Abstract
Heat shock proteins are a significant class of proteins that play a pivotal role in cells undergoing stress (heat/chemical). They also perform certain important functions in cells during normal conditions. Majority of heat shock proteins execute the role of molecular chaperones by enabling the unfolded protein to fold properly. Heat shock protein 60 (HSP 60) plays a significant role in protein homeostasis inside the mitochondria. Aberrant expression of HSP 60 has been observed to result in various disorders in humans. It is also responsible for various cell survival and apoptotic pathways. A wide cell survival program is orchestrated by HSP 60 and this process can be particularly explored for carcinogenesis. Therefore it can act as a lucrative target for treatment of various types of cancers and other diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Molecular chaperones are ubiquitously present in all living organisms. They execute a significant role in protecting the cells from intrinsic and extrinsic stresses. In pathological conditions, these cyto-protective molecules assist in folding of proteins, repair, refolding of peptides that are misfolded, and also in degradation of proteins that cannot be repaired. They are constitutively expressed in ideal growth conditions; however, under heat shock or any other environmental insults, there is an up regulation of many of these chaperones. Thus such chaperones are also known as stress or heat shock proteins (Hsp ). Their primary function is in establishing conformational quality control of the proteome. These molecules achieve the conformational stability of non native polypeptides by interacting with them. A number of other cellular functions have also been attributed to these molecular chaperones. Based on amino acid composition, designated function and molecular weight, these molecular chaperones have been classified into two groups; the high molecular weight and the small molecular weight heat shock proteins. The high molecular weight heat shock proteins have a molecular weight ranging between 60 and 110 kDa. They are dependent on ATP and primarily operate by binding and enabling the nascent polypeptides to fold through ATP-dependent allosteric organization. The small molecular weight heat shock proteins have a molecular weight ranging between 15 and 43 kDa (Creagh et al. 2000). They perform their function in an ATP independent manner. This type of molecular chaperones have been observed to act in various embryonic development pathways, formation of respiratory organs such as cardiac muscles, as biomarkers for formation of tumor, in exercise induced stress, as well as in protein folding .
The elemental capability of Heat shock proteins to perpetuate the longevity of cell is correlative to suppression of caspase activation and apoptosis that could usually be based on their chaperoning capabilities (Beere 2004). They are known to enhance the survival of cell by inhibition of mitochondrial cell death initiated by apoptosome, stabilizing the survival effectors, and inactivating p53. For example, the anti apoptotic activity of HSP 90 might become selectively exploited in case of carcinoma (Whitesell and Lindquist 2005) and may execute a pivotal part in maintenance of tumor cell (Ghosh et al. 2008; Isaacs et al. 2003).
2 Heat Shock Protein 60
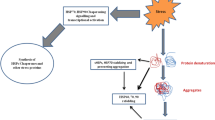
Heat Shock Protein 60 (HSP 60), along with its co-chaperonin HSP10 (Zhao et al. 2002), is an evolutionarily conserved stress response chaperone system in Eukaryotes. Human HSP 60 is also known as the 60 kDa chaperonin (Cpn 60). It is a homolog of bacterial GroEL. It is usually localized within the mitochondria and has been referred to as the mitochondrial molecular chaperone . HSP 60 and HSP10 (Fig. 4.1) also are known to occupy extracellular space, cytosol, and nucleus. The mitochondrial compartmentalized HSP 60 and HSP 10 together form a heptameric ring assembly. A folding chamber is formed due to stacking of these heptameric rings (Nielsen et al. 1999; Nisemblat et al. 2014). HSP 60 executes cardinal functions such as organelle biogenesis and folding/refolding of imported pre-proteins. A number of studies carried out on HSP 60 have pointed out their role in a number of tumor series correlating with disease outcome. The role of heat shock proteins has been discerned in regulation of a significant cancer related gene called survivin. Survivin participates in defence against apoptosis. It is also responsible for controlling mitosis in transformed cells.
3 HSP60 and Human Diseases
HSP 60 plays a significant role in protein homeostasis inside the mitochondria (Meng et al. 2018). A number of diseases have been associated to mutations that occur in HSP 60 (also called HSPD1), e.g. a rare and non treatable hereditary neurodegenerative disorder called spastic paraplegia SPG13 has been discerned to occur due to a mutation V981 in HSP 60. This disorder exhibits its effect by causing spasticity and weakness of lower limbs (Meng et al. 2018). This mutation results in reduction of refolding ability of HSP 60 client proteins. MitCHAP-60 disease is another neurodegenerative disorder that is autosomal recessive in nature. It is designated by hypomyelination and leukodystrophy in the brain. This devitalizing disease occurs due to mutation in HSP 60 (Magen et al. 2012). The underlying mechanism contributing to this disease is the less stability of D3G mutant in formation of heptameric and tetradecameric oligomers in comparison to the wild type. The decreased stability is coupled with deterioration in the refolding ability and ATPase activity.
In addition to the mutations occurring in the HSP 60, aberrant expression of HSP 60 has also culminated in various diseases. The involvement of in various inflammatory responses and immune reactions HSP 60 has also been pointed out (Pockley 2003). Thus these physiopathological pathways can be modulated by the level of expression of HSP60. For instance, the expression of HSP 60 in skin allografts can mediate the rejection by host for these allografts. The increased expression in this case, results in augmented dismissal in non obese diabetic mice. HSP 60 is able to play the role as an auto-antigen and the HSP 60 autoimmunity could be mediated in non obese diagnostic mice by injecting mouse HSP 60 peptides subcutaneously. This vaccination protects against allograft rejection. Mechanistically, this HSP 60 vaccination pathway apparently is involved in shifting the phenotype of the T cell in response to self HSP 60 from a pro-inflammatory Th1 kind of reciprocation to a Th2 regulatory type of reciprocation. The idea of endogenously present HSP 60 being able to execute the role of auto-antigen which further produces anti-HSP 60 antibody has also been corroborated in humans. People suffering from spondyloarthritis or periodontitis possess higher concentration of HSP 60 antibody than normal healthy volunteers. Nevertheless, concentration of human serum anti-HSP 60 appears to be disconnected of predicting kidney allograft rejection (Meng et al. 2018). The autoimmunity opposing HSP 60 might perform as a preventive measure against the occurrence of atherosclerosis with ageing. The functioning of HSP 60 as an auto-antigen also executes a role in the progression of various other autoimmune diseases (e.g., Hashimoto’s thyroiditis, myasthenia gravis, inflammatory bowel diseases, chronic obstructive pulmonary diseases (COPD)). The participation of HSP60 in various autoimmune disorders is extremely intriguing as one of the pharmacologically utilized immunosuppressant mizoribine targets HSP 60.
4 Heat Shock Proteins in Apoptosis
Apoptosis is a morphologically well defined, genetically programmed, cell death. It involves energy dependent biochemical mechanisms. This programmed cell death is a significant part of number of biochemical processes such as normal cell turnover, development and function of the immune system, hormone reliant atrophy, embryonic development and chemical induced cell death. Abnormal apoptosis i.e. too low or too high results in neurodegenerative diseases, ischemic damage, autoimmune disorders and numerous kinds of carcinomas in Homo sapiens. Numerous genes have been established that act as either positive or negative modulators of apoptosis. An increase in the production of inducible heat shock proteins results in hike in resistance to apoptosis that was induced by diversified cytotoxic agents and is engrossed in chemotherapeutic resistance of tumors and carcinogenesis (Creagh et al. 2000). Various studies in the past explored the underlying mechanism involved in apoptosis. The regulatory effects of heat shock proteins in apoptotic death are very well ascertained.
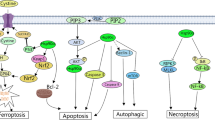
A strict modulation of proliferation, differentiation and death processes is essential for proper cell development and maintenance. Development of any abnormality in any of these processes culminates in serious complications including disorders like leukemia, autoimmunity, viral infections, allergic and neurodegenerative disorders. Aberrant expression of heat shock proteins has been implicated as one of the significant factors responsible for deregulation of development and maintenance of cell. The death of cells in eukaryotes can take place in two principally different ways; necrosis or apoptosis. Intense damage or harm to the cell leads to intense dysfunctioning of cell which subsequently leads to Necrosis. It is characterized by inert and disorderly processes that cause the cell to lose its control over the ionic transport. These disruptive processes results in cytolysis due to swelling of cell and the cellular organelles which takes place due to water uptake by the cell. A local inflammatory response is generated as the cell contents get released into the extracellular tissues present in the vicinity of the cell. On the other hand, apoptosis involves activation of intrinsic cascade leading to cell death. Apoptosis is genetically regulated and a number of external signals can modulate the cascade leading to cell death as shown in Fig. 4.2. This is a sequential step wise process that is initiated by shrinking of cells followed by blebbing of membranes, condensation of chromatin, fragmentation of inter-nucleosomal DNA and apoptotic body formation.
The occurrence of apoptosis or necrosis is dependent on the stress condition present in the cell. Necrosis takes place in harsh situations where the cell is unable to regulate the activation of stepwise programmed cell death i.e. the apoptosis. However, when the surrounding environment is not much harsh, the cells are able to initiate the cascade of apoptosis as shown in Fig. 4.3. Apoptosis is carried out by the aspartate specific cysteine proteases called caspases activity. These caspases split to make the target substrates either active or inactive. A cascade is initiated by the caspases during which preliminary caspases show an interaction with specific adaptor molecules which mediates their own autocatalytic processing. This further leads to cleavage and activation of the downstream caspases that execute the proteolytic disorganization of the cell (Fig. 4.2). Sometimes when the cell faces with a low enough level of stress, cells employ a distinct response pathway to sustain. This process is characterized by inhibition of synthesis of proteins inside the cells; however expression of heat shock proteins is simultaneously induced as a consequence of which the cell enters a thermo tolerant phase transiently. These proteins are classified on the basis of their size e.g. the HSP 27, HSP 60, HSP 70, HSP 90 and HSP 100 families (Beere 2004).
The role of heat shock protein in stress regulation is dependent on a single elemental feature which is their capacity of interaction with substrates that are proteins/polypeptides in nature. HSP 70 and HSP 90 proteins are composed of two parts: a highly conserved amino end that comprises the ATPase and a Carboxy end which constitutes the polypeptide-binding site. There are four amino acids, EEVD, at the carboxy end which is responsible for mediating the inter-domain interaction and binding ability of proteins, and are significant for modulating defence against heat induced stress. On the other hand, HSP27 does not possess an ATPase domain. Mitogen activated protein (MAP)-kinase dependent phosphorylation and self-oligomerization regulate the defense against stress in this case.
The chaperoning property of the heat shock proteins is regulated by a reaction cycle of binding of ATP, hydrolysis and nucleotide exchange to initiate a series of rapid association and dissociation cycles in-between the Heat shock proteins and their target polypeptides. The heat shock proteins bound to ATP, bind and release peptide quickly, which results in decreased overall affinity. On the other hand, the heat shock proteins bound to ADP, bind to the peptide at a slower pace but have more stable interactions. Presence of additional co factors/co chaperones responsible for catalyzing the conversion between ATP and ADP further regulates the capacity of these chaperones. HSP 40 (HDJ-1 and HDJ-2), HSP 70-interacting protein (Hip) and HSP 70-HSP 90 organizing protein (Hop) represent such classes of co-chaperones.
When the stress is eliminated from the cell surrounding, there is again a depreciation in the quantum of heat shock proteins and their number is maintained as present during the normal conditions of the cell. However, paradoxically the presence of high level of heat shock proteins during these stress conditions makes the cell inimical to the presence of various toxic agents. In case of tumor cells, the quantum of heat shock proteins is quite high as they are constitutively expressed at increased level. This increased level of heat shock proteins in tumor cells proves anomalous as these cells are protected from toxic agents which results in development of resistance in these tumor cells against chemotherapies and carcinogenesis. The elevated levels of heat shock proteins apparently lead to rapid growth of tumors that are resistant to therapies.
5 HSP60 in Apoptosis of Tumor Cells
HSP 60 coupled with its co chaperone HSP10, has been discerned to be evolutionarily conserved chaperone that is involved in defending cell against various stresses. It is mostly present inside mitochondria and executes a significant part in biogenesis of the cellular organelle and folding/refolding of proteins that are imported inside the mitochondria (Asea and Brown 2008). Thus it’s able to prevent aggregating of proteins which is essential for the cells in stress environment. There is an upregulation of HSP 60 during different kind of human cancers. It displays anti-apoptotic properties and supports formation, progression, invasion and metastases of tumor, progression. It is also responsible for therapeutic resistance and decreased survival. HSP 60 is deposited on the exterior of mitochondria during carcinogenesis, in the cytosol, plasma membrane, and in secretory vesicles thereby imparting protection to tumor cells from external environmental stress, which further promotes proliferation of cells. HSP 60 also shows participation in permeabilization of mitochondrial membrane by interacting with the cyclophilin D which is responsible for regulation of mitochondrial permeability transition pore.
HSP 60 mediates a cyto-protective cascade based on imparting stability to the quantum of survivin and restraints the functioning of p53 as shown in Fig. 4.4. On the other hand, acute extraction of HSP 60 leads to depreciation in the survivin reserves present in the mitochondria. Survivin is particularly designated for inhibition of the programmed cell death i.e. apoptosis, simultaneous increase in p53 expression, and activating p53 dependent apoptosis in tumor cells. These cyto-protective properties of HSP 60 is carefully explored in tumors in vivo, wherein HSP 60 is subjected to selective upregulation, in comparison to normal cells, and deficit of HSP 60 in normal cells is not linked to dysfunctioning of mitochondria or death of cells.
Survivin is established as a cardinal carcinogenic gene with binary function in division of cell and reticence of apoptosis. A crucial prerequisite of this procedure is the existence of a reserve of survivin occupying the mitochondria, generally in tumor cells, and excreted into the cytoplasm in return to stimulus of cell death. A number of evidences point out that a specific molecule designated for inhibition of apoptosis is provided by the survivin present in the mitochondria, thereby straightly exasperating the growth of tumor in vivo. This cascade is modulated by isolated phosphorylation taking place in mitochondria and associating differentially with the X chromosome-linked inhibitor of apoptosis which is an anti-apoptotic cofactor. Although there is an absence of a cleavable, amino end mitochondrial import sequence, survivin is vigorously transported to the mitochondrial compartment. A number of molecular chaperones associated to survivin occupying the cytoplasm might also be contributing to this cascade. These molecular chaperones include HSP 90 and/or AIP molecules that are involved in the import of pre-proteins into the mitochondria. Inside the mitochondria, survivin might need to associate with HSP 60 to form a complex in order to reinstate the optimum refolding after it’s translocated across the membrane enveloping the mitochondria. This step of translocation includes unfolding of protein. Also, reports have pointed out that siRNA depletion of HSP 60 leads to destabilizing of quantum of survivin present and approximately total depletion of the deposits of survivin present in the mitochondria, thereby abolishing the comeback contrary to the apoptosis.
Besides, stabilizing the level of survivin present in the mitochondria, HSP 60 also imparts cyto-protection by another mechanism which includes the forming of a complex by association of HSP 60 with p53 that inhibits the functioning of p53 tumor cells. Molecular chaperones have precession in inhibiting functioning of p53. Mortalin, a mitochondrial HSP 70, has been reported to participate in binding to and sequestering p53 in the cytoplasm, thereby inhibiting its translocation into the nucleus and centrosomes, wherein such interplay invalidates a checkpoint for duplication of centrosomes that is dependent on p53. Modulation of p53 by HSP 60 is not implicated in bringing about modifications in the Mdm-2 which is a modulator of p53. Therefor the role of HSP 60 is different in comparison to role of inducible HSP 70 that is involved in counteracting the cell death brought about by p53. Based on their interaction with various pathways responsible for maintaining tumor (Whitesell and Lindquist 2005) and their frequent over-expression in carcinoma, molecular chaperones are robustly explored for new cancer diagnostics (Isaacs et al. 2003).
6 Regulation of Apoptosis by Mitochondrial Lon
Human Lon protease is a protein occupying the mitochondrial matrix. It performs a number of functions like degradation of proteins, binding of mitochondrial DNA, and chaperoning activity. Lon is a protease that has emerged as a significant modulator of tumorigenesis accorded by the mitochondria. There is increase in the expression of Lon in the cancer cells. It is an extremely conserved ATPase that is responsible for a number of cellular functions. It plays a cardinal role in ATP dependent proteolytic binding of DNA and chaperoning activity. Lon in eukaryotes participates in the protein quality control mechanism. It is also employed in significant cellular processes such as functioning of mitochondria, homeostasis and biogenesis. As already discussed, proteins are susceptible to getting inactivated by virtue of misfolding, unfolding, or aggregation when cells undergo stress conditions. In such circumstances, protein quality control mechanism, chaperones and proteases, protect the cell and enable its proper functioning. The two components coordinate to grant stability to the misfolded proteins and enable their refolding. They even expel these aggregated/misfolded proteins to evade the detrimental consequences of aggregation of proteins (Kao et al. 2015).
Mitochondria coordinates the life and death of cells. Thus, it employs a crucial regulation of signaling that enables the cell to survive, especially in the intrinsic pathway of senescence. The quantum of Lon present is responsible for regulating mitochondrial actions responsible for the fate of cell. Downregulation of Lon causes the mitochondria to lose its function. It also results in early embryonic mortality, decrease in proliferating cells, and programmed cell death. On the other hand, upregulation of Lon is significant for the cancer cells to survive. The upregulation of Lon also plays a role in tumorigenesis by modulating response to oxidation induced stress (Cheng et al. 2013; Quiros et al. 2014; Gibellini et al. 2014). The signature stress conditions of cancer cells including hypoxia, oxidative and mitochondrial unfolded protein induces the expression of Lon protein (Kao et al. 2015. Lon upregulation takes place in hypoxic conditions due to factor-1α (which is induced by hypoxia). It takes part in the pathway that responds to lower level of oxygen availability. The Lon protein is responsible for enabling cancer cells to get accustomed to hypoxia. Lon protein is also capable of showing chaperoning activity besides the proteolytic activity. Lon is also involved in promotion of assembling 4 to 1 subunits of cytochrome c oxidase (COX), (Hori et al. 2002; Fukuda et al. 2007; Ngo and Davies 2009) signifying the chaperoning properties of Lon protein in yeast and mammalian cells. Therefore, Lon protein occupying the mitochondria might be a protein chaperone to enable cells to sustain and accommodate to a number of stress conditions associated to oncogenesis.
7 Mitochondrial Lon in Regulation Apoptosis via the Interaction with HSP 60
Lon has been observed to increase the stability of proteins and levels of heat shock proteins HSP 60 and mitochondrial HSP 70 in response to cellular stress. Also, during increased stress the enhanced Lon expression was linked to lowering or enhancement in cleaved caspase 3 after hydrogen peroxide treatment, signifying that when Lon is upregulated it defends the cells from senescence/apoptosis and when Lon is downregulated it causes induction of programmed cell death while recovering from stress induced due to oxidation. It has been suggested when the Lon is upregulated, there is enhancement in the stability of proteins HSP 60–mitochondrial HSP 70 which subsequently provides protection to cell from apoptosis due to stresses, which is achieved when Lon binds to HSP 60 or mitochondrial HSP 70. The down regulated expression of HSP 60 by shRNA showed that the signals of pro apoptotic proteins such as Bax, cleaved caspase 3, cleaved PARP, p53, and phosphorylated p53Ser46, were subjected to activation after UV or hydrogen peroxide treatment in FADU cells, denoting that the treated cells underwent apoptosis. The quantum of pro apoptotic proteins decreases and apoptosis is suppressed in the cells that over-express Lon post stress that suggests the significance of Lon protein for regulating apoptosis under environmental stresses. On the other hand, knocking down the HSP 60 expression, increases the quantum of apoptotic proteins in the cells that over-expressing Lon protein in stress. These observations are indicative of the role played by HSP 60 during the stress conditions in the regulation of apoptotic processes which is mediated by Lon (Kao et al. 2015). The part played by HSP 60 in apoptosis modulated by the Lon protein has also been observed using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay. The cells that were TUNEL positive cells are lesser in number in cells where there is over-expression of Lon cells. Also, the TUNEL positive cells were greatly elevated in the cells that over-expressed Lon when HSP 60 was knocked down. These observations are indicative of the fact that increase in Lon protein defends cell from apoptotic pathway in stress conditions by virtue of interplay with and stabilizing HSP 60.
Mitochondrial Lon is responsible for modulating the organelle function, cell proliferation, and apoptosis. The increase in level of Lon is cardinal for survival of cells undergoing oxidative and hypoxic stress. Decrease in Lon results in decrease in number HSP 60 and mitochondrial HSP 70 in cell undergoing oxidative stress, depicting that the stability of proteins HSP 60–mitochondrial HSP 70 complex is reliant on Lon. Also, loss of HSP 60 results in the instability of Lon–mitochondrial HSP 70 interaction. Through binding with HSP 60–mitochondrial HSP 70 complex, increased mitochondrial Lon suppresses apoptosis and is conducive for survival of cell undergoing environment induced stresses, by which they are inclined to perform significant action in a cyto-protective chaperone network. On the basis of analysis of Lon related proteins purified from in solution digestion shotgun proteomics, these, significant mitochondrial proteins have been grouped into five functional classes: (1) mitochondrial chaperones, (2) cellular metabolism and energy, (3) Redox regulation, (4) cell death and survival, and (5) mitochondrial DNA stability. NDUFS8 (which is a NADH-ubiquinone iron-sulfur (Fe-S) 23 kDa subunit) HSP 60, and mitochondrial HSP70 among these are able to bind to Lon. Lon interplays with NDUFS8 of mitochondrial complex I that participates in the generation of ROS under induction by Lon.11 NDUFS8 is a subunit of mitochondrial NADH oxido-reductase (Complex I) that takes part in transferring of electrons from NADH to the respiratory chain in the mitochondrial inner membrane. Also, mitochondrial Fe-S proteins in complex I, II, and III, that is, SDHB (complex II) and Rieske (complex III), and COX 4–1 in complex IV are potential substrates of Lon protease. In case of yeast Lon modulates transport of electrons by disintegrating the subunits of complexes III–V in yeast.
8 Mechanism of Mitochondrial Lon Regulated Apoptosis
HSP60 coupled with mitochondrial HSP 70 is a binding associate. The quantum and stability of HSP 60 and mitochondrial HSP 70 is dependent on the quantum of Lon undergoing oxidative stress. Also, the capability of elevated Lon-deteriorated apoptosis significantly depends upon HSP 60. The mechanism involved in cell sustenance is modulated by Lon by the maintaining the stability of HSP 60 and mitochondrial HSP 70. HSP 60 displays anti-apoptotic as well as pro-apoptotic properties which is dependent on the background of cell type and situation. Mitochondrial HSP 60 is able to inhibit apoptotic pathway by enhancing the stability of survivin, refraining functioning of p53, opposing mitochondrial permeability transition which is cyclophilin D-dependent, and conserving generation of ATP for the complex IV. Also, mitochondrial HSP60 is an inducer of apoptotic pathway by increasing the maturity level of pro-caspase-3. Firstly, mitochondrial Lon stabilizes HSP60 and mitochondrial HSP 70 allowing them the execution of anti-apoptotic properties. Infact, over-expression of HSP 60 and mitochondrial HSP 70 in human tumor cells, has been depicted to oppose p53 and abrogate its apoptotic properties in cancer cells. Secondly, enhanced Lon stabilizes the complex made by association of HSP 60, mitochondrial HSP 70 and Lon to sequestrate HSP 60 in mitochondria, protecting it from activating pro-caspase-3 and cytoplasmic translocation that makes the cell sensitive to apoptotic pathway. Thirdly, in order to maintain the integrity of mitochondria, Lon protein depicts chaperoning properties in order to show cooperation with HSP 60 and mitochondrial HSP 70 complex and maintaining the protein homeostasis in mitochondria during stresses, which is backed by mitochondrial HSP 70 that might assist Lon chaperone misfolded proteins to conserved mitochondria functioning in yeast (Kao et al. 2015). Thus, the loss in the equilibrium between Lon and HSP 60 in the complex formed by Lon, HSP 60 and mitochondrial HSP 70 affects the apoptotic activation and the sustenance of cell. These quanta of Heat shock proteins are raised in different kinds of human tumors, which exhibits significant chaperone functioning to enhance the survival of stress in stress conditions.
9 Heat Shock Protein 60 Modulators and Inhibitors
As the complex formed by HSP 60 and HSP 10 chaperone is significant for maintenance of homeostasis in mitochondria. It executes a cardinal role in cardiovascular disorders including autoimmune diseases and carcinomas. Development of small molecules that can act as regulators of HSP 60 by targeting it can be used in various therapeutic treatment of diseases. Such small molecule regulators act as significant tools to further throw light on the biological functioning of HSP 60 in several contexts (Meng et al. 2018). A number of natural and synthetic compounds have flourished that can target HSP 60. Mechanistically, the HSP 60 inhibitors developed are grouped into two classes:
-
Type I: These inhibitors are responsible for blocking binding and hydrolysis of ATP. This affects the ATP-dépendent conformational changes as a result of which the refolding activity of complex formed by the HSP 60 and HSP 10 is inhibited.
-
Type II: These inhibitors incorporate compounds that show covalent reactions with particular cysteine residues in HSP 60.
Mizoribine is the first small organic molecule that has been known to inhibit HSP 60 inhibitor. It is an imidazole nucleoside antibiotic (Fig. 4.5) and has been isolated from Eupenicillium brefeldianum. Though it lacks anti-microbial activity, it does have potential of acting as immunosuppressor (Mizuno et al. 1974) as a result of which it is widely used in case of renal transplantation (Tajima et al. 1984). The direct binding of mizoribine inhibits the chaperoning properties of the binary complex formed by HSP 60 and HSP 10. Mizoribine also blocks the ATPase activity of HSP 60, which simultaneously results in more stable interaction of HSP 10 and HSP 60.
Epolactaene (Fig. 4.6) is another naturally found compound that inhibits the activity of HSP 60. Epolactaene (2, 2) has been isolated from the fungal strain Penicillium sp. BM 1689-P. It is capable of promoting outgrowth of neurites in SH-SY5Y cells (Meng et al. 2018). Another naturally found molecule capable of inhibiting the activity of HSP 60 is myrtucommulone (MC), (Fig. 4.7). It is a non-prenylated acylphloroglucinol possessing different bio-activities, such as anti-bacterial, anti-oxidant, anti-inflammatory, and anti-tumor properties. It has been shown to affect the mitochondria that have been isolated from human leukaemia cells, and it also alters the mitochondrial functioning at sub-micromolar concentrations, which includes loss of mitochondrial membrane potential.
10 Conclusions
The cyto-protective property might be a general characteristic of various molecular chaperones, including HSP 60. This eventually results in enhancing an anti-apoptotic threshold in case of tumor cells in vivo. Interestingly, this property of molecular chaperones is particularly exploited in transformed cells and not in normal tissues. Additionally, a stark contrast in expression of the molecular chaperones, i.e., HSP 60, in case of oncogenesis in comparison to normal tissues in vivo, depicts that there are several other factors contributing to the selective use of this cascade in tumor cells. These might incorporate qualitative variations in chaperoning properties, as has been exhibited by HSP 90 ATPase function, or link with cancer genes distinctively expressed in cancer, e.g. for functional survivin-chaperone complexes . Despite the cyto-protection provided by these chaperones might enhance the sustenance of tumor cells and favor drug resistance, the distinctive expression or functional exploitation of this cascade in tumor cells might be suitaleble for wider, chaperone-administered anticancer approaches. This concept is further validated by molecular or pharmacologic targeting of complexes between survivin and HSP 90, mortalin and p53, and HSP 60 and survivin/p53 which is reconciled with selective induction of mitochondrial cell death in tumor cells sans any impact on normal cell types, including hematopoietic progenitor cells (Fortugno et al. 2003; Kang and Altieri 2006; Plescia et al. 2005). The concentration of HSP 60 and mitochondrial HSP 70 is reliant on Lon, and Lon-modulated programmed cell death is monitored by the HSP 60–mitochondria HSP 70 complex. Lon interactome, states that a Lon is a versatile protein involved in the regulation of mitochondrial chaperones, survival and death of cells, and mitochondrial DNA stability.
Abbreviations
- COX:
-
cytochrome c oxidase
- Cpn:
-
chaperonin
- Hip:
-
HSP 70-interacting protein
- Hop:
-
HSP 70-HSP 90 organizing protein
- HSP:
-
heat shock proteins
- MAP:
-
mitogen activated protein
- PARP:
-
Poly (ADP ribose) polymerase
- SPG:
-
spastic paraplegia
- shRNA:
-
short hairpin RNA
- TUNEL:
-
transferase-mediated dUTP nick-end labeling
References
Asea AAA, Brown IR (2008) Heat shock proteins and the brain: Implications for neurodegenerative diseases and neuroprotection. Springer Nature
Beere HM (2004) The stress of dying: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651
Cheng CW, Kuo CY, Fan CC, Fang WC, Jiang SS, Lo YK, Wang TY, Kao MC, Lee AYL (2013) Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis 4:e681
Creagh EM, Sheehan D, Cotter TG (2000) Heat shock proteins – Modulators of apoptosis in tumor cells. Leukemia 14:1161–1173
Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, Sessa WC, Altieri DC (2003) Regulation of survivin function by Hsp90. Proc Natl Acad Sci U S A 100:13791–13796
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129:111–122
Ghosh JC, Takehiko Dohi T, Kang BH, Altieri DC (2008) Hsp60 regulation of tumor cell apoptosis. J Biol Chem 283:5188–5194
Gibellini L, Pinti M, Boraldi F, Giorgio V, Bernardi P, Bartolomeo Nasi M, Biasi SD, Missiroli S, Carnevale G, Losi L, Tesei A, Pinton P, Quaglino D, Cossarizza A (2014) Silencing of mitochondrial Lon protease deeply impairs mitochondrial proteome and function in colon cancer cells. FASEB J 24:5122–5135
Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D, Tohyama M, Stern DM, Ogawa S (2002) Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J Cell Biol 157:1151–1160
Isaacs JS, Xu W, Neckers L (2003) Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 3:213–217
Kang BH, Altieri DC (2006) Regulation of survivin stability by the aryl hydrocarbon receptor-interacting protein. J Biol Chem 281:24721–24727
Kao T-Y, Chiu Y-C, Fang W-C, Cheng C-W, Kuo C-Y, Juan H-F, Wu S-H, Lee AY-L (2015) Mitochondrial Lon regulates apoptosis through the association with Hsp60–mtHsp70 complex. Cell Death Dis 6:e1642
Magen MJ, David JA, Michelhaugh SK, Schmidt CJ, Bannon MJ (2012) Increased heat shock protein 70 gene expression in the brains of cocaine-related fatalities may be reflective of post-drug survival and intervention rather than excited delirium. J Forensic Sci 57(6):1519–1523
Meng Q, Li BX, Xiangshu X (2018) Toward developing chemical modulators of Hsp60 as potential therapeutics. Front Mol Biosci 5:35
Mizuno K, Tsujino M, Takada M, Hayashi M, Atsumi K (1974) Studies on bredinin. I. Isolation, characterization and biological properties. J Antibiot 27:775–782
Ngo JK, Davies KJ (2009) Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med 46:1042–1048
Nielsen KL, Lennan NM, Masters M, Cowan NJ (1999) A single-ring mitochondrial chaperonin (Hsp60-Hsp10) can substitute for GroEL-GroES in vivo. J Bacteriol 181:5871–5875
Nisemblat S, Parnas A, Yaniv O, Azem A, Frolow F (2014) Crystallization and structure determination of a symmetrical ‘football’ complex of the mammalian mitochondrial Hsp60–Hsp10 chaperonins. Acta Crystallogr F Struct Biol Commun 70:116–119
Plescia J, Salz W, Xia F, Pennati M, Zaffaroni N, Daidone MG, Meli M, Dohi T, Fortugno P, Nefedova Y, Gabrilovich DI, Colombo G, Altieri DC (2005) Rational design of shepherdin, a novel anticancer agent. Cancer Cell 7:457–468
Pockley AG (2003) Heat shock proteins as regulators of the immune response. Lancet 362:469–476
Quiros PM, Espanol Y, Acin-Perez R, Rodriguez F, Barcena C, Watanabe K, Calvo E, Loureiro M, FernAndez-Garcia MS, Fueyo A, VAzquez J, Enríquez JA, Lopez-Otin C (2014) ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep 8:542–556
Tajima A, Hata M, Ohta N, Ohtawara Y, Suzuki K, Aso Y (1984) Bredinin treatment in clinical kidney allografting. Transplantation 38:116–118
Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5:761–772
Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ (2002) A mitochondrial cell response in mammalian cells. EMBO J 21:4411–4419
Acknowledgements
The authors acknowledge the assistance and support provided by Amity Institute of Biotechnology, Amity University, Noida, Uttar Pradesh, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chaudhuri (Chattopadhyay), P., Rashid, N. (2019). HSP60 as Modulators of Apoptosis. In: Asea, A., Kaur, P. (eds) Heat Shock Protein 60 in Human Diseases and Disorders. Heat Shock Proteins, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-030-23154-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-23154-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23153-8
Online ISBN: 978-3-030-23154-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)