence and survival outcomes, suggesting that RT-PCR positivity may not provide clinically valuable prognostic information [69, 70]. As such, histologic examination using a combination of H&E and IHC remains the gold standard for SLN assessment.
Summary
-
SLNB is a technique to evaluate the pathologic status of the regional nodal basin in patients diagnosed with clinically node-negative malignant melanoma. It is not performed for patients with melanoma in situ or those with clinically-evident nodal metastases.
-
The evidence for performing SLNB is strongest for patients diagnosed with intermediate-thickness melanomas (>1.0–4.0 mm in Breslow thickness). MSLT-1 demonstrated improved disease-free survival, but no difference in melanoma-specific survival, in patients with melanoma 1.2–3.5 mm in thickness. Among patients with nodal metastases, there was improved melanoma-specific survival associated with early nodal intervention in this population.
-
SLNB should also be offered to patients with thick melanomas (>4.0 mm), for which SLN status is strongly associated with disease-specific survival outcomes.
-
In patients with thin melanomas <0.8 mm with high-risk features (ulceration, very high mitotic rate, lymphovascular invasion, or a combination) or those ≥0.8–1.0 mm in Breslow thickness, SLNB may be considered.
-
Lymphoscintigraphy with radiolabeled colloid should be performed prior to SLNB in order to accurately identify the regional draining nodal basin. In patients with melanoma involving the head and neck, SPECT-CT may improve identification of the draining basin.
-
Intradermal injection of blue dye may be used in conjunction with the radioactive tracer for SLN identification. All blue nodes, grossly abnormal nodes, or nodes with at least 10% of the ex vivo maximum radiotracer count of the hottest node are removed.
-
For thorough pathologic evaluation, SLNs should be bivalved and serially sectioned.
-
A combination of H&E and IHC are used to identify nodal metastases. Stains for S-100, HMB-45, and MART-1 are typically utilized.
-
SLN specimens should be evaluated for location of the tumor deposit within the LN (subcapsular, trabecular or intraparenchymal), presence or absence of extracapsular invasion, and size of the tumor deposit.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eight edition cancer staging manual. CA Cancer J Clin. 2017;67:472–92.
Morton DL, Wen D-R, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9.
Balch CM, Soong S-J, Bartolucci AA, et al. Efficacy of an elective regional lymph node dissection of 1 to 4 mm thick melanomas for patients 60 years of age and younger. Ann Surg. 1996;224:255–63.
Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F, Melanoma Programme WHO. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. Lancet. 1998;351:793–6.
Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17.
Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609.
Balch CM, Buzaid AC, Soong S-J, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48.
Wong SL, Faries MB, Kennedy EB, et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American Society of Clinical Oncology and Society of Surgical Oncology clinical practice guideline update. Ann Surg Oncol. 2018;25:356–77.
Coit DG, Thompson JF, Albertini MR, et al. NCCN clinical practice guidelines in oncology: cutaneous melanoma version 2.2019. 2019.
Bartlett EK, Gupta M, Datta J, Gimotty PA, Guerry D, Xu X, Elder DE, Czerniecki BJ, Fraker DL, Karakousis GC. Prognosis of patients with melanoma and microsatellitosis undergoing sentinel lymph node biopsy. Ann Surg Oncol. 2014;21:1016–23.
Kachare SD, Singla P, Vohra NA, Zervos EE, Wong JH, Fitzgerald TL. Sentinel lymph node biopsy is prognostic but not therapeutic for thick melanoma. Surgery. 2015;158:662–8.
van der Ploeg APT, Haydu LE, Spillane AJ, Quinn MJ, Saw RPM, Shannon KF, Stretch JR, Uren RF, Scolyer RA, Thompson JF. Outcome following sentinel node biopsy plus wide local excision versus wide local excision only for primary cutaneous melanoma: analysis of 5840 patients treated at a single institution. Ann Surg. 2014;260:149–57.
Sperry SM, Charlton ME, Pagedar NA. Association of sentinel lymph node biopsy with survival for head and neck melanoma: survival analysis using the SEER database. JAMA Otolaryngol Head Neck Surg. 2014;140:1101–9.
Kachare SD, Brinkley J, Wong JH, Vohra NA, Zervos EE, Fitzgerald TL. The influence of sentinel lymph node biopsy on survival for intermediate-thickness melanoma. Ann Surg Oncol. 2014;21:3377–85.
Bartlett EK, Peters MG, Blair A, et al. Identification of patients with intermediate thickness melanoma at low risk for sentinel lymph node positivity. Ann Surg Oncol. 2016;23:250–6.
Chang JM, Kosiorek HE, Dueck AC, et al. Stratifying SLN incidence in intermediate thickness melanoma patients. Am J Surg. 2018;215:699–706.
Hanna AN, Sinnamon AJ, Roses RE, Kelz RR, Elder DE, Xu X, Pockaj BA, Zager JS, Fraker DL, Karakousis GC. Relationship between age and likelihood of lymph node metastases in patients with intermediate thickness melanoma (1.01–4.00 mm): a National Cancer Database study. J Am Acad Dermatol. 2019;80:433–40.
Ribero S, Osella-Abate S, Sanlorenzo M, Balagna E, Senetta R, FIerro MT, Macripo G, Macri L, Sapino A, Quaglino P. Sentinel lymph node biopsy in thick-melanoma patients (N=350): what is its prognostic role? Ann Surg Oncol. 2015;22:1967–73.
Boada A, Tejera-Vaquerizo A, Ribero S, et al. Sentinel lymph node biopsy versus observation in thick melanoma: a multicenter propensity score matching study. Int J Cancer. 2018;142:641–8.
de Oliveira Filho RS, da Silva AM, de Oliveira DA, Oliveira GG, Nahas FX. Sentinel node biopsy should not be recommended for patients with thick melanoma. Rev Col Bras Cir. 2013;40:127–9.
Gershenwald JE, Mansfield PF, Lee JE, Ross MI. Role for lymphatic mapping and sentinel lymph node biopsy in patients with thick (> or = 4 mm) primary melanoma. Ann Surg Oncol. 2000;7:160–5.
Ferrone CR, Panageas KS, Busam K, Brady MS, Coit DG. Multivariate prognostic model for patients with thick cutaneous melanoma: importance of sentinel lymph node status. Ann Surg Oncol. 2002;9:637–45.
Gajdos C, Griffith KA, Wong SL, Johnson TM, Chang AE, Cimmino VM, Lowe L, Bradford CR, Rees RS, Sabel MS. Is there a benefit to sentinel lymph node biopsy in patients with T4 melanoma? Cancer. 2009;115:5752–60.
Yamamoto M, Fisher KJ, Wong JY, et al. Sentinel lymph node biopsy is indicated for patients with thick clinically lymph node-negative melanoma. Cancer. 2015;121:1628–36.
Mozillo N, Pennacchioli E, Gandini S, Caraco C, Crispo A, Botti C, Secondo L, Barberis M, Verrecchia F, Testori A. Sentinel node biopsy in thin and thick melanoma. Ann Surg Oncol. 2013;20:2780–6.
Karakousis GC, Gimotty PA, Bartlett EK, Sim M-S, Neuwirth MG, Fraker DL, Czerniecki BJ, Faries MB. Thin melanoma with nodal involvement: analysis of demographic, pathologic, and treatment factors with regard to prognosis. Ann Surg Oncol. 2017;24:952–9.
Sinnamon AJ, Neuwirth MG, Yalamanchi P, et al. Association between patient age and lymph node positivity in thin melanoma. JAMA Dermatol. 2017;153:866–73.
Conic RRZ, Ko J, Damiani G, Funchain P, Knackstedt T, Vij A, Vidimos A, Gastman BR. Predictors of sentinel lymph node positivity in thin melanoma using the National Cancer Database. J Am Acad Dermatol. 2019;80:441–7.
Cordeiro E, Gervais M-K, Shah PS, Hong NJL, Wright FC. Sentinel lymph node biopsy in thin cutaneous melanoma: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23:4178–88.
Piazzalunga D, Ceresoli M, Allievi N, et al. Can sentinel node biopsy be safely omitted in thin melanoma? Risk factor analysis of 1272 multicenter prospective cases. Eur J Surg Oncol. 2019;45:820–4. https://doi.org/10.1016/j.ejso.2018.11.022.
Karakousis GC, Gimotty PA, Botbyl JD, et al. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol. 2006;13:533–41.
Balch CM, Soong S-J, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013;20:3961.
Balch CM, Soong S-J, Thompson JF, et al. Age as a predictor of sentinel node metastasis among patients with localized melanoma: an inverse correlation of melanoma mortality and incidence of sentinel node metastasis among young and old patients. Ann Surg Oncol. 2014;21:1075–81.
Holmes EC, Moseley HS, Morton DL, Clark W, Robinson D, Urist MM. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186:481–90.
White DC, Schuler FR, Pruitt SK, Culhane DK, Seigler HF, Coleman RE, Tyler DS. Timing of sentinel lymph node mapping after lymphoscintigraphy. Surgery. 1999;126:156–61.
Rossi CR, De Salvo GL, Trifiro G, et al. The impact of lymphoscintigraphy technique on the outcome of sentinel node biopsy in 1,313 patients with cutaneous melanoma: an Italian multicentric study (SOLISM-IMI). J Nucl Med. 2006;47:234–41.
Alazraki N, Glass EC, Castronovo F, Valdes Olmos RA, Podoloff D. Procedure guideline for lymphoscintigraphy and the use of intraoperative gamma probe for sentinel lymph node localization in melanoma of intermediate thickness 1.0. J Nucl Med. 2002;43:1414–8.
Vermeeren L, Valdes Olmos RA, Klop MC, van der Ploeg IMC, Nieweg OE, Balm AJM, van den Brekel MWM. SPECT/CT for sentinel lymph node mapping in head and neck melanoma. Head Neck. 2011;33:1–6.
Bilde A, von Buchwald C, Mortensen J, Marving J, Hamilton Therkildsen M, Kirkegaard J, Charabi B, Specht L. The role of SPECT-CT in the lymphoscintigraphic identification of sentinel nodes in patients with oral cancer. Acta Otolaryngol. 2006;126:1096–103.
Stoffels I, Boy C, Poppel T, Kuhn J, Klotgen K, Dissemond J, Schadendorf D, Klode J. Association between sentinel lymph node excision with or without preoperative SPECT/CT and metastatic node detection and disease-free survival in melanoma. JAMA. 2012;308:1007–14.
Trinh BB, Chapman BC, Gleisner A, Kwak JJ, Morgan R, McCarter MD, Gajdos C, Kounalakis N. SPECT/CT adds distinct lymph node basins and influences radiologic findings and surgical approach for sentinel lymph node biopsy in head and neck melanoma. Ann Surg Oncol. 2018;25:1716–22.
Daley MD, Norman PH, Leak JA, et al. Adverse events associated with the intraoperative injection of isosulfan blue. J Clin Anesth. 2004;16:332–41.
Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J Surg Oncol. 2012;106:41–5.
Sondak VK, King DW, Zager JS, et al. Combined analysis of phase III trials evaluating [99mTc]tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node-negative cutaneous melanoma. Ann Surg Oncol. 2013;20:680–8.
Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30:2912–8.
McMasters KM, Reintgen DS, Ross MI, Wong SL, Gershenwald JE, Krag DN, Noyes RD, Viar V, Cerrito PB, Edwards MJ. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed. Ann Surg Oncol. 2001;8:192–7.
Ariyan S, Ali-Salaam P, Cheng DW, Truini C. Reliability of lymphatic mapping after wide local excision of cutaneous melanoma. Ann Surg Oncol. 2007;14:2377–83.
Evans HL, Krag DN, Teates D, et al. Lymphoscintigraphy and sentinel node biopsy accurately stage melanoma in patients presenting after wide local excision. Ann Surg Oncol. 2003;10:416–25.
Gannon CJ, Rousseau DL, Ross MI, Johnson MM, Lee JE, Mansfield PF, Cormier JN, Prieto PA, Gershenwald JE. Accuracy of lymphatic mapping and sentinel lymph node biopsy after previous wide local excision in patients with primary melanoma. Cancer. 2006;107:2647–52.
Leong SPL, Thelmo MC, Kim RP, Gokhale R, Rhee JY, Achtem TA, Morita E, Allen RE, Kashani-Sabet M, Sagebiel RW. Delayed harvesting of sentinel lymph nodes after previous wide local excision of extremity melanoma. Ann Surg Oncol. 2003;10:196–200.
Hodges M, Jones E, Jones T, Pearlman N, Gajdos C, Kounalakis N, McCarter M. Analysis of melanoma recurrence following a negative sentinel lymph node biopsy. Melanoma Manag. 2015;2:285–94.
Lee DY, Huynh KT, Teng A, Lau BJ, Vitug S, Lee J-H, Stern SL, Foshag LJ, Faries MB. Predictors and survival impact of false-negative sentinel nodes in melanoma. Ann Surg Oncol. 2016;23:1012–8.
Scolyer RA, Murali R, McCarthy SW, Thompson JF. Pathologic examination of sentinel lymph nodes from melanoma patients. Semin Diagn Pathol. 2008;25:100–11.
Cochran AJ, Wen D-R, Morton DL. Occult tumor cells in lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol. 1988;12:612–8.
Mitteldorf C, Bertsch HP, Zapf A, Neumann C, Kretschmer L. Cutting a sentinel lymph node into slices is the optimal first step for examination of sentinel lymph nodes in melanoma patients. Mod Pathol. 2009;22:1622–7.
Jannink I, Fan M, Nagy S, Rayudu G, Dowlatshahi K. Serial sectioning of sentinel nodes in patients with breast cancer: a pilot study. Ann Surg Oncol. 1998;5:310–4.
Association of Directors of Anatomic and Surgical Pathology (ADASP). ADASP recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Surg Pathol. 2001;25:961–3.
Messina JL, Glass LF, Cruse CW, Berman C, Ku NK, Reintgen DS. Pathologic examination of sentinel lymph node in malignant melanoma. Am J Surg Pathol. 1999;23:686–90.
Prieto VG, Clark SH. Processing of sentinel lymph nodes for detection of metastatic melanoma. Ann Diagn Pathol. 2002;6:257–64.
Cibull M. Handling sentinel lymph node biopsy specimens. Arch Pathol Lab Med. 1999;123:620–1.
Shidham VB, Qi DY, Acker S, Kampalath B, Chang C-C, George V, Komorowski R. Evaluation of micrometastases in sentinel lymph nodes of cutaneous melanoma: higher diagnostic accuracy with Melan-A and MART-1 compared with S-100 protein and HMB-45. Am J Surg Pathol. 2001;25:1039–46.
Chakera AH, Hesse B, Burak Z, et al. EANM-EORTC general recommendations for sentinel node diagnostics in melanoma. Eur J Nucl Med Mol Imaging. 2009;36:1713–42.
Karimipour DJ, Lowe L, Su L, Hamilton T, Sondak VK, Johnson TM, Fullen D. Standard immunostains for melanoma in sentinel lymph node specimens: Which ones are most useful? J Am Acad Dermatol. 2004;50:759–64.
Cook MG, Green MA, Anderson B, Eggermont AM, Ruiter DJ, Spatz A, Kissin MW, BWEM P, EORTC Melanoma Group. The development of optimal assessment of sentinel lymph nodes for melanoma. J Pathol. 2003;200:314–9.
Murray CA, Leong WL, McCready DR, Ghazarian DM. Histopathological patterns of melanoma metastases in sentinel lymph nodes. J Clin Pathol. 2004;57:64–7.
Lobo AZC, Tanabe KK, Luo S, Muzikansky A, Sober AJ, Tsao H, Cosimi B, Duncan LM. The distribution of microscopic melanoma metastases in sentinel lymph nodes: implications for pathology protocols. Am J Surg Pathol. 2012;36:1841–8.
van der Ploeg APT, van Akkooi ACJ, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol. 2011;29:2206–14.
Scoggins CR, Ross MI, Reintgen DS, et al. Prospective multi-institutional study of reverse transcriptase polymerase chain reaction for molecular staging of melanoma. J Clin Oncol. 2006;24:2849–57.
Temple CL, Snell LJ, Power SM, et al. Clinical significance of the RT-PCR positive sentinel node in melanoma. J Surg Oncol. 2007;95:546–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Song, Y., Bruce, A.N., Tieniber, A.D., Xu, X., Karakousis, G.C. (2021). Regional Nodal Staging: Clinically Node Negative. In: Lee, D., Faries, M. (eds) Practical Manual for Dermatologic and Surgical Melanoma Management. Springer, Cham. https://doi.org/10.1007/978-3-030-27400-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-27400-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27399-6
Online ISBN: 978-3-030-27400-9
eBook Packages: MedicineMedicine (R0)