Abstract
Age-related macular degeneration (AMD) continues to be the leading cause of visual impairment for the elderly in developed countries. It is a complex, multifactorial, progressive disease with diverse molecular pathways regulating its pathogenesis. One of the cardinal features of the early clinical subtype of AMD is the accumulation of lipid- and protein-rich deposits within Bruch’s membrane, called drusen, which can be visualized by fundus imaging. Currently, multiple in vitro and in vivo model systems exist, which can be used to help tease out mechanisms associated with different molecular pathways driving disease initiation and progression. Given the lack of treatments for patients suffering from the dry form of AMD, it is imperative to appreciate the different known morphological endpoints associated with the various pathogenic pathways, in order to derive further insights, for the ultimate purpose of disease modeling and development of effective therapeutic interventions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Age-related macular degeneration
- Lipid metabolism
- Inflammation
- Quick-freeze/deep etch
- Retinal pigment epithelial cells
- Cholesterol
- Apolipoprotein
1 Introduction
Age-related macular degeneration (AMD) is a progressive and complex age-related disease. It is the leading cause of vision loss among people over 50 years of age in the Western world. Since the principle risk factor for AMD is advanced age, the number of people afflicted with AMD is estimated to rise to 288 million people by 2040 (Wong et al. 2014). A better understanding of the pathogenesis of AMD is crucial in identification and/or development of preclinical models, ultimately leading to effective therapeutics that may prevent or reverse this disease.
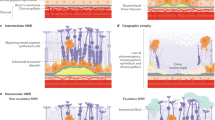
To date, several classification schemes of AMD have been described, based on in vivo imaging using color fundus photos and optical coherence tomography. The Age-Related Eye Disease Study (AREDS) is one of the most well-known systems of classification (Age-Related Eye Disease Study Research 2000). It classifies AMD into early, intermediate, and late stages. A major clinical feature of the disease is extracellular deposition of lipids and proteins underneath the retinal pigment epithelium (RPE) known as drusen. Early-stage “dry” AMD is characterized by the presence of medium-sized drusen (>63 μm; <125 μm) and pigmentary abnormalities; intermediate-stage “dry” AMD is defined by the presence of at least one large druse (>125 μm) and numerous medium-sized drusen, or RPE atrophy excluding the macular region. Advanced-stage AMD can manifest in two forms that may coexist: (1) geographic atrophy (GA) or late “dry,” affecting 85–90% of patients, characterized by several large drusen and RPE atrophy extending to the center of the macula and (2) exudative AMD, affecting 10–15% of patients, defined by choroidal neovascularization and any of its associated sequelae such as subretinal fluid, hemorrhage, RPE detachment, and/or fibrotic scarring (Malek and Lad 2014). A subset of exudative AMD patients respond to antiangiogenic treatment targeting vascular endothelial growth factor (VEGF), whereas the quest for an effective therapy for GA remains elusive to date, due to its diverse and complex pathology, which involves multiple mechanisms including but not limited to dysregulation of lipid metabolism and transport, inflammation, complement pathway dysregulation, extracellular matrix (ECM) remodeling, cell death, and cell adhesion. The focus of this mini review is on the modeling of the pathobiology of dry AMD.
2 Pathobiology of AMD
The pathogenesis of AMD is influenced by cross talk between components of the retinal microenvironment, namely, photoreceptors, RPE cells, Bruch’s membrane (BrM), choriocapillaris, and the outer choroid. Though complement has been shown to play an important part in regulating the health of the choriocapillaris as well as the RPE (Chirco et al. 2016), several other pathways have been shown to regulate the early stages of AMD including lipid metabolism and transport, inflammation, and ECM remodeling, whereas the late “dry” AMD seems to converge into pathogenic pathways such as cell senescence and death (Miller et al. 2017). Thus it would be valuable to develop a targeted approach to understanding modeling of disease phenotypes as well as identify quantifiable endpoints targeting these pathways.
2.1 Lipid Metabolic Dysregulation in AMD
One of the defining characteristics of early “dry” AMD is the accumulation of lipid-rich deposits between the RPE and BrM, as well as within BrM, which vary in size, thickness, and confluence (Klein et al. 1991). It has been shown that at least 40% of drusen volume is comprised of lipids (Wang et al. 2010). Components of drusen are derived from the retina, RPE, and to a lesser extent, the choroidal circulation. This retention of lipids causes formation of a variety of deposits, not only drusen but also basal laminar deposits (BLamD, between RPE and its plasma membrane) and basal linear deposits (BLinD, between the RPE and inner collagenous layer of BrM) (Curcio et al. 2011). Furthermore, genome-wide association studies (GWAS) of AMD patients have led to the identification of multiple lipid metabolism-related genes like ABCA1, ABCA4, APOE, CETP, and LIPC (Yu et al. 2011; Merle et al. 2013). Complementary to the GWAS reports, several epidemiological studies that have investigated the role of statins and AMD, point toward an association between circulating lipid levels and drusen formation (Klein et al. 2014; Vavvas et al. 2016). Possible mechanisms of statin therapy have been postulated, including changes in lipoprotein metabolism, improvement in lipid efflux, lipid clearing by macrophages, and anti-inflammatory and protective effects on RPE cells. Collectively, these studies suggest that AMD may be reversible anatomically and functionally, and establishes lipid metabolism and transport as a viable target for disease modeling and therapy development.
Key players in cholesterol transport and lipid metabolism are apolipoproteins (apo), proteins that have been shown to accumulate in drusen. Accordingly, multiple studies have been conducted to date investigating the role of apolipoproteins using in vivo modeling, often incorporating an additional stressor such as dietary manipulation. For example, apo∗E3-Leiden mice (modeling human type III hyperlipoproteinemia) when fed a high-fat diet for 9 months developed BLamD, composed of electron dense material similar to that seen in human AMD and immunoreactivity toward apoE, supporting apoE’s involvement in BLamD development (Kliffen et al. 2000). Further, aged mice expressing the human APOE4 allele maintained on a high cholesterol diet developed AMD-like pathology, including diffuse sub-RPE deposits, thickened BrM, and RPE atrophy (Malek et al. 2005). The apolipoprotein (apo) A-I mimetic peptide 4F, a small anti-inflammatory and antiatherogenic agent, when delivered via intravitreal route in apoEnull mice displayed improvement in BrM’s health and lessened the esterified cholesterol levels in BrM (Rudolf et al. 2018). Since apoB lipoprotein particles have been detected in BrM in early-stage “dry” AMD, transgenic mice expressing human apoB100 have been generated. High-fat diet and photooxidative injury in these transgenic mice resulted in loss of basal infoldings and cytoplasmic vacuoles in the RPE, in addition to accumulation of BLamD and long-spacing collagen in BrM (Espinosa-Heidmann et al. 2004; Fujihara et al. 2009). Additionally mice expressing mouse apoB100 develop lipoprotein accumulations in their BrM (Fujihara et al. 2014). These studies are consistent with the hypothesis that under conditions of hyperlipidemia, the RPE may secrete apoB100-rich lipoproteins to counter lipotoxicity, which may lead to the formation of lipid-rich deposits as seen in early-stage “dry” AMD. This may explain the aging effect, which in combination with photooxidative injury, leads to lipid accumulation, culminating in the formation of sub-RPE deposits. The accumulation of drusen can be exacerbated by inflammation, as discussed in the next section.
2.2 Inflammation in AMD
Inflammation and immune dysfunction are other pathogenic mechanisms associated with AMD development and central to early stages of the disease. With aging, the retina suffers from a low-grade chronic oxidative insult, which it reportedly sustains for decades. Therefore, the inflammation/“para-inflammation” may at a given point cross a threshold, become pathogenic, and lead to disease development. Noteworthy is that human histological/biochemical evaluation of para-inflammation in the AMD eye is limited to studies that have shown involvement of CD163+, CD68,+ and complement factor-3+ cells in AMD specimens (Lad et al. 2015; Wang et al. 2015; Natoli et al. 2017). In light of the “para-inflammation” hypothesis, the potential for modulating the inflammatory response is currently being evaluated as a therapeutic avenue in AMD.
An element of inflammation that has been postulated to regulate AMD pathogenesis is the NACHT, LRR, and PYD domains containing protein 3 (NLRP3) inflammasome. It is a large multiprotein complex within the immune cells that functions in the innate immune response pathway as a molecular platform for activation of caspase-1 and subsequent maturation and secretion of biologically active interleukin-1β (IL-1β) and IL-18 (Schroder and Tschopp 2010). Terallo et al. described that accumulation of Alu RNA transcripts in RPE following DICER1 loss primed and activated the NLRP3 inflammasome in RPE, leading to IL-1β- and IL-18-mediated RPE degeneration (Tarallo et al. 2012).
3 Potential Endpoints for Preclinical Studies
This mini review has attempted to present some aspects of dry AMD pathobiology, with a focus on the role of lipid metabolism and inflammation in the progression of the disease. Transgenic mouse models are an effective tool to study the contribution of specific molecular pathways to the pathogenicity of AMD. Targeting molecular pathways necessitates the use of unambiguous endpoints to elucidate the role of a particular pathway. For example, characterization of lipids present in mouse BrM requires staining reagents to establish the normal baseline, which then may be compared against genetic or dietary manipulations. Oil red O is a histological tool that may be employed to stain a class of lipids called neutral lipids which encompasses triglycerides (TG), esterified cholesterol (EC), and fatty acids (FA). Furthermore, EC and unesterified cholesterol (UC) can be distinguished by a fluorescent, polyene antibiotic filipin stain. Successful filipin staining has been shown in human tissue sections and cultured cells (Curcio et al. 2011). Ultrastructural studies using transmission electron microscopy (TEM) have long been a benchmark to study deposits in human tissue and animal models. It provides detailed visualization of photoreceptor outer segments, RPE cells and BrM. Using TEM, studies have illustrated electron dense materials below the RPE, RPE pigmentary changes, BrM thickening, and changes in the choriocapillaris. Detailed views of ocular lipoprotein particles and ECM changes have also been made possible by quick-freeze/deep etch, a tissue preparation technique used in conjunction with electron microscopy that can produce three-dimensional images of tissue structure and macromolecular elements while preventing the introduction of post-processing artifacts (Ismail et al. 2017). Finally, immunohistochemical stains may be used to visualize lipid accumulations using antibodies against apoB, apoE, perilipin, and E06, which bind to proteins covalently modified by oxidized phospholipids (Malek et al. 2003; Harris et al. 2013).
Assessment of inflammation is also important as an in vitro, ex vivo, or in vivo endpoint and may be performed by surveying for immune cells. Retinal and RPE-choroid flatmounts are an excellent way to get an overview of the resident microglial cells to establish a baseline. The number and density of microglia can be used to evaluate the effect of injury/insult or transgene expression. Various antibodies can be employed for this purpose, namely, Iba1 (ionized calcium-binding adaptor molecule 1), F4/80, CD68, and CD45. In addition to the number/density of immune cells, the morphological characterization of microglia/macrophage may be used as a measure of inflammatory status. Macrophages can differentiate into either classically activated M1 phenotype, characterized by the expression and production of pro-inflammatory mediators including IL-1β, TNF-α, and IL-6 as well as an increased expression of surface markers such as CD16/32, CD86, CD40, and inducible nitric oxide synthase, which have been reported to drive the inflammatory process. Additionally, the M2 macrophages express high levels of arginase-1 and IL-10 but low levels of IL-12 and IL-23 and are usually induced by anti-inflammatory cytokines IL-4 and IL-13.
4 Summary
Investigation into the pathological mechanisms of a complex and multifactorial disease such as AMD may entail the use of an assortment of model systems, each of which may target distinct disease-associated pathways. It is imperative to develop a comprehensive understanding of potential endpoints associated with each model system in order to delve deeper into mechanistic questions as well as develop effective therapeutic modalities.
References
Age-Related Eye Disease Study Research G (2000) Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 107:2224–2232
Chirco KR, Tucker BA, Stone EM et al (2016) Selective accumulation of the complement membrane attack complex in aging choriocapillaris. Exp Eye Res 146:393–397
Curcio CA, Johnson M, Rudolf M et al (2011) The oil spill in ageing Bruch membrane. Br J Ophthalmol 95:1638–1645
Espinosa-Heidmann DG, Sall J, Hernandez EP et al (2004) Basal laminar deposit formation in APO B100 transgenic mice: complex interactions between dietary fat, blue light, and vitamin E. Invest Ophthalmol Vis Sci 45:260–266
Fujihara M, Bartels E, Nielsen LB et al (2009) A human apoB100 transgenic mouse expresses human apoB100 in the RPE and develops features of early AMD. Exp Eye Res 88:1115–1123
Fujihara M, Cano M, Handa JT (2014) Mice that produce ApoB100 lipoproteins in the RPE do not develop drusen yet are still a valuable experimental system. Invest Ophthalmol Vis Sci 55:7285–7295
Harris LA, Skinner JR, Wolins NE (2013) Imaging of neutral lipids and neutral lipid associated proteins. Methods Cell Biol 116:213–226
Ismail EN, Ruberti JW, Malek G (2017) Quick-freeze/deep-etch electron microscopy visualization of the mouse posterior pole. Exp Eye Res 162:62–72
Klein R, Davis MD, Magli YL et al (1991) The Wisconsin age-related maculopathy grading system. Ophthalmology 98:1128–1134
Klein R, Myers CE, Buitendijk GH et al (2014) Lipids, lipid genes, and incident age-related macular degeneration: the three continent age-related macular degeneration consortium. Am J Ophthalmol 158:513–524 e513
Kliffen M, Lutgens E, Daemen MJ et al (2000) The APO(∗)E3-Leiden mouse as an animal model for basal laminar deposit. Br J Ophthalmol 84:1415–1419
Lad EM, Cousins SW, Van Arnam JS et al (2015) Abundance of infiltrating CD163+ cells in the retina of postmortem eyes with dry and neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 253:1941–1945
Malek G, Lad EM (2014) Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell Mol Life Sci 71:4617–4636
Malek G, Li CM, Guidry C et al (2003) Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol 162:413–425
Malek G, Johnson LV, Mace BE et al (2005) Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A 102:11900–11905
Merle BM, Maubaret C, Korobelnik JF et al (2013) Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: the Alienor study. PLoS One 8:e79848
Miller JW, Bagheri S, Vavvas DG (2017) Advances in age-related macular degeneration understanding and therapy. US Ophthalmic Rev 10:119–130
Natoli R, Fernando N, Jiao H et al (2017) Retinal macrophages synthesize C3 and activate complement in AMD and in models of focal retinal degeneration. Invest Ophthalmol Vis Sci 58:2977–2990
Rudolf M, Mir Mohi Sefat A, Miura Y et al (2018) ApoA-I Mimetic Peptide 4F reduces age-related lipid deposition in Murine Bruch's membrane and causes its structural remodeling. Curr Eye Res 43:135–146
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832
Tarallo V, Hirano Y, Gelfand BD et al (2012) DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149:847–859
Vavvas DG, Daniels AB, Kapsala ZG et al (2016) Regression of some high-risk features of Age-related Macular Degeneration (AMD) in patients receiving intensive statin treatment. EBioMedicine 5:198–203
Wang L, Clark ME, Crossman DK et al (2010) Abundant lipid and protein components of drusen. PLoS One 5:e10329
Wang JC, Cao S, Wang A et al (2015) CFH Y402H polymorphism is associated with elevated vitreal GM-CSF and choroidal macrophages in the postmortem human eye. Mol Vis 21:264–272
Wong WL, Su X, Li X et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106–e116
Yu Y, Reynolds R, Fagerness J et al (2011) Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci 52:4663–4670
Acknowledgments
This study was supported by NEI grants: R01EY027802 (GM), R01EY028160 (GM), and EY005722 (Duke Eye Center) and Research to Prevent Blindness core grant (Duke Eye Center).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Choudhary, M., Malek, G. (2019). A Review of Pathogenic Drivers of Age-Related Macular Degeneration, Beyond Complement, with a Focus on Potential Endpoints for Testing Therapeutic Interventions in Preclinical Studies. In: Bowes Rickman, C., Grimm, C., Anderson, R., Ash, J., LaVail, M., Hollyfield, J. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 1185. Springer, Cham. https://doi.org/10.1007/978-3-030-27378-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-27378-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27377-4
Online ISBN: 978-3-030-27378-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)