Abstract
Early bird plumage is well known primarily due to numerous discoveries of specimens preserving feathers from Early Cretaceous deposits in China. Remiges and rectrices are most commonly preserved with rectrices showing the greatest variation. The long boney-tailed Jeholornis has a unique tail plumage employing two anatomically distinct rectricial pterylae serving both aerodynamic and ornamental functions. Basal pygostylians show disparate tail plumages that are reflected by differences in pygostyle morphology. Sapeornis has a proportionately shorter pygostyle wielding a fan-shaped array of rectrices, whereas the robust pygostyle of Confuciusornis is associated with a pair of elongate rachis-dominated feathers in some specimens, considered indicative of sexual dimorphism. The latter morphology is also present in many enantiornithines. Members of this diverse clade have primarily ornamental tail morphologies, whereas the earliest members of the Ornithuromorpha all possess tail morphologies that appear to be primarily aerodynamic. Body feathers in Archaeopteryx and adult enantiornithines trapped in amber are pennaceous suggesting that reported rachis-less body feathers in Jehol birds may be taphonomic artifacts. Rarely preserved, well-developed pennaceous crural feathers are present in Archaeopteryx and some enantiornithines, whereas crural feathers are short in the Confuciusornithiformes. Their preserved absence in nearly all Jehol ornithuromorph specimens most-likely reflects the smaller available sample size. Crural feathers in many basal ornithuromorphs were probably reduced, as in Yanornis and extant aquatic and semiaquatic birds. Overall, early birds show a trend towards the reduction of the distal hindlimb feathers present in closely related nonavian dinosaurs. However, well-developed tarsometatarsal feathers are present in Sapeornis and two exceptionally well-preserved enantiornithine specimens indicate this group was diverse in the distal extent of their hindlimb plumage, including at least one lineage with feathered pedal digits. Although remarkably modern in many aspects, early bird plumage still differed from that of their modern counterparts including extinct morphotypes and differences in ontogenetic patterns.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Institutional AbbreviationsBMNHC, Beijing Museum of Natural History, Beijing, China; DIP-V, Dexu Institute of Palaeontology, Chaozhou, China; DNHM, Dalian Natural History Museum, Dalian, China; GSGM, Gansu Geological Museum, Lanzhou, Gansu Province, China; HPG, Hupoge Amber Museum, Tenchong, China; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China; LH, Museo de las Ciencias de Castilla-La Mancha, Cuenca, Spain; MCCMLH, Museo de las Ciencias de Castilla-La Mancha, Cuenca, Spain; SDM, Shandong Museum, Jinan, China; STM, Shandong Tianyu Museum of Nature, Pingyi, Shandong, China; UFRJ-DG, Universidade Federal do Rio de Janeiro, Department of Geology collection, Rio de Janeiro, Brazil.
9.1 Introduction
The study of fossil birds begins with feathers. Described in 1861, the original holotype of Archaeopteryx lithographica, still the oldest and most primitive known avian taxon, consists of an isolated feather (von Meyer 1861). At this time, only birds were known to possess this unique integumentary structure, and thus this Late Jurassic feather was identified as avian. Skeletal specimens were later referred to Archaeopteryx based on the co-occurrence of preserved feathers arranged on the forelimbs forming wings (Dames 1884; Owen 1863). However, with the exception of Archaeopteryx, fossil feathers associated with skeletal remains are otherwise very rare in the Mesozoic fossil record and, until the discovery of feathered nonavian dinosaurs and birds in the rich Early Cretaceous Jehol Biota in the late 1990s (Chen et al. 1998; Hou et al. 1996; Ji et al. 1998), the plumage of early birds was largely unknown. Many specimens from the Lower Cretaceous volcanolacustrine deposits in northeastern China that produce the Jehol Biota preserve traces of soft tissues normally rare in the fossil record (Zhou et al. 2003). Thousands of specimens preserving remnants of feathers have been uncovered, most still waiting to be described. These discoveries provide an ever-increasing wealth of data on the plumage of early birds (Zhang et al. 2006). However, because most data comes primarily from a single Biota, albeit one that persisted for over 10 million years (Pan et al. 2013), evolutionary trends across greater periods of time are poorly understood, and it is difficult to make broad statements regarding clades not limited to the Jehol.
The Jehol avifauna accounts for approximately half of all known Mesozoic avian diversity, with representatives from every known Early Cretaceous clade (Zhou and Zhang 2006). It occurs in three formations: the 130.7 Ma Protopteryx-horizon of the Huajiying Formation, the 125 Ma Yixian Formation, and the 120 Ma Jiufotang Formation (the latter two strictly forming the Jehol Group) (Pan et al. 2013). Avian diversity and numbers increase through time, being highest in the Jiufotang Formation where long boney-tailed birds (Jeholornis) live alongside primitive pygostylians (Sapeornithiformes and Confuciusornithiformes) and a diversity of ornithothoracines (the clade formed by Enantiornithes and Ornithuromorpha) (Zhou and Zhang 2006) (Fig. 9.1). The second oldest avian-bearing deposits in the world, the Protopteryx-horizon of the Huajiying Formation, records the earliest members of the Enantiornithes (Wang et al. 2014c), the dominant clade of Cretaceous avians, and the Ornithuromorpha (Wang et al. 2015a), the lineage that includes Neornithes nested within, as well as basal confuciusornithiforms (Navalón et al. 2018; Zhang et al. 2008a; Zheng et al. 2017). Although birds are rare in this horizon (only seven taxa reported represented by a total of ten specimens), the remains of feathers preserved in these specimens are among the best known from China (Wang et al. 2014c; Liu et al. 2019).
Besides Archaeopteryx, nearly all skeletal specimens associated with feather remains known from outside the China are referable to the Enantiornithes with the exception of feathers associated with the holotype of Ambiortus dementjevi from Early Cretaceous deposits in central Mongolia (Kurochkin 1985) and potentially purported feather traces in a specimen of Parahesperornis from the Late Cretaceous of Kansas (Martin 1984; Williston 1896). Elsewhere in China, feathered specimens have also been collected from the Changma locality of the Aptian Xiagou Formation (e.g., enantiornithines Feitianius paradisi and GSGM-07-CM-001, and specimens of the ornithuromorph Gansus yumenensis) (O’Connor et al. 2012a, 2016a; You et al. 2006). In addition, the only reported Late Cretaceous bird, the holotype of the enantiornithine Parvavis chuxiongensis from the Jiangdihe Formation, preserves feather remains (Wang et al. 2014b). Outside China, several enantiornithines preserving feathers are known from the Early Cretaceous of Spain: the holotype of Eoalulavis hoyasi (Sanz et al. 1996), the pellet LH 11386 consisting of several juveniles (Sanz et al. 2001), and the isolated wing MCCMLH31444, all from the 125 Ma Las Hoyas Konservat-Lagerstätten (Navalón et al. 2015) and the holotype of Noguerornis gonzalezi from the Upper Berriasian–Lower Barremian “La Pedrera de Meiá” Limestones (Chiappe and Lacasa-Ruiz 2002; Lacasa-Ruiz 1988). The partial specimens representing the holotype materials of Hoboltia ponomarenkoi from Mongolia (Zelenkov and Averianov 2016) and Enantiophoenix electrophyla from Lebanon (Cau and Arduini 2008; Dalla Vecchia and Chiappe 2002) also preserve feather traces, but these are uninformative. An indeterminate juvenile (UFRJ-DG 031Av) from the Aptian Crato Formation in Brazil preserves excellent tail feathers (de Souza Carvalho et al. 2015), and most recently, associated skeletal and integumentary remains including feathers have been found preserved in mid-Cretaceous Cenomanian (~99 Ma) amber from Myanmar (Xing et al. 2016, 2017, 2018b). The latter specimens contribute important morphological information typically inaccessible from two-dimensional specimens. The cumulative knowledge distilled from these discoveries is summarized here.
9.2 Descriptions
9.2.1 Archaeopteryx
Impressions of feathers are preserved in several specimens of Archaeopteryx lithographica (Foth et al. 2014; Mayr et al. 2005). The plumage of this important fossil avian is thoroughly described in the literature, including a chapter in this volume; thus, this summary will be concise. The short, broad wings are surprisingly modern in morphology (Elzanowski 2002). The primary feathers are asymmetrical, a feature absent in the wings of nonavian dinosaurs (with the exception of the volant dromaeosaurid Microraptor). Although the leading-edge vane displays barb angles comparable to modern birds, those of the trailing edge vane are markedly smaller, also documented in basal pygostylians Sapeornis and Confuciusornis (Feo et al. 2015). There are 10–12 primaries (Foth et al. 2014) and an estimated 11 secondaries (Elzanowski 2002) which is within the range of living birds (most neognathus birds have 10 primaries with secondaries showing a greater range) (Gill 2007). The longest primary is less than twice the length of the manus. The dorsal coverts are half the length of the primaries, similar to living birds (Foth et al. 2014). The body coverts are symmetrical pennaceous feathers with thin, gently curved rachises. The crural feathers are roughly half the length of the tibia (Foth et al. 2014). Very short feathers, approximately one quarter the length of the crural feathers, are present on the proximal tarsometatarsus (Foth et al. 2014). The elongate boney tail bears symmetrical pennaceous feathers perpendicularly oriented from the bone surface along its entire length. Melanosomes sampled from the holotype, identified as a wing covert, suggest this feather was black (Carney et al. 2012).
9.2.2 Jeholornis and Kin (the Jeholornithiformes)
The Jeholornithiformes are the only definitive long-tailed birds currently recognized other than Archaeopteryx (O’Connor et al. 2011) (Fig. 9.2). Remnants of feathers are preserved in approximately a third of all known specimens (38 out of 95 reported specimens), but only the tail has been described (O’Connor et al. 2013). Notably, body feathers are only preserved in a single specimen (STM2-11) along the dorsal surface of the neck and nowhere else (O’Connor et al. 2013). The wings (incomplete but best preserved in STM2-37, STM2-8, STM2-23, and STM2-18) appear to be proportionately longer than in Archaeopteryx (longest primary twice the length of the hand) but are similarly broad (Fig. 9.2a). Two rows of dorsal coverts are clearly present over the secondaries in STM2-8 (Fig. 9.2b); the longer layer is half the length of the secondaries and the shorter layer is half that. The tail consists of two rectricial pterylae: a short proximal fan and a distally restricted “frond” (Fig. 9.2). The proximal pteryla is located over the short proximal caudal vertebrae and consists of six rectrices roughly 10 cm long with broad distal ends overlapping to form a fan-shaped surface (O’Connor et al. 2013). This surface is considered capable of generating lift to supplement the wings. The distal frond consists of five or six pairs of slightly shorter feathers perpendicularly oriented from the lateral surface of the distal vertebrae and a single feather attaching to the distal margin of the last caudal. Compared with the proximal tail feathers, these distal tail feathers are much more narrow, sharply tapered, and strongly curved so that their craniolateral surfaces are concave (Fig. 9.2c) (O’Connor et al. 2012b). The strong curvature and tapered morphology of these feathers suggests they were primarily ornamental. The tapered and narrow morphology of these feathers would also have decreased any incurred aerodynamic cost of this ornamentation (Møller and Hedenström 1999). However, the small cohesive surface formed by the proximal overlap of these distal tail feathers together with the overall elongated boney tail of Jeholornis (27 vertebrae compared to 22 in Archaeopteryx) suggests the distal frond also functioned as a stabilizer, generating pitching moments when the tail was raised or depressed (O’Connor et al. 2013). The feathers are considerably elongated past the point where they are aerodynamically functional, suggesting the distal rectricial pteryla was shaped by both natural and sexual selection (Fitzpatrick 1999). Compared with the “frond-like” morphology present in Archaeopteryx, the two rectricial tracts in Jeholornis are inferred to have both contributed less to the total body mass and incurred less drag, while at the same time generating approximately the same amount of lift (O’Connor et al. 2013). Due to the absence of a pygostyle, the complex caudal plumage of Jeholornis is unlike that of any living bird. However, the presence of a tail crafted through both natural and sexual selective forces parallels observations in neornithines (Thomas 1997).
Photographs of specimens of the long boney-tailed bird Jeholornis: (a) STM2-37 preserving wings and proximal tail feathers; (b) STM 2-8 preserving remiges and wing coverts and both tail tracts; (c) SDM 20090109.1 preserving proximal and distal tail feathers. Scale bars equal 2 cm. The two rows of secondary wing coverts are indicated by solid arrows; the proximal tail tract is indicated by an open arrow
9.2.3 Confuciusornis and Kin (Pygostylia: Confuciusornithiformes)
The basal pygostylian Confuciusornis sanctus is the most common species found in the Jehol Group, known from hundreds (if not thousands) of specimens mostly from the Yixian Formation (Chiappe et al. 1999; Hou et al. 1995). Roughly 300 preserve feather remains (Zheng et al. 2013b). Their narrow wings are proportionately much more elongate than in other early birds. The longest primaries are roughly three times the length of the hand, whereas they are only about twice the length of the hand in Jeholornis. The primaries are much longer than the secondaries forming a novel wing shape not observed in living birds (Falk et al. 2016). Descriptions of their rachis as thin and too weak to support powered flight are considered incorrect, based on measurements not representing the full rachis (Falk et al. 2016; Nudds and Dyke 2010; Zheng et al. 2010). Similar to rachis-dominated feathers, the rachis of the remiges preserves a faint, medially placed longitudinal stripe (STM 13-327). This may represent a ventral groove the same as that observed in the primaries of extant birds (Stettenheim 2000). However, the medial stripe appears to be visible in both ventral and dorsal view, which is somewhat inconsistent with this interpretation. The outermost primary is less than half the length of the second. The third through fifth outermost feathers are the longest (peaking in length in the fourth outermost primary), approximately 20–25% longer than the second primary, after which they decrease in length (IVPP V13156) (Fig. 9.3). The wing preserved in IVPP V13156 consists of 10 asymmetric and sharply tapered primaries (Falk et al. 2016). The secondaries are approximately half the length of the longest primary; they are symmetrical with rounded distal margins (IVPP V13156). Two layers of wing coverts are clearly preserved in Eoconfuciusornis STM7-144 (Zheng et al. 2017). The greater coverts are roughly half the length of the secondaries, and the lesser coverts are half the length of the greater coverts (Fig. 9.3). The distal ends of the secondaries and greater coverts in Eoconfuciusornis preserve dark spots that appear to be more heavily melanized, which both resists wear and serves as ornamentation (Fig. 9.3b) (Zheng et al. 2017). Some specimens of Confuciusornis also preserve direct evidence of their plumage patterns, preserving similar dark circular spots on the tips of some wing feathers. An alula is not preserved in any Confuciusornis (Chiappe et al. 1999) although Eoconfuciusornis STM7-144 preserves a single short pennaceous feather on the alular digit, probably representing a contour feather (Zheng et al. 2017).
Photographs of Confuciusornithiformes: (a) Confuciusornis sanctus IVPP V13156 showing the right wing in dorsal view and the proximal ends of the paired rachis dominated tail feathers; (b) Eoconfuciusornis sp. STM7-144 preserving dark spots on the tips of the greater coverts and secondaries—note the preservation of ovarian follicles and absence of elongate tail feathers, proximal ends of which are visible in (a). Scale bar equals 3 cm
Body feathers cover the dorsal and ventral neck and skull except the rostrum, which was presumably covered in a horny beak although this is very rarely preserved and even then only visible as a faint impression (Hou et al. 1999; Falk et al. 2019). Although only three specimens preserve traces of this feature, the impressions vary considerably in shape suggesting at least some different species were characterized by differences in beak shape (Falk et al. 2019). The dorsal coronal body feathers are longer than the ventral submalar feathers. In Eoconfuciusornis STM7-144 phaeomelanosomes were only recovered in the submalar tract suggesting a rufous throat patch (Zheng et al. 2017). However, samples within a single feather of Confuciusornis show both brown phaeomelanosomes and black eumelanosomes suggesting complex coloration (Zhang et al. 2010). Crural feathers decrease in length distally along the tibiotarsus and end proximal to the ankle (Fig. 9.3). They are reported to be pennaceous (Zheng et al. 2013b); although this interpretation is most likely correct, the elongate feathers assigned to the crus by Zheng et al. (2013b) and those on the proximal tarsometatarsus are reinterpreted here as remnants of the underlying wing feathers. Body feathers cover the dorsal and ventral surfaces of the body including the pygostyle (Fig. 9.3). Some specimens additionally preserve a pair of elongate rachis-dominated feathers (Chiappe et al. 1999), so named because the rachis is proportionately wide although this characteristic is more strongly developed in enantiornithines (Wang et al. 2014c) (Fig. 9.3). The feathers preserve a faint longitudinal stripe that extends nearly the entire length of the rachis (O’Connor et al. 2012a). This has been interpreted as a longitudinal groove, but it is now clear from three-dimensionally preserved RDFs found in amber that the entire rachis was extremely thin and concave and that the dark stripe represents a dorsal groove/ventral ridge in the cortex (Xing et al. 2018a; Carroll et al. 2019). The pennaceous vane in the paired tail feathers is distally restricted; referred to as racket plumes, this morphology is also present in numerous living birds (e.g., Momotidae, racket-tailed drongo). In Confuciusornis the distal “racket” is narrow with a gently tapered distal margin. Although described as symmetrical (Chiappe et al. 1999), the medial vade is typically preserved wider than the lateral vane (IVPP V13156). The vane proximally tapers and is continuous with a very narrow dark strip that lines the rachis for most of its length; this has been hypothesized to represent short barbs that were not differentiated through apoptosis (O’Connor et al. 2012a). Specimens preserving these elongate tail feathers are generally regarded as males (Hou et al. 1996). Interpretations regarding sexual dimorphism are supported by the absence of racket plumes in one specimen of Eoconfuciusornis preserving ovarian follicles (Zheng et al. 2017). Gender identification relying on purported medullary bone in a rectrix-less specimen of Confuciusornis is not supported here (Chinsamy et al. 2013; O’Connor et al. 2018).
9.2.4 Sapeornis and Kin (the Sapeornithiformes)
Sapeornis chaoyangensis is the largest pygostylian bird in the Early Cretaceous with proportionately elongate forelimbs (Zhou and Zhang 2002, 2003). Specimens, most of which come from the Jiufotang Formation, rarely preserve any remnants of their plumage (20 of 106 specimens) (Zheng et al. 2014b). The wings preserved in DNHM D3038, a young subadult specimen, consist of 11–13 elongate strongly asymmetrical primaries and approximately 11 secondaries (Gao et al. 2012). The outermost feather along the cutting edge of the wing is approximately half the length of the second, and the third-fourth outermost primaries are the longest after which the primaries reduce in length. The longest primary is approximately twice the length of the hand (Fig. 9.4a). The secondaries are shorter and subequal (Gao et al. 2012). An alula cannot be observed in this or any other known specimen. Aerodynamic analysis suggests Sapeornis utilized its broad wings for thermal soaring (Serrano and Chiappe 2017). Wing coverts are present, but details are poorly known, and they are described as small and thin (Gao et al. 2012). The tail is preserved in a single described specimen preserving what appears to be an elongate, graduated tail fan in lateral view (Wang et al. 2014c; Zheng et al. 2013b) (Fig. 9.4b). Two additional specimens confirm this interpretation and indicate the tail fan was formed by 8–10 medially striped rectrices (Wang and O’Connor 2017). This case is consistent with their proportionately short and plough-shaped pygostyle with a pygostyle lamina, features otherwise only present in ornithuromorphs and consistent with the presence of specialized rectricial musculature (Wang and O’Connor 2017). Although not preserved in any specimen, body feathers clearly would have been present. Two subadult specimens preserve feathers on the distal hindlimb projecting off of the caudal margin of the intertarsal joint (Zheng et al. 2013b). The feathers roughly extend the distal half of the tibiotarsus and the full length of the tarsometatarsus, becoming shorter distal on the hindlimb (Fig. 9.4b). They are curved, and their distal ends are splayed, suggesting these hindlimb feathers had ornamental qualities, whereas the preservation of these ankle feathers relative to other body feathers may suggest some aerodynamic qualities (O’Connor and Chang 2015). Most likely these feathers were shaped under both natural and sexual selection.
9.2.5 The Diverse Enantiornithes (Aves: Ornithothoraces)
The enantiornithines have a global record that extends from the 130.7 Ma Huajiying Formation until the end Cretaceous and includes data from every continent with the exception of Antarctica (O’Connor et al. 2011). Their diversity and abundance is reflected by the available data regarding their plumage. Over a thousand specimens at the STM reportedly preserve some feather remains and most have yet to be studied (Zheng et al. 2013b). Remains of the remiges are preserved in the holotype specimens of Chiappeavis magnapremaxillo (O’Connor et al. 2016b), Cruralispennia multidonta (Wang et al. 2017), Dapingfangornis sentisorhinus (Li et al. 2006), Eoenantiornis houi (Zhou et al. 2005), Eopengornis martini (Wang et al. 2014c), Fortunguavis xiaotaizicus (Wang et al. 2014a), Junornis houi (Liu et al. 2017), Grabauornis lingyuanensis (Dalsätt et al. 2014), Longirostravis hani (Hou et al. 2004), Noguerornis gonzalezi (Lacasa-Ruiz 1988), Orienantius ritteri (Liu et al. 2019),Parapengornis eurycaudatus (Hu et al. 2015), Protopteryx fengningensis (Zhang and Zhou 2000), Shanweiniao cooperorum (O’Connor et al. 2009), and Zhouornis hani (Zhang et al. 2013), as well as in a bohaiornithid CUGB P1012 (Peteya et al. 2017), and two indeterminate specimens, DNHM D2884 (O’Connor 2009) and MCCMLH31444 (Navalón et al. 2015); three-dimensional specimens encased in amber include two partial wings (Xing et al. 2016), two partial skeletons (Xing et al. 2017, 2018b), a feathered foot (Xing et al. 2019a), and an isolated hindlimb with remiges—the holotype of Elektorornis chenguangi (Xing et al. 2019b). Most completely preserved in Eopengornis martini STM24-1 (Fig. 9.5a), the wings are broad, formed by asymmetrical rectrices with medially striped rachises (Wang et al. 2014c). The outermost primary is two-thirds the length of the next, which is two-thirds the length of the third outermost primary. These are followed by two longer primaries, after which the medial primaries are shorter. The longest primaries are twice as long as the hand. Unlike Confuciusornis, the secondaries are not significantly shorter than the primaries in Eopengornis, forming a broader wing with a larger surface area (Figs. 9.3a and 9.5a). The primaries display both leading and trailing edge vane with barb angles comparable to living birds (Feo et al. 2015). So far there is no strong evidence that the caudal margin of the wing was notched in any taxon. A distally incomplete but well preserved isolated partial wing from the Early Cretaceous of Spain preserves 8–9 primaries and 10–12 secondaries (Navalón et al. 2015). Orienantius BMNHC PH 1156 preserves the calami for 10 primary remiges visible in the post-patagium (Liu et al. 2019). Nine primaries form the wing in the more complete of the two amber specimens (Xing et al. 2016) but only eight are present in the hatchling HPG-15-1 (Xing et al. 2017). An alula is preserved in several specimens (e.g., Eoalulavis hoyasi, Eoenantiornis houi, Protopteryx fengningensis, and DIP-V-15100) and is considered plesiomorphic to Ornithothoraces and present throughout the clade (O’Connor 2009; Xing et al. 2016; Zhang and Zhou 2000; Zhou et al. 2005). As in some living birds, the alula is formed by two feathers that end approximately level with the distal end of the major digit (Xing et al. 2016) and a shorter proximal feather (Sanz et al. 1996). Wing coverts are more heavily obscured by overlap. Three rows are reported in DIP-V-15100 (Xing et al. 2016). The longest coverts appear to be approximately half the length of the remiges (Fig. 9.5a). The Burmese amber specimens further reveal that the contour feathers in at least some enantiornithines had proximodistally specialized barb morphologies, being plumaceous at the base as in living birds (Xing et al. 2016). Analysis of the wings preserved in Junornis houi suggests this taxon engaged in bounding flight, typical of extant arboreal passerines (Liu et al. 2017).
Two pengornithid enantiornithines: (a) Eopengornis martini from the 130.7 Ma Protopteryx-horizon of the Huajiying Formation; (b) Chiappeavis magnapremaxillo from the 120 Ma Jiufotang Formation. The tail in the older taxon consists of a pair of elongate rachis-dominated streamers, whereas that of the younger taxon consists of a fan-shaped tail formed by eight rectrices. Scale bars equal 2 cm
Nearly complete body feathers are present in some specimens, but preservation results in so much overlap that very little information is available regarding most pterylae. Body feathers are clearest along the neck, tail, and tibiotarsus, where there is less anatomical overlap (Fig. 9.5a). Generally, body feathers vary somewhat in length being longer on the dorsal surface of the body and around the pygostyle and shorter on the ventral surface of the body (a pattern generally observed in all Mesozoic birds). They are reported to be rachis-less with the exception of the crural feathers (O’Connor 2009; Zhang and Zhou 2004; Zhang et al. 2006) although this is likely a taphonomic artifact (see Sect. 9.3). Where preserved, these extend the full length of the tibiotarsus. Although crural feathers are short and weakly decrease in length distally in some taxa (e.g., Eopengornis; Fig. 9.5a) (Wang et al. 2014c), in others they are longer (40–60% the length of the tibiotarsus) and subequal the entire length of the tibiotarsus (e.g., Longipteryx, IVPP V13939, Cathayornis STM7-50) (Zhang and Zhou 2004; Zheng et al. 2013b). In some specimens, the crural feathers are preserved nearly perpendicular to the tibiotarsus, which has led some authors to infer an aerodynamic function for these feathers (Zheng et al. 2013b). However, body feathers on other parts of the body are similarly oriented (e.g., coronal and neck feathers), and this is interpreted as the result of compression during burial (Foth 2012), and not indicative of the presence of airfoils.
In one specimen (BMNHC-Ph1061B, Huajiying Formation), the hindlimb feathers extend onto the proximal two-thirds of the tarsometatarsus, where they continue to decrease in length distally (Chiappe and Meng 2016). An isolated foot trapped in amber (DIP V15105) from Burma preserves pennaceous feathers that extend onto the pedal digits themselves, present on digits III and IV and reaching the ungual phalanx on digit IV (Xing et al. 2019b). The pedal scales in DIP V15105, HPG-15-1 and Elektorornis chenguangi additionally have unusual projecting filaments; referred to as scutellate scale filaments, these structures are reportedly white, which may hinder their preservation and/or observation in most two-dimensional specimens (Xing et al. 2017, 2019a, 2019b). It has been suggested these scutellate scale filaments may have had a sensory function (Xing et al. 2019b).
Bizarre feathers, described as having a “proximally wire-like part with a short filamentous distal tip,” preserved on the wing and hindlimb in Cruralispennia from the Huajiying Formation (Wang et al. 2017) may alternatively represent pin feathers (developing feathers still encased in the feather shaft) (O’Connor et al. 2020) or unusual taphonomic artifacts resultant from the lacustrine depositional environment (Kundrát 2004). Alternative interpretations are more consistent with their anatomical location in that ornamental feathers projecting craniolaterally from the wrist would presumably impede flight. Although their identification remains equivocal, the unusual feathers preserved in Cruralispennia strongly resemble pin feathers preserved in a juvenile enantiornithine STM34-1 (Zheng et al. 2012) (O’Connor et al. 2020). These feathers are preserved on several parts of the body. In STM34-1, the pin feathers are clearest in the caudal region and on the tibiotarsus but are also present on the forelimb, as in Cruralispennia, and on the dorsal surface of body. Notably, STM34-1 also appears to preserve some of the original patterning of the feathers; the distal portions of the remiges are preserved as a distinctly darker color suggesting the proximal and distal portions of the feathers were colored different in vivo.
Of the feather pterylae, the morphology of the rectrices is best documented and shows considerable diversity. Most commonly, as in Confuciusornis, a pair of rachis-dominated feathers is present, although compared with Confuciusornis, the rachis is proportionately even wider in enantiornithines (Wang et al. 2014c). Although for some taxa all that is known is a faint incomplete impression of the enlarged rachis (e.g., Bohaiornis guoi and Protopteryx fengningensis), enantiornithine rachis-dominated feathers show greater diversity than the confuciusornithiforms (which appear to share a single morphology): both streamers (fully pennaceous) and racket plumes (distally pennaceous) are documented (the fully pennaceous morphology is currently only documented in two species of the Pengornithidae, Eopengornis martini and Parapengornis eurycaudatus) (Hu et al. 2015; Wang et al. 2014c) (Fig. 9.5a). Within the racket-plume morphology, there is further diversity in the overall length of the feathers relative to the body, the number of pairs (one specimen reportedly preserves two, the holotype specimen of Paraprotopteryx zhengi, and Shanweiniao cooperorum is interpreted as having a similar morphology) (O’Connor et al. 2016b; Zheng et al. 2007) and the morphology of the distal “racket” (O’Connor et al. 2012a). In some taxa, the pennaceous portion is restricted to the distal most portion of the rachis (Junornis), rapidly expanding with a rounded distal margin (spoon-shaped as in Dapingfangornis), whereas the pennaceous portion expands gradually and extends for a greater proportion of the feather in others (e.g., GSGM-07-CM-001, an unnamed enantiornithine from the Xiagou Formation). Many specimens preserve an absence of rectrices (holotype specimens of Avimaia schweitzeri, Cruralispennia multidonta, Fortunguavis xiaotaizicus, Eoenantiornis houi, Grabauornis lingyuanensis, Longipteryx chaoyangensis, and Zhouornis hani and an indeterminate enantiornithine preserving follicles STM29-8), having only wispy body feathers preserved around the pygostyle. As in confuciusornithiforms, these specimens are regarded as females, similarly supported by the preservation of female reproductive tissue in one specimen (Zheng et al. 2013a) and the presence of an egg in Avimaia IVPP V25371 (Bailleul et al. 2019).
Outside China, rachis-dominated tail feathers are also preserved in “Cratoavis” UFRJ-DG 031 Av from Brazil (de Souza Carvalho et al. 2015) and numerous pairs of rachis-dominated feathers have been found in Burmese amber (Xing et al. 2018a; Carroll et al. 2019) indicating that this morphology was widespread and persisted in enantiornithines for over 30 million years. This specimen preserves the feathers primarily as a mold, confirming that the rachis is grooved along at least one of its surfaces, with the enlarged portion of the rachis on either side of the groove forming convex surfaces (de Souza Carvalho et al. 2015). Three-dimensional specimens preserved in amber indicate that the traces in UFRJ-DG 031 are dorsally exposed and flattened. The amber specimens reveal that the wide rachis observed in compression fossils in vivo was C shaped with a thickness between 3 and 10 microns (Xing et al. 2018a; Carroll et al. 2019). Unusual spots on the rachis suggest these ornamental tail feathers bore striking color patterns (de Souza Carvalho et al. 2015). These tail feathers have been documented in numerous young juvenile specimens (e.g., UFRJ-DG 031 Av, STM34-7, STM34-9, IVPP V15564) indicating that unlike living birds, enantiornithines developed sexually dimorphic ornaments at a very early ontogenetic age, before the onset of both sexual and skeletal maturity (Zheng et al. 2012). One specimen in amber (HPG-15-1) suggests that these feathers may even appear as early as the first postnatal molt (Xing et al. 2017).
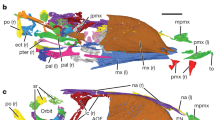
Two specimens clearly preserve more than four elongate rectrices, the holotype specimens of Feitianius paradisi (O’Connor et al. 2016a) and Chiappeavis magnapremaxillo (O’Connor et al. 2016b). The latter is a pengornithid preserving a short, graded fan-shaped tail consisting of ten overlapping rectrices (Fig. 9.5b). This tail is considered to have aerodynamic qualities and be capable of generating lift although the presence of rectricial bulbs like those of neornithines is unlikely, being confirmed absent using UV light in several other enantiornithines (Liu et al. 2019; Chiappe and Meng 2016) and thus this tail may have been incapable of adjusting its width to suit aerodynamic demands during flight (O’Connor et al. 2016b). Feitianius paradisi GSGM-05-CM-004 preserves a tail consisting of at least three different rectricial morphologies, none of which are considered to have any strong aerodynamic properties (Fig. 9.6). Together they are inferred to form a sizeable tail display, the likes of which are only observed among living birds in males of polygamous species (O’Connor et al. 2016a). Chiappeavis and Feitianius represent the opposite extremes observed within the Enantiornithes, although notably ornamental morphologies dominate the spectrum. A recent study on the pygostyle has suggested that the dorsally excavated morphology typical of enantiornithines may have accommodated an enlarged caudal levator muscle, which it may have used to raise the tail in display similar to extant phasianids (Wang and O’Connor 2017) (Fig. 9.6c). Cruralispennia apparently had a small, “ornithuromorph-like” plough-shaped pygostyle but preserves only short caudal coverts (Wang et al. 2017). Additional specimens are required to clarify the pygostyle morphology in this unusual taxon.
A nearly complete juvenile trapped in amber (HPG-15-1) preserves the most complete plumage of any enantiornithine, documenting the early juvenile plumage present in at least one lineage of enantiornithines (Xing et al. 2017). This specimen reveals well-developed remiges (with banded color pattern) combined with sparse body feathers that consist of both pennaceous and filamentous morphotypes. The head and neck are covered with short, dark tufts consisting of 3–4 basally joined filaments which may represent newly erupting contour feathers. Body contour feathers appear to be proximally plumaceous and distally pennaceous. The femur and tibiotarsus have neoptile feathers consisting of basally joined plumaceous barbs with a short or absent rachis with additional isolated bristlelike filaments also present on the crus (Xing et al. 2017). The scutellae scales on the tarsometatarsus bear filamentous projections also present in some extant juvenile birds (Xing et al. 2017). The relatively sparse covering of body feathers is reminiscent of altricial hatchlings, whereas the mature remiges is consistent with the extreme precocial strategy inferred for the Enantiornithes (Zhou and Zhang 2004). Primitive filamentous morphotypes reminiscent of proto-feathers present in basal theropods may be lost during enantiornithine ontogeny or alternatively cannot be discerned in two-dimensionally preserved fossils due to overlap.
Thus far, only two specimens have been sampled for melanosomes: the holotype of Cruralispennia and an indeterminant bohaiornithid (Peteya et al. 2017; Wang et al. 2017). The latter analysis suggested the presence of iridescent plumage, as in the dromaeosaurid Microraptor (Li et al. 2012).
9.2.6 Earliest Members of the Ornithuromorpha (Aves: Ornithothoraces)
In the Jehol ornithuromorphs are generally less common than enantiornithines and thus comparatively less is known regarding their plumage. Remnants of remiges lacking anatomical detail are preserved in several specimens (the holotype specimens of Hongshanornis longicresta, Jianchangornis multidonta, Juehuaornis zhangi, Longicrusavis houi, Piscivoravis lii, Schizooura lii, Tianyuornis cheni, Yixianornis grabaui, and Xinghaiornis lini as well as referred specimens of Yanornis STM 9-15 and 9-49) (Wang et al. 2013, 2015b; Zheng et al. 2014a, c; Zhou et al. 2009, 2012, 2013; Zhou and Zhang 2001, 2005). Uninformative feather traces are also preserved in some specimens of Gansus yumemensis from the Xiagou Formation (Wang et al. 2015c; You et al. 2006) and the holotype of Ambiortus dementjevi from Mongolia (Kurochkin 1985). In no specimen is the wing complete and clearly preserved well enough to allow the number of remiges to be to be determined. The wing is best preserved in the two known specimens of the hongshanornithid Archaeornithura meemanni, the only ornithuromorph known from the Protopteryx-horizon (Wang et al. 2015a). The primaries are broad, strongly asymmetrical and bluntly tapered at their distal ends (Fig. 9.7). At least one short primary (possibly two) forms the leading edge of the wing; this outermost primary (or primaries) is narrower than the others and roughly two-fifths the length of the next primary, which is followed by two longer subequal feathers, after which the primaries decrease in length medial in the wing. The longest primary is approximately 2.5 times the length of the hand, proportionately longer than observed in other Early Cretaceous birds with the exception of Confuciusornis, whose manual digits are not reduced (Fig. 9.3). This morphology is confirmed, as least for hongshanornithids, in a well-preserved referred specimen of Hongshanornis (DNHM D2945/6) (Chiappe et al. 2014). The well-preserved primary feathers in Archaeornithura STM7-145 reveal the presence of a medial longitudinal stripe in the rachis, as in Confuciusornis (Wang et al. 2015a). Because the specimen is torn between two slabs with feather remains in both, it is unclear which surface bore the stripe, although this feature is more clearly visible in the main slab in which the skeleton is in dorsal view. A layer of coverts, approximately the length of the outermost primary, is present, but details of their morphology are obscured by overlap (Fig. 9.7). The morphology of these specimens indicates hongshanornithids had broad, long, and tapered wings. The alula (best preserved in specimens of Archaeornithura) is formed by three short asymmetrical feathers, within the range of modern birds (Lovette and Fitzpatrick 2004), ending proximal to the distal end of the major digit (proportionately shorter than in enantiornithines) (Wang et al. 2015a).
Body feathers are known on the head and neck (Fig. 9.7) but notably are not clearly documented on the tibiotarsus in any specimen except one, despite the fact that crural feathers are widespread in living members (Neornithes) and present in other groups of Early Cretaceous birds. Given that crural feathers are rarely preserved even in Confuciusornis, their absence in basal ornithuromorphs most likely reflects the comparatively smaller number of available specimens (Zheng et al. 2013b). Crural feathers are only preserved in a single exceptionally well-preserved specimen of Yanornis STM9-5, in which the feathers appear to be short and taper off above the ankle—the pes is clearly covered in scales (Zheng et al. 2013b). This is consistent with the semiaquatic ecology inferred for Yanornis and most basal ornithuromorphs—neornithines in aquatic and semiaquatic habitats commonly have proximally restricted and/or reduced crural feathers. The capital coverts in the holotype of Hongshanornis longicresta project dorsally, giving the species its specific name (Zhou and Zhang 2005); although incomplete preservation above the skull roof makes interpretations of a crest equivocal, the feathers are clearly visible tapering rostrally from mid-orbit, ending level with the rostral margin of the antorbital fenestra. A similar crest is not observed in any other hongshanornthid specimen (Chiappe et al. 2014).
Morphology of the rectrices and pygostyle itself suggest that rectricial bulbs were present in even basal members of the Ornithuromorpha (Clarke et al. 2006). All well-preserved rectricial morphologies display undeniable aerodynamic qualities. Most taxa preserve round rectricial fans (Archaeornithura meemanae, Hongshanornis longicresta, Piscivoravis lii, Tianyuornis zhengi, Yanornis martini, Yixianornis grabaui) (Chiappe et al. 2014; Clarke et al. 2006; Wang et al. 2015a; Zheng et al. 2014a, c; Zhou et al. 2013), although Schizooura lii preserves a forked tail, considered to be the optimal aerodynamic condition (Thomas 1993; Zhou et al. 2012). One specimen of Archaeorhynchus spathula preserves a pin-tail, a fan-shaped tail consisting of 12 medially striped feathers paired with two medial streamers of indeterminate distal morphology (Wang et al. 2018). No unequivocal primarily ornamental tail morphologies are documented (Zhou et al. 2014). However, all tail morphologies observed appear to be also somewhat shaped under the influence of sexual selection: rounded tail fans are considered to be ornamental relative to a square-shaped tail fans; elongate forked tails, like that in Schizooura, are less functional (and thus considered also to function as ornamentation) relative to short forked tails; and pin-tails readily serve both ornamental and aerodynamic functions, with the ornamental pins being narrow to reduce their aerodynamic cost (Fitzpatrick 1999; Møller and Hedenström 1999). In the absence of taxa highly specialized for aerodynamic performance at this grade in avian evolution, the tails of the earliest ornithuromorphs appear to be, as in other groups, shaped by both natural and sexual selection. Wispy body feathers, possibly poorly preserved tail coverts, are preserved with the pygostyle in some specimens (e.g., Yanornis STM9-15). Notably, there is no clear evidence than any specimen lacked elongate rectrices in vivo, as in some enantiornithine and confuciusornithiform specimens.
Melanosomes have only been sampled in two specimens, the holotype of Changzuiornis ahgmi (potentially a junior synonym of Juehuaornis zhangi) and a referred specimen of Gansus yummenensis (Barden et al. 2011; Huang et al. 2016). Samples from both specimens revealed eumelanosomes, which suggest the feathers were at least partially black.
Younger and more derived ornithuromorph specimens (those referable to Ornithurae) thus far do not preserve any remnants of their plumage. One hesperornithiform, a Late Cretaceous clade of diving ornithurines, purportedly preserves feathers on the foot (Williston 1896); although the impressions of scales are clear in the illustration, the reported feathers are not readily identifiable, and no description of these traces accompanied a later study, in which the specimen was recognized as distinct from Hesperornis and assigned to Parahesperornis (Martin 1984). Instead, the hespeornithiform toes are described as, “probably lobed and scutellate-reticulate” (Martin 1984: p. 147).
9.3 Discussion
The plumage of the earliest known birds from the Late Jurassic and Early Cretaceous is remarkably well known despite the enormous taphonomic filter presented by the fossilization process and over a hundred million years of geologic time. Among known specimens, there is recognizable inter- and intraclade diversity in the wings, tail, and hindlimb feathers (O’Connor et al. 2016a; Zheng et al. 2013b). Not unexpectedly, cumulative data still pales in comparison to the diversity of feather morphologies, plumages, molts, and patterns recognized among living birds. The numerous flight styles, ecologies, and reproductive strategies utilized by the over 10,000 species of living birds provide for a vast diversity of feather structures, plumages, and wing and tail shapes (Gill 2007). Although studies suggest that by the Late Cretaceous enantiornithines had evolved a diversity of flight styles comparable to neornithines (Dyke and Nudds 2008), specimens preserving feathers from the well-sampled Jehol Biota appear to occupy a more limited ecospace including a limited range of body sizes. Many basal taxa appear to be fairly generalist (e.g., Archaeopteryx, Confuciusornis), whereas enantiornithines show arboreal features and ornithuromorphs show features indicative of semiaquatic ecologies (O’Connor et al. 2011). Morphological specializations of the skeleton for flight and specialized environments do not reach the grade observed in Neornithes. Similarly, one would not expect to find in early birds the same degree of plumage variation observed in living taxa.
Basal birds further lacked many of the advanced biological characteristics that set living birds apart from other living reptiles, such as their rapid growth, high metabolic rates, and advanced pneumatic respiratory system, all paired with an extremely lightweight skeleton highly adapted for aerodynamic activity (Chinsamy et al. 1995; Gill 2007). Non-ornithuromorph birds lacked an enlarged, keeled sternum, notarium, well-developed synsacrum, and other compound bones that form the lightweight and rigid avian skeleton, and grew more slowly (Chinsamy et al. 1995; O’Connor et al. 2011). As in other aspects of their biology, several observable differences are present between the plumage of basal birds and that of neornithines. These include at least one feather morphotype that no longer exists, plumages consisting of feather combinations not observed in living birds, tail morphologies unknown in living birds (some due to the absence of a pygostyle in the most primitive birds), and ontogenetic differences in the appearance of sexually dimorphic ornaments. This information, continuously built upon by new discoveries, helps us to begin to understand the early evolution of the diverse and often highly specialized plumage observed in modern birds.
At least one feather morphotype, the rachis-dominated rectrix, is recognized as extinct and is so far found only in Early Cretaceous confuciusornithiforms and enantiornithines (O’Connor et al. 2012a; Zhang et al. 2006), and is documented in specimens from China, Myanmar, and Brazil (de Souza Carvalho et al. 2015; Carroll et al. 2019; Xing et al. 2018a). Both streamer and racket-plume morphologies are documented in the Enantiornithes, whereas only racket plumes are observed in the Confuciusornithiformes. The rachis is proportionately wider in enantiornithines compared with the Confuciusornithiformes, and these tail feathers are inferred to have evolved independently in these two clades (Wang et al. 2014c). Potentially, rachis-dominated rectrices may also be present in Epidexipteryx (Zhang et al. 2008b), a member of the Scansoriopterygidae—a group of volant nonavian maniraptorans of uncertain phylogenetic affinity (O’Connor and Sullivan 2014; Xu et al. 2015). Notably, the poorly preserved remains of four potentially rachis-dominated (or ribbonlike) rectrices is the only evidence for the presence of feathers with a rachis (presumably pennaceous) in this group (Xu et al. 2015; Wang et al. 2019). This may suggest that the tail ornaments in Epidexipteryx are not modified pennaceous feathers at all. Similarities between these tail feathers may turn out to be a product of the poor preservation of this feature in the only specimen of Epidexipteryx. Given their phylogenetic distribution, these tail ornaments are inferred to have evolved in parallel in scansoriopterygids and pygostylian birds (Wang et al. 2014c).
Recently our understanding of rachis dominated feathers has been transformed by the discovery of three-dimensional rachis dominated feathers preserved in 99 Ma amber from Myanmar. Based on lithic compression fossils, these feathers appeared to consist of a mediolaterally enlarged rachis that bears either a dorsal or ventral longitudinal groove (potentially both), considered to have functioned to strengthen these elongate feathers. The presence of a groove was inferred from the presence of a dark medial longitudinal stripe on the rachis and confirmed through the discovery of a three-dimensional cast of one surface of the tail feathers in the juvenile enantiornithine Cratoavis. They are interpreted as modified pennaceous feathers (O’Connor et al. 2012a; Prum and Brush 2002), supported by the discovery of fully pennaceous “streamer” forms and the presence of a medial longitudinal stripe in the remiges of confuciusornithiforms, unmodified rectrices of Sapeornis, and the remiges and unmodified rectrices of ornithothoracines (Wang et al. 2014c, 2015a). Rachis dominated feathers in amber have shown that the wide, flat morphology actually formed a C-shaped cross-section (Xing et al. 2018a). The dark medial stripe represents a ventral ridge and a dorsal groove and may have represented the so called rachial ridge (Xing et al. 2018a; Carroll et al. 2019). The rachis measures only 3–10 microns thick and completely lacks medullary pith. The absence of a medullary pith suggests that these feathers are not modified modern pennaceous feathers and likely formed through completely different developmental pathways (Carroll et al. 2019). It is unclear whether the medial stripe observed in the rachis of the flight feathers of some well-preserved basal birds (e.g., Eopengornis, Archaeornithura) is indicative of a similar pith-less structure, which would in turn indicate that the feathers of basal birds were far more primitive that previously thought, or if the medial stripe in these flight feathers results from a different morphology. The remiges in three-dimensional amber specimens do not show any medial stripe or groove (Xing et al. 2017).
A large number of bizarre feather morphologies have been described from feathered paravians (the group of maniraptorans that includes birds, dromaeosaurids, and troodontids) found in Jehol deposits (Wang et al. 2017; Xu and Guo 2009; Xu et al. 2010). Within Pennaraptora, the monophyletic clade of maniraptoran dinosaurs with pennaceous feathers (formed by Oviraptorosauria and Paraves), descriptions of the body feathers as rachis-less (unshafted) are widespread (O’Connor et al. 2012a; Zhang et al. 2006). However, the identification of these unusual feathers in Early Cretaceous Jehol birds is at odds with the presence of pennaceous body feathers in the basal Late Jurassic Archaeopteryx (Foth et al. 2014) and in some mid-Cretaceous Burmese enantiornithines (Xing et al. 2016, 2017). Feathers in these specimens are notably preserved as molds and in their entirety, respectively. In contrast, feather traces preserved in Jehol specimens typically consist of decay-resistant melanosomes, organelles that are often absent from the rachis (Zhang et al. 2010). The rachis is thus preserved as a gap between the melanosome-rich vanes, although often marred by a dark medial stripe of uncertain identity. Overlap and slight taphonomic distortion may well be obscuring the presence of a vane in the small body feathers of Jehol birds making them only appear non-pennaceous (Foth 2012). Water also obscures feather morphology; hence, even features in isolated feathers can be controversial (Kundrát 2004), as in the case of the isolated feathers preserved in the holotype of Protopteryx fengningensis, which have been interpreted both as shafted and unshafted (Foth 2012; Zhang et al. 2006). Therefore, the existence of rachis-less body feathers as an extinct morphotype cannot be confirmed until taphonomic factors are explored further (Saitta et al. 2018). Based on observations from Archaeorhynchus STM7-11, the absence of an observable rachis in the body feathers of many specimens may be due to the fact this structure is proportionately thin and delicate (Wang et al. 2018). If a rachis was indeed present in the body feathers of Jehol birds, which seems most likely, it appears in some feathers it would have been basally restricted with very long barbs, giving the feathers a downy appearance (Zhang and Zhou 2000). Such a morphology is reminiscent of neoptile feathers in living birds (Foth 2011; Kundrát 2004) that have also been observed in Burmese enantiornithines trapped in amber (Xing et al. 2017). It is possible the downy morphology observed in the body feathers of Jehol birds and nonavian dinosaurs may be an adaptation for a cooler climate, like that inferred for the paleoenvironment of the Jehol region based on isotope data (Amiot et al. 2011). The presence of proximally plumaceous–distally pennaceous body contour feathers in 99 Ma enantiornithines trapped in amber favors a taphonomic interpretation; however, given the bizarre combination of feather morphotypes revealed by these specimens, similarly unusual plumages in Jehol birds cannot be ruled out. Enantiornithines in amber indicate that primitive unshafted feather morphologies reminiscent of those present in more primitive theropods persisted into the Ornithothoraces at least during early ontogenetic stages (Xing et al. 2017).
Observations from super-precocial enantiornithine juveniles suggest this clade is characterized by a lesser degree of ontogenetic variation between molts compared with neornithines and even some nonavian dinosaurs (Xu et al. 2010). Like living super precocial birds (the Megapodidae) (Starck and Ricklefs 1998), the natal plumage in enantiornithines is characterized by fully developed remiges together with downy body feathers (Xing et al. 2016, 2017; Zhou and Zhang 2004). However, ornamental tail feathers are also documented in several juvenile specimens (de Souza Carvalho et al. 2015; Zheng et al. 2012). Thus, unlike the condition in living birds, in enantiornithines sexually dimorphic ornaments preceded the advent of sexual maturity, being present in the juvenile and possibly even natal plumage. This may suggest that the rachis-dominated racket plumes had a minimal detrimental effect on the overall flight performance in enantiornithines, as already suggested by their morphology (Wang et al. 2014c). This discovery also further supports the hypothesis that more complicated molting patterns are derived within Aves (Gill 2007).
Differences in plumage coloration certainly existed, but these have only just begun to be explored in Cretaceous birds. Mostly melanosome-based coloration is being studied—only six taxa have currently been sampled: two confuciusornithiforms, referred specimens of Eoconfuciusornis (Zheng et al. 2017) and Confuciusornis (Zhang et al. 2010); two enantiornithines, the holotype of Cruralispennia (Wang et al. 2017) and a bohaiornithid enantiornithine (Peteya et al. 2017); and two ornithuromorphs, the holotype of Changzuiornis (Huang et al. 2016) and isolated feathers referred to Gansus (Barden et al. 2011). Preliminary results mostly reveal eumelanosomes (Barden et al. 2011; Huang et al. 2016; Wang et al. 2017); so far, phaeomelanosomes have only been recovered in confuciusornithiforms (Zhang et al. 2010; Zheng et al. 2017). Variation in melanosome distributions within a single feather of Confuciusornis (Zhang et al. 2010) strongly suggests that high-density sampling is required to accurately reconstruct coloration through this method. However, this would be highly destructive and still yield only a partial understanding of coloration, limited to that formed by melanosomes. Only one specimen has been heavily sampled (although still not to the degree necessary to capture detailed patterns)—a female Eoconfuciusornis, revealing a dark plumage with a rufous throat patch (Zheng et al. 2017). The predominance of dark plumages recovered for early birds (including Archaeopteryx) (Carney et al. 2012) and the inability of melanosome morphology to distinguish visibly preserved color patterns (e.g., the spots in Eoconfuciusornis) (Zheng et al. 2017) both suggest the use of melanosomes to reconstruct plumage color is limited. Other pigments utilized by birds are proven to fossilize but have yet to be demonstrated in any Mesozoic bird (Thomas et al. 2014b; Vinther 2015). It is possible carotenoid pigmentation did not evolve in Aves until after the end-Cretaceous extinction (Thomas et al. 2014a).
Although individual basal lineages independently evolved advanced and sometimes complex plumages (e.g., the tail of Jeholornis and the hindlimb feathers in Sapeornis), among Cretaceous birds, there is a trend toward more advanced integumentary features in more derived clades. The flight apparatus in enantiornithines and ornithuromorphs is better suited for powered flight than that of more primitive clades, showing advanced aerodynamic features in both the pectoral girdle and plumage. Although asymmetrical feathers are an apomorphy of Aves, as increased data comes to light, detailed studies of feather anatomy indicate that vane asymmetry to the degree observed in modern birds did not evolve until the Ornithothoraces (Feo et al. 2015). The presence of an alula, the so-called bastard wing, also characterizes this latter clade. This feathery structure on the leading edge of the wing allows for greater angles of attack, mostly used to aid in take-off and landing and during slow flight (Wang et al. 2015a; Zhang and Zhou 2000). The Ornithuromorpha is characterized by the appearance of numerous advanced features that characterize living birds, but Early Cretaceous members still retained a few primitive traits, such as manual claws, gastralia, and distally contacting pubes (O’Connor et al. 2011). Similarly, their wings are proportionately longer than in most early birds, but wings and tail feathers show a limited diversity of shapes, and potentially they retained primitive “rachis-less” body feathers (Wang et al. 2015a) and lacked medullary pith. One major difference between the two ornithothoracine clades is the morphology of the pygostyle, which in basal ornithuromorphs appears nearly modern in structure, whereas enantiornithines retain a primitive morphology similar to that present in Confuciusornis and have similar tail feathers. In contrast, ornithuromorphs do not preserve primitive tail morphologies such as the absence of rectrices or rachis-dominated feathers like that observed in confuciusornithiforms and enantiornithines. Even the earliest-known member of the Ornithuromorpha (130.7 Ma Archaeornithura meemanni) possesses a fan-shaped tail (Wang et al. 2015a) and all taxa in which the tail is known preserve more than four feathers (most enantiornithines have four or less with the exception of Chiappeavis and Feitianius) arranged to form an overlapping surface capable of generating aerodynamic forces. A plough-share-shaped pygostyle similar to that of ornithuromorphs evolved in parallel in the Sapeornithiformes and co-occurs with a long, fan-shaped tail strongly suggesting these two features coevolved (Wang and O’Connor 2017).
The Early Cretaceous Jehol avifauna reveals that early birds were diverse in their plumage (Fig. 9.1), particularly with regard to the tail, a conclusion supported by finds from other deposits (Fig. 9.5). Flight feathers are more commonly preserved than those that cover and protect the body (although in some cases only body feathers are preserved—the taphonomy of each specimen is unique and generally poorly understood). The feathers of the wing are often obscured by overlap, particularly when the forelimb is preserved folded. As a result, most feather data pertain to the tail. Among living volant birds, rectrices show more morphological variation than any other pteryla (Fitzpatrick 1999; Thomas 1997). Remiges are more strictly limited by the functional morphospace of volant behavior (plumage is unknown in all flightless Cretaceous birds), whereas the tail only serves to supplement the wing apparatus (Gill 2007) and was not crucial to flight in early pygostylians, as evidenced by the absence of elongate tail feathers in female confuciusornithiforms and at least some female enantiornithines. However, early members of the Ornithuromorpha do not share neornithine morphological diversity and all known Early Cretaceous members preserve tail morphologies that appear to be primarily aerodynamic in function (primarily, because as discussed tail morphologies are produced by complex interactions between natural and sexual selection). The fact that such morphologies nearly only occur in groups with a plough-share-shaped pygostyle (the Ornithuromorpha and Sapeornithiformes) supports the hypothesis that without the soft tissue and musculature to control the spread of the tail fan (e.g., the rectricial bulbs and m. bulbi rectricium), such a feature may not be greatly beneficial (O’Connor et al. 2016b). In contrast, most members of the Enantiornithes preserve tails that appear primarily ornamental in function. One taxon apparently possessed an extravagant tail plumage comparable to that observed in polygamous neornithines alive today (e.g., Phasianidae, Paradisaeidae) (O’Connor et al. 2016a). This adds to growing evidence that early in the evolution of feathers, sexual selection was a major driving force (O’Connor and Chang 2015). The robust enantiornithine pygostyle, which like Confuciusornithiformes lacks a dorsal lamina, may have been unsuited for supporting large rectricial bulb-like soft tissue, a hypothesis supported by the absence of significant soft tissue located lateral to the pygostyle as revealed by UV light (Chiappe and Meng 2016; Liu et al. 2019). However, it appears well suited for expanded caudal levator and depressor muscles, suggesting that these birds may have engaged in tail displays similar to extant phasianids (Wang and O’Connor 2017). The only known tail that can readily be considered aerodynamic is that preserved in the holotype of the pengornithid Chiappeavis magnapremaxillo (O’Connor et al. 2016b). This group is characterized by a pygostyle that is relatively shorter and less robust than that of other enantiornithines and in this regard is more similar to that of ornithuromorphs relative to other enantiornithines. Notably, the tail fan is shorter than in sympatric ornithuromorphs, likely due to the increase in muscular force required to control longer tail fans (Thomas and Balmford 1995). The expanded pygostyle present in phasianids, strongly reminiscent of the enantiornithine pygostyle (Wang and O’Connor 2017), still possesses rectricial bulbs, although they are just somewhat limited in dorsal expansion by the dorsal platform. The absence of a blade-like dorsal lamina and the presence of expanded dorsolateral and ventrolateral margins of the pygostyle in confuciusornithiforms and enantiornithines suggest strong levator and depressor musculature but do not rule out the presence of rectricial bulbs. Only the strong co-occurrence of a dorsal lamina and tail fans in Sapeornis and ornithuromorphs, the absence of taxa utilizing aerodynamic morphologies in the Confuciusornithiformes and Enantiornithes (with the exception of Chiappeavis), and the absence of significant soft tissue preserved surrounding the pygostyle in Confuciusornis (IVPP V13156) (Fig. 9.3a) and some enantiornithines (Chiappe and Meng 2016; Liu et al. 2019) suggest this feature was absent in the latter clades. Other members of the Pengornithidae preserve rachis-dominated streamers, indicating that intraclade plumage diversity was high, as in living birds (Gluckman 2014).
Hindlimb feathers in early birds are regarded with considerable significance due to the idea that avian flight evolved through a four-winged stage (Zheng et al. 2013b). This historical hypothesis (Beebe 1915) received new life with the discovery of Microraptor zhaoianus, a dromaeosaurid dinosaur that clearly utilized hind-wings in some form of volant behavior (Xu et al. 2003; Pei et al. 2014). However, the presence of hindlimb feathers themselves is not unusual: crural feathers are present in all known living birds, although they are proximally restricted in some taxa, particularly those in aquatic and semiaquatic environments (e.g., Ardeidae, Scolopacidae). Metatarsal feathers are present in raptorial birds, and many owls additionally have feathered toes (Kelso and Kelso 1936). Hindlimb feathers extend onto the metatarsus in Archaeopteryx (Foth et al. 2014), Sapeornis (Zheng et al. 2013b), and some enantiornithines (Chiappe and Meng 2016). In Archaeopteryx and some enantiornithines, the symmetrical crural feathers are roughly half the length of the tibia, but below the ankle, these feathers are small and short. In Sapeornis hindlimb, feathers are only preserved around the ankle, although presumably the proximal portion of the hindlimb was also feathered. Potentially the longer crural feathers in Archaeopteryx and some enantiornithines and the ankle feathers in Sapeornis could somehow have assisted flight; however, this hypothesis requires greater study given that living birds both with and without elongate crural feathers engage in foot-braking during landing (Pennycuick 1968, 1971). Regardless, the condition in Aves differs considerably from the unique condition in Microraptor in which the metatarsal feathers are asymmetrical and the longest on the hindlimb, mimicking to the condition in winged forelimbs in which the feathers on the distalmost element (the hand) are the longest and asymmetrical (Xu et al. 2003). In comparison, the hindlimb feathers in Sapeornis chaoyangensis (Zheng et al. 2013b) are more proximally located (around the ankle rather than entirely on the tarsometatarsus), proportionately shorter, curved, and with a splayed morphology, which all together suggest a primarily ornamental function (O’Connor and Chang 2015). However, there is no doubt the cohesive surface formed by the proximal ends of the feathers would have had some effect on aerodynamics. The crural feathers in Archaeopteryx are very similar to those observed in some enantiornithines (Zhang and Zhou 2004), whereas in others (e.g., Eopengornis), the crural feathers are overall shorter and decrease in length distal on the leg, as in Confuciusornis. However, some enantiornithines had hindlimb feathering more extensive than Archaeopteryx, extending onto the toes, a condition that evolved in parallel to some neornithine lineages (e.g., Strix, Lagopus) (Xing et al. 2019b). Crural feathers are not preserved in any specimen of Jeholornis suggesting they were not likely endowed with strong aerodynamic qualities, and they are clearly short and taper distally in confuciusornithiforms. If distal hindlimb feathers were involved in the evolution of avian flight, their contribution ceased very early in avian evolution, with secondary reduction being the overall predominate trend. Given that asymmetrical distal hindlimb feathers are only present in Microraptor zhaoianus, it is likely that this dromaeosaurid independently evolved its volant abilities in parallel to those of early birds and uniquely utilized a tetrapteryx strategy (Foth et al. 2014), further highlighting the developmental plasticity of feathers.
References
Amiot R, Wang X, Zhou Z-H, Wang X-L, Buffetaut E, Lecuyer C, Ding Z-L, Fluteau F, Hibino T, Kusuhashi N, Mo J-Y, Suteethorn V, Wang Y-Q, Xu X, Zhang F-S (2011) Oxygen isotopes of East Asian dinosaurs reveal exceptionally cold Early Cretaceous climates. Proc Natl Acad Sci USA 108:5179–5183
Bailleul AM, O’Connor J, Zhang S-K, Li Z-H, Wang Q, Lamanna M, Zhu X-F, Zhou Z-H (2019) An Early Cretaceous enantiornithine (Aves) preserving an unlaid egg and probable medullary bone. Nat Commun 10(1275):1–10
Barden HE, Wogelius RA, Li D-Q, Manning PL, Edwards NP, van Dongen BE (2011) Morphological and geochemical evidence of eumelanin preservation in the feathers of the Early Cretaceous bird, Gansus yumenensis. PLoS One 6:e25494
Beebe CW (1915) A tetrapteryx stage in the ancestry of birds. Zoologica 2:38–52
Carney RM, Vinther J, Shawkey MD, D’Alba L, Ackermann J (2012) New evidence on the colour and nature of the isolated Archaeopteryx feather. Nat Commun 3:1–8
Carroll NR, Chiappe LM, Bottjer DJ (2019) Mid-cretaceous amber inclusions reveal morphogenesis of extinct rachis-dominated feathers. Sci Rep 9(18108):1–8
Cau A, Arduini P (2008) Enantiophoenix electrophyla gen. et sp. nov. (Aves, Enantiornithes) from the Upper Cretaceous (Cenomanian) of Lebanon and its phylogenetic relationships. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 149:293–324
Chen P-J, Dong Z, Zhen S (1998) An exceptionally well-preserved theropod dinosaur from the Yixian Formation of China. Nature 391:147–152
Chiappe LM, Lacasa-Ruiz A (2002) Noguerornis gonzalezi (Aves: Ornithothoraces) from the Early Cretaceous of Spain. In: Chiappe LM, Witmer LM (eds) Mesozoic birds: above the heads of dinosaurs. University of California Press, Berkeley, CA, pp 230–239
Chiappe LM, Meng Q-J (2016) Birds of stone. JHU Press, Pittsburgh
Chiappe LM, Ji S, Ji Q, Norell MA (1999) Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of northeastern China. Bull Am Mus Nat Hist 242:1–89
Chiappe LM, Zhao B, O’Connor JK, Gao C-H, Wang X-R, Habib M, Marugan-Lobon J, Meng Q-J, Cheng X-D (2014) A new specimen of the early cretaceous bird Hongshanornis longicresta: insights into the aerodynamics and diet of a basal ornithuromorph. PeerJ 2:1–28
Chinsamy A, Chiappe LM, Dodson P (1995) Mesozoic avian bone microstructure: physiological implications. Paleobiology 21:561–574
Chinsamy A, Chiappe LM, Marugán-Lobón J, Gao C-H, Zhang F-J (2013) Gender identification of the Mesozoic bird Confuciusornis sanctus. Nat Commun 4:1–5
Clarke JA, Zhou Z, Zhang F (2006) Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui. J Anat 208:287–308
Dalla Vecchia FM, Chiappe LM (2002) First avian skeleton from the Mesozoic of northern Gondwana. J Vertebr Paleontol 22:856–860
Dalsätt J, Ericson PGP, Zhou Z-H (2014) A new Enantiornithes (Aves) from the Early Cretaceous of China. Acta Geol Sin 88:1034–1040
Dames W (1884) Über Archaeopteryx. Palaeontologische Abhandlungen 3:119–196
de Souza Carvalho I, Novas FE, Agnolin FL, Isasi MP, Freitas FI, Andrade JA (2015) A Mesozoic bird from Gondwana preserving feathers. Nat Commun 6:1–5
Dyke GD, Nudds RL (2008) The fossil record and limb disparity of enantiornithines, the dominant flying birds of the Cretaceous. Lethaia 42:248–254
Elzanowski A (2002) Archaeopterygidae (Upper Jurassic of Germany). In: Chiappe LM, Witmer LM (eds) Mesozoic birds: above the heads of dinosaurs. University of California Press, Berkeley, CA, pp 129–159
Falk AR, Kaye TG, Zhou Z-H, Burnham DA (2016) Laser fluorescence illuminates the soft tissue and life habits of the Early Cretaceous bird Confuciusornis. PLoS One 11:e0167284
Falk AR, O’Connor J, Wang M, Zhou Z-H (2019) On the preservation of the beak in Confuciusornis (Aves: Pygostylia). Diversity 11(212):1–8
Feo TJ, Field DJ, Prum RO (2015) Barb geometry of asymmetrical feathers reveals a transitional morphology in the evolution of avian flight. Proc R Soc B Biol Sci 282:20142864
Fitzpatrick S (1999) Tail length in birds in relation to tail shape, general flight ecology and sexual selection. J Evol Biol 12:49–60
Foth C (2011) The morphology of neoptile feathers: ancestral state reconstruction and its phylogenetic implications. J Morphol 272:387–403
Foth C (2012) On the identification of feather structures in stem-line representatives of birds: evidence from fossils and actuopalaeontology. Paläontol Z 86:91–102
Foth C, Tischlinger H, Rauhut OWM (2014) New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 511:79–82
Gao C-H, Chiappe LM, Zhang F-J, Pomeroy DL, Shen C-Z, Chinsamy A, Walsh MO (2012) A subadult specimen of the Early Cretaceous bird Sapeornis chaoyangensis and a taxonomic reassessment of sapeornithids. J Vertebr Paleontol 32:1103–1112
Gill FB (2007) Ornithology, 3rd edn. W.H. Freeman and Company, New York, p 758
Gluckman T-L (2014) Pathways to elaboration of sexual dimorphism in bird plumage patterns. Biol J Linn Soc 111:262–273
Hou L, Zhou Z-H, Martin LD, Feduccia A (1995) A beaked bird from the Jurassic of China. Nature 377:616–618
Hou L, Martin LD, Zhou Z, Feduccia A (1996) Early adaptive radiation of birds: evidence from fossils from northeastern China. Science 274:1164–1167
Hou L, Martin LD, Zhonghe Z, Feduccia A, Zhang F (1999) A diapsid skull in a new species of the primitive bird Confuciusornis. Nature 399:679–682
Hou L, Chiappe LM, Zhang F, Chuong C-M (2004) New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften 91:22–25
Hu H, O’Connor JK, Zhou Z-H (2015) A new species of Pengornithidae (Aves: Enantiornithes) from the Lower Cretaceous of China suggests a specialized scansorial habitat previously unknown in early birds. PLoS One 10:e0126791
Huang J-D, Wang X, Hu Y-C, Liu J, Peteya JA, Clarke JA (2016) A new ornithurine from the Early Cretaceous of China sheds light on the evolution of early ecological and cranial diversity in birds. PeerJ 4:e1765
Ji Q, Currie PJ, Norell MA, Ji S-A (1998) Two feathered dinosaurs from northeastern China. Nature 393:753–761
Kelso L, Kelso EH (1936) The relation of feathering of feet of American owls to humidity of environment and to life zones. Auk 53:51–56
Kundrát M (2004) When did theropods become feathered? Evidence for pre-Archaeopteryx feathery appendages. J Exp Zool 302B:1–10
Kurochkin EN (1985) A true carinate bird from Lower Cretaceous deposits in Mongolia and other evidence of Early Cretaceous birds in Asia. Cretac Res 6:271–278
Lacasa-Ruiz A (1988) An Early Cretaceous fossil bird from Montsec Mountain (Lleida, Spain). Terra Nova:45–46
Li L, Duan Y, Hu D, Wang L, Cheng S, Hou L (2006) New eoentantiornithid bird from the Early Cretaceous Jiufotang Formation of western Liaoning, China. Acta Geologica Sinica (English Edition) 80:38–41
Li Q-G, Gao K-Q, Meng Q-J, Clarke JA, Shawkey MD, D’Alba L, Pei R, Ellison M, Norell MA, Vinther J (2012) Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335:1215–1219
Liu D, Chiappe LM, Serrano FJ, Habib M, Zhang Y-G, Meng Q-J (2017) Flight aerodynamics in enantiornithines: information from a new Chinese Early Cretaceous bird. PLoS One 12:e0184637
Liu D, Chiappe LM, Zhang Y-G, Serrano FJ, Meng Q-J (2019) Soft tissue preservation in two new enantiornithine specimens (Aves) from the Lower Cretaceous Huajiying Formation of Hebei Province, China. Cretac Res 95:191–207
Lovette IJ, Fitzpatrick JW (2004) The handbook of bird biology. Princeton University Press, Princeton, NJ
Martin LD (1984) A new hesperornithid and the relationships of the Mesozoic birds. Trans Kans Acad Sci 87:141–150
Mayr G, Pohl B, Peters DS (2005) A well-preserved Archaeopteryx specimen with theropod features. Science 310:1483–1486
Møller AP, Hedenström A (1999) Comparative evidence for costs of secondary sexual characters: adaptive vane emargination of ornamented feathers in birds. J Evol Biol 12:296–305
Navalón G, Marugán-Lobón J, Chiappe LM, Sanz JL, Buscalioni AD (2015) Soft-tissue and dermal arrangement in the wing of an Early Cretaceous bird: implications for the evolution of avian flight. Sci Rep 5:14864
Navalón G, Meng Q-J, Marugán-Lobón J, Zhang Y-G, Wang B-P, Xing H, Liu D, Chiappe LM (2018) Diversity and evolution of the Confuciusornithidae: evidence from a new 131-million-year-old specimen from the Huajiying Formation in NE China. J Asian Earth Sci 152:12–22
Nudds RL, Dyke GD (2010) Narrow primary feather rachises in Confuciusornis and Archaeopteryx suggest poor flight ability. Science 328:887–889
O’Connor JK (2009) A systematic review of Enantiornithes (Aves: Ornithothoraces). In: Geological sciences. Dissertation, University of Southern California, Los Angeles, pp 600
O’Connor JK, Chang H-L (2015) Hindlimb feathers in paravians: primarily ‘wings’ or ornaments? Biol Bull 42:1–6
O’Connor JK, Sullivan C (2014) Reinterpretation of the Early Cretaceous maniraptoran (Dinosauria: Theropoda) Zhongornis haoae as a scansoriopterygid-like non-avian, and morphological resemblances between scansoriopterygids and basal oviraptorosaurs. Vertebrata Palasiatica 52:3–30
O’Connor JK, Wang X-R, Chiappe LM, Gao C-H, Meng Q-J, Cheng X-D, Liu J-Y (2009) Phylogenetic support for a specialized clade of Cretaceous enantiornithine birds with information from a new species. J Vertebr Paleontol 29:188–204
O’Connor JK, Chiappe LM, Bell A (2011) Pre-modern birds: avian divergences in the Mesozoic. In: Dyke GD, Kaiser G (eds) Living dinosaurs: the evolutionary history of birds. Wiley, Hoboken, NJ, pp 39–114
O’Connor JK, Chiappe LM, Chuong C-M, Bottjer DJ, You H-L (2012a) Homology and potential cellular and molecular mechanisms for the development of unique feather morphologies in early birds. Geosciences 2:157–177
O’Connor JK, Sun C-K, Xu X, Wang X-L, Zhou Z-H (2012b) A new species of Jeholornis with complete caudal integument. Hist Biol 24:29–41
O’Connor JK, Wang X-L, Sullivan C, Zheng X-T, Tubaro PL, Zhang X-M, Zhou Z-H (2013) The unique caudal plumage of Jeholornis and complex tail evolution in early birds. Proc Natl Acad Sci USA 110:17404–17408
O’Connor JK, Li D-Q, Lamanna M, Wang M, Harris JD, Atterholt JA, You H-L (2016a) A new Early Cretaceous enantiornithine (Aves: Ornithothoraces) from northwestern China with elaborate tail ornamentation. J Vertebr Paleontol 36:e1054035
O’Connor JK, Wang X-L, Zheng X-T, Hu H, Zhang X-M, Zhou Z-H (2016b) An enantiornithine with a fan-shaped tail, and the evolution of the rectricial complex in early birds. Curr Biol 26:114–119
O’Connor J, Erickson GM, Norell MA, Bailleul AM, Hu H, Zhou Z-H (2018) Medullary bone in an Early Cretaceous enantiornithine (Aves) and discussion regarding its identification in fossils. Nat Commun 9:1–8
O’Connor J, Falk AR, Wang M, Zheng X-T (2020) First report of immature feathers in juvenile enantiornithines from the Early Cretaceous Jehol avifauna. Vert PalAs
Owen R (1863) On the Archaeopteryx of von Meyer, with a description of the fossil remains of a long-tailed species, from the lithographic stone of Solenhofen. Philos Trans R Soc Lond 153:33–47
Pan Y-H, Sha J-G, Zhou Z-H, Fürsich FT (2013) The Jehol Biota: definition and distribution of exceptionally preserved relicts of a continental Early Cretaceous ecosystem. Cretac Res 44:30–38
Pei R, Li Q-G, Meng Q-J, Gao K-Q, Norell MA (2014) A new specimen of Microraptor (Theropoda: Dromaeosauridae) from the Lower Cretaceous of western Liaoning, China. Am Mus Novit 3821:1–28
Pennycuick CJ (1968) A wind-tunnel study of gliding flight in the pigeon Columba livia. J Exp Biol 49:509–526
Pennycuick CJ (1971) Control of gliding angle in Rüppell’s Griffon Vulture Gyps rüppellii. J Exp Biol 55:39–46
Peteya JA, Clarke JA, Li Q-G, Gao K-Q, Shawkey MD (2017) The plumage and colouration of an enantiornithine bird from the Early Cretaceous of China. Palaeontology 60:55–71
Prum RO, Brush AH (2002) The evolutionary origin and diversification of feathers. Q Rev Biol 77:261–295
Saitta ET, Clapham C, Vinther J (2018) Experimental subaqueous burial of a bird carcass and compaction of plumage. PalZ:1–6
Sanz JL, Chiappe LM, Pérez-Moreno BP, Buscalioni AD, Moratalla JJ, Ortega F, Poyato-Ariza FJ (1996) An Early Cretaceous bird from Spain and its implications for the evolution of avian flight. Nature 382:442–445
Sanz JL, Chiappe LM, Fernández-Jalvo Y, Ortega F, Sánchez-Chillon B, Poyato-Ariza FJ, Pérez-Moreno BP (2001) An Early Cretaceous pellet. Nature 409:998–999
Serrano FJ, Chiappe LM (2017) Aerodynamic modelling of a Cretaceous bird reveals thermal soaring capabilities during early avian evolution. J R Soc Interface 14:20170182
Starck JM, Ricklefs RE (1998) Patterns of development: the altricial-precocial spectrum. In: Starck JM, Ricklefs RE (eds) Avian growth and development. Oxford University Press, New York City, pp 3–30
Stettenheim PR (2000) The integumentary morphology of modern birds—an overview. Am Zool 40:461–477
Thomas ALR (1993) On the aerodynamics of birds’ tails. Philos Trans R Soc Lond B Biol Sci 340:361–380
Thomas ALR (1997) On the tails of birds. Bioscience 47:215–225
Thomas ALR, Balmford A (1995) How natural selection shapes bird’s tails. Am Nat 146:848–868
Thomas DB, McGraw KJ, Butler MW, Carrano MT, Madden O, James HF (2014a) Ancient origins and multiple appearances of carotenoid-pigmented feathers in birds. Proc R Soc B Biol Sci 281:1–9
Thomas DB, Nascimbene PC, Dove CJ, Grimaldi DA, James HF (2014b) Seeking carotenoid pigments in amber-preserved fossil feathers. Sci Rep 4:1–6
Vinther J (2015) A guide to the field of palaeo color. BioEssays 37:643–656
von Meyer H (1861) Archaeopteryx litographica (Vogel-Feder) und Pterodactylus von Solenhofen. Neues Jahrbuch für Mineralogie, Geognosie, Geologie, und Petrefakten-kunde: 678–679
Wang W, O’Connor JK (2017) Morphological coevolution of the pygostyle and tail feathers in Early Cretaceous birds. Vertebrata Palasiatica 55:289–314
Wang X-R, Chiappe LM, Teng F-F, Ji Q (2013) Xinghaiornis lini (Aves: Ornithothoraces) from the Early Cretaceous of Liaoning: an example of evolutionary mosaic in early birds. Acta Geologica Sinica English Edition 87:686–689
Wang M, O’Connor JK, Zhou Z-H (2014a) A robust enantiornithine bird from the Lower Cretaceous of China with scansorial adaptations. J Vertebr Paleontol 34:657–671
Wang M, Zhou Z-H, Xu G-H (2014b) The first enantiornithine bird from the Upper Cretaceous of China. J Vertebr Paleontol 34:135–145
Wang X-L, O’Connor JK, Zheng X-T, Wang M, Hu H, Zhou Z-H (2014c) Insights into the evolution of rachis dominated tail feathers from a new basal enantiornithine (Aves: Ornithothoraces). Biol J Linn Soc 113:805–819
Wang M, Zheng X-T, O’Connor JK, Lloyd GT, Wang X-L, Wang Y, Zhang X-M, Zhou Z-H (2015a) The oldest record of Ornithuromorpha reveals heterogeneous rates of morphological evolution among Early Cretaceous birds. Nat Commun 6:6987
Wang R-F, Wang Y, Hu D-Y (2015b) Discovery of a new ornithuromorph genus, Juehuaornis gen. nov. from Lower Cretaceous of western Liaoning, China. Global Geology 34:7–11
Wang Y-M, O’Connor JK, Li D-Q, You H-L (2015c) New information on postcranial skeleton of the Early Cretaceous Gansus yumenensis (Aves: Ornithuromorpha). Hist Biol 28:666–679
Wang M, O’Connor JK, Pan Y-H, Zhou Z-H (2017) A bizarre Early Cretaceous enantiornithine bird with unique crural feathers and an ornithuromorph plough-shaped pygostyle. Nat Commun 8:1–12
Wang X, O’Connor JK, Maina JN, Pan Y, Wang M, Wang Y, Zheng X, Zhou Z (2018) Archaeorhynchus preserving significant soft tissue including probable fossilized lungs. Proc Natl Acad Sci USA. Published online, 1–6. https://doi.org/10.1073/pnas.1805803115
Wang M, O’Connor J, Xu X, Zhou Z-H (2019) A new Jurassic scansoriopterygid and the loss of membranous wings in theropod dinosaurs. Nature 569(7755):256–259
Williston SW (1896) On the dermal covering of Hesperornis. Kansas University Quarterly 5:53–54
Xing L-D, McKellar RC, Wang M, Bai M, O’Connor JK, Benton MJ, Zhang J-P, Wang Y, Tseng K-W, Lockley M, Li G, Zhang W-W, Xu X (2016) Mummified precocial bird wings in mid-Cretaceous Burmese amber. Nat Commun 7:12089
Xing L-D, O’Connor JK, McKellar RC, Chiappe LM, Tseng K-W, Li G, Bai M (2017) A mid-Cretaceous enantiornithine (Aves) hatchling preserved in Burmese amber with unusual plumage. Gondwana Res 49:264–277
Xing L-D, Cockx P, McKellar RC, O’Connor J (2018a) Ornamental feathers in Cretaceous Burmese amber: resolving the enigma of rachis-dominated feather structure. J Palaeogeogr 7(13):1–18
Xing L-D, O’Connor JK, McKellar RC, Chiappe LM, Bai M, Tseng K-W, Zhang J, Yang H-D, Fang J, Li G (2018b) A flattened enantiornithine in mid-Cretaceous Burmese amber: morphology and preservation. Sci Bull
Xing L-D, McKellar RC, O’Connor JK, Bai M, Tseng K-W, Chiappe LM (2019a) A fully feathered enantiornithine foot and wing fragment preserved in mid-Cretaceous Burmese amber. Sci Rep 9(927):1–9
Xing L-D, O’Connor J, Chiappe LM, McKellar RC, Carroll N, Hu H, Bai M, Lei F-M (2019b) A new enantiornithine with unusual pedal proportions found in amber. Curr Biol 29(14):2396–2401
Xu X, Guo Y (2009) The origin and early evolution of feathers: insights from recent paleontological and neontological data. Vertebrata Palasiatica 47:311–329
Xu X, Zhou Z, Wang X, Kuang X, Du X (2003) Four-winged dinosaurs from China. Nature 421:335–340
Xu X, Zheng X-T, You H-L (2010) Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature 464:1339–1341
Xu X, Zheng X-T, Sullivan C, Zhang F-C, O’Connor JK, Wang X-L (2015) A bizarre Jurassic maniraptoran theropod with preserved evidence of membranous wings. Nature 521:70–73
You H-L, Lamanna MC, Harris JD, Chiappe LM, O’Connor J, Ji S-A, Lü J-C, Yuan C-X, Li D-Q, Zhang X, Lacovara KJ, Dodson P, Ji Q (2006) A nearly modern amphibious bird from the Early Cretaceous of northwestern China. Science 312:1640–1643
Zelenkov NZ, Averianov AO (2016) A historical specimen of enantiornithine bird from the Early Cretaceous of Mongolia representing a new taxon with a specialized neck morphology. J Syst Palaeontol 14:319–338
Zhang F, Zhou Z (2000) A primitive enantiornithine bird and the origin of feathers. Science 290:1955–1960
Zhang F, Zhou Z (2004) Leg feathers in an Early Cretaceous bird. Nature 431:925
Zhang F, Zhou Z, Dyke GJ (2006) Feathers and ‘feather-like’ integumentary structures in Liaoning birds and dinosaurs. Geol J 41:395–404
Zhang F, Zhou Z, Benton MJ (2008a) A primitive confuciusornithid bird from China and its implications for early avian flight. Sci China Ser D Earth Sci 51:625–639
Zhang F-C, Zhou Z-H, Xu X, Wang X-L, Sullivan C (2008b) A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 455:1105–1108
Zhang F-C, Kearns SL, Orr PJ, Benton MJ, Zhou Z-H, Johnson D, Xu X, Wang X-L (2010) Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature 463:1075–1078
Zhang Z-H, Chiappe LM, Han G, Chinsamy A (2013) A large bird from the Early Cretaceous of China: new information on the skull of enantiornithines. J Vertebr Paleontol 33:1176–1189
Zheng X, Zhang Z, Hou L (2007) A new enantiornitine bird with four long rectrices from the Early Cretaceous of northern Hebei, China. Acta Geologica Sinica (English Edition) 81:703–708
Zheng X-T, Xu X, Zhou Z-H, Miao D, Zhang F-C (2010) Comment on “Narrow primary feather rachises in Confuciusornis and Archaeopteryx suggest poor flight ability”. Science 330:320
Zheng X-T, Wang X-L, O’Connor JK, Zhou Z-H (2012) Insight into the early evolution of the avian sternum from juvenile enantiornithines. Nat Commun 3:1–8
Zheng X-T, O’Connor JK, Huchzermeyer FW, Wang X-L, Wang Y, Wang M, Zhou Z-H (2013a) Preservation of ovarian follicles reveals early evolution of avian reproductive behaviour. Nature 495:507–511
Zheng X-T, Zhou Z-H, Wang X-L, Zhang F-C, Zhang X-M, Wang Y, Wei G-J, Wang S, Xu X (2013b) Hind wings in basal birds and the evolution of leg feathers. Science 339:1309–1312
Zheng X-T, O’Connor JK, Huchzermeyer FW, Wang X-L, Wang Y, Zhang X-M, Zhou Z-H (2014a) New specimens of Yanornis indicate a piscivorous diet and modern alimentary canal. PLoS One 9:e95036
Zheng X-T, O’Connor JK, Wang X-L, Wang M, Zhang X-M, Zhou Z-H (2014b) On the absence of sternal elements in Anchiornis (Paraves) and Sapeornis (Aves) and the complex early evolution of the avian sternum. Proc Natl Acad Sci USA 111:13900–13905
Zheng X-T, O’Connor JK, Wang X-L, Zhang X-M, Wang Y (2014c) New information on Hongshanornithidae (Aves: Ornithuromorpha) from a new subadult specimen. Vertebrata Palasiatica 52:217–232
Zheng X-T, O’Connor JK, Wang X-L, Pan Y-H, Wang Y, Wang M, Zhou Z-H (2017) Exceptional preservation of soft tissue in a new specimen of Eoconfuciusornis and its biological implications. Natl Sci Rev 4:441–452
Zhou Z, Zhang F (2001) Two new ornithurine birds from the Early Cretaceous of western Liaoning, China. Chin Sci Bull 46:1258–1264
Zhou Z, Zhang F (2002) Largest bird from the Early Cretaceous and its implications for the earliest avian ecological diversification. Naturwissenschaften 89:34–38
Zhou Z, Zhang F (2003) Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Can J Earth Sci 40:731–747
Zhou Z, Zhang F (2004) A precocial avian embryo from the Lower Cretaceous of China. Science 306:653
Zhou Z, Zhang F (2005) Discovery of an ornithurine bird and its implication for Early Cretaceous avian radiation. Proc Natl Acad Sci USA 102:18998–19002
Zhou Z-H, Zhang F-C (2006) Mesozoic birds of China—a synoptic review. Vertebrata Palasiatica 44:74–98
Zhou Z, Barrett PM, Hilton J (2003) An exceptionally preserved Lower Cretaceous ecosystem. Nature 421:807–814
Zhou Z, Chiappe LM, Zhang F (2005) Anatomy of the Early Cretaceous bird Eoenantiornis buhleri (Aves: Enantiornithes) from China. Can J Earth Sci 42:1331–1338
Zhou Z-H, Zhang F-C, Li Z-H (2009) A new basal orithurine (Jianchangornis microdonta gen. et sp. nov.) from the Lower Cretaceous of China. Vertebrata Palasiatica 47:299–310
Zhou S, Zhou Z-H, O’Connor JK (2012) A new toothless ornithurine bird (Schizooura lii gen. et sp. nov.) from the Lower Cretaceous of China. Vertebrata Palasiatica 50:9–24
Zhou S, Zhou Z-H, O’Connor JK (2013) A new piscivorous ornithuromorph from the Jehol Biota. Hist Biol 26:608–618
Zhou S, O’Connor JK, Wang M (2014) A new species from an ornithuromorph dominated locality of the Jehol Group. Chin Sci Bull 59:5366–5378
Acknowledgements
I would like to thank X-T. Zheng of the STM, H-L. Chang of the Henan Geological Museum, and C-L. Gao of the DNHM for access to specimens. I would also like to thank C. Sullivan and T. Stidham of the IVPP for useful discussions and M. Rothman for use of his art work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
O’Connor, J. (2020). The Plumage of Basal Birds. In: Foth, C., Rauhut, O. (eds) The Evolution of Feathers. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-27223-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-27223-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-27222-7
Online ISBN: 978-3-030-27223-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)