Abstract
Imaging studies are integral for the identification of potential surgical candidates and planning hepatic resection in patients with colorectal liver metastases (CRLM). Modalities such as ultrasound (US), multidetector computed tomography (MDCT), magnetic resonance imaging (MRI), and positron emission tomography (PET) are used for CRLM and hepatic assessment. This chapter will discuss the current role of these imaging modalities in the management of patients with potentially resectable CRLM.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Imaging features of CRLM
- Surgical planning for CRLM
- Preoperative chemotherapy
- Vascular assessment
- Liver volumetry
- Future liver remnant
Introduction
Assessment of CRLM resection has rapidly changed over the years, as more lesions are deemed operable due to the expanding criteria for resectability [1]. Liver resection with curative intent necessitates addressing or removing all detectable disease sites. Preoperative imaging is essential to determine the location and extent of CRLM, proximity of lesions to vascular structures, predicting future liver remnant and identifying extrahepatic disease [2]. Moreover, full imaging assessment such as with MRI is important prior to chemotherapy, as treatment can change the appearance of metastatic lesions and affect hepatic parenchyma reducing sensitivity of CT and/or PET for CRLM evaluation [3]. In this chapter, we discuss the integral role of current imaging techniques in the management of patients with potentially resectable CRLM.
Role of Imaging in Detection of CRLM
Ultrasound
Conventional ultrasound (US) is widely used for the assessment of CRLM due to its low cost, absence of radiation exposure, and wide availability. US provides a pooled sensitivity of 63% and specificity of 97.6% for CRLM detection [4]. However, it is the addition of contrast-enhanced US (CEUS) with agents that has dramatically increased the sensitivity for CRLM detection up to 80–90%, which is comparable to that by computed tomography [5, 6]. US contrast agents consist of tiny microbubbles that carry a strong safety profile and can be used in patients with impaired renal function [7]. In patients who have contraindications to iodinated contrast material, CEUS is a viable alternative and superior to non-contrast-enhanced CT in the characterization of focal liver pathology [8].
On US, CRLM often appear as hypoechoic lesions and less commonly may have similar or higher echogenicity relative to normal hepatic parenchyma [9]. CRLM, which are not visualized during preoperative imaging assessment, may be detected with the use of real-time ultrasound intraoperatively [10]. Intraoperative US (IOUS) also facilitates localization of deep-seated, nonpalpable lesions while mapping major hepatic veins, providing real-time guidance during surgery [10]. Patients with multiple CRLM benefit greatly from IOUS due to its high specificity (94–98%) and detection rate of tumors missed on prior imaging [11]. Additionally, contrast-enhanced IOUS may identify small tumor residuals, which is important to ensure microscopically margin-negative resection [12].
While some limitations regarding lesion characterization can be overcome with CEUS, accurate diagnosis is highly examiner dependent and requires experienced operator training [13]. Evaluation of multiple lesions simultaneously is also challenging with CEUS [14]. Neoadjuvant chemotherapy (NAC) can significantly alter liver echotexture (lesion and parenchyma) interfering with sensitivity and specificity of US [14]. Other challenges include limited spatial resolution of transcutaneous probes, assessing patients with obese habitus, high-lying diaphragm, and uncooperative patients [13].
Computed Tomography (CT)
Multidetector CT (MDCT) is the preferred modality for CLRM assessment in the USA due to its ability to image the liver and potential sites of extrahepatic metastases (e.g., chest, nodes, and peritoneum) in one examination [15]. MDCT is also widely available, reliable, and able to accommodate patients with body habitus limitations (e.g., obesity). Improved resolution and faster scanning speed with MDCT has enabled imaging of the entire abdomen and pelvis during a single breath hold, effectively eliminating respiratory motion artifacts [16]. Acquisition of thin slices allows for reconstruction of high-quality images in different planes and rendering reformatted images are now integral to any CT protocol [17]. The use of iodinated contrast media (ICM) to adequately detect and characterize CRLM has been advocated and is recommended by the current national comprehensive cancer network (NCCN) guidelines [18]. A recent meta-analysis reported 82% sensitivity and 73.5% specificity for CRLM detection using contrast-enhanced CT (CECT) [19] (Table 5.1). Oral contrast media is desirable but not mandatory, as it helps with better evaluation of extrahepatic pathologies, such as eliminating false-positive peritoneal metastases.
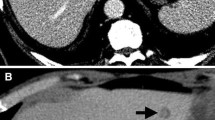
Dynamic contrast-enhanced CT (CECT) involves multi-phase acquisition including true non-contrast, arterial, portal-venous and delayed phase. CRLM are typically hypovascular lesions and appear hypoattenuating compared to normal liver tissue with heterogeneous enhancement on portal venous phase [17]. Since CRLM are hypovascular, arterial phase is typically used for presurgical planning [20]. On delayed images, CRLM can show wash-out contrary to benign pathologies (e.g., hemangiomas) [17]. Newer generation dual-energy CT scanners (DECT), which are becoming mainstream, have further bolstered the role of CT with novel post-processing techniques that improve lesion conspicuity by enhancing the liver-to-lesion contrast [21, 22] (Fig. 5.1).
Dual-Energy CT to improve lesion conspicuity. An 85-year-old male with multiple CRLM. Post-contrast axial reconstructions of material density-iodine in gray scale (a) and color-overlay (b) show better conspicuity of a hepatic lesion (arrows), which is hard to visualize on conventional single-energy CT image (c)
Limitations of CT include low sensitivity for detecting small lesions (<10 mm) and identifying CRLM in patients receiving NAC (Tables 5.1 and 5.2) [19, 23]. NAC significantly decreases the sensitivity of CRLM detection as treated lesions shrink in size [3]. Furthermore, NAC may also cause liver steatosis which reduces hepatic parenchymal attenuation leading to diminished lesion to liver contrast [24]. Patients with a history of iodinated contrast media allergies or impaired renal function are not ideal candidates for CECT [25]. In addition, ionizing radiation exposure for oncologic patients who often require repeated imaging can be of concern. These limitations can be addressed to some extent with DECT scanners, which can minimize radiation exposure by eliminating the need for true non-contrast images and allow for reduced contrast media dose while maintaining high image quality [26,27,28]. Moreover, DECT applications are empowering CT with better diagnostic capabilities for small lesion detection and characterization [29, 30].
Magnetic Resonance Imaging (MRI)
MRI is the modality of choice for evaluating CRLM and has proven to be highly effective for hepatic parenchymal assessment. Compared to CT, MR images offer superb soft tissue contrast, which improves diagnoses of intrahepatic lesions due to greater liver-to-lesion contrast difference (Fig. 5.2) [17]. MRI also excels in detecting small lesions (<10 mm) and identifying CRLM in patients undergoing NAC or have hepatic steatosis (Tables 5.2 and 5.3) [19, 23, 31]. Dynamic contrast-enhanced MRI (CE-MRI) with hepatobiliary agents and diffusion-weighted imaging (DWI) has further improved CRLM detection and characterization of indeterminate lesions, especially for small lesions [32,33,34,35]. Gadoxetate disodium (Gd-EOB-DTPA, Eovist in the USA) is the most commonly used hepatobiliary agent with MRI for CRLM detection with a pooled sensitivity of 93.1% (Table 5.3) [19].
Value of MRI for detection of metastasis in background of fatty liver. A 58-year-old male with CRLM and diffuse hepatic steatosis. Post-contrast T1-weighted axial MR images (a, b) reveal peripherally enhancing hypointense lesions (arrows) that are not discernible on post-contrast axial CT (c, d) images due to uniformly low attenuation of background liver parenchyma suggesting diffuse hepatic steatosis
MRI sequences for CRLM assessment usually include a combination of T1-weighted (T1W), T2-weighted (T2W), and diffusion-weighted images (DWI) [36]. CRLM typically appear hypointense in precontrast T1W images and hyperintense in T2W images [36]. On dynamic CE-MRI, CRLM appear mostly hypointense, similar to CECT [36]. Eovist allows for further assessment of intrahepatic lesions with hepatobiliary (HB) phase (20 minutes after contrast injection) [37]. On HB phase, normal hepatocytes appear hyperintense due to eovist uptake, whereas CRLM do not retain contrast remaining hypointense and appear more conspicuous [37]. On DWI, CRLM become hyperintense compared to the normal liver due to diffusion restriction [38]. However, some benign lesions may also appear hyperintense (T2 shine-through effect) on DWI and can be differentiated from CRLM by the use of apparent diffusion coefficients (the “ADC” map) [35] (Fig. 5.3).
Advantage of DWI for distinguishing benign vs. malignant lesions. A 45-year-old female with CRLM. Post-contrast axial T1 weighted (a) reveal tiny hypoenhancing lesions (red arrow) in segment VIII and a larger nodular peripherally enhancing lesion in segment VII (yellow arrow). All lesions appear hyperintense on DWI (b). Corresponding ADC map (c) shows segment VIII lesions as hypointense suggestive of restricted diffusion that indicates metastasis, whereas the segment VII lesion remains hyperintense suggestive of T2 shine through indicating benign etiology
Limitations of MRI include contraindications such as imaging patients with metal implants and claustrophobia. Increased patient compliance such as longer breath holds and lying still for a lengthy period is required to avoid motion-related imaging artifacts, which can adversely affect diagnostic image quality [36]. Additionally, DWI have inherent limitations due to low spatial resolution and poor signal-to-noise ratio [39]. However, technological advancements in MRI scanners with more dedicated software can address some of these limitations to improve image quality [39].
Positron Emission Tomography (PET)
PET with 18-fluoride deoxyglucose (FDG) analogue is a whole-body imaging technique providing molecular and metabolic information for CRC evaluation [40]. The main advantage of PET imaging is the superior detection of extrahepatic metastases, which can eliminate unnecessary surgical intervention (Table 5.4) [40,41,42,43]. FDG-PET has also demonstrated great value in clarifying equivocal findings for the presence of metastases on CT or MRI [40,41,42,43]. On FDG-PET, diagnosis of metastases is based on focal FDG uptake exceeding the uptake of the surrounding tissue (Fig. 5.4) [44]. Currently, FDG-PET is routinely performed with CT (PET-CT) to improve localization of lesions and detection of CRLM [44, 45]. Through the detection of additional liver CRLM and/or extrahepatic disease, PET-CT was shown to change planned surgical management in 24% of patients [46]. FDG-PET with MRI (PET-MRI) is a relatively newer technique that has shown great potential in CRLM detection [47, 48]. In a recent comparative study, PET-MRI had significantly higher sensitivity and diagnostic accuracy (92.2% and 96.1%, respectively) than PET-CT (67.8% and 82.4%, respectively) [49]. This is likely due to the inherent advantages of MRI in differentiating tissue contrast and lesion detection. However, literature data regarding diagnostic performance are limited, as PET-MRI is available only in a few highly specialized centers.
Limitations with PET imaging include reduced sensitivity for small lesion detection and assessing lesions in patients receiving NAC [50, 51]. The physiologic hepatic parenchymal uptake of FDG along with the inherent low resolution of PET can limit the sensitivity for small lesion detection [51]. Additionally, patients receiving NAC within 4 weeks of their PET scan may have high false-negative results due to reduction in lesion size and tumor metabolic activity [50]. Hypermetabolic processes such as chronic infection or inflammation may also lead to false-positive FDG uptake [52]. Studies have reported that 5–8% of CRC patients are falsely upstaged by PET-CT preoperatively [53]. Patient compliance factor such as respiratory motion is an additional challenge when performing PET-CT [54].
Role of Imaging in Surgical Planning for CRLM
Vascular Assessment
The localization of CRLM relative to vascular structures is important to determine feasibility and planning of hepatic resection. A preoperative vascular “road map” can identify anomalous or aberrant hepatic vascular supply to avoid inadvertent injury to the liver (Fig. 5.5). In addition, knowledge of the vascular map aids in determining the best hepatectomy plane. CT angiography (CTA) is preferred over MR angiography, especially for complex hepatic resection due to rapid acquisition, less susceptibility to motion artifacts, and thin collimation [55]. High-quality multiplanar reconstruction (MPR) and three-dimensional images can be obtained with CT to display the liver vascular anatomy in any desired plane [55]. The proximity of liver lesions to the hepatic vasculature can then be illustrated to decide the best surgical approach [56].
Image post processing for Vascular Mapping. A 36-year-old male with CRLM. Coronal reformatted CT angiography in maximum intensity projection (MIP) reconstruction shows replaced right hepatic artery (RHA) arising from the superior mesenteric artery (SMA). LHA left hepatic artery, SPL A splenic artery, GDA gastroduodenal artery
Liver Volumetry
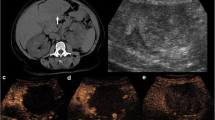
A crucial factor for planning surgical resection is predicting the future liver remnant (FLR) volume to avoid risk of hepatic insufficiency and subsequent mortality [57]. Preoperative estimation of the FLR is used to determine whether the functional reserve is enough to meet metabolic demands of the liver after surgery [57]. Three-dimensional reconstruction images can be used to generate precise volume measurements of the liver lobes [57, 58]. Approximately 25–30% and 40% of the preoperative liver volume should be preserved for those with normal and abnormal parenchyma (e.g., steatosis, fibrosis), respectively [59]. When FLR is deemed inadequate and CRLM is unresectable, portal vein embolization (PVE) can be performed. PVE induces hypertrophy in the remaining liver to maximize the remnant liver volume and increase resectability rate with high clinical success (Fig. 5.6) [60]. A recent meta-analysis showed that 96.1% of patients who undergo PVE develop sufficient liver lobe hypertrophy [60]. CT performed 1 month following embolization can then estimate any increase in FLR [61]. Any remaining CRLM lesions in the non-resected lobe can be treated with other methods such as radiofrequency ablation (Fig. 5.7) [62]. An area of high density or signal intensity on non-contrast CT and thin regular peripheral rim enhancement are expected after ablative therapy. Complete non-enhancement of the ablated lesions is considered evidence of full tumor necrosis at subsequent follow-up.
CT volumetric analysis of the liver. A 56-year-old female with liver metastases who underwent portal vein embolization prior to right hepatectomy. CECT axial (a) image and corresponding 3D volumetric analysis (b) shows the pre-embolization volume of the right lobe (volume = 1552 cm) and left lobe with caudate (volume = 566 cm). Digital subtraction angiography image (c) demonstrates successful embolization of the anterior and posterior branches of right portal vein with particles and coils. (c) Axial CT (d) and three-dimensional volume-rendered CT image (e) obtained 3 months after embolization and resection reveal left hepatic lobe hypertrophy (volume = 1408 cm3). Note: Blue = right hepatic lobe; Gold = left hepatic lobe with caudate
Imaging of ablation zone. A 56-year-old male status pre and post left hepatectomy for CRLM. Post-contrast CT in axial (a) and coronal (b) planes show multiple liver metastases in both lobes of the liver. Left hepatectomy was performed and right lobe lesions in segment VII were treated with radio-frequency ablation (c, d). Follow-up CT, after 3 months, (e, f) shows two non-enhancing hypodense regions suggestive of normal post ablation zone changes with no evidence of residual tumor
Conclusion
Imaging studies play a crucial role in the detection and characterization of CRLM to identify potential surgical candidates. The role of ultrasound has improved with the addition of microbubble contrast agents and remains a valuable tool for intraoperative decision-making. MDCT is most commonly used for initial CRLM detection and staging. MRI is the modality of choice for CRLM assessment, particularly for characterizing and detecting small lesions. MRI is also superior to CT and PET for hepatic evaluation in patients receiving NAC or have underlying hepatic steatosis. High-quality 3D images can be reconstructed from CT or MRI for vascular assessment and FLR estimation to guide the surgical approach. PET has proven essential prior to surgery for its superior extrahepatic disease detection. While each modality has its advantages for patient assessment, a multi-modal imaging approach is required to maximize safety and efficacy of CRLM resection.
References
House MG. Safely expanding the criteria for resectability of hepatic colorectal metastases. Ann Surg. 2011;253:1080–1.
Legou F, Chiaradia M, Baranes L, et al. Imaging strategies before beginning treatment of colorectal liver metastases. Diagn Interv Imaging. 2014;95(5):505–12.
Robinson PJ. The effects of cancer chemotherapy on liver imaging. Eur Radiol. 2009;19(7):1752–62.
Florani I, Torri V, Rulli E, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–31.
Larsen LP, Rosenkilde M, Christensen H, et al. Can contrast-enhanced ultrasonography replace multidetector-computed tomography in the detection of liver metastases from colorectal cancer? Eur J Radiol. 2009;69:308–13.
Cantisani V, Ricci P, Erturk M, et al. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue® low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med. 2010;31:500–5.
Piscaglia F, Bolondi L, Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–75.
Sawhney S, Wilson SR. Can ultrasound with contrast enhancement replace nonenhanced computed tomography scans in patients with contraindication to computed tomography contrast agents? Ultrasound Q. 2017;33(2):125–32.
D’Onofrio M, Crosara S, De Robertis R, et al. Contrast-enhanced ultrasound of focal liver lesions. AJR Am J Roentgenol. 2015;205(1):W56–66.
Lucchese AM, Kalil AN, Schwengber A, Suwa E, Rolim de Moura GG. Usefulness of intraoperative ultrasonography in liver resections due to colon cancer metastasis. Int J Surg. 2015;20:140–4.
Ellebæk SB, Fristrup CW, Mortensen MB, et al. Intraoperative ultrasound as a screening modality for the detection of liver metastases during resection of primary colorectal cancer-a systematic review. Ultrasound Int Open. 2017;3(2):E60–8.
Ruzzenente A, Conci S, Iacono C, et al. Usefulness of contrast-enhanced intraoperative ultrasonography (CE-IOUS) in patients with colorectal liver metastases after preoperative chemotherapy. J Gastrointest Surg. 2013;17:281–7.
Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34(1):11–29.
Dietrich CF, Ignee A, Greis C, et al. Artifacts and pitfalls in contrast-enhanced ultrasound of the liver. Ultraschall Med. 2014;35:108–25.
Kaur H, Hindman NM, Al-Refaie WB, et al. ACR appropriateness criteria® suspected liver metastases. J Am Coll Radiol. 2017;14:S314–25.
Ong KO, Leen E. Radiological staging of colorectal liver metastases. Surg Oncol. 2007;16(1):7–14.
Tirumani S, Kim KW, Nishino M, Howard SA, Krajewski KM, Jagannathan JP. Update on the role of imaging in management of metastatic colorectal cancer. Radiographics. 2014;34:1908–28.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Colon Cancer (version 1. 2017). Accessed 30 Apr 2018. Available from: http://www.nccn.org.
Choi SH, Kim SY, Park SH, et al. Diagnostic performance of CT, gadoxetate disodium-enhanced MRI, and PET/CT for the diagnosis of colorectal liver metastasis: systematic review and meta-analysis. J Magn Reson Imaging. 2018;47(5):1237–50.
Wicherts DA, de Haas RJ, van Kessel CS, et al. Incremental value of arterial and equilibrium phase compared to hepatic venous phase CT in the preoperative staging of colorectal liver metastases: an evaluation with different reference standards. Eur J Radiol. 2011;77:305–11.
Agrawal MD, Pinho DF, Kulkarni NM, Hahn PF, Guimaraes AR, Sahani DV. Oncologic applications of dual-energy CT in the abdomen. Radiographics. 2014;34(3):589–612.
Leng S, Yu L, Fletcher JG, McCollough CH. Maximizing iodine contrast-to-noise ratios in abdominal CT imaging through use of energy domain noise reduction and virtual monoenergetic dual-energy CT. Radiology. 2015;276(2):562–70.
Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257(3):674–84.
Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11(7):860–8.
Nicola R, Shaqdan KW, Aran S, Mansouri M, Abujudeh HH. Contrast-induced nephropathy: identifying the risks, choosing the right agent, and reviewing effective prevention and management methods. Curr Probl Diagn Radiol. 2015;44(6):501–4.
Chung YE, You JS, Lee HJ, et al. Possible contrast media reduction with low keV monoenergetic images in the detection of focal liver lesions: a dual-energy CT animal study. PLoS One. 2015;10(7):e0133170.
Zhang LJ, Peng J, Wu SY, et al. Liver virtual non-enhanced CT with dual-source, dual-energy CT: a preliminary study. Eur Radiol. 2010;20:2257–64.
De Cecco CN, Buffa V, Fedeli S, et al. Dual energy CT (DECT) of the liver: conventional versus virtual unenhanced images. Eur Radiol. 2010;20(12):2870–5.
Robinson E, Babb J, Chandarana H, et al. Dual source dual energy MDCT: comparison of 80 kVp and weighted average 120 kVp data for conspicuity of hypo-vascular liver metastases. Investig Radiol. 2010;45(7):413–8.
Yamada Y, Jinzaki M, Tanami Y, Abe T, Kuribayashi S. Virtual monochromatic spectral imaging for the evaluation of hypovascular hepatic metastases: the optimal monochromatic level with fast kilovoltage switching dual-energy computed tomography. Investig Radiol. 2012;47(5):292–8.
Kulemann V, Schima W, Tamandl D, et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol. 2011;79(2):e1–6.
Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aube C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur Radiol. 2016;26(12):4595–615.
Zech CJ, Korpraphong P, Huppertz A, et al. Randomized multicentre trial of gadoxetic acid-enhanced MRI versus conventional MRI or CT in the staging of colorectal cancer liver metastases. Br J Surg. 2014;101(6):613–21.
Kim HJ, Lee SS, Byun JH, et al. Incremental value of liver MR imaging in patients with potentially curable colorectal hepatic metastasis detected at CT: a prospective comparison of diffusion-weighted imaging, gadoxetic acid-enhanced MR imaging, and a combination of both MR techniques. Radiology. 2015;274(3):712–22.
Colagrande S, Castellani A, Nardi C, Lorini C, Calistri L, Filippone A. The role of diffusion-weighted imaging in the detection of hepatic metastases from colorectal cancer: a comparison with unenhanced and Gd-EOB-DTPA enhanced MRI. Eur J Radiol. 2016;85:1027–34.
Siegelman ES, Chauhan A. MR characterization of focal liver lesions: pearls and pitfalls. Magn Reson Imaging Clin N Am. 2014;22:295–313.
Owen JW, Fowler KJ, Doyle MB, Saad NE, Linehan DC, Chapman WC. Colorectal liver metastases: disappearing lesions in the era of Eovist hepatobiliary magnetic resonance imaging. HPB (Oxford). 2016;18:296–303.
Hardie AD, Egbert RE, Rissing MS. Improved differentiation between hepatic hemangioma and metastases on diffusion-weighted MRI by measurement of standard deviation of apparent diffusion coefficient. Clin Imaging. 2015;39:654–8.
Shenoy-Bhangle A, Baliyan V, Kordbacheh H, Guimaraes AR, Kambadakone A. Diffusion weighted magnetic resonance imaging of liver: principles, clinical applications and recent updates. World J Hepatol. 2017;9(26):1081–91.
Papathanassiou D, Bruna-Muraille C, Liehn JC, et al. Positron emission tomography in oncology: present and future of PET and PET/CT. Crit Rev Oncol Hematol. 2008;72:239–54.
Maas M, Rutten IJ, Nelemans PJ, Lambregts DM, Cappendijk VC, Beets GL, Beets-Tan RG. What is the most accurate whole-body imaging modality for assessment of local and distant recurrent disease in colorectal cancer? A meta-analysis : imaging for recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2011;38:1560–71.
Cantwell CP, Setty BN, Holalkere N, et al. Liver lesion detection and characterization in patients with colorectal cancer: a comparison of low radiation dose non-enhanced PET/CT, contrast-enhanced PET/CT, and liver MRI. J Comput Assist Tomogr. 2008;32:738–44.
Wiering B, Adang EM, van der Sijp JR, et al. Added value of positron emission tomography imaging in the surgical treatment of colorectal liver metastases. Nucl Med Comm. 2010;31:938–44.
Agarwal A, Marcus C, Xiao J, et al. FDG PET/CT in the management of colorectal and anal cancers. AJR Am J Roentgenol. 2014;203:1109–19.
Pelosi E, Messa C, Sironi S, et al. Value of integrated PET/CT for lesion localization in cancer patients: a comparative study. J Comput Assist Tomogr. 2005;29(4):554–9.
Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D. Diagnostic accuracy and impact on management of 18F-FDG PET and PET/CT in colorectal liver metastasis: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging. 2015;42:152–63.
Lee DH, Lee JM, Hur BY, Joo I, Yi NJ, Suh KS, et al. Colorectal cancer liver metastases: diagnostic performance and prognostic value of PET/MR imaging. Radiology. 2016;280:782–92.
Lee DH, Lee JM. Whole-body PET/MRI for colorectal cancer staging: is it the way forward? J Magn Reson Imaging. 2017;45(1):25–35.
Beiderwellen K, Geraldo L, Ruhlmann V, et al. Accuracy of [18F]FDG PET/MRI for the detection of liver metastases. PLoS One. 2015;10:e0137285.
Kong G, Jackson C, Koh DM, et al. The use of 18F-FDG PET/CT in colorectal liver metastases–comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging. 2008;35:1323–9.
Adie S, Yip C, Chu F, Morris DL. Resection of liver metastases from colorectal cancer: does preoperative chemotherapy affect the accuracy of PET in preoperative planning? ANZ J Surg. 2009;79:358–61.
Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol. 2011;38:55–69.
Ruers TJ, Wiering B, Joost R, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with 18F-FDG PET: a randomized study. J Nucl Med. 2009;50:1036–41.
Fayad H, Schmidt H, Wuerslin C, et al. Reconstruction-incorporated respiratory motion correction in clinical simultaneous PET/MR imaging for oncology applications. J Nucl Med. 2015;56:884–9.
Sahani D, Mehta A, Blake M, Prasad S, Harris G, Saini S. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24:1367–80.
Catalano OA, Singh AH, Uppot RN, et al. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008;28:359–78.
Karlo C, Reiner CS, Stolzmann P, et al. CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol. 2010;75(1):e107–11.
D’Onofrio M, De Robertis R, Demozzi E, Crosara S, Canestrini S, Pozzi Mucelli R. Liver volumetry: is imaging reliable? Personal experience and review of the literature. World J Radiol. 2014;6:62–71.
Ferrero A, Vigano L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643–51.
van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36(1):25–34.
Ulla M, Ardiles V, Levy-Yeyati E, Alvarez FA, Spina JC, Garcia-Mónaco RD, De Santibañes E. New surgical strategy to induce liver hypertrophy: role of MDCT-volumetry to monitor and predict liver growth. Hepato-Gastroenterology. 2013;60:337–42.
Evrard S, Rivoire M, Arnaud J-P, et al. Unresectable colorectal cancer liver metastases treated by intraoperative radiofrequency ablation with or without resection. Br J Surg. 2012;99:558–65.
Acknowledgments
The authors would like to thank Anushri Parakh, MD, for her feedback during the editing process of the manuscript and assistance with the liver volumetry application.
Disclosures
Grant support was received from GE Healthcare and the Advisory Board of Allena Pharma; royalties are received from Elsevier Publishing for textbook on Abdomen Imaging.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shaqdan, K.W., Pourvaziri, A., Sahani, D.V. (2020). Role of Imaging in the Management of Patients with Potentially Resectable CRLM. In: Correia, M., Choti, M., Rocha, F., Wakabayashi, G. (eds) Colorectal Cancer Liver Metastases. Springer, Cham. https://doi.org/10.1007/978-3-030-25486-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-25486-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25485-8
Online ISBN: 978-3-030-25486-5
eBook Packages: MedicineMedicine (R0)