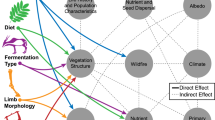
Abstract
Woody plants and grasses are two functionally distinct food groups that pose different mechanical, nutritional, and ecological challenges to herbivores. Accordingly, herbivores have evolved an array of morphological, physiological, and behavioural life history traits that reflect each species’ primary dietary niche. The prevalence of convergences across distantly related groups is evidence that many of these traits are adaptive. Most evaluations are, however, necessarily correlational, and so the functional relevance of many traits is still being debated. The last 2 decades has seen the emergence of larger, more representative, and quantitative datasets, which, along with statistical developments in evolutionary biology, means that a revised set of analyses is warranted. In this chapter we present a collection of updated datasets for almost 100 anatomical and physiological characteristics from 188 species. These data are subjected to phylogenetically-constrained analyses of relationships with diet niches (using %grass in the diet as a niche indicator). Results of these analyses highlight not only the extraordinary amount of convergence within this animal group, but also the constraints that morpho-physiology places on diet niches. To separate correlation from functional significance, we advocate an approach that considers the correlations between traits as part of each species’ “bauplan”, and highlight how this approach has already been used to link trends and outliers with mechanism in various datasets. While some questions about functional relevance require experimental manipulations that will almost certainly never be realized, synergies between experimental and correlational analyses are rapidly changing our understanding of how foraging adaptations, from locating and biting, to chewing and digesting food, have shaped the evolutionary diversification of mammal herbivores.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
4.1 Introduction
Woody plants and grasses are two functionally, and ecologically distinct, components of terrestrial vegetation. For herbivores, these represent two distinct food groups—browse and grass, which differ in spatial distribution, architecture, height above ground, physico-mechanical, biochemical and fermentative properties, presenting different challenges and constraints to the animals that feed on them. Browse includes forbs and other non-woody dicots like herbs because, in many ways, these are structurally and biochemically similar to corresponding parts of woody plants. That large mammal herbivores, restricting our discussion to members of the ‘ungulate ’ orders Proboscidea , Hyracoidea, Perissodactyla , and Artiodactyla , differ in feeding styles, with respect to whether species consume primarily browse or grass, was recognized early on, based on field studies of species’ diet compositions in free-ranging environments (Van Zyl 1965; Gwynne and Bell 1968). But it was the work of Hofmann (Hofmann and Stewart 1972; Hofmann 1973, 1989) that truly formalized concepts that browser and grazer species have unique morphophysiological traits representing evolutionary adaptations to foraging differentially on browse or grass.
The evolutionary significance of browsing and grazing is generally understood, and debated, within the context of a morphophysiological adaptive landscape. Traits including body size, tooth and skull anatomy, morphophysiology of the gastrointestinal tract, and even various behavioural and ecological characteristics of large mammal herbivores, have been linked, both functionally and statistically, with browsing and grazing diet niches (Fortelius 1985; Gordon and Illius 1988; Janis 1988; Janis and Ehrhardt 1988; Hofmann 1989; Spencer 1995; Reed 1996; Owen-Smith 1997; Brashares et al. 2000; Clauss and Lechner-Doll 2001; Clauss et al. 2002; Mendoza et al. 2002; Clauss et al. 2003b; Mendoza and Palmqvist 2006; Clauss et al. 2008a; Mendoza and Palmqvist 2008; Clauss et al. 2009c, 2010a; Codron and Clauss 2010; Fraser and Theodor 2011; Hummel et al. 2011; Kaiser et al. 2013; Dittmann et al. 2015; Lazagabaster et al. 2016; Meier et al. 2016). Traits are often found to be convergent, across unrelated taxa or clades, making the ungulates one of the most conspicuous examples of adaptive radiation in the animal kingdom.
Some views have favoured an alternative model for herbivore differentiation, a diet quality -based niche structure driven by differences in feeding selectivity linked to body size (Gordon and Illius 1994; Robbins et al. 1995; Gordon and Illius 1996; Pérez-Barbería and Gordon 2001; Pérez-Barbería et al. 2001; Codron et al. 2007; Clauss et al. 2013). However, such a concept is mostly reconcilable with a browser-grazer based differentiation if one accepts that feeding selectivity (selective/unselective), and botanical composition of the diet do not covary, but that there may be unselective browsers and selective grazers (Demment and Longhurst 1987). Allowing for variability in selectivity within the botanical niche largely adds to our understanding of browser-grazer differences (Codron et al. 2007).
The process of large mammals acquiring nutrients from plant foods starts with ingestion (locating and biting food), followed by extensive oral processing, and finally achieving a level of digestive fermentation which is more intense than occurs in any other animal group (Karasov and Martínez del Rio 2007). The number of traits that have been discussed in this context is vast (in compiling data for this Chapter alone we collected data for a total of 155 variables!). However, the functional and/or statistical relevance (in terms of diet niche ) of many of these characteristics is not always clear, and in fact dubious in many instances. A comprehensive review of the topic was presented more than a decade ago (Clauss et al. 2008b), which recognized multiple mismatches, in that several concepts linking traits to dietary function, while contributing substantially to the overall narrative, were not supported by empirical/statistical evidence. In this Chapter, we present a large set of statistical analyses of an up-to-date collection of datasets, allowing us to revisit old hypotheses, and to address recent developments over the past decade. This Chapter, therefore, complements the discussion presented in Clauss et al. (2008b), and we encourage readers to consult both to gain a complete perspective on herbivore adaptation. We reflect on specific anatomical and physiological features of browsing and grazing herbivores that allow them to overcome foraging challenges associated with each of the three stages, in succession, of foraging—ingestion, oral processing, and digestion .
4.2 Linking Form and Function
Any predicted link between an anatomical (or physiological) trait and diet niche , i.e., an association derived from the expected functional relevance of the trait, can be interrogated through one of two approaches: experimental or comparative. Experimental approaches seek qualitative changes in traits following manipulation, from feeding experiments and, potentially, defaunation, up to surgical removal of organs, to determine their function by observing the effect of their diminution or absence (Trautmann and Schmitt 1935; Sakaguchi et al. 1981; Tahas et al. 2017, 2018). Such approaches offer the most definitive support for functional significance of traits, for example, demonstrating that tooth wear in non-ruminants is more strongly influenced by external (grit) rather than internal (silica in forages) abrasives (Müller et al. 2014, 2015; and see Saarinen Chap. 2). But, in this example, the experiments do not tell us whether wear-resistant features of herbivore teeth evolved, in response to a shift in diet (to include foods with a higher grit load), or to prevailing environmental conditions. In any case, for mostly logistical reasons, only a few traits have, so far, been subject to such experimental manipulation. A more convenient hypothetico-deductive approach is the comparative method, a widely-used approach for studying adaptive trends in evolutionary biology (Harvey and Pagel 1991). Here, the relationship between traits and diet niches , across species, are evaluated statistically, and rejection of the null hypothesis allows us to conclude the value of the trait is an adaptive response to diet, or to something for which diet is a proxy.
4.2.1 Definition of Herbivore Diet Niches
Statistical evaluations of niche-trait associations, in herbivores, have relied on one of two ways to categorize diet niches. On the one hand, a categorical distinction can be made between browser and grazer species. This categorical usage is typical of most earlier studies (reviewed in Clauss et al. 2008b). In this scheme, mixed- or intermediate-feeders, species that regularly feed on both browse and grass, or switch diets across habitats and/or seasons, can be treated as a third category of feeding style. A categorical approach is not only heuristically valuable in reducing complex diets to simple rules, but has the advantage of being relatively non-sensitive to dietary variations within any one species. On the other hand, diet niches can be viewed as a continuum along the browser-grazer axis, typically depicted by the average percentage grass in the natural diet of each species (Janis 1995; Van Wieren 1996a; Clauss et al. 2003b; Pérez-Barbería et al. 2004). A continuous classification of diets has been favoured by most recent studies, especially following the introduction of new methods, such as stable carbon isotope analysis, that provide rapid estimates of proportions of browse:grass consumption, at least in subtropical savanna environments dominated by C4 grasses (Cerling et al. 2003; Sponheimer et al. 2003; Codron et al. 2007, 2008b; Lazagabaster et al. 2016). The advantage of such an approach is that arbitrary boundaries between niche categories do not need to be established a priori; in a categorical scheme, the question is always at which level of browse/grass intake can a species be classified as a browser or grazer (75%?; 90%?).
In this Chapter we adopt the latter approach, representing the current state of the field, but also because the ‘continuous diet niche’ relaxes the assumption that species’ niches (sensu Hutchinson 1959) are fixed. That is, we recognize that adoption of a specific trait does not prevent a species from feeding in a different niche space, depending upon circumstance. It simply means the species should be more efficient, i.e., more competitive, in the primary niche space to which it is adapted (Codron and Clauss 2010; Damuth and Janis 2011). At the same time, it should be noted that any morpho-physiological trait that may have evolved as an adaptation to a particular diet niche, does not necessarily reflect the average of that niche. In many instances, the trait may simply represent a threshold, above or below which a herbivore may become more efficient in a particular niche. While testing predictions about such nonlinear relationships requires the development of trait-specific models, outside the scope of a large-scale set of analysis such as we present here, treating diet as a continuous variable is a step closer towards achieving this ultimate goal.
4.2.2 Adaptive Value of Morphophysiological Traits
When dealing with a large number of traits, some authors have employed multivariate statistics to determine whether these can distinguish between herbivore feeding styles (Spencer 1995; Mendoza et al. 2002; Mendoza and Palmqvist 2006; Fraser and Theodor 2011), and indeed between diet niches , of other taxonomic groups (Stayton 2006; Martin et al. 2016). These methods offer cursory insights of relative species positions over the adaptive ‘morphospace’ landscape, but cannot link any single trait with a specific function, and so cannot explicitly resolve the relevance of particular traits. Univariate analyses, on the other hand, may be misleading in this regard, in cases where traits have developed as compensatory characteristics that evolved to accommodate features that do have a direct link to feeding behaviour (Raia et al. 2010). Actually, such compensation is also likely to raise, spuriously, the ‘goodness-of-fit’ of multivariate models as well. We have tried here to limit our discussion to only those traits for which there is a specific hypothesis about their functional relevance as a feeding tool, with some exceptions, which we note below. Additionally, we provide some examples of correlations between individual traits that we consider meaningful.
Comparative analyses, with species as the biological unit of interest, are performed on datasets that are inherently non-independent because species’ traits are inherited from common ancestors (Garland et al. 2005). A common way to overcome this is to estimate the correlation between a trait/s and the study group’s phylogenetic tree (Pagel 1999; Garland et al. 2005; Lajeunesse 2009). We follow the method employed by most recent studies on browser-grazer adaptation, using Phylogenetic Least Squares Regression (PGLS) of the caper package for R (Orme et al. 2013). Note, though, that from a deductive perspective, PGLS differs from non-phylogenetically controlled analysis, e.g., Generalized Least Squares (GLS), primarily in that only the former can be used to infer evolutionary convergence. By contrast, lack of a significant relationship between mean percentage grass in the diet with a specific trait does not necessarily mean lack of an adaptive response: the trait may well have evolved as a response to diet, but only within a specific clade that dominates the dataset. Therefore, we also provide results from GLS when these differ from PGLS.
4.2.3 Body Size
Body size is one of the most fundamental biological traits of species, influencing not only variables that reflect size, but also many characteristics related to physiology , shape and even ratios of two variables. In some approaches, particularly those expecting an effect of diet quality , body size has been treated as an alternative to diet niche as an explanatory factor for trait differentiation (Gordon and Illius 1994; Pérez-Barbería and Gordon 2001). Others take body size as a factor determining diet niche itself, with browsers being small and grazers being large, although recognition that browsers are represented across the herbivore size spectrum means that such an approach is not always substantiated. Figure 4.1 shows the typical pattern—while among very small species, there are no strict grazers, and among larger species, there are less browsers, there is no clear constraint put on feeding type by body mass . Here we treat body size as a covariate in all analyses, using body mass (log10-transformed) as a proxy for size. This approach not only allows for patterns to be inferred while controlling for body size variations across species, but—for traits which are also log-transformed—provides estimates of allometric scaling exponents (slopes in log-log regressions). Detailed discussion of differences in allometric scaling is outside our scope, but presentation of these results should be useful for stimulating further discussion about herbivore adaptations.
4.2.4 Data Compilation and Analysis
We compiled a database, from the published literature, of anatomical and physiological measures, as well as mean percentage grass in the natural diet. Additionally, we collated previously unpublished ruminant data on measures of the palate, the cranial and caudal rumen pillar thickness, the area of the intraruminal, ruminoreticular and reticuloomasal orifices (IRO, RRO, and ROO, respectively; calculated as ovals from two length measurements), the height of the Papillae unguiculiformes, the larger curvature of the abomasum, and liver mass. In total, data for 188 large mammal herbivore species and 95 craniodental, skeletal, and soft tissue characteristics that have been (or can be) hypothesized to differ across diet niches were analyzed. The number of species included varied between datasets, ranging from 10 to 135. The complete dataset, including traits not included as part of our statistical analyses, is included as an electronic supplement with the online version of this Chapter. For PGLS, a single mammalian ‘supertree’ was used for phylogenetic correlation (Fritz et al. 2009b), pruned to incorporate the species included in each data set. Lambda (λ), depicting the strength of the phylogenetic signal (0 = no signal, values approaching 1 = strong phylogenetic correlation), is estimated using maximum likelihood estimates. We use a species’ mean body mass (BM, in kg) as a proxy for body size, taking values reported in each study (where available) or species’ means as reported in a global dataset (Smith et al. 2003).
Herbivore digestive strategies are dichotomously distributed between ruminants and non-ruminants (Clauss et al. 2015). Among large herbivores, non-ruminants are primarily hindgut fermenters , barring the Hippopotamidae. Traits related, not only to digestive physiology , but even biting and chewing of foods, are expected to respond differently between ruminants and non-ruminants. In many instances, therefore, studies have either been restricted to analysis of one group only (typically ruminants, or even single families within the Ruminantia, e.g., Bovidae), or analyses have been repeated on data subsets comprising species only from one group (Pérez-Barbería et al. 2004; Codron et al. 2008b; Lazagabaster et al. 2016). Here, we adopt a nesting approach, with digestive strategy treated as a binary variable within which BM and percentage of grass are nested. Thus, we test explicitly for differences in adaptive responses (differences in slopes) to percentage of grass between ruminants and non-ruminants, and reduce Type I error rates that would otherwise be inflated by conducting multiple analyses of overlapping datasets.
Ultimately, three models (four if both ruminants and non-ruminants are included in the dataset) are tested for each trait: BM and percentage grass in the diet as single effects, and BM + percentage grass as covariates. Model fits are compared by an Information Theoretic approach, Akaike’s Information Criterion (AIC), applying corrections for small sample sizes (denoted by the subscript c). Only models with ΔAICc ≤ 2, where ΔAICc is the difference in AICc of a candidate model from the lowest AICc in the set, are reported when assembling tables of results (Burnham and Anderson 2001; Burnham and Anderson 2002). Thus, if factors like percentage grass, or ruminant vs. non-ruminant, do not feature in the best-supported models, we exclude them as effects driving variation in the specific trait.
4.3 Ingestion
4.3.1 Searching: Perception and Posture
One of the few advantages of being an herbivore, as compared to a carnivore , is that food is relatively easy to find, and it does not run away. This does not mean that plants are defenceless against herbivory , just that herbivores do not require too much in terms of perception and mobility when it comes to finding food. Nevertheless, browse plants and grasses are distributed differently at landscape, patch, and bite scales, presenting different searching and biting constraints for herbivores (Gross et al. 1993; Shipley 2007). Whereas grasses grow more-or-less continuously within a landscape or patch matrix, woody plants are more patchily dispersed. Browsers should, therefore, spend more time searching and moving between foraging patches than grazers. Whether browsers and grazers differ in sensory perception, as it may be associated with locating different food types, has so far received very little attention in empirical studies (Gordon 2003). Intuitively, we would expect that visual, hearing, and olfactory senses of herbivores are more likely to represent predator-detection systems rather than peculiar adaptations for locating stationary food items. Accordingly, brain and eye size, and indeed maximum visual acuity (determined by the number of cones per degree of visual angle), is not related to the percentage grass in species’ diets (Table 4.1).
When suggesting that grazers have a higher density of lingual taste buds, Hofmann (1988) also suggested that browsers should rely more on their sense of smell. This hypothesis matches the finding that the area of the ethmoid bone is negatively related to percentage grass intake (Table 4.1). An increased ethmoid area means a larger area for nerves to be conducted to the nose and, ultimately, a more acute sense of smell. The resulting interpretation that, for diet selection, grazers rely more on their taste and browsers more on their smell, matches the concept that grasses contain less anti-digestive or toxic substances, and hence may not exert a strong selective pressure to evolve a pre-ingestive detection system (Fowler 1983; Mlambo et al. 2015).
Given the difference in spatial distribution of their foods, browsers and grazers could also be expected to differ in mobility . While limbs, particular hind limbs, tend to be longer amongst grazers (Table 4.1), such results should be treated with caution because many elements of limb morphology, including length, likely reflect the habitats in which species live. That is, grazers typically live in more open habitats and thus should be expected to take flight more often than browsers, which may often avoid predation simply by hiding. On the other hand, grasses are a seasonal resource, dying back in dry seasons (Tainton 1999). For this reason, grazing species are more often migratory, moving several hundred, or thousand, km to alternate foraging areas during limiting periods (Avgar et al. 2014); a phenomenon which could, in part, explain their longer limbs.
4.3.2 Biting: Face, Mouth, Lips, Tongue, and Palate
Browse and grass occur at different heights above ground. With the evident exception of bamboo, grasses are generally at, or near, ground-level, whereas browse foods are more heterogeneously distributed in vertical space, from trees of several metres in height to forbs located at or even below the grass layer (Tainton 1999). A vertical feeding height stratification amongst browser species accounts for, in part, the massive variation in body size of these animals (du Toit 1990). Grasses, however, are somewhat bimodally distributed in this regard, between tall, and short or ‘lawn’ grasses, and a feeding height stratification of grazers has been hypothesized from field studies (Bell 1971; Prins and Olff 1998; Murray and Illius 2000), and from investigations of craniodental morphology (Codron et al. 2008b). The various spatial arrangements of leaves, and fruit, of woody plants also means a more heterogeneous architecture than in grasses, in terms of individual bites presented to herbivores. Hence, grazers are typically expected to be less selective foragers, taking larger—and probably more nutritionally homogeneous—bites than browsers. A possible exception is amongst grazers feeding on low quality grasses—often the taller, more fibrous grass taxa. In these cases, leaf:stem ratios are lower than amongst lawn grasses, meaning a lower nutritional value overall (because grass stems are generally tougher, more fibrous, and less proteinaceous than grass leaves) (Macandza et al. 2004; Benvenutti et al. 2006), and a possible requirement for smaller, more selective bite sizes in grazers feeding on this resource.
A more regular ground-level feeding behaviour of grazers is best reflected in the braincase angle—the angle between the basioccipital bone and the palate (Lazagabaster et al. 2016). A more acute angle translates into a steeper orientation of the jaw relative to the skull, and hence a strong negative relationship exists between the braincase angle and percentage grass in the diet (Table 4.2).
Feeding height differences between grazers and browsers are reflected, not only in head posture , but also the shape and size of the face. Grazers are expected to have a longer face than browsers, allowing the former to crop bites from short grasses, even during die-back in the dry season (Spencer 1995; Schuette et al. 1998). Even after accounting for species differences in body size, face depth, represented by the region in front of the orbit (measured as the distance between the orbit and the premolar-molar transition), increases across species with higher percentage grass in the diet, but less so in ruminants than in non-ruminants (Table 4.2). Also, amongst ruminants, browsers have a wider distance between the last molars than do grazers (see max palate width, Table 4.2), suggesting a more pointed face shape in browsers. A classic explanation for this finding is a typical bauplan constraint (e.g., Janis 1995): in order to accommodate the larger teeth of more hypsodont species (mostly grazers, see below), the orbita has to be moved posterior to the tooth row, leading, for example, to a negative relationship between the hypsodonty index and the masseteric fossa:face length ratio (Fig. 4.2a). Another measure that could be considered related to face depth, the length of the palate (either as total length, or as the length of the rugated portion), available for ruminants only, did not show a relationship with percentage grass intake (Table 4.2), which is in line with these measurements being independent from the orbita’s position. This can be interpreted as an indication that it is the mentioned bauplan constraint, and not a general requirement for a snout, that leads to longer skulls in grazers.
Proxies for face depth (e.g., masseter:fossa face length ratio) are negatively related to M3 size (a—the hypsodonty index) and to each other (b—diastema length vs. palate length), and hypsodonty is also correlated with cheek tooth row length in ruminants, but not in non-ruminants (c) and with the size (mass) of the masseter (d). Diastema, palate and tooth row lengths are presented relative to BM0.33, and masseter mass relative to BM0.75
The length of the rugated portion of the palate (anterior to the maxillary tooth row) corresponds somewhat to the length of the diastema (the distance between the base of the third incisor and the most anterior premolar present; Fig. 4.2b). The diastema has been hypothesized to be longer in grazers (Mendoza et al. 2002), although the functional relevance of this is unclear. We hypothesize that the diastema is linked to a functional dichotomy of the dental apparatus (Hemae 1967)—cropping by the incisors on the one hand, which requires that incisors can be brought into occlusion without impediment from the cheek teeth , and grinding movements of the cheek teeth on the other hand, which should not lead to concurrent incisor attrition. In species in which interlocking canines prevent a wide lateral grinding chewing stroke, such as suids, peccaries, hippos or tapirs (Kiltie 1981; Fortelius 1985; Herring 1985), this functional dilemma does not apply. The evolutionary loss of upper incisors in ruminants (including camelids), and the extremely loose fit of overhanging ‘hinged’ upper canines in those ruminant species that have them (Aitchison 1946), can be interpreted as adaptations to avoid disruption of the lateral chewing stroke by the front teeth. The cropping function of the anterior teeth is possible in species with a transverse chewing stroke because the mandible is generally less wide than the maxilla, and cheek teeth, therefore, are not in full occlusion when the incisors are. One important aspect of the transverse chewing stroke, in those herbivores that have it, is that it is not a strictly horizontal lateral movement, but, due to the inclination of the cheek teeth surface, a movement with both horizontal and vertical components. A longer diastema will increase the distance between front teeth due to the vertical component of the chewing stroke. If we accept that grazers generally have a lower occlusal relief in their cheek teeth (Fortelius and Solounias 2000), i.e., potentially a lesser vertical deflection during the chewing stroke, a longer diastema would, theoretically, compensate for this, ensuring the distance between the front teeth. If this was a major function of the diastema, we would also expect this effect to be larger in non-ruminants (with upper incisors) than in ruminants (without upper incisors). However, although the corresponding interaction terms indicated a shorter diastema in ruminants, diastema length was only significantly related to diet in GLS but not in PGLS, and neither was the diastema:cheek tooth row length ratio (Table 4.2), suggesting that it is not an important correlate of diet.
The shorter, more pointed face and mouth of browsers can be linked to a more selective feeding behaviour, aiming for leaves or fruits of relatively higher nutritional value, while attempting to avoid less nutritious stems, and defensive spines, within the available browse matrix. Consequently, browsers also have a relatively longer mobile portion of the tongue (Table 4.2), assisting with selective biting and with stripping leaves from spiny branches (Meier et al. 2016). Clearly, non-selective browsers such as elephants (although these animals also eat grass, and are more often classified as intermediate-feeders) lack this type of facial ‘pointiness’, and while their trunks do add to dexterity while foraging, they rely more on bulk intake of even low quality items like bark (Codron et al. 2006; Pretorius et al. 2016).
The biting apparatus of grazers reflects cropping of resources near the ground. Grazers have wider incisors, and a more protrusive incisor arcade (Table 4.2), that acts almost as a spade during cropping (Gordon and Illius 1988; Pérez-Barbería and Gordon 2001), and have a wider muzzle (represented by the absolute width of the premaxilla, the ratio of dentary length:breadth, or the ratio of muzzle:palate width, all showing the same relationship to diet; Table 4.2). Within ruminants, both the palate and the tongue showed a maximum:minimum width ratio (i.e., an hourglass shape) that increased with percentage grass intake (Table 4.2). However, the relationship only occurs in GLS for the tongue, and the two measures are not correlated with each other (Pearson’s r = −0.125, p = 0.398), hence the relevance, if any, of the hourglass shape for the natural diet is not clear.
Amongst non-ruminants, the response of the face depth/muzzle width complex to percentage grass in the diet is stronger than amongst ruminants (steeper slopes for non-ruminants; Table 4.2). In an analysis that evaluated relationships between craniodental metrics with a presumed feeding height proxy amongst grazers, Codron et al. (2008b) showed that wide muzzles, and a steeper mandibular angle (a more direct measure of jaw position relative to the horizontal plane than the braincase angle), are most extreme amongst short/lawn grass grazers (principally the bovid tribe Alcelaphini—wildebeest (Connochaetes spp.), hartebeest and their kin). By contrast, members of the Hippotragini (specifically roan antelope Hippotragus equiinus and sable antelope H. niger) and Reduncini (waterbuck and reedbuck) have less-derived versions of these traits, almost resembling browsers. Subsequently, the evolution of at least two distinct evolutionary pathways to grazing (including between the Alcelaphini vs. Hippotragini/Reduncini clades) was detected in a phylogenetic analysis of African bovid diet niches (Louys and Faith 2015). If one extends this concept to include non-ruminants, the stronger response to percentage grass intake can be explained as a function of many non-ruminant grazers being large-bodied, ground-level ‘lawnmowers’, that use their wide mouths and prehensile lips to crop large mouthfuls, as especially evident in the square-lipped rhinoceros Ceratotherium simum or the common hippopotamus Hippopotamus amphibius (Owen-Smith 2013).
4.3.3 Intake Amount and Feeding Time
So far, we have mentioned intake quantity mainly as a function of variations in potential bite size. Such functions are not trivial, since bite size is the basic unit of intake for herbivores (Shipley 2007; for carnivores , ecological roles are determined more by the number of prey individuals taken). Nonetheless, for herbivores, for which the relatively low nutritional quality and digestibility of food is a limiting factor, achieving large amounts of total biomass intake is the primary foraging goal. In herbivores, intake scales higher than metabolic rate (i.e., at a scaling exponent higher than 0.75), which is possible because gut capacity also scales higher than metabolic rate (Müller et al. 2013; and see Table 4.3). Indeed, daily dry matter intake of mammalian herbivores is an order of magnitude greater than for similar-sized carnivores (Codron et al. 2016), the latter consuming diets of higher digestibility and digestible energy content. Consequently, herbivores require digestive tracts of larger volume (Chivers and Hladik 1980; Clauss et al. 2017). If one postulates a difference in digestible energy content between browse and grass, then one would also expect a corresponding difference in intake levels and gut capacity between browsers and grazers. So far, the available data for food intake from feeding experiments do not suggest such a difference (Table 4.3). This most likely reflects the fact that, although grass often contains higher digestible energy levels than browse (Hummel et al. 2006), regional, seasonal, plant species and plant part variability is so high (Paine et al. 2018) that food-specific intake levels would be senseless. Additionally, intake is often not aimed at meeting maintenance, but at maximizing energy gain, and, therefore, increases with forage diet quality in experimental settings (Van Soest 1965; Meyer et al. 2010). To judge whether intake really differs systematically between browser and grazer species, systematic feeding experiments aimed at specifically this question would probably be required.
By contrast, another characteristic of browse and grass, namely the speed at which the material can be fermented, has a more evident effect: browse typically reaches its maximum fermentation gain faster than grass (Hummel et al. 2006). Therefore, it makes sense that ruminant browsers have a higher frequency of feeding bouts, to faster replace the material that is digested quicker, whereas ruminant grazers have fewer, longer feeding bouts (Table 4.3). Differences in bout frequency between browsers and grazers also reflect differences in digestive strategies (Hummel et al. 2006; and see below), such as larger forestomach capacities in ruminant grazers.
As far as we are aware, differences in intake requirements of short- vs. tall-grass grazers have not been investigated. Theoretically, however, these two grazer types should achieve different instantaneous intakes at different feeding heights, with species like wildebeest achieving higher intake at lower sward levels than sympatric medium- or tall-grass grazers like hartebeest (Alcelaphus and Damaliscus spp.) (Murray 1993; Murray and Illius 2000), or even Reduncini and Hippotragini. Amongst non-ruminants, however, no difference in instantaneous intake was achieved by horses of three body size classes feeding at varying sward heights (Fleurance et al. 2009). Taking ever-increasing bite sizes during cropping might be a feature unique to ruminant grazers, and could explain the smaller effect of percentage grass in the diet on traits like muzzle width than in non-ruminants. Similarly, differences in cheek tooth anatomy—although these function mainly in chewing —reflect differences in bite sizes. In particular, ruminant grazers have reduced premolars compared to ruminant browsers (Table 4.4: premolar:molar row length ratio), possibly enabling larger bite sizes (Janis and Constable 1993; Reed 1996; Codron et al. 2008b; Copeland et al. 2009; Lazagabaster et al. 2016). The emphasis of mastication is then shifted to the back of the mouth (see below). Amongst tall-grass ruminant grazers, however, the premolar row is not reduced in this way, probably because of the smaller bite sizes these species achieve while cropping (Codron et al. 2008b). Similarly, non-ruminants do not have reduced premolars, which has been hypothesized as reflecting a requirement for higher food intake with instantaneous ingestive mastication than in ruminant grazers (Janis and Constable 1993). We view the relatively large premolars of non-ruminant grazers and of tall-grass ruminant grazers as convergent, both not necessarily reflecting a greater intake, but smaller, more consistent bite sizes, and an evenness of the distribution of chewing emphasis throughout the front and back of the mouth. This would also explain why intake rates of horses did not vary across sward heights.
4.4 Oral Processing
4.4.1 Chewing
Mammalian herbivores , more than any other animal group, rely on chewing as a necessary process in preparing food for digestion (Reilly et al. 2001). The main purpose of extensive mastication is to reduce ingesta to smaller particle sizes, enabling passage through the gastrointestinal tract and, more importantly, increasing the total surface area for fermentation by microbiota in the gut. Therefore, reducing digesta particle size is a means for herbivores to reduce the need for long digesta retention times (Clauss et al. 2009d), a trade-off particularly evident when comparing non-chewing herbivorous reptiles that have large digesta particles and long retention times, with herbivorous mammals that chew their ingesta and have smaller digesta particles and shorter retention times (Fritz et al. 2010; Franz et al. 2011). Among mammals, ruminants achieve distinctively finer particles in the lower digestive tract than non-ruminants (Fritz et al. 2009a; Clauss et al. 2015). Although a first study with zoo animals indicated a more distinct particle size reduction in large ruminant grazers compared to browsers (Clauss et al. 2002), no similar difference was found between grazing and browsing rhinos (Steuer et al. 2010), and further studies with naturally feeding ruminant species did not reveal any difference in particle size between feeding types (Hummel et al. 2008; Lechner et al. 2010), indicating that ruminant browsers are only less efficient chewers on diets fed to them in captivity.
4.4.2 Cheek Teeth
Herbivores have evolved large, robust cheek teeth (premolars and molars) for chewing , and an extraordinary level of morphological diversity , across species and clades, is seen, even in early Eocene forms (Jernvall et al. 1996; and see Saarinen Chap. 2). Cheek tooth morphology has received perhaps the most research attention, in terms of traits that represent adaptations to various diet niches (Fortelius 1985; Janis 1988; Archer and Sanson 2002; Heywood 2010b; Kaiser et al. 2010).
One of the most common classifications of herbivore cheek tooth morphology is to separate species with low-crowned ‘brachydont ’ from those with high-crowned ‘hypsodont’ teeth. Hypsodonty, usually measured as the ratio of the enamel crown height:occlusal width ratio of the M3 (the hypsodonty index), is associated with wear-resistance—an adaptation to ensure that sufficient enamel remains in occlusion throughout the life of the animal (reviewed in Damuth and Janis 2011). Therefore, hypsodonty is expected to be higher in species that consume more abrasive foods, as well as species in which attrition (wear that occurs from tooth-on-tooth contact during occlusion) is higher, although the latter has not been measured empirically. A positive relationship between the hypsodonty index and percentage grass in the diet (Table 4.4), based on the presumption that grasses are more abrasive foods than browse, has been shown repeatedly (Cerling et al. 2003; Sponheimer et al. 2003; Codron et al. 2007; Clauss et al. 2008b; Hummel et al. 2011; Kaiser et al. 2013; Lazagabaster et al. 2016). The hypsodonty index is probably the most widely-used proxy for interpreting diet niches of fossil mammals (Janis 1995; Palmqvist et al. 2003; Cerling et al. 2005; Janis 2008; Damuth and Janis 2011; and see Saarinen Chap. 2); a correlation between changes in percentage grass in the diet (or inferred from grass availability) and hypsodonty, over geological time scales, has even been found within lineages (Feranec 2003; Strömberg 2006). The development of a high-crowned M3 itself need not be interpreted as a factor restricting the diet niche; in most datasets , many intermediate-feeding species have as high a hypsodonty index, or even higher, than many grazers (Janis 1988; Copeland et al. 2009). Thus, even if the evolution of hypsodonty evolved at faster rates amongst grazing species, the trait does not preclude browsing, and so a high molar crown is probably an attractive option for all herbivores, to facilitate a broader niche (Feranec 2007; Rivals et al. 2010; Damuth and Janis 2011).
One factor driving the association between the hypsodonty index and percentage grass intake is that plant silica bodies (phytoliths ), which are more abundant in grasses (Hodson et al. 2005), wear down tooth enamel. There has been discussion on whether phytoliths are harder than tooth enamel or not, and whether they can lead to tooth wear (for this reason, or irrespective of a hardness difference), or whether external abrasives (dust, grit) are more likely causative agents for cheek tooth wear (Baker et al. 1959; Mainland 2003; Sanson et al. 2007; Damuth and Janis 2011; Lucas et al. 2013; Erickson 2014; Rabenold and Pearson 2014; Xia et al. 2015). On the one hand, dental wear was caused in feeding experiments, in non-ruminants, by both diets with high phytolith and high external abrasives content (Müller et al. 2014, 2015). A similar result was produced in an in vitro study—using a chewing machine with horse teeth (Karme et al. 2016), and in an experiment with sheep—sand added to a hay diet led to changes in tooth microwear (Hoffman et al. 2015). On the other hand, other experimental findings suggest that external abrasives have less of an effect on the teeth of ruminants than expected, based on the findings in non-ruminants (Merceron et al. 2016; Ackermans et al. 2018). It has been suggested that ruminant digestive physiology reduces the effect of dust and grit on molar wear, because these external abrasives are probably washed off the plant material in the rumen before it is regurgitated for rumination. This hypothesis could explain why ruminants chew more cursorily during ingestion and more systematically during rumination (Dittmann et al. 2017), and why ruminant grazers are generally less hypsodont than non-ruminant grazers (Table 4.4), but this hypothesis awaits direct testing.
The higher hypsodonty index of grazers is interpreted as a response to i) ground-level feeding and ii) changing environmental conditions, with hypsodonty being greater in more arid and ‘dusty’ environments (Mendoza and Palmqvist 2008; Damuth and Janis 2011; but see results in Sanson and Read 2017 for lack of differences in buffalo Syncerus caffer tooth wear across substrates). As such, the hypsodonty index has been interpreted as a trait linked to species’ habitats, rather than diet niches per se (Mendoza and Palmqvist 2008; Kaiser et al. 2013), and it has been promoted as a proxy for resolving palaeoclimatic conditions (Eronen et al. 2009, 2010).
Only one study has demonstrated an empirical link between hypsodonty, percentage grass in the diet, and total levels of silica consumed in free-ranging conditions (Hummel et al. 2011), but that study did not distinguish between silica of exogenous versus endogenous origin. In their study of feeding height stratification amongst grazers, Codron et al. (2008b) showed that the hypsodonty index is highest amongst short-grass grazers, which also points to an exogenous factor (there is no reason, at this stage, to believe levels of internal abrasives differ between short and tall grasses).
Regardless of the relative strength of diet versus environmental effects, a higher hypsodonty index, because this metric is measured on the third molar, reflects a shift in chewing emphasis from homogeneously distributed throughout the mouth (in browsers and in most non-ruminants) to the back of the mouth (in grazers, especially ruminant grazers). Such a shift, along with a reduced premolar row in many ruminant grazers (see above), may help reduce torsional forces during mastication (Greaves 1991). Actually, in ruminants there is a negative correlation between the lower cheek tooth row length and the hypsodonty index (Fig. 4.2c), adding weight to the functional interpretation of increased M3 hypsodonty shifting chewing towards the posterior part of the tooth row. However, in tall-grass ruminant grazers (e.g., Hippotragini and Reduncini), such a shift in chewing strategy has not occurred, as their premolars are far more pronounced, and their M3 hypsodonty index is much lower, than short-grass grazers (Codron et al. 2008b). Whether this difference reflects a difference in diet niche as well requires further investigation; measures of total enamel volume, rather than the hypsodonty of a single tooth, should answer this question. Although enamel volume is related positively with percentage grass intake, as is mandibular molar row length, and, also, the combined length of all internal enamel structures (Table 4.4), no quantitative comparisons between presumed short- and tall-grass grazers have yet been made based on these traits.
Regardless of whether the majority of chewing is located at the back, or throughout, the mouth, differences in masticatory traits between browsers and grazers should also reflect differences in the fracture properties of these food types. Fracture properties of leaves are associated with patterns of leaf venation, with polygonal particles emanating from browse and elongate particles from grass (Kelly and Sinclair 1989; Sanson 1989; Nultsch 2000). Thus, it is not only the volume of enamel, but variations in its structural distribution that differs between browsers and grazers (Archer and Sanson 2002). In line with the more heterogeneous fracture properties of browse, a more homogeneous cusp wear pattern was found in grazing rhinos compared with browsing species (Taylor et al. 2013). On a broader taxonomic scale, the development of fused cusps in Bovidae (antelope and buffalo), and the absence of this trait in Cervidae (deer), was proposed as an explanation for the fact that a strictly grazing diet niche is comparatively rare amongst cervids (Heywood 2010a).
The positioning of enamel ridges along the cheek teeth of herbivores also appears to reflect diet niche differences, in that a greater proportion of occlusal enamel is aligned at acute angles to the direction of the chewing plane in grazers (Heywood 2010b; Kaiser et al. 2010; and see Table 4.4). One interpretation is that, although chewing strokes are generally transverse in all herbivores (Fortelius 1985), grazers have more anterior-posterior jaw movements during chewing than do browsers. In the latter movement pattern, the alignment of occlusal enamel ridges in grazers could represent compensation for the straighter alignment of grass blades between occlusal surfaces (Kaiser et al. 2010).
4.4.3 Musculature, Teeth and Chewing Intensity
Adaptations for fracturing highly resistant plant foods include not only large, robust teeth, but also powerful chewing muscles, of which the masseter is the most pronounced (Hendrichs 1965). Presumably as a response to the more fracture-resistant properties of grass as compared with browse (see Paine et al. 2018), the size (mass) of the masseter is positively related to percentage grass intake (Table 4.4; Clauss et al. 2008a). Skeletal features of the mandible itself also reflect this trend, including the size of the masseteric ridge and insertion areas, and the area dimensions of the mandibular lever (Table 4.4). The latter is seen as a direct indicator that the masseteric action in grazers entails a higher workload than browsers, as does the fact that grazers have a longer moment arm, measured as the mean angle of the master relative to the zygomatic arch (Varela and Fariña 2015).
The mechanical advantages of larger masseters, with greater areas of action, implies that bite forces of grazers are greater than those of browsers. Such adaptations operate alongside the differences in cheek tooth morphology described above. Correspondingly, there are positive correlations between masseter and cheek tooth variables (Figs. 4.2a and d; see also Fraser and Rybczynski 2014). Notably, these traits would not preclude animals that have them (typically, grazers) from a browsing niche, but do appear to limit the niches of browsers.
Comparative investigations of chewing intensity in herbivores have been guided mostly by hypothesized differences between non-ruminants and ruminants. Indeed, early observations on the size of masseters showed that this muscle accounted for substantially less of the total jaw musculature of ruminants than non-ruminants (Hendrichs 1965). Overall oral chewing intensity is expected to be greater amongst non-ruminants (Turnbull 1970; Fortelius 1985), because, in ruminants, much of the mechanical breakdown of food, into smaller particles, is achieved through chewing by rumination (Trudell-Moore and White 1983; McLeod and Minson 1988). Experimental data provided some evidence for a more regular chewing pattern during ingestion in horses than in cattle and camels (Dittmann et al. 2017), and also for a greater chewing intensity amongst horses than cattle—although the latter result could not be supported statistically, because of a small sample size (Janis et al. 2010). The studies of Janis et al. (2010), and of Fletcher et al. (2010), did, however, forward a hypothesis that the more complex (and robust) chewing apparatus of non-ruminants, compared to that of ruminants, is a function of higher workloads amongst non-ruminants. This concept is corroborated by the finding of a lower strain measured in goats during rumination as compared to ingestive mastication (Williams et al. 2011). Again, the fact that the majority of particle size-reducing chewing occurs in ruminants during rumination, presumably on material that has likely been washed free of dust/grit, as well as being somewhat ‘softened’ by its residence in the rumen (Janis et al. 2010; Mihlbachler et al. 2016; Dittmann et al. 2017), is part of this hypothesis. A comparison of the properties of swallowed ingesta in ruminants with material regurgitated for rumination is required to address these hypotheses. We can nevertheless provide corroborative evidence from the comparative approach for a difference in chewing workloads of non-ruminants vs. ruminants: the responses of craniodental traits like the hypsodonty index, cheek tooth volume, and molar row length, as well as the masseteric ridge length and ratio of the masseteric fossa to face length (negative relationship) to percent grass intake are all stronger (with steeper slopes) amongst non-ruminants than ruminants (Table 4.4). Hence, the different digestive strategies of non-ruminants and ruminants differ not only in terms of how an herbivore adapts to make a living out of feeding on low quality diets, but also in terms of solutions to processing foods of increasing toughness. Under this scenario, the entire forestomach complex of ruminants can be seen as a feature relaxing the selective pressure on their chewing apparatus (and chewing intensity). This view differs from a traditional outlook that sees rumination as an advantageous digestive strategy, against which non-ruminants can only be competitive with if they achieve higher intake levels and thereby extract more nutrients per day than ruminants (Janis 1976; Duncan et al. 1990). Rather, we propose that lower demands on oral chewing apparatus is one of the key advantages to being a ruminant, and may even be the factor giving ruminants a competitive edge over non-ruminants that, ultimately, resulted in the former replacing the latter as the most speciose terrestrial herbivores since the Oligocene (Janis et al. 1994; Janis 2008). Indeed, changes in diversity patterns of non-ruminants and ruminants in the fossil record are broadly co-incident with the replacement of browsers by intermediate-feeders and grazers through the Miocene (Janis et al. 2000), and subsequently higher rates of speciation amongst ruminant grazers (Janis 2008; Codron In review).
4.5 Digestion
4.5.1 General Digestive Tract Capacity and Digesta Retention
With a past focus on comparative digestive anatomy of browsers and grazers among ruminants, few generalized expectations exist that apply to all large mammal herbivores, i.e., to include non-ruminants. Because browse ferments at a faster rate than grass, we would expect generally longer mean retention times (MRT) of digesta particles and fluids in the digestive tract of grazers, and a corresponding larger gut capacity (Hummel et al. 2006). The available data, however, does not corroborate this concept across herbivores (see results for gut contents wet mass in Table 4.3, and MRT in Table 4.5). However, across herbivores, the MRT difference between small particles and fluids (called the selectivity factor), in the whole digestive tract, increases significantly with percentage grass intake (Table 4.5; and see Steuer et al. 2010). For the ruminant forestomach, however, the expected effects can be demonstrated: a more capacious reticulorumen in grazers (results for reticulorumen wet contents in Table 4.3, and rumen height in Table 4.6) with longer MRT of small particles (but not of fluids) at this site (Table 4.5), and a resulting distinct increase in the MRT difference between small particles and fluids (Table 4.5). The effect of body mass on measures of MRT is much lower than previously suggested (reviewed in Clauss et al. 2013), and is even absent in the case of fluid MRT in the reticulorumen (Table 4.5). As a result of the larger rumen capacities and longer particle MRT, ruminant grazers have been reported to digest fibre better than ruminant browsers (Pérez-Barbería et al. 2004). Corresponding analyses that include both ruminants and non-ruminants are lacking, but the effect has been demonstrated across rhino species (Steuer et al. 2010). The shorter particle MRT in the reticulorumen of browsing ruminants might explain why more material, with residual fermentability, reaches their large intestine, leading to higher concentrations of volatile fatty acids at this site (Table 4.6).
These results emphasize the difference between particle and fluid MRT for herbivores. It has been hypothesized that a differential movement of fluid and particles leads to a ‘washing’ of the digesta with a removal of a part of the microbiota (Müller et al. 2011). In foregut fermenters , including ruminants, this should theoretically lead to a higher inflow of microbes into the glandular stomach and small intestine (Dittmann et al. 2015; Hummel et al. 2015). This advantage has no relevance in hindgut fermenters , which might be the reason why the characteristic is generally more pronounced in ruminants (interaction effects for MRT in Table 4.5). For any herbivore (ruminant and non-ruminant), however, removal of microbes by ‘washing’ is bound to shift the metabolism of the microbiota from maintenance towards growth, putatively increasing their fermentative efficiency, and shifting fermentative processes from the production of CO2 and methane towards microbial cell mass (reviewed in Clauss and Hummel 2017). Why such an option, which is in theory favourable for any kind of herbivore, may be not available to some browsers, is explained in the next section.
4.5.2 Ruminant Forestomach Morphophysiology
For a historical overview of the development of interpretations of observed differences in forestomach morphophysiology between ruminant browsers and grazers see Clauss and Hummel (2017). At first, differences were mainly linked to putative differences in fibre content between browse and grass (Hofmann 1989), but data on the crude fibre concentration of rumen contents do not support this interpretation (Table 4.6). Similarly, no difference in the pH, or the volatile fatty acid concentration, in the rumen contents are evident between browsers and grazers (Table 4.6). One of the components of the primary concept of rumen physiology had focused on the reticular groove—the anatomical structure facilitating the bypass of milk in suckling ruminants, and thus preventing it from entering the rumen. This structure was thought to remain functional in adult browsers (Rowell-Schäfer et al. 2001). Limited experimental data, however, did not corroborate this concept (Lechner et al. 2009).
In a second step, the focus shifted to another original observation by Hofmann (1989), namely a difference in the stratification of rumen contents between browsers and grazers (Clauss et al. 2003b), which was introduced by Hofmann et al. (2008), and in the predecessor to the current Chapter (Clauss et al. 2008b). A series of studies demonstrated this difference, using as proxies, either directly a difference in the dry matter concentration (the reciprocal of the moisture content) between dorsal (upper) and ventral (lower) rumen contents (with a higher difference in grazers; Table 4.6; and see Clauss et al. 2009b), a difference in the presence of a gas dome in the dorsal rumen (Tschuor and Clauss 2008), or indirectly the intraruminal papillation pattern (because stratified rumen contents should be linked to an inhomogenous papillation in the rumen; Clauss et al. 2009c, corresponding to a less homogenous papillation in grazers; Table 4.6). It was assumed that a higher fluid input in, and throughput through, the rumen leads to a more pronounced stratification in grazers. The correlations between the different stratification proxies (Fig. 4.3a–c) support this interpretation. Thus, a major difference between browsers and grazers is the ratio of small particle to fluid MRT in the reticulorumen in the latter (indicating a faster fluid turnover), and this ratio significantly increases with percentage grass in the diet (Table 4.5). In browsers, less fluid is put through the reticulorumen, and the rumen fluid is more viscous (Clauss et al. 2009a, b; Lechner et al. 2010), putatively trapping some of the fermentation gases, and resulting in less stratified rumen contents and an even intraruminal papillation . In grazers, the clear stratification comprises a dorsal gas dome, and a fibre ‘raft’ or ‘mat’ on top of a liquid layer (Hummel et al. 2009).
Correlations between three proxies for rumen stratification (a–c)—the difference in dry matter (DM) of dorsal and ventral rumen contents, the proportion of surface papilla in the atrium relative to the dorsal rumen (SEF), and the selectivity factor (SF) of the reticulorumen contents (i.e., the ratio of particle:solute retention time). Coping with differences in fluid throughput also results in a correlation between parotid gland mass (expressed relative to BM0.75) and omasum size (OLSA = omasal leaf surface area, expressed relative BM0.67) (d)
Focusing on the stratification creates a logically coherent interpretation of different observations. Significantly larger salivary glands in browsers than in grazers (Table 4.7; and see Hofmann et al. 2008), are no longer equated with a higher saliva output; larger glands are considered to produce saliva with a higher protein content (and hence viscosity), the proteins representing a defence against plant secondary metabolites, in particular against tannins (Austin et al. 1989; Hagermann and Robbins 1993; Fickel et al. 1998). The necessity to add these proteins to the saliva is considered a constraint on the amount of saliva that can be produced, and, therefore, smaller salivary glands—without the need to produce these proteins—are equated with higher amounts of saliva (Hofmann et al. 2008). The significantly thicker rumen muscles (the pillars) in grazers (Table 4.6) are considered an adaptation to the fibre ‘mat’ that putatively requires more force for mixing peristalsis than do homogenous rumen contents (Clauss et al. 2003b). Similarly, the larger intraruminal orifice in grazers (Table 4.6) may be better suited to allow a mixing of the fibre ‘mat’, which might tend to block a more narrow opening more easily. The significantly lower reticular crests in browsers (Table 4.6) are considered to lead to an incomplete emptying of the reticulum during contractions (Clauss et al. 2010a), which may be important because with the putatively more viscous rumen fluid and lower moisture saturation in the rumen of browsers, re-filling of the reticulum with fluid might be slower. Fluid reticulum contents are a prerequisite of the ruminant particle sorting mechanism, which operates on particle buoyancy and sedimentation (Lechner-Doll et al. 1991). Finally, grazers, with the higher fluid throughput through the reticulorumen, require larger omasa (Table 4.6)—with the main function of omasa being the resorption of fluid, to prevent too diluted digesta reaching the sites of auto-enzymatic digestion (Clauss et al. 2006). Therefore, across ruminants, we would expect a negative relationship between salivary gland size (with smaller glands producing higher amounts of salivary fluid) and omasum size (with larger omasa absorbing more of the fluid), as indicated in Fig. 4.3d. A thicker acid-producing fundic mucosa of the abomasum of browsers (Table 4.6; note that this is only the case in GLS but not in PGLS, and in contrast to the pyloric mucosa) is interpreted as an adaptation to putatively higher bicarbonate contents in the rumen fluid of browsers, due to the higher viscosity and CO2 entrapment, which might require higher amounts of acid for acidification (Clauss et al. 2008b). No functional relevance, linked to the fluid throughput and viscosity, is attributed to the ruminoreticular orifice, or the reticuloomasal orifice, which both do not differ between browsers and grazers (Table 4.6); these openings do not have to accommodate the fibre ‘mat’ but pass on only a selection of rumen contents (ruminoreticular orifice), or those particles intended for passage into the lower digestive tract (reticuloomasal orifice). Neither the size of the reticulum nor that of the abomasum differ between the feeding types (Table 4.6), and are not linked to the concept of fluid throughput.
Thus, characteristics of grazers were interpreted as enhancing the stratification of the rumen contents (Clauss et al. 2008b), reinforcing a difference that was thought to exist between browse (with little propensity to stratify) and grass, based on in vitro experiments (Sutherland 1988; Wattiaux et al. 1992; Clauss et al. 2001). Note that this interpretation is still focused on a general difference between the diets, which is putatively amplified by the morphophysiological characteristics. The hypothesized adaptive value was supposed to lie in an enhanced ‘filter bed effect’, i.e., the entrapment of smaller particles in the fibre ‘mat’, with a corresponding longer particle retention and digestion, which appeared suitable for grass forage; additionally, it was thought that the greater amount of low-viscosity fluid, with a pre-sorting of particles already in the rumen, would facilitate a more efficient particle sorting mechanism (that should also lead to more intensive rumination in grazers; Clauss et al. 2003b). The, presumably, larger particles escaping the forestomach of browsers (Clauss et al. 2002) were considered an indication for a less efficient sorting mechanism, and the larger papillae unguiculiformes—papillae around the reticuloomasal orifice—in browsers (Table 4.6) were interpreted as a compensatory straining mechanism that partially prevents the escape of large particles (Nygren et al. 2001).
However, while the basis of fluid throughput as the main determinant of these morphophysiological interrelationships remains unchallenged, a revision of the interpretation of its adaptive value became necessary. During an experimental study, with fistulated non-domestic ruminants , in which differences in rumen contents stratification, rumen fluid viscosity and the ratio of particle to fluid MRT in the reticulorumen were demonstrated, no difference in the sorting mechanism (of large vs. small particles), or the size of particles escaping the forestomach, were evident, suggesting that these factors were not affected by adaptations related to fluid throughput and stratification (Lechner et al. 2010). The previously documented differences in particle size were related to the use of artificial diets in captivity (Hummel et al. 2008; Lechner et al. 2010), and the previous assumption that browse does not tend to stratify by particle size in in vitro systems was corrected (Clauss et al. 2009b; Lechner et al. 2010). Additionally, a ‘filter-bed’ effect could be detected on grass vs. browse diets, irrespective of the feeding type of the animals (Clauss et al. 2011a; Lauper et al. 2013), and no general difference in the time spent in rumination appears to exist between browsers and grazers (Table 4.5).
Therefore, the interpretation of the adaptive value of the various characteristics underwent another revision, and is currently focused on the relevance of the high fluid throughput itself. In order to avoid semantic circular reasoning, two digestive strategies in ruminants were defined (the ‘moose-type’ and the ‘cattle-type’), that can then be compared to the feeding types of browsers and grazers (Clauss et al. 2010b). As stated above, a high fluid throughput most likely increases the harvest of microbes from the forestomach and, thus, makes this system more efficient, and should, therefore, be an adaptation in any ruminant, irrespective of feeding type (Clauss et al. 2010b; Clauss and Hummel 2017). Note that now, corresponding adaptations are not linked to a diet property, but to a putative optimization of the digestive tract itself. While any diet, that does not comprise extreme amounts of browse, appears to allow such adaptations, strict browsers, due to their salivary defence mechanisms, cannot use the advantages of a high fluid throughput (Codron and Clauss 2010). Correspondingly, while ‘moose-type’ ruminants appear to be constrained to pure browse diets (and are browsers), ‘cattle-type’ ruminants have a much broader diet spectrum. In particular, this new concept explains the lack of ‘escalation’ of morphophysiological adaptations from intermediate-feeding to grazing, i.e., many traits considered typical for grazers, including the selectivity factor, do not become more pronounced from intermediate-feeders to grazers (see also discussion on hypsodonty index above). Indeed, many of those species that show particularly extreme ‘cattle-type’ characteristics, such as the Bovini or the muskox (Ovibos moschatus), with a very high fluid throughput, a distinct rumen contents stratification, and intraruminal papillation pattern, and extremely large omasa, are not the strictest grazers, but often intermediate-feeders (Clauss and Hofmann 2014). The reasons for the differences within ‘cattle-type’ ruminants, as in the various characteristics linked to fluid throughput, are not considered direct reflections of a diet niche in this scenario, but expressions of different degrees of ‘escalation’ (sensu Vermeij 1994, 2013), during the evolutionary optimization of their digestive physiology .
4.5.3 Organ Size
The original suggestion that liver size is related to diet, with browsers putatively requiring larger livers for the detoxification of secondary plant compounds (Hofmann 1989; Duncan et al. 1998), was detectable in GLS, but not confirmed in PGLS in the current dataset, also not for ruminants (Table 4.7, interaction). However, the finding that among ruminants, the proportion of connective (i.e., non-functional) liver tissue increased significantly with percentage grass in the diet (Table 4.7), supports the concept that browsers require more functional liver tissue. The masses of the heart, kidney or lung show no relationship with diet (Table 4.7; even though it could be expected that some reduction in organ size compensates for larger rumens of grazers; Clauss et al. 2003a). The general tendencies for a lower organ size increase in ruminants compared to non-ruminants (Table 4.7, interaction) might correspond to such a concept of organ reduction in ruminants, in compensation for large forestomachs (Mortolaa and Lanthier 2005). However, more detailed data would be required to corroborate these findings.
4.6 Conclusions: Where to from Here?
The morphological and physiological traits of herbivores reflect different constraints imposed by browse- versus grass-based diet niches . The most prominent complex is that of putatively diet-specific adaptations in orocranial (and especially dental) anatomy. Morphophysiological characteristics of the digestive tract also show significant correlations with diet, even after accounting for the phylogenetic structure of datasets. Clearly, large mammalian herbivores remain an important model for our understanding of key macroevolutionary concepts such as adaptive radiation, and convergence.
An aspect of digestive physiology that has received comparatively little attention so far, in spite of an enormous increase in the availability of methodological approaches in recent years, is the bacterial, archaeal, fungal and protozoan microbiome of herbivores. Available data (e.g., wild ruminants; Henderson et al. 2015) has not yet been evaluated, either with respect to feeding type (browser vs grazer) or digestion type (‘moose-type’ vs ‘cattle-type’); our own lack of methodological expertise prevented us from exploring those data here. Given that the microbiota may be involved in detoxification or defence processes, more studies would be highly interesting. To our knowledge, the only systematic comparison, with respect to the microbiome, is restricted to literature data (gained from traditional microscopic analyses) of the composition of the protozoal microbiome (Clauss et al. 2011b) in ruminants of different dietary niches . The case of rumen protozoa can serve as an illustration of why a detailed look at the data, and at the conceptual design of studies, is necessary to prevent fallacies and meaningless findings in the following paragraphs.
A subjective, put potentially widely shared impression, when reading literature about browsers and grazers, is that the categorisation of a species into a feeding type is often presented as an aim in itself, as if it had relevant heuristic value. Therefore, the impression is that studies involving morphophysiological measurements are often justified by their use of proxies to facilitate that categorisation. To single out—admittedly unfairly—a particular study as an example, it appears conceptually awkward to attempt to categorize a ruminant species, such as the mouflon (Ovis ammon musimon), in the feeding type spectrum by analysing its rumen protozoa as a proxy (Obidziński et al. 2017). When dealing with extant species, this approach has the evident flaw that the real proxy for a feeding type is the botanical investigation of the diet consumed; in the case of mouflon, stating that they are intermediate-feeders based on a review of their natural diet (Marchand et al. 2013) is the superior conceptual approach. The additional heuristic value of investigating their protozoal microbiome lies in revealing effects of differences in the actually consumed diet on that microbiome (as done very elegantly by Obidziński et al. 2017).
When dealing with extinct species, proxy-based approaches are the only ones available and very common, particularly for dental anatomical characters and dental wear proxies, often supplemented by stable isotope measurements (we refrain from reviewing that literature, but see Saarinen Chap. 2 for more detail). For dental anatomy and isotope values, such reconstructions often under-emphasize the degree of uncertainty linked to these proxies (for example, as mentioned above, that many extant ruminant intermediate-feeders have a higher hypsodonty index than strict grazers, and that the degree of wear experienced by herbivores differs between ruminants and non-ruminants), and the use of wear-related proxies is mostly not based on experimentally derived results.
The actually observed pattern of the distribution of a character, across feeding types, is highly relevant for a functional understanding of digestive morphology and physiology . For example, differences in the ratio of small particle to fluid MRT in the reticulorumen (selectivity factor, Table 4.5; Dittmann et al. 2015), and in the composition of rumen protozoa (Clauss et al. 2011b), could be correctly summarized by the statement “in ruminants, browsers and grazers differ in characteristics of rumen fluid throughput and rumen protozoa”. However, such a superficial view omits the fact that the seeming thresholds of change for the respective characteristic differs dramatically between the two datasets (Fig. 4.4a and b): while the selectivity factor increases dramatically at a threshold of 15–20% grass in the natural diet (as does the intraruminal papillation pattern; Codron and Clauss 2010), the proportion of Entodiniinae to Diplodiniinae protozoa changes at a threshold of about 80% grass in the natural diet. Consequently, the two measurements—the selectivity factor and the relative proportion of Entodiniinae—do not correlate well with each other (Fig. 4.4c). This closer look informs us that different mechanisms may be at play in these two complexes; at the moment we do not have a detailed understanding of these processes.
Discordance in how morphophysiological traits respond to diet. The ratio of particle:solute mean retention time in the reticulorumen (called SF RR) increases dramatically in species with >15–20% grass in the natural diet (a), whereas the proportion of gut protozoa represented by the Entodiniinae decreases dramatically at around 80% grass in the natural diet (b; note that the proportion of Diplodiniinae increases at more or less the same rate at this diet). Consequently, the two traits are not correlated (c)
Another important aspect of comparative analyses is that statistical support for a link between a species’ traits and their dietary niches should not lead to rigid views of exclusive solutions. Nor, on the other hand, should such links be interpreted to exclude species from certain niches (Codron and Clauss 2010). Rather, taxon-specific peculiarities need to be acknowledged that may deviate from general trends and might, depending on the number of corresponding species present in a dataset, allow, or prevent, the detection of these trends. For example, differences exist in the method of cropping plant material, whereby some species (like goats) use their lips, while others (like cattle) use their tongue more extensively (Meier et al. 2016). This does not invalidate the statistical finding that, across a certain dataset, ruminant browsers differ in tongue morphology from grazers in having higher proportions of a “freely mobile” part of their tongue. However, the outlier of cattle in this respect precludes the opposite conclusion, that species with a highly mobile tongue must be browsers, and also precludes the interpretation that grazers cannot have mobile tongues. Additionally, it highlights that conclusions might change, depending on the nature of the dataset: in a hypothetical dataset with mainly bovine grazers and only goat-type browsers, the analysis might have found a more mobile tongue in grazers than in browsers.
Other important examples are that, amongst grazers, some species have extremely wide muzzles (grazing rhinos, hippos, Alcelaphini), whereas others (Equidae, Hippotragini) have more pointed faces. Determining whether these differences reflect different dietary niches , for instance foraging at different sward heights, or merely different solutions to the same problem, requires taxon-specific hypotheses and, ideally, experimentation. The existence of outliers, for instance blackbuck (Antilope cervicapra), which have a high particle to fluid MRT ratio in the reticulorumen (i.e., a high fluid thoughput; Hummel et al. 2015), but a very small omasum (Sauer et al. 2016), emphasizes that general trends are not obligatory. Amongst ruminant browsers, cervids and giraffids are typically better represented in datasets than are bovids, and hence might unduly influence current interpretations. In particular, comparative physiological data for Tragelaphini are lacking in many datasets, but may represent an alternate solution to browsing from giraffids and cervids, as indicated by their deviation from the typical salivary gland size-diet relationship observed across ruminants (Robbins et al. 1995; Hofmann et al. 2008).
Apart from the self-evident request for more data on more species, and for additional corroboration of once-only measurements that are then analysed again and again with new statistical approaches, we believe that, in order to interrogate the question of exclusivity in trait-niche relationships, our description of diet niches themselves needs to shift from one based on species’ means to a more inclusive approach depicting niche breadths. One such approach considers whether morphophysiological characteristics are more closely aligned with minimum or maximum levels of grass in species’ natural diets (Codron and Clauss 2010), potentially allowing us to define thresholds around which species may or may not be excluded from certain niches. For example, there appears to be no continuous relationship between the amount of browse/grass in the diet and the digestion type (‘moose-type’ vs ‘cattle-type’) amongst ruminants, but rather a dichotomy between species that do, and do not, eat grass. Similarly, the hypsodonty index is not continuously distributed along a percentage grass in the diet axis; rather, species that lack sufficiently large tooth crowns typically do not eat grass, whereas species that have large tooth crowns may occupy almost any diet niche (Damuth and Janis 2011). These statements echo the one by Van Wieren (1996b) that “browsers are non-grazers”. Broadening our representation of niches, to include ‘niche breadths’, would allow investigations not only of exclusiveness in adaptation, but also potentially raises important questions that could lead to a more complete functional interpretation. A potentially interesting addition to this niche breadth approach is to include the niche history of species, i.e., a measure of how long the species has existed in a given dietary niche space. Examples do exist of diet niche shifts within lineages, sometimes coupled with evidence for morphological shifts (Strömberg 2006; Feranec 2007; Codron et al. 2008a; Cerling et al. 2015; Ecker et al. 2018). These studies differ with respect to whether dietary niches were interpreted to have become more specialized or generalized over evolutionary time, further emphasizing a lack of dietary niche exclusivity.
What is also lacking from many discussions, and a topic we have only sporadically addressed here, is the covariance amongst traits. For the most part, investigations of multiple traits have attempted only to define species’ distributions in multivariate morphospace. We expect, however, that covariance emanates because certain traits/organ systems have evolved as functional units, or as anatomical trade-offs. The relative size of salivary glands is an important example of this; only when considered together with the size of the omasum does the functional relevance of variations in both organ systems become clear. Thus, correlations between traits should be studied, and interpreted, in terms of both the overall bauplan of species, and the way in which components interact to make the organism more efficient at exploiting a particular niche. In doing so, we must be open to the outcome that what we considered an adaptation to a particular diet, or a particular characteristic of a diet, might ultimately be an adaptation to a different characteristic of the same diet, or an adaptation to a nondietary selective pressure that only correlated with the dietary niche .
References
Ackermans NL et al (2018) Controlled feeding experiments with diets of different abrasiveness reveal slow development of mesowear signal in goats (Capra aegagrus hircus). J Exp Biol 221:186411
Aitchison J (1946) Hinged teeth in mammals: a study of the tusks of muntjacs (Muntiacus) and Chinese water deer (Hydropotes inermis). Proc Zool Soc Lond 116:329–338
Archer D, Sanson G (2002) Form and function of the selenodont molar in southern African ruminants in relation to their feeding habits. J Zool 257:13–26. https://doi.org/10.1017/S0952836902000614
Austin PJ, Suchar LA, Robbins CT, Hagermann AE (1989) Tannin-binding proteins in saliva of deer and their absence in saliva of sheep and cattle. J Chem Ecol 15:1335–1347
Avgar T, Street G, Fryxell JM (2014) On the adaptive benefits of mammal migration. Can J Zool 92:481–490. https://doi.org/10.1139/cjz-2013-0076
Baker G, Jones LHP, Wardrop ID (1959) Cause of wear in sheep’s teeth. Nature 184:1583–1584
Bell RHV (1971) A grazing ecosystem in the Serengeti. Sci Am 225:86–93
Benvenutti MA, Gordon IJ, Poppi DP (2006) The effect of the density and physical properties of grass stems on the foraging behaviour and instantaneous intake rate by cattle grazing an artificial reproductive tropical sward. Grass Forage Sci 61:272–281. https://doi.org/10.1111/j.1365-2494.2006.00531.x
Brashares JS, Garland T, Arcese P (2000) Phylogenetic analysis of coadaptation in behavior, diet, and body size in the African antelope. Behav Ecol 11:452–463
Burnham KP, Anderson DR (2001) Kullback-Leibler information as a basis for strong inference in ecological studies. Wildl Res 28:111–119
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cerling TE, Harris JM, Passey BH (2003) Diets of east African Bovidae based on stable isotope analysis. J Mammal 84:456–470
Cerling TE, Harris JM, Leakey MG (2005) Environmentally driven dietary adaptations in African mammals. In: Ehleringer JR, Cerling TE, Dearing MD (eds) A history of atmospheric CO2 and its effects on plants, animals and ecosystems. Springer, New York, pp 258–272
Cerling TE et al (2015) Dietary changes of large herbivores in the Turkana Basin, Kenya from 4 to 1 ma. Proc Natl Acad Sci U S A 112:11467–11472. https://doi.org/10.1073/pnas.1513075112
Chivers DJ, Hladik CM (1980) Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J Morphol 166:337–386
Clauss M, Hofmann RR (2014) The digestive system of ruminants, and peculiarities of (wild) cattle. In: Melletti M, Burton J (eds) Ecology, evolution and behaviour of wild cattle: implications for conservation. Cambridge University Press, Cambridge, pp 57–62
Clauss M, Hummel J (2017) Physiological adaptations of ruminants and their potential relevance for production systems. Rev Bras Zootec 46:606–613
Clauss M, Lechner-Doll M (2001) Differences in selective reticulo-ruminal particle retention as a key factor in ruminant diversification. Oecologia 129:321–327
Clauss M, Lechner-Doll M, Behrend A, Lason K, Lang D, Streich WJ (2001) Particle retention in the forestomach of a browsing ruminant, the roe deer (Capreolus capreolus). Acta Theriol 46:103–107
Clauss M, Lechner-Doll M, Streich WJ (2002) Faecal particle size distribution in captive wild ruminants: an approach to the browser/grazer-dichotomy from the other end. Oecologia 131:343–349
Clauss M et al (2003a) The maximum attainable body size of herbivorous mammals: morphophysiological constraints on foregut, and adaptations of hindgut fermenters. Oecologia 136:14–27
Clauss M, Lechner-Doll M, Streich WJ (2003b) Ruminant diversification as an adaptation to the physicomechanical characteristics of forage. A reevaluation of an old debate and a new hypothesis. Oikos 102:253–262
Clauss M et al (2006) The macroscopic anatomy of the omasum of free-ranging moose (Alces alces) and muskoxen (Ovibos moschatus) and a comparison of the omasal laminal surface area in 34 ruminant species. J Zool 270:346–358
Clauss M, Hofmann RR, Streich WJ, Fickel J, Hummel J (2008a) Higher masseter muscle mass in grazing than in browsing ruminants. Oecologia 157:377–385
Clauss M, Kaiser TM, Hummel J (2008b) The morphophysiological adaptations of browsing and grazing mammals. In: Gordon IJ, Prins HHT (eds) The ecology of browsing and grazing. Springer, Heidelberg, pp 149–178
Clauss M et al (2009a) Physical characteristics of rumen contents in two small ruminants of different feeding type, the mouflon (Ovis ammon musimon) and the roe deer (Capreolus capreolus). Zoology 112:195–205
Clauss M et al (2009b) Physical characteristics of rumen contents in four large ruminants of different feeding type, the addax (Addax nasomaculatus), bison (Bison bison), red deer (Cervus elaphus) and moose (Alces alces). Comp Biochem Physiol A Mol Integr Physiol 152:398–406
Clauss M, Hofmann RR, Fickel J, Streich WJ, Hummel J (2009c) The intraruminal papillation gradient in wild ruminants of different feeding types: implications for rumen physiology. J Morphol 270:929–942
Clauss M, Nunn C, Fritz J, Hummel J (2009d) Evidence for a tradeoff between retention time and chewing efficiency in large mammalian herbivores. Comp Biochem Physiol A Mol Integr Physiol 154:376–382
Clauss M, Hofmann RR, Streich WJ, Fickel J, Hummel J (2010a) Convergence in the macroscopic anatomy of the reticulum in wild ruminant species of different feeding types and a new resulting hypothesis on reticular function. J Zool 281:26–38
Clauss M, Hume ID, Hummel J (2010b) Evolutionary adaptations of ruminants and their potential relevance for modern production systems. Animal 4:979–992
Clauss M et al (2011a) The effect of size and density on the mean retention time of particles in the reticulorumen of cattle (Bos primigenius f. taurus), muskoxen (Ovibos moschatus) and moose (Alces alces). Br J Nutr 105:634–644
Clauss M, Müller K, Fickel J, Streich WJ, Hatt J-M, Südekum K-H (2011b) Macroecology of the host determines microecology of endobionts: protozoal faunas vary with wild ruminant feeding type and body mass. J Zool 283:169–185
Clauss M, Steuer P, Müller DWH, Codron D, Hummel J (2013) Herbivory and body size: allometries of diet quality and gastrointestinal physiology, and implications for herbivore ecology and dinosaur gigantism. PLoS One 8:e68714
Clauss M et al (2015) Faecal particle size: digestive physiology meets herbivore diversity. Comp Biochem Physiol A Mol Integr Physiol 179:182–191
Clauss M et al (2017) Reconstruction of body cavity volume in terrestrial tetrapods. J Anat (Lond) 230:325–336
Codron D (In review) Evolution of large mammal herbivores. In: Scogings P (ed) Herbivores and woody plants in savannas. Wiley
Codron D, Clauss M (2010) Rumen physiology constrains diet niche: linking digestive physiology and food selection across wild ruminant species. Can J Zool 88:1129–1138
Codron J, Lee-Thorp JA, Sponheimer M, Codron D, Grant RC, De Ruiter DJ (2006) Elephant (Loxodonta africana) diets in Kruger National Park, South Africa: spatial and landscape differences. J Mammal 87:27–34
Codron D, Lee-Thorp JA, Sponheimer M, Codron J, de Ruiter D, Brink JS (2007) Significance of diet type and diet quality for ecological diversity of African ungulates. J Anim Ecol 76:526–537
Codron D, Brink JS, Rossouw L, Clauss M (2008a) The evolution of ecological specialization in southern African ungulates: competition or physical environmental turnover? Oikos 117:344–353
Codron D et al (2008b) Functional differentiation of African grazing ruminants: an example of specialized adaptations to very small changes in diet. Biol J Linn Soc 94:755–764
Codron D, Codron J, Sponheimer M, Clauss M (2016) Within-population isotopic niche variability in savanna mammals: disparity between carnivores and herbivores. Front Ecol Evol 4:15. https://doi.org/10.3389/fevo.2016.00015
Copeland SR, Sponheimer M, Spinage CA, Lee-Thorp JA, Codron D, Reed KE (2009) Stable isotope evidence for impala Aepyceros melampus diets at Akagera National Park. Rwanda Afr J Ecol 47:490–501
Damuth J, Janis CM (2011) On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol Rev (Camb) 86:733–758
Demment MW, Longhurst WH (1987) Browsers and grazers: constraints on feeding ecology imposd by gut morphology and body size. Proceedings of the IVth international conference on goats. Brazilia, Brazil, pp 989–1004
Dittmann MT et al (2015) Digesta kinetics in gazelles in comparison to other ruminants: evidence for taxon-specific rumen fluid throughput to adjust digesta washing to the natural diet. Comp Biochem Physiol A Mol Integr Physiol 185:58–68. https://doi.org/10.1016/j.cbpa.2015.01.013
Dittmann MT, Kreuzer M, Runge U, Clauss M (2017) Ingestive mastication in horses resembles rumination but not ingestive mastication in cattle and camels. J Exp Zool A Ecol Integr Physiol 327:98–109
du Toit JT (1990) Feeding-height stratification among African browsing ruminants. Afr J Ecol 28:55–61
Duncan P, Foose TJ, Gordon IJ, Gakahu CG, Lloyd M (1990) Comparative nutrient extraction from forages by grazing bovids and equids: a test of the nutritional model of equid/bovid competition and coexistence. Oecologia 84:411–418
Duncan P, Tixier H, Hofmann RR, Lechner-Doll M (1998) Feeding strategies and the physiology of digestion in roe deer. In: Andersen R, Duncan P, Linell JDC (eds) The European roe deer: the biology of success. Scandinavian University Press, Oslo, pp 91–116
Ecker M, Brink JS, Rossouw L, Chazan M, Horwitz LK, Lee-Thorp JA (2018) The palaeoecological context of the Oldowan-Acheulean in southern Africa. Nat Ecol Evol. https://doi.org/10.1038/s41559-018-0560-0
Erickson KL (2014) Prairie grass phytolith hardness and the evolution of ungulate hypsodonty. Hist Biol 26:737–744
Eronen JT, Evans AR, Fortelius M, Jernvall J (2009) The impact of regional climate on the evolution of mammals: a case study using fossil horses. Evolution 64:398–408
Eronen JT et al (2010) Precipitation and large herbivorous mammals II: application to fossil data. Evol Ecol Res 12:235–248
Feranec RS (2003) Stable isotopes, hypsodonty, and the paleodiet of Hemiauchenia (Mammalia: Camelidae): a morphological specialization creating ecological generalization. Paleobiol 29:230–242
Feranec RS (2007) Ecological generalization during adaptive radiation: evidence from Neogene mammals. Evol Ecol Res 9:555–577
Fickel J, Göritz F, Joest BA, Hildebrandt T, Hofmann RR, Breves G (1998) Analysis of parotid and mixed saliva in roe deer (Capreolus capreolus L.). J Comp Physiol B 168:257–264
Fletcher TM, Janis CM, Rayfield EJ (2010) Finite element analysis of ungulate jaws: can mode of digestive physiology be determined? Palaeontol Electron 13:21A
Fleurance G, Fritz H, Duncan P, Gordon IJ, Edouard N, Vial C (2009) Instantaneous intake rate in horses of different body sizes: influence of sward biomass and fibrousness. Appl Anim Behav Sci 117:84–92
Fortelius M (1985) Ungulate cheek teeth: developmental, functional and evolutionary interrelations. Acta Zool Fenn 180:1–76
Fortelius M, Solounias N (2000) Functional characterization of ungulate molars using the abrasion-attrition wear gradient: a new method for reconstructing paleodiets. Am Mus Novit 3301:1–36
Fowler ME (1983) Plant poisoning in free-living wild animals: a review. J Wildl Dis 19:34–43
Franz R, Hummel J, Müller DWH, Bauert M, Hatt J-M, Clauss M (2011) Herbivorous reptiles and body mass: effects on food intake, digesta retention, digestibility and gut capacity, and a comparison with mammals. Comp Biochem Physiol A Mol Integr Physiol 158:94–101
Fraser D, Rybczynski N (2014) Complexity of ruminant masticatory evolution. J Morphol 275:1093–1102
Fraser D, Theodor JM (2011) Comparing ungulate dietary proxies using discriminant function analysis. J Morphol 272:1513–1526. https://doi.org/10.1002/jmor.11001
Fritz J, Hummel J, Kienzle E, Arnold C, Nunn C, Clauss M (2009a) Comparative chewing efficiency in mammalian herbivores. Oikos 118:1623–1632
Fritz SA, Bininda-Emonds ORP, Purvis A (2009b) Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol Lett 12:538–549
Fritz J, Hummel J, Keinzle E, Streich WJ, Clauss M (2010) To chew or not to chew: faecal particle size in herbivorous reptiles and mammals. J Exp Zool A Ecol Integr Physiol 313:579–586
Garland TJ, Bennett AF, Rezende EL (2005) Phylogenetic approaches in comparative physiology. J Exp Biol 218:3015–3035
Gordon IJ (2003) Browsing and grazing ruminants: are they different beasts? For Ecol Manag 181:13–21
Gordon IJ, Illius AW (1988) Incisor arcade structure and diet selection in ruminants. Funct Ecol 2:15–22
Gordon IJ, Illius AW (1994) The functional significance of the browser-grazer dichotomy in African ruminants. Oecologia 98:167–175
Gordon IJ, Illius AW (1996) The nutritional ecology of African ruminants: a reinterpretation. J Anim Ecol 65:18–28
Greaves W (1991) A relationship between premolar loss and jaw elongation in selenodont artiodactyls. Zool J Linnean Soc 101:121–129
Gross JE, Hobbs NT, Wunder BA (1993) Independent variables for predicting intake rate of mammalian herbivores: biomass density, plant density, or bite size? Oikos 68:75–81
Gwynne MD, Bell RHV (1968) Selection of vegetation components by grazing ungulates in the Serengeti National Park. Nature 220:390. https://doi.org/10.1038/220390a0
Hagermann AE, Robbins CT (1993) Specifity of tannin-binding salivary proteins relative to diet selection by mammals. Can J Zool 71:628–633
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Hemae KM (1967) Masticatory function in the mammals. J Dent Res 46:883–893
Henderson G et al (2015) Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep 5:14567
Hendrichs H (1965) Vergleichende Untersuchung des Wiederkauverhaltens. Biol Zentralbl 84:681–751
Herring SW (1985) Morphological correlates of masticatory patterns in peccaries and pigs. J Mammal 66:603–617
Heywood JJN (2010a) Explaining patterns in modern ruminant diversity: contingency or constraint? Biol J Linn Soc 99:657–672
Heywood JJN (2010b) Functional anatomy of bovid upper molar occlusal surfaces with respect to diet. J Zool 281:1–11
Hodson MJ, White PJ, MEad A, Broadley MR (2005) Phylogenetic variation in the silicon composition of plants. Ann Bot 96:1027–1046
Hoffman JM, Fraser D, Clementz MT (2015) Controlled feeding trials with ungulates: a new application of in vivo dental molding to assess the abrasive factors of microwear. J Exp Biol 218:1538–1547
Hofmann RR (1973) The ruminant stomach. East African Literature Bureau, Nairobi
Hofmann RR (1988) Morphological evolutionary adaptations of the ruminant digestive system. In: Dobson A, Dobson MJ (eds) Aspects of digestive physiology in ruminants. Comstock Publishing Associates, Cornell University Press, Ithaca, NY
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78:443–457
Hofmann RR, Stewart DRM (1972) Grazer or browser: a classification based on the stomach-structure and feeding habit of east African ruminants. Mammalia 36:226–240
Hofmann RR, Streich WJ, Fickel J, Hummel J, Clauss M (2008) Convergent evolution in feeding types: salivary gland mass differences in wild ruminant species. J Morphol 269:240–257
Hummel J, Südekum K-H, Streich WJ, Clauss M (2006) Forage fermentation patterns and their implications for herbivore ingesta retention times. Funct Ecol 20:989–1002
Hummel J et al (2008) Differences in fecal particle size between free-ranging and captive individuals of two browser species. Zoo Biol 27:70–77
Hummel J et al (2009) Physical characteristics of reticuloruminal contents of cattle in relation to forage type and time after feeding. J Anim Physiol Anim Nutr 93:209–220
Hummel J et al (2011) Another one bites the dust: faecal silica levels in large herbivores correlate with high-crowned teeth. Proc R Soc Lond B Biol Sci 278:1742–1747. https://doi.org/10.1098/rspb.2010.1939
Hummel J, Hammer S, Hammer C, Ruf J, Lechenne M, Clauss M (2015) Solute and particle retention in a small grazing antelope, the blackbuck (Antilope cervicapra). Comp Biochem Physiol A Mol Integr Physiol 182:22–26
Hutchinson GE (1959) Homage to Santa Rosalia, or why are there so many kinds of animals? Am Nat XCIII(870):137–145
Janis CM (1976) The evolutionary strategy of the Equidae and the origins of rumen and caecal digestion. Evolution 30:757–774
Janis CM (1988) An estimation of tooth volume and hypsodonty indices in ungulate mammals and the correlation of these factors with dietary preferences. In: Russell DE, Santoro J-P, Signogneau-Russell D (eds) Teeth revisited. Proceedings of the VIIth international symposium on dental morphology. Mémoires du Muséum national d'Histoire Naturelle, Paris (serie C), vol 53, pp 367–387
Janis CM (1995) Correlations between craniodental morphology and feeding behavior in ungulates: reciprocal illumination between living and fossil taxa. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge, pp 76–98
Janis CM (2008) An evolutionary history of browsing and grazing ungulates. In: Gordon IJ, Prins HHT (eds) The ecology of browsing and grazing. Springer, Heidelberg, pp 21–45
Janis CM, Constable E (1993) Can ungulate craniodental features determine digestive physiology? J Vertebr Paleontol 13:43A
Janis CM, Ehrhardt D (1988) Correlation of relative muzzle width and relative incisor width with dietary preference in ungulates. Zool J Linnean Soc 92:267–284
Janis CM, Gordon IJ, Illius AW (1994) Modelling equid/ruminant competition in the fossil record. Hist Biol 8:15–29
Janis CM, Damuth J, Theodor JM (2000) Miocene ungulates and terrestrial primary productivity: where have all the browsers gone? Proc Natl Acad Sci U S A 97:7899–7904
Janis CM, Constable EC, Houpt KA, Streich WJ, Clauss M (2010) Comparative ingestive mastication in domestic horses and cattle: a pilot investigation. J Anim Physiol Anim Nutr 94:e402–e409. https://doi.org/10.1111/j.1439-0396.2010.01030.x
Jernvall J, Hunter JP, Fortelius M (1996) Molar tooth diversity, disparity, and ecology in Cenozoic ungulate radiations. Science 274:1489–1492
Kaiser TM, Fickel J, Streich WJ, Hummel J, Clauss M (2010) Enamel ridge alignment in upper molars of ruminants in relation to their natural diet. J Zool 281:12–25
Kaiser DP, Müller DWH, Fortelius M, Schulze E, Codron D, Clauss M (2013) Hypsodonty and tooth facet development in relation to diet and habitat in herbivorous ungulates: implications for understanding tooth wear. Mammal Rev 43:34–46. https://doi.org/10.1111/j.1365-2907.2011.00203.x
Karasov WH, Martínez del Rio C (2007) Physiological ecology: how animals process energy, nutrients, and toxins. Princeton University Press, Princeton, NJ
Karme A, Rannikko J, Kallonen A, Clauss M, Fortelius M (2016) Mechanical modelling of tooth wear. J R Soc Interface 13. https://doi.org/10.1098/rsif.2016.0399
Kelly KE, Sinclair BR (1989) Size and structure of leaf and stalk components of digesta regurgitated for rumination in sheep offered five forage diets. N Z J Agric Res 32:365–374
Kiltie RA (1981) The function of interlocking canines in rain forest peccaries (Tayassuidae). J Mammal 62:459–469
Lajeunesse MJ (2009) Meta-analysis and the comparative phylogenetic method. Am Nat 174:369–381
Lauper M et al (2013) Rumination of different-sized particles in muskoxen (Ovibos moschatus) and moose (Alces alces) on grass and browse diets, and implications for rumination in different ruminant feeding types. Mamm Biol 78:142–152
Lazagabaster IA, Rowan J, Kamilar JM, Reed KE (2016) Evolution of craniodental correlates of diet in African Bovidae. J Mamm Evol 23:385–396
Lechner I et al (2009) No ‘bypass’ in adult ruminants: passage of fluid ingested vs. fluid inserted into the rumen in fistulated muskoxen (Ovibos moschatus), reindeer (Rangifer tarandus) and moose (Alces alces). Comp Biochem Physiol A Mol Integr Physiol 154:151–156
Lechner I et al (2010) Differential passage of fluids and different-sized particles in fistulated oxen (Bos primigenius f. taurus), muskoxen (Ovibos moschatus), reindeer (Rangifer tarandus) and moose (Alces alces): rumen particle size discrimination is independent from contents stratification. Comp Biochem Physiol A Mol Integr Physiol 155:211–222
Lechner-Doll M, Kaske M, von Engelhardt W (1991) Factors affecting the mean retention time of particles in the forestomach of ruminants and camelids. In: Tsuda T, Sasaki Y, Kawashima R (eds) Physiological aspects of digestion and metabolism in ruminants. Academic, San Diego, CA, pp 455–482
Louys J, Faith JT (2015) Phylogenetic topology mapped onto dietary ecospace reveals multiple pathways in the evolution of the herbivorous niche in African Bovidae. J Zool Syst Evol Res 53:140–154
Lucas PW et al (2013) Mechanisms and causes of wear in tooth enamel: implications for hominin diets. J R Soc Interface 10:2012.0923
Macandza VA, Owen-Smith N, Cross PC (2004) Forage selection by African buffalo in the late dry season in two landscapes. S Afr J Wildl Res 34:113–121
Mainland IL (2003) Dental microwear in grazing and browsing Gotland sheep (Ovis aries) and it implications for dietary reconstruction. J Archaeol Sci 30:1513–1527
Marchand P, Redjadj C, Garel M, Cugnasse J-M, Maillard D, Loison A (2013) Are mouflon (Ovis gmelini musimon) really grazers? A review of variation in diet composition. Mamm Rev 43:275–291
Martin SA, Alhajeri BH, Steppan SJ (2016) Dietary adaptations in the teeth of murine rodents (Muridae): a test of biomechanical predictions. Biol J Linn Soc 119:766–784
McLeod MN, Minson DJ (1988) Large particle breakdown by cattle eating ryegrass and alfalfa. J Anim Sci 66:992–999
Meier AR, Schmuck U, Meloro C, Clauss M, Hofmann RR (2016) Convergence of macroscopic tongue anatomy in ruminants and scaling relationships with body mass or tongue length. J Morphol 277:351–362
Mendoza M, Palmqvist P (2006) Characterizing adaptive morphological patterns related to diet in Bovidae (Mammalia: Artiodactyla). Acta Zool Sin 52:988–1008
Mendoza M, Palmqvist P (2008) Hypsodonty in ungulates: an adaptation for grass consumption or for foraging in open habitat? J Zool 274:134–142
Mendoza M, Janis CM, Palmqvist P (2002) Characterizing complex craniodental patterns related to feeding behaviour in ungulates: a multivariate approach. J Zool 258:223–246
Merceron G et al (2016) Untangling the environmental from the dietary: dust does not matter. Proc R Soc Lond B Biol Sci 283:20161032
Meyer K, Hummel J, Clauss M (2010) The relationship between forage cell wall content and voluntary food intake in mammalian herbivores. Mammal Rev 40:221–245. https://doi.org/10.1111/j.1365-2907.2010.00161.x
Mihlbachler MC, Campbell D, Ayoub M, Chen C, Ghani I (2016) Comparative dental microwear of ruminant and perissodactyl molars: implications for paleodietary analysis of rare and extinct ungulate clades. Paleobiology 42:98–116
Mlambo V, Marume U, Gajana CS (2015) Utility of the browser’s behavioural and physiological strategies in coping with dietary tannins: are exogenous tannin-inactivating treatments necessary? S Afr J Anim Sci 45:441–451
Mortolaa JP, Lanthier C (2005) Breathing frequency in ruminants: a comparative analysis with non-ruminant mammals. Respir Physiol Neurobiol 145:265–277
Müller DWH et al (2011) Phylogenetic constraints on digesta separation: variation in fluid throughput in the digestive tract in mammalian herbivores. Comp Biochem Physiol A Mol Integr Physiol 160:207–220. https://doi.org/10.1016/j.cbpa.2011.06.004
Müller DWH et al (2013) Assessing the Jarman–Bell principle: scaling of intake, digestibility, retention time and gut fill with body mass in mammalian herbivores. Comp Biochem Physiol A Mol Integr Physiol 164:129–140
Müller J et al (2014) Growth and wear of incisor and cheek teeth in domestic rabbits (Oryctolagus cuniculus) fed diets of different abrasiveness. J Exp Zool 321A:283–298
Müller J et al (2015) Tooth length and incisal wear and growth in Guinea pigs (Cavia porcellus) fed diets of different abrasiveness. J Anim Physiol Anim Nutr 99:591–604
Murray MG (1993) Comparative nutrition of wildebeest, hartebeest and topi in the Serengeti. Afr J Ecol 31:172–177
Murray MG, Illius AW (2000) Vegetation modification and resource competition in grazing ungulates. Oikos 89:501–508
Nultsch W (2000) Allgemeine Botanik (General Botany), 11th edn. Georg Thieme Verlag, Stuttgart
Nygren K, Lechner-Doll M, Hofmann RR (2001) Influence of papillae on post-ruminal regulation of ingesta passage in moose (Alces alces). J Zool 254:375–380
Obidziński A, Miltko R, Bolibok L, Wajdzik M, Skubis J, Nasiadka P (2017) Variation of natural diet of free ranging mouflon affects their ruminal protozoa composition. Small Rumin Res 157:57–64
Orme D et al. (2013) Caper: comparative analyses of phylogenetics and evolution in R. R package version 0.5.2. https://CRAN.R-project.org/package=caper
Owen-Smith N (1997) Distinctive features of the nutritional ecology of browsing versus grazing ruminants. Z Säugetierkd 62:176–191
Owen-Smith N (2013) Megaherbivores. In: Levin S (ed) Encyclopedia of biodiversity, 2nd edn. Academic
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Paine OCC et al (2018) Grass leaves as potential hominin dietary resources. J Hum Evol 117:44–52
Palmqvist P, Gröcke DR, Arribas A, Fariña RA (2003) Paleoecological reconstruction of a lower Pleistocene large mammal community using biogeochemical (δ13C, δ15N, δ18O, Sr:Zn) and ecomorphological approaches. Paleobiology 29:205–229
Pérez-Barbería FJ, Gordon IJ (2001) Relationships between oral morphology and feeding style in the Ungulata: a phylogenetically controlled evaluation. Proc R Soc Lond B Biol Sci 268:1021–1030
Pérez-Barbería J, Gordon I, Illius A (2001) Phylogenetic analysis of stomach adaptation in digestive strategies in African ruminants. Oecologia 129:498–508
Pérez-Barbería FJ, Elston DA, Gordon IJ, Illius AW (2004) The evolution of phylogenetic differences in the efficiency of digestion in ruminants. Proc R Soc Lond B Biol Sci 271:1081–1090. https://doi.org/10.1098/rspb.2004.2714
Pretorius Y et al (2016) Why elephant have trunks and giraffe long tongues: how plants shape large herbivore mouth morphology. Acta Zool 97:246–254. https://doi.org/10.1111/azo.12121
Prins HHT, Olff H (1998) Species-richness of African grazer assemblages: towards a functional explanation. In: Newbery DM, Prins HHT, Brown N (eds) Dynamics of tropical communities. Blackwell Science, Oxford, pp 449–490
Rabenold D, Pearson OM (2014) Scratching the surface: a critique of Lucas et al. (2013)’s conclusion that phytoliths do not abrade enamel. J Hum Evol 74:13–133
Raia P, Carotenuto F, Meloro C, Piras P, Pushkina D (2010) The shape of contention: adaptation, history, and contingency in ungulate mandibles. Evolution 64:1489–1503
Reed KE (1996) The palaeoecology of Makapansgat and other African Plio-Pleistocene hominid localities. Unpublished PhD thesis, University of Michigan, Michigan
Reilly SM, McBrayer LD, White TD (2001) Prey processing in amniotes: biomechanical and behavioral patterns of food reduction. Comp Biochem Physiol A Mol Integr Physiol 128:397–415
Rivals F, Semprebon GM, Solounias N (2010) Advances in ungulate dental wear techniques reveal new patterns of niche breadth and expansion throughout the Cenozoic. J Vertebr Paleontol SVP Program and Abstracts Book:151A–152A
Robbins CT, Spalinger DE, Van Hoven W (1995) Adaptation of ruminants to browse and grass diets: are anatomical-based browser-grazer interpretations valid? Oecologia 103:208–213
Rowell-Schäfer A, Lechner-Doll M, Hofmann RR, Streich WJ, Güven B, Meyer HHD (2001) Metabolic evidence of a “rumen bypass” or a “ruminal escape” of nutrients in roe deer (Capreolus capreolus). Comp Biochem Physiol A Mol Integr Physiol 128:289–298
Sakaguchi E, Itoh J, Shinohara H, Matsumoto T (1981) Effects of removal of the forestomach and caecum on the utilization of dietary urea in golden hamsters (Mesocricetus auratus) given two different diets. Br J Nutr 46:503–512
Sanson GD (1989) Morphological adaptations of teeth to diets and feeding in the Macropodoidea. In: Grigg G, Jarman PJ, Hume I (eds) Kangaroos, Wallabies and Rat-kangaroos. Surrey-Beatty, Sydney, pp 151–168
Sanson GD, Read SKJ (2017) Dietary exogenous and endogenous abrasives and tooth wear in African buffalo. Biosurf Biotribol 3:211–223
Sanson GD, Kerr SA, Gross KA (2007) Do silica phytoliths really wear mammalian teeth? J Archaeol Sci 34:526–531
Sauer C, Bertelsen MF, Hammer S, Lund P, Weisbjerg MR, Clauss M (2016) Macroscopic digestive tract anatomy of two small antelopes, the blackbuck (Antilope cervicapra) and the Arabian sand gazelle (Gazella subgutturosa marica). Anat Histol Embryol 45:392–398
Schuette JR, Leslie DMJ, Lochmiller RL, Jenks JA (1998) Diets of hartebeest and roan antelope in Burkina Faso: support of the long-faced hypothesis. J Mammal 79:426–436
Shipley LA (2007) The influence of bite size on foraging at larger spatial and temporal scales by mammalian herbivores. Oikos 116:1964–1974
Smith FA et al (2003) Body mass of late quaternary mammals. Ecology 84:3403. (Ecological Archives: E3084-3094)
Spencer LM (1995) Morphological correlates of dietary resource partitioning in the African Bovidae. J Mammal 76:448–471
Sponheimer M et al (2003) Diets of southern African Bovidae: stable isotope evidence. J Mammal 84:471–479
Stayton CT (2006) Testing hypotheses of convergence with multivariate data: morphological and functional convergence among herbivorous lizards. Evolution 60:824–841
Steuer P et al (2010) Comparative investigations on digestion in grazing (Ceratotherium simum) and browsing (Diceros bicornis) rhinoceroses. Comp Biochem Physiol A Mol Integr Physiol 156:380–388
Strömberg CAE (2006) Evolution of hypsodonty in equids: testing a hypothesis of adaptation. Paleobiology 32:236–258. https://doi.org/10.1666/0094-8373(2006)32[236:eohiet]2.0.co;2
Sutherland TM (1988) Particle separation in the forestomach of sheep. In: Dobson A, Dobson MJ (eds) Aspects of digestive physiology in ruminants. Cornell University Press, Ithaca, NY, pp 43–73
Tahas SA et al (2017) Gross measurements of the digestive tract and visceral organs of addax antelope (Addax nasomaculatus) following a concentrate or forage feeding regime. Anat Histol Embryol 46:282–293
Tahas SA et al (2018) Microanatomy of the digestive tract, hooves and some visceral organs of addax antelope (Addax nasomaculatus) following a concentrate or forage feeding regime. Anat Histol Embryol 47:254–267. https://doi.org/10.1111/ahe.12351
Tainton NM (1999) The ecology of the main grazing lands of South Africa: the savanna biome. In: Tainton NM (ed) Veld management in South Africa. University of Natal Press, Pietermaritzburg, pp 23–53
Taylor LA et al (2013) Detecting inter-cusp and inter-tooth weat patterns in rhinocerotids. PLoS One 8:e80921
Trautmann A, Schmitt I (1935) Experimentelle Untersuchungen zur Frage der Psalterfunktion (Experimental investigations on omasum function). Dtsch Tierarztl Wochenschr 12:177–179
Trudell-Moore J, White RG (1983) Physical breakdown of food during eating and rumination in reindeer. Acta Zool Fenn 175:47–49
Tschuor A, Clauss M (2008) Investigations on the stratification of forestomach contents in ruminants: an ultrasonographic approach. Eur J Wildl Res 54:627–633
Turnbull WD (1970) Mammalian masticatory apparatus. Fieldiana Geol 18:147–356
Van Soest PJ (1965) Symposium on factors influencing the voluntary intake of herbage by ruminants: voluntary intake in relation to chemical composition and digestibility. J Anim Sci 24:834–843
Van Wieren SE (1996a) Browsers and grazers: foraging strategies in ruminants. In: Van Wieren SE (ed) Digestive strategies in ruminants and nonruminants. Thesis Landbouw, University of Wageningen, Wageningen, pp 119–146
Van Wieren SE (1996b) Digestive strategies in ruminants and non-ruminants. PhD thesis. University of Wageningen, Wageningen
Van Zyl JHM (1965) The vegetation of the S.A. Lombard nature reserve and its utilization by certain antelope. Zool Afr 1:55–71
Varela L, Fariña RA (2015) Masseter moment arm as a dietary proxy in herbivorous ungulates. J Zool 296:295–304
Vermeij GJ (1994) The evolutionary interaction among species: selection, escalation, and coevolution. Annu Rev Ecol Syst 25:219–236
Vermeij GJ (2013) On escalation. Annu Rev Earth Planet Sci 41:1–19
Wattiaux MA, Satter LD, Mertens DR (1992) Effect of microbial fermentation on functional specific gravity of small forage particles. J Anim Sci 70:1262–1270
Williams SH, Stover KK, Davis JS, Montuelle SJ (2011) Mandibular corpus bone strains during mastication in goats (Capra hircus): a comparison of ingestive and rumination chewing. Arch Oral Biol 56:960–971
Xia J et al (2015) New model to explain tooth wear with implications for microwear formation and diet reconstruction. Proc Natl Acad Sci U S A 112:10669–10672. https://doi.org/10.1073/pnas.1509491112
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Codron, D., Hofmann, R.R., Clauss, M. (2019). Morphological and Physiological Adaptations for Browsing and Grazing. In: Gordon, I., Prins, H. (eds) The Ecology of Browsing and Grazing II. Ecological Studies, vol 239. Springer, Cham. https://doi.org/10.1007/978-3-030-25865-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-25865-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25864-1
Online ISBN: 978-3-030-25865-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)