Abstract
The Ultrasonic velocity (U), density (ρ), and viscosity (η) have been measured experimentally for the ternary liquid mixtures of ortho methoxy phenol (omp), 1 butanol and n hexane at various temperatures viz., 303 K, 308 K and 313 K at constant frequency 2 MHz. for different concentrations ranges from 0.001 M to 0.01 M. The thermodynamic and acoustical parameters such as adiabatic compressibility (β), Rao constant (R), absorption coefficient (α/f2), internal pressure (πi), cohesive energy (CE), free volume (Vf), free length (Lf), acoustic impedance (z), available volume (Va), viscous relaxation time and Lenard Jones potential were calculated from the experimental data. The various excess properties including excess Ultrasonic velocity, excess acoustic impedance, excess free length, excess adiabatic compressibility, excess free volume and excess internal pressure were also computed. These parameters in accordance with their ultrasonic velocities corresponding to different concentrations of the mixture have been discussed. The molecular interactions were predicted based on the results obtained for ultrasonic velocities of different concentrations of the ternary mixtures at different temperatures.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Molecular interactions
- Ultrasonic velocity
- Ternary liquid mixture
- Excess adiabatic compressibility
- Internal pressure
- Excess acoustic impedance
1 Introduction
The ultrasonic study is one of the most important studies in diagnosing the interactions of the molecules [1]. In particular, the ultrasonic waves when it hits the system which is under investigation causes the interaction because of which there is the change with respect to structure, forces of attraction and thermochemical aspects in between the constituent molecules [2, 3]. The previous studies such as NMR, IR and UV by which the structural changes or nature of bonding can be enunciated for even small chemical reactions occurred but this ultrasonic study may be substantiated even for extremely less concentrated solutions (in order 10−4 M) since the ultrasonic waves are highly sensitive [4,5,6]. Also the special characters possessed by the donor which is ortho methoxy phenol in this ternary system of OMP+1 butanol+n hexane belongs to a derivative of phenolic ether whose chemical, physical properties and its applications stimulate the authors to probe the present study.
OMP used in many organic reactions including electrophilic aromatic substitution reaction, regioselective (hydroxy methylation) reactions etc., This compound provides best smell to whisky and other substances due to which it finds applications in perfumery industries and in the preparation of vanillin. The synthesis of vanillin involves unwanted inorganic by products and more cost, this prompted the author to carry out the present study.
Hydrogen bonding plays the most important role in molecular interactions. Eventually, the ternary system which is under investigations containing 1 butanol possess hydrogen bonding along with OMP (donor) wherein the ethereal oxygen has enough tendency to form hydrogen bonding that makes the interactions, strong.
Although a large number of investigations are carried in liquid mixtures having cyclo hexane (or) benzene as one of the components, it is found that no work has been made so for to measure the ultrasonic velocity of the ternary mixtures of ortho methoxy phenol, 1 butanol and n hexane. Furthermore, for many practical purposes it is necessary to predict the excess properties of a multi component liquid mixture from the properties of the pure components rather than the normal acoustical properties.
Therefore, the present study has been undertaken by the authors to provide useful information regarding the molecular interactions possessed by the system of ortho methoxy phenol, 1 Butanol and n- hexane at different temperatures.
2 Materials and Methods
The mixtures (OMP+1 butanol+n hexane) of various concentrations in mole fraction were prepared by taking analytical reagent grade and spectroscopic reagent grade chemicals with minimum assay of 99.9% and obtained from E Merck Ltd (India). All the component liquids were purified by the standard methods [7]. The density, viscosity, and ultrasonic velocity were measured for various concentrations viz., 0.001–0.01 M at different temperatures viz. 303 K, 308 K, and 313 K keeping constant frequency of 2 MHz. Ultrasonic velocity measurements were made using an ultrasonic interferometer (Model F-81, supplied by M/S Mittal Enterprises, New Delhi) with the accuracy of ±0.1 m·s−1. Water at desired temperature is circulated through the outer jacket of the double-walled measuring cell containing the experimental liquid. The densities of the mixture were measured using a 10-ml specific gravity bottle by relative measurement method with an accuracy of ± 0.01 kg·m−3. An Oswald viscometer (10 ml) with an accuracy of ±0.001 Ns·m−2 was used for the viscosity measurement. The flow time was determined using a digital racer stopwatch with an accuracy of ±0.1 s.
2.1 Theory and Calculations
Intermolecular free length (Lf), is calculated using the standard expression
Where K is a temperature dependent constant known as Jacobson constant and β is the adiabatic compressibility that can be calculated from the speed of sound (U) and the density of the medium (ρ) as
The relation for free volume in terms of ultrasonic velocity and the viscosity (η) of liquid as
Expression for the determination of internal pressure πi by the use of free volume as
Where b stands for cubic packing which is assumed to be 2 for liquids and K is a dimensionless constant independent of temperature and nature of liquids and its value is 4.281 × 109, T is the absolute temperature and Meff is the effective molecular weight. The viscous relaxation time was obtained using the relation
Gibbs free energy is calculated from the relation
Where τ is the viscous relaxation time, K the Boltzman’s constant, T, the absolute temperature and h is the Planck’s constant.
The acoustic impendance is given by,
Where U and ρ are the velocity and density of liquid, respectively.
In order to study the non-ideality of the liquid mixtures, namely excess parameters (AE) of all the acoustic parameter were computed by
Where Aid = ∑nAiXi, Ai is any acoustical parameters and Xi the mole fraction of the liquid components of I.
3 Results and Discussions
The experimentally measured values of density, viscosity and ultrasonic velocity for the mixtures at 303 K 308 K and 313 K are presented in Table 1. Table 2 represents the excess values of acoustic impedance and ultrasonic velocity. The excess adiabatic compressibility and excess free length are depicted in Table 3. Excess values of free volume and internal pressure for the mixture are presented in Table 4.
From the Table 1, it was observed that the ultrasonic velocity of the ternary liquid mixtures increases with increasing concentration of the mixture at 303 K which suggests that weak interactions due to dipole-dipole nature of the OMP and 1 butanol but at 308 K and 313 K an uneven trend is observed due to dipole-induced dipole nature of system while the viscosity and density is found to be decreases/increases with increasing concentration at all the working temperatures. But it is noted that when the temperature increases for a particular concentration of the mixture, the ultrasonic velocity decreases due to thermal agitation.
In order to understand more about the interaction between the components of liquid mixtures, it is necessary to discuss the same in terms of excess parameters [8] rather than the calculated values of normal acoustic properties like adiabatic compressibility, free length, free volume, internal pressure and acoustic impedance whose values were tabulated from Tables 5, 6, 7, 8 and 9 respectively for which no discussions were provided in this study. The Table 10 revealed Experimental values of Density, Viscosity and Ultrasonic velocity for each pure component present in the ternary mixture and those values are found to be in good agreement with the theoretical values. The excess properties can yield an idea about the linearity or non- linearity of the system as association or other type of interactions. It can be seen from Tables 2, 3 and 4.
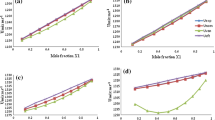
Table 2, the acoustic impedance increases with increasing concentration of the mixture at irrespective of the temperatures. The negative values show the strong interactions present between the component molecules. The corresponding plot is given in Fig. 1.
Table 2, it is noticed that the values of excess velocity increases with increase in concentration at all the temperatures under which investigation is done. Further the negative values predict the linear behavior of the system and weak to moderate interactions exhibits by the system. The corresponding plot is given in Fig. 2.
Table 3, the adiabatic compressibility is the decrease in volume per unit increase in pressure when no heat flows in or vice versa. It is indirectly proportional to the square of the density and ultrasonic velocity. But with respect to excess properties the values obtained are negative. This negative values strongly support the nature of the interactions which is found to be strong that attributed to the closed packed nature of the molecules. Similar conclusions were also arrived by Islam and Quadri [9]. In our present investigation the excess adiabatic values (magnitude alone) increase while the concentration increases at irrespective of the working temperatures under the influence of ultrasonic sound. The corresponding plot is given in Fig. 3.
Table 3, the Excess free length decreases with increase of concentration which predicts the presence of specific molecular interaction between the molecules of the liquid mixture. The adiabatic compressibility and free length are the deciding factors of the ultrasonic velocity in liquid systems. The values of excess inter molecular free length follows the same trend as that of βE (adiabatic compressibility). The values of excess inter molecular free length (L Ef ) are negative. The negative deviation of excess free length is an indication of the existence of strong interaction between the components. This is due to dipole – dipole, dipole-induced dipole and charge transfer complex formation owing to hydrogen bonding. The corresponding plot is given in Fig. 4.
Table 4, Free volume is the space available in the system when the constituent molecules come closure for interaction. In the present study the values of excess free volume show an uneven trend when the concentration of the mixture increases at irrespective of temperatures. But along the temperature at 308 K the values show higher than at 303 K and lower than at 313 K for all the concentration (except 0.001 M). This clearly indicated that there is a stronger interactions experienced by the molecules at 303 and 313 K rather than at 308 K for which weaker interactions are predicted. The corresponding plot is given in Fig. 6.
Table 4, the internal pressure is important factor in deciding the thermodynamic properties of liquids [10]. The internal pressure is the cohesive force, which is a resultant of force of attraction and force of repulsion between the molecules. Internal pressure also gives an idea of the solubility characteristics. In the present study the negative excess internal pressure π Ei over the entire range of the concentration of the system also supports the presence of interaction. The corresponding plot is given in Fig. 5.
4 Conclusions
The strong molecular association arises due to dipole-dipole interaction and the polar/non polar nature of different molecular entities in the mixture. The values of excess parameters support the existence of interactions due to hydrogen bonding and –I effect of –OCH3 group and the formation of charge transfer complex. In this present study it is observed that the chemical reaction will be allowed due to dipole-diplole interactions and dipole – induced dipole interactions between OMP and 1 - butanol in n hexane.
References
Arul, G., Palaniappan, L.: Ultrasonic study of 1-butanol in pyridine with benzene. Ind. J. Pure. Apple Phys. 43, 755–758 (2005)
Kannappan, V., Jaya Shanthi, R.: Ultrasonic studies of induced dipole-dipole interactions in binary liquids mixtures. Ind. J. Pure. Appl. Phys. 43, 750–754 (2005)
Kannappan, A.N., Rajendran, V.: Acoustic parameters of some ternary liquid mixtures. Ind. J. Pure. Appl. Phys. 30, 240–242 (1992)
Ali, A., Nain, A.A.K., Hyder, S.: Molecular interactions in formamide+isomeric butanols: an ultrasonic and volumetric study. J. Solution Chem. 32(10), 865–877 (2003)
Aralaguppi, M.I., Barragi, J.C.: Physicochemical and excess properties of the binary mixtures of methylcyclohexane + ethanol + propan-1-ol + propan-2-ol, + butan-1-ol, + 2-methyl-1-propanol or 3-methyl-1-butanol at T = 298.15, 303.15 and 308.15 K. J. Chem. Therm. 38, 434–442 (2006)
Niham, P.S., Kapade, V.M., Hasan, M.: Molecular interactions in binary mixtures of bromobenzene with normal alkanols C1-C4: an ultrasonic study. Ind. J. Pure Appl. Phys. 38, 170–173 (2000)
Furniss, B.S., Hannaford, A.J., Smith, P.W.G., Tatchell, A.R.: Vogel’s Text book of Practical Organic Chemistry, 5th edn. Longman Scientific & Technical, New York (1989)
Sridevi, U., Samatha, K., Viswanatha, Sarma A.: Excess thermodymamic properties in binary liquids. J. pure Appl. Ultrason. 26, 1–11 (2004)
Islam, M.R.: Quadri, ultrasonic velocity and viscosity of binary liquid mixtures. Thermo. Chim. Acta. 115, 335–340 (1987)
Rajendran, V., Marikani, A.: Investigation of thermodynamic properties of amine-alcohol mixtures at 303.15 K. Acoustics Lett. 18, 90–94 (1994)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Ibrahim, P.S.S., Chidambaravinayagam, S., Murugan, J.S., Jeyeakumar, J.E. (2019). Acoustical and Excess Properties on Ternary Liquid Mixtures of Ortho Methoxy Phenol, 1 Butanol and n-Hexane at Different Temperatures. In: Rajan, M., Anand, K., Chuturgoon, A. (eds) Proceedings of the International Conference on Nanomedicine (ICON-2019). ICON 2019. Springer Proceedings in Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-25135-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-25135-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25134-5
Online ISBN: 978-3-030-25135-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)